
GCMS-Based Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Triterpenoid-Rich Hydroethanolic Extract and Fractions of Ficus Sur (Forrsk) Stem Bark
Uchenna Benjamin Okeke*, Patrick Igbinaduwa, Joseph Adetunji Aladesanmi, Vuyisa Mzozoyana, and Ibrahim Oluwatobi KehindePublished Date : October 21, 2025
DOI : https://doi.org/10.12982/NLSC.2026.012
Journal Issues : Number 1, January-March 2026
Abstract Ficus sur is employed widely in folklore medicine for the mitigation of several disease conditions. The study aims to validate the phytochemical composition, antioxidant, and anti-inflammatory activities of hydroethanolic extract and fractions (dichloromethane, ethyl acetate, and n-butanol) of F. sur stem bark. Phytochemical profiling of the extract and fractions was carried out by established methods and gas chromatography-mass spectroscopy (GC-MS) technique, while their total phenolic (TPC) and flavonoid contents (TFC) were assessed by the Folin-Ciocalteu reagent and aluminium chloride complex formation methods, respectively. In vitro antioxidant activity was carried out by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging, ferric reducing antioxidant power (FRAP), and total antioxidant capacity (TAC) assays. The carrageenan-induced paw oedema protocol was employed in the evaluation of the anti-inflammatory activity of the extract and fractions. Phytochemicals such as terpenoids, phenolics, flavonoids, and glycosides were found to be present in the extract and fractions. However, GC-MS analysis of the extract revealed the presence of majorly oleanane-type and phytosterol triterpenoids, while polyphenolic compounds and fatty acids existed in small abundances. The extract and fractions showed considerably high TAC and TFC values, and substantial antioxidant activities in the DPPH, FRAP, and TAC antioxidant assays. The groups treated with n-butanol fraction and the standard drug (diclofenac) exhibited the highest anti-inflammatory effects with maximum percentage inhibitions of 62.05% and 75.63% of paw oedema in the rats, respectively. The study revealed that F. sur stem bark possessed rich content of triterpenoids and polyphenols, and demonstrated considerable antioxidant and anti-inflammatory properties as suggested by ethnomedicine.
Keywords: Anti-inflammatory activity, Antioxidant, Phytochemical screening, Flavonoids, Phenolic compounds
Graphical Abstract:

Citation: Okeke, U.B., Igbinaduwa, P., Aladesanmi, J.A., Mzozoyana, V., and Kehinde, I.O. 2026. GCMS-based phytochemical profiling, antioxidant and anti-inflammatory activity of triterpenoid-rich hydroethanolic extract and fractions of Ficus Sur (Forrsk) stem bark. Natural and Life Sciences Communications. 25(1): e2026012.
INTRODUCTION
Plants, with their rich and diverse array of natural products, are a vital source of various therapeutic agents that are continuously being explored to develop novel drugs (Dzobo et al., 2022). Some pharmaceutical products originate from natural products after thorough in vitro and in vivo pharmacological evaluation and validation, which is required before they are approved for use against various disease conditions and disorders (Chaachouay et al., 2024). Phytochemicals, such as alkaloids, flavonoids, lignins, tannins, and terpenoids, are constituent bioactive non-nutrient plant compounds found in plants that possess intriguing biological properties, which include antioxidant, antimicrobial, anti-inflammatory, and anticancer actions, as seen in several herbal medicines, some of which are now formulated as alternative medicine products. Polyphenols, terpenoids, and other associated secondary metabolites significantly reduce the damaging effects of reactive oxygen species (ROS), which are produced from oxidative stress (Rudrapal et al., 2022).
During metabolic processes, living cells produce free radicals as a natural part of their functions (Phaniendra et al., 2015). These free radicals, which can be derived from oxygen or nitrogen, are highly reactive and can damage proteins, lipids, carbohydrates, and DNA (Lobo et al., 2010), ultimately having a detrimental effect on human health. When there is an imbalance between reactive oxygen species and antioxidants, it can lead to oxidative stress, which causes cellular damage and can result in a variety of diseases in humans, including cancer, diabetes, cardiovascular issues, and infertility.
Antioxidants are substances that prevent, inhibit, or reduce oxidation processes. Phytochemicals such as polyphenols, tannins, terpenoids, and some alkaloids can delay or inhibit the production of free radicals, making them a free radical scavenger (Pizzino et al., 2017). Due to their capability to neutralise free radicals and shield the human body from diseases linked to oxidative stress, plants are regarded as vital sources of natural antioxidants that promote health (Lobo et al., 2010; Al-Breiki et al., 2018; Chaachouay and Zidane, 2024). These secondary plant metabolites found in medicinal plants significantly influence essential physiological and biochemical functions in humans (Kumar et al., 2023; Zandavar et al., 2023). Plant-derived polyphenolic and terpenoid compounds are crucial for dietary and nutraceutical applications due to their powerful radical scavenging capabilities (Pandey and Rizvi, 2009; Tsao, 2010).
Inflammation is a defensive mechanism triggered by harmful foreign stimuli, such as pathogens, viruses, dust particles, irritants, and damaged cells, to initiate healing (Chen et al., 2017). It comprises various steps, starting with an induction phase, continuing with a peak of inflammation, and ending with the resolution phase (Schett and Neurath, 2018). The induction phase is needed for effective host defence. It is caused due to external and endogenous noxious stimuli resulting from mechanical, chemical, or biological cell destruction (Harvanová et al., 2025). The resolution phase is necessary for reducing inflammation and restoring cell homeostasis after removing the noxious stimuli. During an inflammatory state, immune cells experience increased ROS levels, producing oxidative stress (Hussain and Harris, 2007; Reuter et al., 2010). Elevated ROS release can activate a signalling cascade within the intracellular matrix, amplifying the gene expression of proinflammatory mediators (Lingappan, 2008). Several studies have linked inflammation to oxidative stress that results in numerous highly interrelated pathophysiological occurrences (Biswas, 2016). Inflammation has been linked to the onset and progression of various chronic conditions, including cardiovascular and gastrointestinal diseases, diabetes, cancer, infertility, and arthritis (Chen et al., 2017; Furman et al., 2019). The use of anti-inflammatory agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), has been successful in preventing or treating most pathological inflammatory processes. Although these medications are effective and easily accessible, they can cause negative outcomes, including gastric ulcers, pain, hepatic and renal dysfunctions, hypersensitivity, and central nervous system effects (Drini, 2017; Bindu et al., 2020).
Ficus sur Forrsk, a species of the genus Ficus, Moraceae family, commonly known as broom cluster fig, cape fig, and bush fig, is a fast-growing deciduous or evergreen tree, widely distributed in tropical African countries like Angola, Cameroon, Ghana, Nigeria, and South Africa (Murugesu et al., 2021; Sieniawska et al., 2022). It has many applications in traditional medicine, where different parts of the plant have been used for the treatment of several disease conditions, such as eye problems, pains, sexually transmitted diseases, anti-emetic, urinary problems, swellings, infertility, fever, cough, and gastrointestinal disorders (Sieniawska et al., 2022). Different parts of F. sur from preceding studies revealed the existence of numerous classes of secondary plant metabolites, which include alkaloids, triterpenes, flavonoids, and glycosides (Saloufou et al., 2018; Ogunlaja et al., 2022; Sieniawska et al., 2022). Also, several biological studies carried out on the different parts of F. sur include antioxidant and antibacterial activities (Adebayo-Tayo and Odeniyi 2012; Ramde-Tiendrebeogo et al., 2012; Omoregie and Okugbo, 2014; Eluka et al., 2015; Akachukwu and Uchegbu, 2016), diuretic activity (Ayele et al., 2020), larvicidal activities (Olayemi et al., 2017), and anticonvulsant studies (Abdulrazaq et al., 2018). Previous studies, particularly regarding the total phenolic and flavonoid levels, as well as the antioxidant and anti-inflammatory properties of the plant sample, has predominantly focused on the crude extract. Accordingly, this study intended to investigate the phytochemical composition, antioxidant capacity, and anti-inflammatory effects of the extract and solvent fractions from Ficus sur stem bark.
MATERIALS AND METHODS
Equipment and instruments
Analytical weighing balance (Metler Toledo, USA), Rotary Evaporator (DLAB RE-100 Pro, China), Thermostat oven (DHG-9123-A, China), Gas chromatograph (7890B, Agilent Technologies), Magnetic heater with stirrer (Biobase, China), UV-VIS spectrophotometer (Biobase BK-D560, China), Fourier Transform Infrared spectrophotometer (FTIR) (Thermo Fisher, USA).
Chemicals and reagents
n-hexane (Loba Chemie, India), Dichloromethane (GHTech, China), Ethyl acetate (Loba Chemie, India), Methanol, analytical reagent grade (Merck, Germany), Ethanol (Loba Chemie, India), n-Butanol (Riedel-deHaën®, Germany), Sulphuric acid (Molychem, India), L-ascorbic acid (Eurostar, UK), Gallic acid (Riedel-deHaën®, Germany), Quercetin (Sigma-Aldrich, Germany), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (Molychem, India), 2,4,6-Tri(2-pyridyl)-1,3,5-triazine (TPTZ) (Loba Chemie, India), Ammonium molybdate (Molychem, India), Trisodium phosphate (Loba Chemie, India), Folin-Ciocalteu reagent (Loba Chemie, India), Aluminium chloride (Molychem, India), Potassium acetate (Loba Chemie, India), Ferric chloride, FeCl3.6H2O (Molychem, India), Ferrous sulphate, FeSO4.7H2O (GHTech, China), Carrageenan (CDH, India).
Plant materials collection and extraction
The stem bark of Ficus sur was collected from a bush in Ibadan, Oyo State, Nigeria, by Mr. T.K. Odewo, a Taxonomist at the Forest Research Institute of Nigeria (FRIN), Ibadan. The plant was authenticated at FRIN, where the herbarium specimen (FHI113940) was deposited. The air-dried and powdered stem bark of F. sur (5 kg) was extracted with 80% ethanol (7.5 L) through maceration at room temperature (26 – 32 °C) for 3 days. The solution was filtered using Whatman No. 4 filter paper, and the filtrate was concentrated to dryness in vacuo at 50°C to obtain the crude hydroethanolic extract (286 g, 5.72% w/w).

Figure 1. Ficus sur in its natural habitat (A – stem bark and fruits; B – peeled and dried stem bark)
Fractionation of F. sur crude extract
The modified Kupchan method of solvent partitioning, as described by Van-Wagenen et al. (1993), was employed for this process. The extract (250 g) was initially suspended in 200 mL of distilled water in a 2 L separating funnel and then sequentially partitioned into different solvents in the following order: dichloromethane, ethyl acetate, and n-butanol. Each partitioned fraction was subsequently concentrated at 45°C under vacuum, except the n-butanol fraction, which was concentrated on a thermostatic water bath at 50°C. This yielded dichloromethane (DCM), ethyl acetate (EtOAc), and n-butanol (BuOH) fractions.
Phytochemical analysis
Standard methods, as described by Sofowora (1993) and Trease and Evans (2002), were used to conduct the phytochemical screening of the extract and its fractions. They were screened for alkaloids, flavonoids, phenolics and tannins, terpenoids, glycosides, saponins, anthraquinones, carbohydrates, and proteins.
Experimental animals
Male albino rats weighing between 180 and 220 g were used in the biological study. The rats were housed in well-ventilated cages at a temperature of 22°C (± 3°C) and subjected to a 12-hour natural light and daylight cycle for 14 days before the experiment, allowing them to adapt to the laboratory conditions. They were provided with rodent chow (Topfeed® finisher pellets) and unlimited water access. All experimental procedures adhered to ethical guidelines for the care and welfare of research animals (National Research Council, 2011), and ethical approval was granted by the Ethics Committee of the Faculty of Pharmacy, University of Benin, Benin City, Nigeria (EC/FP/024/02).
Gas chromatography-mass spectrometry (GC-MS) analysis of F. sur stem bark extract
GC–MS analysis of the stem bark extract of Ficus sur was carried out using the 7890B gas chromatograph coupled to 5977E inert mass spectrometer with electron impact source (Agilent Technologies) equipped with RTX1ms capillary column coated with 5% of phenyl methyl siloxane (15 m length × 0.25 mm diameter × 0.25 μm film thickness) (RESTEK). Pure helium gas (99.99%) was used as the carrier gas at a constant flow rate of 1.2 mL/min, an initial nominal pressure of 9.7853 psi, and at an average velocity of 39.923 cm/sec. One microliter of the samples was injected in splitless mode at an injection temperature of 300°C. The oven was initially programmed at 50°C, then ramped at 13°C/min to 300°C for 10 minutes. The run time was 19.231 minutes, with a 3-minute solvent delay. The mass spectrometer was operated in electron-impact ionisation mode at 70 eV with an ion source temperature of 230°C, a quadrupole temperature of 150°C, and a transfer line temperature of 280°C. Scanning of possible compounds was performed from m/z 50 to 550 amu at a 2.62s/scan rate, and the compounds were identified by comparing the measured mass spectral data with those in the National Institute of Standards and Technology (NIST) 23 Mass Spectral Library (Edeoga et al., 2005).
Total phenolic content
The Folin-Ciocalteu reagent (FCR) method, as described by Singleton (1999), was used to determine the total phenolic content (TPC) of the extract. Gallic acid served as the standard to create the calibration curve. Both gallic acid at various concentrations and the extract at 1 mg/mL were prepared in triplicate. Subsequently, 1 mL of the standard and test solutions was mixed with 2 mL of Folin-Ciocalteu reagent (diluted ten times with water) and allowed to stand for 3 min, then 2 mL of 7.5% sodium carbonate. After mixing, the solution was agitated and kept in the dark at room temperature for 30 min. Afterwards, the absorbance was taken spectrophotometrically at 760 nm against a blank consisting of all the reagents without the standard and extract. A standard gallic acid calibration curve was developed from which the total phenolic content of the samples was determined in milligrams of gallic acid equivalent per gram of dry extract (mgGAE/g dry sample), and expressed as mean value and standard error of the mean (SEM).
Total flavonoid content
The aluminium chloride complex formation assay as described by Mervat et al. (2009) was employed to evaluate the extract's total flavonoid content (TFC). Quercetin, a known flavonoid used as the standard, was prepared in graded concentrations in methanol. Subsequently, 0.1 mL of 10% aluminium chloride and 0.1 mL of 1 M potassium acetate, prepared in methanol, were added to 1 mL of the standard, extract, and fractions (1 mg/mL), respectively, in triplicate. The mixture was thoroughly mixed and left in the dark for 30 minutes to allow the reaction to proceed. Thereafter, the absorbance of the solution was measured at 420 nm using a UV-Visible spectrophotometer. The extract's flavonoid content was determined in milligrams of quercetin equivalent (QE) per gram of dry sample (mgQE/g dry sample).
In vitro antioxidant assay
DPPH radical scavenging assay
The method described by Sanchez-Moreno et al. (1998) was employed in this assay. The test solutions (1 mL) and l-ascorbic acid at graded concentrations (3.13, 6.25, 12.50, 25.00, 50.00, and 100.00 μg/mL) were each combined with a 1 mL portion of DPPH solution prepared in methanol (0.05 mg/mL) in triplicate. Following this, the samples were kept in a dark closure for 30 minutes, after which their absorbances were measured spectrophotometrically at 517 nm. A blank of methanol was used for comparison. The percentage inhibition of DPPH radical by the extract, fractions and control was then calculated based on the equation below:
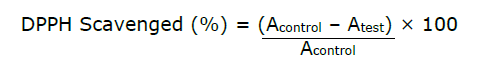
Where ‘Acontrol’ is the absorbance shown by the control (0.05 mg/mL DPPH solution) and ‘Atest’ is the absorbance by the test sample (extract + 0.05 mg/mL DPPH solution).
Ferric reducing antioxidant power (FRAP)
The assay followed the procedure described by Benzie and Strain (1996). To each test sample, 3 mL of the FRAP solution was added to a total volume of 100 µl. Subsequently, the reaction mixture was placed in a dark cabinet and incubated at 37°C for 30 minutes. The absorbance was then measured at 593 nm using a UV-visible spectrophotometer (Biobase BK-D560). A blank solution was prepared with 2 mL of FRAP solution and 1 mL of distilled water. A standard curve for FRAP was generated by mixing varying concentrations of FeSO4.7H2O (0, 100, 200, 400, 600, 800, and 1,000 µM) with the FRAP reagent, following the same procedure as the test samples. The FRAP value was calculated based on the extract's ability to reduce ferric ions, extrapolated from the linear calibration curve, and expressed as millimole (mmol) of FeSO4 equivalents per gram dry weight of the sample.
Total antioxidant assay (TAC)
The phosphomolybdenum method, as described by Prieto et al. (1999), was employed in this assay. To 0.3 mL (1 mg/mL) of the extract, fractions solutions, and graded concentrations of l-ascorbic acid, were mixed with 3 mL of a phosphomolybdenum reagent solution that included 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate. Afterwards, the mixture was incubated for 90 minutes at a temperature of 95°C. After the incubation, the tubes were allowed to cool to room temperature, and the absorbance of the resulting reaction mixture was measured at 695 nm using a blank solution (0.3 mL of phosphomolybdenum reagent + 3 mL of methanol). Ascorbic acid (AA) was utilised as the positive reference standard to create the standard calibration curve. Subsequently, the estimation of ascorbic acid equivalents of the extract and fractions was calculated using this standard calibration curve. The experiment was carried out in triplicate, and the results were expressed as the equivalent of ascorbic acid in mg per gram of extract.
Anti-inflammatory activity
The carrageenan-induced paw oedema protocol, as described by Jia et al. (2005), was utilised. Fifty-five albino rats were divided into eleven groups, each comprising five animals. Group 1 (negative control) was administered 1 mL/kg of normal saline (0.9%). Group 2 received the diclofenac standard drug at a dose of 10 mg/kg body weight, while Groups 3, 4, and 5 were given the extract at 100, 200, and 400 mg/kg body weight, respectively. Groups 6 and 7 were administered 200 and 400 mg/kg body weight of dichloromethane fraction, whereas Groups 8 and 9 received 200 and 400 mg/kg body weight of ethyl acetate fraction. Lastly, groups 10 and 11 were given 200 and 400 mg/kg body weight of n-butanol fraction. Subsequently, 0.1 ml of freshly prepared carrageenan suspension (1% w/v in 0.9% normal saline) was injected into the subplantar region of each rat's right hind paw. The circumference of the rat paw was measured using a vernier calliper immediately before and at 1, 2, 3, 4, and 5 hours after the carrageenan injection. The percentage of oedema inhibition was calculated in comparison to the control group that was administered the vehicle, using the formula:

The difference in paw thickness values (mm) was calculated by comparing the volumes of the left and right paws.
Statistical analysis
Statistical comparisons between groups were conducted using One-way ANOVA. To compare the means between the test and control groups, the post-hoc Duncan’s test was utilised. The results were presented as mean ± standard error of the mean (SEM), and the significance level was set at P<0.05. All statistical analyses were done using GraphPad Prism Software Version 9.0 (GraphPad Software Inc., United States).
RESULTS
Percentage yield from extraction and partitioning
As shown in Table 1, the percentage yield of F. sur crude hydroethanolic extract obtained from the dry powdered stem bark of the plant was 5.72%. The n-butanol fraction had the highest yield (52.69 g; 21.08%) among the partitioned fractions, while the dichloromethane fraction had the lowest yield (15.80 g; 6.32%).
Table 1. The overall yield and percentage of F. sur stem bark extract and fractions.
|
Extract/Fraction |
Character |
Weight (g) |
Percentage yield (%) |
|
Crude |
Brown powder |
286 |
5.72 |
|
Dichloromethane |
Pale yellow semisolid |
15.80 |
6.32 |
|
Ethyl acetate |
Yellowish brown semisolid |
41.96 |
16.78 |
|
n-Butanol |
Brown powder |
52.69 |
21.08 |
Phytochemical constituents of the extract and fraction
Preliminary phytochemical analysis revealed the presence of alkaloids, saponins, tannins, phenolics, flavonoids, and terpenoids in both the hydroethanolic extract, EtOAc, and BuOH fractions of F. sur stem bark (Table 2). Terpenoids were the only phytochemicals found in the DCM fraction. Alkaloids were present only in the crude extract. Alkaloids are a diverse group of nitrogen-containing plant metabolites known for their pharmacological properties. Flavonoids, phenolics, and tannins are renowned for their antioxidant capacity and potential to mitigate oxidative stress-related diseases. Saponins were identified in the crude extract, ethyl acetate, and n-butanol fractions, which contain higher polar constituents. Glycosides were found in the crude extract and all the partitioned fractions, indicating the presence of sugar-bound secondary metabolites that may have physiological effects such as antioxidant, anti-inflammatory, and anticancer properties.
Table 2. Preliminary phytochemical analysis of F. sur stem bark extract and fractions.
|
Phytochemical constituent |
Hydroethanolic extract |
DCM |
EtOAc |
BuOH |
|
Alkaloid Dragendorff’s Wagner’s |
+ + |
- - |
- - |
- - |
|
Flavonoid |
+ |
- |
+ |
+ |
|
Phenolic and tannin |
+ |
- |
+ |
+ |
|
Saponin |
+ |
- |
- |
- |
|
Anthraquinone |
- |
- |
- |
- |
|
Cardiac glycoside |
+ |
- |
+ |
+ |
|
Terpenoid |
+ |
+ |
+ |
+ |
|
Phlobatannin |
- |
- |
- |
- |
|
Carbohydrate |
+ |
- |
+ |
+ |
|
Amino acids |
- |
- |
- |
- |
|
Amino acids |
- |
- |
- |
- |
Note: +: presence; −: non presence; DCM: dichloromethane fraction; EtOAc: ethyl acetate fraction; BuOH: n-butanol fraction.
Total phenolic content
Phenolic compounds are important plant constituents with antioxidant activity due to the presence of hydroxyl groups that facilitate free radical scavenging action (Platzer et al., 2022). The total phenolic content varied from 802 ± 53 to 1,557 ± 75 mg GAE/g of dry sample across the extract and fractions (Table 4). The mean phenolic content of the stem bark extract was 628.46 ± 2.13 mg GAE/g of dry extract. The ethyl acetate fractions had the highest TFC of 686.65 ± 4.19 among the fractions, which followed the order: ethyl acetate fraction ˃ n-butanol fraction ˃ stem bark extract ˃ dichloromethane fraction.
Total flavonoid content
Flavonoids are among the major groups of phenolic compounds in medicinal plants, with a broad spectrum of chemical and biological activities, particularly radical scavenging and anti-inflammatory activities. In this study, the flavonoid contents of the extract and fractions of F. sur showed a similar trend to the total phenolic contents. The mean concentrations of flavonoids in the stem bark extract and fractions obtained based on the quercetin calibration curve (y = 0.0083x + 0.0826, R2 = 0.9994) showed that the ethyl acetate fraction had the highest TFC of 9.81 ± 1.27, and decreased in the order of ethyl acetate ˃ n-butanol ˃ hydroethanolic extract ˃ DCM (Table 4).
GC-MS phytochemical profiling of F. sur extract
GC-MS analysis of F. sur stem bark extract revealed the phytoconstituents with varying abundance based on the percentage area of the various peaks in the chromatogram. Compounds identified in the extract include pentacyclic triterpenes (urs-12-en-24-oic acid (28.24%), 12-oleanen-3-yl acetate (12.36%), and alpha-amyrin (2.25%)); phytosterols (β-sitosterol (11.28%), stigmasterol (1.30%), campesterol (0.79%)); fatty acids (oleic acid (14.93%), n-hexadecanoic acid (6.12%), 9-octadecenoic acid (2.43%), 9-octadecenoic acid (0.87%)); and benzene derivatives (isophthalic acid) (8.27%), catechol (0.15%), vanillin (0.19%)) (Table 3).
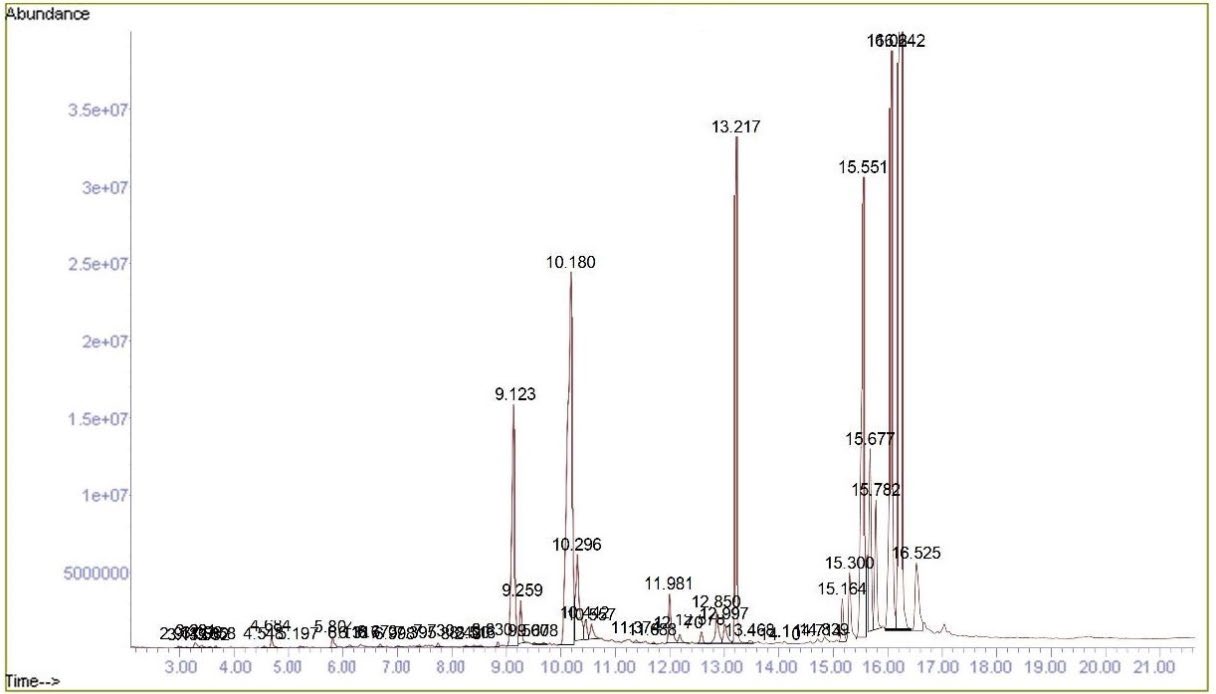
Figure 2. GC-MS Chromatogram of F. sur hydroethanolic stem bark extract.
Table 3. List of phytochemical constituents present in F. sur stem bark extract by GC-MS analysis.
|
S. No. |
RT (Min) |
Name of compound |
M/F |
M/W |
%PA |
Type/Class |
|
1. |
2.977 |
Benzoic acid |
122.12 |
0.07 |
Aromatic carboxylic acid |
|
|
2. |
3.113 |
Tetraacetyl-d-xylonic nitrile |
343.29 |
0.03 |
Nitrile |
|
|
3. |
3.281 |
Catechol |
110.11 |
0.15 |
Aromatic alcohol |
|
|
4. |
3.532 |
4-(4-Methyl-[1,3,2]dioxaborinan-2-yloxy)-phenol |
208.02 |
0.03 |
Phenol ether |
|
|
5. |
3.658 |
Cinnamaldehyde |
132.16 |
0.04 |
Cinnamaldehydes |
|
|
6. |
4.548 |
Benzaldehyde |
106.12 |
0.05 |
Aromatic aldehydes |
|
|
7. |
4.684 |
4-Hydroxy-3-methoxybenzaldehyde (Vanillin) |
152.15 |
0.19 |
Phenolic aldehyde |
|
|
8. |
5.197 |
N,N-Dimethylacetamide |
87.12 |
0.04 |
Acetamides |
|
|
9. |
5.804 |
(3R)-8-hydroxy-3-methyl-3,4-dihydro-1H-2-benzopyran-1-one {(-)-Mellein} |
178.18 |
0.22 |
3,4-Dihydroisocoumarin |
|
|
10. |
6.118 |
5-Methylsalicylic acid |
152.15 |
0.07 |
Benzoic acids |
|
|
11. |
7.395 |
3,4-Dihydroxyphenylglycol |
170.16 |
0.03 |
Phenolic |
|
|
12. |
7.730 |
Tetradecanoic acid |
228.37 |
0.08 |
Saturated Fatty acid |
|
|
13. |
8.243 |
Dibutyl phthalate |
278.34 |
0.04 |
Benzoic acid esters |
|
|
14. |
8.400 |
Pentadecanoic acid |
242.4 |
0.04 |
Saturated Fatty acid |
|
|
15. |
9.123 |
n-Hexadecanoic acid |
256.42 |
6.12 |
Saturated Fatty acid |
|
|
16. |
10.180 |
Oleic Acid |
282.5 |
14.93 |
Unsaturated fatty acid |
|
|
17. |
10.296 |
9-Octadecenoic acid |
282.5 |
2.43 |
Unsaturated fatty acid |
|
|
18. |
10.442 |
Octadecanoic acid |
284.5 |
0.38 |
Saturated Fatty acid |
|
|
19. |
10.557 |
9,12-Octadecadienoic acid |
280.4 |
0.44 |
Unsaturated fatty acid |
|
|
20. |
11.374 |
18-Nonadecenoic acid |
296.5 |
0.05 |
Unsaturated fatty acid |
|
|
21. |
11.981 |
Hexadecanoic acid |
256.42 |
1.18 |
Saturated Fatty acid |
|
|
22. |
12.170 |
Phthalic acid |
166.13 |
0.21 |
Aromatic dicarboxylic acid |
|
|
23. |
12.578 |
1,4-Benzenedicarboxylic acid |
166.13 |
0.28 |
Aromatic dicarboxylic acid |
|
|
24. |
12.850 |
9-Octadecenal |
266.5 |
0.87 |
Fatty aldehyde |
|
|
25. |
12.997 |
2- Chloropropionic acid |
108.52 |
0.56 |
Carboxylic acid |
|
|
26. |
14.714 |
1,2-Benzenediol |
110.11 |
0.06 |
Phenolics |
|
|
27. |
14.839 |
dl-alpha-Tocopherol |
430.7 |
0.15 |
Tocopherols |
|
|
28. |
15.164 |
Campesterol |
400.7 |
0.79 |
Phytosterol |
|
|
29. |
15.300 |
Stigmasterol |
412.7 |
1.30 |
Phytosterol |
|
|
30. |
15.551 |
beta-Sitosterol |
414.7 |
11.28 |
Phytosterol |
|
|
31. |
15.551 |
gamma-Sitosterol |
414.7 |
11.28 |
Phytosterol |
|
|
32. |
15.677 |
alpha-Amyrin |
426.7 |
3.34 |
Pentacylic triterpenoid |
|
|
33. |
16.064 |
Olean-12-en-3-ol, acetate |
C32H52O2 |
468.75 |
12.36 |
Pentacylic triterpenoid |
|
34. |
16.242 |
Urs-12-en-24-oic acid |
468.7 |
28.24 |
Pentacylic triterpenoid |
|
|
35. |
16.525 |
A'-Neogammacer-22(29)-en-3-ol, acetate, (3.beta.,21.beta.) |
C32H52O2 |
468.8 |
2.14 |
Pentacylic triterpenoid |
Note: RT: Retention Time; M/F: Molecular Formula; MW: Molecular Weight (g/mol); PA: Peak Area
In vitro antioxidant activities
In vitro antioxidant activity of F. sur extract and fractions was evaluated by DPPH scavenging activity (expressed in percentage inhibition and IC50), FRAP (expressed as mmol FeSO4 per g of dry extract), and TAC (expressed as mg of ascorbic acid equivalents per g of dry extract) assays (Figure 3).
DPPH radical scavenging activity
The ability of F. sur stem bark extract and fractions to scavenge DPPH free radicals at different concentrations ranging from 3.125 to 100 µg/mL are shown in Figure 3A. The extract, fractions and standard demonstrated a concentration-dependent DPPH scavenging activity, with IC50 values of 15.32 ± 1.02, 51.68 ± 2.34, 14.55 ± 1.03, 2.40 ± 0.18, and 2.40 ± 0.18 µg/mL for the extract, DCM, EtOAc, and BuOH fractions, and l-ascorbic acid, respectively. The DPPH (1,1-diphenyl-2-picrylhydrazyl) antioxidant assay is based on the capacity of a potential antioxidant to quench the DPPH radical, a stable free radical that exhibits strong absorption at 517 nm. DPPH is converted into a more stable molecule after accepting an electron or a hydrogen atom from an antioxidant scavenger, resulting in a purple colouration in the solution.
Ferric reducing antioxidant power
The ability of the plant extract and fractions to reduce Fe3+ to Fe2+ by electron donation was assessed using the ferric reducing power activity (FRAP) assay, another indicator of antioxidant activity. The principle of the FRAP assay is based on the ability of an antioxidant compound to reduce Fe3+ to Fe2+. The amount of Fe2+ complex in the sample is determined by measuring the development of Perl's blue at 695 nm, followed by reduction by an antioxidant. A rise in absorbance typically corresponds to an increase in reductive capacity. The FRAP value as a measure of the antioxidant activity of the extract was obtained from a linear regression equation of the l-ascorbic acid standard curve (Figure 3B), expressed in milligrams of ascorbic acid equivalent per gram of extract (mgAAE/g). In this study, the extract and fractions demonstrated substantial FRAP outcome, as shown in Table 4. EtOAc and BuOH fractions showed comparable FRAP values of 3.22 ± 0.12 and 3.21 ± 0.05 mmol FeSO4 equivalent/g of dry extract, respectively, while the dichloromethane fraction had the least FRAP activity of 1.35 ± 0.04 mmol FeSO4 equivalent/g of dry extract.
Total antioxidant capacity
The phosphomolybdenum assay was used to assess the total antioxidant capacity (TAC) of the extract and fractions. TAC assay is based on a potential antioxidant reducing MoVI to MoV and the subsequent formation of a green phosphate/MoV complex at acid pH. In the present study, BuOH exhibited the highest TAC activity among all the fractions, with a value of 329.42 ± 3.97 mg ascorbic acid equivalents per gram of dry extract. The TAC activity obtained was in this order: n-butanol fraction > hydroethanolic extract > ethyl acetate fraction > dichloromethane (Figure 3C).
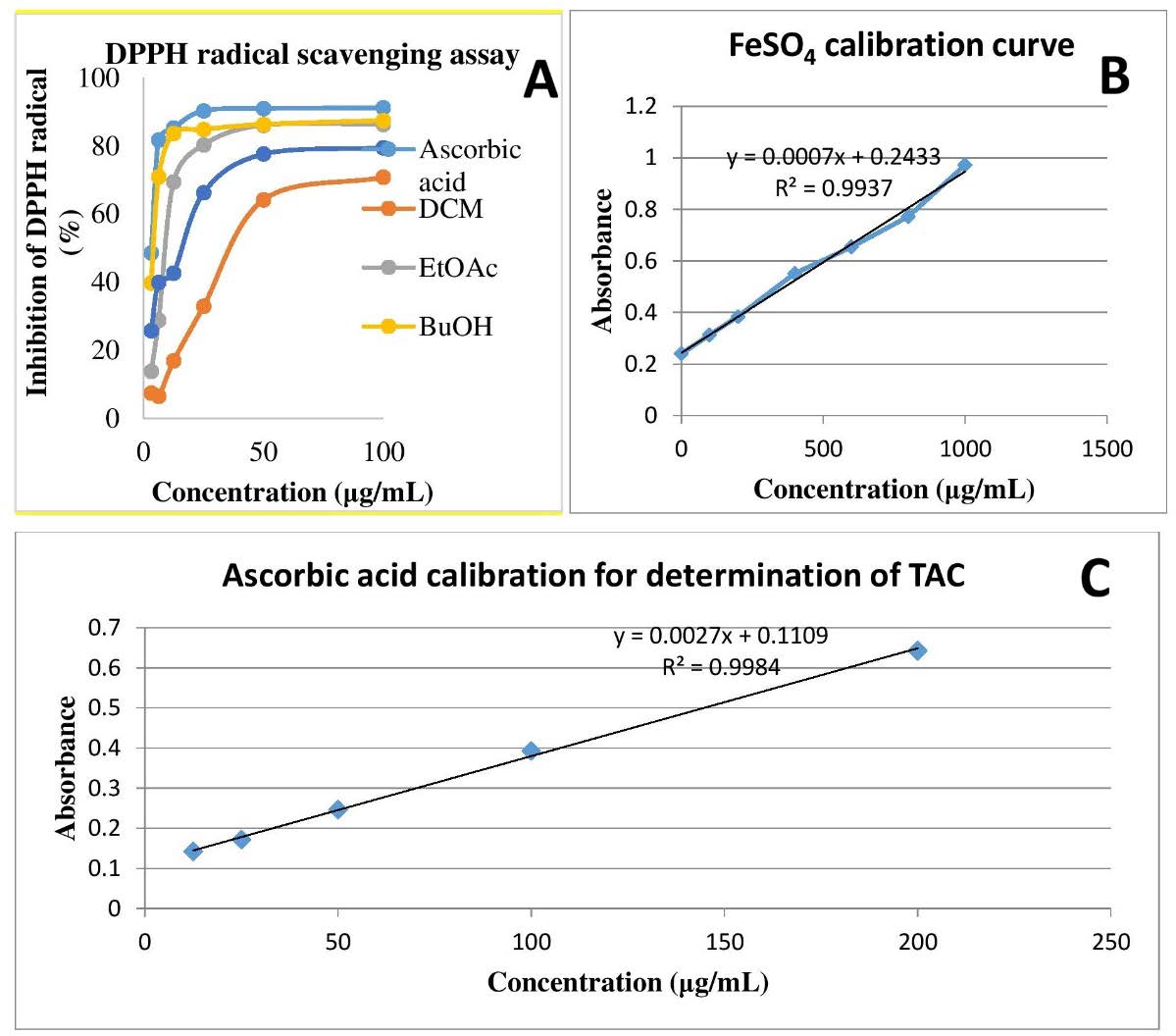
Figure 3. In vitro antioxidant activities of F. sur crude extract and fractions.
Table 4. Quantitative phytochemical and antioxidant activity of F. sur stem bark extract and fractions.
|
Plant sample |
Total phenolic content (TPC) |
Total flavonoid Content (TFC) |
DPPH |
FRAP |
TAC |
|
Hydroethanolic extract |
628.46 ± 2.13 |
5.19 ± 0.52 |
15.32 ± 1.02 |
2.63 ± 0.29 |
215.71 ±. 6.42 |
|
DCM fraction |
447.99 ± 3.22 |
3.58 ± 0.76 |
51.68 ± 2.34 |
1.35 ± 0.04 |
177.07 ± 5.13 |
|
EtOAc fraction |
732.01 ± 5.14 |
9.81 ± 1.27 |
14.55 ± 1.03 |
3.22 ± 0.12 |
178.92 ± 3.16 |
|
BuOH fraction |
686.65 ± 4.19 |
9.41 ± 0.29 |
2.40 ± 0.18 |
3.21 ± 0.05 |
329.42 ± 3.97 |
|
l-Ascorbic acid |
ND |
ND |
2.22 ± 0.11 |
NA |
NA |
Note: NA: Not Applicable; ND: Not Determined;
DCM: dichloromethane fraction; EtOAc: ethyl acetate fraction; BuOH: n-butanol fraction; TPC: expressed as mg gallic acid equivalents (mgGAE)/g dry weight sample;
TFC: Expressed as mg quercetin equivalents (mgQE)/g dry weight sample;
DPPH: Expressed as IC50 value; FRAP: Expressed as mmol FeSO4 per g of dry extract;
TAC: Expressed as mg of ascorbic acid equivalents per g of dry extract
In-vivo anti-inflammatory activities of F. sur extract and fractions
The anti-inflammatory potential of F. sur extract and fractions (DCM, EtOAc, and BuOH) was evaluated in male albino rats using the carrageenan-induced paw oedema model. The injection of carrageenan into the rat's paw caused a significant increase in paw thickness. The average paw volume of the rats was significantly (P˂0.05) increased in both the extract fractions-treated groups compared to the normal (untreated) group. The extract and both fractions caused a dose-dependent and non-significant (P ˃0.05) decrease in inhibition of rats’ paw oedema compared to the positive control group that received diclofenac (Figures 4A and B). The decreased rat paw oedema in the extract and fractions-treated groups was more distinct from the 3rd after carrageenan injection. The highest effect of percentage inhibition of paw oedema in the rats was observed in the standard control group, followed by the group treated with 400 mg/kg body weight of n-butanol fraction at the 5th hour of measurement.
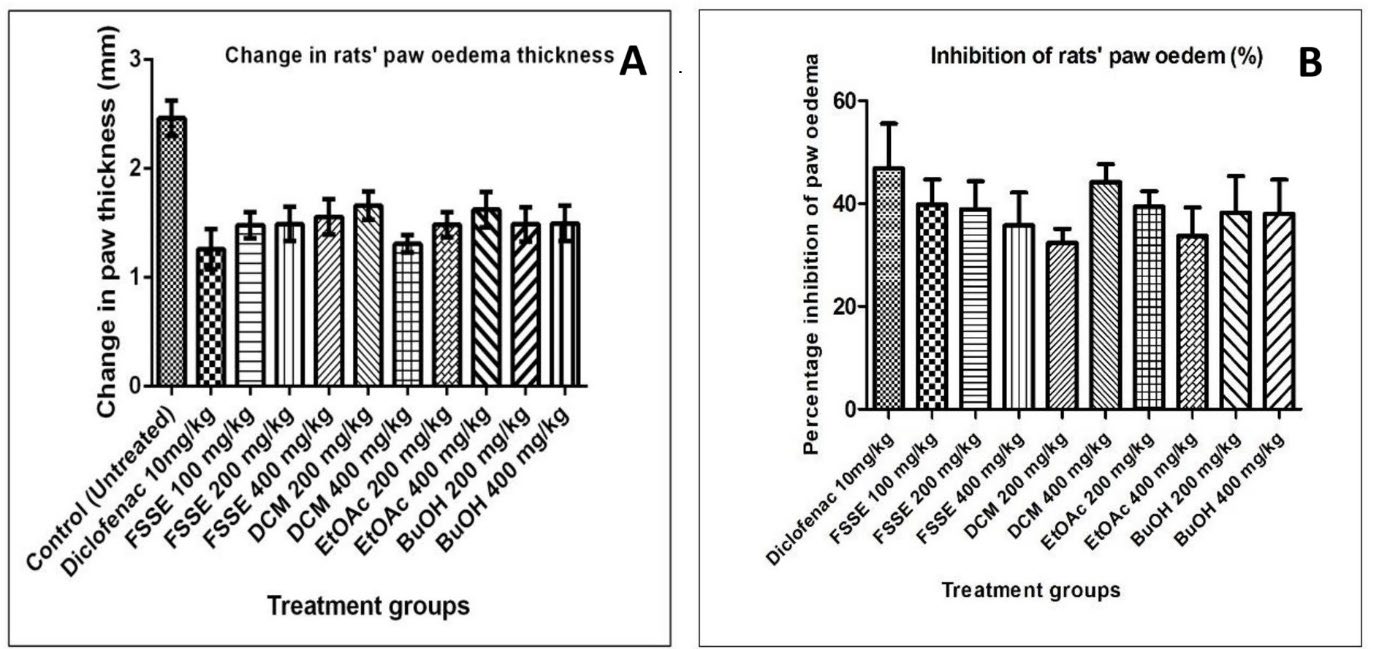
Figure 4. Effect of Ficus sur hydroethanolic extract on change in paw thickness (A) and percentage inhibition of paw oedema (B) in carrageenan-induced anti-inflammatory assay. Data represent mean ± SEM (n = 6); (P < 0.05). DCM: dichloromethane fraction; EtOAc: ethyl acetate fraction; n-BuOH: n-butanol fraction.
DISCUSSION
Ficus sur has been extensively used in folk medicine to treat various ailments. Several studies have demonstrated multiple biological activities of different parts of the plant (Sieniawska et al., 2022; Diatta et al., 2024). The extraction of plant materials involves isolating and recovering the constituent chemicals or substances from plant matter, employing solvents and other advanced separation techniques to dissolve and extract bioactive compounds from various parts of plants, such as fruits, flowers, seeds, leaves, roots, stems, and whole plants. The choice of solvents in the extraction process is dictated by the type of compounds or constituents desired. Extraction with polar solvents like ethanol and methanol may be attributed to their polarity index and ability to permeate through plasma membranes, facilitating the timely release of cellular contents into the solvent, which ultimately yields a high mixture of compounds, also referred to as crude extract. Our findings regarding the yield and subsequent bioactivities of the ethanolic extract are supported by previous reports on bioassays in differential solvent systems (Adebayo et al., 2017; Akma and Hafizah, 2021; Sieniawska et al., 2022).
Studies have shown that different phytochemical compositions can induce various biological activities in organisms, such as antioxidant, anti-inflammatory, antibacterial, and anti-tumour activities (Zhang et al., 2024). The type, content, and interaction of these phytochemical compositions affect the biological activity of extracts (Kumar et al., 2023; Fais and Era, 2024; Zhang et al., 2024). The extract of F. sur was partitioned into different solvents of varying polarity to analyse their phytochemical content and potential bioactivities. Phenolics, flavonoids, tannins, cardiac glycosides, and terpenoids found in the extract and fractions (Tables 2 and 3) may be responsible for the pharmacological activities, which include anticancer, antimicrobial, antioxidant, and anti-inflammatory activities, observed in the plant.
Most herbal medicines contain high levels of polyphenols, flavonoids, and terpenoids with powerful antioxidant activity, which gives them various protective and disease-fighting abilities. Phenolic compounds are plant secondary metabolites considered important because they have one or more hydroxyl groups on their aromatic ring. They serve many biological functions, including protecting against oxidative damage (Kumar et al., 2019; Zhang et al., 2022). Triterpenes have shown significant antioxidant properties that enable them to scavenge free radicals and boost the body's antioxidant defenses. This activity makes them a promising focus for research into health benefits, including conditions related to inflammation and reactive oxygen species (ROS) (González-Burgos and Gómez-Serranillos, 2012). Flavonoids have strong antioxidant effects by interacting with reactive oxygen species such as hydroxyl radicals, superoxide anions, and lipid peroxy radicals (Rudrapal et al., 2022; Jomova et al., 2025). Besides their antioxidant roles, flavonoids also modulate the anti-inflammatory process and inhibit ROS-induced apoptosis and inflammation, mainly due to their numerous free hydroxyl groups, which increase their reactivity with oxidants (Panche et al., 2016).
GC-MS analysis of the stem bark extract from F. sur revealed various phytoconstituents present in differing quantities, as indicated by the percentage area of the peaks in the chromatogram shown in Figure 2. The total ion chromatogram (TIC) provided details on peak number, retention time (RT), compound name, molecular formula, molecular weight (MW), and area percentage. Compounds were identified by comparing their mass spectra with entries in the mass spectral libraries, specifically the National Institute of Standards and Technology (NIST) 14 Mass Spectral Library, to confirm their identities, molecular weights, and structures (Table 3). The main compounds identified include pentacyclic triterpenoids (urs-12-en-24-oic acid, 12-oleanen-3-yl acetate, and alpha-amyrin), phytosterols (β-sitosterol, γ-sitosterol, stigmasterol, and campesterol), fatty acids, and benzene derivatives. Notably, gamma-sitosterol and oleanane-type triterpenoids (12-oleanen-3-yl acetate and urs-12-en-24-oic acid) were identified for the first time in Ficus sur stem bark extract, although related compounds have been reported in other studies. Nwanisobi et al. (2021) identified olean-12-en-3-ol acetate from Ficus sur seed oil. Dongmo et al. (2022) isolated olean-12-en-3-one from the fruits of Ficus sur. Feleke and Brehane (2025) reported the presence of oleanane and ursine-type pentacyclic triterpenoids (3-acetoxy-α-amyrin and 3-acetoxy-β-amyrin). Mouelle et al. (2025) also isolated various triterpenoids, including α-amyrin acetate, β-amyrin acetate, 3β-acetoxy-olean-12-en-11-one, lupeol, lupenyl acetate, taraxastan-3,20-diol, stigmasterol, and stigmasterol glycoside from Ficus sur stem bark extract. The GC-MS analysis confirms the presence of a diverse range of triterpenoids in the hydroethanolic stem bark extract of F. sur. These findings align with previous studies on this plant, which have also identified various triterpenoids (Nwanisobi et al., 2021; Ogunlaja et al., 2022; Sieniawska et al., 2022; Mouelle et al., 2025).
The total phenolic and flavonoid contents of the hydroethanolic extract and fractions of F. sur stem bark align with their antioxidant and anti-inflammatory activities, as observed in this study. The higher levels of phenolics and flavonoids recorded here are attributed to the presence of polar constituents, such as polyphenols and some terpenoids. This explains the widespread traditional use of the plant stem bark in numerous herbal medicines, as also documented in previous studies (Saloufou et al., 2018; Sieniawska et al., 2022). Sieniawska et al. (2022) found higher phenolic and flavonoid contents in the methanolic and aqueous extracts of F. sur stem bark, confirming its rich polyphenolic profile.
Natural antioxidants in plants play a vital role in inhibiting or preventing the harmful effects of oxidative stress. Antioxidants are essential substances that protect the body from damage caused by free radicals and oxidative stress. The antioxidant activity of the extract and fractions was evaluated using the DPPH, FRAP, and TAC assays. These methods are convenient for assessing the antioxidant potential of medicinal plants. The presence of antioxidant compounds (with hydrogen-donating groups) such as flavonoids and phenols, causes changes like the color shift of the methanolic DPPH solution due to stable, non-radical compound formation; the reduction of Fe3+ to a blue Fe2+ complex in FRAP; and the reduction of MoVI to MoV, resulting in a green phosphate/MoV complex in TAC (Vasudevara and Sravanthi, 2017). The extract and fractions demonstrated significant, concentration-dependent antioxidant activities, as shown in Table 4. The high antioxidant activity observed suggests they contain a substantial amount of polar constituents and antioxidant compounds. Additionally, other secondary metabolites, such as alkaloids and glycosides present in the stem bark extract, have also been reported to possess notable antioxidant activity (Khanal et al., 2022; Hilal et al., 2024).
To assess the potential anti-inflammatory properties of F. sur stem bark extract and its fractions, the carrageenan-induced inflammation assay was employed. This method is widely recognised as the most common animal model for evaluating the anti-inflammatory effects of compounds and pharmaceutical preparations (Mansouri et al., 2015; Patil et al., 2018). In this study, F. sur extract and the various fractions (DCM, EtOAc, and BuOH) demonstrated significant, dose-dependent inhibition of the rats’ paw oedema throughout the measurement period. This indicates their capacity to counteract both early and delayed stages of inflammation. The response pattern observed in the extract and fractions suggests strong peripheral anti-inflammatory properties, likely attributable to the presence of terpenoids, polyphenols, and flavonoids, which can inhibit leukotriene B4 (LTB4) formation. These compounds are involved in leukocyte chemotaxis, including that of macrophages and neutrophils, at inflammatory sites (Yoon and Baek, 2005). According to Panache et al. (2016), flavonoids are known to be potent enzyme inhibitors, including xanthine oxidase (XO), cyclooxygenase (COX), lipoxygenase, and phosphoinositide 3-kinase, which participate in inflammatory processes. β-Sitosterol, a phytosterol, and one of the main phytochemicals found in F. sur, have been found to possess antioxidant and anti-inflammatory activities in several studies (Sun et al., 2020; Zhang et al., 2023). Triterpenes, based on multiple in vivo and in vitro studies, have demonstrated inhibitory activity against inflammatory enzymes such as 5-lipoxygenase, elastase, and phospholipase A2, while also suppressing various inflammatory pathways like interleukin release, lipid peroxidation, free radical-mediated processes, corticosteroid metabolism, and activities of the complement system and protein kinases (Rios et al., 2000; Yadav et al., 2010; Ramos et al., 2021; Mantiniotou et al., 2025). This study has demonstrated the anti-inflammatory potential of F. sur stem bark hydroethanolic extract and its various partitioned fractions.
CONCLUSION
The hydroethanolic extract of Ficus sur stem bark and its solvent fractions demonstrated substantial anti-inflammatory and antioxidant effects. These effects are likely due to the abundant presence of bioactive terpenoids and polyphenols. The plant's anti-inflammatory characteristics make it a promising candidate for studies focused on developing effective bioactive compounds to tackle disease conditions that are caused or worsened by inflammatory processes. This research lays the groundwork for future investigations into the molecular mechanisms underlying the biological effects of the phytochemicals present in this plant.
REFERENCES
Abdulrazaq, S., Halimatu S.H., Yahaya M.S., Aliyu M.M., Umar, U.P., Mohamed, G.M., Maryam, A.M., Amina J.Y., and Maria, M.M. 2018. Preliminary phytochemical and anticonvulsant studies on the root extracts of Ficus capensis Thunb. (Moraceae). Journal of Pharmacy and Bioresources. 15(1): 1-9. https://doi.org/10.4314/jpb.v15i1.1
Adebayo, M.A., Enitan, S.S., Owonikoko, W.M., Igogo, E., and Ajeigbe, K.O. 2017. Haematinic properties of methanolic stem bark and fruit extracts of ficus surin rats pre-exposed to phenylhydrazine-induced haemolytic anaemia. African Journal of Biomedical Research. 20: 85-92.
Adebayo-Tayo, B.C. and Odeniyi, A.O. 2012. Phytochemical screening and microbial inhibitory activities of Ficus capensis. African Journal of Biomedical Research. 15(1): 35-40.
Akachukwu, D. and Uchegbu, R.I. 2016. Phytochemical, antimicrobial and free radical scavenging activity of Ficus capensis thunb leaves. Journal of Complementary and Alternative Medical Research. 1(4): 1-7. https://doi.org/10.9734/JOCAMR/2016/29851
Akma, I.M. and Hafizah, Z.N. 2021. Pharmacology of Ficus: A review. p.118-134. In: Recent advances in Ficus research. Noor Publishing, Moldova.
Al-Breiki, A.M., Al-Brashdi, H.M., Al-Sabahi, J.N., and Khan, S.A. 2018. Comparative GC-MS analysis, in-vitro antioxidant and antimicrobial activities of the essential oils isolated from the peel of omani lime. Chiang Mai Journal of Science. 45(4): 1782-1795.
Ayele, M., Makonnen, E., Ayele, A.G., and Tolcha, Y. 2020. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ficus sur forssk (Moraceae) leaves in saline-loaded rats. Journal of Experimental Pharmacology. 12: 619-627. https://doi.org/10.2147/JEP.S283571
Benzie, I.F. and Strain, J.J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Analytical Biochemistry. 239: 70-76. https://doi.org/10.1006/abio.1996.0292
Bindu, S., Mazumder, S., and Bandyopadhyay, U. 2020. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical Pharmacology. 180: 114147. https://doi.org/10.1016/j.bcp.2020.114147
Biswas, S.K. 2016. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Medicine and Cellular Longevity. 2016: 5698931. https://doi.org/10.1155/2016/5698931
Chaachouay, N. and Zidane, L. 2024. Plant-derived natural products: A source for drug discovery and development. Drugs and Drug Candidates. 3(1): 184-207. https://doi.org/10.3390/ddc3010011
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., and Zhao, L. 2017. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 9(6): 7204-7218. https://doi.org/10.18632/oncotarget.23208
Diatta, K., Diatta, W., Mbaye, A.I., Sarr, A., Dieng, S.I.M., Maiga, H., and Fall, A.D. 2024. Monographic on Ficus sur forssk (Moraceae): A review on its traditional uses, phytochemistry and pharmacology. Asian Journal of Research in Botany. 7(2): 347-356. https://doi.org/10.9734/ajrib/2024/v7i2235
Dongmo, F.L.M., Kamsu, G.T., Nanfack, A.R.D., Ndontsa, B.L., Farooq, R., Bitchagno, G.T.M., and Atia-tul-Waha, T.M. 2022. Chemical constituents and antibacterial activity from the fruits of Ficus sur Forssk. Investigational Medicinal Chemistry and Pharmacology. 5(1): 64. https://doi.org/10.31183/imcp.2022.00064
Drini, M. 2017. Peptic ulcer disease and nonsteroidal anti-inflammatory drugs. Australian Prescriber. 40(3): 91-93. https://doi.org/10.18773/austprescr.2017.037
Dzobo, K. 2022. The role of natural products as sources of therapeutic agents for innovative drug discovery. Comprehensive Pharmacology. 408-2022. https://doi.org/10.1016/B978-0-12-820472-6.00041-4
Edeoga, H.O., Okwu, D.E., and Mbaebie, B.O. 2005. Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology. 4: 685-688. https://doi.org/10.5897/AJB2005.000-3127
Eluka, P., Nwodo, F., Akah, P., and Onyeto, C. 2015. Anti-ulcerogenic and antioxidant properties of the aqueous leaf extract of Ficus capensis in Wistar albino rats. Merit Research Journal of Medicine and Medical Sciences. 3(1): 022-026.
Fais, A. and Era, B. 2024. Phytochemical composition and biological activity. Plants. 13(3): 331. https://doi.org/10.3390/plants13030331
Feleke, S. and Brehane, A. 2005. Triterpene compounds from the latex of Ficus sur. Bulletin of the Chemical Society of Ethiopia. 19(2): 307-310. https://doi.org/10.4314/bcse.v19i2.21137
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D.W., Fasano, A., Miller, G.W., et al. 2019. Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 25(12): 1822-1832. https://doi.org/10.1038/s41591-019-0675-0
González-Burgos, E. and Gómez-Serranillos, M.P. 2012. Terpene compounds in nature: A review of their potential antioxidant activity. Current Medicinal Chemistry. 19(31): 5319-5341. https://doi.org/10.2174/092986712803833335
Harvanová, G. and Duranková, S. 2025. Inflammatory process: Factors inducing inflammation, forms and manifestations of inflammation, immunological significance of the inflammatory reaction. Alergologia Polska - Polish Journal of Allergology. 12(1): 54-61. https://doi.org/10.5114/pja.2025.147674
Hilal, B., Khan, M.M., and Fariduddin, Q. 2024. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: Phenolics, terpenes, and alkaloids. Plant Physiology and Biochemistry (PPB). 211: 108674. https://doi.org/10.1016/j.plaphy.2024.108674
Hussain, P.S. and Harris, C.C. 2007. Inflammation and cancer: An ancient link with novel potentials. International Journal of Cancer. 121: 2373-80. https://doi.org/10.1002/ijc.23173
Jia, J.M., Wu, C.F., Liu, W., Yu, H., Hao, Y., Zheng, J.H., and Ji, Y.R. 2005. Anti-inflammatory and analgesic activities of the tissue culture of Saussurea involucrata. Biological and Pharmaceutical Bulletin. 28(9): 1612-1614. https://doi.org/10.1248/bpb.28.1612
Jomova, K., Alomar, S.Y., Valko, R., Liska, J., Nepovimova, E., Kuca, K., and Valko, M. 2025. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chemico-Biological Interactions. 413: 111489. https://doi.org/10.1016/j.cbi.2025.111489
Khanal, L.N., Sharma, K.R., Pokharel, Y.R., and Kalauni, S.K. 2022. Phytochemical analysis and in vitro antioxidant and antibacterial activity of different solvent extracts of Beilschmiedia roxburghiana nees stem barks. Scientific World Journal. 2022: 6717012. https://doi.org/10.1155/2022/6717012
Kumar, A.P.N., Kumar, M., Jose, A., Tomer, V., Oz, E., Proestos, C., Zeng, M., Elobeid, T.K.S., and Oz, F. 2023. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules. 28(2): 887. https://doi.org/10.3390/molecules28020887
Kumar, N. and Goel, N. 2019. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports. 24: e00370. https://doi.org/10.1016/j.btre.2019.e00370
Kumar, S., Korra, T., Thakur, R., Arutselvan, R., Kashyap, A.S., Nehela, Y., Chaplygin, V., Minkina, T., and Keswani, C. 2023. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress. 8: 100154. https://doi.org/10.1016/j.stress.2023.100154
Lingappan, K. 2008. NF-κB in oxidative stress. Current Opinion in Toxicology. 7: 81-86. https://doi.org/10.1016/j.cotox.2017.11.002
Liu, R.H. 2013. Health-promoting components of fruits and vegetables in the diet. Advances in Nutrition. 4(3): 384S. https://doi.org/10.3945/an.112.003517
Lobo, V., Patil, A., Phatak, A., and Chandra, N. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4(8): 118-26. https://doi.org/10.4103/0973-7847.70902
Mansouri, M.T., Hemmati, A.A., Naghizadeh, B., Mard, S.A., Rezaie, A., and Ghorbanzadeh, B. 2015. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian Journal of Pharmacology. 47(3): 292-298. https://doi.org/10.4103/0253-7613.157127
Mantiniotou, M., Athanasiadis, V., Kalompatsios, D., Bozinou, E., and Lalas, S.I. 2025. Therapeutic capabilities of triterpenes and triterpenoids in immune and inflammatory processes: A Review. Compounds. 5(1): 2. https://doi.org/10.3390/compounds5010002
Mervat, M.M., Far, E., Hanan, A., and Taie, A. 2009. Antioxidant activities, total anthrocynins, phenolics and flavonoid contents of some sweet potato genotypes under stress of different concentration of sucrose and sorbitol. Australian Journal of Basic Applied Science. 3(4): 3609-3616.
Mouelle, E.N.M., Nsangou, F.M., Fofack, H.M.T., Mboutchak, D., Koliye, P.R., Ateba, A.B., Ntie-Kang, F., Akone, S.H., and Happi, N.E. 2025. In vitro and in silico studies of the biological activities of some secondary metabolites belonging to Ficus sur forssk (Moraceae): Towards optimization of wighteone metabolite. Chemistry & Biodiversity. 22(1): e202401270. https://doi.org/10.1002/cbdv.202401270
Murugesu, S., Selamat, J., and Perumal, V. 2021. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants. 10: 2749. https://doi.org/10.3390/plants10122749
National Research Council. 2011. Guide for the care and use of laboratory animals: Eighth edition. The National Academies Press, Washington, DC. https://doi.org/10.17226/12910.
Nwanisobi, G.C., Aghanwa, C.I., and Ezeagu, C.U. 2021. Fatty acid composition of Ficus sur seed oil (Moraceae) obtained in Enugu State, Nigeria. Journal of Chemical Society of Nigeria. 46(6): 1055-1061. https://doi.org/10.46602/jcsn.v46i6.686
Ogunlaja, O.O., Moodley, R., Baijnath, H., and Jonnalagadda, S.B. 2022. Antioxidant activity of the bioactive compounds from the edible fruits and leaves of Ficus sur forssk. (Moraceae). African Journal of Science. 118:(3/4). https://doi.org/10.17159/sajs.2022/9514
Olayemi, I.K., Samuel, O.M., Ukubuiwe, A.C., Ande, A.T., Adeniyi, K.A., and Shittu, K.O. 2017. Larvicidal activities of leaf extracts of Adansonia digitata L. (Malvales: Malvaceae) and Ficus sur forssk (Rosales: Moraceae) against Culex quinquefasciatus Mosquito (Diptera: Culicidae). Journal of Mosquito Research. 7(15): 115-124. https://doi.org/10.5376/jmr.2017.07.0015
Omoregie, E.S. and Okugbo, O.T. 2014. In vitro antioxidant activity and phytochemical screening of methanol extracts of Ficus capensis and Dacryodes edulis leaves. Journal of Pharmacy and Bioresources. 11(2): 66-75. https://doi.org/10.4314/jpb.v11i2.6
Panche, A.N., Diwan, A.D., and Chandra, S.R. 2016. Flavonoids: An overview. Journal of Nutritional Science. 5: e47. https://doi.org/10.1017/jns.2016.41
Pandey, K.B. and Rizvi, S.I. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2(5): 270-8. https://doi.org/10.4161/oxim.2.5.9498
Patil, K.R., Mahajan, U.B., Unger, B.S., Goyal, S.N., Belemkar, S., Surana, S.J., Ojha, S., and Patil, C.R. 2018. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. International Journal of Molecular Sciences. 20(18): 4367. https://doi.org/10.3390/ijms20184367
Phaniendra, A., Jestadi, D.B., and Periyasamy, L. 2015. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry. 30(1): 11-26. https://doi.org/10.1007/s12291-014-0446-0
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., and Bitto, A. 2017. Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017:8416763. https://doi.org/10.1155/2017/8416763
Platzer, M., Kiese, S., Tybussek, T., Herfellner, T., Schneider, F., Schweiggert-Weisz, U., and Eisner, P. 2022. Radical scavenging mechanisms of phenolic compounds: A Quantitative structure-property relationship (QSPR) study. Frontiers in Nutrition. 9: 882458. https://doi.org/10.3389/fnut.2022.882458
Prieto, P., Pineda, M., and Aguilar, M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry. 269:337. https://doi.org/10.1006/abio.1999.4019
Ramde-Tiendrebeogo, A., Tibiri, A., Hilou, A., Lompo, M., Millogo-Kone, H., Nacoulma, O.G., and Guissou I.P. 2012. Antioxidative and antibacterial activities of phenolic compounds from Ficus sur Forssk and Ficus sycomorus L. (Moraceae): Potential for sickle cell disease treatment in Burkina Faso. International Journal of Biological and Chemical Sciences. 6(1): 328-336. https://doi.org/10.4314/ijbcs.v6i1.29
Ramos, G.F., Amponsah, I.K., Harley, B.K., Jibira, Y., Baah, M.K., Adjei, S., Armah, F.A., and Mensah, A.Y. 2021. Triterpenoids mediate the antimicrobial, antioxidant, and anti-inflammatory activities of the stem bark of Reissantia indica. Journal of Applied Pharmaceutical Science. 11(05): 039-048.
Reuter, S., Gupta, S.C., Chaturvedi, M.M., and Aggarwal, B.B. 2010. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine. 49: 1603-16. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Rios, J.L., Recio, M.C., Maáñez, S., and Giner, R.M. 2000. Natural triterpenoids as anti-inflammatory agents. Studies in Natural Products Chemistry. 22(C): 93-143. https://doi.org/10.1016/S1572-5995(00)80024-1
Rudrapal, M., Khairnar, S.J., Khan, J., Dukhyil, A.B., Ansari, M.A., Alomary, M.N., Alshabrmi, F.M., Palai, S., Deb, P.K., and Devi, R. 2022. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Frontiers in Pharmacology. 13: 806470. https://doi.org/10.3389/fphar.2022.806470
Saloufou, K.I., Boyode, P., Simalou, O., Eloh, K., Idoh, K., Melila, M., Toundou, O., Kpegba, K., and Agbonon, A. 2018. Chemical composition and antioxidant activities of different parts of Ficus sur. Journal of HerbMed Pharmacology. 7(3): 185-192. https://doi.org/10.15171/jhp.2018.30
Sanchez-Moreno, C., Larrauri, J.A., and Saura-Calixto, F. 1998. A procedure to measure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture. 76: 270-276. https://doi.org/10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9
Schett, G. and Neurath, M.F. 2018. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nature Communications. 9: 3261. https://doi.org/10.1038/s41467-018-05800-6
Sieniawska, E., Świątek, Ł., Sinan, K.I., Zengin, G., Boguszewska, A., Polz-Dacewicz, M., Sadeer, N.B., Etienne, O.K., and Mahomoodally, M.F. 2022. Phytochemical insights into Ficus sur extracts and their biological activity. Molecules. 27(6): 1863. https://doi.org/10.3390/molecules27061863
Singleton, V.L., Orthofer, R., and Lamuela-Raventos, R.M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymology. 299: 152-178. https://doi.org/10.1016/S0076-6879(99)99017-1
Sofowora, A. 1993. Screening plants for bioactive agents. p.134-156. In: Medicinal plants and traditional medicine in Africa. Spectrum Books, Ibadan.
Sun, Y., Gao, L., Hou, W., and Wu, J. 2020. β-sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-Exposed BV2 cells. BioMed Research International. 2020: 7532306. https://doi.org/10.1155/2020/7532306
Trease, G.E. and Evans, W.C. 2003. Pharmacognosy. Saunders, London.
Tsao, R. 2010. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2(12): 1231-1246. https://doi.org/10.3390/nu2121231
Van-Wagenen, B.C., Larsen, R., Cardellina, J.H., Randazzo, D., Lidert, Z.C., and Swithenbank, C. 1993. Ulosantion, a potent insecticide from the sponge Ulosa ruetzleri. The Journal of Organic Chemistry. 58: 335-337. https://doi.org/10.1021/jo00054a013
Vasudevara, B. and Sravanthi, D.J. 2017. GC/MS analysis and in-vitro antioxidant activity of methanol extract of Ulothrix flacca and its main constituent dimethyl sulfone. IOSR Journal of Pharmacy and Biological Sciences. 12(1): 93-104. https://doi.org/10.9790/3008-12010193104
Yadav, V.R., Prasad, S., Sung, B., Kannappan, R., and Aggarwal, B.B. 2010. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel). 2(10): 2428-66. https://doi.org/10.3390/toxins2102428
Yoon, H. and Baek, S.J. 2005. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Medical Journal. 46(5): 585-596. https://doi.org/10.3349/ymj.2005.46.5.585
Zandavar, H. and Afshari Babazad, M. 2023. Secondary metabolites: Alkaloids and flavonoids in medicinal plants. IntechOpen, London. https://doi.org/10.5772/intechopen.108030
Zhang, P., Liu, N., Xue, M., Zhang, M., Liu, W., Xu, C., Fan, Y., Meng, Y., Zhang, Q., and Zhou, Y. 2023. Anti-inflammatory and antioxidant properties of β-sitosterol in copper sulfate-induced inflammation in Zebrafish (Danio rerio). Antioxidants (Basel, Switzerland). 12(2): 391. https://doi.org/10.3390/antiox12020391
Zhang, P., Wang, H., Xu, X., Ye, Y., and Zhang, Y. 2024. Correlation analysis between phytochemical composition and biological activities of Artemisia scoparia. Food Bioscience. 62: 105342. https://doi.org/10.1016/j.fbio.2024.105342
Zhang, Y., Cai, P., Cheng, G., and Zhang, Y. 2022. Brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Natural Product Communications. 17(1): 1-14. https://doi.org/10.1177/1934578X211069721
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Uchenna Benjamin Okeke1, 2 *, Patrick Igbinaduwa2, Joseph Adetunji Aladesanmi3, Vuyisa Mzozoyana4, and Ibrahim Oluwatobi Kehinde5
1 Department of Pharmaceutical and Medicinal Chemistry, College of Pharmacy, Afe Babalola University, Ado-Ekiti 360102, Nigeria.
2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Benin, Benin City 300001, Nigeria.
3 Department of Pharmacognosy and Natural Products, College of Pharmacy, Afe Babalola University, Ado-Ekiti 360102, Nigeria.
4 School of Chemistry and Physics, Westville Campus, University of KwaZulu-Natal, Durban 4001, South Africa.
5 Molecular Bio-computation and Drug Design Laboratory, School of Health Sciences, University of KwaZulu-Natal, Durban 4001, South Africa.
Corresponding author: Uchenna Benjamin Okeke, E-mail: okeke.uchenna@abuad.edu.ng
ORCID iD:
Uchenna Benjamin Okeke: https://orcid.org/0000-0003-0384-9408
Patrick Igbinaduwa: https://orcid.org/0000-0002-1527-187X
Vuyisa Mzozoyana: https://orcid.org/0000-0002-6679-1541
Ibrahim Oluwatobi Kehinde: https://orcid.org/0000-0002-9425-0267
Total Article Views
Editor: Wipawadee Yooin,
Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: August 5, 2025;
Revised: September 29, 2025;
Accepted: October 10, 2025;
Online First: October 21, 2025

