
Potential and Characteristic Combinations of Collagen with Natural Polymer-Based Hydrogel for Burn Wound Dressing Applications
Khadijah Zai*, Lintang Kusuma Ratri, Chasna Salsabila Rosydiana, and Khansa Auliya Putri DewantoPublished Date : October 17, 2025
DOI : https://doi.org/10.12982/NLSC.2026.013
Journal Issues : Number 1, January-March 2026
Abstract Burn injuries are a common type of open wound, often caused by exposure to heat sources such as fire, hot liquids, or heated surfaces. Without proper treatment, these injuries can lead to complications like tissue necrosis and eschar formation, which impair healing and increase infection risk. Effective wound management is essential to accelerate recovery and minimize damage. Hydrogel dressings made from hydrophilic polymers have emerged as promising materials due to their ability to maintain moisture, absorb exudate, and provide a cooling effect. Natural polymers such as collagen, κ-carrageenan, chitosan, and alginate are particularly attractive for biomedical applications owing to their biocompatibility, biodegradability, and non-toxicity. In this study, hydrogel film formulations combining natural polymers were developed and optimized using the Simplex Lattice Design (SLD) method to determine the optimal polymer composition. Films were evaluated for swelling ratio, water vapor transmission rate (WVTR), degradation rate, and tensile strength. Among the tested combinations, the collagen–alginate hydrogel film showed the most desirable characteristics, including a swelling ratio of 100.75 ± 6.70%, WVTR of 1070.36 ± 221.43 g/m²·day, 33.44 ± 5.09% degradation over 8 days, and tensile strength of 0.061 ± 0.004 MPa. These values indicate good performance in moisture management, durability, and controlled degradation. Overall, the results demonstrate that collagen–alginate hydrogel films are promising candidates for burn wound management. Future work will focus on incorporating bioactive agents and conducting in vitro and in vivo studies to evaluate safety, efficacy, and wound healing performance.
Keywords: Biodegradable films, Biopolymer, Hydrogel, Chitosan, Wounds, Collagen
Graphical Abstract:

Citation: Zai, K., Ratri, L.K., Rosydiana, C.S., and Dewanto, K.A.P. 2026. Potential and characteristic combinations of collagen with natural polymer-based hydrogel for burn wound dressing applications. Natural and Life Sciences Communications. 25(1): e2026013.
INTRODUCTION
Burn wounds, primarily caused by heat from fire, hot liquids, or solids, can compromise the epidermis and trigger tissue necrosis and eschar formation (Jeschke et al., 2020; Surowiecka et al., 2022). Effective management requires dressings that maintain a moist environment, absorb exudate, dissipate heat, and protect against infection, thereby promoting faster wound healing and reducing complications (Stoica et al., 2020; Ghosh et al., 2021).
Hydrogels are particularly suitable for burn wound dressings due to their hydrophilic polymer networks, which retain water, provide a cooling effect, and promote tissue regeneration (Madaghiele et al., 2014). Hydrogels composed of a single polymer often exhibit limited mechanical strength or gel-forming capacity. Therefore, combining collagen, the predominant protein in connective tissue, with natural polymers such as alginate, chitosan, or κ-carrageenan can enhance structural integrity, swelling capacity, and overall performance (Zhang et al., 2018; Li et al., 2022).
The simplex lattice design (SLD) method was selected for optimization because it is particularly suitable for formulations consisting of two or three component mixtures. In SLD, the total amount of independent variables (formulation components) is held constant, which facilitates the mathematical structuring and interpretation of data (Bolton, 1997; Bolton and Bon, 2010). Incorporating this design enables systematic exploration of polymer ratios and supports the identification of optimum formulations from complex mixtures.
Although collagen–polymer hydrogels have been widely studied, systematic optimization of polymer ratios using Simplex Lattice Design (SLD) and assessment of hydrogel stability after autoclave sterilization have been less explored. In this study, we aim to develop and optimize collagen-based hydrogel films combined with alginate, chitosan, or κ-carrageenan using SLD; characterize the films in terms of swelling ratio, water vapor transmission rate (WVTR), degradation, and tensile strength; and evaluate the structural integrity of hydrogels after sterilization, an essential step for clinical translation.
This approach provides a systematic framework for optimizing collagen–polymer hydrogels and identifies formulations suitable for future in vivo burn wound applications, highlighting both the methodological and practical novelty of this work.
MATERIALS AND METHODS
Materials
Hydrolyzed collagen was purchased from Vinh Wellness. Sodium alginate was purchased from Kimica Co. Chitosan was purchased from Chimultiguna (Indonesia). κ-carrageenan was purchased from Indo Food Chem (Indonesia). Calcium dichloride and acetic acid were purchased from Sigma Aldrich. Potassium chloride, sodium tripolyphosphate (TPP), and sodium chloride were purchased from Xilong Scientific.
Combination of Collagen and Alginate-Based Hydrogel Film
The variation in collagen and alginate concentrations was determined using the simplex lattice design (SLD) method. The lower and upper limits for collagen concentration were established at 1–2.5%, while for alginate, they were set at 2.5–4%. The total concentration of the collagen and alginate combination used was 5%. The SLD produced 8 formulation runs (Table 1).
Tabel 1. Variance of the combination of collagen and alginate.
|
Material |
Formula (% w/v) |
|||||||
|
Run 1 |
Run 2 |
Run 3 |
Run 4 |
Run 5 |
Run 6 |
Run 7 |
Run 8 |
|
|
Collagen |
1 |
1.375 |
1 |
2.125 |
1.75 |
1.75 |
2.5 |
2.5 |
|
Alginate |
4 |
3.625 |
4 |
2.875 |
3.25 |
3.25 |
2.5 |
2.5 |
|
CaCl2* |
0.16 |
0.145 |
0.16 |
0.115 |
0.13 |
0.13 |
0.1 |
0.1 |
|
Water |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
Note: *The use of CaCl2 follows the alginate concentration, with a ratio of 0.1% CaCl2 for every 2.5% alginate.
Combination of collagen and chitosan-based hydrogel film
A hydrogel formulation was carried out by varying the concentrations of collagen, chitosan, and tripolyphosphate (TPP) as a cross-linking agent. The collagen concentration used ranged from 0.2% to 5%, chitosan from 0.5% to 2.5%, and TPP from 1% to 3%. The defined concentration range for collagen was 1.5–3.5%. The total combined concentration of collagen, chitosan, and TPP was 7%. A total of 14 formulation runs were generated using the SLD (simplex lattice design) approach, as shown in Table 2.
Table 2. Variance of the combination of collagen and chitosan.
|
Formula(%W/V) |
||||||||||||||
|
Material |
Run 1 |
Run 2 |
Run 3 |
Run 4 |
Run 5 |
Run 6 |
Run 7 |
Run 8 |
Run 9 |
Run 10 |
Run 11 |
Run 12 |
Run 13 |
Run 14 |
|
Collagen |
2.83 |
3.50 |
3.50 |
2.50 |
3.50 |
3.50 |
3.50 |
2.50 |
2.50 |
3.50 |
1.50 |
2.50 |
2.50 |
2.50 |
|
Chitosan |
1.83 |
2.50 |
1.50 |
1.50 |
0.50 |
2.50 |
1.50 |
2.50 |
1.50 |
2.50 |
1.50 |
2.50 |
1.50 |
2.50 |
|
TPP |
2.30 |
1.00 |
2.00 |
3.00 |
1.00 |
2.00 |
2.00 |
2.00 |
3.00 |
2.00 |
3.00 |
3.00 |
3.00 |
3.00 |
|
Acetic acid |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
|
Distilled water |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
to 100 |
Combination of collagen and κ-carrageenan-based hydrogel film
The hydrogel was prepared by optimizing the concentrations of the hydrogel-forming polymers, namely collagen (0.2–5%), κ-carrageenan (0.8–1.5%), and KCl (0.3–1%) as a crosslinking agent. The total polymer concentration in the formulation was 5%, based on the upper limit of hydrolyzed collagen concentration required to form a gel. The variations in collagen, kappa-carrageenan, and KCl concentrations were then determined using the simplex lattice design (SLD) method (Table 3).
Table 3. Variance of the combination of collagen and κ-carrageenan.
|
Run |
Materials |
||
|
Collagen (%) |
κ-carrageenan (%) |
KCl (%) |
|
|
1 |
3.65 |
0.91 |
0.44 |
|
2 |
3.08 |
0.92 |
1.00 |
|
3 |
3.27 |
0.98 |
0.75 |
|
4 |
3.10 |
1.50 |
0.40 |
|
5 |
2.83 |
1.50 |
0.67 |
|
6 |
3.07 |
1.23 |
0.70 |
|
7 |
3.30 |
1.23 |
0.47 |
|
8 |
3.07 |
1.23 |
0.70 |
|
9 |
3.55 |
1.15 |
0.30 |
|
10 |
2.59 |
1.45 |
0.96 |
|
11 |
3.52 |
0.80 |
0.68 |
|
12 |
3.07 |
1.23 |
0.70 |
|
13 |
2.83 |
1.17 |
1.00 |
|
14 |
3.55 |
1.15 |
0.3 |
|
15 |
2.59 |
1.45 |
0.96 |
|
16 |
3.90 |
0.80 |
0.30 |
|
17 |
3.52 |
0.80 |
0.68 |
Preparation of collagen and alginate-based hydrogel film
Each of the collagens and alginate were dissolved in water until completely dissolved. The two solutions were then mixed under stirring at 100 rpm until a homogeneous mixture was obtained. The mixture was subsequently sonicated at 37°C for 15 minutes to remove trapped air bubbles. Next, the solution was poured into a 9 cm diameter petri dish with a volume of 7.5 mL and incubated at 37°C for 1 hour to allow gel formation. Then, 5 mL of CaCl2 solution was added to the petri dish and incubated for 10 minutes. After this, the hydrogel underwent a series of rinses with water, totaling three cycles, with the objective of eliminating the superfluity of CaCl2. In the final step, the hydrogel was dried at 50°C for three hours in an oven.
Preparation of collagen and chitosan-based hydrogel film
Collagen was dissolved in water and stirred until homogeneous. Chitosan was dissolved in 0.125 M acetic acid solution until completely dissolved. Sodium tripolyphosphate was dissolved in a certain amount of water. Subsequently, the three solutions were mixed in a ratio of 3:3:2 collagen:chitosan:sodium tripolyphosphate. The solution mixture was stirred until homogeneous at 150 rpm. Then, the solution was sonicated at 37°C for 15 min to remove trapped air bubbles. The solution was then poured into a 9 cm diameter petri dish with a thickness of 0.4 cm. Subsequently, the hydrogel was subjected to an incubation at 37°C for a duration of one hour, which served as the crosslinking process. After that, the sample was stored at -10°C for 20 hours and then incubated at room temperature for 4 hours. This cycle was repeated three times. In the final step, the hydrogel was dried at 50°C for 6 hours in an oven.
Preparation of collagen and κ-carrageenan-based Hydrogel Film
κ-carrageenan was dissolved in hot water (80°C). Subsequently, KCl was introduced to the mixture as a crosslinking agent to facilitate the formation of a gel-like substance. Hydrolyzed collagen was dissolved separately in hot water (60°C) under stirring. The κ-carrageenan-KCl solution was then mixed with the hydrolyzed collagen solution and homogenized using an electric stirrer. The resulting solution was poured into a 9 cm diameter Petri dish. The polymer mixture was poured to a thickness of 0.4 cm and then dried at 50°C for 8 hours to form a hydrogel film.
Characterization of hydrogel film
pH value test
The pH measurement of the hydrogel was carried out using a universal pH indicator solution (LobaChemie), with 50 µL dropped onto the hydrogel.
Swelling ratio
The swelling ratio of hydrogel films was determined using a gravimetric method. Samples (1×1 cm2) were dried at 80°C for 3 hours to obtain the initial dry weight (w0). They were then immersed in simulated wound fluid (SWF: 0.368 g CaCl2 + 8.298 g NaCl in 1,000 mL distilled water) at 37°C (Khabbaz et al., 2019). At 15-minute intervals, excess surface fluid was removed with a non-woven polyester cloth, and the swollen hydrogel was weighed (wₜ) until a constant weight was reached. The swelling ratio (%) was calculated using Equation 1. All measurements were performed in triplicate (n = 3).
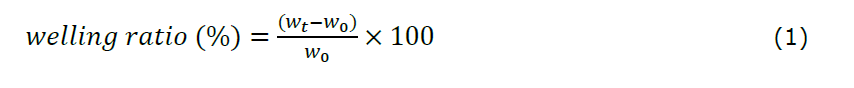
Note:
w0: initial weight (mg)
wt: weight at time (mg)
Water vapor transmission rate (WVTR)
The Water Vapor Transmission Rate (WVTR) test was conducted by measuring the evaporation of water from a bottle sealed with the hydrogel film. The test bottle was first filled with distilled water up to ¾ of its volume from the bottle’s opening, then weighed to obtain the initial weight before incubation (w₁). The hydrogel sample was cut into a circular shape and sealed onto the bottle opening using PTFE tape. The sealed bottle was then placed in an incubator at 37°C for 24 hours. Subsequently, the bottle containing water was subjected to 24 hours of equilibration. Thereafter, the bottle was re-weighed (w2), and the WVTR value was calculated using Equation 2. All experiments were performed in triplicate (n = 3).
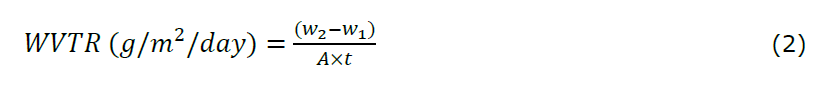
Note:
w1: weight of bottle before incubation
w2: weight of bottle after incubation
A: the mouth area of the bottle
Hydrogel film degradation
Degradation testing was carried out by immersing 1×1 cm hydrogel film samples in 10 mL of simulated wound fluid (SWF) at 37°C. The hydrogel films were first cut into 1×1 cm2 pieces and dried in an oven at 80°C for 3 hours. After drying, the initial dry weight of the hydrogel was recorded. The hydrogel was subsequently submerged in a simulated wound fluid (SWF) maintained at a temperature of 37°C. The weight of the hydrogel was measured at 30-minute intervals until it reached the maximum swelling ratio and showed a decrease in weight. Subsequent measurements were continued until the hydrogel film was completely degraded. The percentage of degradation was calculated based on the weight loss from the point of maximum swelling to the time at which the hydrogel was fully degraded. The degradation ratio percentage was calculated using equation 3. All experiments were performed in triplicate (n = 3).
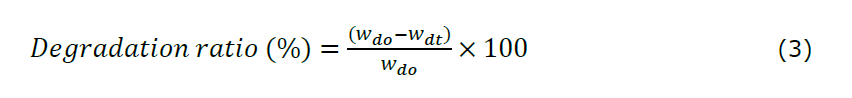
Note:
wdo: initial weight of the sample before degradation (after reaching maximum swelling)
wdt: weight of the sample after degradation
Tensile strength test
Mechanical strength testing was performed on the optimized hydrogel film, both sterilized and non-sterilized. This test was designed to ascertain the tensile strength and elongation at break of the hydrogel films, as these are pivotal parameters that define their mechanical properties. The films were cut into rectangular strips measuring 2.5 × 5 cm2, then tested using a Universal Testing Machine (UTM) at a loading speed of 10 mm/min with a load capacity of 2.5 kN. The values of elongation at break and tensile strength were calculated using Equations 4 and 5. All experiments were performed in triplicate (n = 3).
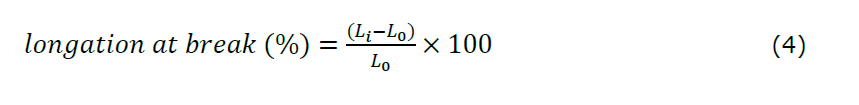
Note:
L0: initial length (mm)
Li: final length (mm)

Note:
Fmax: the maximum force generated at the point of sample fracture (N)
A: cross-sectional area of the sample (mm2)
Determination of The Optimum Formula
The determination of the optimum formulation was based on hydrogel characterization results, including swelling ratio, WVTR, and degradation. Selection of the optimum formulation was performed using the Simplex Lattice Design (SLD) with the criteria shown in Table 4. The formulation that maximized desirability was identified as the optimal formulation.
Table 4. The range of variables and criteria used for optimization.
|
Collagen-carrageenan |
|||||
|
Variable |
Criteria |
Goal |
Lower |
Upper |
|
|
Independent variable |
Collagen (%) |
in range |
2.5 |
3.9 |
|
|
kappa-Carrageenan (%) |
in range |
0.8 |
1.5 |
|
|
|
KCl (%) |
in range |
0.3 |
1 |
|
|
|
Dependent variable |
Swelling ratio (%) |
maximize |
100 |
300 |
|
|
WVTR (g/m2.day) |
in range |
500 |
2,000 |
|
|
|
Degradation (%) |
minimize |
0 |
100 |
|
|
|
Collagen-chitosan |
|||||
|
Independent variable |
Collagen (%) |
in range |
1.5 |
3.5 |
|
|
Chitosan (%) |
in range |
0.5 |
2.5 |
|
|
|
TPP (%) |
in range |
1 |
3 |
|
|
|
Dependent variable |
Swelling ratio (%) |
maximize |
100 |
600 |
|
|
WVTR (g/m2.day) |
in range |
2,000 |
4,000 |
|
|
|
Degradation (%) |
minimize |
20 |
100 |
|
|
|
Collagen-alginate |
|||||
|
Independent variable |
Collagen (%) |
in range |
1 |
2.5 |
|
|
Alginate (%) |
in range |
2.5 |
4 |
|
|
|
Dependent variable |
Swelling ratio (%) |
in range |
100 |
200 |
|
|
WVTR (g/m2.day) |
maximize |
279 |
2,000 |
|
|
|
Degradation (%) |
maximize |
0 |
50 |
|
|
Sterilization of hydrogel film
The optimized hydrogel formulation was sterilized using steam heat sterilization in an autoclave. Steam sterilization is typically conducted at temperatures ranging from 121–130°C for a short duration of 15–20 minutes. In this study, the hydrogel film was sterilized by placing it in an autoclave at 121°C for 15 minutes.
Data Analysis and Optimization
All experiments were performed in triplicate, and the mean ± standard deviation (SD) was calculated for each response (swelling ratio, WVTR, degradation, tensile strength). These values were then entered into the Simplex Lattice Design (SLD) software to generate predictive models, contour plots, and desirability functions. The SLD method was used to identify optimum polymer combinations and ensure that experimental variability was appropriately considered.
RESULTS
Physical properties of hydrogel film
The three hydrogel film formulations differed in visual and physical properties (Figure 1). Collagen–κ-carrageenan hydrogels were clear and smooth, with a thickness of 0.1–0.3 mm and pH 5.0–5.5. Collagen–chitosan hydrogels had a slightly rougher texture, were opaquer, 0.22–0.37 mm thick, and pH ranged from 4.5–5.3. Collagen–alginate hydrogels were thicker (0.33–0.48 mm), with pH between 6.0–7.3.
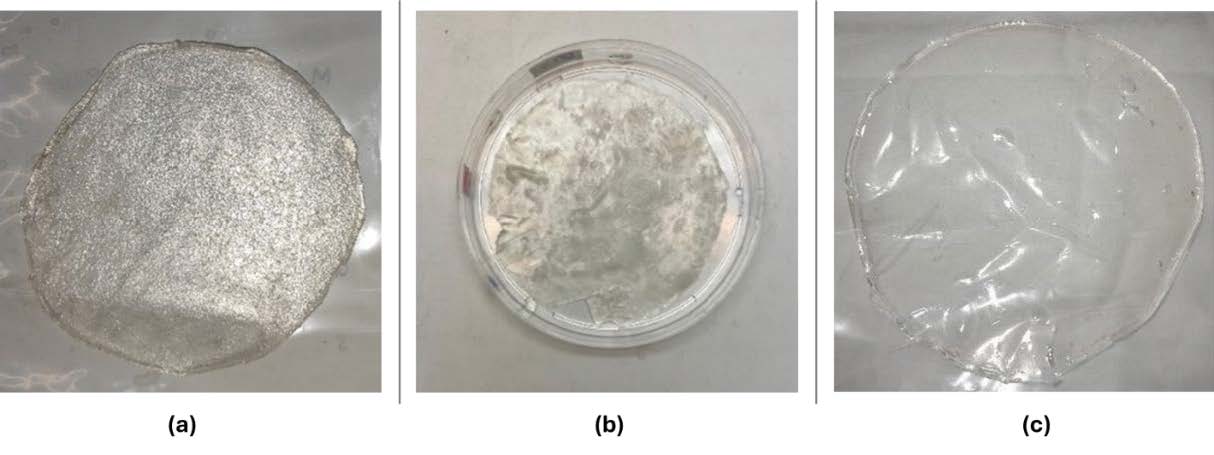
Figure 1. Appearance of hydrogel film a) collagen and κ-carrageenan-based hydrogel film, b) collagen and chitosan-based hydrogel film, c) collagen and alginate-based hydrogel film.
Swelling ratio
All hydrogels exhibited swelling ratios >100%, meeting the criterion for wound dressings (Figure 2). For collagen–κ-carrageenan films, swelling ranged from 124.3 ± 48.2% (Run 1, low κ-carrageenan 0.91%) to 361.9 ± 81.5% (Run 4, high κ-carrageenan 1.5%) (Figure 2a). Collagen–chitosan films showed swelling between 218.2 ± 25.1% (Run 4, low collagen 2.5%, high TPP 3%) and 573.8 ± 17.2% (Run 11, moderate collagen 1.5%, low TPP 3%) (Figure 2b). Collagen–alginate films exhibited swelling from 52.3 ± 9.7% (Run 8, high collagen 2.5%) to 105.6 ± 21.7% (Run 3, low collagen 1%, high alginate 4%) (Figure 2c). All values represent the mean of three independent replicates (n = 3); summary data are provided in Supplementary Tables S1–S3. Contour plots in Figure 2 were generated using the Simplex Lattice Design (SLD) model based on these averages.
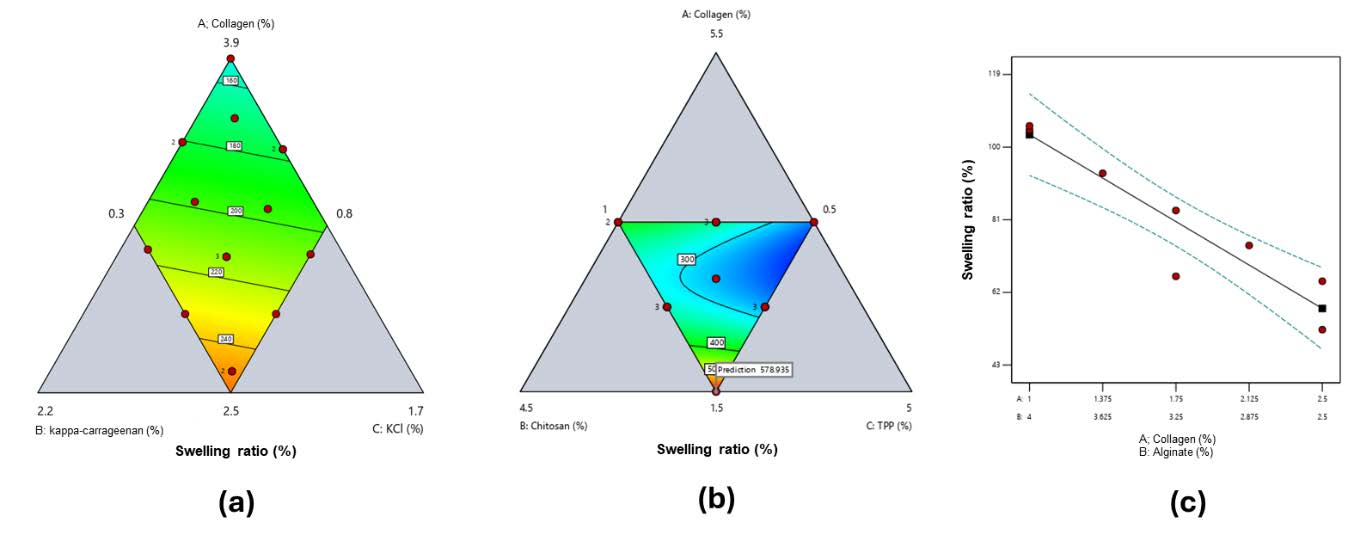
Figure 2. Contour plot response of swelling ratio for a) collagen and κ-carrageenan-based hydrogel film (red to blue green: high to low), b) collagen and chitosan-based hydrogel film (red to blue green: high to low), c) collagen and alginate-based hydrogel film.
Water vapor transmission rate (WVTR)
WVTR values for all hydrogels fell within the suitable range for burn wound care (Figure 3). Collagen–κ-carrageenan films showed a decrease from 1,264 ± 7 g/m²·day (Run 1, low κ-carrageenan 0.91%) to 765 ± 23 g/m²·day (Run 5, high κ-carrageenan 1.5%) (Figure 3a). For collagen–chitosan films, WVTR increased from 2,309 ± 239 g/m²·day (Run 4, low collagen 2.5%, high TPP 3%) to 3,665 ± 170 g/m²·day (Run 5, high chitosan 2.5%, moderate collagen 3.5%) (Figure 3b). Collagen–alginate films exhibited WVTR values ranging from 968 ± 39 g/m²·day (Run 4, high collagen 2.125%) to 1,508 ± 242 g/m²·day (Run 1, low collagen 1%) (Figure 3c). All values are averages of three replicates (n = 3), with summary data in Supplementary Tables S1–S3. Contour plots in Figure 3 were generated using SLD based on these averages.
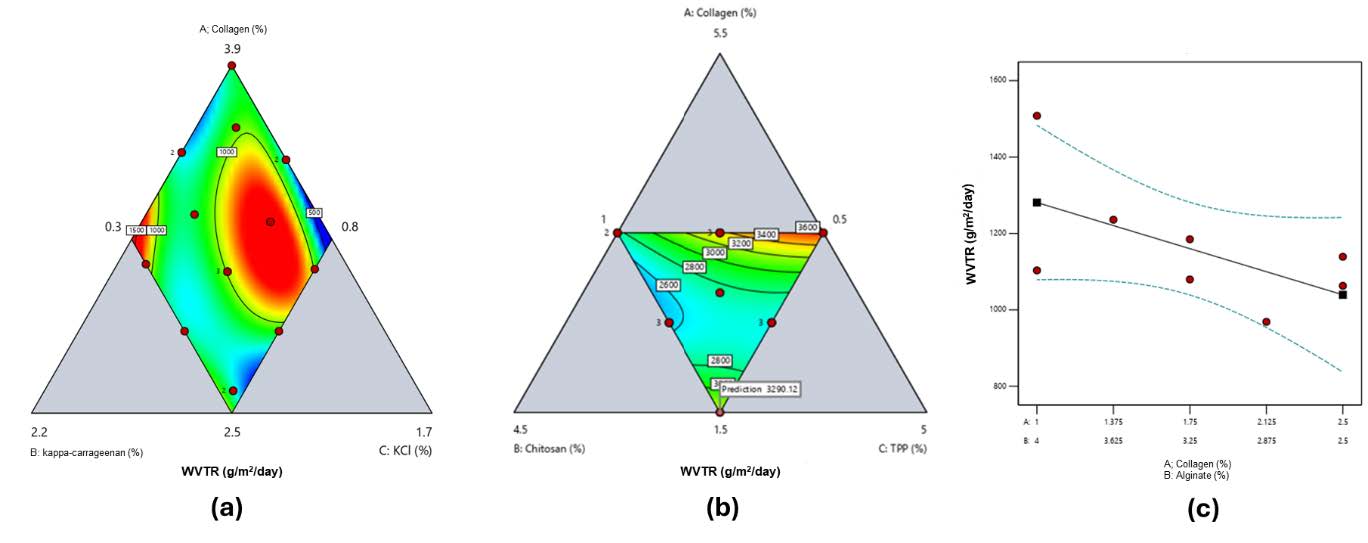
Figure 3. Contour plot response of water vapor transmission rate (WVTR) ratio for a) collagen and κ-carrageenan-based hydrogel film (red to blue green: high to low), b) collagen and chitosan-based hydrogel film (red to blue green: high to low), c) collagen and alginate-based hydrogel film.
Hydrogel film degradation
Degradation patterns were formulation-dependent (Figure 4). In collagen–κ-carrageenan films, degradation ranged from 0 ± 0% (Runs 2–3, high κ-carrageenan) to 100 ± 0% (Run 1, low κ-carrageenan) (Figure 4a). Collagen–chitosan films exhibited degradation from 4.1 ± 3.5% (Run 2, high collagen 3.5%, low TPP 1%) to 100 ± 0% (Run 5, high chitosan 2.5%, high TPP 3%) (Figure 4b). Collagen–alginate films showed non-linear degradation, with values from 0 ± 0% (Run 8, high collagen 2.5%) to 100 ± 0% (Run 4, intermediate collagen 2.125%) (Figure 4c). All results represent means of three independent replicates (n = 3), with summary data in Supplementary Tables S1–S3. Contour plots in Figure 4 were generated using SLD based on these averages.
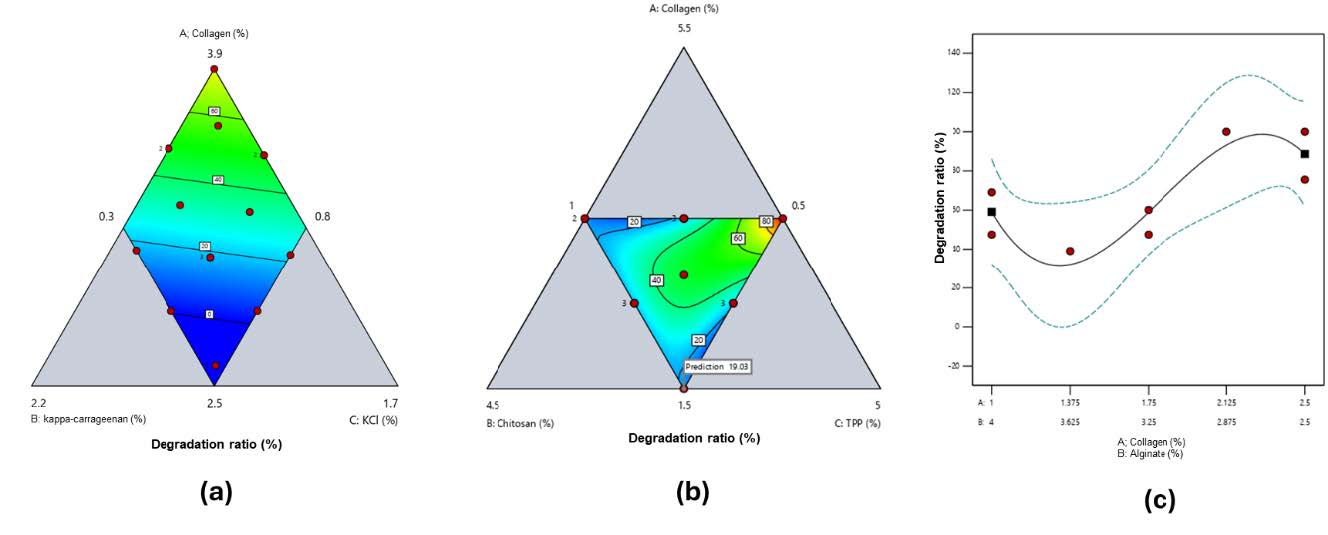
Figure 4. Contour plot response of degradation ratio for a) collagen and κ-carrageenan-based hydrogel film (red to blue green: high to low), b) collagen and chitosan-based hydrogel film (red to blue green: high to low), and c) collagen and alginate-based hydrogel film.
Determination of the optimum formula
Simplex Lattice Design (SLD) provided optimum formulations based on swelling ratio, WVTR, and degradation (Figure 5). Optimal compositions were:
a) Collagen–κ-carrageenan: 2.5% collagen, 1.5% κ-carrageenan, 1% KCl (Desirability = 0.880)
b) Collagen–chitosan: 1.5% collagen, 2.5% chitosan, 3% TPP (Desirability = 0.973)
c) Collagen–alginate: 1% collagen, 4% alginate (Desirability = 0.793)
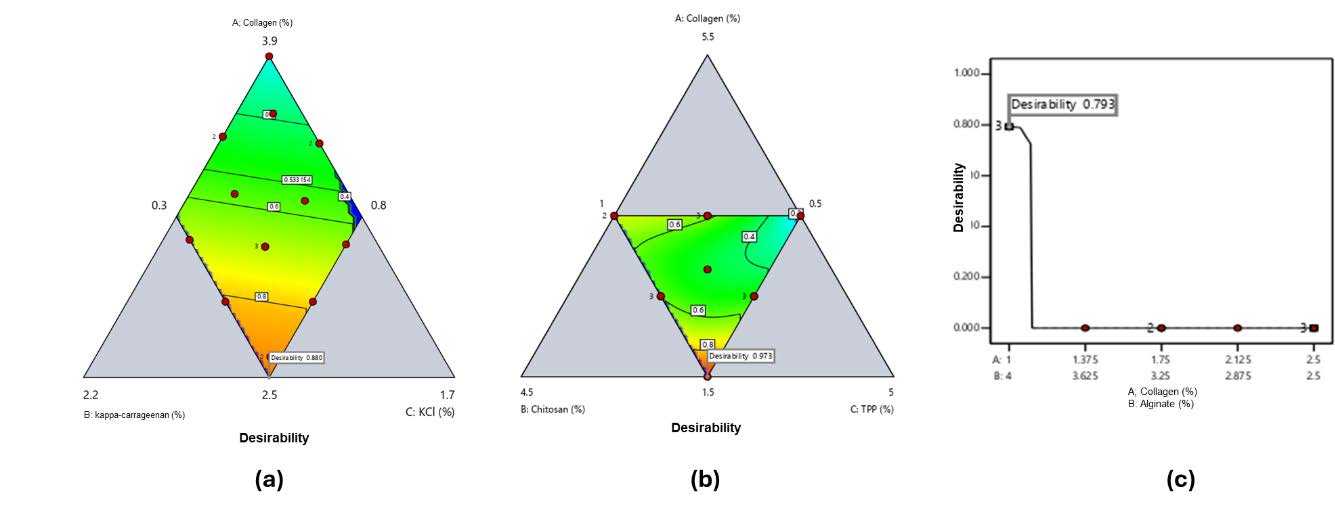
Figure 5. Contour plot response of desirability value for a) collagen and κ-carrageenan-based hydrogel film (red to blue green: high to low), b) collagen and chitosan-based hydrogel film (red to blue green: high to low), and c) collagen and alginate-based hydrogel film.
Table 5 shows that the collagen–chitosan film had the highest swelling (576.70%) but degraded rapidly (100% in 4 hrs). Collagen–alginate films exhibited acceptable swelling (104.99%) and maintained structural integrity for 8 days, with degradation reaching only 45.85 ± 13.35% at the end of the experiment. Collagen–κ-carrageenan films exhibited moderate swelling (216.91%) and complete degradation after 6 hrs, with low tensile strength (0.031 MPa).
Table 5. Characteristics of the optimum hydrogel film formulations (N=3).
|
Parameter |
Collagen-carrageenan |
Collagen-chitosan |
Collagen-alginate |
|
Thickness (mm) |
0.20 ± 0.04 |
0.44 ± 0.04 |
0.47 ± 0.02 |
|
Swelling ratio (%) |
216.912 ± 93.650 |
576.697 ± 22.495 |
104.990 ± 5.203 |
|
WVTR (g/m2.day) |
1,152.220 ± 12.640 |
3,115.877 ± 317.129 |
1,385.005 ± 293.969 |
|
Degradation (%) |
100 (after 6 hr) |
100 (after 4 hr) |
45.850 ± 13.348 (after 8 days) |
|
Tensile strength (MPa) |
0.031 ± 0.001 |
0.073 ± 0.050 |
0.072 ± 0.050 |
Sterilization of hydrogel film
Sterilization via autoclaving (121°C, 15 min) preserved the properties of collagen–κ-carrageenan and collagen–alginate films (Table 6). However, the collagen–chitosan film degraded post-sterilization and was unsuitable for thermal methods.
Table 6. Characteristics of the sterilized optimum hydrogel film formulations (N=3).
|
Parameter |
Collagen-carrageenan |
Collagen-chitosan |
Collagen-alginate |
|
Thickness (mm) |
0.19 ± 0.06 |
- |
0.45 ± 0.02 |
|
Swelling ratio (%) |
240.505 ± 99.265 |
- |
100.750 ± 6.695 |
|
WVTR (g/m2.day) |
1,190.640 ± 45.580 |
- |
1,070.357 ± 221.426 |
|
Degradation (%) |
100 (after 6 hr) |
- |
33.438 ± 5.091 (after 8 days) |
|
Tensile strength (MPa) |
0.054 ± 0.007 |
- |
0.061 ± 0.004 |
DISCUSSION
The physical characteristics presented in Tables 5 and 6 show that all optimum hydrogel films had thickness values below that of the human dermis (0.5–2.0 mm). Specifically, collagen–carrageenan films exhibited the lowest thickness (0.20 ± 0.04 mm), followed by collagen–chitosan (0.44 ± 0.04 mm) and collagen–alginate (0.47 ± 0.02 mm). Such thin structures are advantageous because they can absorb wound exudate more effectively and conform better to the wound bed (Bierhalz et al., 2016). Additionally, it was found that the physical characteristics of the resultant hydrogels were affected by the concentrations of polymer and crosslinker employed. Hydrogels with a high collagen concentration tend to produce films with a smoother texture due to a reduction in pore size within the structure (Tucker and Lai, 2024).
Swelling ratio testing was conducted to evaluate the hydrogel film's ability to absorb wound exudate and maintain a moist wound environment without causing exudate accumulation that could interfere with the healing process (Gonçalves et al., 2020). An ideal wound dressing should enhance exudate drainage while maintaining a moist environment; therefore, a hydrogel used as an effective wound dressing should have a swelling ratio value greater than 100% (Morgado et al., 2015).
Swelling behavior (Figure 2) was highly formulation dependent. The component that most significantly influenced the increase in swelling ratio of the collagen and κ-carrageenan hydrogel film was κ-carrageenan (Figure 2a). This effect is attributed to the enhanced hydrophilicity of the hydrogel caused by increasing concentrations of κ-carrageenan (Hezaveh et al., 2012). In contrast, in the collagen–chitosan-based hydrogel film, an increase in chitosan concentration led to a reduction in the swelling ratio (Figure 2b). This phenomenon can be attributed to a concurrent rise in the concentration of the crosslinking agent, TPP. The interaction between chitosan and collagen in an acidic environment increases the positive charge along the collagen chains. This electrostatic repulsion between chains opens the structure, thereby increasing porosity and swelling (Anindyajati et al., 2020). However, the interaction between collagen and crosslinkers such as TPP may result in a less elastic structure, which is less capable of expanding due to excessive crosslinking, ultimately reducing the swelling ratio (Ghica et al., 2017). Moreover, the swelling ratio of the collagen–alginate-based hydrogel film was highest at the highest alginate concentration and lowest collagen concentration (Figure 2c). A high alginate content in the hydrogel forms a more hydrophilic network, thus improving the swelling capacity of the hydrogel (Ahn et al., 2019).
The moisture permeability of the hydrogel was assessed through the implementation of WVTR testing. As a wound dressing for burn injuries, hydrogel must have WVTR to provide an environment that supports the wound healing process. A hydrogel with an excessively high WVTR can cause rapid water loss, leading to dehydration of the wounded skin. Conversely, a hydrogel with a WVTR that is too low can lead to the accumulation of wound exudate, resulting in maceration of the surrounding healthy tissue and causing pain for the patient (Morgado et al., 2015). Therefore, an ideal wound dressing should have a WVTR value higher than that of normal skin (Ujang et al., 2014). Normal skin has a WVTR of approximately 204 g/m2/day, while the WVTR range for burned skin is about 279 – 5,138 g/m2/day (Ujang et al., 2014; Demeter et al., 2023).
WVTR trends (Figure 3) were consistent with polymer properties. The combination of collagen and κ-carrageenan led to a reduction in the hydrogel's WVTR, as illustrated in Figure 3a. This is due to the hydrophilic nature of κ-carrageenan, which allows the hydrogel to absorb moisture and provide hydration to the skin membrane (Khaliq et al., 2022). On the other hand, the WVTR value increased with the addition of polymer concentration in the collagen–chitosan hydrogel (Figure 3b). The hydrophilic nature of both collagen and chitosan can create open diffusion pathways, thereby increasing WVTR (Andonegi et al., 2020). However, at higher concentrations, a denser structure with fewer pores may form, which can reduce WVTR (Azuri et al., 2023). In contrast, the WVTR of collagen–alginate-based hydrogel films decreased with increasing collagen concentration in the formulation (Figure 3c). At lower collagen levels, the higher proportion of alginate provides an abundance of carboxylate groups for Ca2+ ionic crosslinking, forming a hydrated and porous network that facilitates greater vapor diffusion (Lee and Mooney, 2012; Pawar and Edgar, 2012). As collagen content increases, alginate’s relative fraction falls, reducing crosslinking sites, leading to a denser polymeric matrix with smaller pores and increased hydrogen bonding, all of which minimize hydrophilicity and lower WVTR (Jang et al., 2014; Liu et al., 2018).
Degradation testing of the film was conducted to determine the weight loss of the hydrogel over specific time intervals. During degradation, water molecules react with ester, amide, or ether groups, leading to the cleavage of the polymer chains composing the film. The resulting shorter polymer chains are released via diffusion, causing polymer mass loss (Pan and Brassart, 2022).
Degradation (Figure 4) patterns confirmed that collagen alone degrades quickly, while κ-carrageenan and TPP enhance stability. An increase in κ-carrageenan content resulted in a decrease in degradation percentage, likely due to the enhanced structural strength of the hydrogel film provided by crosslinking between κ-carrageenan and KCl. A similar trend was observed in collagen–chitosan-based hydrogel films, where increasing the concentration of both the polymer and the crosslinking agent (TPP) led to a decrease in degradation percentage (Figure 4b). However, a unique degradation pattern was observed in the collagen–alginate hydrogel films. As shown in Figure 4c, the degradation percentage curve appeared to bend downward and upward. This can be interpreted as follows: the interaction between collagen and alginate increases degradation at intermediate polymer concentrations, while at maximum and minimum concentrations, their interaction reduces the degradation of the hydrogel film.
The optimum formulation for each polymer combination was determined using the SLD method. In this study, an investigation was conducted to assess the influence of two types of polymer combinations, with or without a crosslinking agent, on the swelling ratio, WVTR, and percentage degradation of the resulting hydrogel films. The responses obtained were evaluated against the predefined objectives, either by maximizing or minimizing the target values (Bolton, 1997). Detailed parameters for this study are presented in Table 4.
Based on the parameter settings in Table 4, the SLD method generated a single run solution with the highest desirability value, approaching 1.0, indicating strong alignment between the experimental outcomes and predicted targets. The desirability values for each polymer combination are shown in Figure 5.
By following SLD recommendations, we prepared and characterized hydrogel films for each optimum formula. Table 5 shows that the collagen–chitosan-based hydrogel film exhibited the highest swelling ratio, reaching 576.697 ± 22.495%. However, the collagen–chitosan-based hydrogel film failed to maintain its structural integrity for long periods. Collagen–alginate-based hydrogel film demonstrated the longest ability to maintain its structural integrity and had a good enough swelling ratio (104.99%).
Moreover, hydrogel films must possess good mechanical strength to avoid restricting movement when applied to wound sites (Liu et al., 2023). An appropriate mechanical strength for hydrogel wound dressings ranges from 0.001 to 0.1 MPa, which corresponds to the mechanical properties of soft human tissue (Feiner and Dvir, 2017). All the optimized hydrogel film formulations demonstrated mechanical properties within this suitable range, making them appropriate for use as wound dressings (Table 5). Additionally, WVTR values for all hydrogel films were in a good range for wound burn skin dressings. Therefore, the collagen–alginate-based hydrogel film can be considered the most promising candidate for application in burn wound treatment, offering a balance of swelling ratio, WVTR value, structural integrity, flexibility, and mechanical compatibility with the skin.
Sterilization is a critical requirement for biomaterials intended for implantation or direct contact with living tissues, such as organs or wounds, to minimize the risk of infection and inflammation (Stoppel et al., 2014; Bento et al., 2023). The risk of wound infection increases significantly in the presence of microbial contamination. Therefore, hydrogel wound dressings must undergo sterilization to eliminate or inactivate any microbial contaminants introduced during the fabrication process and to ensure their safety for patient use (Palmer et al., 2012). Therefore, the optimum hydrogel film formulations were sterilized via steam autoclaving. Among available techniques, steam sterilization is widely used due to its practicality, accessibility, and effectiveness compared to gas or radiation methods (Han et al., 2017; de Sousa Iwamoto et al., 2022). However, for particularly sensitive biomaterials, alternative methods such as supercritical CO2 have been shown to preserve structural integrity better than classical sterilization (Bernhardt et al., 2015). Autoclaving, a process that involves the application of high temperatures and humidity, has been identified as a method for the destruction of the structural and metabolic components of microbial cells (Bento et al., 2023). The optimal hydrogel film formulations were sterilized in an autoclave at 121°C for 15 minutes. Following sterilization, the physical characteristics of the hydrogel films were re-evaluated to ensure that the process did not compromise their integrity or functionality.
Table 6 shows that the swelling ratio, WVTR, degradation percentage, and tensile strength of the hydrogel films after sterilization did not exhibit significant differences compared to the characteristics before sterilization based on t-test analysis (Table 5). This finding indicates that steam sterilization using an autoclave did not affect the properties of the collagen–κ-carrageenan and collagen–alginate-based hydrogel films. However, the collagen–chitosan-based hydrogel film was unable to maintain its crosslinked structure after autoclaving, resulting in film disintegration. Collagen, being a protein, is highly thermolabile, and exposure to heat can denature it into gelatin, disrupting the hydrogel structure (Zhang et al., 2020). Additionally, the ionic interactions between chitosan and TPP are not sufficiently strong to maintain hydrogel integrity under autoclave conditions, leading to structural breakdown (Galante et al., 2016). Therefore, it is recommended to use alternative terminal sterilization methods that are safe and compatible with heat-sensitive materials. If such methods are not available, the hydrogel films may be prepared under aseptic conditions to ensure sterility without compromising structural integrity.
Despite the promising results, this study has several limitations that should be considered. First, all experiments were conducted in vitro (non-cell-based experiment), and the biological performance of the hydrogels in vivo, including wound healing efficacy, biocompatibility, and potential immune responses, remains to be evaluated. Second, hydrogel films do not contain any active pharmaceutical or antimicrobial agents; their performance is based solely on polymer composition and physical properties. Third, while the Simplex Lattice Design (SLD) effectively optimized polymer ratios, it is constrained by the predefined concentration ranges and may not capture extreme combinations outside these limits. Finally, all experiments were performed under controlled laboratory conditions, and the influence of real wound environments (e.g., variable exudate, pH, or microbial load) on hydrogel performance was not assessed. These limitations highlight the need for future in vivo studies and potential incorporation of bioactive compounds to further validate the clinical utility of the optimized hydrogels.
CONCLUSION
The combination of collagen and natural polymers (κ-carrageenan, chitosan, and alginate) successfully produced hydrogel films with good characteristics that meet the requirements for burn wound dressings. Among the formulations, the collagen–alginate-based hydrogel film exhibited the most favorable and promising properties for application as a burn wound dressing. Furthermore, the use of terminal sterilization via autoclaving in this study supports the scalability of collagen–alginate hydrogel film production.
AUTHOR CONTRIBUTIONS
Concept – K.Z.; Design – K.Z., L.K.R., C.S.R., K.A.P.D.; Supervision – K.Z.; Resources – K.Z.; Materials – K.Z.; Data Collection and/or Processing – L.K.R., C.S.R., K.A.P.D; Analysis and/or Interpretation – L.K.R., C.S.R., K.A.P.D., K.Z.; Literature Search – L.K.R., C.S.R., K.A.P.D., K.Z.; Writing – L.K.R., C.S.R., K.A.P.D., K.Z.; Critical Reviews – K.Z.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahn, Y., Kim, H., and Kwak, S.-Y. 2019. Self‑reinforcement of alginate hydrogel via conformational control. European Polymer Journal. 116: 480-487. https://doi.org/10.1016/j.eurpolymj.2019.03.017
Andonegi, M., Las Heras, K., Santos‑Vizcaíno, E., Igartua, M., Hernandez, R. M., de la Caba, K., and Guerrero, P. 2020. Structure‑properties relationship of chitosan/collagen films with potential for biomedical applications. Carbohydrate Polymers. 237: 116159. https://doi.org/10.1016/j.carbpol.2020.116159
Anindyajati, T. P., Lastianny, S. P., Yogianti, F., and Murdiastuti, K. 2020. Effect of collagen‑chitosan hydrogel formula combined with platelet‑rich plasma (A study of pH, viscosity, and swelling test). Majalah Kedokteran Gigi Indonesia. 6(3): 123-129. https://doi.org/10.22146/majkedgiind.44391
Azuri, M. S., Forid, M. S., Ishak, W. M. W., Ain, N., and Azhar, A. 2023. Optimization of chitosan concentration on physicochemical properties of polyvinyl alcohol‑based hydrogel. International Journal of Chemical and Biochemical Science. 24(8): 147-157.
Bento, S. A., Gaspar, M. C., Coimbra, P., de Sousa, H. C., and Braga, E. M. 2023. A review of conventional and emerging technologies for hydrogels sterilization. International Journal of Pharmaceutics. 634: 122671. https://doi.org/10.1016/j.ijpharm.2023.122671
Bernhardt, A., Wehrl, M., Paul, B., Hochmuth, T., Schumacher, M., Schütz, K., and Gelinsky, M. 2015. Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS One. 10(8): e0129205. https://doi.org/10.1371/journal.pone.0129205
Bierhalz, A. C. K., Westin, C. B., and Moraes, Â. M. 2016. Comparison of the properties of membranes produced with alginate and chitosan from mushroom and from shrimp. International Journal of Biological Macromolecules. 91: 496-504. https://doi.org/10.1016/j.ijbiomac.2016.05.095
Bolton, S. 1997. Pharmaceutical statistics, practical and clinical applications, 3rd ed. Marcel Dekker, Inc., New York.
Bolton, S. and Bon, C. 2010. Pharmaceutical statistics: Practical and clinical applications, 5th ed. Informa Healthcare, USA. https://doi.org/10.3109/9781420074239
Demeter, M., Scărișoreanu, A., and Călina, I. 2023. State of the art of hydrogel wound dressings developed by ionizing radiation. Gels. 9(1): 55. https://doi.org/10.3390/gels9010055
de Sousa Iwamoto, L.A., Duailibi, M.T., Iwamoto, G.Y., de Oliveira, D.C., and Duailibi, S. E. 2022. Evaluation of ethylene oxide, gamma radiation, dry heat and autoclave sterilization processes on extracellular matrix of biomaterial dental scaffolds. Scientific Reports. 12: 4299. https://doi.org/10.1038/s41598-022-08258-1
Feiner, R. and Dvir, T. 2017. Tissue‑electronics interfaces: From implantable devices to engineered tissues. Nature Reviews Materials. 3: 17076. https://doi.org/10.1038/natrevmats.2017.76
Galante, R., Rediguieri, C. F., Kikuchi, I. S., Vasquez, P. A., Colaço, R., Serro, A. P., and Pinto, T. J. 2016. About the sterilization of chitosan hydrogel nanoparticles. PLoS One. 11(12): e0168862. https://doi.org/10.1371/journal.pone.0168862
Ghica, M. V., Albu Kaya, M. G., Dinu‑Pîrvu, C.‑E., Lupuleasa, D., and Udeanu, D. I. 2017. Development, optimization and in vitro/in vivo characterization of collagen-dextran spongious wound dressings loaded with flufenamic acid. Molecules. 22(9): 1552. https://doi.org/10.3390/molecules22091552
Ghosh B., Mukhopadhyay M., and Bhattacharya D. 2021. Biopolymer-based nanofilms for the treatment of burn wounds. p.311–336. In M. Rai and C.A.D. Santos (eds). Biopolymer-Based Nano Films: Applications in Food Packaging and Wound Healing. Elsevier. https://doi.org/10.1016/B978-0-12-823381-8.00005-3
Gonçalves, M. M., Carneiro, J., Justus, B., Espinoza, J. T., Budel, J. M., Farago, P. V., and Paula, J. P. de. 2020. Preparation and characterization of a novel antimicrobial film dressing for wound healing application. Brazilian Journal of Pharmaceutical Sciences. 56: e18784. https://doi.org/10.1590/s2175-97902020000118784
Han, X., Hung, H.-C., Jain, P., Sun, F., Xu, X., Yang, W., Bai, T., and Jiang, S. 2017. Sterilization, hydration-dehydration and tube fabrication of zwitterionic hydrogels. Biointerphases. 12(2): 02C411. https://doi.org/10.1116/1.4983502
Hezaveh, H., Muhamad, I. I., Noshadi, I., Shu Fen, L., and Ngadi, N. 2012. Swelling behaviour and controlled drug release from cross‑linked κ‑carrageenan/NaCMC hydrogel by diffusion mechanism. Journal of Microencapsulation. 29(4): 368-379. https://doi.org/10.3109/02652048.2011.651501
Jang, J., Seol, Y.J., Kim, H.J., Kundu, J., Kim, S.W., and Cho, D.W. 2014. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. Journal of the Mechanical Behavior of Biomedical Materials. 37: 69-77. https://doi.org/10.1016/j.jmbbm.2014.05.004
Jeschke, M.G., van Baar, M. E., Choudhry, M. A., Chung, K. K., Gibran, N. S., and Logsetty, S. 2020. Burn injury. Nature Reviews Disease Primers. 6(1): 11. https://doi.org/10.1038/s41572-020-0145-5
Khabbaz, B., Solouk, A., and Mirzadeh, H. 2019. Polyvinyl alcohol/soy protein isolate nanofibrous patch for wound‑healing applications. Progress in Biomaterials. 8(3): 185-196. https://doi.org/10.1007/s40204-019-00120-4
Khaliq, T., Sohail, M., Minhas, M. U., Ahmed Shah, S., Jabeen, N., Khan, S., Hussain, Z., Mahmood, A., Kousar, M., and Rashid, H. 2022. Self‑crosslinked chitosan/ κ‑carrageenan‑based biomimetic membranes to combat diabetic burn wound infections. International Journal of Biological Macromolecules. 197: 157-168. https://doi.org/10.1016/j.ijbiomac.2021.12.100
Lee, K.Y. and Mooney, D.J. 2012. Alginate: Properties and biomedical applications. Progress in Polymer Science. 37(1): 106-126. https://doi.org/10.1016/j.progpolymsci.2011.06.003
Li, M., Kamdenlek, P., Kuntanawat, P., Eawsakul, K., Porntaveetus, T., Osathanon, T., and Manaspon, C. 2022. In vitro preparation and evaluation of chitosan/pluronic F‑127 hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai University Journal of Natural Sciences. 21(4): e2022052. https://doi.org/10.12982/CMUJNS.2022.052
Liu, C., Mejia, D.L., Chiang, B., Luker, K.E., and Luker, G.D. 2018. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 75: 213-225. https://doi.org/10.1016/j.actbio.2018.06.003
Liu, X., Qin, S., Xu, L., Fu, G., Huang, Y., Yu, C., Cheng, G., Li, Y., He, Y., Qi, Y., et al. 2023. A tough and mechanically stable adhesive hydrogel for non‑invasive wound repair. Frontiers in Bioengineering and Biotechnology. 11: 1173247. https://doi.org/10.3389/fbioe.2023.1173247
Madaghiele, M., Demitri, C., Sannino, A., and Ambrosio, L. 2014. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns & Trauma. 2(4): 153–161. https://doi.org/10.4103/2321-3868.143616
Morgado, P. I., Aguiar‑Ricardo, A., and Correia, I. J. 2015. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. Journal of Membrane Science. 490: 139-151. https://doi.org/10.1016/j.memsci.2015.04.064
Palmer, I., Clarke, S. A., Nelson, J., Schatton, W., Dunne, N. J., and Buchanan, F. 2012. Identification of a suitable sterilisation method for collagen derived from a marine Demosponge. International Journal of Nano and Biomaterials. 4(2): 148. https://doi.org/10.1504/IJNBM.2012.050306
Pan, Z. and Brassart, L. 2022. Constitutive modelling of hydrolytic degradation in hydrogels. Journal of the Mechanics and Physics of Solids. 167: 105016. https://doi.org/10.1016/j.jmps.2022.105016
Pawar, S.N. and Edgar, K.J. 2012. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials. 33(11): 3279-3305. https://doi.org/10.1016/j.biomaterials.2012.01.007
Ramadass, S. K., Perumal, S., and Madhan, B. 2014. Design and evaluation of a collagen‑based hydrogel for wound healing. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 102(4): 869-878.
Stoica, A. E., Chircov, C., and Grumezescu, A. M. 2020. Hydrogel dressings for the treatment of burn wounds: An up‑to‑date overview. Materials. 13(12): 1. https://doi.org/10.3390/ma13122853
Stoppel, W. L., White, J. C., Horava, S. D., Henry, A. C., Roberts, S. C., and Bhatia, S. R. 2014. Terminal sterilization of alginate hydrogels: Efficacy and impact on mechanical properties. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 102(4): 877-884. https://doi.org/10.1002/jbm.b.33070
Surowiecka, A., Chrapusta, A., Klimeczek Chrapusta, M., Korzeniowski, T., Drukała, J., and Strużyna, J. 2022. Mesenchymal stem cells in burn wound management. International Journal of Molecular Sciences. 23: 15339. https://doi.org/10.3390/ijms232315339
Tucker, J. and Lai, V. 2024. Mechanical characterization of a tunable 3‑D collagen‑hyaluronic acid hydrogel for use in cell culture. Recent Progress in Materials. 6(4): 1-13. https://doi.org/10.21926/rpm.2404028
Ujang, Z., Rashid, A. H. A., Suboh, S. K., Halim, A. S., and Lim, C. K. 2014. Physical properties and biocompatibility of oligochitosan membrane film as wound dressing. Journal of Applied Biomaterials and Functional Materials. 12(3): 155-162. https://doi.org/10.5301/jabfm.5000190
Zhang, J., Xia, W., and Liu, P. 2018. Chitosan/collagen scaffold containing fucoidan for skin tissue engineering. International Journal of Biological Macromolecules. 107: 93-99.
Zhang, X., Xu, S., Shen, L., and Li, G. 2020. Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. Journal of Leather Science and Engineering. 2: 1-29. https://doi.org/10.1186/s42825-020-00033-0
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supplementary
Table S1. Formulation parameters and measured responses (swelling ratio, WVTR, degradation) of collagen and κ-carrageenan-based hydrogel film (n = 3).
|
Run |
Formula |
Swelling Ratio (%) |
WVTR value (g/m2.day) |
Degradation ratio (%) |
||
|
Collagen (%) |
Carrageenan (%) |
KCl (%) |
||||
|
1 |
3.65 |
0.91 |
0.44 |
124.26 ± 48.23 |
1,264.29 ± 7.21 |
100.00 ± 0.00 |
|
2 |
3.08 |
0.92 |
1 |
201.72 ± 16.65 |
1,088.69 ± 20.39 |
0.00 ± 0.00 |
|
3 |
3.27 |
0.98 |
0.75 |
272.88 ± 34.29 |
1,175.97 ± 24.94 |
0.00 ± 0.00 |
|
4 |
3.10 |
1.50 |
0.40 |
361.93 ± 81.45 |
1,168.61 ± 41.71 |
4.42 ± 4.23 |
|
5 |
2.83 |
1.50 |
0.67 |
243.64 ± 148.65 |
765.53 ± 23.11 |
4.25 ± 5.10 |
|
6 |
3.07 |
1.23 |
0.70 |
131.20 ± 3.20 |
1,019.86 ± 36.23 |
48.23 ± 15.66 |
|
7 |
3.30 |
1.23 |
0.47 |
177.92 ± 24.99 |
637.28 ± 47.94 |
38.20 ± 33.27 |
|
8 |
3.07 |
1.23 |
0.70 |
244.13 ± 43.32 |
1,136.31 ± 7.62 |
0.00 ± 0.00 |
|
9 |
3.55 |
1.15 |
0.30 |
163.38 ± 73.07 |
657.73 ± 18.28 |
9.36 ± 7.63 |
|
10 |
2.59 |
1.45 |
0.96 |
201.60 ± 98.38 |
658.98 ± 24.73 |
0.00 ± 0.00 |
|
11 |
3.52 |
0.80 |
0.68 |
251.72 ± 13.51 |
535.06 ± 32.64 |
0.00 ± 0.00 |
|
12 |
3.07 |
1.23 |
0.70 |
266.08 ± 23.51 |
1,078.95 ± 55.84 |
13.44 ± 6.29 |
|
13 |
2.83 |
1.17 |
1 |
261.03 ± 52.48 |
825.11 ± 16.37 |
0.00 ± 0.00 |
|
14 |
3.55 |
1.15 |
0.30 |
205.38 ± 31.26 |
731.49 ± 63.31 |
14.20 ± 1.05 |
|
15 |
2.59 |
1.45 |
0.96 |
230.62 ± 25.72 |
890.26 ± 58.88 |
0.00 ± 0.00 |
|
16 |
3.90 |
0.80 |
0.30 |
132.52 ± 38.29 |
753.19 ± 46.74 |
100.00 ± 0.00 |
|
17 |
3.52 |
0.80 |
0.68 |
105.96 ± 43.95 |
769.26 ± 63.21 |
100.00 ± 0.00 |
Table S2. Formulation parameters and measured responses (swelling ratio, WVTR, degradation) of collagen and chitosan-based hydrogel film (n = 3).
|
Run |
Formula |
Swelling Ratio (%) |
WVTR value (g/m2.day) |
Degradation ratio (%) |
||
|
Collagen (%) |
Chitosan (%) |
TPP (%) |
||||
|
1 |
2.83 |
1.83 |
2.3 |
236.415 ± 7.863 |
3,106.588 ± 181.814 |
47.876 ± 11.698 |
|
2 |
3.5 |
2.5 |
1 |
336.34 ± 10.778 |
3,157.676 ± 25.404 |
4.077 ± 3.512 |
|
3 |
3.5 |
1.5 |
2 |
393.49 ± 18.589 |
3,560.244 ± 39.072 |
14.419 ± 4.305 |
|
4 |
2.5 |
1.5 |
3 |
218.227 ± 25.069 |
2,309.249 ± 239.364 |
29.320 ± 7.037 |
|
5 |
3.5 |
0.5 |
1 |
256.854 ± 58.451 |
3,665.572 ± 169.549 |
100.000 ± 0.000 |
|
6 |
3.5 |
2.5 |
2 |
443.048 ± 33.884 |
2,378.915 ± 191.444 |
30.854 ± 17.862 |
|
7 |
3.5 |
1.5 |
2 |
306.561 ± 17.082 |
2,979.200 ± 157.978 |
17.912 ± 5.483 |
|
8 |
2.5 |
2.5 |
2 |
402.160 ± 90.284 |
2,649.449 ± 98.548 |
5.612 ± 1.580 |
|
9 |
2.5 |
1.5 |
3 |
328.260 ± 24.991 |
2,545.283 ± 86.026 |
37.479 ± 6.322 |
|
10 |
3.5 |
2.5 |
2 |
348.286 ± 33.292 |
3,381.270 ± 133.758 |
41.002 ± 10.844 |
|
11 |
1.5 |
1.5 |
3 |
573.764 ± 17.198 |
3,330.679 ± 359.430 |
19.031 ± 7.023 |
|
12 |
2.5 |
2.5 |
3 |
300.259 ± 38.906 |
2,280.553 ± 209.525 |
22.534 ± 10.027 |
|
13 |
2.5 |
1.5 |
3 |
262.638 ± 14.108 |
2,356.854 ± 188.053 |
27.303 ± 0.689 |
|
14 |
2.5 |
2.5 |
3 |
337.298 ± 57.728 |
3,203.739 ± 328.573 |
22.281 ± 3.029 |
Table S3. Formulation parameters and measured responses (swelling ratio, WVTR, degradation) of collagen and alginate-based hydrogel film (n = 3).
|
Run |
Formula |
Swelling Ratio (%) |
WVTR value (g/m2.day) |
Degradation ratio (%) |
|
|
Collagen (%) |
Alginate (%) |
||||
|
1 |
1 |
4 |
104.490 ± 8.667 |
1,508.113 ± 242.357 |
69.112 ± 9.031 |
|
2 |
1.375 |
3.625 |
93.160 ± 7.772 |
1,235.967 ± 181.673 |
39.046 ± 2.166 |
|
3 |
1 |
4 |
105.560 ± 21.664 |
1,103.327 ± 33.947 |
47.427 ± 4.395 |
|
4 |
2.125 |
2.875 |
74.280 ± 4.908 |
968.668 ± 39.281 |
100.000 ± 0.000 |
|
5 |
1.75 |
3.25 |
66.200 ± 16.679 |
1,079.697 ± 104.035 |
47.472 ± 2.331 |
|
6 |
1.75 |
3.25 |
83.420 ± 9.221 |
1,184.769 ± 50.807 |
60.116 ± 1.801 |
|
7 |
2.5 |
2.5 |
64.920 ± 17.171 |
1,062.934 ± 98.118 |
75.565 ± 8.886 |
|
8 |
2.5 |
2.5 |
52.250 ± 9.676 |
1,138.974 ± 153.553 |
100.000 ± 0.000 |
Khadijah Zai1, *, Lintang Kusuma Ratri2, Chasna Salsabila Rosydiana2, and Khansa Auliya Putri Dewanto2
1 Department of Pharmaceutics, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia.
2 Undergraduate Student Program, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
Corresponding author: Khadijah Zai, E-mail: khadijah03@ugm.ac.id
ORCID iD:
Khadijah Zai: https://orcid.org/0000-0003-4447-6177
Lintang Kusuma Ratri: https://orcid.org/0009-0007-7899-8113
Chasna Salsabila Rosydiana: https://orcid.org/0009-0008-9340-7489
Khansa Auliya Putri Dewanto: https://orcid.org/0009-0003-2567-5258
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: July 27, 2025;
Revised: October 2, 2025;
Accepted: October 10, 2025;
Online First: October 17, 2025

