
Anticancer Effects of Rutin, Rhein, and Their Combination on A549 Lung Cancer Cells
Benjaporn Buranrat, Kamchai Saepang, Tasana Pitaksuteepong, and Supavadee Boontha*Published Date : October 3, 2025
DOI : https://doi.org/10.12982/NLSC.2026.007
Journal Issues : Number 1, January-March 2026
Abstract Lung cancer is a major concern in northern Thailand. Although rutin and rhein have shown cytotoxic effects against A549 lung cancer cells, their comparative anticancer activities remain unexplored. This study aimed to compare the anticancer effects of rutin and rhein on A549 cancer cells. The effects of rutin and rhein on cytotoxicity, colony formation, cell migration, and reactive oxygen species (ROS) production were evaluated using sulforhodamine B (SRB), clonogenic, scratch wound healing assays, and dichlorodihydrofluorescein diacetate (DCF-DA) flow cytometry, respectively. All experiments used a non-treated control group. At 24–72 hours, rutin and rhein exhibited dose- and time-dependent cytotoxicity against A549 cells, with effective concentrations of 0–2,500 μg/mL and 0–50 μg/mL, respectively. The results demonstrated that rhein exhibited greater cytotoxicity (IC50 = 2.70 μg/mL) compared to rutin (IC50 = 817.10 μg/mL) after 72 hours of incubation. It was found that rutin decreased colony formation, slowed down migration, and increased ROS production in A549 cells at doses ranging from 0 to 2,500 μg/mL. When rhein was added to A549 cells at doses ranging from 0 to 50 μg/mL, it inhibited colony formation, slow down migration, and enhanced ROS production. According to the results, rhein is more effective than rutin at killing A549 cancer cells. This suggests that it could be used as an anticancer drug to both prevent and treat lung cancer. The combination of rhein and rutin has the potential to be beneficial in the treatment of lung cancer.
Keywords: Anticancer, Lung cancer, Rhein, Rutin
Graphical Abstract:

Funding: This research was supported by the University of Phayao and the Thailand Science Research and Innovation Fund (Fundamental Fund 2026).
Citation: Buranrat, B., Saepang, K., Pitaksuteepong, T., and Boontha, S. 2026. Anticancer effects of rutin, rhein, and their combination on A549 lung cancer cells. Natural and Life Sciences Communications. 25(1): e2026007.
INTRODUCTION
In 2022, lung cancer was the leading cause of cancer-related morbidity and mortality worldwide, with nearly 2.5 million new cases and over 1.8 million deaths. It accounted for approximately 12.4% of all cancer diagnoses globally and 18.7% of cancer deaths (Bray et al., 2024). In Thailand, lung cancer accounted for 23,494 new cases (12.8%) and 19,864 deaths (16.7%), making it the second most common cause of cancer-related death after liver cancer, particularly in the northern region (Aungkulanon et al., 2016; Virani et al., 2017; Chang et al., 2018; Rankantha et al., 2018; Bray et al., 2024). The main treatments for lung cancer are surgery, radiotherapy, and chemotherapy. Most patients are diagnosed at advanced stages, limiting the effectiveness of surgery (Araghi et al., 2023; Liu et al., 2024). As a result, chemotherapy and targeted therapies are now the preferred options; however, the emergence of drug resistance remains a major challenge, limiting their clinical success (Wang et al., 2019). To improve patient outcomes and quality of life, it is crucial to explore and develop novel therapeutic approaches.
Rutin, also referred to as quercetin-3-O-rutinoside, is a flavonoid abundantly present in various plants (Ganeshpurkar and Saluja, 2017; Ullah et al., 2020). It exhibits a wide range of biological activities, such as antibacterial, antiviral, anti-inflammatory, antioxidant, and antitumor effects, as well as the ability to protect gastric mucosa and lower blood glucose levels (Hao et al., 2024). Furthermore, rutin has been shown to protect against numerous diseases, including cardiovascular disorders, neurodegenerative conditions, and diabetes (AL-Ishaq et al., 2019; Ullah et al., 2020; Satari et al., 2021). Rutin demonstrated in vitro anticancer activity against numerous types of cancer cell, i.e., breast cancer, cervical cancer, lung cancer, colorectal cancer, and liver cancer (Ben Sghaier et al., 2016; Pandey et al., 2021). In in vitro lung cancer studies, it demonstrated anticancer activity in A549 lung cancer cells at concentrations ranging from 20 to 560 μM (Choiprasert et al., 2010), exhibiting an IC50 of 559.83 µM (Ben Sghaier et al., 2016), and in GLC4 lung cancer cells at a dose of 4 μM (Choiprasert et al., 2010). It inhibited cell growth, cell migration, and invasion and induced apoptosis and autophagy (Ben Sghaier et al., 2016; Pandey et al., 2021). Exposure to rutin has been associated with increased caspase-3 and caspase-9 activity, along with increased p53 protein levels, leading to mitochondrial-mediated apoptosis in various cancer types (Guon and Chung, 2016; Yang et al., 2019; Pandey et al., 2021).
Rhein (an anthraquinone), an active compound found in several medicinal herbs such as rhubarb (Rheum rhabarbarum L.), exhibits potent antitumor properties (Henamayee et al., 2020; Wang et al., 2021). Its mechanisms of action include inhibiting tumor cell proliferation, invasion, and metastasis while also inducing apoptosis (Henamayee et al., 2020; Zhang et al., 2023). In addition to its antitumor properties, rhein demonstrates a broad spectrum of pharmacological activities, including anti-inflammatory, antimicrobial, antioxidant, hepatoprotective, and neuroprotective effects (Zhou et al., 2015; Li et al., 2021). The IL-6/STAT3 pathway is crucial in non-small cell lung cancer (NSCLC), and rhein modulates this pathway by upregulating Bax, downregulating Bcl-2, inducing G2/M arrest, and promoting apoptosis, with IC50 values of 24.59 μM in PC-9, 52.88 μM in H460, and 23.9 μM in A549 cells (Yang et al., 2019). Zhen et al. (2013) reported that treatment with rhein lysinate (100 μM) reduced cell proliferation in H460 and A549 cells to 60% and 58%, respectively, compared to untreated control cells.
It has been reported that rutin and rhein can decrease drug resistance and chemotherapeutic side effects (Satari et al., 2021; Liu et al., 2022). Despite their individual therapeutic properties, the comparative anticancer activities of rutin and rhein remain inadequately studied. Furthermore, the potential synergistic effects of their combination in anticancer applications have yet to be investigated. Therefore, this study aimed to evaluate and compare the cytotoxicity, colony formation inhibition, suppression of cell migration, and ROS production induced by rutin and rhein in A549 lung cancer cells. The anticancer potential of their combination was also investigated. To the best of our knowledge, this study is the first to demonstrate and systematically compare the individual and synergistic effects of rutin and rhein on cell viability, colony formation, wound healing, and ROS generation in A549 lung cancer cells, providing novel insights into their anticancer potential.
MATERIALS AND METHODS
Materials
Rutin and rhein, with a purity of 100%, were obtained from Xi’an Mainherb Biotech Co., Ltd. (Shaanxi, China). The Gibco® cell culture medium components were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA), whereas all chemical compounds and solvents were acquired from Sigma Aldrich (Merck KGaA, Darmstadt, Germany).
Cell cultures and sulforhodamine B (SRB) assay
A549 lung cancer cells were grown with Dulbecco’s modified eagle medium (DMEM) plus antibiotics and fetal bovine serum (FBS). For cytotoxic effects of rutin and rhein, an SRB assay was performed as previously described (Boontha et al., 2025). The cells were plated onto a 96-well cultured plate (1×104 cells/well) for 24 hours. After that, the cell lines were exposed to various concentrations of rutin (0-2,500 µg/mL) or rhein (0-50 µg/mL) or in combination and then cultured for 24–72 hours. Further, the cells were exposed to 10% trichloroacetic acid, stained with 0.4% SRB, solubilized the with 200 µL of 10 mM Tris base, and read the optical density at 540 nm.
Colony formation assay
A549 cells were cultured onto a 6-well plate (500 cells/well) for 24 hours. After that, the cell lines were exposed to various concentrations of rutin (0-2,500 µg/mL) or rhein (0-50 µg/mL) or in combination for 24 hours, then cultured for a further 15 days. After the end of the incubation period, the cells were exposed to 100% cold methanol and stained with 0.25% crystal violet. Colonies were captured, counted, and compared to the control group.
Wound healing assay
A549 cells were plated onto a 24-well cultured plate (2.5 × 105 cells/well) for 24 hours. After that, the cell lines were made a wound by 0.2 mL pipette tips, washed with PBS buffer, and exposed to various concentrations of rutin (0-2,500 µg/mL) or rhein (0-50 µg/mL) or in combination for 24 hours. After that, the wound at the time intervals of 0 hour and 24 hours was compared with the control cells. Images were taken using the Olympus CKX53 inverted microscope (×4 magnifications). The wound closure percentage was calculated by photographing the uncovered wound area and comparing it to the treatment and control groups.
Reactive oxygen species (ROS) assay
A549 cells were plated onto a 6-well plate (2.5 × 105 cells/well) for 24 hours. After that, the cell lines were exposed to various concentrations of rutin (0-2,500 µg/mL) or rhein (0-50 µg/mL) or in combination for 24 hours, collected the cells, washed, and stained with 25 µM DFC-DA for 30 min in the dark at 37°C. Subsequently, ROS production was detected by flow cytometry (BD Biosciences, San Jose, CA, USA) using BD Accuri C6 Plus software.
Data analysis
The data were expressed as the mean ± standard deviation (SD). Statistical analysis utilized Sigma Stat software version 3.5 (Systat Software Inc., San Jose, CA, USA), employing a post hoc LSD test following a one-way Analysis of Variance (ANOVA). A P-value of less than 0.05 was considered statistically significant.
RESULTS
Cytotoxicity of rutin and rhein in A549 cells
The cytotoxic effects of rutin and rhein on A549 cancer cells were evaluated using the SRB assay. The results showed a significant increase in A549 cell mortality with rutin treatment at concentrations ranging from 0 to 2,500 µg/mL, with more pronounced effects at higher incubation times (Figure 1A). The half-maximal inhibitory concentration (IC50) values for rutin were 1,328.3 ± 156.5 µg/mL and 817.1 ± 72.2 µg/mL at 48 and 72 hours, respectively. Similarly, rhein treatment at concentrations ranging from 0 to 50 µg/mL resulted in a marked increase in A549 cell mortality, with cytotoxicity increasing over longer incubation periods (Figure 1B). The IC50 values for rhein were 11.5 ± 1.7 µg/mL, 5.8 ± 0.2 µg/mL, and 2.7 ± 0.4 µg/mL at 24, 48, and 72 hours, respectively. These data demonstrate that rhein displayed significantly higher cytotoxicity against A549 cells than rutin. Treatment with rutin at concentrations ranging from 0 to 2,500 µg/mL in combination with rhein at 25 µg/mL resulted in reduced cell viability compared to treatment with rutin alone at the same concentrations (Figure 1C). Similarly, treatment with rhein at concentrations ranging from 0 to 50 µg/mL in combination with rutin at 500 µg/mL resulted in lower cell viability than treatment with rhein alone at the corresponding concentrations (Figure 1D).
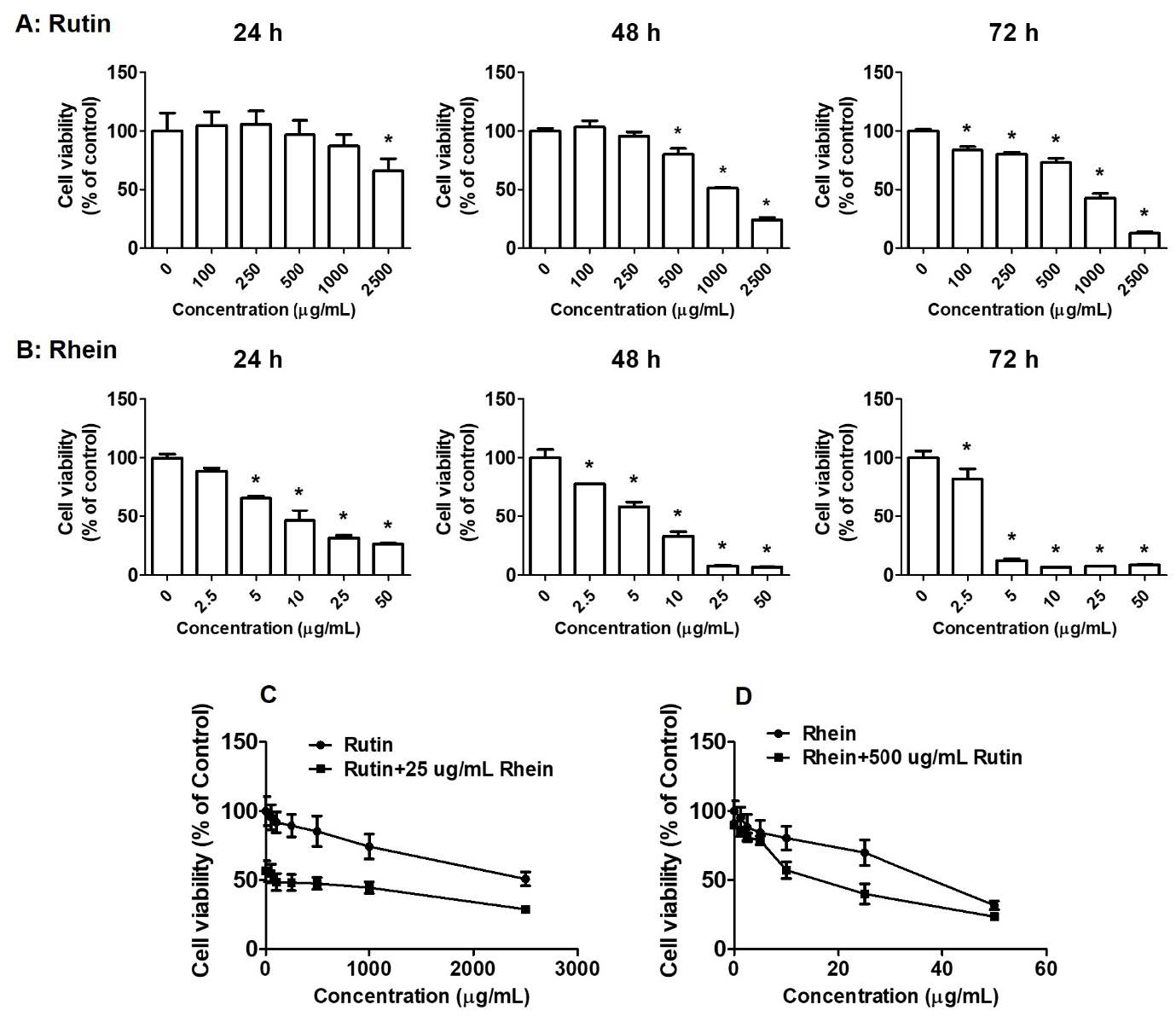
Figure 1. Effects of rutin and rhein on cell proliferation. A549 cells were incubated with various concentrations of rutin (0-2,500 µg/mL, A) or rhein (0-50 µg/mL, B) individually for 24–72 hours or in combination (C-D) for 24 hours. Cell viability was calculated using the sulforhodamine B method. *P < 0.05 versus the control group.
The effects of rutin and rhein on clonogenic inhibition in A549 cells
The clonogenic assay was utilized to evaluate the inhibitory effects of rutin and rhein on A549 cell colony formation over 24, 48, and 72 hours. The results revealed that both rutin and rhein suppressed colony formation in A549 cells in a dose-dependent manner (Figures 2A and 2B). Notably, treatment with rutin at concentrations of 500 µg/mL, 1,000 µg/mL, and 2,500 µg/mL in combination with rhein at 25 µg/mL significantly reduced colony formation compared to the control group (Figure 2C). Similarly, rhein at concentrations of 10 µg/mL, 25 µg/mL, and 50 µg/mL, when combined with rutin at 500 µg/mL, demonstrated significant inhibition of A549 cell colony formation compared to the control group (Figure 2D).
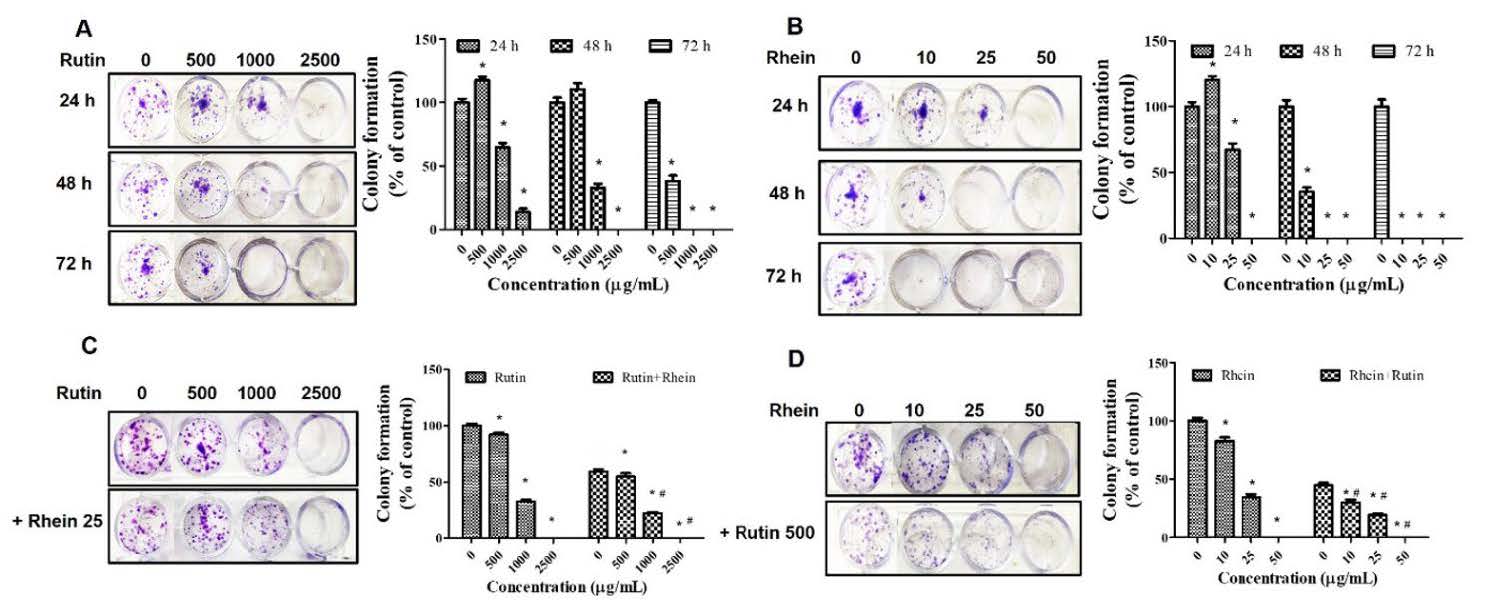
Figure 2. Effects of rutin and rhein on colony formation. A549 cells were incubated with rutin (0-2,500 µg/mL, A) or rhein (0-50 µg/mL, B) individually for 24–72 hours or in combination (C-D) for 24 hours. The cells were then cultured for 15 days, and the colonies were counted. *P<0.05 in comparison to the controls. #P<0.05 compared to rutin or rhein.
Anti-migratory effects of rutin and rhein in A549 cells
A wound healing assay was performed to assess the inhibitory effects of rutin, rhein, and their combination on A549 cell migration. Rutin treatment resulted in A549 wound closure rates of 87.50%, 70.83%, and 50.83% at doses of 500, 1,000, and 2,500 µg/mL, respectively (Figure 3A). Similarly, rhein treatment at 10, 25, and 50 µg/mL resulted in wound closure rates of 86.44%, 74.32%, and 40.23%, respectively (Figure 3B). A significant suppression of A549 cell migration was observed with rutin at a concentration of 1,000 µg/mL (Figure 3A) and with rhein at 10 µg/mL (Figure 3B). The combined effects of rutin (500 µg/mL) and rhein (25 µg/mL) on migration were examined in A549 cells, given their notable cytotoxicity (Figures 1C and 1D) and substantial inhibition of clonogenic growth (Figures 2C and 2D). As shown in Figure 3C, treatment with the combination of rutin (500 µg/mL) and rhein (25 µg/mL) further suppressed A549 wound closure to 45.37%. These findings indicate that the combination of rutin and rhein exerts a synergistic effect in inhibiting A549 cell migration (Figure 3C).
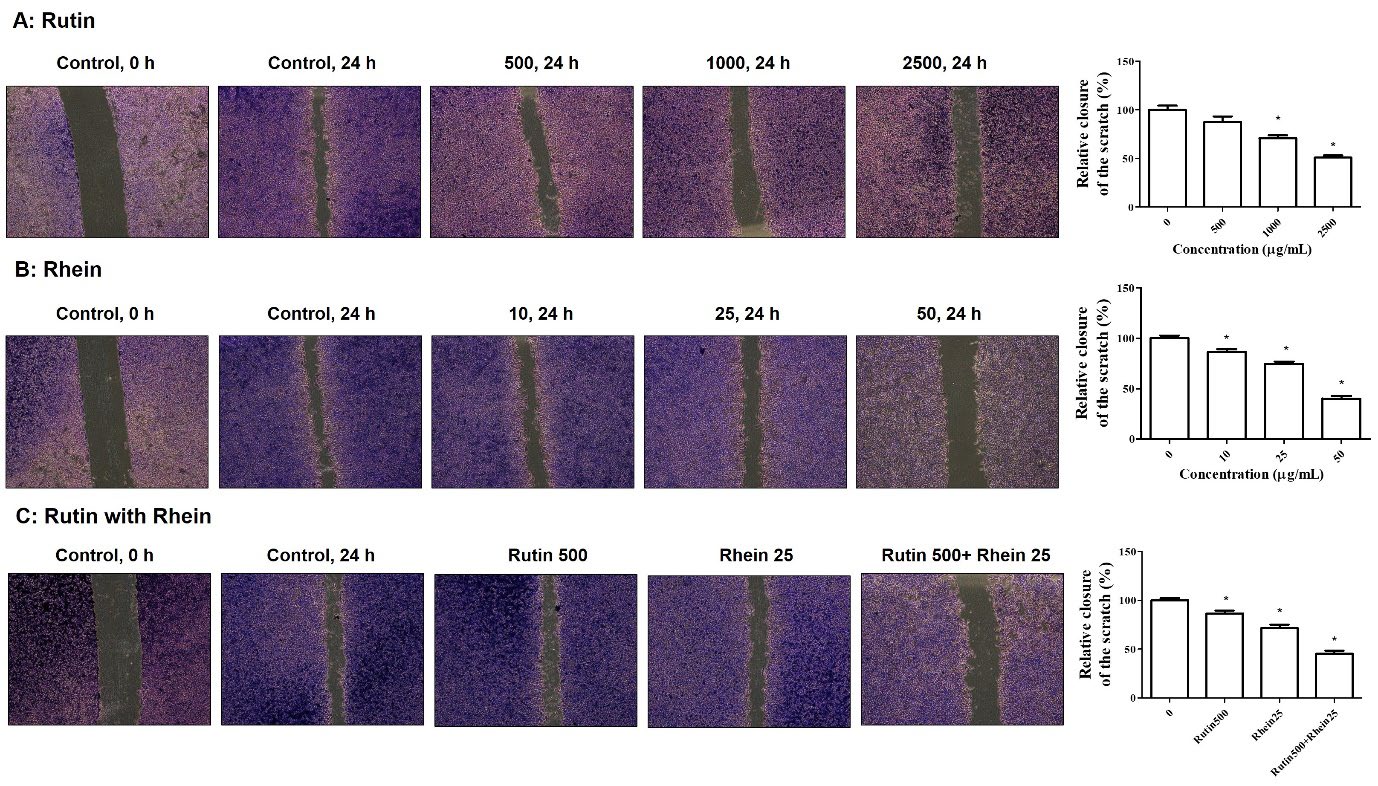
Figure 3. The effects of rutin and rhein on cell migration. After plating A549 cells, a wound was created using 0.2 mL tips. The cells were treated with either rutin (0-2,500 µg/mL, A) or rhein (0-50 µg/mL, B) or a combination of the two (C) for 24 hours. They were then stained with 0.5% crystal violet and observed under an inverted microscope (×4 magnification). *P<0.05 when compared to the controls.
The effects of rutin and rhein on ROS production in A549 cells
To assess ROS production, the fluorescence of DCF-DA was monitored, where a rightward shift in the fluorescence signal reflected an increase in ROS levels. As shown in Figure 4A, rutin at concentrations of 500, 1,000, and 2,500 µg/mL increased ROS production by 11.3%, 20.3%, and 24.7%, respectively. Similarly, rhein at concentrations of 10, 25, and 50 µg/mL increased ROS production by 5.8%, 9.1%, and 15.7%, respectively (Figure 4B). Notably, compared to the ROS production observed in the cells treated with either rutin (500 µg/mL; 9.5%) or rhein (25 µg/mL; 8.9%) alone, the combination of rutin (500 µg/mL) and rhein (25 µg/mL) significantly elevated ROS levels in A549 cells by 24.3% (Figure 4C).
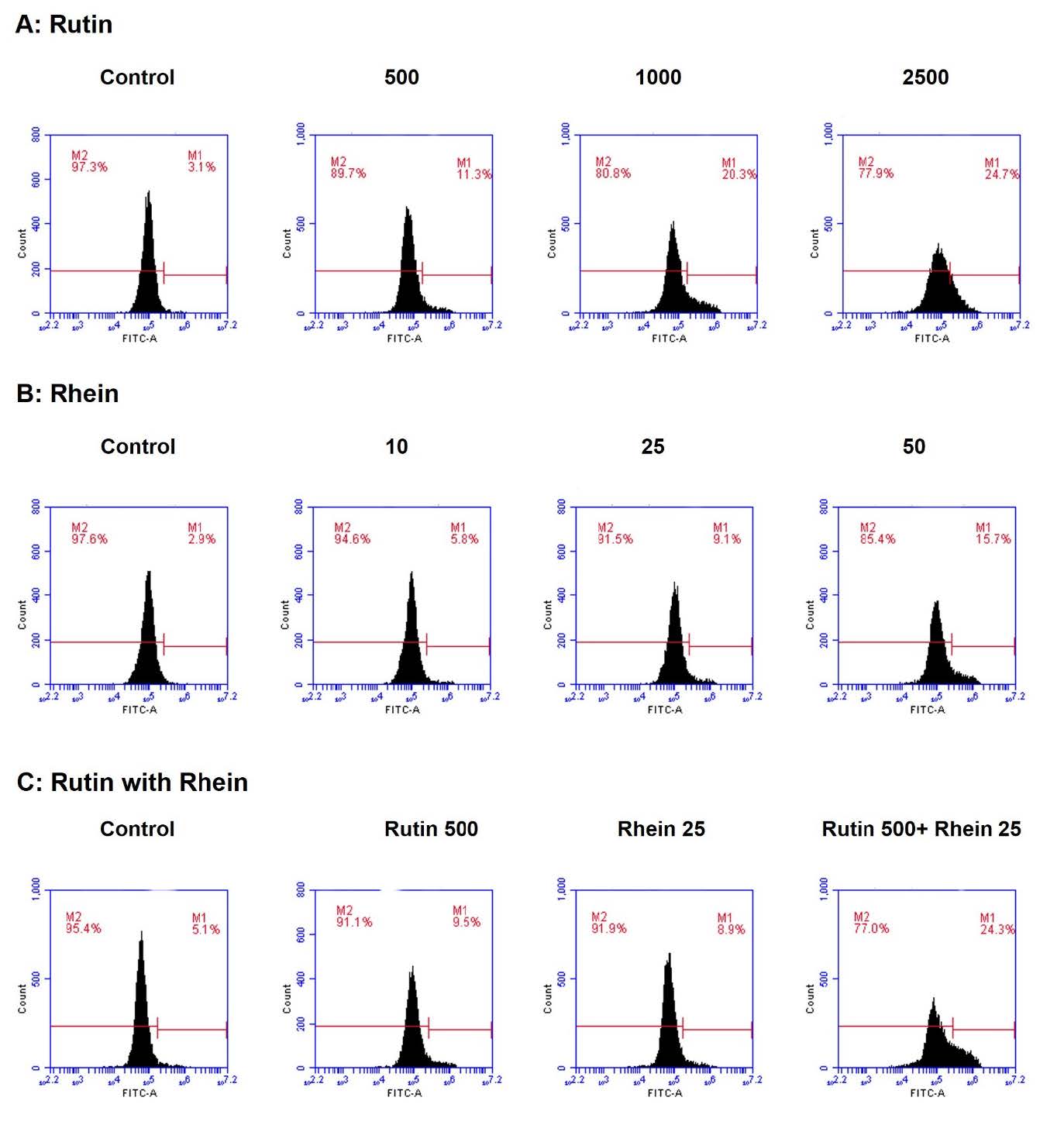
Figure 4. The effects of rutin and rhein on the production of reactive oxygen species (ROS). Following a 24-hour treatment with either rutin (0-2,500 µg/mL, A) or rhein (0-50 µg/mL, B) or a combination of the two (C), A549 cells were stained with DCF-DA fluorescent dye, and the production of ROS was assessed using flow cytometry.
DISCUSSION
Chemotherapy is a principal therapeutic modality for advanced lung carcinoma. Despite the development of novel anticancer medications and therapies, treatment outcomes for certain patients continue to be unsatisfactory. Furthermore, extended chemotherapy frequently diminishes patients' capacity to endure treatment. In addition to adverse effects, drug resistance is a critical element in the failure of cancer treatment, as malignant cells rapidly acquire resistance to chemotherapeutic agents via numerous pathways, diminishing their therapeutic efficacy. Thus, in order to improve lung cancer patients' quality of life and treatment outcomes, new therapeutic approaches are being investigated. Many studies have demonstrated the anticancer properties of rutin and rhein, indicating their potential as therapeutic agents against various cancer cell lines (Ben Sghaier et al., 2016; Henamayee et al., 2020; Pandey et al., 2021; Wang et al., 2021). While rutin and rhein have demonstrated cytotoxic effects on A549 lung cancer cells, their relative anticancer activities remain to be investigated. To date, there has been no comparative study assessing the anticancer effects of rutin, rhein, and their combination in cancer cells. Therefore, this study is the first comparative investigation of their anticancer activity on A549 cells, focusing on cell toxicity, proliferation, migration, and the production of ROS. Beyond cytotoxicity, it is critical to assess the antiproliferative and antimigratory effects of rutin and rhein on A549 cells, as proliferation promotes tumor regrowth and recurrence following treatment. Clonogenic assays are used to evaluate cell proliferation and colony formation, modeling tumor recurrence and aiding drug discovery (Gomes et al., 2023). Metastasis is also dependent on cell migration, which is usually measured using a wound healing scratch assay (Vang Mouritzen and Jenssen, 2018). Additionally, excessive ROS generation induces apoptosis, making ROS production a target for chemotherapeutic agents in cancer treatment (Nakamura and Takada, 2021).
The cytotoxic effects and colony formation inhibition in A549 cells were investigated for two combinations: rutin (0-2,500 µg/mL) with rhein (25 µg/mL) and rhein (0-50 µg/mL) with rutin (500 µg/mL). The results show that all combinations of rutin (0–2,500 µg/mL) with rhein (25 µg/mL) showed significantly greater cytotoxicity in A549 cells than rutin alone. For the combination of rhein (0–25 µg/mL) with rutin (500 µg/mL) (Figure 1C), only 10 µg/mL and 25 µg/mL concentrations of rhein, when paired with 500 µg/mL of rutin, exhibited significantly enhanced cytotoxicity relative to rhein alone (Figure 1D). The selectivity index (SI)—calculated as IC50 for normal cells/IC50 for cancer cells—compares the cytotoxicity of the tested compounds. An SI > 3 indicates excellent selectivity (Mahavorasirikul et al., 2010; Chothiphirat et al., 2019), while an SI > 1.0 suggests higher toxicity to tumor cells than to normal cells (Krzywik et al., 2020). According to previous reports, rutin is more effective in MDA-MB-231 human breast cells, with an SI value of 3.5 (Pravin et al., 2024), and in 786-O human renal cancer cells, with an SI value of 5.5 (Caparica et al., 2020). Regarding the selectivity of rhein in cancer cells, studies have shown that it exhibits anticancer effects on normal human lung fibroblast cell lines, with an SI of approximately 1.0 (Wei et al., 2022). There is limited evidence addressing the selectivity and potential toxicity of rhein and rutin in non-cancerous cells. Future studies should investigate the selectivity of rutin, rhein, and their combination in normal fibroblast cells or normal lung epithelial cells (e.g., normal human bronchial epithelial (NHBE) cells) to confirm their safety profile and delineate an appropriate therapeutic window (Davis et al., 2015).
In the colony formation inhibition study, all combinations of rutin (0–2,500 µg/mL) with rhein (25 µg/mL) exhibited significantly enhanced inhibition of colony formation in A549 cells relative to rutin alone (Figure 2C). All combinations of rhein (0–50 µg/mL) with rutin (500 µg/mL) also demonstrated significantly enhanced inhibition of colony formation compared to rhein alone (Figure 2D). Furthermore, the effects of rutin (500 µg/mL) and rhein (25 µg/mL) on cell migration and ROS production were examined in A549 cells, given their notable cytotoxicity and potent suppression of clonogenic growth. The results suggest that the combination of rutin and rhein more effectively suppresses A549 cell migration and enhances ROS production compared to either compound alone. In this study, the wound healing assay was limited to 24 hours, as the A549 lung cancer cells showed a significant reduction in viability after 48 hours of treatment with rutin (500-2,500 µg/mL) and rhein (10-50 µg/mL), as shown in Figures 1A and 1B. The wound healing process in a cell monolayer depends on both cell proliferation and migration (Bahavar and Tafrihi, 2023; Eizadifard et al., 2023). As only a minor inhibitory effect was observed with rutin (500–2,500 µg/mL) and rhein (10–50 µg/mL) on A549 cell viability after 24 hours of incubation, no substantial reduction in wound closure was detected at these concentrations within the same timeframe. We therefore conclude that the observed reduction in wound closure is likely attributable to impaired cell invasion rather than loss of viability.
ROS are widely recognized as key mediators of oxidative stress-induced cytotoxicity. Increased levels of ROS can initiate apoptosis by promoting mitochondrial dysfunction, DNA damage, and caspase activation; however, excess ROS may also drive other modes of cell death, including necrosis and autophagy (Redza-Dutordoir et al., 2016; Wang et al., 2024). Apoptosis induction is an important anticancer mechanism (Brentnall et al., 2013; Bahavar and Tafrihi, 2023), and the elevated ROS observed in A549 cells may contribute to this process through mitochondrial dysfunction and caspase activation (Redza-Dutordoir et al., 2016). However, other ROS-mediated pathways, such as necrosis or autophagy, cannot be excluded. Although previous studies have demonstrated that rutin and rhein can induce apoptosis in A549 lung cancer cells (Hsia et al., 2009; Wu et al., 2017; Pandey et al., 2021; Huo et al., 2022), our investigation did not include the use of specific apoptosis markers to confirm this mechanism. This is a limitation of this study, and future work employing established apoptotic assays, such as Annexin V staining, caspase activation, and PARP cleavage (Brentnall et al., 2013; Kupcho et al., 2019; Chen et al., 2022), will be necessary to substantiate the involvement of apoptotic pathways.
Our findings suggest that rutin exhibits anticancer activity in A549 cells by inducing cell death through ROS production, inhibiting clonogenic formation, and suppressing cell migration within a concentration range of 0–2,500 µg/mL. Our work demonstrates that rutin causes cytotoxicity and inhibits cell migration in A549 cancer cells, which is similar to earlier findings. However, our findings were predicated on the application of a greater rutin concentration (Ben Sghaier et al., 2016; Ibrahim et al., 2023). Rutin exhibited an IC50 of 1,338.37 μM at 72 hours in our study, which is higher than the previously reported values of 559.83 μM using the MTT assay (Ben Sghaier et al., 2016) and 350.73 μM using the SRB assay (Ibrahim et al., 2023), likely reflecting differences in assay methodology and incubation time. Our study demonstrated a higher IC50 value for rutin (1,338.37 μM at 72 hours) than previously reported in other cancer cell lines, including 500 μM in pancreatic PANC1, 221.57 μM in colorectal CaCO-2, 58.55 μM in breast MCF-7, 91.05 μM in prostate PC3, and 350.73 μM in skin A375 cells (Ibrahim et al., 2023). These differences may reflect variations in cell-type sensitivity to rutin, as well as differences in experimental design, assay conditions, and incubation times. Rutin is generally less potent in A549 cells when used alone (IC50 ~560 µM; Ibrahim et al., 2023), but its anticancer activity can be markedly enhanced through nanoformulation or chemical modification. Notably, nanomicelles reduced its IC50 to 52.32 µM (Ibrahim et al., 2023), a prenanoemulsion achieved an IC50 of 155 µM (Hoai et al., 2020), and rutin linoleate showed an IC50 of ~100 µM in NCI-H23 lung cancer cells (Marcovici et al., 2024). In addition, rutin has consistently been reported to impair A549 cell adhesion and migration, whereas its effects on ROS production appear context-dependent, with studies demonstrating both attenuation of superoxide levels and induction of ROS-mediated cytotoxicity depending on the experimental conditions.
This study showed that rhein had stronger anticancer activity than rutin in A549 cells. At concentrations ranging from 0 to 50 µg/mL, rhein can kill A549 cancer cells by producing ROS, stopping clonogenic growth, and suppressing cell migration. Our study confirmed that rhein can induce cytotoxicity, suppress cell migration, and inhibit colony formation in A549 cancer cells (Yang et al., 2019; Liu et al., 2022), albeit at a lower concentration compared to previous reports. Rhein exhibited an IC50 of 20.41 μM at 48 hours in our study, which is close to a previously reported value
(23.9 μM; Yang et al., 2019) but lower than the 47.3 μM reported by Liu et al. (2022), likely due to variations in assay methodology and incubation duration. Furthermore, our study demonstrated that the combination of rutin (800 µM) and rhein (175.9 µM) exhibited cytotoxic potential against A549 cells comparable to that of the taxol (1 µM) and rhein (100 µM) combination reported by Zhen et al. (2013). Anthraquinones have demonstrated low toxicity toward normal cells, positioning them as promising candidates for the development of novel anticancer agents (Arrousse et al., 2023). Hsia et al. (2009) reported that rhein (50 μM) significantly reduced the cell viability of A549 cells in a dose- and time-dependent manner. Additionally, rhein at the same concentration significantly increased the ROS levels in A549 cells to 23.4% compared to the control group.
CONCLUSION
This study demonstrates that rhein exhibits stronger anticancer activity against lung cancer cells than rutin. The combination of rutin (500 μg/mL) and rhein (25 μg/mL) had the strongest anticancer effects, outperforming the effectiveness of either substance alone. Further research into the anticancer potential and underlying mechanisms of this combination may provide valuable insights into their effectiveness as cancer preventive agents.
AUTHOR CONTRIBUTIONS
Supavadee Boontha designed the study and the experiments, conducted the project, and prepared the manuscript (original draft). Benjaporn Buranrat was responsible for the A549 cell study. Kamchai Saepang assisted in conducting the experiments. Tasana Pitaksuteepong helped to critically review and edit the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
AL-Ishaq, R.K., Abotaleb, M., Kubatka, P., Kajo, K., and Büsselberg, D. 2019. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 9(9): 430. https://doi.org/10.3390/biom9090430
Araghi, M., Mannani, R., Heidarnejad Maleki, A., Hamidi, A., Rostami, S., Safa, S.H., Faramarzi, F., Khorasani, S., Alimohammadi, M., Tahmasebi, S., et al. 2023. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell International. 23(1): 162. https://doi.org/10.1186/s12935-023-02990-y
Arrousse, N., Harras, M.F., El Kadiri, S., Haldhar, R., Ichou, H., Bousta, D., Grafov A., Rais, Z., and Taleb, M. 2023. New anthraquinone drugs and their anticancer activities: Cytotoxicity, DFT, docking, and ADMET properties. Results in Chemistry. 6: 100996. https://doi.org/10.1016/j.rechem.2023.100996
Aungkulanon, S., Tangcharoensathien, V., Shibuya, K., Bundhamcharoen, K., and Chongsuvivatwong, V. 2016. Post universal health coverage trend and geographical inequalities of mortality in Thailand. International Journal for Equity in Health. 15(1): 190. https://doi.org/10.1186/s12939-016-0479-5
Bahavar, P. and Tafrihi, M. 2023. Exploring the anticancer properties of the gum of Ferula gummosa: Impact on cytotoxicity, caspase 3/7 activity and apoptosis, and gene expression in SW-480 cells. International Journal of Environmental Health Research. 34(3): 1810-1823. https://doi.org/10.1080/09603123.2023.2246403
Ben Sghaier, M., Pagano, A., Mousslim, M., Ammari, Y., Kovacic, H., and Luis, J. 2016. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomedicine & Pharmacotherapy. 84: 1972-1978. https://doi.org/10.1016/j.biopha.2016.11.001
Boontha, S., Buranrat, B., Saepang, K., Temkitthawon, P., and Pitaksuteepong, T. 2025. Cytotoxic, cell apoptosis, colony formation and anti-migratory activity of three herbal plant extracts in MCF-7 breast cancer cells. Natural and Life Sciences Communications. 24(1): e2025004. https://doi.org/10.12982/NLSC.2025.004
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R.L., Soerjomataram, I., and Jemal, A. 2024. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 74(3): 229–263. https://doi.org/10.3322/caac.21834
Brentnall, M., Rodriguez-Menocal, L., De Guevara, R.L., Cepero, E., and Boise, L.H. 2013. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology. 14: 32. https://doi.org/10.1186/1471-2121-14-32
Caparica, R., Júlio, A., Araújo, M.E.M., Baby, A.R., Fonte, P., Costa, J.G., and Santos de Almeida, T. 2020. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules. 10(2): 233. https://doi.org/10.3390/biom10020233
Chang, J.T., Jeon, J., Sriplung, H., Yeesoonsang, S., Bilheem, S., Rozek, L., Chitapanarux, I., Pongnikorn, D., Daoprasert, K., Vatanasapt, P., et al. 2018. Temporal trends and geographic patterns of lung cancer incidence by histology in Thailand, 1990 to 2014. Journal of Global Oncology. 4: 1-29. https://doi.org/10.1200/JGO.18.00013
Chen, Q., Ma, K., Liu, X., Chen, S.H., Li, P., Yu, Y., Leung, A.K.L., and Yu, X. 2022. Truncated PARP1 mediates ADP-ribosylation of RNA polymerase III for apoptosis. Cell discovery. 8(1): 3. https://doi.org/10.1038/s41421-021-00355-1
Choiprasert, W., Dechsupa, N., Kothan, S., Garrigos, M., and Mankhetkorn, S. 2010. Quercetin, quercetrin except rutin potentially increased pirarubicin cytotoxicity by non-competitively inhibiting the p-glycoprotein-and MRP1 function in living K562/adr and GLC4/adr cells. American Journal of Pharmacology and Toxicology. 5(1): 24-33. https://doi.org/10.3844/ajptsp.2010.24.33
Chothiphirat, A., Nittayaboon, K., Kanokwiroon, K., Srisawat, T., and Navakanitworakul, R. 2019. Anticancer potential of fruit extracts from Vatica diospyroides symington type SS and their effect on program cell death of cervical cancer cell lines. The Scientific World Journal. 2019: 5491904. https://doi.org/10.1155/2019/5491904
Davis, A.S., Chertow, D.S., Moyer, J.E., Suzich, J., Sandouk, A., Dorward, D.W., Logun, C., Shelhamer, J.H., and Taubenberger, J.K. 2015. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 63(5), 312–328. https://doi.org/10.1369/0022155415570968
Eizadifard, F., Tafrihi, M., and Mohadjerani, M. 2023. Antioxidant, cytotoxic, and genotoxic potentials of the gum of Ferula gummosa Boiss on PC-3 cells. Avicenna Journal of Phytomedicine. 13(3): 316-327.
Ganeshpurkar, A. and Saluja, A.K. 2017. The pharmacological potential of rutin. Saudi Pharmaceutical Journal. 25(2): 149-164. https://doi.org/10.1016/j.jsps.2016.04.025
Gomes, N.P., Frederick, B., Jacobsen, J.R., Chapnick, D., and Su, T.T. 2023. A high throughput screen with a clonogenic endpoint to identify radiation modulators of cancer. Radiation Research. 199(2): 132-147. https://doi.org/10.1667/RADE-22-00086.1
Guon, T.E., and Chung, H.S. 2016. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncology Letters. 11(4): 2463-2470. https://doi.org/10.3892/ol.2016.4247
Hao, B., Yang, Z., Liu, H., Liu, Y., and Wang, S. 2024. Advances in flavonoid research: Sources, biological activities, and developmental prospectives. Current Issues in Molecular Biology. 46(4): 2884-2925. https://doi.org/10.3390/cimb46040181
Henamayee, S., Banik, K., Sailo, B.L., Shabnam, B., Harsha, C., Srilakshmi, S., Vgm, N., Baek, S.H., Ahn, K.S., and Kunnumakkara, A.B. 2020. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules. 25(10): 2278. https://doi.org/10.3390/molecules25102278
Hoai, T.T., Yen, P.T., Dao, T.T.B., Long, L.H., Anh, D.X., Minh, L.H., Anh, B.Q., and Thuong, N.T. 2020. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. Journal of Nanomaterials. 2020: 8867669. https://doi.org/10.1155/2020/8867669
Hsia, T.C., Yang, J.S., Chen, G.W., Chiu, T.H., Lu, H.F., Yang, M.D., Yu, F.S., Liu, K.C., Lai, K.C., Lin, C.C., et al. 2009. The roles of endoplasmic reticulum stress and Ca2+ on rhein-induced apoptosis in A-549 human lung cancer cells. Anticancer research. 29(1): 309–318.
Huo, M., Xia, A., Cheng, W., Zhou, M., Wang, J., Shi, T., Cai, C., Jin, W., Zhou, M., Liao, Y., and Liao, Z. 2022. Rutin promotes pancreatic cancer cell apoptosis by upregulating miRNA-877-3p expression. Molecules. 27(7): 2293. https://doi.org/10.3390/molecules27072293
Ibrahim, R., Kasabri, V., Sunoqrot, S., Shalabi, D., Alkhateeb, R., and Alhiari, Y. 2023. Preparation and characterization of rutin-encapsulated polymeric micelles and studies of synergism with bioactive benzoic acids and triazolofluoroquinolones as anticancer nanomedicines. Asian Pacific Journal of Cancer Prevention. 24(3): 977-989. https://doi.org/10.31557/APJCP.2023.24.3.977
Krzywik, J., Mozga, W., Aminpour, M., Janczak, J., Maj, E., Wietrzyk, J., Tuszyński, J. A., and Huczyński, A. 2020. Synthesis, antiproliferative activity and molecular docking studies of novel doubly modified colchicine amides and sulfonamides as anticancer agents. Molecules. 25(8): 1789. https://doi.org/10.3390/molecules25081789
Kupcho, K., Shultz, J., Hurst, R., Hartnett, J., Zhou, W., Machleidt, T., Grailer, J., Worzella, T., Riss, T., Lazar, D., et al. 2019. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis: An International Journal on Programmed Cell Death. 24(1-2): 184–197. https://doi.org/10.1007/s10495-018-1502-7
Li, G.M., Chen, J.R., Zhang, H.Q., Cao, X.Y., Sun, C., Peng, F., Yin, Y.P., Lin, Z., Yu, L., Chen, Y., et al. 2021. Update on pharmacological activities, security, and pharmacokinetics of rhein. Evidence-based Complementary and Alternative Medicine: eCAM. 2021: 4582412. https://doi.org/10.1155/2021/4582412
Liu, B., Zhou, H., Tan, L., Siu, K.T.H., and Guan, X.Y. 2024. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduction and Targeted Therapy. 9(1): 175. https://doi.org/10.1038/s41392-024-01856-7
Liu, J., Ding, D., Liu, F., and Chen, Y. 2022. Rhein inhibits the progression of chemoresistant lung cancer cell lines via the Stat3/Snail/MMP2/MMP9 pathway. BioMed Research International. 2022: 7184871. https://doi.org/10.1155/2022/7184871
Mahavorasirikul, W., Viyanant, V., Chaijaroenkul, W., Itharat, A., and Na-Bangchang K. 2010. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complementary and Alternative Medicine. 10(55): 1-8. https://doi.org/10.1186/1472-6882-10-55
Marcovici, I., Vlad, D., Buzatu, R., Popovici, R.A., Cosoroaba, R.M., Chioibas, R., Geamantan, A., and Dehelean, C. 2024. Rutin linoleate triggers oxidative stress-mediated cytoplasmic vacuolation in non-small cell lung cancer cells. Life. 14(2): 215. https://doi.org/10.3390/life14020215
Nakamura, H. and Takada, K. 2021. Reactive oxygen species in cancer: Current findings and future directions. Cancer Science. 112(10): 3945-3952. https://doi.org/10.1111/cas.15068
Pandey, P., Khan, F., Farhan, M., and Jafri, A. 2021. Elucidation of rutin's role in inducing caspase-dependent apoptosis via HPV-E6 and E7 down-regulation in cervical cancer HeLa cells. Bioscience Reports. 41(6): BSR20210670. https://doi.org/10.1042/BSR20210670
Pandey, P., Khan, F., Qari, H.A., and Oves, M. 2021. Rutin (bioflavonoid) as cell signaling pathway modulator: Prospects in treatment and chemoprevention. Pharmaceuticals. 14(11): 1069. https://doi.org/10.3390/ph14111069
Pravin, B., Nanaware, V., Ashwini, B., Wondmie, G.F., Jardan, Y.A.B., and Bourhia, M. 2024. Assessing the antioxidant properties of naringin and rutin and investigating their oxidative DNA damage effects in breast cancer. Scientific Reports. 14(1): 15314. https://doi.org/10.1038/s41598-024-63498-7
Rankantha, A., Chitapanarux, I., Pongnikorn, D., Prasitwattanaseree, S., Bunyatisai, W., Sripan, P., and Traisathit, P. 2018. Risk patterns of lung cancer mortality in northern Thailand. BMC Public Health. 18(1): 1138. https://doi.org/10.1186/s12889-018-6025-1
Redza-Dutordoir, M. and Averill-Bates, D.A. 2016. Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta. 1863(12): 2977-2992. https://doi.org/10.1016/j.bbamcr.2016.09.012
Satari, A., Ghasemi, S., Habtemariam, S., Asgharian, S., and Lorigooini, Z. 2021. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evidence-Based Complementary and Alternative Medicine: eCAM, 2021: 9913179. https://doi.org/10.1155/2021/9913179
Ullah, A., Munir, S., Badshah, S.L., Khan, N., Ghani, L., Poulson, B.G., Emwas, A.H., and Jaremko, M. 2020. Important flavonoids and their role as a therapeutic agent. Molecules. 25(22): 5243. https://doi.org/10.3390/molecules25225243
Vang Mouritzen, M., and Jenssen, H. 2018. Optimized scratch assay for in vitro testing of cell migration with an automated optical camera. Journal of Visualized Experiments. 138: 57691. https://doi.org/10.3791/57691-v
Virani, S., Bilheem, S., Chansaard, W., Chitapanarux, I., Daoprasert, K., Khuanchana, S., Leklob, A., Pongnikorn, D., Rozek, L.S., Siriarechakul. S., et al. 2017. National and subnational population-based incidence of cancer in Thailand: Assessing Cancers with the highest burdens. Cancers. 9(8): 108. https://doi.org/10.3390/cancers9080108
Wang, D., Wang, X. H., Yu, X., Cao, F., Cai, X., Chen, P., Li, M., Feng, Y., Li, H., and Wang, X. 2021. Pharmacokinetics of Anthraquinones from Medicinal Plants. Frontiers in Pharmacology. 12: 638993. https://doi.org/10.3389/fphar.2021.638993
Wang, M., Yu, F., Zhang, Y., and Li, P. 2024. Programmed cell death in tumor immunity: Mechanistic insights and clinical implications. Frontiers in Immunology. 14: 1309635. https://doi.org/10.3389/fimmu.2023.1309635
Wang, X., Zhang, H., and Chen, X. 2019. Drug resistance and combating drug resistance in cancer. Cancer Drug Resistance. 2(2): 141-160. https://doi.org/10.20517/cdr.2019.10
Wei, M.X., Zhou, Y.X., Lin, M., Zhang, J., and Sun, X. 2022. Design, synthesis and biological evaluation of rhein-piperazine-dithiocarbamate hybrids as potential anticancer agents. European Journal of Medicinal Chemistry. 241: 114651. https://doi.org/10.1016/j.ejmech.2022.114651
Wu, F., Chen, J., Fan, L.M., Liu, K., Zhang, N., Li, S.W., Zhu, H., and Gao, H.C. 2017. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Experimental and Therapeutic Medicine. 14(1): 127–130. https://doi.org/10.3892/etm.2017.4494
Yang, L., Li, J., Xu, L., Lin, S., Xiang, Y., Dai, X., Liang, G., Huang, X., Zhu, J., and Zhao, C. 2019. Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway. Cancer Management and Research. 11: 1167–1176. https://doi.org/10.2147/CMAR.S171517
Yang, L., Lin, S., Kang, Y., Xiang, Y., Xu, L., Li, J., Dai, X., Liang, G., Huang, X., and Zhao, C. 2019. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. Journal of Experimental & Clinical Cancer Research. 38(1): 31. https://doi.org/10.1186/s13046-018-1015-9
Zhang, H., Ma, L., Kim, E., Yi, J., Huang, H., Kim, H., Raza, M A., Park, S., Jang, S., Kim, K., et al. 2023. Rhein induces oral cancer cell apoptosis and ROS via suppresse AKT/mTOR signaling pathway in vitro and in vivo. International Journal of Molecular Sciences. 24(10): 8507. https://doi.org/10.3390/ijms24108507
Zhen, Y.Z., Hu, G., Zhao, Y.F., Yan, F., Li, R., Gao, J.L., and Lin, Y.J. 2013. Synergy of Taxol and rhein lysinate associated with the downregulation of ERK activation in lung carcinoma cells. Oncology Letters. 6(2): 525–528. https://doi.org/10.3892/ol.2013.1398
Zhou, Y.X., Xia, W., Yue, W., Peng, C., Rahman, K., and Zhang, H. 2015. Rhein: A review of pharmacological activities. Evidence-Based Complementary and Alternative Medicine. 2015: 578107. https://doi.org/10.1155/2015/578107
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Benjaporn Buranrat1, 2, Kamchai Saepang1, 3, Tasana Pitaksuteepong4, and Supavadee Boontha1, 3, *
1 Research Group in Herbal and Development of Formulation and Delivery Systems for Elderly Adults and Cancer Treatment, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
2 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand.
3 Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
4 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Supavadee Boontha, E-mail: supavadee.bo@up.ac.th
ORCID iD:
Supavadee Boontha: https://orcid.org/0000-0003-3335-6081
Benjaporn Buranrat: https://orcid.org/0000-0002-1554-6327
Kamchai Saepang: https://orcid.org/0000-0002-4426-7570
Tasana Pitaksuteepong: https://orcid.org/0000-0001-5641-5430
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: April 3, 2025;
Revised: August 30, 2025;
Accepted: September 29, 2025;
Online First: October 3, 2025

