
Development of Mangiferin-Loaded Anisotropic Emulsion for Cosmetic Applications: Structural Characterization of Lamellar System
Paveena Wongtrakul, Warunee Leesajakul, Waranya Neimkhum, Wicharn Janwitayanuchit, and Parapat Sobharaksha*Published Date : October 1, 2025
DOI : https://doi.org/10.12982/NLSC.2026.004
Journal Issues : Number 1, January-March 2026
Abstract Lamellar emulsions have emerged as promising delivery systems for bioactive compounds due to their ability to improve physical stability and enhance skin hydration. Mangiferin, a polyphenol extracted from mango leaves, exhibits potent antioxidant and anti-inflammatory properties and was incorporated as a natural active ingredient in the studied emulsions. This study aimed to develop lamellar emulsions containing mangiferin extract, formulated using surfactant mixtures. To characterize the structural properties of the resulting formulations, advanced analytical techniques, including polarized light microscopy, differential scanning calorimetry (DSC), small-angle X-ray scattering (SAXS), and wide-angle X-ray scattering (WAXS), were employed. The emulsions were prepared by blending sorbitan-based and sucrose-based surfactants in varying ratios to identify the optimal combination. It was found that a 4:1 ratio of sorbitan palmitate to sucrose palmitate resulted in a well-organized lamellar structure. The formulated emulsions exhibited a creamy texture, good spreadability, and desirable viscosity characteristics. Polarized light microscopy revealed an anisotropic structure with distinct Maltese cross patterns, while DSC analysis demonstrated specific phase transition temperatures that shifted upon mangiferin incorporation. Furthermore, SAXS and WAXS analyses confirmed the presence of a periodic lamellar structure in the emulsions. In conclusion, lamellar emulsions containing mangiferin extract were successfully developed using a blend of sorbitan palmitate and sucrose palmitate, with the 4:1 ratio proving to be particularly effective in achieving a stable and well-structured formulation.
Keywords: Lamellar emulsion, Mangiferin, Light polarized microscopy, DSC, SAXS, WAXS
Graphical Abstract:

Funding: The authors are grateful for the research funding provided by Cosmetic and Herbal Innovation Center (CHIC), Faculty of Pharmaceutical Sciences, Huachiew Chalermprakiet University and the support from Prof. Dr. Hideki Sakai and Dr. Koji Tsuchiya at Department of Pure and Applied Chemistry, Faculty of Science and Technology and Research Institute for Science and Technology, Tokyo University of Science for SAXS measurement.
Citation: Wongtrakul, P., Leesajakul, W., Neimkhum, W., Janwitayanuchit, W., and Sobharaksha, P. 2026. Development of mangiferin-loaded anisotropic emulsion for cosmetic applications: Structural characterization of lamellar system. Natural and Life Sciences Communications. 25(1): e2026004.
INTRODUCTION
Topical formulations play a crucial role in delivering active ingredients through the skin for therapeutic and cosmetic purposes. Among those, emulsions are a fundamental component in dermatological formulations, offering a multitude of benefits for skin health and appearance. These biphasic systems, typically comprising oil and water phases stabilized by emulsifiers, serve as effective delivery vehicles for active ingredients, enhance skin hydration, and improve barrier function. The water phase provides immediate moisture, while the oil phase forms a protective barrier on the skin's surface, preventing moisture loss and effectively enhancing skin hydration while reducing transepidermal water loss (TEWL) (Roso et al., 2023). The dual-phase nature of emulsions allows for the dispersion of hydrophobic actives within oil droplets and hydrophilic actives within the aqueous phase, ensuring optimal delivery and efficacy. For instance, a study on a resveratrol-based emulsion demonstrated significant anti-aging effects, including reduced skin wrinkling, increased firmness, and decreased redness. Participants reported improved skin smoothness and moisture levels, highlighting the emulsion's efficacy in delivering active ingredients to target sites within the skin (Schulte to Brinke et al., 2021). A study focused on developing oil-in-water (O/W) dermato-cosmetic emulsions containing a mixture of vegetable oils as the dispersed phase, combined with the active compounds of a bioactive complex based on a plant-derived monoterpene phenol and a peptide, demonstrated that the emulsions could be used independently or incorporated into dermato-cosmetic formulations intended to combat skin oxidative stress. (Barna et al., 2023). Furthermore, the presence of surfactants as emulsifiers in formulations facilitates the penetration of active ingredients into the deeper layers of the skin, enhancing their bioavailability and therapeutic effects. Non-ionic surfactants have the potential to integrate into the intercellular domains of the stratum corneum (SC) lipid matrix, enhancing the fluidity of barrier lipids and reducing the SC’s barrier function against active compounds. (Strati et al., 2021). However, conventional emulsions are inherently thermodynamically unstable, which can result in phase separation, creaming, or alterations in their texture and visual appearance during storage. Lamellar liquid crystal emulsions exhibit greater stability compared to conventional emulsions, as their ordered structure helps to prevent phase separation, creaming, and other forms of instability over time (Lee et al., 2019). Additionally, lamellar emulsions have emerged as a superior alternative to conventional emulsions in skincare formulations, offering enhanced benefits due to their structural similarity to the skin's natural lipid barrier. They enhance skin hydration by reinforcing the skin barrier and reducing TEWL. (da Rocha-Filho et al., 2016). A study demonstrated that a lamellar-like skin emulsion cream improved overall median skin hydration by 55% over four weeks, compared to a 37% improvement with a conventional moisturizing emulsion (Kircik, 2014). A clinical trial evaluating combined therapy with a multi-lamellar emulsion containing pseudoceramide in patients with atopic dermatitis demonstrated significant improvements in skin barrier function, alleviation of AD symptoms, increased SC hydration, and reduced TEWL compared to the conventionally treated group (Lee et al., 2024).
Amphiphilic molecules such as surfactants, lipids, and fatty alcohols are well known for their ability to spontaneously self-assemble in aqueous environments into organized bilayer structures, where hydrophilic headgroups orient toward water, while hydrophobic tails pack inward. These periodic lamellar liquid crystalline phases can be created under suitable conditions, particularly with the use of specific surfactant types and concentrations—as demonstrated by Fameau et al. (2024), who showed that lamellar gel networks from ionic surfactant, cetyltrimethylammonium chloride, and long-chain (C16, C18) fatty alcohol mixtures exhibit lamellar assemblies with strong viscoelastic properties. The fatty alcohols influence the lateral packing of the lamellar phase, with an orthorhombic packing observed for the C18 system and hexagonal packing detected in C16 and C16/C18 samples. In biphasic emulsion systems, these amphiphilic molecules predominantly accumulate at oil-water interfaces, with lamellar phase formation driven by the same fundamental forces of hydrophobic tail segregation from water and hydrophilic head orientation toward aqueous phases. However, the presence of oil introduces additional complexity by providing hydrophobic environment that can alter the partitioning behavior of amphiphiles and promote lamellar domain formation either at interfaces or within water-rich regions. Unlike in bulk aqueous systems, where bilayer stacks tend to be continuous, lamellar structures in emulsions are typically confined to localized regions such as interfacial multilayers or discrete domains within dispersed phase. These lamellar organizations significantly enhance long-term dispersion stability of emulsion and enable controlled release mechanisms that particularly valuable in pharmaceutical and cosmetic applications (Kuwabara et al., 2024).
The demand for natural ingredients has been steadily rising, driven by consumer preferences for safer and more sustainable products. Plant extracts and natural compounds have been extensively studied for their beneficial properties, including photoprotection (Michalak, 2023), UV filtration (He et al., 2021), wound healing (Shedoeva et al., 2019), and anti-skin aging effects (Abdelfattah et al., 2022; Poomanee et al., 2023). Mangiferin, a bioactive compound extracted from mango leaves, bark, or roots, possesses potent antioxidant properties that help protect the skin from oxidative stress induced by free radicals (Prommajak et al., 2014). Additionally, it possesses anti-inflammatory effects, making it beneficial for soothing irritated or inflamed skin (Pleguezuelos-Villa et al., 2019), as well as antimicrobial activity, which helps combat infection-causing bacteria (Yang et al., 2020). Mangiferin-loaded phospholipid vesicles effectively counteracted skin lesion formation and promoted wound healing. (Pleguezuelos-Villa et al., 2024). Mangiferin is classified as a Biopharmaceutics Classification System (BCS) Class IV compound due to its poor aqueous solubility and limited intestinal permeability (Barakat et al., 2022). These physicochemical properties present significant challenges for its incorporation into emulsion-based delivery systems. Consequently, mangiferin was chosen as a representative natural model compound for studying its integration into emulsions.
The objective of this study was to develop lamellar liquid crystal emulsions formulated with a blend of surfactants, incorporate the extracted mangiferin, and evaluate their lamellar structural characteristics for potential topical application. The morphology of the lamellar structure was characterized utilizing a polarized light microscope. Small-angle and wide-angle X-ray scattering spectroscopy (SAXS and WAXS) were used for further characterization of liquid crystal structure, and differential scanning calorimetry (DSC) measurements were carried out to evaluate the thermal behavior of the emulsion samples.
MATERIALS AND METHODS
Materials
Mangiferin was extracted from mango leaves as described in our previous study (Tessiri et al., 2021). High-performance thin-layer chromatography (HPTLC) analysis determined the purity of the extracted mangiferin to be 95.02% ± 0.064. The emulsifiers including sorbitan stearate, sucrose palmitate, and sucrose stearate were purchased from Chanjao Longevity Co., Ltd. Stearyl alcohol from Emery Oleochemicals, caprylic/capric triglycerin from Inolex Chemical Company, cyclomethicone form Summit Chemical Co., Ltd., and sorbitan palmitate from Sigma-Aldrich were purchased from local distributors. Butylene glycol and guar gum were purchased from Chemipan Corporation Co., Ltd. The choice of using ingredients of this grade aimed at best featuring real industrial conditions.
Preparation of emulsion containing mangiferin extract
Various emulsifier systems, combining different ratios of high and low hydrophilic-lipophilic balance (HLB) surfactants, were employed to explore the optimal mixture for creating lamellar emulsions. The sorbitan-based surfactants, sorbitan palmitate (HLB = 6.7), sorbitan stearate (HLB = 4), polyoxyethylene sorbitan monostearate (HLB = 14.9) and sucrose-based surfactant, sucrose palmitate (HLB =16) were used in this study. The formulations presented in Table 1, expressed as weight by weight percent, comprised an oil phase consisting of stearyl alcohol, caprylic/capric triglyceride, and cyclomethicone, along with a water phase containing butylene glycol, guar gum and purified water. Guar gum was employed as the thickening agent. The total amount of emulsifiers was adjusted to 6% w/w according to the preliminary study (Sobharaksha et al., 2024). The emulsions were prepared using standard procedures. Guar gum was first hydrated in water to prepare a stock dispersion at a concentration of 20% w/w. The amount of this stock dispersion added to the prepared emulsion was adjusted to achieve a final dry guar gum content of 0.6% w/w. This was to ensure a complete hydration of gum polymer. The oil phase and water phase were individually weighed in glass beakers and heated to 75°C using a water bath. Subsequently, the oil phase was gradually incorporated into the water phase under continuous stirring until the obtained emulsion reached room temperature (30 ± 2°C). The mangiferin extract was then homogeneously mixed into the emulsions at a concentration of 0.02% w/w. The amount of mangiferin incorporated into the developed formulations was designed based on our preliminary study on its free radical scavenging activity. The IC50 value against DPPH radicals was found to be 7.21 ± 0.49 µg/mL. To facilitate quantitative analysis of mangiferin in the samples, approximately 30 times the IC50 value was included in the formulations. The prepared samples were stored in a refrigerator until further experiments were carried out.
Table 1. Formulations of the studied emulsions containing different emulsifiers.
|
Ingredients (%w/w) |
Phase |
F #1 |
F #2 |
F #3 |
F #4 |
F #5 |
|
Mangiferin extract |
|
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Stearyl alcohol |
o |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
Caprylic/capric triglyceride |
o |
4.00 |
4.00 |
4.00 |
4.00 |
4.00 |
|
Cyclomethicone |
o |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
|
Butylene glycol |
w |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
|
Guar gum |
w |
0.60 |
0.60 |
0.60 |
0.60 |
0.60 |
|
Sorbitan palmitate |
o |
- |
- |
4.50 |
4.80 |
- |
|
Sorbitan stearate |
o |
4.50 |
4.80 |
- |
- |
4.00 |
|
Sucrose palmitate |
w |
1.50 |
1.20 |
1.50 |
1.20 |
- |
|
Polyoxyethylene- sorbitan stearate |
w |
- |
- |
- |
- |
2.00 |
|
Purified Water to |
w |
100 |
100 |
100 |
100 |
100 |
|
Total HLB |
|
7.00 |
6.40 |
9.00 |
8.60 |
7.60 |
Note: o = oil phase, w = water phase
Characterization of the studied emulsion
The prepared emulsions were retained for microstructure examination, utilizing a light polarized microscopy, a differential scanning calorimeter, and small-angle X-ray, wide-angle X-ray scattering equipment. The release of mangiferin from the emulsions was also performed using a dialysis method. The rheological characteristics of the emulsions were examined using viscometer.
Investigation using light polarized microscopy
Polarized optical microscopy was conducted using a microscope (ECLIPSE 50i, Nikon Corporation, Tokyo, Japan), to observe the morphology of the samples under a polarized light source. Undiluted samples were placed directly onto microscope slides and gently covered with coverslips. The slide was then placed under the microscope lens. The sample was observed under regular light initially. The dark field was adjusted and polarized light was turned on to observe the optical properties under the polarized light.
X-ray scattering analysis
The measurement was carried out following the method previously described by Misono, (2015). Small-angle X-ray scattering measurements were performed using a SAXSess instrument (Anton Paar, Austria) equipped with a PW3830 sealed glass bulb X-ray source (PANalytical, Netherland; Cu-Kα, λ = 0.154 nm). The samples were enclosed in a sealed paste cell and measured at 25°C. The X-ray generator was operated under standard conditions at an accelerating voltage of 40 kV and a beam current of 50 mA. Each measurement was acquired with a fixed exposure time of 20 minutes. Scattered intensities were detected using an imaging plate positioned at a calibrated sample-to-detector distance. Due to the translucent beam stop configuration, the collected raw scattering profiles exhibited attenuated primary beam intensity at q = 0. All of the data were normalized to the same incident primary-beam intensity for the transmission calibration and were corrected for background scattering from the capillary and cell windows.
= 0.154 nm). The samples were enclosed in a sealed paste cell and measured at 25°C. The X-ray generator was operated under standard conditions at an accelerating voltage of 40 kV and a beam current of 50 mA. Each measurement was acquired with a fixed exposure time of 20 minutes. Scattered intensities were detected using an imaging plate positioned at a calibrated sample-to-detector distance. Due to the translucent beam stop configuration, the collected raw scattering profiles exhibited attenuated primary beam intensity at q = 0. All of the data were normalized to the same incident primary-beam intensity for the transmission calibration and were corrected for background scattering from the capillary and cell windows.
Differential scanning calorimetry
Differential scanning calorimetry was performed using a DSC 200F3 instrument from Netzsch, Selb, Germany, to detect the phase transition temperature of the samples. The emulsion samples (4 mg) were sealed in a weighted alumina crucible, with a same empty crucible serving as control. The temperature was raised from 25 to 300°C at 10°C min-1, under nitrogen gas purging at 50 mL/min and protective gas at 100 mL/min.
In vitro release of mangiferin
The release of mangiferin from the emulsions was evaluated using the dialysis method (Hu et al., 2021). This experiment, along with other studies, aimed to investigate the differences in mangiferin release characteristics between formulations #4 and #5, which may be influenced by the structural environment surrounding mangiferin within the emulsions, resulting in the difference in release profiles. Purified water was used as the release medium, with the concern that concentration of the released mangiferin did not exceed 20% of its water solubility during the experiment. (Acosta et al., 2016). In our previous study, the solubility of mangiferin in water at 27 ± 2°C was determined to be 0.1744 ± 0.0005 mg/mL (Tessiri et al., 2021). Fourteen grams of emulsions containing mangiferin of 0.0028 g were loaded into a dialysis bag (2.2 x 4.5 cm) with molecular weight cutoff of 12,000-14,000 (Cellu Sep T4, USA), which was then immersed in 400 mL of water. The amount of the investigated sample (14 g), when completely dispersed in 400 mL of water, provided a mangiferin concentration of 0.007 mg/mL. This concentration was less than 20% of mangiferin's solubility in water (0.034 mg/mL), ensuring that sink conditions were maintained throughout the experiment. The release medium was maintained at a constant temperature of 27 ± 0.5°C and stirred at a speed of 400 rpm. At specified time points of 1, 2, 4, and 6 hours, the three-milliliters of the medium was withdrawn for mangiferin analysis by using the ultraviolet spectrophotometer (UV-1,800, Shimadzu, Japan) at a wavelength of 317 nm to obtain the absorbance of the released mangiferin. The three-milliliters of fresh medium was added to maintain the constant volume of the medium. The absorbances were then converted to mangiferin content (Ct) using a standard curve covering standard mangiferin concentrations ranging from 0.46 to 30 µg/mL. The linearity of the equation was observed to be Y = 0.0374X + 0.0044 with the correlation coefficient of r = 0.998. The total mangiferin was denoted as Co. The percentage of the released mangiferin was calculated as (Ct/Co) × 100%. The released amount of mangiferin was recorded as a function of time. Testing samples prepared from emulsions without mangiferin were processed in the manner to achieve a mangiferin concentration within the range of the standard curve. These samples were scanned for UV absorbance to confirm the absence of interference from other ingredients during mangiferin content analysis. The results indicated no absorption peaks at 317 nm. The release experiments were performed in triplicate. The statistical level of significance was set at 5%. Statistical analyses were performed using Microsoft Excel 365.
Viscosity measurement
Flow behaviors of the emulsions were determined using a viscometer (Brookfield model DV-II+, Middleboro MA, USA). The measurements were carried out using spindle SC-25 and small sample adapter at room temperature (25 ± 2°C). The speeds of rotation were increased from 0.3 to 100 rpm and subsequently decreased to 0.0 rpm. The shear stress, shear rate and viscosity were recorded and used to prepare viscosity profile.
RESULTS
Preparation of emulsions containing mangiferin
The studied emulsions were prepared using different ratios of blended emulsifiers, as detailed in Table 2. Based on our preliminary investigations, a combination of sorbitan-based and sucrose-based surfactants yielded the anisotropic emulsions (Sobharaksha et al., 2024). For comparison, F #5, comprising a typical blend of sorbitan stearate and polyoxyethylene sorbitan stearate, was prepared alongside the developed LC emulsions (F #1 – F #4). Blank formulations exhibited a white, creamy semisolid appearance, while those containing 0.02% w/w of mangiferin appeared as off-yellow creamy semisolids. Emulsions containing a high concentration of sorbitan stearate (F #1 and F #2) left a persistent white residue on the skin upon application. Sorbitan stearate is an ester derived from sorbitol and stearic acid. Its long hydrophobic tail, attributed to stearic acid, consists of an 18-carbon saturated fatty acid. In contrast, sorbitan palmitate contains a 16-carbon fatty acid. Due to its higher molecular weight, sorbitan stearate may form a thicker emulsion, leading to a more substantial film on the skin, which could result in an undesirable user experience. Therefore, sorbitan palmitate was selected as the preferred emulsifier for our formulation.
Table 2. Mixed surfactant ratios in the studied formulations.
|
Formulation
|
Sorbitan palmitate HLB = 6.7 |
Sorbitan stearate HLB = 4.0 |
Sucrose palmitate HLB =16 |
Polyoxyethylene sorbitan stearate HLB =14.9 |
|
F #1 |
- |
3 |
1 |
- |
|
F #2 |
- |
4 |
1 |
- |
|
F #3 |
3 |
- |
1 |
- |
|
F #4 |
4 |
- |
1 |
- |
|
F #5 |
- |
2 |
- |
1 |

Figure 1. Images of the mangiferin-containing emulsions (F #1-F #5) in glass bottles.
Microscopic evaluation of the studied emulsions
Figure 1 and figure 2, 3 showed the photographic and microscopic images of the studied emulsions, respectively. Emulsions formulated with a combination of sorbitan-based and sucrose-based surfactants displayed a clear anisotropic structure under a light polarized microscopy, which is a characteristic of lamellar liquid crystal structure (Zhang and Liu, 2013). The Maltese cross pattern originates from the self-assembly of amphiphilic molecules within emulsion system, where lamellar structure are oriented either parallel or perpendicular to the plane of polarized light. This directional arrangement refract the light into two different directions, creating bright and dark regions under polarized light microscopy. The characteristic Maltese crosses were scarcely observed in F #5, suggesting a typical O/W emulsion. However, the emulsions containing sorbitan stearate and sucrose palmitate (F #1 and F #2) exhibited fewer Maltese cross patterns compared to those formulated with sorbitan palmitate and sucrose palmitate (F #3 and F #4). Moreover, owing to its tendency to leave a white residue on the skin upon application, the emulsion systems containing sorbitan stearate and sucrose palmitate were not selected for further study. Since the Maltese crosses were clearly observed in the emulsion containing sorbitan palmitate and sucrose palmitate at a 4:1 ratio, the F #4 was selected for further investigations.
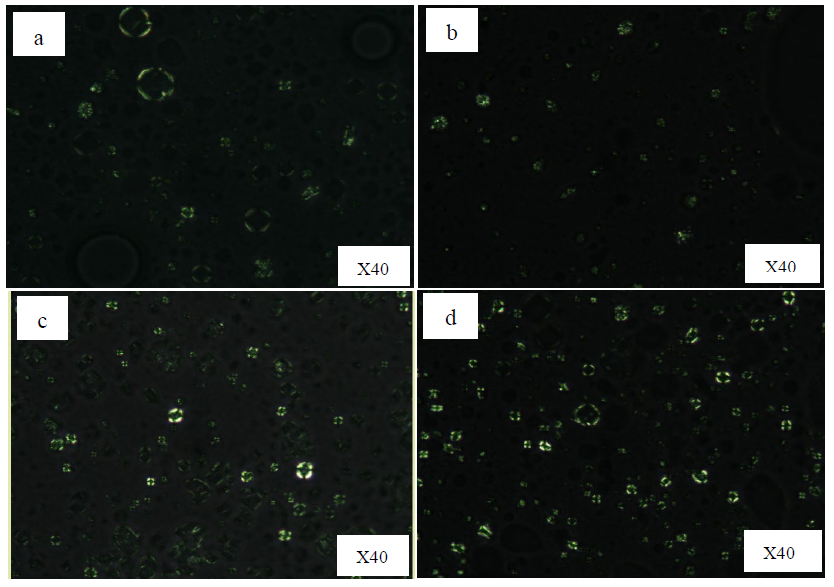
Figure 2. Microscopic images under a light polarized microscopy of a) F #1; sorbitan stearate: sucrose palmitate (3:1), b) F #2; sorbitan stearate: sucrose palmitate (4:1), c) F #3; sorbitan palmitate: sucrose palmitate (3:1), d) F #4; sorbitan palmitate: sucrose palmitate (4:1)
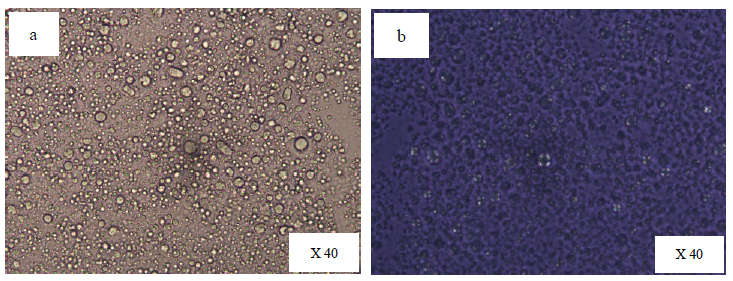
Figure 3. Microscopic images of F #5 containing sorbitan stearate: polyoxyethlene sorbitan stearate (2:1) a) Normal image b) Light polarized image
SAXS and WAXS measurements
SAXS and WAXS results (Figure 4) revealed distinct differences between the mangiferin-loaded lamellar (F #4) and O/W (F #5) emulsions. Particularly, the SAXS pattern of F #4 displayed a prominent peak at around q = 0.9 nm-1, whereas F #5 exhibited a broad peaks. The distance value, d, which could be deduced from the equation, d = 2 / q, using the peaks positions in the X-ray scattering signals, was assigned at 67 Å. Single broad peaks were observed from both formulations (F #4 and F #5) in the wide-angle range measurement at around q = 15 nm-1.
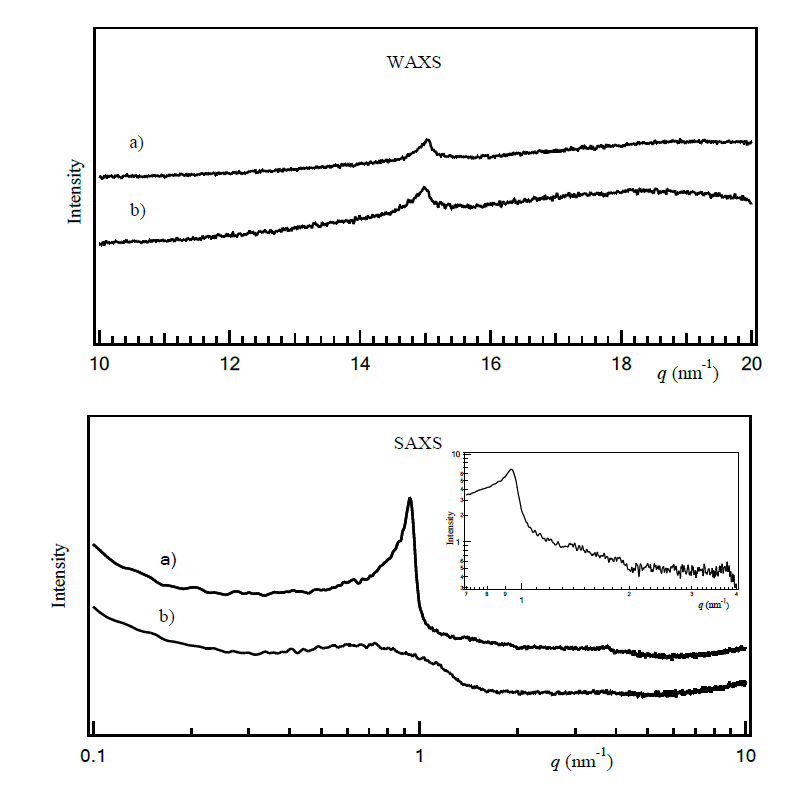
Figure 4. WAXS and SAXS diffractograms: a) F #4 with mangiferin containing sorbitan palmitate: sucrose palmitate at 4:1, b) F #5 with mangiferin containing sorbitan stearate and polyoxyethlene sorbitan sterate at 2:1
Differential scanning calorimetry analysis
DSC investigations were performed to assess the thermal properties of F #4 and F #5 with and without mangiferin, as shown in Figure 5. The results revealed that the inclusion of mangiferin into both lamellar and o/w emulsions resulted in shifts in phase transition temperatures. However, this shift pattern differed between the lamellar and o/w emulsions. The addition of mangiferin into the lamellar emulsion caused a decrease in phase transition temperatures, while its incorporation in the non-lamellar emulsion led to an increase in phase transition temperatures.
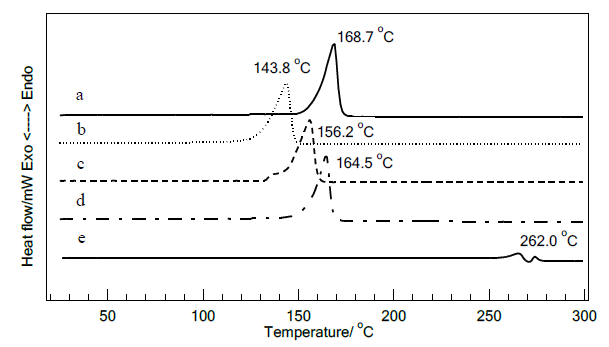
Figure 5. DSC thermatograms of F #4 and F #5, a) F #4 without mangiferin, b) F #4 with mangiferin, c) F #5 without mangiferin, d) F #5 with mangiferin, e) Mangiferin
In vitro release studies
The release of mangiferin from the investigated emulsions was conducted using dialysis method (Figure 6). The release rate of mangiferin in the lamellar emulsion, F #4, was observed to be slower than that of F #5, indicating a sustained release characteristic of lamellar emulsions. The percentage release of mangiferin over a 6-hour period from F #4 and F #5 were 28.39 ± 6.06 and 33.35 ± 3.34, respectively.
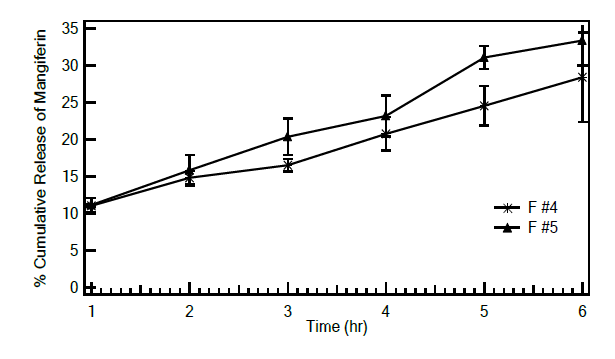
Figure 6. Cumulative release of mangiferin from F #4 (*) and F #5 (æ) (n=3).
Viscosity measurements
The rheological characteristics of the studied emulsions were investigated using a viscometer. The viscosity of the emulsions decreases with the increase of shear rate, exhibiting the shear thinning property. The shear stress and shear rate curve indicated pseudoplastic behavior of the studied emulsion. As shown in Figure 7, both F #4 and F #5 exhibited pseudoplastic behavior with a small hysteresis area, indicative of thixotropy characteristics.
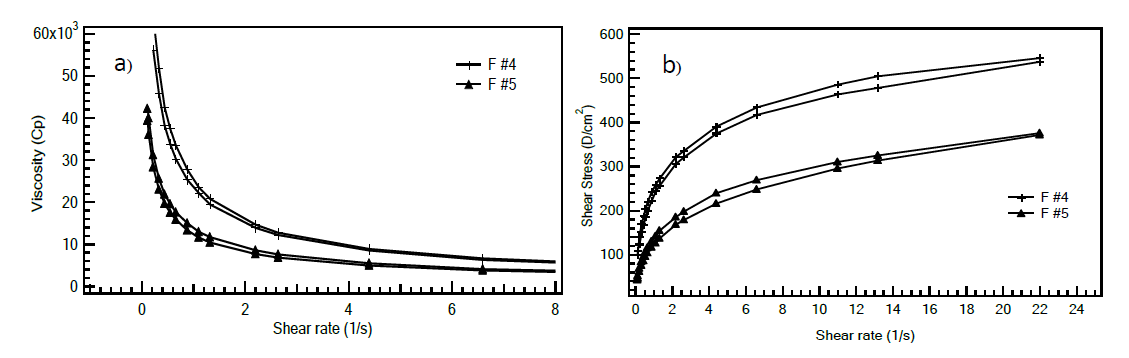
Figure 7. Flow behaviors of F #4 (+) and F #5 (æ) a) Viscosity- shear rate profile and b) Shear stress – shear rate profile.
DISCUSSION
An emulsion is a dispersed system in which two immiscible liquids, typically oil and water, are dispersed into each other with the aid of an emulsifying agent or emulsifier to form a stable mixture. Cream is a semi-solid dosage form fundamentally based on an emulsion structure but includes ingredients to modify texture, viscosity, and stability. The oil phase consists of lipophilic (oil-soluble) ingredients. These can include oils, waxes, and oil-soluble active ingredients. The water phase consists of hydrophilic (water-soluble) ingredients, typically water and other polar substances. Emulsifiers allow these two liquids to mix and remain dispersed, preventing them from separation into distinct layers. Emulsifiers are amphiphilic molecules that create a protective barrier around oil droplets in oil-in-water emulsions or water droplets in water-in-oil emulsions, thereby stabilizing the mixture. They can be classified into primary emulsifiers and secondary emulsifiers or co-emulsifiers. Primary emulsifiers are essential surfactants in emulsion formulations, forming a protective layer around the dispersed droplets. Secondary emulsifiers are additional emulsifying agents utilized alongside primary emulsifiers to enhance stability and to modify the viscosity and sensory characteristics of the emulsions. While a primary emulsifier is responsible for reducing the interfacial tension between the oil and water phases, the co-emulsifier works synergistically with it to improve the overall emulsification process. Additionally, employing two surfactants with different Hydrophilic-Lipophilic Balance (HLB) values is a strategy for achieving optimal emulsion stability (Singpanna and Charnvanich, 2021). Surfactants with high HLB values are predominantly hydrophilic, whereas those with low HLB values are more lipophilic. This distinction influences their molecular configurations, enabling the formation of complex interfacial films at the oil-water boundary.
Generally, the emulsifiers form a monolayer or a thin bilayer film around the dispersed droplets. However, by selecting and combining surfactants with secondary emulsifiers, more complex and ordered structures can be created at the oil-water interface. These include various structured phases, such as lamellar (layer) or cubic (three-dimensional) structures. Certain types of surfactants and co-emulsifiers, based on their combined molecular geometry and concentration, can facilitate the formation of these ordered structures. Svoboda et al. (2021) demonstrated that in aqueous systems containing appropriate combinations of surfactants and fatty alcohols, bilayer structures can form when the concentration of emulsifiers exceeds that required to form a monomolecular film at the oil droplet interface. This type of structure dramatically stabilizes emulsion system and effectively entraps a huge amount of water, allowing maximum skin hydration (Jia et al., 2017). To develop these ordered structures, a selection of mixed surfactants and fatty alcohols was undertaken. Stearyl alcohol, also known as octadecan-1-ol, was selected as an agent to enhance the viscosity of the formulations. This compound is a long-chain primary fatty alcohol characterized by an unbranched, saturated carbon chain with 18 carbon atoms and a hydroxyl group located at the first carbon (C-1). As amphiphilic molecules, fatty alcohols commonly function as secondary emulsifiers. Surfactants with long hydrocarbon chains, acting as primary emulsifiers, have been shown to improve emulsion stability (Maulvi et al., 2017). Based on this principle, surfactants containing 16 and 18 carbon atoms were chosen. A combination of surfactants with high and low HLB values was explored to identify the optimal pairing for creating an ordered structure at the oil–water interface used in this study. This ordered arrangement of the emulsifiers at oil-water interphase displays anisotropic properties, which can be observed using a polarized light microscopy (Duncke et al., 2016). An anisotropic sample, such as lamellar structure, divides a ray of light into two separate rays, creating an optical interference pattern known as birefringence, which manifests as a bright Maltese cross pattern. The degree of birefringence depends on the thickness, orientation, and composition of the lamellae.
By varying the types and ratios of surfactants, while maintaining a 6% emulsifier content by weight and 1% stearyl alcohol, a lamellar structure was successfully formed. The ratios and concentrations of surfactants were preliminary designed to achieve a total HLB value in the range of 8 to 12, which is positioned in the middle of the HLB scale (1-20) in order to facilitate the formation of lamellar structure. All of the studied emulsions observed under a light polarized microscopy displayed the Maltese cross sign, although to varying degrees. It has been reported that a combination of polyoxyethylene sorbitan based surfactants (such as Tween® series) and sorbitan based surfactants (such as Span® series) can facilitate the formation of lamellar structures in emulsions. However, achieving these structures often necessitates relatively high concentrations of these surfactants (Mahdi et al., 2011). This may explain the limited observation of Maltese cross images in F #5, which contains sorbitan stearate and polyoxyethylene sorbitan stearate. This formulation was intentionally formulated as a non-lamellar emulsion. In contrast, a combination of sorbitan stearate or sorbitan palmitate with sucrose palmitate resulted in the formation of lamellar structures, as evidenced by the clearer observation of Maltese cross patterns. The clear detection of the lamellar structure may be attributed to the architectural arrangement of both emulsifiers with reverse structures at the oil-water interface, thereby creating a mixed critical packing parameter (CPP) close to that suitable for lamellar formation, which is in the range of 0.5-1 (Huang and Gui, 2018). When comparing formulations containing sorbitan stearate (F #1 - F #2) with those containing sorbitan palmitate (F #3 - F #4), the formulations with sorbitan palmitate exhibited a more pronounced Maltese cross pattern under polarized light microscopy. This observation suggested that the similar carbon chain lengths in sorbitan palmitate and sucrose palmitate resulted in a critical packing parameter value much closer to 0.5–1. The obvious detection was observed in the formulation containing sorbitan palmitate and sucrose palmitate at 4:1 ratio. The crystalline packing and ordered molecular orientation of surfactant chains and stearyl alcohol contribute to the observed birefringence.
X-ray scattering technique is the most appropriate technique for analyzing the microstructure of surfactant arrangements at the oil-water interface. A single sharp SAXS peak of F #4 indicated a single, periodic length scale within the sample. The SAXS diffractogram at q = 0.93 nm⁻¹ revealed that the stearyl alcohol and mixed surfactants in F #4 organized into a periodic structure, which is typically characteristic of a lamellae or bilayer, arising from the regular spacing between the layers at 67 Å. This interlayer spacing corresponds to the thickness of one complete lamellar repeat unit, comprising a surfactant bilayer plus the aqueous layer. The absence of higher-order reflections (as shown in the upper inset of the SAXS diffractogram) indicates limited long-range order within the lamellar structure. The missing second-order quasi-Bragg peak, which would typically appear at q ≈ 2 nm⁻¹, results from disrupted long-range periodicity caused by variable bilayer spacing. This structural disorder arises from the complex formulation composition, including mixed surfactants, the presence of guar gum polymer (0.6%), and high water content (~84%) creating heterogeneous interlayer regions. These compositional factors introduce fluctuations in the lamellar d-spacing, which broaden and suppress higher-order reflections, while preserving the fundamental lamellar periodicity. Despite the absence of long-range order, the observation of significant birefringence under polarized light microscopy confirms the presence of ordered molecular orientation within individual bilayers. This local molecular order indicates crystalline packing of the lipophilic chains from the surfactant components including sorbitan palmitate, sucrose palmitate, and stearyl alcohol.
In contrast, this specific structural arrangement was not observed in F #5. For both formulations, the single sharp peaks observed in the WAXS diffractogram near q = 15 nm-1 can be attributed to tight molecular packing, with the regular spacing between alkyl chains corresponding to a d-spacing of approximately 42 Å. It could be described that the molecular assemblies formed a well-ordered structure, with the strong interaction between alkyl chains of stearyl alcohol and surfactants arranged in a hexagonal manner (Karl et al., 2015).
In conclusion, analysis of optical microscopy, combined with SAXS and WAXS data, suggested that the amphiphilic molecules at the interface in F #4 were organized into a lamellar structure characterized by a layer spacing of approximately 67 Å and an alkyl chain packing length of about 42 Å. In contrast, the system in F #5 exhibited an isotropic monolayer arrangement with an alkyl chain packing length of 42 Å. Both formulations contained sorbitan-based surfactants (sorbitan palmitate), but the paired surfactant differed: F #4 included sucrose palmitate, while F #5 used polyoxyethylene sorbitan stearate. Sucrose palmitate (580 g/mol) has a rigid hydrophilic head group, whereas polyoxyethylene sorbitan stearate (~1,310 g/mol) features a bulkier head group with higher flexibility, leading to distinct interfacial packing arrangement. Sucrose palmitate, when combined with a suitable co-surfactant, is more likely to form lamellar aggregations, whereas polyoxyethylene sorbitan stearate tends to favor the formation of monolayer. This distinction may explain the larger droplet size observed in F #4, which was measured at 7.45 ± 4.09 μm (n=100) by using an optical microscope, compared to the smaller droplet size of F #5, which was 5.77 ± 2.04 μm (n=100).
Differential Scanning Calorimetry is one of the most commonly used technique of thermal characterization of system transformations. The DSC investigations of all freshly prepared emulsion samples exhibited a single transition peak at rather high temperature. Karl et al. (2015) reported that the pure long-chain alcohols, stearyl alcohol, exhibited a unique endothermic peak corresponding to the melting of the fatty chains at 60°C, while its ternary cream, which were homogeneously mixture of stearyl alcohol, surfactant (CTAC) and water exhibited endothermic peak at higher transition temperature. Similar finding were also reported by Okamoto et al. (2016), who observed that stearyl alcohol/surfactants/water system exhibited a singular DSC peak with transition temperatures higher than those of their individual components. They proposed that this phenomenon resulted from the formation of molecular assemblies within the samples. Likewise our investigation, a singular sharp DSC peak at high transition temperature was observed from F #4 and F #5. Stearyl alcohol has a melting point of 58 to 60°C, while the primary emulsifiers—sorbitan stearate, sorbitan palmitate, sucrose palmitate, and polyoxyethylene sorbitan stearate—exhibit melting points of 54 to 57°C, 47°C, 152 to 156°C, and 45 to 50°C, respectively. The DSC peaks observed at 168.7°C for F #4 and 156°C for F #5 may be indicative of phase transitions related to a well-ordered structure, such as a crystalline phase. These transitions are likely influenced by the interactions between the hydrophobic tails of the surfactants and the fatty alcohol, affecting phase change behavior rather than the melting of the individual components. The DSC results suggested the presence of tightly packed molecular structures undergoing phase transitions. None of endotherm peaks at lower temperature were observed, indicating no free emulsifiers appeared in the emulsion systems. When mangiferin was incorporated into the emulsions, the corresponding transition temperature of aggregations shifted, which may be attributed to the influences of mangiferin molecules to the molecular assemblies of stearyl alcohol and the surfactants, affecting F #4 and F #5 in different ways. The absence of mangiferin thermal peak also implied to environment change owing to the incorporation of mangiferin in the system. Mangiferin is a C-glucosyl xanthone that exhibits both hydrophilic and hydrophobic characteristics. When incorporated into the emulsion system, it can interact with other amphiphilic molecules, thereby altering their thermal behaviors. In F #4, mangiferin may disrupt the ordered molecular packing, leading to a reduction in the energy required for phase transitions. Conversely, in F #5, the presence of mangiferin may restrict the molecular mobility of the isotropic aggregates, resulting in increased energy requirements for phase transitions. These experimental findings also indicated differences in the microstructural arrangements between the studied formulations.
In vitro release experiments were carried out to investigate the release characteristics of the studied emulsion. Differences in the release rate of mangiferin were noticed between F #4 and F #5. The release rate of mangiferin from the lamellar emulsion of F #4 was observed to be slower than that of F #5. This finding suggested that mangiferin was slightly more tightly packed within the microstructure formed in F #4 compared to that in F #5. Similar findings were observed in the liquid crystal emulsions containing a model drug, ketoprofen, with a drug release rate much slower than that in traditional emulsions. (Hu et al., 2021).
The viscosity behavior of the emulsion is an important factor for skin application. Both formulations, F #4 and F #5, exhibited pseudoplastic behavior with a small hysteresis area, indicative of thixotropy characteristics. This rheological behavior allows the emulsions to spread easily when applied to the skin (Kwak et al., 2015). As the compositions of F #4 and F #5 were largely similar except for the emulsifier systems, the observed differences in viscosity were primarily attributed to the type of mixed surfactants used. It has been reported that the types and concentrations of surfactants are significant factors affecting the rheological properties of emulsions. (Teeranachaideekul et al., 2023).
CONCLUSION
The lamellar structure has been reported to significantly stabilize emulsion systems and effectively trap water, allowing for maximum skin hydration- an essential benefit for cosmetic application. This structure was successfully formed in the studied emulsion system containing stearyl alcohol and a mixture of surfactants, specifically sorbitan palmitate – sucrose palmitate at a ratio of 4:1. Confirmation of the obtained structures was conducted using various instruments, including SAXS, WAXS, DSC, and a polarized light microscopy. Instead of utilizing pre-mixed liquid crystal-making surfactants, which are costly raw materials, the suitable surfactant mixtures can be employed to lower production costs. Furthermore, mangiferin, an ingredient with an amphiphilic structure, can be incorporated into this emulsion. However, some potential benefits of its topical application, such as enhancing skin hydration, improving skin permeation, and preserving the stability of active substances, remain unexplored. Further investigations on these topics are expected to provide conclusive answers.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Pharmaceutical Sciences, Huachiew Chalermprakiet University for providing material and instrumentation used in this study.
AUTHOR CONTRIBUTIONS
Paveena Wongtrakul and Parapat Sobharaksha assisted in conducting the experiments, performed data visualization and wrote the manuscript. Warunee Leesajakul designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdelfattah, M.A.O., Dmirieh, M., Ben Bakrim, W., Mouhtady, O., Ghareeb, M.A., Wink, M., and Sobeh, M. 2022. Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidence from in silico, in vitro and in vivo studies. Journal of Ethnopharmacology. 292: 115187. https://doi.org/10.1016/j.jep.2022.115187
Acosta, J., Sevilla, I., Salomón, S., Nuevas, L., Romero, A., and Amaro, D. 2016. Determination of mangiferin solubility in solvents used in the biopharmaceutical industry. Journal of Pharmacy and Pharmacognosy Research. 4(2): 49-53. https://doi.org/10.56499/jppres15.099_4.2.49
Barakat, S., Nasr, M., Ahmed, R.F., Badawy, S., and Mortada, N. 2022. Recent formulation advances of mangiferin. Revista Brasileira de Farmacognosia. 32: 871-882. https://doi.org/10.1007/s43450-022-00297-z
Barna, A.S., Maxim, C., Trifan, A., Blaga, A.C., Cimpoesu, R., Turcov, D., and Suteu, D. 2023. Preliminary approaches to cosmeceuticals emulsions based on N-prolylpalmitoyl tripeptide-56 acetat-bakuchiol complex intended to combat skin oxidative stress. International Journal of Molecular Sciences. 24: 7004. https://doi.org/10.3390/ijms24087004
da Rocha-Filho, P.A., Maruno, M., Ferrari, M., and Topan, J.F. 2016. Liquid crystal formation from sunflower oil: Long term stability studies. Molecules. 21(6): 680. https://doi.org/10.3390/molecules21060680
Duncke, A.C., Marinho, T.O., Barbato, C.N., Freitas, G.B., Oliveira, MCK., and Nele, M. 2016. Liquid crystals observations in emulsion fractions from Brazilian crude oils by polarized light microscopy. Energy Fuels. 30(5): 3815-3820. https://doi.org/10.1021/acs.energyfuels.5b02943
Fameau, A.L., Veronico, L., Le Coeur, C., Mahmoudi, N., and Gentile, L. 2024. Shear and cooling effects on lamellar gel network structure: Insights from Rheo-SANS. Journal of Molecular Liquids. 414: 126283. https://doi.org/10.1016/j.molliq.2024.126283
He, H., Li, A., Li, S., Tang, J., Li, L., and Xiong, L. 2021. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomedicine and Pharmacotherapy. 134: 111161. https://doi.org/10.1016/j.biopha.2020.111161
Hu, J., Ni, Z., Zhu, H., Li, H., Chen, Y., Shang, Y., Chen, D., and Liu, H. 2021. A novel drug delivery system-Drug crystallization encapsulated liquid crystal emulsion. International Journal of Pharmaceutics. 607: 121007. https://doi.org/10.1016/j.ijpharm.2021.121007
Huang, Y. and Gui, S. 2018. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. Royal Society of Chemistry Advances. 8(13): 6978-6987. https://doi.org/10.1039/C7RA12008G
Jia, B., Zhang, Q., Zhang, Z., Chen, M., and Zhang, W. 2017. Preparation of liquid crystal emulsion and its application performance study. Journal of Dispersion Science and Technology. 39(1): 100-105. https://doi.org/10.1080/01932691.2017.1297721
Karl, W., Perla, R., Gérard, C., Franck, C., Luc, N.-M., Hayat, B., and Denis, F. 2015. Effect of surfactant on structure thermal behavior of cetyl stearyl alcohols. Journal Thermal Analysis Calorimetry. 123(2): 1411-1417. https://doi.org/10.1007/s10973-015-5074-2
Kircik, L.H. 2014. Effect of skin barrier emulsion cream vs a conventional moisturizer on transdermal water loss and corneometry in atopic dermatitis: A pilot study. Journal of Drugs in Dermatology. 13(12): 1482-1484.
Kuwabara, H., Ogura, T., Tsuchiya, K., Akamatsu, M., Arakawa, K., Sakai., K., and Sakai, H. 2024. Structural analysis of interfacial films of oil/water emulsions prepared by emulsification via lamellar gels. Journal of Oleo Science. 12: 1467-1477. https://doi.org/10.5650/jos.ess24181
Kwak, M.S., Ahn, H.J., and Song, K.W. 2015. Rheological investigation of body cream and body lotion in actual application conditions. Korea-Australia Rheology Journal. 27(3): 241-251. https://doi.org/10.1007/s13367-015-0024-x
Lee, J.B., Noh, M., Kim, S.J., and Jang, J. 2019. Liquid crystal emulsions containing high content ceramides for improved skin barrier functions. Korean Journal of Cosmetic Science. 1(1): 9-29. https://doi.org/10.3390/cosmetics6010009
Lee, S.Y., Park, J.S., Kim, D., Jeong, W., Hwang, C., Kim, H.O., Park, C.W., and Chung, B.Y. 2024. Effcacy of a multi‑lamellar emulsion containing a synthetic sphingosine kinase 1 activator and pseudoceramide in patients with atopic dermatitis: A randomized controlled trial. Dermatology and Therapy. 14: 2591-2605. https://doi.org/10.1007/s13555-024-01254-5
Mahdi E.S., Sakeena M., Abdulkarim M., Abdullah G., Abdul Sattar, M.Z., and Noor A.M. 2011. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Design, Development and Therapy. 5: 311-323. https://doi.org/10.2147/DDDT.S15698
Maulvi, F.A., Desai, A.R., Choksi, H.H., Patil, R.J., Ranch, K.M., Vyas, B.A., and Shah, D.O. 2017. Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses. International Journal of Pharmaceutics. 524(1-2): 193-204. https://doi.org/10.1016/j.ijpharm.2017.03.083
Michalak, M. 2023. Plant extracts as skin care and therapeutic agents. International Journal of Molecular Sciences. 24: 15444. https://doi.org/10.3390/ijms242015444
Misono, T., Sekihara, R., Endo, T., Sakai, K., Abe, M., and Sakai, H. 2015. Ternary phase behavior of phytosterol ethoxylate, water, and imidazolium-based ionic liquid systems - Lyotropic liquid crystal formation over a wide range of compositions. Colloids and Surfaces: A Physicochemical and Engineering Aspects. 472: 117-123. https://doi.org/10.1016/j.colsurfa.2015.01.083
Okamoto, T., Tomomasa, S., and Nakajima, H. 2016. Preparation and thermal properties of fatty alcohol/surfactant/oil/water nanoemulsions and their cosmetic applications. Journal of Oleo Science. 65(1): 27-36. https://doi.org/10.5650/jos.ess15183
Pleguezuelos-Villa, M., Nácher, A., Hernández, M.J., Ofelia Vila Buso, M.A., Ruiz Sauri, A., and Díez-Sales, O. 2019. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. International Journal of Pharmaceutics. 564: 299-307. https://doi.org/10.1016/j.ijpharm.2019.04.056
Pleguezuelos-Villa, M., Castangia, I., Octavio Diez-Sales, Manca, M.L., Manconi, M., Sauri, A.R., Tal'ens-Visconti, R., and Nacher, A. 2024. Control of skin damages caused by oxidative stress using mangiferin and naringin co-loaded in phospholipid vesicles. Journal of Drug Delivery Science and Technology. 91: 105261. https://doi.org/10.1016/j.jddst.2023.105261
Poomanee, W., Yaowiwat, N., Pattarachaidaecharuch, T., and Leelapornpisid, P. 2023. Optimized multiherbal combination and in vivo anti-skin aging potential: A randomized double blind placebo controlled study. Scientific Reports. 13(1): 5633. https://doi.org/10.1038/s41598-023-32738-7
Prommajak, T., Kim, S.M., Pan, C.H., Kim, S.M., Surawang, S., and Rattanapanone, N. 2014. Identification of antioxidants in young mango leaves by LC-ABTS and LC-MS. Chiang Mai University Journal of Natural Sciences. 13(3): 317-330. https://doi.org/10.12982/CMUJNS.2014.0038
Roso, A., Kern, C., Cambos, S., and Garcia, C. 2023. Diversity challenge in skin care: Adaptations of a simple emulsion for efficient moisturization across multiple geographies. Applied Sciences. 13(24): 13175. https://doi.org/10.3390/app132413175
Schulte to Brinke, A., Janssens-Böcker, C., and Kerscher, M. 2021. Skin anti-aging benefits of a 2% resveratrol emulsion. Journal of Cosmetics, Dermatological Sciences and Applications. 11(2): 155-168. https://doi.org/10.4236/jcdsa.2021.112015
Shedoeva, A., Leavesley, D., Upton, Z., and Fan, C. 2019. Wound healing and the use of medicinal plants. Evidence-based Complementary and Alternative Medicine. 22: 2684108. https://doi.org/10.1155/2019/2684108
Strati, F., Neubert, R.H.H., Opálka, L., Kerth, A., and Brezesinski, G. 2021. Non-ionic surfactants as innovative skin penetration enhancers: Insight in the mechanism of interaction with simple 2D stratum corneum model system. European Journal of Pharmaceutical Sciences. 157: 105620. https://doi.org/10.1016/j.ejps.2020.105620
Singpanna, K. and Charnvanich, D. 2021. Effect of the hydrophilic-lipophilic balance values of non-ionic surfactants on size and size distribution and stability of oil/water soybean oil nanoemulsions. The Thai Journal of Pharmaceutical Sciences. 45(6): 487-491. https://doi.org/10.56808/3027-7922.2530
Sobharaksha, P., Mahamongkol, H., Channarong, S., Tansathien, K., and Wongtrakul, P. 2024. Development of mangiferin-loaded anisotropic emulsion for cosmetic applications: A comprehensive study on formulation, antioxidant activity, stability, and skin compatibility. Science, Engineering and Health Studies. 18: 24050022. https://doi.org/10.69598/sehs.18.24050022
Svoboda, M., Jiménez, S.M.G., Kowalski, A., Cooke, M., Mendoza, C., and Lísa, M. 2021. Structural properties of cationic surfactant-fatty alcohol bilayers: Insights from dissipative particle dynamics. Soft Matter. 17(43): 9967-9984. https://doi.org/10.1039/D1SM00850A
Teeranachaideekul, V., Soontaranon, S., Sukhasem, S., Chantasart D., and Wongrakpanich, A. 2023. Infuence of the emulsifer on nanostructure and clinical application of liquid crystalline emulsions. Scientific Reports. 13(1): 4185. https://doi.org/10.1038/s41598-023-31329-w
Tessiri, C., Channarong, S., and Wongtrakul, P. 2021. Development of aqueous formulation containing the extracted mangiferin. Key Engineering Materials. 201: 40-47. https://doi.org/10.4028/www.scientific.net/KEM.901.40
Yang, S., Zhou, Q., Zhang, B., Zhang, L., Yang, D., Yang, H., Du, G., and Lu, Y. 2020. Screening, characterization and evaluation of mangiferin polymorphs. Natural Products and Bioprospecting. 10: 187-200. https://doi.org/10.1007/s13659-020-00247-z
Zhang, W. and Liu, L. 2013. Study on the formation and properties of liquid crystal emulsion in cosmetic. Journal of Cosmetics, Dermatological Sciences and Applications. 3: 139-144. https://doi.org/10.4236/jcdsa.2013.32022
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Paveena Wongtrakul1, 2, Warunee Leesajakul1, 2, Waranya Neimkhum1, 2, Wicharn Janwitayanuchit2, 3, and Parapat Sobharaksha1, 2, *
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Huachiew Chalermprakiet University, Samut Prakan 10540, Thailand.
2 Cosmetic and Herbal Innovation Center (CHIC), Faculty of Pharmaceutical Sciences, Huachiew Chalermprakiet University, Samut Prakan 10540, Thailand.
3 Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Huachiew Chalermprakiet University, Samut Prakan 10540, Thailand.
Corresponding author: Parapat Sobharaksha, E-mail: parapat.sob@hcu.ac.th
ORCID iD:
Parapat Sobharaksha: https://orcid.org/0009-0000-3947-1000
Paveena Wongtrakul: https://orcid.org/0009-0009-1760-7670
Waranya Neimkhum: https://orcid.org/0000-0002-1627-2794
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: March 17, 2025;
Revised: September 15, 2025;
Accepted: September 18, 2025;
Online First: October 1, 2025

