
Role of Stingless Bee Honey in Ameliorating Cognitive Dysfunction in an Aluminium Chloride and D-Galactose Induced Alzheimer’s Disease Model in Rats
Shah Rezlan Shajahan, Hussin Muhammad, Mohd Zulkifli Mustafa, Yatinesh Kumari, Imrana Jazuli, Azlina Zulkapli, Norshafarina SK, Amanina Nurjannah Atan Hamdan, Nazatul Syawany Wahid, and Muhammad Danial Che Ramli*Published Date : June 13, 2025
DOI : https://doi.org/10.12982/NLSC.2025.053
Journal Issues : Number 3, July-September 2025
Abstract Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and behavioural changes. With limited treatments available, the search for alternative therapeutic approaches is critical. Stingless bee honey (SBH) has been recognized for its medicinal properties, making it a promising candidate for addressing AD-related symptoms. This study investigates the therapeutic effects of SBH on cognitive abilities, neuronal integrity, and biochemical pathways in rats modelled with AD induced by aluminium chloride (AlCl3) and D-galactose (D-gal). Behavioural studies were conducted using the Open field test (OFT) and the Morris water maze (MWM). Histological analysis and neurotransmitter levels, including dopamine, corticosterone, acetylcholinesterase, and serotonin, were measured using ELISA. Results indicated that SBH significantly enhanced cognitive performance, reduced neuronal damage, and altered key bio-indicators, suggesting a therapeutic effect against AD. Histological examination showed preservation of hippocampal cells, and ELISA results indicated normalization of neurotransmitter levels and stress hormone reduction due to AlCl3 and D-gal activity in the brain. These findings suggest that SBH can improve cognitive and memory functions in AD patients without causing harm to vital organs during short-term use. This study supports the therapeutic benefits of SBH and highlights the need for further research on its long-term effects and mechanisms.
Keywords: Alzheimer's disease, Stingless bee honey, Cognitive dysfunction, Aluminium chloride, D-galactose
Funding: The authors are grateful for the research grant provided by Management & Science University. Grant ID: SG-020-022023-FHLS.
Citation: Shajahan, S.R., Muhammad, H., Mustafa, M.Z., Kumari, Y., Jazuli, I., Zulkapli, A., SK, N., Hamdan, A.N.A., Wahid, N.S., and Ramli, M.D.C. 2025. Role of stingless bee honey in ameliorating cognitive dysfunction in an aluminium chloride and D-galactose induced Alzheimer’s disease model in rats. Natural and Life Sciences Communications. 24(3): e2025053.
INTRODUCTION
Alzheimer's Disease (AD) is a significant public health issue and a progressive neurological disorder that impairs memory and cognitive functions. It is the most common form of dementia, accounting for up to 70% of cases, and is characterized by the accumulation of tau tangles and amyloid-beta (Aβ) plaques in the brain. Alzheimer’s disease is the most common type of dementia; approximately one in eight people over the age of 65 years are at risk of developing Alzheimer’s disease (Panachamnong et al., 2014). Projections indicate that the number of AD cases will triple by 2050, creating a substantial burden on healthcare systems and families worldwide (Lane et al., 2018; D’Haese et al., 2020).
AD is marked by a gradual decline in memory, behavioural changes, and alterations in personality. The disease develops slowly over many years, and symptoms can vary greatly among individuals. Early indicators may include language difficulties, challenges with daily activities, and trouble remembering recent events. As the disease progresses, individuals may experience personality changes, agitation, and mobility issues (Lane et al., 2018; D’Haese et al., 2020).
As of 2024, over 55 million people globally are living with dementia, with nearly 10 million new cases reported annually. Alzheimer's disease is the leading cause of dementia, making up 60-70% of cases. The number of individuals affected by dementia is anticipated to rise to 78 million by 2030 and 139 million by 2050, with a notable increase expected in low- and middle-income countries. Currently, 60% of people with dementia reside in these regions, and this proportion is projected to increase to 71% by 2050 (Alzheimer’s International, 2023).
The economic impact of dementia is substantial, with the global cost estimated at over $1.3 trillion annually. This figure is anticipated to rise to $2.8 trillion by 2030, encompassing costs from informal care, direct social care, and direct medical care. Informal care, often provided by family members, accounts for about 40% of these costs globally (Alzheimer’s International, 2023).
The treatment of Alzheimer's Disease (AD) remains challenging, with current medical practices offering only limited symptomatic relief rather than a cure. Available treatments, including cholinesterase inhibitors and memantine, can improve cognitive function and slow disease progression but do not address the underlying causes of AD. Recent research highlights the potential of medicinal plants as alternative treatments for AD (Cummings, 2021). The leaves of Aloe vera were exploited in the discovery of bioactive natural products for the treatment of Alzheimer’s disease (Bendjedid et al., 2020). Berberine, an alkaloid found in plants such as Argemone mexicana (prickly poppy) and Coptis chinensis (Chinese goldthread), has demonstrated anti-inflammatory, anti-cancer, anti-diabetic, and memory-enhancing properties (Cummings, 2021).
In light of this, the current study seeks to explore natural remedies as safer, patient-friendly alternatives to conventional medications. Natural products may offer effective options when it comes to drug efficacy standards. For example, substances like stingless bee honey, which contains flavonoids and catechins, have been shown to inhibit tumor growth, inflammation, oxidative stress, cognitive decline, and other age-related neurological dysfunctions, including those associated with Alzheimer's disease (Lane et al., 2018; Zulkifli et al., 2023). Research has demonstrated that honey can influence various biological processes related to Alzheimer's, including the expression of genes involved in neuroinflammation and amyloid-beta formation (Majtan et al., 2012). However, most studies have focused on traditional types of honey, leaving a gap in understanding the unique properties and mechanisms of stingless bee honey in the context of Alzheimer's disease.
This study aims to fill that gap by investigating the potential of stingless bee honey as a natural alternative for treating AD, focusing on its absence of side effects. The study used a combination of aluminium chloride and D-galactose-induced cognitive impairment model in Sprague Dawley rats to assess its effects on neuronal damage and cholinergic deficits (Damodaran et al., 2020). The results offer a deeper understanding of how the active components in stingless bee honey may improve memory and prevent neurodegenerative effects. Cognitive abilities related to Alzheimer's disease were evaluated using several tests: the Open Field Test (OFT) for locomotor activity and the Morris Water Maze (MWM) for memory and learning, and neurodegeneration assessment via Haematoxylin & Eosin (H&E) staining.
MATERIALS AND METHODS
Source of honey
The multiflora stingless bee honey (SBH), native to Southeast Asia, was sourced from a bee farm located in the Kelantan region of Kelantan. This honey was produced by the stingless bee species H. itama (Hymenoptera: Apidae: Meliponini). The samples are stored at 4°C until they are analysed.
Animal design
The study involved 48 adult Sprague-Dawley rats, aged 8 weeks, with body weights ranging from 180 to 200 grams. The animals were provided by the Institute for Medical Research (IMR), Malaysian Ministry of Health. They were housed under standard laboratory conditions at 22 ± 2°C, with a 12:12 hour light-dark cycle (0700-1900) and ad libitum access to food and water (Frisbee et al., 2015; Abidin et al., 2024).
Before the study began, the rats were acclimatized to the animal house environment for one week. On the day of the experiment, the rats were transferred to the laboratory at least an hour prior to the start of the procedures. The experiments were conducted between 9:00 a.m. and 6:00 p.m. (Frisbee et al., 2015; Abidin et al., 2024).
This study was conducted following ethical guidelines and approved by the Management and Science University Research Ethics Committee under approval number MSU-RMC-021-FR01-08-C3/017.
Table 1. Animal design.
|
Group |
n |
Treatment |
Experimental design |
|
1 |
8 |
Normal |
Saline |
|
2 |
8 |
Negative (AD) |
AlCl3 (150 mg/kg) + D-gal (300 mg/kg) |
|
3 |
8 |
Positive (AD + Donepezil) |
AlCl3 (150 mg/kg) + D-gal (300 mg/kg) + Donepezil (1.5 mg/kg) |
|
4 |
8 |
Treatment 1 |
AlCl3 (150 mg/kg) + D-gal (300 mg/kg) + SBH (500 mg/kg) |
|
5 |
8 |
Treatment 2 |
AlCl3 (150 mg/kg) + D-gal (300 mg/kg) +SBH (750 mg/kg) |
|
6 |
8 |
Treatment 3 |
AlCl3 (150 mg/kg) + D-gal (300 mg/kg) +SBH (1,000 mg/kg) |
In this study, rats were systematically divided into six distinct groups, each consisting of eight individuals. Group 1 served as the normal control group, while Group 2 functioned as the negative control, also known as the AD-like model. Group 3 was designated as the positive control, or AD + Donepezil group, and Groups 4, 5, and 6 were designated as treatment groups. For the experimental procedures, Donepezil (DPZ) was prepared in distilled water, and aluminium chloride (AlCl3) and D-galactose (D-gal) were dissolved in 0.9% saline.
The experimental protocol involved administering treatments for a total of 14 days. Group 1, the normal control group, was given saline (1 ml/kg) for the entire duration. Group 2, representing the AD model, was subjected to daily administration of AlCl3 (150 mg/kg) and D-galactose (300 mg/kg) for 7 days to induce Alzheimer’s disease-like symptoms. Group 3, the positive control group, also received AlCl3 and D-galactose for 7 days but was treated with Donepezil (1.5 mg/kg) for 14 days to assess its efficacy.
Groups 4, 5, and 6, which were the treatment groups, followed a similar induction regimen with AlCl3 and D-galactose for 7 days. These groups then received treatment with stingless bee honey at varying doses 500 mg/kg, 750 mg/kg, and 1,000 mg/kg, respectively for 14 days (Topuz et al., 2020).
Upon completion of the treatment period, cognitive functions were assessed using the Open Field Test (OFT) and Morris Water Maze (MWM) to evaluate locomotor activity, memory, and learning abilities. Following these tests, the rats were euthanized, and their brains were carefully extracted. The hippocampus from each brain was isolated and preserved in a 10% formaldehyde solution for subsequent histological analysis (Tian et al., 2019; Topuz et al., 2020).
This comprehensive experimental design was intended to thoroughly investigate the effects of various treatments on Alzheimer's disease-like symptoms and to explore potential alternatives to conventional therapies.
Validation of Alzheimer’s disease model
To ensure the successful induction of Alzheimer’s disease (AD) in the rodent model, a series of behavioural, biochemical, and histopathological assessments were conducted as validation markers.
Behaviourally, the Open Field Test (OFT) and Morris Water Maze (MWM) were used to evaluate locomotor activity, anxiety-like behaviour, spatial learning, and memory performance. A significant decrease in exploratory activity in the OFT, along with increased escape latency and reduced time spent in the target quadrant during the MWM, indicated cognitive impairments consistent with AD pathology.
Biochemical validation involved measuring acetylcholinesterase (AChE) activity in brain tissues, as increased AChE activity reflects cholinergic dysfunction, a hallmark of AD. Additionally, neurotransmitter levels including serotonin and dopamine, as well as corticosterone levels, were quantified to assess neurochemical changes associated with AD progression.
Histopathological analysis of hippocampal sections was performed using hematoxylin and eosin (H&E) staining to identify neuronal degeneration. Key pathological features observed included neuronal shrinkage, cytoplasmic vacuolization, and disorganized cellular architecture, which further confirmed the establishment of an AD-like state.
Together, these markers validated the rodent model as a reliable representation of Alzheimer’s disease, providing a robust foundation to evaluate the therapeutic effects of stingless bee honey in this study.
Behavioural experiments
To evaluate the behavioural responses of the animals to aluminium chloride (AlCl3) and D-galactose (D-gal) administration, two behavioural assays were conducted sequentially. The initial assessment involved measuring locomotor activity using the Open Field Test (OFT), followed by an evaluation of memory and learning capabilities through the Morris Water Maze (MWM) test. Prior to testing, the animals were acclimated to the experimental room in the animal facility for at least one hour. Testing sessions were scheduled between 9 a.m. and 1 p.m. to ensure consistency (Tian et al., 2019).
Open field test (OFT)
The Open field test is designed to assess potential changes in emotional response and exploratory behaviour under mildly stressful conditions. The test was conducted in a square box measuring 80 x 80 x 40 cm. The box featured red walls and a white polished floor, which was marked with black lines to create 16 equal squares, each measuring 4 x 4 cm. Each rat was individually placed in the centre of the box and observed for a duration of 3 minutes (Tian et al., 2019).
During the test, several parameters were monitored to detect alterations in exploratory behaviour. These included the frequency of movement and the number of instances where the rat stood on its hind legs to explore its surroundings. Emotional responses were evaluated by observing the frequency of grooming behaviours and analysing faecal pellet counts, which provided insights into the rat's stress levels and emotional state (Topuz et al., 2020).
Morris water maze (MWM)
The water maze utilised for assessing the abilities in memory and spatial learning. MWM is made up of a pool with a 45 cm depth and 1.5 metre diameter. The platform (10x10) be positioned in the centre of the southwest quadrant of the tank, which be divided into four equal quadrants. A non-toxic black dye be used to make the water opaque, and the platform be hidden 2 cm below the water surface. Various figures and objects that might serve as clues for the rats will be hung on the walls of the testing chamber, which be illuminated with consistent light intensity during
the studies. A video camera mounted on the ceiling are film all swimming trials (Topuz et al., 2020).
All of the rats undergo acquisition and probing tests during the first and second phases of the trial. Rats from various quadrants are placed into the pool for 4 trials each day for 4 days as part of the acquisition test. In each experiment, the rats swam for 60 seconds and take out from the pool. When the rats find the platform, they were permitted to stay there for 15 seconds before taken off. The rats be placed on the platform for 15 seconds if they cannot locate it after 60 seconds of searching the platform. 24 hours following the most recent acquisition experiment, the probe test was conducted. The platform is removed from the tank for the probing test, allowed the rats swam freely for 60 seconds (Topuz et al., 2020).
Histology study
Tissue processing
Specimens were placed in a liquid fixing agent, known as fixative. The fixative slowly penetrated, hardened, preserved, and protected the tissue during the subsequent processing steps. Melted paraffin wax was hydrophobic; therefore, water from the specimen needed to be removed before it was infiltrated with paraffin wax. This process was known as dehydration. This technique was carried out by submerging brain specimens in a series of alcohol solutions of increasing concentration until pure, water-free alcohol was achieved. The standard dehydration order was (1) 75% alcohol, (2) 80% alcohol, (3) 95% alcohol, (4) absolute ethanol, and (5) absolute ethanol. Tissues were water-free, but alcohol and wax were immiscible. The intermediate solvent, xylene, was used as a clearing agent to replace alcohol in the tissue. Xylene was also essential for removing fat from the tissue, which hindered wax penetration. The typical clearing steps for the specimens were xylene 1 and xylene 2. Paraffin wax-based histological waxes were the most commonly used. Typically, the wax was liquid at 60°C, and this temperature allowed it to penetrate tissues (Slaoui and Fiette, 2011; Gurina and Simms, 2024).
Embedding, sectioning & mounting
The mould was filled with molten wax, and the specimen was placed into it. The specimen was carefully oriented in the mould, as the placement of the sample determined the plane of the section. A cassette was placed on top of the mould, and more wax was added to stabilize the mould. The mould was placed on a cold plate and allowed to solidify. After the block had hardened, the cassette was removed, and the block was trimmed using a microtome until the tissue was exposed. The tissue was cut, and the ribbons were floated in a water bath. The slide was immersed using a fishing technique to pick up the ribbon. The slide was kept in an incubator for drying (Slaoui and Fiette, 2011; Gurina and Simms, 2024).
Haematoxylin & eosin staining
Since most dyes used to visualize cells were water-soluble, the embedded wax was removed before staining. The resin was dissolved in xylene two times for 5 minutes. After de-waxing, the slide passed through several alcohols to remove the xylene and was rinsed with running tap water. When the section was hydrated, the aqueous reagents easily penetrated the cells and tissue elements. The slide was stained with haematoxylin dye for 10 minutes and rinsed with water. The section was treated with a weak alkaline solution, potassium acetate, which converted haematoxylin to blue. Eosin was then applied as a counterstain for 5 minutes, staining non-nuclear elements in shades of pink. The slide passed through several changes of alcohol concentration to remove water. Lastly, a thin layer of polystyrene mountant was applied with a glass coverslip (Slaoui and Fiette, 2011; Gurina and Simms, 2024).
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to quantify key biomarkers related to Alzheimer’s disease, including dopamine, corticosterone, acetylcholinesterase, and serotonin, in serum samples. Commercial ELISA kits (as described in Alhajj et al., 2023) were employed, following the manufacturer’s protocols.
Briefly, serum samples, standards, and controls (50 µL each) were added to antibody-coated microplate wells, followed by the addition of detection antibody. Plates were incubated at 37°C for 1 hour, washed 3–5 times, and then treated with 100 µL of substrate solution. After 30 minutes of incubation at room temperature in the dark, 50 µL of stop solution was added to terminate the reaction, producing a color change. Absorbance was measured at 450 nm using a microplate reader (Alhajj et al., 2023).
Biochemical analysis
To evaluate the therapeutic effects of stingless bee honey (SBH) on neurodegenerative changes in the Alzheimer’s disease (AD) rat model, biochemical parameters were assessed using enzyme-linked immunosorbent assay (ELISA). The assays targeted key neurochemical markers associated with cognitive function, stress regulation, and neuroinflammation.
Neurotransmitter and hormonal analysis
ELISA was used to quantify serum levels of dopamine, serotonin, acetylcholinesterase (AChE), and corticosterone. These markers were chosen due to their critical roles in AD-related pathology:
Acetylcholinesterase (AChE): Elevated AChE activity is a hallmark of AD and correlates with reduced acetylcholine availability. In this study, SBH-treated groups showed a significant reduction in AChE levels compared to the untreated AD group, suggesting improved cholinergic function.
Dopamine and Serotonin: These neurotransmitters regulate mood, motivation, and cognitive processing. AD is associated with their depletion. SBH administration resulted in increased serum levels of both dopamine and serotonin, implying its neuromodulator effects likely due to its antioxidant and anti-inflammatory properties.
Corticosterone: As a stress hormone, elevated corticosterone contributes to hippocampal atrophy and cognitive decline in AD. A significant reduction in corticosterone levels was observed in SBH-treated rats, indicating an attenuation of stress response and potential neuroprotection.
Mechanistic implications
The observed improvements in neurotransmitter and hormone levels suggest that SBH exhibits antioxidant, anti-inflammatory, and neurodegenerative properties. These effects are potentially mediated by bioactive compounds in SBH, such as flavonoids and phenolic acids, known for modulating intracellular signalling pathways, reducing oxidative stress, and protecting neuronal integrity.
Furthermore, these biochemical outcomes align with the behavioural improvements observed in the Morris Water Maze and Open Field Test, as well as with histopathological findings indicating preserved hippocampal architecture in treated groups.
Data analysis
The gathered data were reported in the form of mean plus standard error of the mean ± SEM. For the purpose of determining the statistical significance, one-way or a two-way analysis of variance (ANOVA) was performed, and then either the Tukey or Dunnett post hoc test was performed, depending on the circumstances. Each comparison that yielded a P value less than < 0.05 was deemed to be statistically significant. The software programme GraphPad Prism, version 9, was utilised in order to carry out the analysis (Zulkifli et al., 2023).
RESULTS
Effects of co-administration of different doses of SBH on locomotor activity
On the designated test day, locomotor activity was evaluated using the Open Field Test (OFT) to ensure that SBH administration did not cause motor impairment or hyperactivity that could confound behavioural outcomes. As shown in Figure 1, there were no statistically significant differences in locomotor activity among the groups, including the normal control, negative control (AlCl₃ + D-gal), positive control (donepezil), and SBH-treated groups (500, 750, and 1,000 mg/kg). One-way ANOVA analysis confirmed that SBH treatment, at all tested doses, did not significantly alter general locomotor behaviour (P > 0.05). This suggests that subsequent cognitive and behavioural findings are unlikely to be influenced by changes in baseline motor function.

Figure 1. Effect of co-administration of different doses of SBH on locomotor activity in rats using the open field test.
The open field test was used to assess exploratory behaviour and general locomotor activity, which can indirectly reflect anxiety-like behaviour in the Alzheimer's disease (AD) rat model.
The experimental groups included:
• Normal control
• Negative control (AlCl₃ 150 mg/kg + D-gal 300 mg/kg)
• Positive control (AlCl₃ + D-gal + donepezil 1.5 mg/kg)
• Treatment groups:
Treatment 1 (SBH 500 mg/kg)
Treatment 2 (SBH 750 mg/kg)
Treatment 3 (SBH 1000 mg/kg)
Data are presented as mean ± SEM (n = 8 per group). Although the treatment groups exhibited trends toward increased locomotor activity compared to the negative control group, no statistically significant differences were observed among the groups (P > 0.05).
This result suggests that while SBH treatment may exert mild anxiolytic or stimulatory effects, especially at 750 mg/kg, these effects were not strong enough to reach statistical significance. The absence of significant changes could also reflect variability in behavioural responses or limitations of the model to detect subtle improvements in locomotor function. Therefore, these findings should be interpreted cautiously and considered alongside more sensitive cognitive assessments such as the Morris Water Maze.
Effects of co-administration of different doses of SBH on the morris water maze test
To determine whether the rodents exhibited cognitive impairments, a behavioural test was conducted to assess hippocampal-dependent learning and memory (Topuz et al., 2020). In this study, the Morris Water Maze (MWM) test was utilized to evaluate spatial learning and memory capabilities by analysing the rodents swimming trajectories, escape latency, and residence time in the target quadrant (Scearce-Levie, 2011; Sánchez et al., 2023).
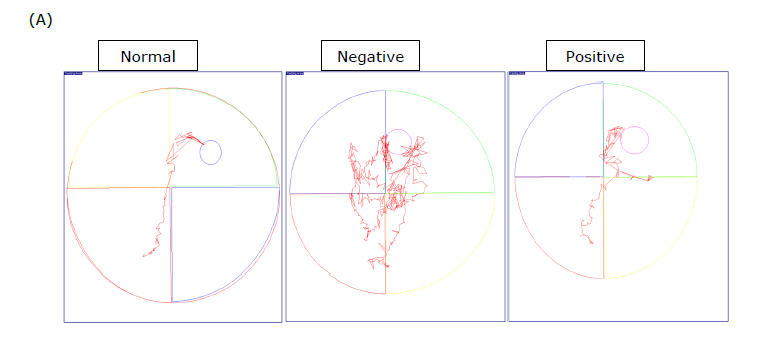
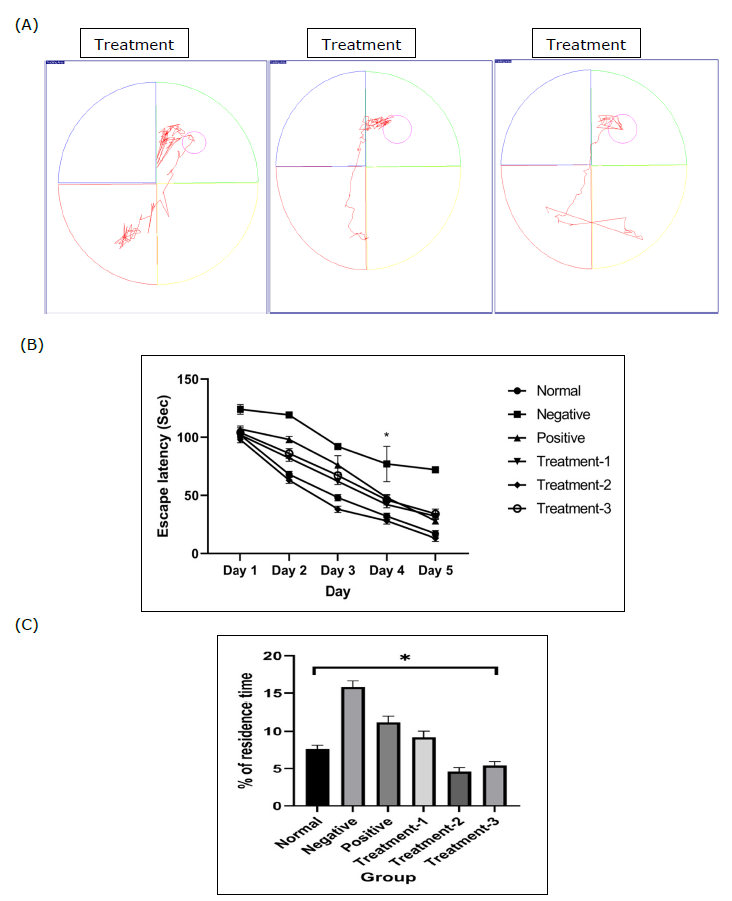
Figure 2. Effect of stingless bee honey (SBH) on spatial learning and memory in rats using the Morris Water Maze (MWM) test. (A) Representative swim paths of each group on day 4. (B) Mean escape latency to find the hidden platform during the 4-day navigation test. (C) Percentage of time spent in the target quadrant during the probe trial. Data are expressed as mean ± SEM (n = 8). Statistical analyses were performed using two-way ANOVA with Tukey post hoc for (B) and one-way ANOVA with Tukey post hoc for (C). *P < 0.05 considered statistically significant.
Figure 2(A) shows the representative swimming trajectories of the six groups on the final day of the navigation test. A two-way ANOVA was conducted to evaluate the impact of control groups and different SBH dosages on the time required to locate the escape platform over four days, revealing a significant interaction between treatment and duration.
According to Figure 2(B), the Tukey post hoc test (P < 0.05) indicated that Treatment 2 and Treatment 3 significantly differed from the control group and Treatment 1, although no significant difference was observed between Treatment 2 and Treatment 3.
In the probe trial test depicted in Figure 2(C), a one-way ANOVA revealed significant differences between the groups. The Tukey post hoc test showed that Treatment 2 and Treatment 3 spent significantly less time in the target quadrant compared to the control group and Treatment 1 group.
Histological examinations

Figure 3. Photomicrograph represent light microscope images of H&E stained sections from the hippocampus of an AD-like model and stingless bee honey (SBH) treated rats. (40x magnification).
In the AD (B) and AD+DPZ (C) groups, neurons exhibit darkly stained nuclei (long arrows) and irregular, tangle-like shapes with cytoplasmic vacuolation and edema (arrow heads). Conversely, neurons in the SBH treated groups (D, E, and F) and the normal control group (A) show intact structures with clear nuclei (long arrows). Particularly in the AD + SBH 750 mg/kg group (E), neurons display nearly normal morphology with reduced cytoplasmic vacuolation. Extensive neuronal damage and degeneration of neurons and surrounding cells are evident in the AD group (B).
Table 2. Comparative analysis of three different doses of stingless bee honey.
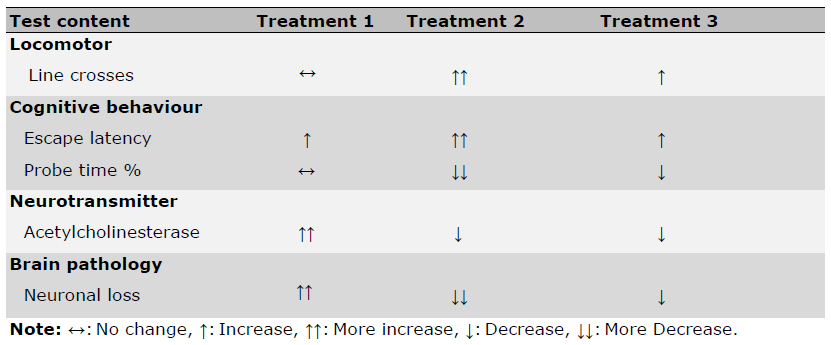
The table above summarizes the effects of three different doses of stingless bee honey on various parameters in the treatment groups.
Enzyme-linked immunosorbent assay (ELISA)
Dopamine
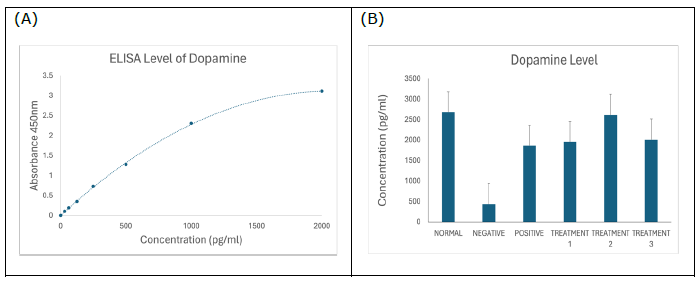
Figure 4. (A) Standard curve of dopamine. Results are expressed as the mean ± SD of standard curves. (B) Dopamine ELISA assay showing significantly reduced dopamine concentration in negative SD rats (P < 0.0001), which was ameliorated by treatment with SBH.
Corticosterone
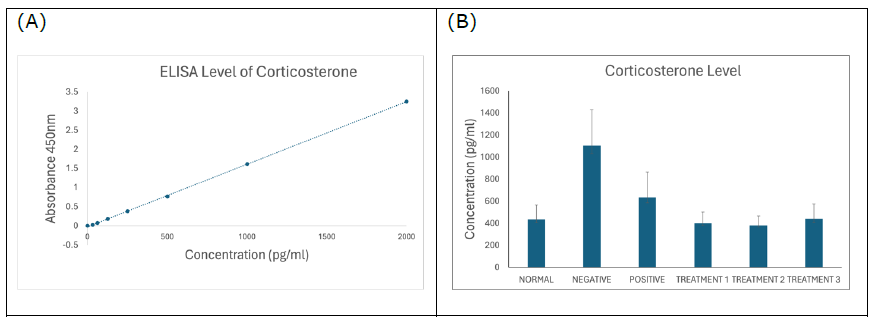
Figure 5. (A) Standard curve of corticosterone. Results are expressed as the mean ± SD of standard curves. (B) Corticosterone ELISA assay showing significantly increased corticosterone concentration in negative SD rats (P < 0.0001), which was decreased concentration of corticosterone by the treatments of SBH.
Acetylcholinesterase
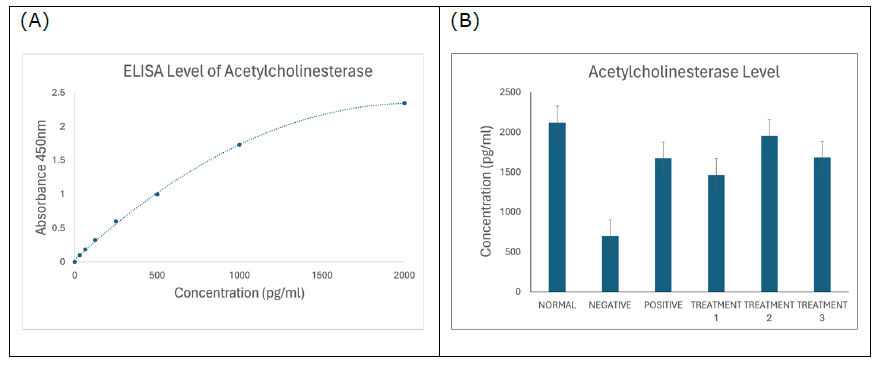
Figure 6. (A) Standard curve of acetylcholinesterase. Results are expressed as the mean ± SD of standard curves. (B) Acetylcholinesterase ELISA assay showing significantly reduced acetylcholinesterase concentration in negative SD rats (P < 0.0001), which was ameliorated by the treatments of SBH.
Serotonin
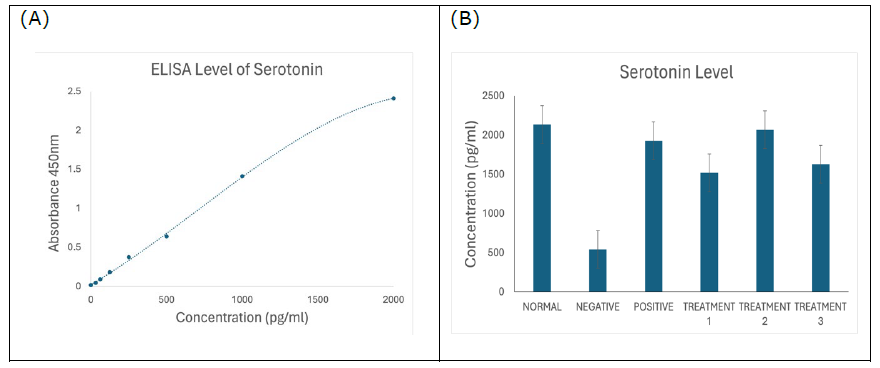
Figure 7. (A) Standard curve of serotonin. Results are expressed as the mean ± SD of standard curves. (B) Serotonin ELISA assay showing significantly reduced serotonin concentration in negative SD rats (P < 0.0001), which was ameliorated and increased by the treatments of SBH.
Alzheimer’s disease (AD) is a progressive neurodegenerative condition characterized by subtle yet persistent declines in cognitive function, mood, and daily functioning. Although the precise aetiology of AD remains elusive, key contributing factors such as cholinergic dysfunction, oxidative stress, and reduced neuronal metabolism have been frequently cited in the literature (Chen et al., 2022). Among these, oxidative stress-induced neurotoxicity is considered an early and central event in the pathogenesis of AD, with significant involvement in neuronal cell death triggered by Aβ deposition (Esmaeili et al., 2022). Given its potent antioxidant properties, stingless bee honey (SBH) is hypothesized to counteract these pathogenic mechanisms. The present study aimed to assess the therapeutic potential of SBH in a rat model of AD induced by aluminum chloride (AlCl₃) and D-galactose.
In this study, rats subjected to combined AlCl₃ and D-gal exposure were subsequently treated with SBH. Behavioural tests were used to evaluate the efficacy of SBH in mitigating AD-like symptoms. Donepezil (DPZ), an FDA-approved drug for mild to moderate AD, served as the positive control. DPZ acts by selectively inhibiting acetylcholinesterase activity via reversible competitive and non-competitive mechanisms (Haider et al., 2020). The AD model used in this study replicated common clinical features such as cognitive impairment, spatial disorientation, and memory loss. Similar symptoms have been linked to hippocampal neurodegeneration, particularly within the medial and temporal brain regions (Olajide et al., 2021).
To determine whether SBH impairs general motor function, the present study employed the Open Field Test (OFT). The results (Figure 1) showed no statistically significant differences in locomotor activity across all treatment groups. These findings suggest that SBH does not adversely affect motor functions at the tested doses. This is critical because treatments that impair motor performance may confound behavioural assessments of cognition (Barker et al., 2021). Notably, the negative control group (AlCl₃ + D-gal) demonstrated reduced line crossings, reflecting impaired exploration and neurotoxicity, which is consistent with previous findings on the motor-inhibiting effects of neurotoxic agents (Bustamante-Barrientos et al., 2023; Luo et al., 2024).
In contrast, the positive control group treated with DPZ showed behavioural recovery, aligning with prior studies that link cholinesterase inhibition to improved behavioural and cognitive outcomes in neurodegenerative models (Pentkowski et al., 2021; Walczak-Nowicka and Herbet, 2021; Nakamura et al., 2023). Similarly, SBH-treated groups did not show motor impairments, further supporting the safety of SBH in terms of locomotor function. This finding is important when assessing therapeutic agents for CNS disorders, as emphasized in earlier research (Karvandi
et al., 2023; Gadge et al., 2024). The high flavonoid content in SBH, noted for its antioxidant and anti-inflammatory actions, likely contributes to this neuroprotective effect without introducing locomotor deficits.
In the Morris Water Maze (MWM) test, rats treated with SBH demonstrated improved spatial memory, evidenced by greater time spent in the target quadrant and increased crossings, relative to the AD model group. In contrast, the negative control group exhibited longer escape latencies and thigmotaxis behaviour, staying near the wall and showing erratic swimming, characteristic of cognitive deficits reported in similar AD models (Xiao et al., 2011; Luo et al., 2024). These behavioural patterns corroborate findings by Wang et al. (2014) and Tian et al. (2019), who observed similar impairments in learning and memory in AD-induced rodents. Accordingly, our data support that SBH treatment ameliorates cognitive dysfunction induced by AlCl₃ and D-gal.
ACh is essential for memory and learning, and its hydrolysing enzyme, acetylcholinesterase (AChE), is a marker for cholinergic neuron activity. In this study, SBH significantly affected AChE levels in treated rats. Previous work has shown that AlCl₃ and D-gal increase AChE activity, thereby disrupting cholinergic transmission and promoting neurodegeneration (Chiroma et al., 2019; Haider et al., 2020; de la Monte and Tong, 2024). Our findings are consistent with these reports, as the negative control group showed elevated AChE levels, indicative of cholinergic dysfunction. However, SBH treatment significantly reduced AChE levels, suggesting its restorative effect on cholinergic signalling, a result aligned with findings from Walczak-Nowicka and Herbet (2021) and Shaikh et al. (2024).
Histological analysis in the present study provided further confirmation of neurodegeneration in the AD model. The hippocampi of negative control rats exhibited darkly stained neurons, vacuolization, and perinuclear spaces, consistent with oxidative stress and neurodegeneration (Miyata et al., 2011; Olajide et al., 2021). These findings align with those of Çoban et al. (2015), who observed similar neuronal degeneration in AD-induced rats. Importantly, SBH-treated rats displayed notably fewer histological abnormalities, indicating that SBH ameliorates neurodegenerative damage. In contrast, DPZ-treated rats still showed signs of degeneration, supporting recent studies that suggest DPZ does not halt structural neuronal damage despite symptomatic relief (Haider et al., 2020).
Following 28 days of SBH treatment, histological comparisons among groups revealed that the Treatment 1 group (750 mg/kg) showed the least impairment, both behaviourally and histologically. Conversely, Treatment 2 and 3 groups (500 mg/kg and 1,000 mg/kg, respectively) exhibited more pronounced AD-like symptoms. Table 2 confirms that the therapeutic impact of SBH is dose-dependent, with 750 mg/kg appearing to be the most effective.
The ELISA test results further supported SBH’s therapeutic potential. Plasma dopamine levels were significantly lower in the negative control group compared to both normal and SBH-treated groups (Figure 4B). This aligns with previous literature showing dopamine dysregulation in AD models (de la Torre, 2021). SBH treatment significantly elevated dopamine levels, potentially contributing to the observed cognitive and behavioural improvements (Shaikh et al., 2023, 2024). These findings support the role of SBH as a neuroprotective agent capable of restoring dopaminergic function.
Similarly, corticosterone levels were significantly elevated in the negative control group but were reduced in all SBH-treated groups (Figure 5B). Elevated corticosterone is associated with stress-related neurodegeneration and cognitive deficits in AD (Jeong et al., 2006; Hendrickson et al., 2018). The reduction in corticosterone following SBH treatment suggests an anxiolytic and neuroprotective effect, in line with previous studies emphasizing the need to manage stress hormone levels in AD management (Ekici et al., 2022; Koutentaki et al., 2023).
The ELISA test for AChE activity (Figure 6B) demonstrated that SBH-treated groups had higher AChE activity than the AD group, suggesting restoration of cholinergic function. This observation contrasts with the significantly reduced AChE levels in the negative control group, affirming the cholinergic deficits in AD (Hampel et al., 2018). Increased AChE levels in SBH groups support the hypothesis that SBH can help restore cholinergic balance, which is vital for cognition (Walczak-Nowicka and Herbet, 2021; Shaikh et al., 2024).
Finally, serotonin levels measured via ELISA (Figure 7B) revealed significantly lower levels in the negative control group. This decline reflects serotonergic dysfunction, a hallmark of mood and cognitive disturbances in AD (Pan et al., 2013; Shah et al., 2023). SBH treatment significantly elevated serotonin levels, indicating its potential to improve both cognitive and neuropsychiatric symptoms. These findings are in agreement with prior studies suggesting serotonergic restoration is key in AD management (Trillo et al., 2013; Kisby et al., 2019; Shah et al., 2023; Shaikh et al., 2023; Zulkifli et al., 2023).
CONCLUSION
This study demonstrates the promising potential of stingless bee honey (SBH) as a neurotherapeutic agent in the early stages of Alzheimer's Disease (AD). SBH effectively alleviates neurobehavioral symptoms and enhances cognitive, executive, and psychological functions in rats. The findings indicate that SBH significantly reduces cognitive impairments and helps restore neurotransmitter levels in an AD model, suggesting its potential role in mitigating neurodegeneration. Given its natural origin and therapeutic effects, SBH could emerge as a valuable compound for addressing behavioural and cognitive disturbances associated with AD. These results pave the way for further research to explore its underlying mechanisms and possible clinical applications in treating neurodegenerative disorders.
ACKNOWLEDGEMENTS
The authors would like to thank the Faculty of School Graduate Studies, Management & Science University (MSU) for providing the instruments and facilities necessary for conducting this research. Finally, we appreciate all the authors for their contributions and approval of the final manuscript.
AUTHOR CONTRIBUTIONS
Shah Rezlan Shajahan conducted the entirety of the experimental work, performed all the data analysis, and wrote the initial manuscript. Muhammad Danial Che Ramli, as the supervisor provided thorough oversight, reviewed all aspects of the work and manuscript. Hussin Muhammad and Azlina Zulkapli supplied the experimental rats, while Mohd Zulkifli Mustafa provided the stingless bee honey used in the treatment. Yatinesh Kumari and Imrana Jazuli collaborated on the behavioural analysis, specifically with the use of software to track and measure the results. Norshafarina Shari, Amanina Nurjannah Atan Hamdan and Nazatul Syawany Wahid offered continuous feedback and guidance throughout the research process. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abidin, İ., Keser, H., Şahin, E., Öztürk, H., Başoğlu, H., Alver, A., and Aydin-Abidin, S. 2024. Effects of housing conditions on stress, depressive-like behavior and sensory-motor performances of C57BL/6 mice. Laboratory Animal Research. 40(3): 6.
Alhajj, M., Zubair, M., and Farhana, A. 2023. Enzyme linked immunosorbent assay. In StatPearls. StatPearls Publishing. 72(1): 4-15.
Alzheimer’s International. 2023. Dementia statistics. Alzheimer’s Disease International. 23(1): 1-95.
Barker, L.A., James, E., and Wilson, M.A. 2021. Behavioral assessments of locomotor and cognitive functions in animal models of neurodegeneration. Frontiers in Behavioral Neuroscience. 15(1): 652659.
Bendjedid, S., Djelloul, R., Tadjine, A., Bensouici, C., and Boukhari, A. 2020. In vitro assessment of total bioactive contents, antioxidant, anti-Alzheimer and antidiabetic activities of leaves extracts and fractions of Aloe vera. Natural and Life Sciences Communications. 19(3): 469-485.
Bustamante-Barrientos, F.A., Luque-Campos, N., Araya, M.J., Lara-Barba, E., de Solminihac, J., Pradenas, C., Molina, L., Herrera-Luna, Y., Utreras-Mendoza, Y., Elizondo-Vega, R., et al. 2023. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. Journal of Translational Medicine. 21 (12): 613.
Chen, Z.R., Huang, J.B., Yang, S.L., and Hong, F.F. 2022. Role of cholinergic signaling in alzheimer’s disease. Molecules. 27(1): 1816.
Chiroma, S.M., Baharuldin, M.T.H., Mat Taib, C.N., Amom, Z., Jagadeesan, S., Ilham Adenan, M., Mahdi, O., and Moklas, M.A.M. 2019. Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their hippocampus. International Journal of Molecular Sciences. 20(1): 1871.
Çoban, J., Doğan-Ekici, I., Aydın, A.F., Betül-Kalaz, E., Doğru-Abbasoğlu, S., and Uysal, M. 2015. Blueberry treatment decreased D-galactose-induced oxidative stress and brain damage in rats. Metabolic Brain Disease. 30(1): 793–802.
Cummings, J. 2021. New approaches to symptomatic treatments for Alzheimer’s disease. Molecular Neurodegeneration. 16 (1): 2.
Damodaran, T., Cheah, P.S., Murugaiyah, V., and Hassan, Z. 2020. The nootropic and anticholinesterase activities of Clitoria ternatea Linn. root extract: Potential treatment for cognitive decline. Neurochemistry International. 139(27): 104785.
de la Monte, S.M., and Tong, M. 2024. Dysregulated mTOR networks in experimental sporadic Alzheimer’s disease. Frontiers in Cellular Neuroscience. 18: 1432359.
de la Torre, J.C. 2021. Deciphering Alzheimer’s disease pathogenic pathway: Role of chronic brain hypoperfusion on p-Tau and mTOR. Journal of Alzheimer’s Disease. 79(12): 1381–1396.
D’Haese, P.F., Ranjan, M., Song, A., Haut, M.W., Carpenter, J., Dieb, G., Najib, U., Wang, P., Mehta, R.I., Chazen, J.L., et al. 2020. β-amyloid plaque reduction in the hippocampus after focused ultrasound-induced blood–brain barrier opening in Alzheimer’s disease. Frontiers in Human Neuroscience. 14(1): 23.
Ekici, Ö., Aslan E., Aladağ T., Güzel H., Korkmaz Ö. A., Bostancı A., Sadi G., and Pektaş M.B. 2022. Masseter muscle and gingival tissue inflammatory response following treatment with high-fructose corn syrup in rats: Anti-inflammatory and antioxidant effects of kefir. Journal of Food Biochemistry. 46(3): e13732.
Esmaeili, Y., Yarjanli, Z., Pakniya, F., Bidram, E., Łos, M.J., Eshraghi, M., Klionsky, D.J., Ghavami S., and Zarrabi A. 2022. Targeting autophagy, oxidative stress, and ER stress for neurodegenerative disease treatment. Journal of Controlled Release. 345(13): 147–175.
Frisbee, J.C., Brooks, S.D., Stanley, S.C., and d’Audiffret, A.C. 2015. An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes, and negative health outcomes in rodents. Journal of Visualized Experiments. 106 (1): 165-177.
Gadge, A.S., Shirsat, D.V., Soumia, P.S., Pote, C.L., Pushpalatha, M., Pandit, T.R., Dutta, R., Kumar, S., Ramesh, S. V., Mahajan, V., et al. 2024. Physiochemical, biological, and therapeutic uses of stingless bee honey. Frontiers in Sustainable Food Systems. 7(1): 123-132
Gurina, T.S., and Simms, L. 2023. Histology, Staining. In StatPearls. StatPearls Publishing.
Haider, S., Liaquat, L., Ahmad, S., Batool, Z., Siddiqui, R., A., Tabassum, S., Shahzad. S., Rafiq, S., and Naz, N. 2020. Naringenin protects AlCl3/D-galactose induced neurotoxicity in a rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS One. 15: e0227631.
Hampel, H., Mesulam, M., Cuello, A.C., Farlow, M.R., Giacobini, E., Grossberg, G.T., Khachaturian, A.S., Vergallo, A., Cavedo, E., Snyder, P.J., et al. 2018. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 141(1): 1917–1933.
Hendrickson, R.C., Raskind, M.A., Millard, S.P., Sikkema, C., Terry, G.E., Pagulayan, K.F., Li, G., and Peskind, E.R. 2018. Evidence for altered brain reactivity to norepinephrine in veterans with a history of traumatic stress. Neurobiology of Stress. 8(2): 103–111.
Jeong, Y.H., Park, C.H., Yoo, J, Shin, K.Y., Ahn, S., Kim, H., Lee, S.H., Emson, P.C., and Suh, Y. 2006. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APP V717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB Journal. 20(12): 729–731.
Karvandi, M.S., Sheikhzadeh, H.F., Aref, A.R., and Mahdavi, M. 2023. The neuroprotective effects of targeting key factors of neuronal cell death in neurodegenerative diseases: The role of ER stress, oxidative stress, and neuroinflammation. Frontiers in Cellular Neuroscience. 17(12): 123-134.
Kisby, B., Jarrell, J.T., Agar, M.E., Cohen, D.S., Rosin, E.R., Cahill, C.M., Rogers, J.T., and Huang, X. 2019. Alzheimer’s disease and its potential alternative therapeutics. Journal of Alzheimer’s Disease and Parkinsonism. 9(12): 187-198.
Koutentaki, E., Basta, M., Antypa, D., Zaganas, I., Panagiotakis, S., Simos, P., and Vgontzas, A.N. 2023. IL-6 enhances the negative impact of cortisol on cognition among community-dwelling older people without dementia. Healthcare. 11(12): 951.
Lane, C.A., Hardy, J., and Schott, J.M. 2018. Alzheimer’s disease. European Journal of Neurology. 25(12): 59–70.
Luo, L., Yan, T., Yang, L., and Zhao, M. 2024. Aluminum chloride and D-galactose induced a zebrafish model of Alzheimer’s disease with cognitive deficits and aging. Computational and Structural Biotechnology Journal. 23(11): 2230–2239.
Majtan, J., Klaudiny, J., Bohova, J., Kohutova, L., Dzurova, M., Sediva, M., Bartosova, M., and Majtan, V. 2012. Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: Possible therapeutic implications. Fitoterapia. 83(1): 671–677.
Miyata, T., Takizawa, S., and van Ypersele de S.C. 2011. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: Novel therapeutic targets. American Journal of Physiology-Cell Physiology. 300(16): C226–C231.
Nakamura, Y., Kim, R., Nishiyama, K., Kikuchi, T., Ishikawa, I., and Aoki, H. 2023. Efficacy and safety of a transdermal donepezil patch in patients with mild-to-moderate Alzheimer's disease: A 24-week, randomized, multicenter, double-blind, parallel group, non-inferiority study. Geriatrics & Gerontology International. 23(12): 275–281.
Olajide, O.J., Gbadamosi, I.T., Yawson, E.O., Arogundade, T., Lewu, F.S., Ogunrinola, K.Y., Adigun, O.O., Bamisi, O., Lambe, E., Arietarhire, L.O., et al. 2021. Hippocampal degeneration and behavioral impairment during Alzheimer-like pathogenesis involves glutamate excitotoxicity. Journal of Molecular Neuroscience. 71(23): 1205–1220.
Pan, W.D., Yoshida, S., Liu, Q., Wu, C.L., Wang, J., Zhu, J., and Cai, D.F. 2013. Quantitative evaluation of severity of behavioral and psychological symptoms of dementia in patients with vascular dementia. Translational Neurodegeneration. 2(12): 9.
Panachamnong, N., Methapatara, P., Sungkarat, S., Taneyhill, K., and Intasai, N. 2014. Clusterin as a blood biomarker for diagnosis of mild cognitive impairment and Alzheimer’s disease. Natural and Life Sciences Communications. 13(19): 331-344.
Pentkowski, N.S., Rogge-Obando, K.K., Donaldson, T.N., Bouquin, S.J., and Clark, B.J. 2021. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neuroscience and Biobehavioral Reviews. 127(12): 647–658.
Sánchez, C.Q., Schmitt, F.W., Curdt, N., Westhoff, A.C., Bänfer, I.W.H., Bayer, T.A., and Bouter, Y. 2023. Search strategy analysis of 5xFAD Alzheimer mice in the morris water maze reveals sex- and age-specific spatial navigation deficits. Biomedicines. 11(11): 599.
Scearce-Levie, K. 2011. Monitoring spatial learning and memory in Alzheimer's disease mouse models using the Morris Water Maze. Methods in Molecular Biology. 670(65): 191–205.
Shah, H., Dehghani, F., Ramezan, M., Gannaban, R.B., Haque, Z.F., Rahimi, F., Abbasi, S., and Shin, A.C. 2023. Revisiting the role of vitamins and minerals in Alzheimer's disease. Antioxidants. 12(12): 415.
Shaikh, A., Ahmad, F., Teoh, S.L., Kumar, J., and Yahaya, M.F. 2023. Honey and Alzheimer’s disease: Current understanding and future prospects. Antioxidants. 12(11): 427.
Shaikh, A., Ahmad, F., Teoh, S.L., Kumar, J., and Yahaya, M.F. 2024. Unveiling the therapeutic potential of Kelulut (stingless bee) honey in Alzheimer’s disease: Findings from a rat model study. Antioxidants. 13(1): 926.
Slaoui, M., and Fiette, L. 2011. Histopathology procedures: From tissue sampling to histopathological evaluation. Methods in Molecular Biology. 691(12): 69–82.
Tian, H., Ding, N., Guo, M., Wang, S., Wang, Z., Liu, H., Yang, J., Li, Y., Ren, J., Jiang, J., et al. 2019. Analysis of learning and memory ability in an Alzheimer’s disease mouse model using the Morris Water Maze. Journal of Visualized Experiments. 152(32): 154-162.
Topuz, R.D., Gunduz, O., Tastekin, E., and Karadag, C.H. 2020. Effects of hippocampal histone acetylation and HDAC inhibition on spatial learning and memory in the Morris water maze in rats. Fundamental & Clinical Pharmacology. 34(12): 222–228.
Trillo, L., Das, D., Hsieh, W., Medina, B., Moghadam, S., Lin, B., Dang, V., Sanchez, M.M., De Miguel, Z., Ashford, J.W., and Salehi, A. 2013. Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neuroscience & Biobehavioral Reviews. 37(1): 1363–1379.
Walczak-Nowicka, L.J., and Herbet, M. 2021. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. International Journal of Molecular Sciences. 22: 9290.
Wang, C., He, L., Yan, M., Zheng, G.Y., and Liu, X.Y. 2014. Effects of polyprenols from pine needles of Pinus massoniana on ameliorating cognitive impairment in a D-galactose-induced mouse model. Age. 36(12): 9676.
Xiao, F., Li, X.-G., Zhang, X.-Y., Hou, J.-D., Lin, L.-F., Gao, Q., and Luo, H.-M. 2011. Combined administration of D-galactose and aluminium induces Alzheimer like lesions in brain. Neuroscience Bulletin. 27(12): 143–155.
Zulkifli, N.A., Hassan, Z., Mustafa, M.Z., Azman, W.N.W., Hadie, S.N.H., Ghani, N., and Mat Zin, A.A. 2023. The potential neuroprotective effects of stingless bee honey. Frontiers in Aging Neuroscience. 14(31): 1048028.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Shah Rezlan Shajahan1, Hussin Muhammad2, Mohd Zulkifli Mustafa3, Yatinesh Kumari4, Imrana Jazuli4, Azlina Zulkapli5, Norshafarina SK1, Amanina Nurjannah Atan Hamdan1, Nazatul Syawany Wahid1, and Muhammad Danial Che Ramli6, *
1 School of Graduate Studies, Postgraduate Centre, Management & Science University, Shah Alam 40100, Selangor, Malaysia.
2 Toxicology & Pharmacology Unit, Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Shah Alam 40170, Selangor, Malaysia.
3 Department of Neuroscience, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150 Kota Bharu, Kelantan, Malaysia.
4 Neurological Disorder & Aging Research Group (NDA), Neuroscience Research Strength (NRS), Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, 47500 Selangor, Malaysia.
5 Laboratory Animal Resource Unit, Special Resource Centre, Institute for Medical Research, National Institute of Health, Jalan Pahang, 50588 Kuala Lumpur, Malaysia.
6 Faculty of Health & Life Sciences, Management & Science University, Shah Alam 40100, Selangor, Malaysia.
Corresponding author: Muhammad Danial Che Ramli, E-mail: muhddanial_cheramli@msu.edu.my
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: March 11, 2025;
Revised: May 21, 2025;
Accepted: June 4, 2025;
Online First: June 13, 2025

