
Quantification of Phenylbutanoids and Terpinen-4-ol in Zingiber montanum Oils: Comparison of Hydro-distillation and Deep-frying Extraction Methods and the Effect of Temperature
Wudtichai Wisuitiprot, Wuttichai Jaidee, Pitsaphun Werayingyong, Somsak Kreechai, Nipon Keawtai, and Vanuchawan Wisuitiprot*Published Date : May 23, 2025
DOI : https://doi.org/10.12982/NLSC.2025.047
Journal Issues : Number 3, July-September 2025
Abstract The aim of this study was to determine the quantities of (E)-1-(3,4-dimethoxyphenyl) butadiene (DMPBD), (E)-4-(3’,4’-dimethoxyphenyl) but-3-en-1-ol (compound D), and terpinen-4-ol in Zingiber montanum oils and to investigate the effect of temperature on the extraction efficiency of deep-frying method. Typically, Z. montanum oil is prepared by deep-frying with plant-fixed oil or by hydro-distillation. However, the quantities of compound D, DMPBD, and terpinen-4-ol in both types of oil have not been fully clarified. The results revealed that DMPBD and terpinen-4-ol were effectively extracted by hydro-distillation, while compound D, DMPBD, and terpinen-4-ol were found in the oil prepared by deep-frying with plant-fixed oil. Temperature was shown to have a significant impact on the quantities of active compounds in fried oil. The lowest amounts of compound D (0.75 ± 0.031 mg/mL), was quantified in the oil prepared by deep-frying at 200°C, while the highest amounts of compound D (0.90 ± 0.016 mg/mL) was observed in the oil prepared by deep-frying at 100°C. The highest amount of terpinene-4-ol (1.08 ± 0.004 mg/mL) and DMPBD (0.95 ± 0.045 mg/mL) were presented in hydro-distillation oil. Both Z. montanum oils show potential as ingredients for topical application, particularly in the treatment of muscle or skeletal inflammation. However, optimization of the preparation process needs to be considered.
Keywords: Zingiber montanum, Compound D, DMPBD, Terpinen-4-ol, Elevated temperature
Funding: This study was supported by the Thai Traditional Medical Knowledge Fund, Ministry of Public Health, Thailand.
Citation: Wisuitiprot, W., Jaidee, W., Werayingyong, P., Kreechai, S., Keawtai, N., and Wisuitiprot, V. 2025. Quantification of phenylbutanoids and terpinen-4-ol in Zingiber montanum oils: Comparison of hydro-distillation and deep-frying extraction methods and the effect of temperature. Natural and Life Sciences Communications. 24(3): e2025047.
INTRODUCTION
Zingiber montanum (J. Koenig) Link ex A. Dietr is a medicinal plant in the Zingiberaceae family. It is found in Thailand and other South and Southeast Asian countries: India, Malaysia, Bangladesh, and Indonesia. Z. montanum has been used as a single plant or as an ingredient in traditional herbal recipes for treating several symptoms such as abdominal pain, muscle and skeletal pain, flatulence, and inflammation (Ministry of Public Health Thailand 2025). Studies have revealed that Z. montanum rhizomes contain many pharmacological active compounds, and the prominent compounds are curcuminoids, terpenoids, and phenylbutanoids, which have anti-inflammatory activity (Han et al., 2021; Verma et al., 2018). Terpinen-4-ol, a monoterpene, is a promising anti-inflammatory agent because it can decrease inflammation by suppressing the production of inflammatory cytokines in monocytes and macrophages, such as TNF-α, IL-1β, and PGE2 (Hart et al., 2000; Ninomiya et al., 2013). Moreover, terpinen-4-ol is a member of the oxygenated monoterpenes that inhibits inflammation induced by free radical chain reactions (Insuan et al., 2021). Phenylbutanoids such as (E)-1-(3,4-dimethoxyphenyl) butadiene (DMPBD), (E)-4(3’,4’-dimethoxyphenyl) but-3-en-1-ol (compound D) are of particular interest because they have shown potent anti-inflammatory activity by inhibiting cyclooxygenase activity similarly to non-steroidal anti-inflammatory drugs (NSAIDs drug) (Panthong et al., 1997; Jeenapongsa et al., 2003; Han et al., 2005). A report has also revealed that phenylbutanoids limit initial inflammation by reducing the expression of IL-1β in both pro- and active forms (Chaiwongsa et al. 2012). A study also indicated that the inflammatory cytokine cascading, stimulated by nuclear factor-kappa B, signal transducer and activator of transcription 3 (Stat3), and mitogen-activated protein kinase (MAPK) pathways, was diminished by Z. montanum oil (Truong et al., 2021). Based on the bioactivity of active compounds associated with anti-inflammatory properties, Z. montanum extract or oil shows potential for development as pharmaceutical and cosmeceutical products. For pharmaceutical applications, Z. montanum oil has been shown to be an effective anti-inflammatory product for treating or preventing muscle inflammation and pain (Cheechareoan et al., 2016; Manimmanakorn et al., 2016; Wisuitiprot et al., 2019). A cosmeceutical product for preventing photo-induced skin aging was also inspired by a study that revealed Z. montanum oil, prepared by solvent-free microwave extraction, suppressed metalloproteinase (MMP) expression and decreased elastase enzyme activity in UVB-irradiated human dermal fibroblast cells (Navabhatra et al., 2022). This anti-photoaging activity was further supported by an in vivo study, which reported significant inhibition of metalloproteinase-1 expression, free radical formation, and tyrosinase activity (Tyas et al., 2024). Additional bioactivities related to cosmeceutical uses, such as antioxidant activity, collagen synthesis promotion, and anti-tyrosinase activity, were also reported (Li et al., 2019).
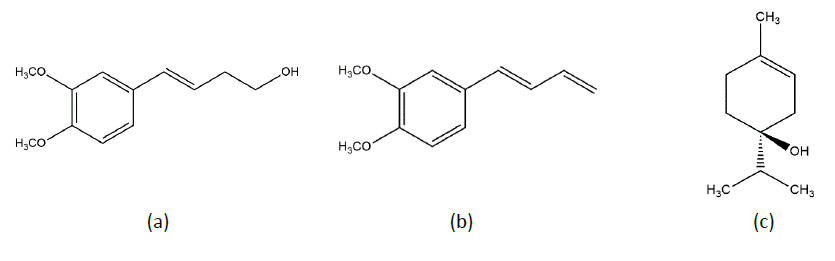
Figure 1. Chemical structure of compound D (a), DMPBD (b), terpinen-4-ol.
Although the raw material of Z. montanum consists of two types: crude extract obtained by solvent extraction and oil produced by either distillation or frying, both containing beneficial bioactive compounds (Sukatta et al., 2009; Han et al., 2021; Nishidono et al., 2024), the oil preparation is notable for its green process, which is free of organic solvents (Martins et al. 2023). Z. montanum oils, commonly incorporated into health products, are typically obtained by water distillation or deep-frying with plant-fixed oils. The Z. montanum oils that have been traditionally used for a long time are recommended for treating muscle and skeletal disorders in The Thailand National List of Essential Medicines. Some studies have attempted to develop extraction methods to improve the effectiveness of producing high amounts of terpenoids and phenylbutanoids. They found that elevated temperatures, by using a microwave, pressurized techniques, or a soxhlet apparatus, improve the extraction efficiency of compound D and DMPBD more than conventional solvent maceration (Kaewchoothong, 2009; Chienthavorn et al., 2011; Koparde and Magdum, 2014). Hydro-distillation is the traditional technique for producing essential oil or distilled oil containing a large number of terpenoids, particularly terpinen-4-ol (Chienthavorn et al., 2011; Sukatta et al., 2009). Additionally, Z. montanum oil has been prepared by deep-frying the rhizome with plant-fixed oil. In Asia, this method has been widely used for preparing the herb oil incorporated in traditional recipes. Z. montanum oil prepared by deep-frying with plant-fixed oil was shown to contain substantial amounts of terpenoids (Singsai et al., 2022). However, the amount of phenylbutanoids was reported to be scarce in both Z. montanum oils. The purpose of this study was to investigate the effect of temperature on the extraction efficiency of deep-frying method. The quantity of each compound D, DMPBD and terpinen-4-ol in Z. montanum oils produced by distilling with water or deep-frying with refined coconut oil were analyzed and compared.
MATERIALS AND METHODS
Materials
Methanol (HPLC grade), acetic acid (AR grade) and hexane (AR grade) were purchased from Labscan Asia Co. Ltd. (Bangkok, Thailand). Refined coconut oil was purchased from Patum Vegetable Oil Co., Ltd. (Prathumthani, Thailand). Standard terpinen-4-ol and carbon tetrachloride were obtained from Sigma-Alrich (St. Louis, USA). Rhizomes of Z. montanum were purchased from Tubyaychiang Community Enterprise organic farm (Phisanulok, Thailand).
Z. montanum extract and oils preparation
The plant collection method and experimental use followed all the relevant guidelines. The specimens were identified by Chonchid Kumpun, the botanist of the Botanical Garden of Sirindhorn College of Public Health Phitsanulok. The voucher specimen, collection number 04293, was deposited at the Thai traditional medicine herbarium, also located at the Sirindhorn College of Public Health Phitsanulok. The rhizomes were sliced into tiny pieces and then dried at 50°C for 24 hours. The dried rhizomes were extracted using a Soxhlet apparatus with hexane for 4 hours. The extract solution was filtered through Whatman No.1 filter paper before undergoing rotary evaporation at 40°C. The extract was stored in an amber bottle at -20°C and protected from light until used.
The essential oil was prepared by the hydro-distillation method in which 100 grams of fresh Z. montanum rhizome was chopped into tiny pieces and placed in a round-bottomed flask containing 500 mL of water. The essential oil was extracted using a distillation apparatus. The distilled oil was collected in an amber vial and stored at -20°C. The fried oil was prepared by frying the sliced fresh rhizomes with refined coconut oil at a ratio of 1:1 (w/w; rhizomes to refined coconut oil). In this experiment, 1,000 g of freshly sliced rhizome was fried with 1,000 g of refined coconut oil. The frying temperature was controlled at 100°C, 160°C, and 200°C using a hot plate (IKA C-MAG HS7, IKA® Werke GmbH & Co., Staufen, Germany). The amount of compound D, DMPBD and terpinen-4-ol was determined from oils collected at 15, 30, 45, 60, and 90 minutes of preparation to observe the extraction profiles. Extraction time of fried oils was 120 minutes approximately.
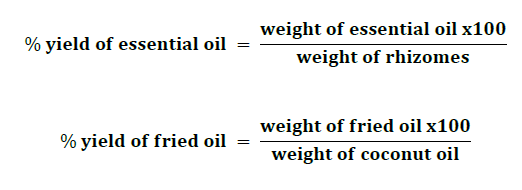
The percent yield of essential oil and fried oil was calculated regarding these equations below;
Isolation of compound D and DMPBD by column chromatography
Compound D and DMPBD were separated from the crude Z. montanum extract (PI) (9.39 g) using flash chromatography (Select silica cartridge, 120 g, 5% EtOAc-n-hexane), yielding 8 subfractions (PI-1 to PI-8). They further purified fraction PI-1 (1.51 g) through flash chromatography (Select silica cartridge, 25 g, 3-5% EtOAc-n-hexane) to isolate DMPBD (46.9 mg). Similarly, they subjected fraction PI-7 (971.0 mg) to flash chromatography (Select silica cartridge, 120 g, 10-50% EtOAc-n-hexane) to obtain Compound D (54.9 mg). The team then determined the chemical structures of Compound D and DMPBD using NMR spectra obtained with a Bruker Avance 600 MHz spectrometer.
Determination of compound D, DMPBD and terpinen-4-ol by HPLC
Compound D and DMPBD in Z. montanum oils were analyzed using the RP-HPLC system with an Agilent 1260 Infinity system (Agilent Technologies, USA) and detection at 254 nm. A Luna Phenomenex analytical column (250 mm x 7 mm, 5-µm particle size) (Phenomenex, Deerfield, IL, USA), at a flow rate of 1 mL/min, and an injection volume of 10 µL, was used for this experiment. The mobile phase consisted of a gradient elution of 2% acetic acid in ultrapure water (A) and methanol (B), with the following proportions: 60 to 50% of A for 0–5 minutes, 50 to 30% of A for 5–15 minutes, 30 to 20% of A for 15–25 minutes, 20 to 50% of A for 25–30 minutes, 50 to 60% of A for 30–32 minutes, and 60% of A for 32–40 minutes. For terpinen-4-ol, the analysis condition consisted of a mobile phase containing methanol and water in a ratio of 90:10, with a flow rate of 1.0 mL/min and absorbance measured at 202 nm. The column was incubated and maintained at a temperature of 40°C.
The HPLC system was validated according to AOAC guideline recommendations. Validation studies were performed following the Association of Official Analytical Chemists (AOAC) guidelines, including the parameters of linearity, limits of detection and quantification (LOD and LOQ), precision, and accuracy (Association of Official Analytical Collaboration (AOAC) International 2002). Linearity of the detector response for both compound D and DMPBD standards was obtained from nine concentrations ranging between 1.95 and 500 µg/mL in methanol, while the concentration of terpinen-4-ol was 0.78–200.00 µg/mL. The calibration curves and linearity equations of compound D, DMPBD, and terpinen-4-ol standards were determined by linear least square regression analysis using the data of peak area versus concentration and evaluated by the correlation coefficient (R2). The repeatability (intra-day) of HPLC analysis was determined from three dilutions of 20 mg of Z. montanum oil in 1 mL, injected three times throughout the day in triplicate. The intra-day precision was obtained by performing the same procedure over three consecutive days. The relative standard deviation (%RSD) was used as a criterion for evaluating the variation of intra- and inter-day precision. The accuracy of the analytical method was determined by enriching the Z. montanum oil with aliquots of standard solutions of compound D, DMPBD, and terpinen-4-ol with known concentrations.
RESULTS
Z. montanum extract was successfully performed using hexane reflux extraction. Compound D and DMPBD were isolated through column chromatography and used as standard markers to control the quality of Z. montanum oils using the HPLC method. The essential oil was obtained through the distillation method, yielding 3.75% ± 0.78 (w/w), while the deep-fried oil yielded 86.24% ± 2.56 (w/w).
Isolated compound D and DMPBD from Z. montanum extract
Compound D and DMPBD were isolated from Z. montanum extract. Structure of these two compounds was elucidated by spectra analysis which were similar to previous reports (Suksaeree et al. 2014; Tangyuenyongwatana 2017).
Compound D: a pale-yellow oils. 1H-NMR (CDCl3, 600 MHz): δ = 6.92 (1H, d, J = 2.0 Hz, H-2), 6.89 (1H, dd, J = 8.2, 2.0 Hz, H-6), 6.80 (1H, d, J = 8.2 Hz, H-5), 6.43 (1H, d, J = 16.0 Hz, H-7), 6.07 (1H, dt, J = 16.0, 7.2 Hz, H-8), 3.89 (3H, s, OMe-11), 3.87 (3H, s, OMe-12), 3.75 (2H, t, J = 6.3 Hz, H-10), 2.47 (2H, q, J = 6.8 Hz, H-9).
DMPBD: a pale-yellow oils. 1H-NMR (CDCl3, 600 MHz): δ = 6.96 (1H, d, J = 2.0 Hz, H-2), 6.93 (1H, dd, J = 8.2, 2.0 Hz, H-6), 6.82 (1H, d, J = 8.2 Hz, H-5), 6.67 (1H, dd, J = 15.6, 10.6 Hz, H-7), 6.51 (1H, d, J = 10.6, 6.8 Hz, H-8), 6.45 (1H, d, J = 10.6, 6.8 Hz, H-9), 5.30 (1H, dd, J = 15.4, 1.7 Hz, H-10a), 5.13 (1H, dd, J = 10.6, 0.7 Hz, H-10b), 3.91 (3H, s, OMe-11), 3.89 (3H, s, OMe-12).
Determination of compound D and DMPBD by HPLC method
The HPLC method successfully achieved the chromatographic separation of compound D, DMPBD, and terpinen-4-ol. The chromatograms of compound D, DMPBD, and terpinen-4-ol are presented in Figures 2 and 3. The peak area versus concentration was plotted to construct standard curves for compound D, DMPBD, and terpinen-4-ol, showing a good linear relationship. The parameters of HPLC method validation were presented in Table 1.
Table 1. Analytical parameters of HPLC method validation.
|
Parameters |
Compound D |
DMPBD |
Terpinen-4-ol |
|
Linearity (mg/mL) |
1.95 – 500.00 |
1.95 – 500.00 |
0.78 – 400.00 |
|
LOD (mg/mL) |
4.46 |
9.22 |
4.69 |
|
LOQ (mg/mL) |
13.51 |
27.94 |
13.51 |
|
R2 |
1.0000 |
0.9999 |
0.9996 |
|
% Recovery 1a |
102.78 ± 1.25b |
98.45 ± 3.12 |
96.77 ± 1.85 |
|
2 |
98.78 ± 4.11 |
103.45 ± 2.45 |
101.01 ± 3.11 |
|
3 |
99.56 ± 2.45 |
99.12 ± 0.78 |
104.56 ± 0.93 |
|
Equations of calibration curve |
y=52.482x+274.580 |
y=28.683x-20.512 |
y=18.863x+86.976 |
|
Equations of calibration curve |
y=52.482x+274.580 |
y=28.683x-20.512 |
y=18.863x+86.976 |
Note: a Concentrations of compound D, DMPBD and terpinen-4-ol in samples 1, 2 and 3 were 30 µg/mL, 100 µg/mL and 150 µg/ml respectively. b %Recovery ± SD.
Regard to Table 1, for compound D the coefficient of correlation (R2) = 1.0, for DMPBD, R2 = 0.9999, and for terpinen-4-ol, R2 = 0.9996, indicating a high correlation and demonstrating a good fit to the regression curve. The precision of the method, as indicated by the intra-day and inter-day RSD values, did not exceed 5%, demonstrating the high reproducibility of the method. The concentrations of the recovered analytes indicated the high efficiency of the test method. The quantity of active compounds indicated that the method of preparation obviously affected the number of those compounds extracted from Z. montanum rhizomes. Both phenylbutanoids were effectively extracted by deep-frying; the amounts of compound D in fried oil prepared at 100°C, 160°C, and 200°C were 0.91 ± 0.016 mg/mL, 0.80 ± 0.022 mg/mL, and 0.75 ± 0.031 mg/mL, while DMPBD was 0.23 ± 0.072 mg/mL, 0.13 ± 0.041 mg/mL, and 0.14 ± 0.022 mg/mL, respectively. Terpinen-4-ol was determined in both fried oil and distilled oil; the highest amount of terpinene-4-ol was measured in distilled oil (1.08 ± 0.004 mg/mL); however, only compound D was undetectable in distilled oil. Regarding Figures 2b-2c and 3b-3c, the chromatograms of DMPBD and terpinene-4-ol are presented with peak shifts and overlapping with other peaks. This could be due to the effect of the oil vehicle in the sample that might alter the solubility proportion of those compounds in the mobile phase during the elution process. (Haidar Ahmad, 2017). However, each peak was verified by adding increasing amounts of standard compounds to aliquots of the sample in order to identify the correct peaks (Figure 2d-2e, Figure 3d-3e) (Moldoveanu and David, 2017).
The effect of temperatures on quality of compound D, DMPBD and terpinene-4-ol in Z. montanum oils
Both Z. montanum oils were successfully prepared by frying with coconut oil and distilling with water. The quantities of compound D, DMPBD, and terpinen-4-ol in the oils were measured using the HPLC method (Table 2). Fried Z. montanum oil prepared at various temperatures revealed different amounts of compound D, DMPBD, and terpinen-4-ol. The oil prepared by deep-frying with coconut oil at 200°C showed the lowest quantity of compound D, DMPBD, and terpinen-4-ol, while the oil prepared at 100°C showed the highest quantity of those compounds. The amount of the three compounds in fried Z. montanum oil tended to decrease with an increase in frying temperature. In contrast, no quantity of compound D was determined in distilled Z. montanum oil; however, the highest amount of DMPBD and terpinen-4-ol was observed.
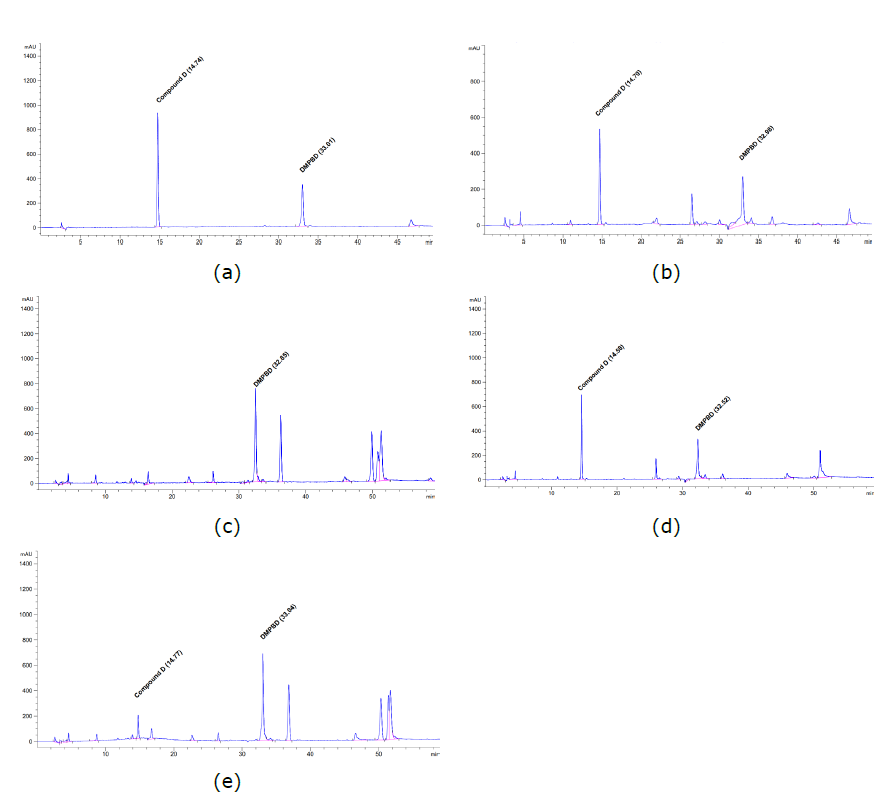
Figure 2. Chromatogram of isolated (E)-4-(3', 4'-dimethoxyphenyl)-but-3-en-1-ol (compound D) and (E)-1-(3,4-dimethoxyphenyl) butadiene (DMPBD) (a); chromatogram of compound D; DMPBD in fried Z. montanum oil (b) and chromatogram of compound D and DMPBD in distilled Z. montanum oil (c); chromatogram of compound D and DMPBD in fried Z. montanum oil spiked with compound D and DMPBD standards 100 µg/mL (d) and chromatogram of compound D and DMPBD in distilled Z. montanum oil spiked with compound D and DMPBD standards 100 µg/mL (e).
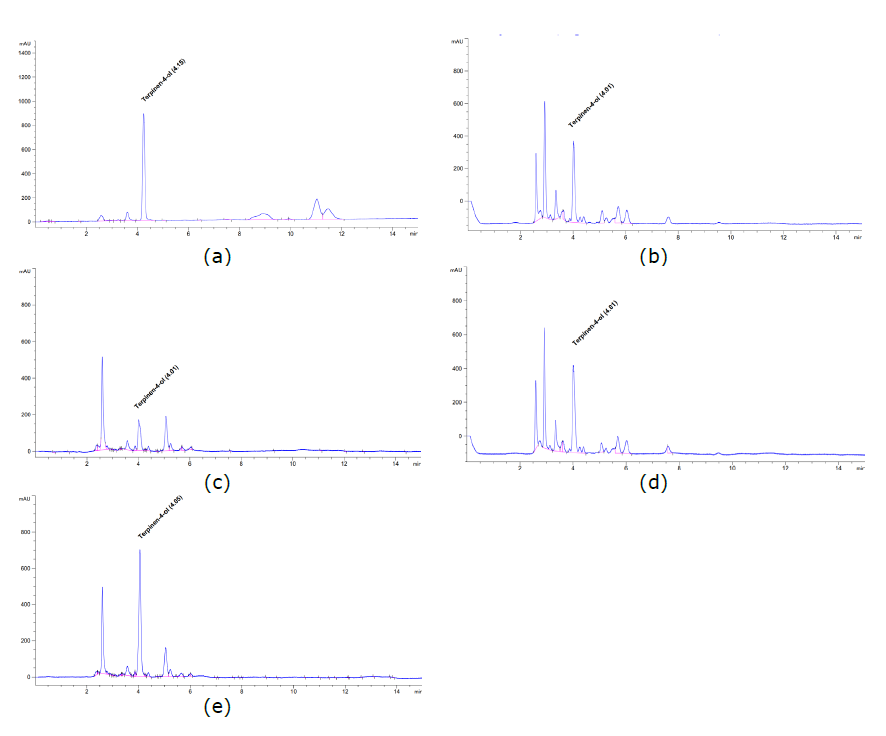
Figure 3. Chromatogram of standard terpinen-4-ol (a); chromatogram of terpinene-4-ol in fried Z. montanum oil (b) and chromatogram of terpinen-4-ol in distilled Z. montanum oil (c); chromatogram of terpinene-4-ol in fried Z. montanum oil spiked with terpinen-4-ol standard 100 µg/mL (d); chromatogram of terpinen-4-ol in distilled Z. montanum oil spiked with terpinen-4-ol standard 100 µg/mL (e).
Table 2. Quantity of compound D, DMPBD and terpinen-4-ol in Z. montanum oils prepared by deep-frying and hydro-distillation (n≥3).
|
Z. montanum oils |
Compound D |
DMPBD |
Terpinen-4-ol |
|||
|
Amount (mg/mL ±SD) |
Percent* |
Amount (mg/mL ±SD) |
Percent* |
Amount (mg/mL ±SD) |
Percent* |
|
|
Z. montanum oil (100°C) |
0.900 ± 0.016 |
0.90 ± 0.08a |
0.230 ± 0.072 |
0.20 ± 0.03b |
0.060 ± 0.003 |
0.06 ± 0.01c |
|
Z. montanum oil (160°C) |
0.800 ± 0.022 |
0.81 ± 0.03a,d |
0.130 ± 0.041 |
0.15 ± 0.06 |
0.070 ± 0.005 |
0.07 ± 0.05e |
|
Z. montanum oil (200°C) |
0.750 ± 0.031 |
0.67 ± 0.18a,d |
0.140 ± 0.022 |
0.17 ± 0.01b |
0.010 ± 0.000 |
0.02 ± 0.08c,e |
|
Z. montanum oil (Distillation) |
- |
- |
0.950 ± 0.045 |
1.06 ± 0.02b |
1.080 ± 0.004 |
1.05 ± 0.02 c,e |
Note: a, b, c, d, e indicating the significantly different P value <0.05; analyzed by ANOVA with Turkey’s HSD; *Percent was calculated based on the weight of fried oil or essential oil.
The extraction profile of compound D, DMPBD and terpinen-4-ol during oil preparation
During oil preparation, an oil sample was collected every 15 minutes to determine the quantity of compound D, DMPBD, and terpinen-4-ol. The extraction profile was presented as the amount of compound D, DMPBD and terpinen-4-ol released from the rhizome over time (Figures 4 and 5). In the fried oil preparation, compound D and DMPBD were released more rapidly from the rhizome within 15 minutes of the initiation process at 100°C and 160°C. Their quantities tended to increase gradually, except in the fried oil prepared at 200°C, where the amount of compound D dramatically decreased after 45 minutes of preparation, and DMPBD dramatically decreased after 30 minutes of preparation (Figure 4 (c)). Figures 4(a), (b), and (c) illustrate that compound D was released more rapidly than DMPBD from the rhizome. Moreover, elevated temperature had a significant effect on the quantity of both compound D and DMPBD (Figure 4(d)).
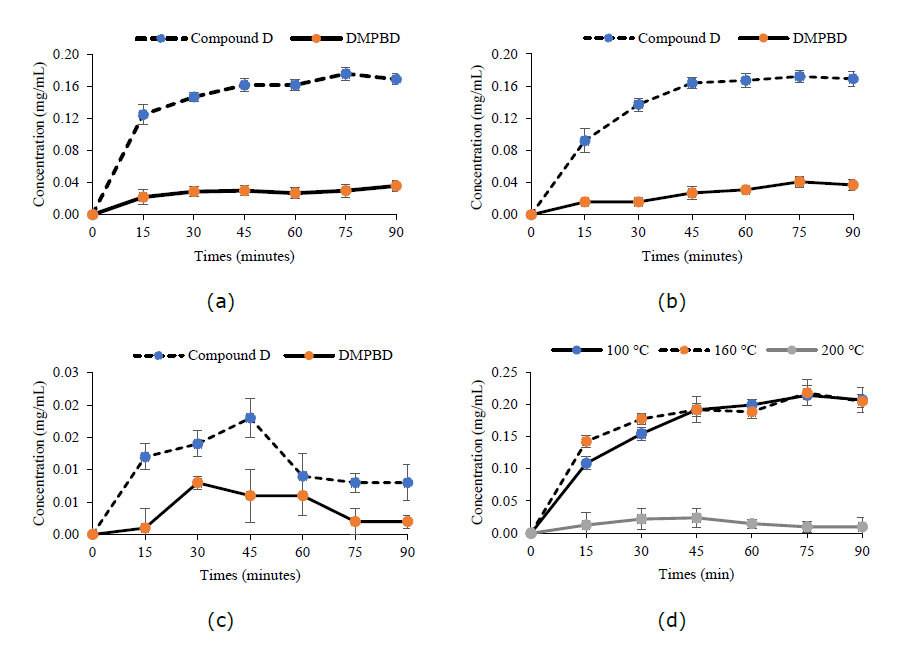
Figure 4. The extraction profiles of compound D and DMPBD during frying with coconut oil comparing 100°C (a), 160°C (b) and 200°C (c) and the release profile of phenybutanoids (the sum quantity of compound D and DMPBD) during frying with coconut oil, comparing temperatures of 100°C, 160°C, and 200°C (d).
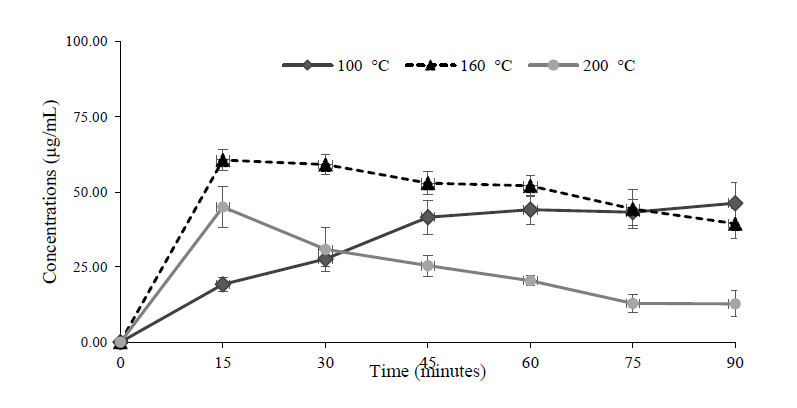
Figure 5. The release profile of terpinen-4-ol during frying with coconut oil comparing 100°C, 160°C and 200°C.
The extraction profile of terpinen-4-ol in fried oil shows that the release of terpinen-4-ol was observed within 15 min after the initiation point. However, the elevated temperature also played a crucial role in terpinen-4-ol extraction. The quantity of terpinen-4-ol tended to decrease after 15 min when prepared by frying at 160°C and 200°C. On the contrary, the release of terpinen-4-ol from the rhizome increased regularly when prepared by frying at 100°C (Figure 5). As illustrated in Table 2, compound D was not detected in the distilled Z. montanum oil. The extraction profiles indicated only DMPBD and terpinen-4-ol (Figure 6) which were both gradually released from the rhizome. The cumulative concentration of both compounds and times showed linear relationships.
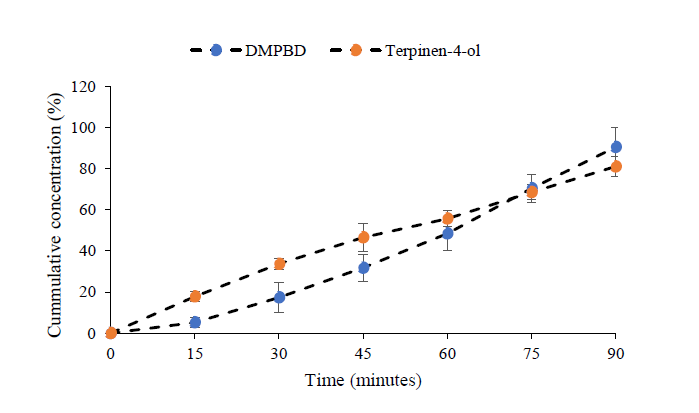
Figure 6. The extraction profiles of DMPBD and terpinen-4-ol in Z. montanum oil during distillation process.
DISCUSSION
Zingiber montanum is a medicinal plant that has traditionally been used as an anti-inflammatory agent. Compound D and DMPBD, phenylbutanoids presenting potent anti-inflammatory activity, are found in Z. montanum rhizomes. Similarly, terpinen-4-ol, a terpenoid, is also prominent and presents promising bioactivity associated with inflammation. Z. montanum oil has been traditionally prepared by deep-frying with plant-fixed oil or distilling with water. Both oils have been used as a recipe for treating muscle and skeletal disorders (Manimmanakorn et al., 2016; Wisuitiprot et al., 2019).
The current study successfully isolated compound D and DMPBD from Z. montanum rhizome extract. The isolated phenylbutanoids were used as standard markers for Z. montanum oils standardization. Regarding the percent yield of both oils, fried oil presented a huge percentage of yield because the fried oil was covered with coconut oil, while essential oil was only extracted from the rhizomes. The results indicated that compound D, DMPBD and terpinen-4-ol are found in both Z. montanum oils, while essential oils are present only in DMPBD and terpinen-4-ol. The difference in the amount of compound D and DMPBD present in fried oil and distilled oil might result from the chemical structure differently affecting the stability of both compounds. DMPBD might be more stable than compound D. DMPBD contains two conjugated double bonds connected to a 3,4-dimethoxyphenyl group. The conjugation between the phenyl ring and the butadiene system allows for delocalization of π-electrons across the structure, increasing the molecule's overall stability (Bruice, 2020). Compound D has only a single double bond in the but-3-en-1-ol chain, meaning there is less conjugation compared to DMPBD. The lack of extended conjugation decreases the electron delocalization across the molecule. Moreover, the presence of a hydroxyl group also makes compound D more susceptible to degradation when exposed to elevated temperatures. Although compound D could be detected after being prepared under high temperatures, its solubility in water might also be the influencing factor causing compound D to be less present in the essential oil. In the case of the distilled oil, compound D might partition or solubilize into the water phase during distillation because the hydroxyl group allows partial solubility in water (Cisneros et al., 2017). Therefore, compound D was not detected in the distilled oil. Although elevated temperatures allow for the efficient extraction of compound D and DMPBD, high temperatures also induce the degradation of natural components (ElGamal et al., 2023). The amount of compound D decreases dramatically by more than 25%, and DMPBD by more than 15%, when the preparation temperature is increased from 100°C to 200°C. This is because DMPBD is more thermally stable due to its extensive conjugation, which provides better delocalization of electrons and resistance to heat-induced bond cleavage (Steenson and Min, 2000). Although both compound D and DMPBD were found to degrade under elevated temperature, both of them seem to be extracted from the rhizome effectively in fried oil due to heat-assisted extraction (Jovanović et al., 2023). The extraction profile in fried Z. montanum oil shows that compound D was extracted from the rhizome within the first 15 minutes of preparation and then increased gradually, while the quantity of DMPBD remained relatively steady after the initial 15 minutes. This difference is likely due to the variation in melting points between the two compounds. DMPBD is expected to have a higher melting point due to its rigid and conjugated structure, whereas compound D has a lower melting point because the presence of a hydroxyl group increases flexibility and weakens intermolecular interactions (Bruice, 2020). As a result, compound D partitioned into the hot oil more easily than DMPBD and was extracted first. Although DMPBD is more stable than compound D, its degradation is also evident. This could be explained by the thermal degradation of DMPBD, which contains a terminal double bond that is sensitive to oxidation reactions induced by high temperatures (Kuroki et al., 1983; Sawaguchi and Senō, 1996; Che Sulaiman et al., 2017). Generally, oxygen molecules have limited diffusion into the oil phase at elevated temperatures, but the oxidation reaction is accelerated by thermal activation (Chen et al., 2011). Therefore, the terminal double bond of DMPBD is degraded by oxidation.
Raising the temperature to 200°C impacted the release of phenylbutanoids from the rhizome, as compound D began to decrease after 45 minutes of preparation, while the decrease in DMPBD was noticeable after 30 minutes. This may be due to the degradation of DMPBD, driven by oxidation reactions at elevated temperatures. DMPBD is more susceptible to oxidation because of its conjugated double bonds and terminal double bond, that are prone to oxidative cleavage, whereas compound D has a single double bond that is less reactive than a conjugated system. Although the hydroxyl group in compound D can undergo oxidation, this process is generally slower and less likely compared to the oxidation of double bonds. Therefore, the extraction profile of compound D and DMPBD showed a dramatic decrease when preparing Z. montanum fried oil at 200°C. In the case of terpinen-4-ol, the release pattern resembled that of DMPBD when fried at 100°C, but the extraction profile increased sharply at 160°C. However, the quantity of terpinen-4-ol decreased significantly when fried at 200°C, likely due to the increased vaporization rates and degradation of monoterpenes at higher temperatures (Mondello et al., 2022; Yang et al., 2015). The extraction profile of terpinen-4-ol during distillation also showed a gradual increase over time. The approximate temperature for the distillation of monoterpenes using water distillation typically ranges from 100°C to 150°C, which accelerates monoterpene vaporization (Rafiq et al., 2024).
CONCLUSION
Fried oil prepared by deep-frying with coconut oil contained compound D, DMPBD, and terpinen-4-ol, while essential oils present prominently as DMPBD and terpinen-4-ol. Fried oil shows the highest amount of compound D, while essential oil shows the highest amount of DMPBD and terpinen-4-ol. The elevated temperature obviously influences the quantity of compound D, DMPBD and terpinen-4-ol extracted by deep-frying. Therefore, the oils of Z. montanum are promising ingredients that could potentially be used as topical applications or raw materials for topical products targeting inflammation, due to their content of compound D, DMPBD and terpinen-4-ol, which demonstrate potent anti-inflammatory properties. However, the standardization of the preparation method requires further investigation and optimization, as high temperature significantly influences the quantity and stability of the active compounds.
ACKNOWLEDGEMENTS
The author also acknowledges Mr. Roy I Morien, an English Language expert and native speaker, for his editing of the grammar, syntax and general English expression in this manuscript.
AUTHOR CONTRIBUTIONS
Conceptualization, V.W. (Vanuchawan Wisuitiprot), W.J. (Wuttichai Jaidee), and W.W. (Wudtichai Wisuitiprot); methodology and experimental design, V.W., N.K. (Nipon Keawtai), W.J., and W.W.; Validation, V.W. and W.W.; formal analysis, V.W., W.J. and W.W.; investigation, V.W., W.J., W.W.; resources, V.W., S.K. (Somsak Kreechai)., P.W. (Pitsaphun Werayingyong) and W.W.; data curation and interpretation, V.W., W.J. and W.W.; writing-original draft preparation, V.W., W.J. and W.W.; writing-review and editing, V.W., W.J., N.W. and W.W.; visualization, V.W. and W.W.; supervision, V.W., W.J. and W.W.; project administration, W.W., S.K. and P.W.; funding acquisition, W.W. and S.K. All authors have read and agree to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Association of Official Analytical Collaboration (AOAC) International. 2002. AOAC guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals.
Bruice, P.Y. 2020. Delocalized electrons: Their effect on stability, pka, and the products of a reaction - aromaticity and electronic effects: An introduction the reactions of benzene tutorial: Drawing resonance contributors. Organic chemistry. 8 ed. Canada: Pearson/Prentice Hall. p. 358-421.
Chaiwongsa, R., Ongchai, S., Boonsing, P., Kongtawelert, P., Panthong, A., and Reutrakul, V. 2012. Active compound of Zingiber cassumunar roxb. Down-regulates the expression of genes involved in joint erosion in a human synovial fibroblast cell line. African Journal of Traditional, Complementary and Alternative Medicines. 10(1): 40-48.
Che Sulaiman, I.S., Basri, M., Fard Masoumi, H.R., Chee, W.J., Ashari, S.E., and Ismail, M. 2017. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans lindau leaves by response surface methodology. Chemistry Central Journal. 11(1): 54.
Cheechareoan, S., Pathanawiriyasirikul, T., Manmee, C., and Janpol, K. 2016. Efficacy of plai cream in adult patients with muscle strain: A randomized, double-blind, placebo-controlled trial. Journal of the Medical Association of Thailand. 99 Supplement 2: S147-S152.
Chen, B., McClements, D.J., and Decker, E.A. 2011. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Critical Reviews in Food Science and Nutrition. 51(10): 901-916.
Chienthavorn, O., Poonsukcharoen, T., and Pathrakorn, T. 2011. Pressurized liquid and superheated water extraction of active constituents from Zingiber cassumunar roxb. rhizome. Separation Science and Technology. 46(4): 616-624.
Cisneros, J.A., Robertson, M.J., Mercado, B.Q., and Jorgensen, W.L. 2017. Systematic study of effects of structural modifications on the aqueous solubility of drug-like molecules. ACS medicinal chemistry letters. 8(1): 124-127.
ElGamal, R., Song, C., Rayan, A.M., Liu, C., Al-Rejaie, S., and ElMasry, G. 2023. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy. 13(6): 1580.
Haidar Ahmad, I.A. 2017. Necessary analytical skills and knowledge for identifying, understanding, and performing HPLC troubleshooting. Chromatographia. 80(5): 705-730.
Han, A.R., Kim, M.S., Jeong, Y.H., Lee, S.K., and Soe, E.K. 2005. Cyclooxygenase-2 inhibitory phenylbutenoids from the rhizomes of Zingiber cassumunar. Chemical & Pharmaceutical Bulletin. 53: 1466-1468.
Han, A.R., Kim, H., Piao, D., Jung, C.H., and Seo, E.K. 2021. Phytochemicals and bioactivities of Zingiber cassumunar roxb. Molecules. 26(8): 2377.
Hart, P.H., Brand, C., Carson, C.F., Riley, T.V., Prager, R.H., and Finlay-Jones, J.J. 2000. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflammation Research: Official Journal of the European Histamine Research Society. 49(11): 619-626.
Insuan, O., Thongchuai, B., Chaiwongsa, R., Khamchun, S., and Insuan, W. 2021. Antioxidant and anti-inflammatory properties of essential oils from three eucalyptus species. Natural and Life Sciences Communications. 20(4): e2021091.
Jeenapongsa, R., Yoovathaworn, K., Sriwatanakul, K.M., Pongprayoon, U., and Sriwatanakul, K. 2003. Anti-inflammatory activity of (e)-1-(3,4-dimethoxyphenyl) butadiene from Zingiber cassumunar roxb. Journal of Ethnopharmacology. 87: 143-148.
Jovanović, M.S., Milutinović, M., Lazarević, Z., Mudrić, J., Matejić, J., Kitić, D., and Šavikin, K. 2023. Heat- and microwave-assisted extraction of bioactive compounds from Gentiana asclepiadea L. underground parts: Optimization and comparative assessment using response surface methodology. Journal of Applied Research on Medicinal and Aromatic Plants. 34: 100483.
Kaewchoothong, A. 2009. Preparation and quality control of Zingiber cassumunar extract with high-yielded anti-inflammatory active compounds. Master Dissertation. Prince of Songkla University, Songkla, Thailand.
Koparde, A.A., and Magdum, C. 2014. Conventional and microwave assisted extraction of Eulophia ochreta lindl and Zingiber cassumunar roxb: An comparitive account. Research Journal of Pharmacy and Technology. 7(7): 746-748.
Kuroki, T., Sawaguchi, T., Suzuki, K., Ide, S., and Ikemura, T. 1983. Distribution of double bonds in thermally degraded polyisobutylene. Polymer. 24(4): 428-432.
Li, M.X., Bai, X., Ma, Y.P., Zhang, H.X., Nama, N., Pei, S. J., and Du, Z.Z. 2019. Cosmetic potentials of extracts and compounds from Zingiber cassumunar roxb. rhizome. Industrial Crops and Products. 141: 111764.
Manimmanakorn, N., Manimmanakorn, A., Boobphachart, D., Thuwakum, W., Laupattarakasem, W., and Hamlin, M.J. 2016. Effects of Zingiber cassumunar (plai cream) in the treatment of delayed onset muscle soreness. Journal of Integrative Medicine. 14(2): 114-120.
Martins, R., Barbosa, A., Advinha, B., Sales, H., Pontes, R., and Nunes, J. 2023. Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes. 11(8): 2255.
Ministry of Public Health Thailand. 2025. Musculoskeletal medications. p. 86-89. In Department of Political Drugs [ed] National list of essential medicine 2025 (Herbal Medicine). Ministry of Public Health, Nonthaburi.
Moldoveanu, S.C., and David, V. 2017. Chapter 4 - basic information regarding the hplc techniques. p. 87-187. In: Moldoveanu SC, David V, editors. Selection of the hplc method in chemical analysis. Elsevier, Boston.
Mondello, F., Fontana, S., Scaturro, M., Girolamo, A., Colone, M., Stringaro, A., Vito, M.D., and Ricci, M.L. 2022. Terpinen-4-ol, the main bioactive component of tea tree oil, as an innovative antimicrobial agent against Legionella pneumophila. Pathogens. 11(6): 682.
Navabhatra, A., Maniratanachote, R., and Yingngam, B. 2022. Antiphotoaging properties of Zingiber montanum essential oil isolated by solvent-free microwave extraction against ultraviolet B-irradiated human dermal fibroblasts. Toxicological Research. 38(2): 235-248.
Ninomiya, K., Hayama, K., Ishijima, S.A., Maruyama, N., Irie, H., Kurihara, J., and Abe, S. 2013. Suppression of inflammatory reactions by terpinen-4-ol, a main constituent of tea tree oil, in a murine model of oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro. Biological and Pharmaceutical Bulletin. 36(5): 838-844.
Nishidono, Y., Saifudin, A., and Tanaka, K. 2024. Characterization of the volatile constituents of plai (Zingiber purpureum) by gas chromatography–mass spectrometry. Molecules. 29(6): 1216.
Panthong, A., Kanjanapothi, D., Niwatananant, W., Tuntiwachwuttikul, P., and Reutrakul, V. 1997. Anti-inflammatory activity of compound d {(e)-4-(3′,4′-dimethoxyphenyl)but-3-en-2-ol} isolated from Zingiber cassumunar roxb. Phytomedicine. 4(3): 207-212.
Rafiq, A., Manzoor, B., Nayeem, M., Jabeen, A., Amin, QA. 2024. Extraction of essential oils. p. 279-298. In: SM. Jafari and S. Akhavan-Mahdavi [eds] Extraction processes in the food industry. Woodhead Publishing, Cambridge.
Sawaguchi, T. and Senō, M. 1996. Thermal degradation of polyisobutylene: Effect of end initiation from terminal double bonds. Polymer Degradation and Stability. 54(1): 33-48.
Singsai, K., Charoongchit, P., and Utsintong, M. 2022. The comparison of the oil types in plai (Zingiber cassumunar) oil extraction and analysis of the chemical constituents in plai oil by gas chromatography-mass spectrometry technique. Health Science, Science and Technology Reviews. 15(3): 18-28.
Steenson, D.F., and Min, D.B. 2000. Effects of β-carotene and lycopene thermal degradation products on the oxidative stability of soybean oil. Journal of the American Oil Chemists' Society. 77(11): 1153-1160.
Sukatta, U., Rugthaworn, P., Punjee, P., Chidchenchey, S., and Keeratinijakal, V. 2009. Chemical composition and physical properties of oil from plai (Zingiber cassumunar roxb.) obtained by hydro distillation and hexane extraction. Agriculture and Natural Resources. 43(5): 212-217.
Suksaeree, J., Monton, C., Charoenchai, L., Madaka, F., and Chusut, T. 2014. Determination of (E)-4-(3’,4’-dimethoxyphenyl)-but-3-en-1-ol content in Zingiber cassumunar roxb. (plai) patches. International Journal of Pharmacy and Pharmaceutical Sciences. 6(5): 434-436.
Tangyuenyongwatana, P. 2017. HPTLC analysis of (E)-4-(3ʹ,4ʹ-dimethoxyphenyl)but-3-en-1-ol in Zingiber cassumunar roxb rhizome extract: Method validation and its application on studying compound-genetic relationship. Thai Journal of Pharmaceutical Sciences. 41(3): 1-6.
Truong, V.L., Manochai, B., Pham, T.T., and Jeong, W.S. 2021. Antioxidant and anti-inflammatory activities of Zingiber montanum oil in HEPG2 cells and lipopolysaccharide-stimulated raw 264.7 macrophages. Journal of Medicinal Food. 24(6): 595-605.
Tyas, D., Wijayanti, N., Nuringtyas, T., and Wahyuono, S. 2024. Protective effects of Zingiber cassumunar roxb. extract against UVB‐induced oxidative stress in wistar albino rats (Rattus novergicus berkenhout, 1769). Indonesian Journal of Biotechnology. 29(1): 25.
Verma, R.S., Joshi, N., Padalia, R.C., Singh, V.R., Goswami, P., Verma, S.K., Iqbal, H., Chanda, D., Verma, R.K., Darokar, M.P. et al. 2018. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. Journal of the Science of Food and Agriculture. 98(1): 321-327.
Wisuitiprot, V., Bumrungchaichana, W., Kaewtai, N., Rawangking, A., Saiphanit, S., Lasongmuang, K., Meekai, N., and Wisuitiprot, W. 2019. Effectiveness of a plai oil prepared by Thai traditional medicine process in the treatment of myofascial pain syndrome: A randomized placebo controlled trial: Effectiveness of plai oil in myofascial pain syndrome. Journal of Health Science and Medical Research. 37(3): 207-215.
Yang, Z., Xiao, Z., and Ji, H. 2015. Solid inclusion complex of terpinen-4-ol/β-cyclodextrin: Kinetic release, mechanism and its antibacterial activity. Flavour and Fragrance Journal. 30(2): 179-187.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Wudtichai Wisuitiprot1, 2, Wuttichai Jaidee3, Pitsaphun Werayingyong5, Somsak Kreechai5, Nipon Keawtai1, and Vanuchawan Wisuitiprot4, 6, *
1 Sirindhorn College of Public Health Phitsanulok, Faculty of Public Health and Allied Health Sciences, Praboromarajchanok Institute of Heath Workforce Development, Ministry of Public Health, Phitsanulok 65130, Thailand.
2 Herbal and Research Unit, Faculty of Public Health and Allied Health Sciences, Praboromarajchanok Institute of Heath Workforce Development, Ministry of Public Health, Phitsanulok 65130, Thailand.
3 Medicinal Plants Innovation Center of Mae Fah Luang University, Chiang Rai 57100, Thailand.
4 Cosmetics and Natural Products Research Center, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
5 Department of Thai Traditional Medicine and Alternative Medicine, Ministry of Public Health, Nonthaburi 11000, Thailand.
6 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Vanuchawan Wisuitiprot, E-mail: vanuchawan@hotmail.com
ORCID:
Vanuchawan Wisuitiprot: https://orcid.org/0000-0003-2213-8951
Wudtichai Wisuitiprot: https://orcid.org/0000-0001-8072-1382
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: September 9, 2024;
Revised: April 28, 2025;
Accepted: May 8, 2025;
Online First: May 23, 2025

