
Effects of Vitamin A Supplementation on Reproductive Function and Estrus Synchronization Response of Kamphaeng Saen Heifers Raised under Restrictive Feeding
Sangphet Leelachayakun, Sutisa Majarune, Wisut Maitreejet, Charkrit Borirak, Tatphicha Rongthong, Anchalee Khongpradit, Suriya Sawanon, and Phoompong Boonsaen*Published Date : May 2, 2025
DOI : https://doi.org/10.12982/NLSC.2025.042
Journal Issues : Number 3, July-September 2025
Abstract During the dry season in tropical areas, green pastures are limited. Heifers or cows fed primarily hay or rice straw are at risk of insufficient ß-carotene (provitamin A). Vitamin A (VitA) is crucial for reproductive functions. This study investigated the influence of VitA supplementation on the reproductive performance of Kamphaeng Saen beef heifers raised under restrictive feeding (n = 18; body condition score [BCS] = 3.5) over 48 days. The animals were equally divided into two groups: 1) the control group (fed a basal diet) and 2) the VitA group (fed the basal diet plus 100,000 IU of VitA per head per day). After 28 days of VitA supplementation, all heifers underwent estrus synchronization protocol. Reproductive tract and ovarian components were examined using ultrasonography to evaluate the size of the cervix, uterine horn, ovaries, follicles, and corpus luteum (CL) on Days 13 and 20 of the estrus synchronization protocol. Blood samples were collected to assess VitA and progesterone concentrations in serum. The results indicated that heifers supplemented with VitA tended to have more compact CL (P = 0.06). However, VitA did not significantly improve overall reproductive tract components or blood parameters between the control and VitA groups (P > 0.05). These findings suggest that VitA supplementation can enhance CL quality with short-term supplementation, but long-term VitA supplementation requires further investigation.
Keywords: Beef cattle, Kamphaeng Saen Heifers, Reproductive performance, Restrictive feeding, Vitamin A
Funding: The authors are grateful for the research funding provided by Kasetsart University through the Graduate School Fellowship Program.
Citation: Leelachayakun, S., Majarune, S., Maitreejet, W., Borirak, C., Rongthong, T., Khongpradit, A., Sawanon, S., and Boonsaen, P. 2025. Effects of vitamin a supplementation on reproductive function and estrus synchronization response of Kamphaeng Saen heifers raised under restrictive feeding. Natural and Life Sciences Communications. 24(3): e2025042.
INTRODUCTION
The beef cattle farming industry has expanded significantly in recent years, leading to the development of various technologies aimed at enhancing the efficiency of beef cattle production. Reproductive technologies in female cattle, including artificial insemination (AI) and hormone-based estrus and ovulation induction, can potentially raise farm productivity (Velazquez, 2023). Proper estrus and ovulation are essential for managing the estrous cycle and facilitating rapid herd breeding management. In Thailand, embryo transfer (ET) and AI have been used to disseminate the genetic material of superior bulls and cows (Techakumphu et al., 2001). Several factors affect the success of ET or AI, including semen quality, embryo viability, the reproductive health status of the heifer/cow/recipient, AI techniques, transfer methods, and nutrient management in the breeding herd (Muhaidat et al., 2023). Nutrient intake plays an important role in the reproduction of cows and heifers, with the efficiency of the reproductive system relying on both macronutrients (protein and energy) and micronutrients (vitamins and minerals) (Ikeda et al., 2005; Cho et al., 2014; NASEM, 2016).
In tropical regions, green pasture is scarce during the dry season, necessitating the preservation of forages such as silage, hay, or rice straw for ruminant animals. Thailand is a major producer and exporter of rice, ranking sixth globally in production (Sowcharoensuk, 2024). Consequently, rice straw, a by-product of rice production, serves as a primary roughage during the summer. However, the quality of rice straw is poor, characterized by low digestibility and nutrient content (Wanapat et al., 2009; NASEM, 2016; Srisaikham and Lounglawan, 2020). Additionally, rice straw and other dried forages contain low levels of ß-carotene (Arikan and Rodway, 2001; Katamoto et al., 2003; Thongun et al., 2021), which is essential for synthesizing vitamin A (VitA) in cattle (Mitsuishi and Yayota, 2024).
VitA is vital for embryonic development and reproductive functions, existing in the form of carotenoids (ß-carotene) in green forage tissues (Haliloglu et al., 2002; Mitsuishi and Yayota, 2024). This fat-soluble vitamin supports reproductive system health in preparation for mating (Clagett-Dame and Knutson, 2011). Numerous studies have reported that retinoids play a role in oocyte maturation, follicular growth, fertilization, and early embryonic development. The level of VitA in ovarian follicular fluid may reliably indicate follicle health, with healthy follicles strongly correlated with elevated estradiol levels. Follicular disorders are indicated by low serum VitA levels (Brown et al., 2003; Abouzaripour et al., 2018). Thus, VitA is crucial for the reproductive system, stimulating and promoting the development of the corpus luteum (CL) by enhancing StAR gene transcription, which increases cholesterol availability for progesterone synthesis in luteal cells (Wickenheisser et al., 2005; Manna et al., 2015). Progesterone facilitates implantation and gestation, playing a critical role in preparing the uterine endometrium for embryo implantation (Hafez et al., 2000; Clagett-Dame and Knutson, 2011; Halasz and Szekeres-Bartho, 2013). Many reports indicate that CL is rich in VitA, which is essential for steroidogenesis and CL formation (Arıkan and Rodway, 2001; Haliloglu et al., 2002; Wickenheisser et al., 2005; Pasquariello et al., 2022; Mitsuishi and Yayota, 2024). The low serum VitA concentrations in cows are associated with increased uterine horn diameter and infertility (Ganguly and Mukherjee, 1988; Raila et al., 2017). Furthermore, the quality of embryos in the cows receiving VitA supplements has been shown to improve (Shaw et al., 1995).
In breeding programs or ET procedures, the preparation of the heifer/cow or recipient is essential. A healthy cow or heifer with good fertility must receive adequate nutrition and maintain a favorable BCS. For ET or fixed-time AI, synchronization programs are required to prepare the uterine environment for pregnancy after ET or to induce ovulation (Bó and Baruselli, 2014; Mebratu, 2020). The effects of VitA or ß-carotene on the reproduction of cows or heifers have been suggested (Haliloglu et al., 2002; Ikeda et al., 2005; Cho et al., 2014; Gouvêa et al., 2018; Mitsuishi and Yayota, 2024). However, the impact of low VitA or ß-carotene consumption on crossbred heifers or cows raised in tropical conditions has not been reported. Therefore, this study aims to determine the effect of VitA supplementation in the diet on the reproductive function of Kamphaeng Saen heifers fed a low VitA or ß-carotene diet under restrictive feeding in tropical conditions in Thailand.
MATERIALS AND METHODS
Study area, animal housing and management
This study was conducted at a beef cattle farm, Department of Animal Science, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Nakhon Pathom, Thailand. Eighteen heifers (440 ± 47 kg body weight, average age 23 months) were individually housed in open-air pens (2.5 × 5 m) with a concrete floor, experiencing average temperatures of 24–34 °C during the rainy season (August to October). The animals were selected based on uniformity of BCS >3.5 and were examined via ultrasonography for a normal reproductive system from the replacement heifers of the Kamphaeng Saen beef cattle herd (BCS: emaciated = 1, thin = 2, ideal = 3, fat = 4, overfat = 5, scaled according to the method described by Edmonson et al., 1989). Kamphaeng Saen beef cattle is the first synthetic breed developed in Thailand (Bos taurus; 50% Charolais and Bos indicus; 25% Brahman × 25% Thai native) (Sawanon et al., 2011). The animals were treated for intestinal and external parasites with Aben-15® (150 mg Albendazole; F.E. Pharma Company Limited®, Bangkok, Thailand), Ivomec® Plus (1% Ivermectin and 10% Clorsulon, Merial® Inc., Duluth, GA, USA), and vaccinated against foot-and-mouth disease. The animals were restrictively fed 3 kg/head/day of rice straw as roughage (low VitA or provitamin A content) and a concentrate diet (16% CP) at 3 kg/head/day, twice daily (7:00 a.m. and 6:00 p.m.). All animals had free access to fresh water. The animal study protocol was approved by The Animal Usage and Ethics Committee of Kasetsart University, Thailand (ACKU64-AGK-013).
Experimental design
Heifers were randomly assigned to two experimental groups: 1) control (n = 9), heifers fed a basal diet and 2) VitA group (n = 9), heifers fed a basal diet supplemented with 100,000 IU of VitA per head per day (Vitamin A acetate 1000; 1,000,000 IU/g, Zhejiang NHU Co., Ltd., Zhejiang ICP, China). VitA was supplemented using a top-dressing method (50 mg/head/meal) for each meal throughout the experiment. The quantity of supplementation was recommended in the Guidelines for OVN Optimum Vitamin Nutrition® in ruminants, DSM, 2022. This experiment was conducted from August to October, with a 14-day adaptation period before treatment commenced.
Feed chemical composition analysis
The nutrient compositions and ß-carotene concentrations of the diets are shown in Table 1. The concentrate diet and rice straw were analyzed for crude protein (CP), ether extract (EE), neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) using the Association of Official Analytical Chemists (AOAC) procedures (2016). The concentration of ß-carotene (provitamin A) in the diet was analyzed using high-performance liquid chromatography (HPLC) following the In-house method WI-TMC-11, as described in Munzuroglu et al. (2003).
Table 1. Chemical compositions (%DM) and ß-carotene concentration of concentrate diet/1 and rice straw.
|
Items |
Concentrate |
Rice straw |
|
Dry matter (%) |
89.08 |
91.30 |
|
Crude protein |
16.89 |
5.90 |
|
Ether extract |
4.61 |
1.50 |
|
Neutral detergent fiber |
26.51 |
74.00 |
|
Acid detergent fiber |
14.05 |
44.50 |
|
Ash |
7.11 |
12.10 |
|
Calcium |
0.85 |
0.53 |
|
Phosphorus |
0.73 |
0.12 |
|
ß-carotene (µg/100 g) |
61.30 |
|
|
Note: /1Commercial concentrate diet in powder form containing 16% crude protein. LOQ is the limit of quantification. |
||
Estrus synchronization (ES) and detection
All heifers underwent synchronization using procedures described in the Operational Manual for Bovine Embryo Transfer Procedures (Bureau of Biotechnology in Livestock Production, 2013), as scheduled in Figure 1. On Day 0, the ovaries were examined via rectal ultrasonography (SonoScape S2 ultrasonography, B-mode 2-16 MHz rectal probe, Medical Corp, China). The dominant follicles were destroyed using a GnRH hormone treatment (Receptal®, 0.004 mg/mL buserelin acetate, Merck & Co., Inc., Rahway, NJ, USA) via a 2.5 mL intramuscular injection, followed by the insertion of a progesterone hormone device (Controlled internal drug release device; CIDR®, 1.38 g progesterone/device, Zoetis, 10 Sylvan Way Parsippany, NJ, USA) into a vagina. On Day 10, PGF2α (Estrumate®, 500 mcg cloprostenol, Merck & Co., Inc., Rahway, NJ, USA) was administered via a 2 mL intramuscular injection in the morning. On Day 11, the progesterone device was removed in the morning and the ovaries were examined via rectal ultrasonography. On Day 13, the behavior and signs (such as clear mucus discharge from the vulva, mounting, and standing heat) and the ovaries (examined via rectal ultrasonography) of the heifers were recorded.
A penile translocation bull was used to detect estrus behavior of the hiefers twice daily (6:00–7:00 a.m. and 4:00–5:00 p.m.).
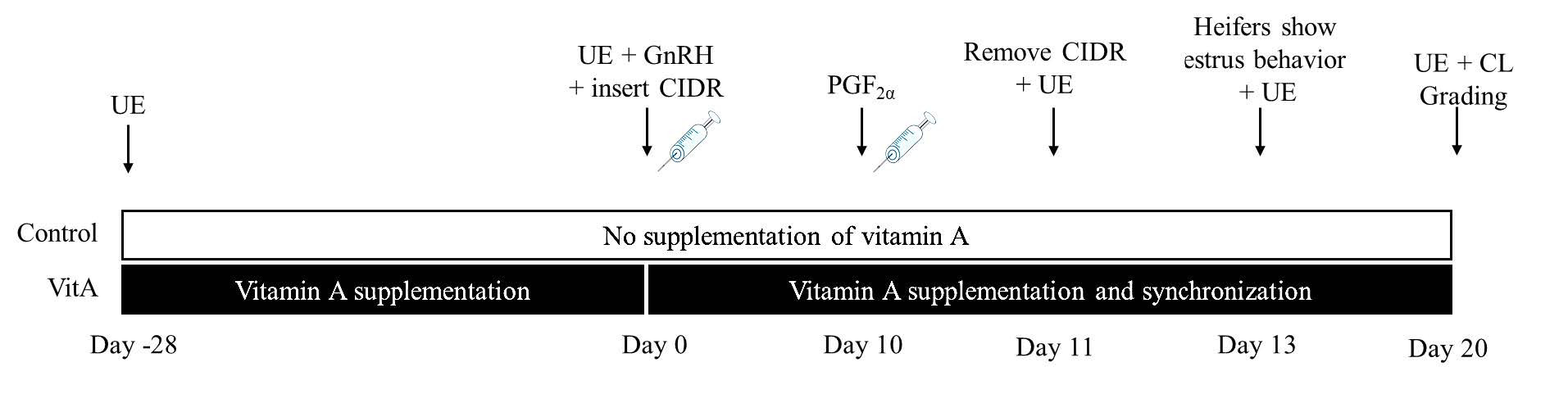
Figure 1. Vitamin A supplementation and synchronization protocol schedule. UE = Ultrasonography evaluation, GnRH = GnRH injection, PGF2α = Prostaglandin2α injection, P4 = Progesterone, CIDR = Controlled internal drug release device.
Evaluation of reproductive parameters
Reproductive tract and ovarian components were measured (ultrasonography evaluation; UE) as scheduled in Figure 1 using ultrasound (SonoScape S2 ultrasonography, B-mode 2-16 MHz rectal probe, Medical Corp, China) via a transrectal probe via a B-mode screen to determine the diameter of the cervix, uterine horn, preovulatory follicle, and CL on Days 0, 11, 13 and 20 of the ES protocol. CL quality was graded using the method described in Vieira et al. (2014). On Day 20 of the ES protocol, CL grades were classified as follows: 1) grade A: CL represents 75% of the ovary volume, 2) grade B: CL represents 50% of the ovary volume, and 3) grade C: CL represents 25% of the ovary volume.
Blood collection and hormone assay
Blood samples were collected to assess the concentrations of progesterone and ß-carotene in serum. The collection was via the jugular vein using vacuum blood collection tubes containing a clot activator (two tubes with 4 mL each). All blood samples were allowed to clot for 2 hours at room temperature, then centrifuged for 15 minutes at 1,000×g. The serum was transferred into sterilized 2 mL microtubes and stored at -80°C. The concentration of VitA in serum was measured using the HPLC method (Kanďár et al., 2014). The concentration of progesterone in serum was measured using an enzyme immunoassay method (Brown et al., 2004).
Statistical analysis
The student’s t-test was applied to evaluate the effects of VitA supplementation on the diameter and area of the ovary, dominant follicle preovulatory follicle (POF), and CL. Normal distribution was checked using descriptive statistics, including mean, minimum, maximum, frequency, and standard deviation. Data on estrus expression and insemination rates were analyzed using Fisher's exact test with SAS® on Demand for Academics (SAS Institute, 2023). Significance was declared at a P-value of < 0.05, with tendencies declared at P-values between 0.05 and 0.10.
RESULTS
Vitamin A and ß-carotene intake of heifers
The ß-carotene concentration in the concentrate diet and rice straw (Table 1) indicated that the concentrate diet contained ß-carotene 1,839 µg/100 g of the diet. In contrast, the rice straw contained ß-carotene below the limit of quantification (40 µg/100 g). Therefore, the heifers ingested the primary source of VitA from the treatment, while the control group received ß-carotene only from the basal diet.
Effect of vitamin A on estrus expression and ovulation rate of Kamphaeng Saen heifers on Day 13 of synchronization
This study investigated the impact of VitA supplementation on estrus expression and ovulation rate in Kamphaeng Saen heifers. The results are shown in Table 3. They indicated no significant difference between the control and the VitA groups.
Table 2. Effect of vitamin A on estrus expression and ovulation rate of Kamphaeng Saen heifers on Day 13 of the synchronization protocol.
|
Items |
Control |
Vitamin A |
SEM |
P-value |
|
|
Number of heifers (n) |
9 |
9 |
|
||
|
Estrus expression (%) |
89 (8/9) |
100 (9/9) |
0.11 |
0.33 |
|
|
Standing heat after removing a CIDR (%) |
78 (7/9) |
89 (8/9) |
1.18 |
0.92 |
|
|
Ovulation rate (%) |
78 (7/9) |
89 (8/9) |
1.18 |
0.92 |
|
|
Note: SEM = Standard error of mean; in parentheses () is the number of animals expressing estrus/total number of animals. CIDR = Controlled internal drug release device |
|||||
Effect of vitamin A on the diameter of the reproductive tract and ovarian components on Day 13 of the estrus synchronization protocol in Kamphaeng Saen heifers
The measurements of the diameters and areas of the reproductive tract and ovarian components using ultrasonographic images (Figure 2) on Day 13 of the ES protocol in Kamphaeng Saen heifers are shown in Table 4. There were no significant (P > 0.05) differences in the diameters of the cervix, uterine horn, and ovary, or the areas of the ovary and POF between the groups.
Table 3. Effect of vitamin A on the diameter of the reproductive tract and ovarian components on Day 13 of estrus synchronization protocol in Kamphaeng Saen heifers.
|
Items |
Control |
Vitamin A |
SEM |
P-value |
|
|
Number of heifers (n) |
9 |
9 |
|||
|
Diameter of the cervix (ipsilateral POF, cm) |
1.95 |
2.02 |
0.31 |
0.82 |
|
|
Diameter of uterine horn (ipsilateral POF, cm) |
1.18 |
1.16 |
0.16 |
0.90 |
|
|
Diameter of ovary (ipsilateral POF, cm) |
2.37 |
2.30 |
0.17 |
0.67 |
|
|
Area of ovary (ipsilateral POF, cm2) |
3.19 |
2.91 |
0.39 |
0.47 |
|
|
Diameter of POF |
1.25 |
1.20 |
0.14 |
0.79 |
|
|
Area of preovulatory follicle (ipsilateral POF, cm2) |
0.89 |
0.99 |
0.11 |
0.39 |
|
|
Note: SEM = Standard error of the mean; POF = preovulatory follicle |
|
||||
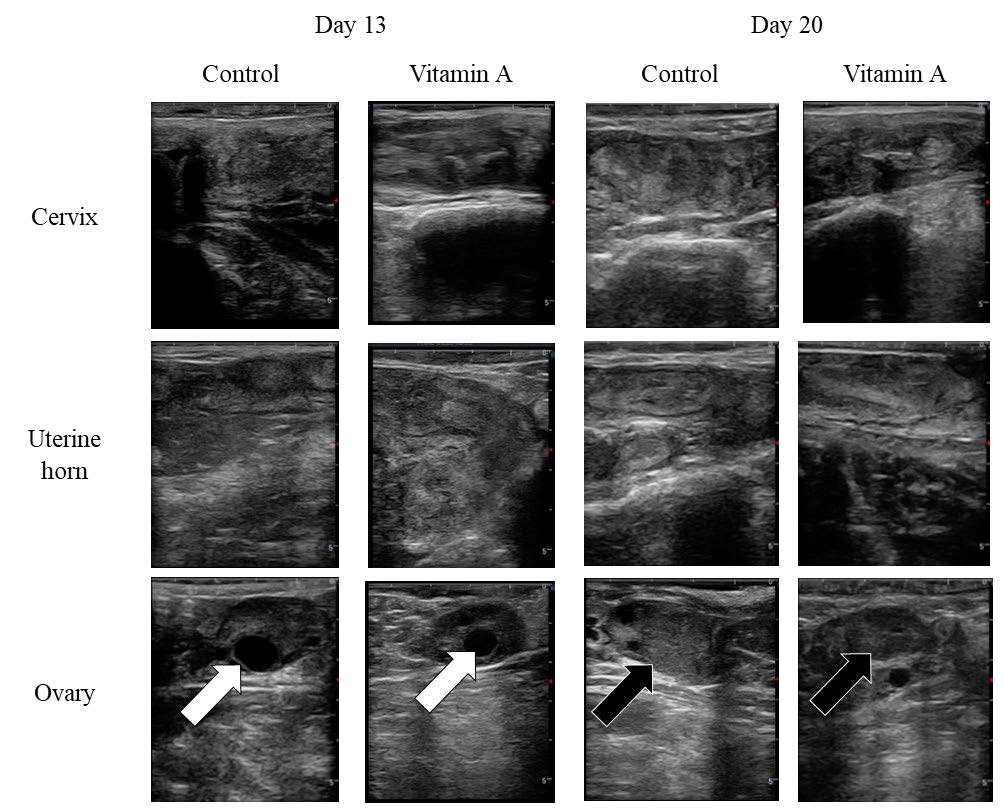
Figure 2. Ultrasonographic images of Kamphaeng Saen heifer reproductive tract on Days 13 and 20 of the ES protocol. White arrows indicate the ovary. Black arrows indicate the CL.
Effect of vitamin A on the diameter of the reproductive tract and ovarian components on Day 20 of the estrus synchronization protocol in Kamphaeng Saen heifers
The measurements of the diameters and areas of the reproductive tract and ovarian components using ultrasonographic images (Figure 2) on Day 20 of the ES protocol in Kamphaeng Saen heifers are shown in Table 5. There were no significant (P > 0.05) differences in the diameter of the cervix, diameter of the uterine horn, diameter of the ovary, area of the ovary, diameter of the CL, and area of the CL between the groups on Day 13. However, during this period, the CL developed after the ovulation of the dominant follicle.
Table 4. Effect of vitamin A on the diameter of the reproductive tract and ovarian components on Day 20 of the estrus synchronization protocol in Kamphaeng Saen heifers.
|
Items |
Control |
Vitamin A |
SEM |
P-value |
|
Number of heifers (n) |
9 |
9 |
||
|
Diameter of the cervix (ipsilateral CL, cm) |
1.61 |
1.73 |
0.17 |
0.51 |
|
Diameter of uterine horn (ipsilateral CL, cm) |
1.12 |
1.06 |
0.18 |
0.73 |
|
Diameter of ovary (ipsilateral CL, cm) |
1.71 |
1.79 |
0.13 |
0.55 |
|
Area of ovary (ipsilateral CL, cm2) |
3.34 |
3.86 |
0.48 |
0.30 |
|
Diameter of CL (cm) |
1.69 |
1.64 |
0.12 |
0.68 |
|
Area of CL (cm2) |
1.64 |
1.75 |
0.35 |
0.76 |
|
Note: SEM = Standard error of the mean; CL = corpus luteum. |
||||
Effect of vitamin A in synchronization protocol on heifer ovulation
The percentage of the ovulated Kamphaeng Saen heifers was evaluated. The results indicate no significant (P = 0.84) difference in the percentage of heifers ovulating between the control and VitA groups (78% vs. 89%, respectively). In the present study, although the differences in ovulation were not observed between the control and VitA groups, the quality of CL was detected after ovulation as shown in Table 7.
Table 5. Effects of dietary vitamin A supplementation on Kamphaeng Saen heifers ovulation on Day 20 of the estrus synchronization protocol.
|
Items |
Control |
Vitamin A |
SEM |
P-value |
|
Number of heifers (n) |
||||
|
Diameter of the cervix (ipsilateral CL, cm) |
9 |
9 |
||
|
Diameter of uterine horn (ipsilateral CL, cm) |
1.61 |
1.73 |
0.17 |
0.51 |
|
Diameter of ovary (ipsilateral CL, cm) |
1.12 |
1.06 |
0.18 |
0.73 |
|
Area of ovary (ipsilateral CL, cm2) |
1.71 |
1.79 |
0.13 |
0.55 |
|
Diameter of CL (cm) |
3.34 |
3.86 |
0.48 |
0.30 |
|
Area of CL (cm2) |
1.69 |
1.64 |
0.12 |
0.68 |
|
Note: SEM=Standard error of the mean; CL = corpus luteum. |
||||
Effects of vitamin A on quality classes of the corpus luteum in Kamphaeng Saen heifers on Day 20 of the synchronization protocol
The quality and type of CL in Kamphaeng Saen heifers are shown in Table 7. There were no significant (P > 0.05) differences in CL quality between the control and VitA groups. The distribution of CL grades in the control versus VitA group was as follows: Grade A; 44% vs. 56%, Grade B; 22% vs. 33%, Grade C; 11% vs. 0%, respectively. These results suggest a positive effect of VitA supplementation. Regarding CL type, there was no significant (P > 0.05) difference in the presence of cavity CL between the control and VitA groups, with 56% vs. 22%, respectively. However, the occurrence of compact CL (Figure 3) tended to be higher (P = 0.06) in the VitA group compared to the control group (67% vs. 22%, respectively).
Table 6. Effects of dietary vitamin A supplementation on corpus luteum (CL) quality in Kamphaeng Saen heifers on Day 20 of the estrus synchronization protocol.
|
Treatments |
Corpus luteum grade (%) /1 |
Compact CL (%) |
Cavity CL (%) |
||
|
A |
B |
C |
|||
|
Control |
33.33 (3/9) |
22.22 (2/9) |
11.11 (1/9) |
22.22 (2/9) |
55.56 (5/9) |
|
Vitamin A |
55.56 (5/9) |
33.33 (3/9) |
0.00 (0/9) |
66.67 (6/9) |
22.22 (2/9) |
|
P-value |
0.66 |
0.62 |
0.33 |
0.06 |
0.16 |
|
Note: /1 Corpus luteum (CL) grade: Grade A = CL represents 75% of the ovary volume, Grade B = CL represents 50% of the ovary volume, and Grade C = CL represents 25% of the ovary volume. In the parenthesis () is the number of detected animals/total number of animals. |
|||||
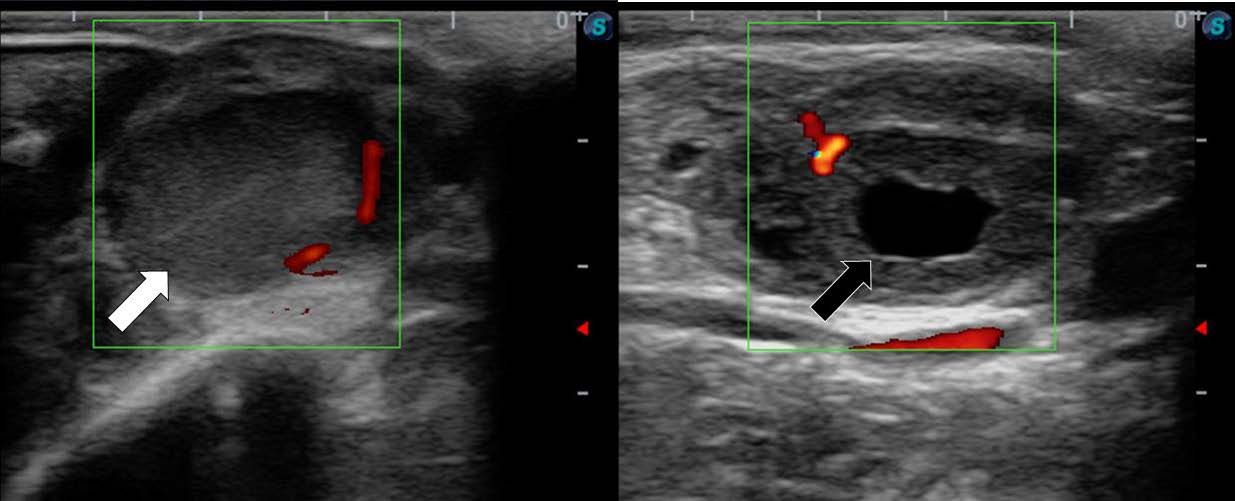
Figure 3. Ultrasonographic images of Kamphaeng Saen heifer ovary showing compact corpus luteum (white arrow) and cavity corpus luteum (black arrow).
Effect of vitamin A on serum progesterone concentration of Kamphaeng Saen heifers in ES protocol
The concentrations of serum progesterone are shown in Figure 4. There was no significant (P > 0.05) difference in serum progesterone levels between the control and the VitA groups at various time points. On Day 0 of the protocol, the concentrations were 3.48 ng/mL and 4.60 ng/mL, respectively (P = 1.49). On Day 10, the concentrations were 4.83 ng/mL and 4.86 ng/mL, respectively (P = 1.08). On Day 13, the concentrations were 1.82 ng/mL and 1.06 ng/mL, respectively (P = 0.51). On Day 20, the concentrations were 3.73 ng/mL and 4.33 ng/mL, respectively (P = 1.29).
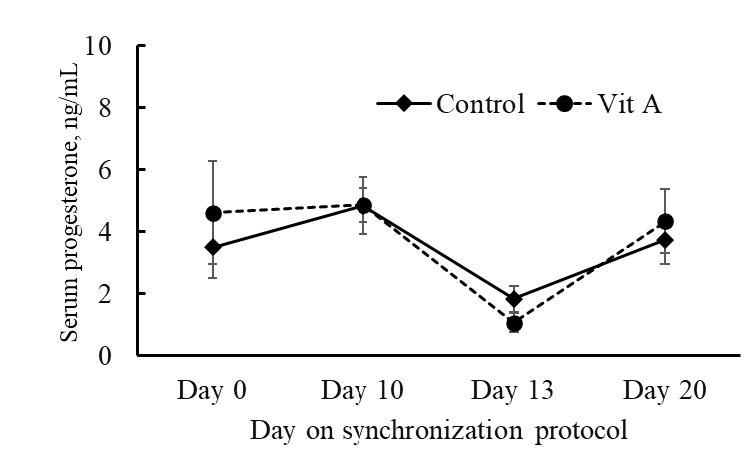
Figure 4. Serum progesterone concentration of Kamphaeng Saen heifers during estrus synchronization
Effect of vitamin A on vitamin A concentration in serum of Kamphaeng Saen heifers at Day 20 of the synchronization protocol
The concentrations of serum VitA of the hiefers were measured. The results indicated no significant (P = 0.05) difference between the control group and VitA groups (0.35 vs. 0.4 mg/L, respectively).
DISCUSSION
This experiment was conducted on Kamphaeng Saen heifers raised in an intensive program under low VitA or ß-carotene consumption and restrictive diets. This situation often occurs in summer when green pasture is limited. Farmers preserve roughages such as grass or corn silage, hay, or rice straw for the long dry season. Particularly, rice straw is a valuable by-product of rice production. However, this material contains low levels of provitamin A or ß-carotene, which has an essential role in reproductive health. The present study shows heifers that ingested VitA or
ß-carotene from both supplementation and the basal diet but in the control group, they ingested only from the basal diet (Table 1). Nonetheless, the basal diet contained a very low ß-carotene. Kawashima et al. (2010) reported that cows fed synthetic β-carotene (2,000 mg/day) for three weeks before calving exhibited apparent luteal activity within three weeks postpartum compared to cows without β-carotene supplementation. β-carotene may also play a crucial role in CL development to maintain pregnancy (Mitsuishi and Yayota, 2024). However, in the present study, the basal diet and roughage contained very low levels of VitA or ß-carotene. In cases of insufficient VitA or provitamin A in a herd, supplementation is essential. VitA activates the StAR and cytochrome P450 genes, accumulating in the form of the retinoic acid receptors and retinol X receptors, which promote StAR gene expression and directly induce transcription, facilitating cholesterol transport for progesterone production (Lee et al., 1999; Suwa et al., 2016). VitA also induces the expression of the cytochrome P450 11α-hydroxylase (CYP11) gene, which is involved in producing the P450scc enzyme and converting cholesterol into pregnenolone, thereby increasing the synthesis in luteal cells (Wickenheisser et al., 2005; Manna et al., 2015). Although the results in these studies did not show significant effects on reproduction, animals supplemented with VitA had higher serum VitA concentrations on Day 20 of the ES protocol.
In the present study, on Day 13, the reproductive tract of Kamphaeng Saen heifers supplemented with VitA did not show significant effects either. Previous studies reported that the diameter of the cervix ranged from 1.5 to 2 cm, the uterine horn from 2 to 3 cm, and the ovary approximately 1.5 cm in crossbred heifers during estrus (Gupta, 1962; Anderson et al., 1991). Although the roles of VitA on the cervix or uterine horn responses have not been extensively reported, the present study found no significant differences in reproductive tract measurements between the control and VitA groups. VitA supplementation was expected to play an important role in estrus expression in heifers. The percentage of estrus expression in the VitA group was higher than in the control group on Day 13. VitA may influence the diameter and area of POF. This vitamin potentially activates and enhances estrogen and progesterone synthesis, regulated by Aldh1a1 and Aldh1a2 genes. These functions improve ovarian and steroid hormone synthesis by increasing the expression of MESP2, which targets StAR and CYP11a1 genes to control the proliferation of ovarian granulosa cells through the PI3K-Akt-hedgehog pathway (Shuang et al., 2023).
The VitA supplementation group exhibited greater serum progesterone concentrations. Rode et al. (1990) and Weiss (1998) reported that VitA is degradable in the rumen by rumen microbes. Therefore, adding VitA to the diet is essential to address the insufficiency of VitA or provitamin A in the cattle herd, especially during the dry season. In the present study, supplementation of 100,000 IU/head/day for 48 days resulted in serum progesterone levels of 4.33 ng/mL. In contrast, Tharnish and Larson (1992) reported that supplementation of VitA in gestating cows at 100,000 vs. 1,000,000 or 2,000,000 IU/head/day for less than three months resulted in serum progesterone levels of 9.89 vs. 8.92 or 6.35 ng/mL, respectively. They also reported that longer supplementation (>5 months) with similar treatments resulted in serum progesterone levels of 11.06 vs. 10.35 or 9.46 ng/mL, respectively. These findings indicate that long-term VitA supplementation could elevate and maintain progesterone levels. The size and type of CL influence progesterone production, making CL quality essential for maintaining gestation. The size and type of CL are determined by POF size, which directly affects CL development. After ovulation, larger POFs lead to better CL quality (size and compactness) (Robinson et al., 2006; Vasconcelos et al., 2001). The development of CL could be categorized into two types: compact and cavity-type. The cavity-type has more quick development than the compact-type. However, the development of cavity-type CL may be limited by blood flow to the CL (Alila and Hansel, 1984; Wiltbank, 1994; Grygar et al., 1997; Jaśkowski, 2019; Jaśkowski et al., 2021). Perez‐Marin (2009) reported that the fertilization rate of cows or heifers with cavity CL was lower than that of cattle with compact CL (42.9% vs. 57.1%, respectively). Although the size of the cavity CL ranges from medium to large, it does not adversely affect progesterone production (Kastelic et al., 1990; Gábor et al., 2004; Jaśkowski, 2019; Jaśkowski et al., 2021). As previously mentioned, VitA may enhance the reproductive performance of beef cattle. To prepare heifers or cows as recipients for ET or the breeding season, sufficient daily VitA intake is necessary. During periods of insufficient green pasture, VitA supplementation in the diet could effectively maintain reproductive system potential.
CONCLUSION
Supplementation of VitA in Kamphaeng Saen beef heifers tends to improve CL quality compared to no VitA supplementation. However, further studies are needed to determine the successful transfer or conception rates of either ET or insemination programs in the production herd, particularly during insufficient green pasture.
ACKNOWLEDGEMENTS
The authors acknowledge the beef cattle farm, Department of Animal Science, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Nakhon Pathom, Thailand. This research was funded by Kasetsart University through the Graduate School Fellowship Program.
AUTHOR CONTRIBUTIONS
S. Leelachayakun: Original manuscript preparation and revision; sample collection and processing; formal analysis; data collection, curation, analysis and interpretation.
S. Majarune: Study conceptualization and design; methodology; formal analysis; data collection, curation, analysis and interpretation; manuscript writing and revision.
W. Maitreejet: Methodology; formal analysis; data collection.
C. Borirak: Data collection.
T. Rongthong: Data collection.
A. Khongpradit: Data collection, curation and analysis.
S. Sawanon: Resources and supervision
P. Boonsaen: Study conceptualization and design; methodology; formal analysis; results; interpretation supervision; project administration; fund acquisition; writing -review and editing the article; critical revision.
All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
Abouzaripour, M., Fathi, F., Daneshi, E., Mortezaee, K., Rezaie, M.J., and Abdi, M. 2018. Combined effect of retinoic acid and basic fibroblast growth factor on maturation of mouse oocyte and subsequent fertilization and development. International Journal of Fertility and Sterility. 12(1): 68.
Alila, H., and Hansel, W. 1984. Origin of different cell types in the bovine corpus luteum as characterized by specific monoclonal antibodies. Biology of Reproduction. 31(5): 1015–1025.
Anderson, K.J., Lefever, D.G., Brinks, J.S., and Odde, K. 1991. The use of reproductive tract scoring in beef heifers. Agricultural Practice. 12: 19–26.
Arikan, Ş., and Rodway, R.G. 2001. Seasonal variation in bovine luteal concentrations of β-carotene. Journal of Veterinary and Animal Sciences. 25: 165–168.
AOAC. 2016. Official methods of the Association of official analytical chemists. Association of Official Analytical Chemists, Washington DC, USA.
Bó, G.A., and Baruselli, P.S. 2014. Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal. 1(Supplement 8): 144–150.
Brown, J., Walker, S., and Steinman, K. 2005. Endocrine manual for reproductive assessment of domestic and non-domestic species: Types of Enzyme immune assays. (pp.12-15). Smithsonian National Zoological Park, Conservation and Research Center. Washington, DC, USA.
Brown, J.A., Eberhardt, D.M., Schrick, F.N., Roberts, M.P., and Godkin, J.D. 2003. Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary. Molecular Reproduction and Development. 64(3): 261–269.
Bureau of Biotechnology in Livestock Production. 2013. Operational manual for bovine embryo transfer procedures. Department of Livestock Development, Thailand.
Cho, S.-R., Kim, U., Kumar, K., Lee, S.-H., Lee, M.-S., Kim, H.-S., Lee, H.-J., and Yang, B.-C. 2014. The effects of vitamin A administration to heifer and pregnant cow on the changes of hormonal and body weight. Journal of Embryo Transfer. 29: 327–331.
Clagett-Dame, M., and Knutson, D. 2011. Vitamin A in reproduction and development. Nutrients. 3(4): 385–428.
Edmonson, A., Lean, I., Weaver, L., Farver, T., and Webster, G. 1989. A body condition scoring chart for Holstein dairy cows. Journal of Dairy Science. 72(1): 68–78.
Gábor, G., Toth, F., and Mezes, M. 2004. Preliminary comparison of luteal cavity size with some serum metabolic parameters in dairy cows. 37th Annual Meeting of Society for the Study of Reproduction. University of British Columbia, Vancouver, Canada.
Ganguly, C., and Mukherjee, K. 1988. Relationship between maternal serum vitamin A and vitamin A status of the corresponding fetuses. Journal of Tropical Pediatrics. 34(6): 313–315.
Gouvêa, V.N., Colli, M.H.A., Junior, W.A.G., Motta, J.C.L, Acedo, T.S., Vasconcellos G. S.F.M., Tamassia, L.F.M., Elliff, F.M., Mingoti, R.D., and Baruselli, P.S. 2018. The combination of β-carotene and vitamins improve the pregnancy rate at first fixed-time artificial insemination in grazing beef cows. Livestock Science. 217: 30–36.
Grygar, I., Kudláč, E., Doležel, R., and Nedbálková, J. 1997. Volume of luteal tissue and concentration of serum progesterone in cows bearing homogeneous corpus luteum or corpus luteum with cavity. Animal Reproduction Science. 49(2-3): 77–82.
Gupta, H.C. 1962. Biochemical and physiological properties of the cervical and uterine fluids of the cow during estrus." LSU Historical Dissertations and Theses. 721. https://repository.lsu.edu/gradschool_disstheses/721
Hafez, E.S.E, Jainudeen, M.R and Rosnina, Y. Hormones, Growth Factors, and Reproduction. In Reproduction in Farm Animals (pp. 31–54). Baltimore, Maryland, USA: Lippincott Williams & Wilkins. Philadelphia, USA.
Halasz, M., and Szekeres-Bartho, J. 2013. The role of progesterone in implantation and trophoblast invasion. Journal of Reproductive Immunology. 97(1): 43–50.
Haliloglu, S., Baspinar, N., Serpek, B., Erdem, H., and Bulut, Z. 2002. Vitamin A and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle. Reproduction in Domestic Animals. 37(2): 96–99.
Ikeda, S., Kitagawa, M., Imai, H., and Yamada, M. 2005. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. Journal of Reproduction and Development. 51(1): 23–35.
Jaśkowski, B. 2019. Corpus luteum with a cavity in cattle: An overview of past and present knowledge. Medycyna Weterynaryjna. 75(6): 340–346.
Jaśkowski, B.M., Bostedt, H., Gehrke, M., and Jaśkowski, J.M. 2021. Ultrasound characteristics of the cavitary corpus luteum after oestrus synchronization in heifers in relation to the results of embryo transfer. Animals. 11(6): 1706.
Kanďár, R., Drábková, P., Myslíková, K.,and Hampl, R. 2014. Determination of retinol and α-tocopherol in human seminal plasma using an HPLC with UV detection. Andrologia. 46(5): 472–478.
Kastelic, J., Bergfelt, D., and Ginther, O. 1990. Relationship between ultrasonic assessment of the corpus luteum and plasma progesterone concentration in heifers. Theriogenology. 33(6): 1269–1278.
Katamoto, H., Yamada, Y., Nishizaki, S., and Hashimoto, T. 2003. Seasonal changes in serum vitamin A, vitamin E and beta-carotene concentrations in Japanese black breeding cattle in Hyogo prefecture. Journal of Veterinary Medical Science. 65(9): 1001–1002.
Kawashima, C., Nagashima, S., Sawada, K., Schweigert, F.J., Miyamoto, A., and Kida, K. 2010. Effect of β-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows. Reproductive in Domestic Animals. 45: e282–e287.
Lee, T.C., Miller, W.L., and Auchus, R.J. 1999. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. Journal of Clinical Endocrinology and Metabolism. 84(6): 2104–2110.
Manna, P.R., Stetson, C.L., Daugherty, C., Shimizu, I., Syapin, P.J., Garrel, G., Cohen-Tannoudji, J., Huhtaniemi, I., Slominski, A.T., Pruitt, K., and Stocco, D.M. 2015. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mechanisms of Ageing and Development. 150: 74–82.
Mebratu, B. 2020. Embryo transfer in cattle production and its principle and applications. International Journal of Pharmacy and Biomedical Research. 7: 40–54.
Mitsuishi, H., and Yayota, M. 2024. The efficacy of β-carotene in cow reproduction: A review. Animals. 14(14): 2133.
Muhaidat, N., Karam, A.M., Nabhan, M.S., Dabbah, T., Odeh, B., Eid, M., Almahallawi, N.J., and Alshrouf, M.A. 2023. Factors affecting the outcomes of first in vitro fertilization and embryo transfer: A retrospective investigation. International Journal of Women's Health. 15: 1537–1545.
Munzuroglu, O., Karatas, F., and Geckil, H. 2003. The vitamin and selenium contents of apricot fruit of different varieties cultivated in different geographical regions. Food Chemistry. 83(2): 205–212.
National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle, 8th ed.; Subcommittee on Beef Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture; National Academy Press: Washington, DC, USA.
Pasquariello, R., Anipchenko, P., Pennarossa, G., Crociati, M., Zerani, M., Brevini, T.A., Gandolfi, F., and Maranesi, M. 2022. Carotenoids in female and male reproduction. Phytochemistry. 204: 113459.
Perez‐Marin, C. 2009. Formation of corpora lutea and central luteal cavities and their relationship with plasma progesterone levels and other metabolic parameters in dairy cattle. Reproduction in Domestic Animals. 44(3): 384–389.
Raila, J., Kawashima, C., Sauerwein, H., Hülsmann, N., Knorr, C., Myamoto, A., and Schweigert, F.J. 2017. Validation of blood vitamin A concentrations in cattle: Comparison of a new cow-side test (icheck™ fluoro) with high-performance liquid chromatography (HPLC). BioMed Central Veterinary Research. 13(1): 126 (2017).
Robinson, R.S., Hammond, A.J., Nicklin, L.T., Schams, D., Mann, G.E., and Hunter, M.G. 2006. Endocrine and cellular characteristics of corpora lutea from cows with a delayed post-ovulatory progesterone rise. Domestic Animal Endocrinology. 31(2): 154–172.
Rode, L., McAllister, T., and Cheng, K.J. 1990. Microbial degradation of vitamin A in rumen fluid from steers fed concentrate, hay or straw diets. Canadian Journal of Animal Science. 70: 227–233.
SAS Inst. Inc. Ondemand for Academic. 2023. Sas ondemand for academic. Ondemand for Academic ed. Cary, NC, USA.: SAS Institute Publication.
Sawanon, S., Boonsaen, P., and Innuruk, P. 2011. Body measurements of male Kamphaengsaen beef cattle as parameters for estimation of live weight. Kasetsart Journal - Natural Science. 45(3): 428–434.
Shaw, D., Farin, P., Washburn, S., and Britt, J. 1995. Effect of retinol palmitate on ovulation rate and embryo quality in superovulated cattle. Theriogenology. 44(1): 51–58.
Shuang, C., Meixia, C., Bangxin, X., Zhekun, Z., Xinyu, W., Jie, L., Huakai, W., Xiangzhou, Z., Shiyan, Q., and Xiangfang, Z. 2023. Retinoic acid enhances ovarian steroidogenesis by regulating granulosa cell proliferation and mesp2/star/cyp11a1 pathway. Journal of Advanced Research. 58: 163–173.
Sowcharoensuk, C. 2024. Industry outlook 2024-2026: Rice industry. [accessed 2024]. https://www.krungsri.com/en/research/industry/industry-outlook/agriculture/rice/io/io-rice-2024–2026.
Srisaikham, S., and Lounglawan, P. 2020. Utilization of sunnhemp meal in beef cattle diet supplemented with urea-treated rice straw. Chiang Mai University Journal of Natural Sciences. 19(4): 879–899.
Suwa, H., Kishi, H., Imai, F., Nakao, K., Hirakawa, T., and Minegishi, T. 2016. Retinoic acid enhances progesterone production via the camp/pka signaling pathway in immature rat granulosa cells. Biochemistry and Biophysics Reports. 8: 62–67.
Techakumphu, M., Sukavong, Y., Yienvisavakul, V., Buntaracha, B., Pharee, S., Intaramongkol, S., Apimeteetumrong, M., and Intaramongkol, J. 2001. The transfer of fresh and frozen embryos in an elite swamp buffalo herd. Journal of Veterinary Medical Science. 63(8): 849–852.
Tharnish, T.A., and Larson, L.L. 1992. Vitamin A supplementation of Holsteins at high concentrations: Progesterone and reproductive responses. Journal of Dairy Science. 75(9): 2375–2381.
Thongun, N., Unchuen, N., Boonsaen, P., and Buaphan, S. 2021. Effect of feeding program on growth performance of wagyu × Thai Holstein Friesian crossbred feedlots. Khon Kaen Agriculture Journal. 49(supplement 2): 698–707. (in Thai)
Vasconcelos, J.L.M., Sartori, R., Oliveira, H.N., Guenther, J.G., and Wiltbank, M.C. 2001. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 56(2): 307–314.
Velazquez, M.A. 2023. Nutritional strategies to promote bovine oocyte quality for in vitro embryo production: Do they really work?. Veterinary Sciences. 10(10): 604.
Vieira, L.M., Rodrigues, C.A., Mendanha, M.F., M.F., Sales, J.N.S., Souza, A.H., Santos, J.E.P., and Baruselli, P.S. 2014. Donor category and seasonal climate associated with embryo production and survival in multiple ovulation and embryo transfer programs in Holstein cattle. Theriogenology. 82(2): 204–212.
Wanapat, M., Polyorach, S., Boonnop, K., Mapato, C., and Cherdthong, A. 2009. Effects of treating rice straw with urea or urea and calcium hydroxide upon intake, digestibility, rumen fermentation and milk yield of dairy cows. Livestock Science. 125(2): 238–243.
Weiss, W.P. 1998. Requirements of fat-soluble vitamins for dairy cows: A review. Journal of Dairy Science. 81(9): 2493–2501.
Wickenheisser, J.K., Nelson-DeGrave, V.L., Hendricks, K.L., Legro, R.S., Strauss, J.F., 3rd, and McAllister, J.M. 2005. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 90(8): 4858–4865.
Wiltbank, M.C. 1994. Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. Journal of Animal Science. 72(7): 1873–1883.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Sangphet Leelachayakun1, 2, Sutisa Majarune1, Wisut Maitreejet1, Charkrit Borirak1, Tatphicha Rongthong1, Anchalee Khongpradit1, Suriya Sawanon1, and Phoompong Boonsaen1, *
1 Department of Animal Science, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Nakhon Pathom 73140, Thailand.
2 The Graduate School, Kasetsart University, Bangkok 10903, Thailand.
Corresponding author: Phoompong Boonsaen, E-mail: fagrppb@ku.ac.th
ORCID ID: Phoompong Boonsaen: https://orcid.org/0000-0003-1696-6136
Total Article Views
Editor: Tonapha Pusadee,
Chiang Mai University, Thailand
Article history:
Received: January 28, 2025;
Revised: April 3, 2025;
Accepted: April 22, 2025;
Online First: May 1, 2025

