
Potential Protective Effects of Silybum marianum Ethanolic Extract Against Thiobencarb-Induced Hepatotoxicity in White Albino Rats
Atef Mohamed Khedr Nassar*, Rasha Ahmed El-Naggar, Eman H. Radwan, Hafsa O. Hashem, Khaled Y. Abdel-Halim, Yehia M. Salim, and Amal Z. GhoneimPublished Date : April 25, 2025
DOI : https://doi.org/10.12982/NLSC.2025.038
Journal Issues : Number 3, July-September 2025
Abstract The seed extracts of milk thistle (Silybum marianum) are commonly known in traditional medicine as cleansers of the liver. However, its potency in preventing or reducing pesticide hepatotoxicity has not been investigated. Therefore, the current study aimed to evaluate the hepatoprotective effects of the ethanolic extract of seeds of S. marianum (ESM) against thiobencarb (TBC)-induced toxicity in males of white albino rats. The animals were grouped and orally received repetitive sublethal doses of TBC (1/20 LD50; 51.65 mg/kg b. w) and ESM (8 mg/kg b. w) for 28 days. Results showed that TBC increased the lipid profile (triglycerides, cholesterol, low-density lipoprotein, and high-density lipoprotein), antioxidant enzymes (catalase and glutathione reductase), and malonaldehyde and glutathione contents in serum samples. Also, the results of TBC treatment showed alterations in the histopathology of hepatocytes and positive expression of the apoptotic proteins including caspase-3 and proliferating cell nuclear antigen. In contrast, the ESM treatment counteracted the adverse effects of TBC in rats to control levels. This ameliorative action of ESM might be attributed to its role in enhancing the antioxidant scavenging activity and avoiding apoptosis of the hepatocytes.
Keywords: Thiobencarb, Short-term exposure, Milk thistle, Rats, Hepatotoxicity, Apoptosis
Citation: Nassar, A.M.K., El-Naggar, R.A., Radwan, E.H., Hashem, H.O., Abdel-Halim, K.Y., Salim, Y.M., and Ghoneim, A.Z. 2025. Potential protective effects of Silybum marianum ethanolic extract against thiobencarb-induced hepatotoxicity in white albino rats. Natural and Life Sciences Communications. 24(3): e2025038.
INTRODUCTION
Silybum marianum L Gaertn is classified as an herbaceous weed plant belonging to the family Compositae and native to the Mediterranean region (Tůmová et al., 2010). Milk thistle has been used for decades to treat hepatic and biliary problems. It was also employed as an antidote to the poisoning of Amantia mushroom and other toxic medications to the liver and kidney (El-Kamary et al., 2009). In Germany, it was recommended as a therapy for dyspeptic symptoms toxicity-induced harm to the liver, hepatic alcoholic liver disease, and supportive treatment for chronic inflammation liver diseases(Tamayo and Diamond 2007). The seed powder extract of S. marianum was suggested as a beneficial treatment of several liver illnesses (degenerative necrosis and functional impairment) and provided hepatoprotection against glucosamine, thioacetamide, halothane, and carbon tetrachloride intoxication (Luper, 1998; Tamayo and Diamond 2007; Kshirsagar et al., 2009). Additionally, it shields hepatocytes from ischemia, radiation, iron toxicity, iron excess, and viral hepatitis (Shaker et al., 2011). Moreover, silymarin, isolated from S. marianum, is utilized in healthcare as a new therapy that protects the liver (Elmowafy et al., 2013; Cordero-Pérez et al., 2013; Raj and Gothandam, 2014).
Milk thistle’s protective effectiveness is determined by its antioxidant capacity, promotion of protein synthesis, effect on lipid metabolism, and stabilization of the membrane phospholipid (Rašković et al., 2011). Furthermore, the seed extracts of S. marianum (ESM) are formed of various flavonolignan isomers (65-80%), with smaller levels of flavonoids and fatty acids (20-35%). Further, it is a mixture of five main compounds: silybinin, isosilychristin, silydianin, and taxifolin (Lee and Liu, 2003; Polyak et al., 2010).
On the other hand, herbicide usage is essential to control weeds and significantly help increase agricultural production (Qu et al., 2021). In addition to their known or suspected carcinogenic or mutagenic effects, carbamate herbicides have shown cumulative effects on the human body that might cause neurological, hormonal, and reproductive complications (Almeida and De Souza, 2023). The herbicide thiobencarb (TBC) kills weeds by inhibiting the production of extremely long-chain fatty acids, which suppress the growth of broadleaf weeds (An et al., 2022). It is used to destroy weeds in rice farming in Egypt and other nations where it kills weeds by preventing cellular division in seedlings (Abba et al., 2007). Although Gramineous weeds are the main target of TBC, but it can be hazardous to non-target species both acutely and chronically (Wang et al., 2021). TBC substance is categorized as low toxic (acute) in humans (Group D; according to EPA classification of toxicity hazard) (USEPA, 1997).TBC residues were detected in tap and rice field water, which accentuates the urgent need to remove them from affected areas (Duc et al., 2023). The TBC is stable for hydrolysis and anaerobic aquatic metabolism (Shimasaki et al., 2013; USEPA, 2000). Additionally, exposure to this herbicide was reported to cause oxidative stress, immunomodulation, disruption of reproductive systems, lower fertility, abnormal abortions, birth defects, and histopathological disorders (Mecdad et al., 2011). Herbicides induce oxidative stress by producing free radicals that cause DNA and cell damage (Al Basher et al., 2020; Nassar et al., 2021; Abuzeid et al., 2022). According to Muniz et al. (2008), oxidative stress causes an imbalance in the amounts of antioxidant defense mechanisms and inflammatory mediators, resulting in programmed cell death (apoptosis) (Abdel-Daim and Abdeen, 2018; Nassar et al., 2021).
However, prior studies showed severe unfavorable effects of TBC and its breakdown products on cultured rats’ hepatocytes (Jinno et al., 1997) and induced oxidative stress in fish, Gambusia affinis (Abdel-Halim and Massoud, 2014; Abdel-Halim et al., 2016). After administrating 44.78 mg/kg of TBC orally three days a week for six weeks, male Wister rats experienced significant reductions in testicular malondialdehyde (MDA), serum testosterone, and testicular total antioxidant capacity (Ahmed, 2017). To the best of our knowledge, information on the protective activity of ESM against herbicide toxicity in mammals is scarce. Therefore, the current study aimed to assess the protective role of ESM against the hepatotoxicity of TBC in male rats following a 28-day exposure.
MATERIAL AND METHODS
Chemicals
Chemicals and reagents J.T. Baker Chemical Co., Philipsburg, N.J. 08805 USA, provided the following chemicals: sodium chloride (NaCl; No. 1-3624), potassium phosphate mono (1-3251)/dibase (1-3252). Hydrochloric acid (HCl; No 56333) was supplied by SDFCL Chem-Limited 315-317, T.V. Industrial Estate, 248, Worli Road, Mumbai-30-India. Trichloroacetic acid (TCA; UN No.: 1839), and sodium azide (NaN3; FW 65.01) were provided by LOBA Chemie, pvt, Ltd, 107, Wodehouse Road, Mumbai, 400005, India. BDH Chemical Ltd Poole, England provided ethylene diamine tetraacetic acid (EDTA; product No. 28025). Methanol (P09337G21) was provided by Carlo Erba Reactifs-SDS Chaussee du Vexin-BP616 F-27106 Valde Reuil. El-Gamhouria Co. for pharmaceuticals and chemicals (Cairo, Egypt) provided maize oil and hydrogen peroxide (H2O2 50%; CAS No. 7733-19-6). Thiobarbituric acid (TBA) (C4H4N2O2S; FW 144.15) (Product No 91584-25G) was provided by ADVENT CHEMBIO PVT LTD®, Mumbai-400701, India. Sigma Chemical Co. (St. Louis, Mo 63178 USA) provided the isoflurane (1,1,1-isoflurane-trifloro-ethane) (C3H2CIF5O; FW 184.5) (247-897-7), 5, 5’-dithiobis-2-nitrobenzoic acid (DTNB; 69-78-3) (C14H8N2O8S2; FW 396.3), haematoxylin (C16H14O6; FW 302.28) (208-237-3), eosin (56360-46-4) (C20H8N2O2Br2; FW 580.1), xylene (95-47-6), and tris-HCl (77-86-1) (C4H11NO3; FW 121.1). The supplier of 96% a.i. thiobencarb (TBC) was Kafr El-Zayat Pesticides and Chemicals Co. in Egypt.
Ethanol extract of S. marianum preparation
Dry seeds of S. marianum were collected during the Spring season (in May) of 2022. In a shaking water bath, the fine seed powder of ESM was extracted overnight in absolute ethanol (Sigma-Aldrich) with continuous agitation at 250 rpm. Ethanol was evaporated at 35°C using a rotary evaporator to a soft yellow slurry (Serçe et al., 2016). Then the ethanol extract was kept at -20°C until dissolved in saline solution for rat administration. Additionally, about 0.1 g of dried extract was redissolved in methanol for the GC-MS analysis.
Gas chromatography-mass spectrometry (GC-MS) analysis
The chemical composition of ESM was conducted using a GC 1310-ISQ mass spectrometer (Thermo Scientific, USA) with a 30 m × 0.25 mm × 0.25 μm film thickness capillary column (HP–5MS). The injector temperature was programmed at 270°C. Helium was used as a carrier gas at a constant flow rate. The column oven temperature was held at 55°C, increased by 5°C/min to 200°C and held for 2 min, and then increased to 300 at 15 °C/min and held for 3 min. The spectral results were collected at 70 eV ionization voltages from 50–600 m/z in full scan mode. The ion source and transfer line temperatures were set at 200 and 250°C, respectively. The phytochemical constituents were identified by their retaining times and mass spectra with those of WILEY 09 and NIST 11 (National Institute of Standards and Technology, Gaithersburg, MD) Mass Spectral databases.
Animals and accommodation
The care of laboratory animals was carried out following the NRC’s (2013) suggested guidelines for this study. Male Rattus norvergicus Wistar rats, weighing 150 ± 10 g at 8-10 weeks of age, were acquired from the Animal House at Alexandria University in Egypt. The animals were housed individually in wire mesh cages made of stainless steel with clean wood husk bedding during the study period. The cages were cleaned and changed every 3 days. During the adaptation period, the animals were kept in cages as groups. The animals were kept under 22 ± 4 °C, 55 ± 15 % RH, artificial illumination cycle set to 12 hours L/D, and the air exchange rate at 10 times per hour. During the pre-test period (one week), animals were given Ground Purina Laboratory Chow (GPLC) and observed concerning weight gain, eating habits, and any clinical signs of disease. Tap water in polyethylene bottles with a capacity of about 300 ml (700 ml during the adaptation period), was available without restriction. The animal care ethics procedure (No. DUFA-2024-12) was approved by the Faculty of Agriculture Institutional Animal Care and Use Committee at Damanhour University.
Experimental design
The study was carried out following the regulatory requirements recommended by the OECD (2008). Five experimental groups (five rats each) were randomly assigned to the animals. The following oral medications were given to the rats: G1, the NC (normal control) group, got 1 ml/100 g b. w. of saline solution; G2, the group receiving the positive control (PC) received 1ml/100 g b. w. of maize oil and G3, the group receiving the ESM treatment, got ESM (8 mg/100 g b. w.) (National Toxicology Program, 2011). The G4, receiving 51.65 mg/kg b. w. (equal 1/20 LD50) of TBC and G5, the rats were administered both TBC and ESM at the previously described doses. The animals were checked twice a day for clinical symptoms and death. The entire clinical assessment was carried out. The animals fasted overnight (for about 17 hrs) on the last day before the introduction of anesthesia. All rats were given isoflurane (inhalation method) anesthesia at the end of the experiment and used for gross necropsy examinations. After 24 hrs of the last dose (on the thirties day), the rats were slaughtered, liver tissues were removed for biochemical quantifications and blood samples were taken from the abdominal aorta for blood biochemistry studies.
Lipid profile assessment
High-density lipoprotein (HDL), triglycerides (TG), and total cholesterol (TC) were measured using the kits bought from Biodiagnostic Co. for diagnostics and research reagents, Egypt, following the techniques of Young (2001), Stein (1987), and Lopes et al. (1977) (www.Biodiagnostic.com). A method of calculating low-density lipoprotein (LDL) was developed by Friedewald et al. (1972).
Oxidative stress enzymes
Catalase (CAT)
Based on a decrease in absorbance at 240 nm that occurs when hydrogen peroxide (H2O2) is consumed, catalase (CAT) activity was determined (Beers and Sizer, 1952). One milliliter (12.5 mM H2O2) (substrate), two milliliters (66.7 mM phosphate buffer, pH 7.0), and two milliliters of enzyme source made up the reaction mixture. The unit of CAT is the quantity of enzyme that releases half of the peroxide oxygen from an H2O2 solution at 25°C, and this unit of measurement is used to express the activity as U/mg protein.
Malondialdehyde (MDA) content
The thiobarbituric acid reactive substances (TBARS) approach was used to perform spectrophotometric determination of malondialdehyde (MDA) level in tissue homogenate (Rice-Evans et al., 1991). An aliquot (250 µl) of homogenate was combined with 2 ml of thiobarbituric acid (TBA; 0.37%), 1 ml of 15% (w/v) trichloroacetic acid (TCA) in 25 mM HCl. After 10 min of boiling, the mixture was rapidly cooled and centrifuged for 5 min at 5000 rpm. At 535 nm, the developing color was measured. The MDA level was calculated as nM/g of tissue using an extinction coefficient of 156 mM-1.
Reduced glutathione (GSH)
A yellow composite, measured at 405 nm, is produced when 5, 5’-dithiobis-2-nitrobenzoic acid (DTNB) is reduced with reduced glutathione (GSH) (Beutler et al., 1963). Precisely, 500 µl of the enzyme source was combined with the same volume of 500 mM TCA, and the mixture was centrifuged for 15 min at 3000 rpm. 500 µl of the supernatant was thoroughly combined with 1 ml of DTNB (1 mM) and 1 ml of phosphate-buffered saline (PBS; 100 mM, pH 7.4). The absorbance was measured at 405 nm compared to the blank after 10 min. The protein expression for GSH was nM/mg.
Glutathione peroxidase (GPx)
The method used to measure GPx enzyme activity was that of Flohe and Gunzler (1984). The enzyme was thoroughly mixed with the phosphate buffer solution (100 mM, pH 7.0), EDTA (50 mM), sodium azide (250 mM), and H2O2 (10 mM). After that, for 40 seconds at 340 nm, the absorbance change was measured every 3 seconds. The activity was reported as mU GPx/mg protein, where a GPx unit is the quantity of enzyme needed to oxidize one microgram of NADPH per minute.
Histopathological examination
The liver tissues were separated, embedded in paraffin wax at (56-58 °C), dehydrated, cleaned, and sectioned at 4 µm using a rotary microtome after being instantly fixed in 10% formalin for 24-48 hrs at room temperature. Hematoxylin and eosin (H & E) stains were used on the sections according to Martinez et al. (2014). To identify any tissue damage, the slides were inspected under a light microscope and microphotographed (De Jesus et al., 2016).
Immunohistochemical expression of caspase-3 and PCNA proteins
The expression patterns of proliferating cell nuclear antigen (PCNA) protein and caspase-3 in liver tissues were measured immunohistochemically using the peroxidase technique (Karen et al., 2002). Formalin-preserved tissues were fixed in paraffin and sectioned into four-millimeter pieces on glass slides coated with poly L-lysine. The slides were immersed in xylene three times, each lasted 5 minutes. Then rehydrated using graded alcohol solutions ranging from 70 to 100%, followed by 30 minutes under tap water. Endogenous peroxidase activity was suppressed by 0.3% H2O2 in methanol for 20 minutes of incubation. After a 5-minute TBS wash and 1-hour treatment with normal blocking serum to prevent non-specific binding sites, the sections were incubated for an additional night with the primary polyclonal antibodies proteins at a dilution of 1:150 (Thermo-scientific). Following a 5-minute TBS wash, the primary antibody enhancer was applied to the slides, and they were then left to incubate for ten minutes at room temperature. After that, it underwent four buffer rinses before being exposed to the secondary antibody conjugated to horseradish peroxidase (HRP) for fifteen minutes at room temperature. They were cleaned as previously mentioned, allowed to develop color in a solution containing peroxidase substrate (a chromogen compatible with peroxidase), and then rinsed four times with deionized water. In order to prepare the slides for light microscopy examination, they were cover-slipped with an aqueous mounting solution and counter-stained with hematoxylin (Jha et al., 2017). Using the Image J software, the positive immune-histochemistry stain for caspases-3 and PCNA proteins was assessed, and the color intensity was estimated.
Statistical analysis
The statistical analysis system (SAS ver. 9.3) was employed to statistically analyze the results. Tukey’s multiple comparison post-hoc test was used to compare the significant means of treatments at a probability level of 0.05 (SAS, 2016). Furthermore, the relevance of the data retrieved from the photos was assessed at the 0.01 and 0.05 probability levels using the least significant difference (LSD) comparison method.
RESULTS
The therapeutic effects of ESM against TBC toxicity in male rats were studied after 28 days of oral administration. Results indicated no observed mortality or toxicological signs on tested animals. Except for TBC-treated rats, which showed a considerable rise in liver weight following scarification of the treated animals, the weight of organs in the treated groups did not differ significantly from the control group. The gross necropsy examination indicated normal morphological patterns in the treated rats, except for the TBC group where morphological defects in the liver and accumulation of extensive lipid vesicles were observed.
Lipid profiles
Alterations in serum lipid profiles were attributed to stress from tested chemicals that might cause injury to liver cells. As illustrated in Figure 1, TBC-treated rats showed the greatest cholesterol level (88.80 mg/dL), with respect to the NC group (56.36 mg/dL). The TBC+ESM-treated rats exhibited the least cholesterol content (49.17 mg/dL), followed by ESM (59.56 mg/dL), and were similar to the NC and PC groups of rats (Figure 1A).
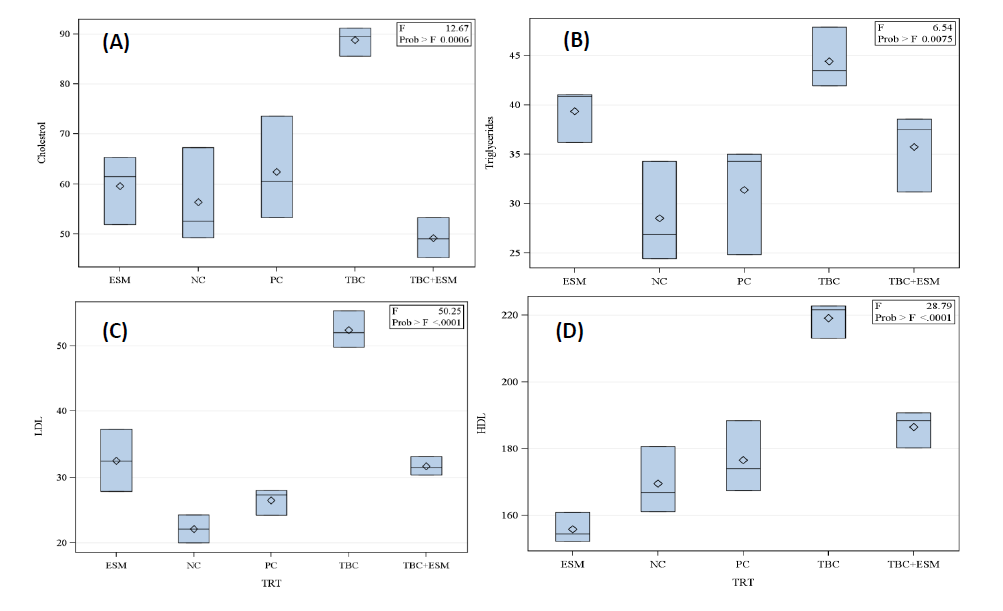
Figure 1. Lipid profiles in serum samples of male albino rats treated with the herbicide, thiobencarb (TBC), ethanol extract of S. marianum (ESM), TBC+ESM, positive (PC), and negative (NC)controls for 28-days orally. [A] cholesterol (mg/dL), [B] triglycerides (mg/dL), [C] LDL (mg/dL), and [D] HDL (mg/dL). Each block represents the mean ± SE at a 5% probability level.
Regarding the triglycerides, TBC-treated rats had the greatest content (44.44 mg/dL) compared to 28.52 mg/dL in samples of the NC group. No significant differences were reported between other treatments, where triglyceride levels ranged from 31.37 to 39.36 mg/dL (Figure 1B). A significant increase in the level of LDL (52.38 mg/dL) was reported in the TBC-treated group compared to the ESM-treated rats (32.53mg/dL). The ESM, TBC+ESM, and PC groups did not statistically differ from one another however, they did significantly differ from the NC rats (Figure 1C). The TBC-treated rats had significantly greater HDL value (219.15 mg/dL) compared with the TBC+ESM (186.49 mg/dL), PC (176.62 mg/dL), NC (169.58 mg/dL), and ESM (155.91 mg/dL). The lowest value in HDL (155.91 mg/dL) was reported in serum samples of the ESM-treated rats, but it was no different from the NC rats (Figure 1D).
Oxidative stress
As shown in Figure 2, the ESM treatment markedly counteracted the elevation in CAT enzyme activity caused by TBC treatment. The CAT activity was 5.14 U/mg protein in samples of TBC rats and was significantly different from all other tested groups. The CAT activities were 3.52, 3.33, 3.08, and 2.72 U/mg protein in rats of PC, TBC+ESM, NC, and ESM groups, respectively, and were not different statistically (Figure 2A). Similarly, the TBC-treated rats remarkedly elevated the GSH content to 1.83 nM/mg protein compared to ESM, TBC+ESM, NC, and PC treatments (Figure 2B). No statistical differences were reported between ESM, TBC+ESM, NC, and PC groups of rats in the GSH activities of 0.116, 0.065, 0.052, and 0.040 nM/mg protein, respectively. TBC+ESM and ESM treatments showed GSH activities similar to negative (NC) and positive (PC) control treatments.
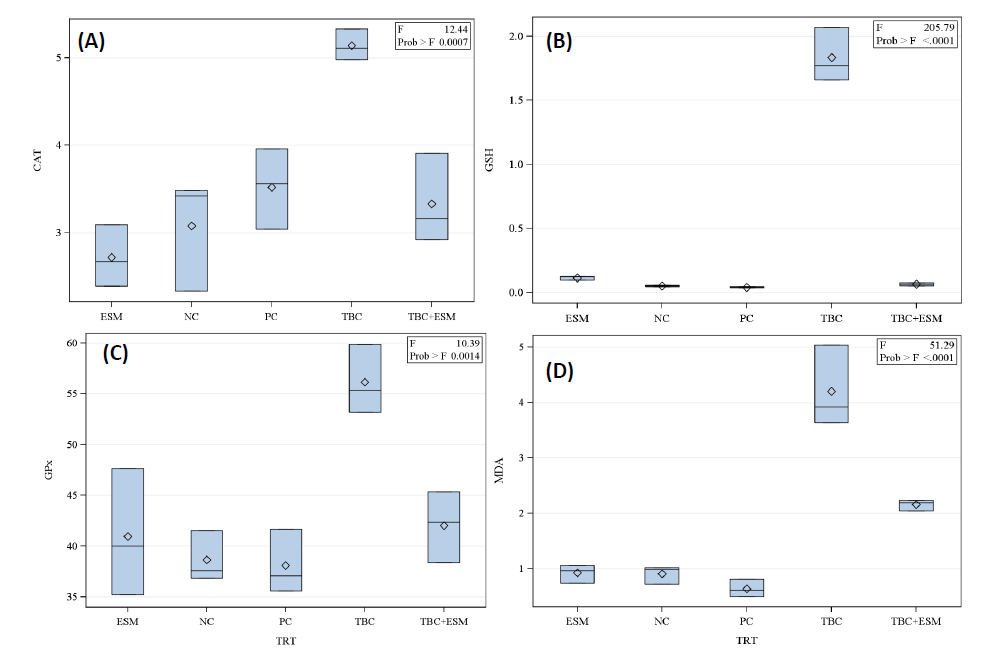
Figure 2. Antioxidative stress enzyme activities of (A) CAT (U/mg protein), (B) GSH (nM/mg protein), (C) GPx (mU/mg protein), and (D) MDA (nM/g tissue) level in the serum samples of male albino rats orally administered sublethal doses of thiobencarb (TBC), ethanol extract of S. marianum (ESM), TBC+ESM, positive (PC), and negative (NC) controls for 28-days. Data presented as mean ±SE at a 5% probability level, n=5.
Also, results presented in Figure 2C showed that TBC treatment significantly increased the GPx activity (56.14 mU/mg protein) compared to other tested treatments. The ESM treatment reversed the increase in the GPx activity to the control levels. About MDA, a remarkable increase (4.20 nM/g tissue) was induced by TBC treatment, followed by TBC+ESM (2.16 nM/g tissue) compared to negative control (0.910 nM/g tissue) and ESM treatment (0.924 nM/g tissue)(Figure 2D).
Milk thistle extract alleviates histological defects in rats
Hepatic histopathological changes indicate the extent of liver injury directly, and routine H & E staining is used to evaluate hepatocyte modifications. As demonstrated in Figure 3A, NC treatment showed normal histological architecture of the liver containing parenchyma (P), central vein (V), and homogenous distribution of nucleus (N). In comparison with the NC group, vacuolated cytoplasm, proliferating blood vessels, ballooning degeneration of hepatocytes, and enlargement of the nucleus (N) appeared in the TBC-treated tissues (Figure 3B). In the case of ESM treatment, liver parenchyma (P), and central vein (V) appeared. Moreover, less vacuolated cytoplasm and numerous sizes of nucleus (N) were noted (Figure 3C). In vehicle treatment (PC), no significant changes were noted compared with the NC group (Figure 3D). However, ESM pretreatment of TBC administration alleviated liver injury as recovered cellular nuclei, not swollen hepatocytes, regular central vein (V), and homogenous nucleus (N) size (Figure 3E).

Figure 3. Photomicrographs of (A) normal histological architecture of liver from control rats, section showed liver parenchyma (P), and homogenous size of nucleus (N), (B) TBC treatment showed vacuolated cytoplasm (stripped right arrow), proliferating blood vessels (yellow arrow), ballooning degeneration of hepatocytes (double arrow), enlargement of the nucleus (N), and in some cases, double nucleus (N*), [C] ESM-treated rats showed liver parenchyma (P), central vein (V), fewer vacuolated cytoplasm, and numerous size of the nucleus (N), [D] maize oil treatment revealed liver parenchyma (P), central vein (V), and homogenous distribution of nucleus (N) (arrow), and [E] TBC+ESM for 28-days of oral gavage showed recovered cellular nuclei, regular central vein, less hepatocellular damage, and homogenous of nucleus size, H&E 40X.
Milk thistle extract reduces expressed apoptosis proteins
The caspase-3 protein was infrequently expressed only in the cells of NC samples of liver tissues (Figure 4A). Rats treated with TBC showed an increased expression pattern of this protein (Figure 4B). At such expression, the mean surface area was 82.95 µm2, which is 30.07 times greater than the mean surface area of 2.89 µm2 of the NC rats (Figure 5A). Moreover, this area showed an integrated density (mean; 679.42) of 11-folds of the NC group (61.71) (Figure 5B). In the case of TBC administration in concomitant with ESM, a decline in the expressed caspase-3 protein was noticed compared with the TBC administration alone (Figure 4C). Such decline indicated a surface area of 32.22 µm2 which was 11.88-folds of the negative control and an integrated density of 111.74 (about 1.81-folds of the PC group). The ESM induced a significant decline in the expressed caspase-3 protein (Figure 4D), showing a mean surface area (26.72 µm2) and an integrated density of 81.45 which was statistically different compared to the PC and NC groups. Rats given the vehicle treatment showed weakly expressed caspase-3 protein (Figure 5A) and an integrated density of 61.05 (Figure 5B).
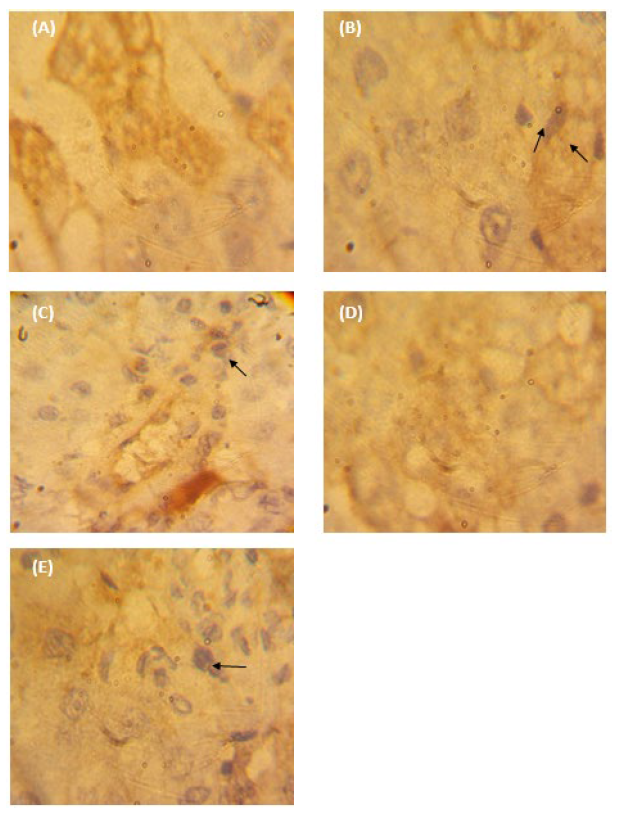
Figure 4. Photographs of paraffin-embedded sections through (A) normal control (NC) liver tissues, (B) TBC-treated rats, (C) TBC+ESM-treated rats, (D) ESM-treated rats, and (E) vehicle-treated rats (corn oil) for 28 days. It illustrated the immuno-stained with caspase-3 antibody and counterstained with hematoxylin. The strong positive expression was obtained with brown color (arrow) [40X].
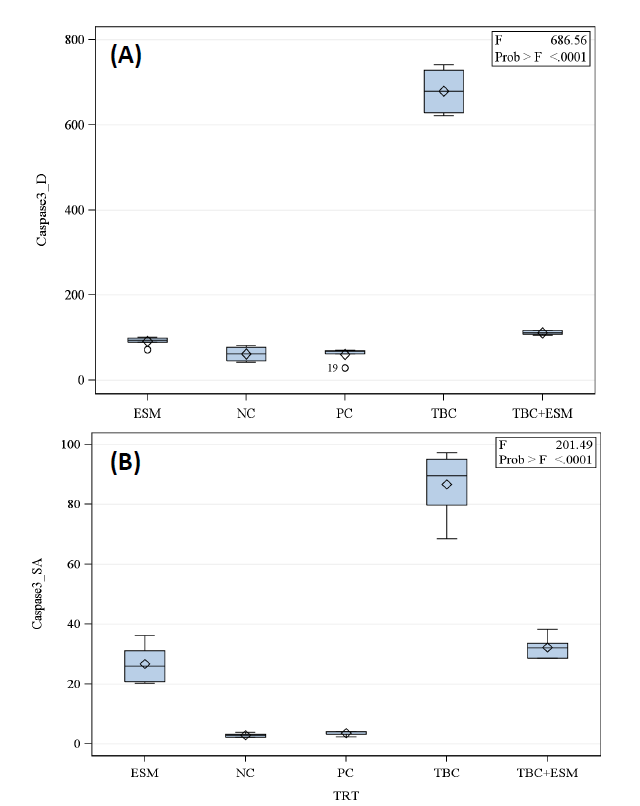
Figure 5. Boxplot of mean ± SE of (A) integrated density and (B) surface area (μm2) of hepato-sections of albino rats for expressed caspase-3 protein in TBC-treated rats in concurrent with the ethanolic extract of S. marianum (TBC+ESM) (orally; 8 mg/100 g b. w) for 28-days. The significant differences were estimated at a 5% probability level according to the Tukey post-hoc test.
Regarding the expressed PCNA protein, weak expressions were found in tissue sections of the NC group (Figure 6A). Rats treated with TBC had the liver tissues with the highest PCNA expression pattern (Figure 6B), with an integrated density of 478.55 (8.15-folds of the NC group) and a mean surface area of 69.27 µm2, which is about 125.95-times that of the NC group but does not exceed 0.549 µm2 (Figure 7B). A significant decline in the expressed protein was noticed in TBC+ESM-treated tissue (Figure 6C), indicating a mean value of surface area of 22.23 µm2 (40.49-fold of NC), and integrated density of 113.15 (1.93-fold of NC). A marked decline in the expressed protein was noticed in ESM-treated rats (Figure 6D), where the mean value of surface area declined to 14.18 µm2 (25.83-fold of NC), and an integrated density did not exceed 115.30 (1.96-fold of NC). No significant differences were obtained for vehicle-treated rats (Figure 6E), which showed a mean surface area of 0.412 µm2 and an integrated density of 69.06 (1.18-fold of NC).
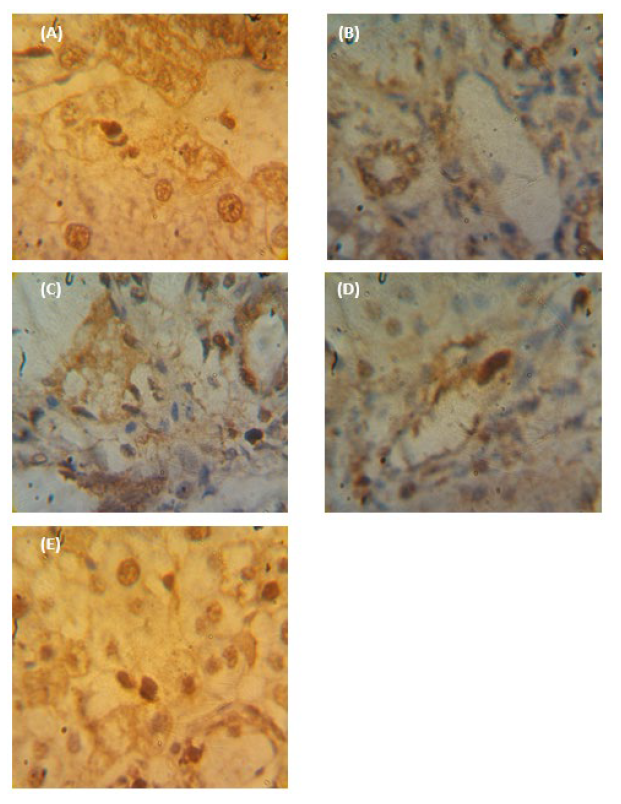
Figure 6. Photographs of paraffin-embedded sections through (A) normal control (NC) liver, (B) TBC-treated rats,(C) TBC-treated rats in concomitant with the ethanolic extract of S. marianum (TBC+ESM), (D) ESM-treated rats, and (E) vehicle-treated rats (corn oil) for 28 days illustrate immuno-stained with PCNA antibody and counterstained with hematoxylin. The strong positive expression was obtained with dark-brown color (arrow) [40X].
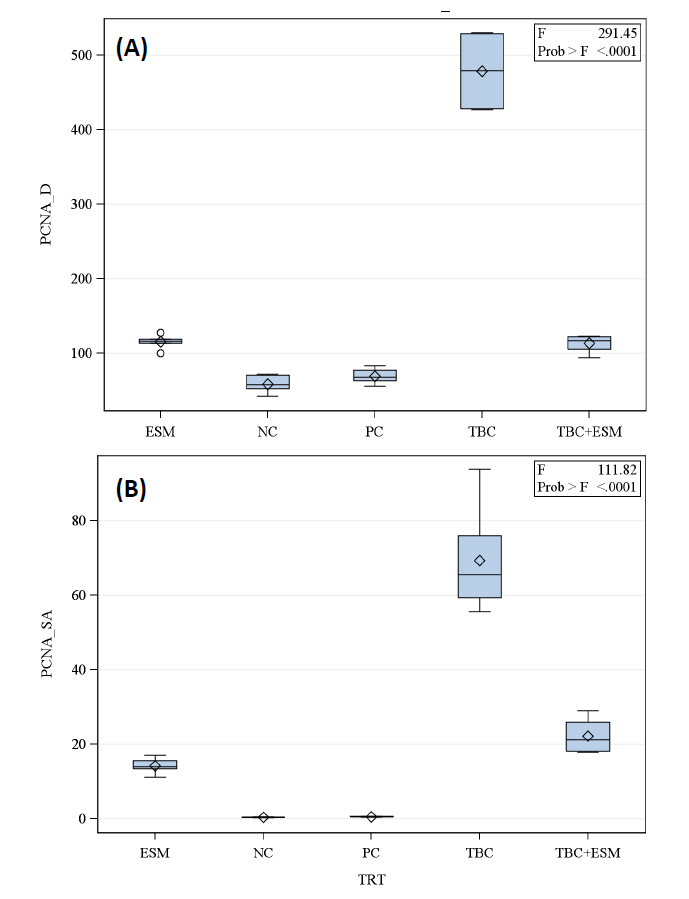
Figure 7. Boxplot of mean ± SE of (A) integrated density and (B) surface area (μm2) of hepato-sections of albino rats for expressed PCNA protein in TBC-treated rats in concurrent with the ethanolic extract of S. marianum (TBC+ESM) (orally; 8 mg/100 g b. w) for 28-days. The significant differences were estimated at a 5% probability level according to the Tukey post-hoc test.
DISCUSSION
The liver is an essential organ for chemical metabolism and the elimination of toxins from the human body (Merrell and Cherrington, 2011). The present study findings showed a protective role of ESM against hepatotoxic sublethal toxicity of the herbicide, TBC. Particularly, it was reported that S. marianum either the whole ground seeds or extracts were used for several years as a liver cleaner and natural hepatoprotective agent against hepatic disorders (Wellington and Jarvis, 2001; Abenavoli et al., 2010).
The current study adds to the literature supporting evidence that ESM could shield the liver against the herbicide (TBC)-induced damage. It ameliorates oxidative stress by elevating the antioxidant enzyme activities, minimizing LPO, and reducing apoptosis induction. Changes in histopathological features and serum biochemical measures serve as clear markers of the liver’s diseased state (Wang et al., 2018). Serum lipid profiles that are elevated following toxicant exposure include cholesterol, triglycerides, LDL, and HDL. These lipid profiles are discharged into the circulation from injured hepatocytes and are linked to severe liver dysfunction (Singh et al., 2016).
Consistent with earlier research, the current investigation demonstrated that repeated administration of sublethal (1/20 of the LD50) dosages of TBC caused serious liver impairment in rats, as seen by significantly higher blood levels of lipid profiles when compared to the NC group (Xing et al., 2015; Ali et al., 2016). Additionally, the results reported in our study also indicated that ESM treatment significantly countered TBC-induced hepatic toxicity by improving hepatic functions. Moreover, the hepatic architecture was clearly damaged (histological investigation), which resulted in significant pathological abnormalities like vacuole formation, infiltration, and localized necrosis due to TBC treatment. On the other hand, the presence of degraded hepatic cells was significantly decreased by ESM treatment. The absence of inflammation and cellular necrosis in the hepato-sections demonstrated the protection provided by ESM.
The ESM was chosen for clinical experiments based on unpublished data where a comparison between different polarity solvents was conducted. The positive ameliorative effects of ESM against TBC hepatotoxicity were due to its richness in phytochemicals (Table 1). The 9, 12-Octadecadienoic acid (Z,Z), linoleic acid ethyl ester, n-hexadecanoic acid hexadecanoic acid ethyl ester, linoelaidic acid, 2-methyl hexadecanal, squalene, 10,13-octadecadienoic acid methyl, furfural, diglycerol, mequinol, trans-isoeugenol, Z,E-7,11-hexadecadien-1-yl acetate, and 1,11-tridecadiene. In general, these bioactive agents that have strong antioxidant capacity might be effective in preventing hepatotoxicity (Cao et al., 2016). By promoting scavenging oxygen free radicals and increasing hepatocyte regeneration, the ESM works as an antioxidant to lower the LPO (Saller et al., 2001; Zhang et al., 2013; Surai, 2015; Feng et al., 2016). Additionally, the flavonoid phenolic structure allows free radicals and ROS to be electronically donated, stabilizing them and preventing LPO by binding with intracellular GSH (Karimi et al., 2011). It was shown that ESM has immune-modulatory, anti-fibrotic, anti-proliferative, and anti-apoptotic qualities (Tsai et al., 2008; Sasu et al., 2015). It has been shown to suppress the expression of tumor necrosis factor-alpha (TNF-α) (Ahmad et al., 2013).
The ESM treatment significantly reduced the production of proliferating cell nuclear antigen proteins and caspase-3, which are both increased in TBC-induced liver damage. These results were verified by the immunohistochemistry results in Figures 5 and 7. The quantitative results of ESM showed fewer positive cells for the expressed proteins that might inhibit the mitochondrial-controlled apoptosis (Islam, 2017) as proposed mechanisms of ESM against TBC-induced hepatotoxicity. The primary source of ROS production is found in the complexes in the mitochondria, and numerous pesticides have been demonstrated to block these complexes (Sherer et al., 2007; Bhat et al., 2015). Endoplasmic reticulum (ER) stress and apoptosis are often linked to mitochondrial malfunction in fish and mammals (Malhotra and Kaufman, 2011; Yang et al., 2020).
Pesticides have been documented to make apoptosis by triggering several signaling pathways including intrinsic pathways linking to the mitochondria and DNA damage as well as extrinsic pathways such as modulation of death receptors (Green and Reed, 1998; Ashkenazi and Dixit, 1998). The present findings are in accordance with those obtained by Wang et al. (2018), where pre-treatment of rats with ESM showed a decline in the expressed caspase-3 and Bcl-2 proteins after oral administration for 7 days. Eid et al. (2021) reported another similar finding, where ESM treatment inhibited liver damage and effectively inhibited the modulation of TNF-α and IL-6 in amiodarone-treated rats (orally administrated for 8 weeks).
Table 1. Major detected chemical constituents of the ethanolic extract of seeds of Silybum marianum using GC-MS and ameliorated thiobencarb herbicide adverse effects to liver parameters of white albino rats.
|
RT (min) |
% of Area |
Compound |
CAS# |
|
4.430 |
0.12 |
Benzoyl isothiocyanate |
000532-55-8 |
|
6.194 |
1.01 |
Furfural |
000098-01-1 |
|
9.439 |
0.02 |
6-Methoxy-2-hexanol, TMS derivative |
1000216-63-4 |
|
9.994 |
0.02 |
Diglycerol |
000627-82-7 |
|
13.007 |
0.01 |
Mequinol |
000150-76-5 |
|
22.711 |
0.06 |
trans-Isoeugenol |
005932-68-3 |
|
26.923 |
0.15 |
4-((1E)-3-Hydroxy-1-propenyl)-2- |
1000297-95-5 |
|
27.175 |
0.51 |
1,11-Tridecadiene |
1000130-76-4 |
|
28.583 |
0.15 |
Hexadecanoic acid, methyl ester |
000112-39-0 |
|
28.753 |
0.01 |
Silane, trimethylphenyl- |
000768-32-1 |
|
29.185 |
6.69 |
Hexadecanoic acid, ethyl ester |
000628-97-7 |
|
29.690 |
5.53 |
9,12-Octadecadienoic acid (Z,Z)- |
000060-33-3 |
|
30.119 |
2.77 |
10,13-Octadecadienoic acid, meth... |
056554-62-2 |
|
30.367 |
6.85 |
n-Hexadecanoic acid |
000057-10-3 |
|
30.773 |
15.81 |
Linoleic acid ethyl ester |
000544-35-4 |
|
31.086 |
45.76 |
9,12-Octadecadienoic acid (Z,Z)- |
000060-33-3 |
|
33.340 |
4.00 |
Linoelaidic acid |
000506-21-8 |
|
34.116 |
0.40 |
Hexadecanal, 2-methyl- |
055019-46-0 |
|
36.268 |
0.27 |
3-n-Butylthiophene-1,1-dioxide |
142076-45-7 |
|
36.828 |
2.64 |
9,12-Octadecadienoic acid (Z,Z)-... |
003443-82-1 |
|
37.118 |
1.41 |
9,12-Octadecadienoic acid (Z,Z)- |
000060-33-3 |
|
38.462 |
2.10 |
Squalene |
000111-02-4 |
|
39.240 |
0.01 |
Z,E-7,11-Hexadecadien-1-yl acetate |
051607-94-4 |
|
39.428 |
0.01 |
Methyl 9,12-heptadecadienoate |
1000336-36-2 |
|
40.019 |
0.38 |
9,12-Octadecadienoic acid (Z,Z)- |
000060-33-3 |
CONCLUSION
The present study reported that short-term administration of ESM exerted pronounced effects against TBC-induced liver injury. The results reported that ESM caused a reduction in LPO and an elevation of the enzymatic antioxidant activity revealing enhanced hepatic antioxidative defense systems, counteracted cell apoptosis, and strengthened liver vitality. However, these findings indicated that ESM may be a potential candidate for treating TBC-induced hepatotoxicity. Extracts of specific members of the reported flavolignans, flavonoids, terpenoids, fatty acids and alcohols, and hydrocarbons should be tested against hepatotoxicity of pesticides.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Agriculture, Damanhour University for granting access to Pesticides Residue Analysis and Toxicity to run various experiments. Also, the authors wish to thank Faculty of Science, Damanhour University for using the Animal House Facility.
AUTHOR CONTRIBUTIONS
Atef Nassar: Conceptualization, Supervision, Writing - review & editing, Visualization, Investigation, Data curation. Rasha El-Naggar: Methodology, Investigation. Yehia Salim: Writing, Methodology. Eman Radwan: Conceptualization, Supervision, Writing, Methodology. Hafsa Hashem: Supervision, Writing, Methodology. Khaled Abdel-Halim: Writing-review, Visualization, Data curation. Amal Ghoneim: Conceptualization, Supervision, Writing, Methodology.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
DECLARATIONS
All co-authors have seen and agree with the manuscript's contents and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
ETHICAL APPROVAL
All experiments on rats were carried out in accordance with the relevant guidelines and regulations approved by the Scientific Committee of Animal Experimentation of the Faculty of Agriculture, Damanhour University (No. DUFA-2024-0012).
REFERENCES
Abba, H.H., Authman, M.M., Abumourad, I., and El Badawi, A.A. 2007. Studies on the effect of thiobencarb herbicide on some biological, physiological, biochemical, histological, and genetic aspects of Nile tilapia, Oreochromis niloticus. Egypt. Journal of Aquatic Biology and Fisheries. 11: 123-150.
Abdel-Daim, M.M. and Abdeen. A. 2018. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food and Chemical Toxicology. 114: 69–77.
Abdel-Halim, K.Y. and Massoud, S.A. 2014. Oxidative stress and protein carbonylation induction in mosquito fish Gambusia affinis as biomarkers of thiobencarb exposure. Sci-Afric Journal of Scientific Issues, Research and Essays. 2(8): 348-353.
Abdel-Halim, K.Y., Abdel-Raouf, M., and Abdo, G.Y. 2016. Aqueous ozonation of herbicide, thiobencarb: Implications for oxidative stress on mosquito fish Gambusia affinis. International Journal of ChemTech Research. 9(4): 91-99.
Abenavoli, L., Capasso, R., Milic, N., and Capasso, F. 2010. Milk thistle in liver diseases: Past, present, future. Phytotherapy Research. 24: 1423-1432.
Abuzeid, M.A.E.S., Nassar, A.M.K., and Khozimy,A. 2022. Atrazine residues in surface water from various agricultural areas of El-Beheira Governorate, Egypt. Alexandria Science Exchange Journal. 43(1): 45-51.
Ahmad, I., Shukla, S., Kumar, A., Singh, B.K., Kumar, V., Chauhan, A.K., Singh, D., Pandey, H.P., and Singh, C. 2013. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chemico-Biological Interactions. 201(1-3): 9-18.
Ahmed, R.A. 2017. Thiobencarb decreases testicular germ cell proliferation, inhibits spermatogenesis, and increases apoptosis: Histopathological and immunohistochemical assessments. European Journal of Pharmaceutical and Medical Research. 4(10): 16-22.
Al Basher, G., Abdel-Daim, M.M., Almeer, R., Ibrahim, K.A., Hamza, R.Z., Bungau, S., and Aleya, L. 2020. Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environmental Science and Pollution Research. 27: 6505–6514.
Ali, M.H.H., Messiha, B.A.S., and Abdel-Latif, H.A.T. 2016. Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats. Pharmaceutical Biology. 54: 1198-1208.
Almeida, E.M.F., and De Souza, D. 2023. Current electroanalytical approaches in the carbamates and dithiocarbamates determination. Food Chemistry. 15(417): 135900.
An, G., Park, J., Lim, W., and Song, G. 2022. Thiobencarb induces phenotypic abnormalities, apoptosis, and cardiovascular toxicity in zebrafish embryos through oxidative stress and inflammation. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 261: 109440.
Ashkenazi, A. and Dixit, V.M. 1998. Death receptors: Signaling and modulation. Science. 281(5381): 1305–1308.
Beers, J.A.R. and Sizer, R.F. 1952. Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry. 195: 133-140.
Beutler, E., Duron, O., and Kelly, B.M. 1963. Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine. 61: 882-888.
Bhat, A.H., Dar, K.B., Anees, S., Zargar, M.A., Masood, A., Sofi, M.A., Ganie, S.A. 2015. Oxidative stress, mitochondrial dysfunction, and neurodegenerative diseases: A mechanistic insight. Biomedicine and Pharmacotherapy. 74: 101–110.
Cao, L.J., Li, H.D., Yan, M., Li, Z.H., Gong, H., Jiang, P., Deng, Y., Fang, P.F., and Zhang, B.K. 2016. The protective effects of isoliquiritigenin and glycyrrhetinic acid against triptolide-induced oxidative stress in HepG2 cells involve Nrf2 activation. Evidence-Based Complementary and Alternative Medicine. 2016: 8912184.
Cordero-Pérez, P., Torres-González, L., Aguirre-Garza, M., Camara-Lemarroy, C., Guzmán-de la Garza, F., Alarcón-Galván, G., Zapata-Chavira, H., de Jesús Sotelo-Gallegos, M., Nadjedja Torres-Esquivel, C., Sánchez-Fresno, E., et al. 2013. Hepatoprotective effect of commercial herbal extracts on carbon tetrachloride-induced liver damage in Wistar rats. Pharmacognosy Research, 5(3), 150–156.
Diplock, A.T., Symons, N.C.R., and Rice-Evans, C.A. 1991. Technique in Free Radical Research. 1st Edition, Volume 22. Elsevier, Amsterdam.eBook ISBN: 9780080858913.
Duc, H.D., Oanh, N.T., and Khanh, N.T.M. 2023. Thiobencarb degradation by Pseudomonas sp. Th1 and CupriavidusoxalaticusTh2 isolated from soil. Current Microbiology. 80(11): 342.
Eid, R.A., Zaki, M.S.A., Al-Shraim, M., Eldeen, M.A., Massoud, E.E.S., Shati, A.A., Kamar, S.S., and Haidara, M.A. 2021. Silymarin's defensive role against hepatotoxicity induced by amiodarone in albino rats. International Journal of Morphology. 39(2): 407-415.
El-Kamary, S.S., Shardell, M.D., Abdel-Hamid, M., Ismail, S., El-Ateek, M., Metwally, M., Mikhail, N., Hashema, M., Mousab, A., Aboul-Fotouhe, A., et al. 2009. A randomized controlled trialto assess the safety and efficacy of silymarin on symptoms, signs, and biomarkers of acute hepatitis. Phytomedicine. 16: 391-400.
Elmowafy M., Viitala, T., Ibrahim,H.M., et al. 2013. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. European Journal of Pharmaceutical Sciences. 50(2):161–171.
Feng, B., Meng, R., Huang, B., Shen, S.M., Bi, Y., and Zhu, D.L.2016. Silymarin alleviates hepatic oxidative stress and protects against metabolic disorders in high-fat diet-fed mice. Free Radical Research. 50: 314-327.
Flohe, L., and Gunzler, W.A.1984. Assays of glutathione peroxidase. In methods of Enzymology. Academic Press, New York, USA.
Friedewald, W.T., Leve,R.I., and Fredrickson, D.S.1972. Estimation of the concentration of low-density lipoprotein separated by three different methods. Clinical Chemistry. 18: 499-502.
Green, D.R. and Reed, J.C. 1998. Mitochondria and apoptosis. Science. 281(5381): 1309–1312.
Islam, M.T. 2017. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurological Research. 39(1): 73–82.
Jha, K., Shukla, M., Kumar, M., Shukla, V.K., and Pandey, M. 2017. Expression of caspase D inhibition of apoptosis lowers survival in breast cancer. World Journal of Surgical, Medical and Radiation Oncology. 6(3): 7–16.
Jinno, H., Haniokap, H., Takahashis, A., Nishimura, T., Toyooka, T., and Ando, M. 1997. Comparative cytotoxicity of the aqueous chlorination products of thiobencarb, a thiocarbamate herbicide, in cultured rat hepatocytes. Toxicology In Vitro. 11: 731-739.
Karen, P., Rosalba, T., and Daisy, J. 2002. Sensitivity of a novel biotin-free detection reagent (power vision+™) for immunohistochemistry. Journal of Histotechnology. 25(4): 247–250.
Karimi, G., Vahabzadeh, M., Lari, P., Rashedinia, M., and Moshiri, M. 2011. Silymarin: A promising pharmacological agent for treatment of diseases. Iranian Journal of Basic Medical Sciences. 14(4): 308-317.
Kshirsagar, A., Ingawale, D., Ashok, P.,and Vyawahare, N. 2009. Silymarin: A comprehensive review. Pharmacognosy Reviews. 3(5): 126-134.
Lee, D.Y.W. and Liu, Y. 2003. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle). Journal of Natural Products. 66: 1171-1174.
Lopes, M., Stone, S., Ellis, S., and Collwell, J. 1977. Cholesterol determined in high-density lipoprotein separated by three different methods. Clinical Chemistry. 23(5): 882-884.
Luper, S. 1998. A review of plants used in the treatment of liver disease: Part I. Alternative Medicine Review. 3: 410-421.
Malhotra, J.D., and Kaufman, R.J. 2011. ER stress and its functional link to mitochondria: Role in cell survival and death. Cold Spring Harbor Perspectives in Biology. 3(9): a004424.
Martinez, A.A., Suárez, P., Ruiz, Y., Vidal, A. and Juan, F.S., 2014. In vitro toxicity and histopathological effects induced in the mantle tissue of males of mytilus galloprovincialis during short-term exposure to a tar mixture. Molluscan Shellfish Safety. pp.229-238.
Mecdad, A.A., Ahmed, M.H., ElHalwagy, M.E.A., and Afify, M.M.M. 2011. A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-sprayers. Egyptian Journal of Forensic Sciences. 1: 93–98.
Merrell, M.D. and Cherrington, N.J. 2011. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metabolism Reviews. 43: 317-334.
Nassar, J.F., McCauley, L., Scherer, J., Lasarev, M., Koshy, M., Kow, Y.W., Nazar-Stewart, V., and Kisby, G.E. 2008. Biomarkers of oxidative stress and DNA damage in agricultural workers: A pilot study. Toxicology and Applied Pharmacology. 227: 97–107.
Nassar, A.M.K., Abdel-Halim, K.Y., and Abbassy, M.A. 2021. Mitochondrial biochemical and histopathological defects induced by the herbicide pendimethalin in tilapia fish (Oreochromis niloticus). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 242: 108949.
National Toxicology Program. 2011. Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (feed studies). National Toxicology Program Technical Report Series. 565: 1-177.
NRC (National Research Council). 2013. Guide for the care and use of laboratory animals, institute of laboratory animal resources, National Academy Press, Washington, DC.
OECD. 2008. Test No. 407: Repeated dose 28-day oral toxicity study in rodents. OECD Guidelines for the Testing of Chemicals. Section 4, OECD Publishing, Paris.
Polyak, S.J., Morishima, C., Lohmann, V., Pal, S., Lee, D.Y.W., Liu, Y., Graf, T.N., and Oberlies, N.H. 2010. Identification of hepatoprotective flavonolignans from silymarin. Proceedings of the National Academy of Sciences. USA. 107: 5995-5999.
Qu, R.Y., He, B., Yang, J.F., Lin, H.Y., Yang, W.C., Wu, Q.Y., Li, Q.X., and Yang, G.F. 2021. Where are the new herbicides? Pest Management Science. 77(6): 2620-2625.
Raj, S. and Gothandam, K.M. 2014. Hepatoprotective effect of polyphenols rich methanolic extract of Amorphophallus commutatus var. wayanadensis against CCl4induced hepatic injury in Swiss albino mice. Food and Chemical Toxicology. 67: 105–112.
Rašković, A., Stilinović, N., Kolarović, J., Vasović, V., Vukmirović, S., and Mikov, M. 2011. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 16: 8601-8613.
Saller, R., Meier, R., and Brignoli, R. 2001. The use of silymarin in the treatment of liver diseases. Drugs. 61: 2035-2063.
SAS (statistical analysis systems). 2016. Version 9.4 of the SAS System for Windows. Cary. NC, USA: SAS Institute Inc.
Sasu, A., Herman, H., Mariasiu, T., Rosu, M., Balta, C., Anghel, N., Miutescu, E., Cotoraci, C., and Hermenean, A. 2015. Protective effects of silymarin on epirubicin-induced mucosal barrier injury of the gastrointestinal tract. Drug and Chemical Toxicology. 38(4): 442-451.
Serçe, A., Toptancı, B.Ç., Tanrıkut, S.E., Altaş, S., Kızıl, G., Kızıl, S., and Kızıl, M.2016. Assessment of the antioxidant activity of Silybum marianum seed extract and its protective effect against DNA oxidation, protein damage and lipid peroxidation. Food Technology and Biotechnology. 54(4): 455-461.
Shaker, M.E., Zalata, K.R., Mehal, W.Z., Shiha, G.E., and Ibrahim, T.M. 2011. Comparison of imatinib, nilotinib and silymarin in the treatment of carbon tetrachloride-induced hepatic oxidative stress, injury, and fibrosis. Toxicology and Applied Pharmacology. 252(2): 165-75.
Sherer, T.B., Richardson, J.R., Testa, C.M., Seo, B.., Panov, A.V., Yagi, T., Matsuno-Yagi, A., Miller, G.W., and Greenamyre, J.T. 2007. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. Journal of Neurochemistry. 100(6): 1469-1479.
Shimasaki, Y., Tsuyama, M., Tasmin, R., Qiu, X., Shimizu, M., Sato, Y., Yamasaki, Y., Kato-Unoki, Y., Nukata, A., Nakashima, T., Ichinose, H., Wariishi, H., Honjo, T., and Oshima, Y.2013. Thiobencarb herbicide reduces growth, photosynthetic activity, and amount of risk iron-sulfur protein in the diatom Thalassiosira pseudonana. Journal of Biochemical and Molecular Toxicology. 27(9): 437-444.
Singh, H., Sidhu, S., Chopra, K., and Khan, M.U.2016. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: Inhibition of hepatic inflammation and fibrosis. Canadian Journal of Physiology and Pharmacology. 94: 879-887.
Stein, E.A. 1987. Lipids, Lipoproteins, and Apolipoproteins. pp. 448-481. In: Tietz NW, ed. Fundamentals of Clin. Chem. 3rded. Philadelphia: WB Saunders.
Surai, P.F. 2015. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants (Basel). 4: 204-247.
Tamayo, C. and Diamond, S. 2007. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integrative Cancer Therapies. 6: 146-157.
Tsai, J.H., Liu, J.Y., Wu, T.T., Ho, P.C., Huang, C.Y., Shyu, J.C., Hsieh, Y.S., Tsai, C.C., and Liu, Y.C.2008. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. Journal of Viral Hepatitis. 15(7): 508-514.
Tůmová, L., Tůma, J., Megušar, K., and Doležal, M. 2010. Substituted pyrazinecarboxamides as abioticelicitors of flavolignan production in Silybum marianum (L.) Gaertn cultures in vitro. Molecules. 15: 331-340.
USEPA. 1997. Reregistration Eligibility Decision (RED) Thiobencarb Epa738-R-97-013, United States Environmental Protection Agency Washington, D.C. 20460.
USEPA. 2000. U.S. Environmental Protection Agency, Thiobencarb Re-registration Eligibility Decision (RED). https://archive.epa.gov/pesticides/reregistration/web/ pdf/2665fact.pdf
Wan, J.M., Sit, W.H., Lee, C.L., Fu, K.H., and Chan, D.K. 2006. Protection of lethal toxicity of endotoxin by Salvia miltiorrhiza BUNGE is via reduction in tumor necrosis factor alpha release and liver injury. International Immunopharmacology. 6: 750-758.
Wang, L., Huang, Q., Li, Y.,Huang, Y.,Xie, J.,Xu, L., Dou, Y., Su, Z., Zeng, H., and Chen J. 2018. Protective effects of silymarin on triptolide-induced acute hepatotoxicity in rats. Molecular Medicine Reports. 17: 789-800.
Wang, R., Bingner, R.L., Yuan, Y., Locke, M., Herring, G., Denton, D., and Zhang, M. 2021. Evaluation of thiobencarb runoff from rice farming practices in a California watershed using an integrated riceWQ-annAGNPS system. Science of The Total Environment. 767: 144898.
Wellington, K. and Jarvis, B. 2001. Silymarin: A review of its clinical properties in the management of hepatic disorders. Biodrugs. 15: 465-489.
Xing, H., Jia, K., He, J., Shi, C., Fang, M., Song, L., Zhang, P., Zhao, Y., Fu, J., and Li, S. 2015. Establishment of the tree shrew as an alcohol induced fatty liver model for the study of alcoholic liver diseases. PLoS One. 10(6): e0128253.
Yang, C., Lim, W., and Song, G. 2020. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology. 234: 108758.
Young, D.S. 2001. Effects of Disease on Clinical Lab. Tests, 4th ed. AACC.
Zhang, W., Hong, R.T., and Tian, T.L. 2013. Silymarin's protective effects and possible mechanisms on alcoholic fatty liver for rats. Biomolecules & Therapeutics. 21: 264-269.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Atef Mohamed Khedr Nassar1, 2, *, Rasha Ahmed El-Naggar3, Eman H. Radwan3, Hafsa O. Hashem4, Khaled Y. Abdel-Halim5, Yehia M. Salim1, and Amal Z. Ghoneim3
1 Department of Plant Protection (Pesticides), Faculty of Agriculture, Damanhour University, Damanhour, El-Beheira, PO Box 59, Egypt.
2 Council of Future Studies and Risk Management, Ministry of Higher Education, Cairo, Egypt.
3 Zoology Department, Faculty of Science, Damanhour University, Alexandria, Egypt.
4 Zoology Department, Faculty of Science, Alexandria University, Alexandria, Egypt.
5 Mammalian & Aquatic Toxicology Department, Central Agricultural Pesticides Laboratory (CAPL), Agricultural Research Center (ARC), 12618-Dokki, Giza, Egypt.
Corresponding author: Atef Mohamed Khedr Nassar, E-mail: atef.nassar@dmu.edu.eg
ORCID ID: Atef Mohamed Khedr Nassar: https://orcid.org/0000-0002-0394-1530
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: December 19, 2024;
Revised: March 6, 2025;
Accepted: March 6, 2025;
Online First: April 25, 2025

