
Formulation and Characterization of Sodium Alginate-based Nanoparticle Doped with Seagrass (Syringodium isoetifolium) Extract: In vitro Anti-inflammatory, Antioxidant and Anti-diabetic Activities
Mahashree Saravanan, Balakrishnan Mahalakshmi, Kudalavagothi Afeeza, Senthilkumaran Subriya, Muruhan Sridevi, and Elangovan Dilipan*Published Date : March 13, 2025
DOI : https://doi.org/10.12982/NLSC.2025.035
Journal Issues : Number 2, April-June 2025
Abstract Sodium alginate is a naturally occurring, biodegradable biopolymer that has been extensively utilized in the formulation of nanoparticles for controlled drug delivery systems. In this study, sodium alginate-based nanoparticles (NaAlgNPs) doped with Syringodium isoetifolium extract were synthesized and characterized to evaluate their antioxidant, anti-inflammatory, and anti-diabetic properties. Fourier-transform infrared (FT-IR) spectroscopy confirmed the presence of hydroxyl, ether, and carboxyl groups, thereby indicating robust interactions between sodium alginate and the seagrass extract. Field emission scanning electron microscopy (FESEM) revealed a well-defined morphology, while energy-dispersive X-ray (EDX) analysis validated the presence of bioactive elements. The antioxidant activity, assessed through 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays, exhibited 60% and 65% radical scavenging at 100 µg/mL, respectively. The anti-inflammatory potential, evaluated through protein denaturation inhibition, demonstrated 80.25% inhibition at 50 µg/mL, exceeding that of Diclofenac sodium, which showed 77.17% inhibition. The anti-diabetic potential, determined via α-amylase and α-glucosidase inhibition assays, revealed 85% and 70% inhibition at 125 µg/mL, respectively, underscoring its significant hypoglycemic effects. These findings indicate that NaAlgNPs incorporated with Syringodium isoetifolium extract exhibit considerable bioactivity, positioning them as promising candidates for pharmaceutical applications. Future research should prioritize in vivo studies and clinical trials to assess biocompatibility and therapeutic efficacy. Furthermore, exploring these nanoparticles for targeted drug delivery, wound healing, and chronic disease management may further augment their biomedical potential, thereby supporting their utilization as eco-friendly and cost-effective therapeutic agents.
Keywords: Nanoparticles, Encapsulation, Sodium alginate, Seagrass, Bioactivity
Citation: Saravanan, M., Mahalakshmi, B., Afeeza, KLG., Subriya, S., Sridevi, M., and Dilipan, E. 2025. Formulation and characterization of sodium alginate-based nanoparticle doped with seagrass (Syringodium isoetifolium) extract: In vitro anti-inflammatory, antioxidant and anti-diabetic activities. Natural and Life Sciences Communications. 24(2): e2025035.
INTRODUCTION
Nanotechnology has significantly transformed various scientific disciplines, particularly biomedical applications, by providing innovative solutions for drug delivery, diagnostics, and therapeutics (Miyazaki and Islam, 2007). Among the diverse array of nanomaterials, biocompatible and biodegradable nanoparticles have gained considerable prominence due to their potential to enhance drug stability, solubility, circulation time, and targeted release capabilities (Bennet and Kim, 2014; Yao et al., 2020). One of the most promising biopolymers for the synthesis of nanoparticles is sodium alginate (NaAlg), a natural polysaccharide derived from brown seaweed. Owing to its biocompatibility, biodegradability, and tunable physicochemical properties, sodium alginate-based nanoparticles (NaAlgNPs) have been extensively investigated as drug delivery carriers (Lee and Mooney, 2012). Various fabrication techniques, including ionic gelation, coacervation, solvent evaporation, and emulsion cross-linking, can be utilized to synthesize NaAlgNPs, with ionic gelation being the most preferred method due to its simplicity, cost-effectiveness, and precise control over particle size (Paques et al., 2014; Ching et al., 2017).
NaAlgNPs exhibit nanometer-scale sizes (100–500 nm), a negatively charged surface, and a highly porous structure, rendering them particularly suitable for biomedical applications (Targhotra and Chauhan, 2020). Their nanoscale dimensions facilitate efficient cellular uptake and enhance bioavailability (Gigliobianco et al., 2018). Moreover, the negatively charged surface mitigates nanoparticle aggregation, ensuring prolonged circulation within biological systems (Hamidi et al., 2008). The porous nature of NaAlgNPs provides an increased surface area, allowing for efficient drug encapsulation and controlled release, thereby making them exemplary candidates for targeted drug delivery systems (Lee and Mooney, 2012). Collectively, these characteristics augment the potential of NaAlgNPs in therapeutic interventions for chronic diseases.
Diabetes represents a global metabolic disorder affecting millions of individuals, with prevailing management strategies predominantly relying on oral hypoglycemic agents (Chaudhury et al., 2017). However, emerging research has indicated a robust association between diabetes and periodontal disease, whereby severe periodontitis may contribute to poor glycemic control and exacerbate diabetes-related complications (Mealey and Oates, 2006; Preshaw et al., 2012). This underscores the necessity for novel antidiabetic agents that offer enhanced therapeutic efficacy while minimizing adverse side effects. Natural compounds derived from marine organisms, particularly seagrasses, have demonstrated promising pharmacological activities attributed to their rich content of secondary metabolites.
Seagrasses, recognized as the sole marine flowering plants, represent an underexplored reservoir of bioactive compounds with potential therapeutic applications (Dilipan et al., 2017). Among these species, Syringodium isoetifolium, a widely distributed seagrass, is noted for its production of flavonoids, polyphenols, terpenoids, and alkaloids, which demonstrate a range of bioactivities, including antioxidant, anti-inflammatory, antimicrobial, and anti-diabetic effects (Ghosh et al., 2012; Subhashini et al., 2013; Deepak et al., 2019). The incorporation of these bioactive metabolites into sodium alginate nanoparticles (NaAlgNPs) may enhance their bioavailability, stability, and therapeutic efficacy, thus offering an environmentally friendly and sustainable nanocarrier system for drug delivery and the management of chronic diseases.
In light of these advantages, the present study aims to synthesize and characterize NaAlgNPs laden with Syringodium isoetifolium extract utilizing Fourier-transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FESEM). Furthermore, the study assesses the antioxidant and anti-diabetic activities of the synthesized nanoparticles via in vitro assays, thereby providing valuable insights into their prospective biomedical applications. This research highlights the importance of marine-derived nanotherapeutics and their potential in the development of cost-effective, sustainable, and efficient drug delivery systems for the management of diabetes and its associated complications.
MATERIALS AND METHODS
Collection of seagrass species
Fresh seagrass samples of Syringodium isoetifolium were collected from Thondi coastal area (9°44’05.99“ N; 79°01’04.10” E), Palk Bay, Tamil Nadu during April 2023. They were collected and transported to the laboratory, where they were cleansed with seawater to remove macrophytes and extraneous material, followed by a rinse with distilled water. The seagrass species were identified using the taxonomic keys provided by Dilipan et al. (2020). After being washed well seagrass was shade dried for 10-16 days. In shade drying, the sample was dried in air at normal room temperature without sunlight to avoid abiotic stress. After drying, the seagrass was continuously monitored and maintained to prevent unwanted things and moisture content.
Extraction of bioactive compounds in Syringodium isoetifolium
The powdered plant material (10 g) was extracted with 100 mL of acetone: hexane: ethanol (5:3:2) using a Soxhlet system and filtered through Whatman No.1 filter paper for further analysis (Dilipan et al., 2023).
Synthesis of sodium alginate nanoparticle
The synthesis of sodium alginate nanoparticles incorporating seagrass extract was conducted through a systematic series of steps. Initially, 0.5 g of sodium alginate was dissolved in 50 mL of deionized water within a beaker, which was subsequently placed on a magnetic stirrer and heated to 50°C while maintaining a stirring speed of 180 rpm (Mohammed et al., 2023). Upon complete dissolution of the sodium alginate, 0.5 mL of glacial acetic acid was added dropwise, followed by the introduction of 0.2 g of calcium chloride, ensuring thorough mixing to facilitate complete dissolution. This process resulted in the formation of a gel-like structure. Subsequently, 2% seagrass extract was incorporated into the solution and continuously stirred for 2 hours to ensure adequate integration. The final solution was then subjected to freeze-drying utilizing a lyophilizer, and the resultant sodium alginate nanoparticles were collected for further analysis.
Characterization of sodium alginate nanoparticle
Fourier-transformed infrared spectrum
A Fourier-transformed infrared (ATR-FTIR) (Bruker Alpha II) spectrophotometer was used to examine and document the functional group of the seagrass extract nanoparticle composed of sodium alginate (Selvamani et al., 2025). With a resolution of 4 cm-1, the FTIR spectrum covered a range of 4000 to 500 cm-1.
Field emission scanning electron microscopy
FESEM with energy dispersive x-ray analysis (EDaX) was used to examine sodium alginate-composed seagrass extract nanoparticles. The nanoparticle was evenly spread over the sample holder using carbon tape and coated with platinum using an ion carbon system for 120 s before SEM observation. SEM-EDX detector discovered nanoparticle elemental composition (Dharshini et al., 2024).
Anti-oxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The DPPH (2.2-diphenyl-1-picrylhydrazyl) assay, developed (Shimada et al., 1992), was used to evaluate a sample's radical scavenging ability. The DPPH solution was tested for optical density at 517 nm after incubation for 30 minutes at 37 degrees Celsius in the dark to estimate absorbance. The percentage of inhibition of scavenging action was determined using ascorbic acid as a positive control.
DPPH scavenging effect (%) = ((A0-A1)/A0) *100
where A0 is the absorbance of control, and A1 is the absorbance of the sample.
2,2’-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS)
The 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay was conducted (Gião et al., 2007). The positive control for ascorbic acid and the percentage of inhibition was calculated using the following formula:
I = A0−A1/A0 × 100, where A0 is the absorbance of control reaction, and A1 is the absorbance of the sample.
Effect on protein denaturation
The protein denaturation experiment was done according to Gupta et al. (2010), with minor changes reported by Gunathilake et al. (2018). The reaction mixture (5 mL) included 0.2 mL of 1% bovine albumin, 4.78 mL of phosphate-buffered saline (PBS, pH 6.4), and 25 to 125 μg of the sample. It was mixed and incubated in a water bath (37°C) for 15 minutes, then heated at 70°C for 5 minutes. A UV/VIS spectrometer (Optima, SP-3000, Tokyo, Japan) assessed turbidity at 660 nm after cooling. The following formula computes the protein denaturation inhibition percentage.
% inhibition of denaturation = 100 × (1 − A2/A1),
Where A1 = absorption of the control sample, and A2 = absorption of the test sample.
Antidiabetic activity
α-Amylase inhibitory activity
The NaAlgNPs facilitated by seagrass were combined with 500 μL of 0.02 M H2SO4 buffer and maintained at 25°C for 10 minutes. After the incubation period, 500 μL of a 1% starch solution in a 0.02 M sodium phosphate buffer (pH 6.9 and containing 0.006 M NaCl) was added to the reaction mixture. Following this, the reaction mixture was incubated at a temperature of 25°C for 10 minutes, after which 1.0 mL of dinitrosalicylic acid (DNSA) was mixed (Kwon et al., 2008). The reaction eventually terminated after five minutes of incubation in boiling water and then cooling to room temperature. The mixture was diluted after the reaction using 10 mL of distilled water, and the absorbance was quantified at a wavelength of 540 nm. The control group consisted of a combination of all reagents and the enzyme except the sample. The percentage inhibition was utilized to express the inhibitory activity of α-amylase.
α-Glucosidase inhibitory activity
A solution containing 100 μL of α-glycosidase at a concentration of 0.5 mg/mL in a 0.1 M phosphate buffer with a pH of 6.9 was incubated for 10 minutes at a temperature of 25°C. Subsequently, a solution comprising 50 μL of 5 M p-nitrophenyl α-D-glucopyranoside in 0.1 M phosphate buffer (pH 6.9) was introduced. The mixtures of reactions were subjected to incubation at a temperature of 25°C for 5 minutes, following which the spectrophotometer was utilized to measure the absorbance at a wavelength of 405 nm. The experimental protocol involved utilizing a control solution comprising all reagents and the enzyme, excluding the sample. The outcomes of the α-glucosidase inhibition activity were quantified as a percentage of inhibition (Kwon et al., 2008).
RESULTS
FTIR
The Fourier-transform infrared (FTIR) spectra of sodium alginate nanoparticles (NaAlgNPs) reveal characteristic absorption bands that correspond to significant functional groups, including hydroxyl (O-H), ether (C-O-C), and carboxyl (C=O) groups (Figure 1). The broad absorption band observed between 3,000 and 3,600 cm⁻¹ serves as an indicator of O-H stretching vibrations, thereby confirming the presence of hydroxyl groups within the alginate structure, which contribute to its hydrophilic properties. The presence of aliphatic C-H stretching vibrations, typically manifesting within the range of 2,920–2,850 cm⁻¹, denotes the hydrocarbon backbone of the polymer.
The asymmetric and symmetric stretching vibrations of carboxylate (COO⁻) groups are observed at 1,649 cm⁻¹ and 1,460 cm⁻¹, respectively, which are characteristic of the carboxylate salt ions in sodium alginate. A broad peak occurring around 3,200–3,500 cm⁻¹ further corroborates the presence of extensive hydrogen bonding interactions within the polymer network. Peaks identified in the 2,800–3,000 cm⁻¹ range correspond to C-H stretching vibrations, signifying the presence of carbon-hydrogen bonds within the polymer matrix.
Furthermore, a distinct absorption peak located within the 1,600–1,750 cm⁻¹ interval is associated with carbonyl (C=O) stretching vibrations, affirming the presence of carboxyl functional groups in the sodium alginate nanoparticle structure. The 1,000–1,150 cm⁻¹ region displays absorption bands that correspond to C-O-C stretching vibrations, indicative of ether or ester linkages within the polymer backbone. These spectral findings substantiate the successful formulation of sodium alginate nanoparticles, which retain their essential functional groups that are critical which retain their essential functional groups that are critical for biocompatibility, stability, and drug delivery applications.
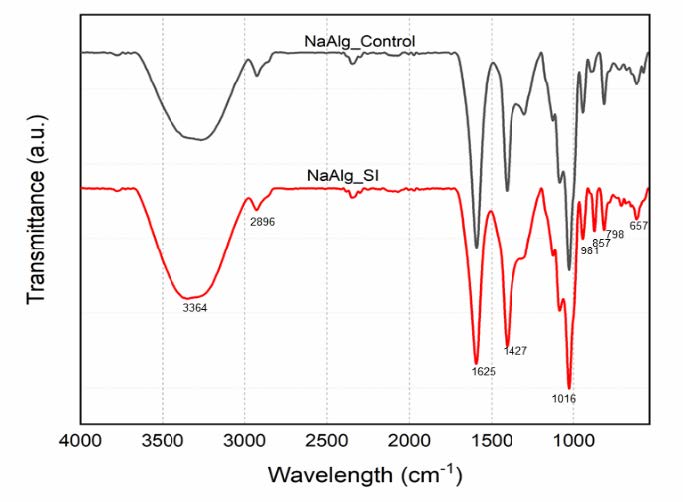
Figure 1. Fourier-transform infrared spectroscopy (FTIR) spectral analysis of Syringodium isoetifolium-mediated sodium alginate nanoparticles (NaAlg_SI) compared to control sodium alginate nanoparticles (NaAlg Control).
FESEM
The Field Emission Scanning Electron Microscopy (FESEM) image illustrates that the surface morphology of sodium alginate nanoparticles (NaAlgNPs) exhibits structured, sharp, and uniform crystalline formations, indicative of a controlled synthesis process. The distinct crystal-like shapes imply well-defined nanoparticle structures (Figure 2a). The Energy Dispersive X-ray (EDX) spectrum elucidates the elemental composition of NaAlgNPs (Figure 2b), highlighting a high concentration of oxygen (O) atoms derived from the sodium alginate matrix, which encompasses hydroxyl and carboxyl functional groups. A notable carbon (C) component is present in the alginate polymer backbone. Calcium (Ca) is detected as a minor element, likely resulting from the crosslinking of alginate during the synthesis process. The spectrum corroborates the presence of these key elements, which are fundamental for the formation of NaAlgNPs.
In contrast, the FESEM image of NaAlgNPs synthesized in the presence of Syringodium isoetifolium extract (Figure 2c) reveals a more aggregated and rounded morphology compared to those depicted in Figure 2a. This plant-mediated synthesis may introduce bioactive compounds that influence nanoparticle morphology, resulting in less defined structures. This alteration suggests a potential capping and stabilizing effect of biomolecules from the extract during the synthesis process. The EDX analysis of the plant-mediated NaAlgNPs (Figure 2d) identifies the presence of sodium (Na), potassium (K), aluminum (Al), phosphorus (P), sulfur (S), and nitrogen (N) elements, attributed to the bioactive compounds in Syringodium isoetifolium. The presence of potassium (K) and sulfur (S) indicates the involvement of plant secondary metabolites in the capping or reduction processes.
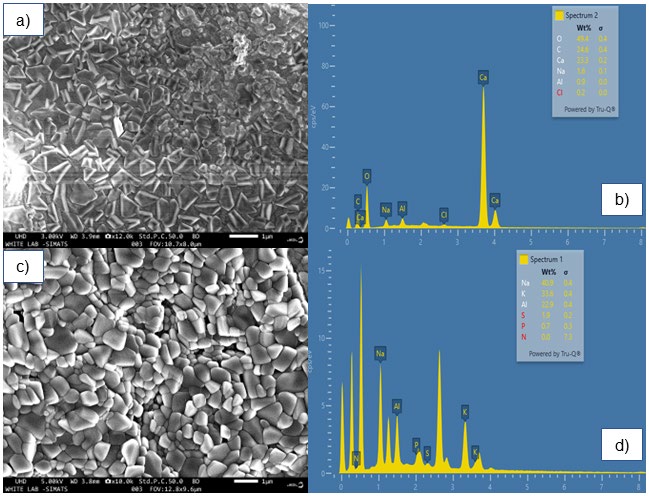
Figure 2. SEM and EDaX image of NaAlgNPs and NaAlgNPs of Syringodium isoetifolium. (a) Field emission scanning electron microscope (FESEM) image of NaAlgNPs, (b) EDaX spectra of NaAlgNPs, (C) FESEM image of Syringodium isoetifolium-mediated NaAlgNPs, and (D) EDaX spectra of Syringodium isoetifolium-mediated NaAlgNPs.
Antioxidant activity
The antioxidant activity of sodium alginate nanoparticles (NaAlgNPs) and seagrass extract-loaded sodium alginate nanoparticles (NaAlgNPs_SI) was evaluated using the DPPH radical scavenging assay at concentrations ranging from 20 to 100 μg/mL (Figure 3). The results indicate a dose-dependent increase in antioxidant activity for both formulations. The NaAlgNPs exhibited relatively low inhibition, with values of approximately 5% at 20 μg/mL, 8% at 40 μg/mL, 15% at 60 μg/mL, 23% at 80 μg/mL, and 30% at 100 μg/mL. In contrast, the NaAlgNPs_SI formulation demonstrated significantly higher antioxidant activity, with inhibition values of approximately 10% at 20 μg/mL, 22% at 40 μg/mL, 42% at 60 μg/mL, 55% at 80 μg/mL, and 65% at 100 μg/mL.
The positive control, ascorbic acid, exhibited the highest antioxidant activity, with inhibition consistently increasing from approximately 18% at 20 μg/mL to nearly 70% at 100 μg/mL. The substantial difference in inhibition between NaAlgNPs and NaAlgNPs_SI suggests that Syringodium isoetifolium extract significantly enhances the antioxidant properties of the nanoparticles. This increased radical scavenging ability is likely attributable to the presence of bioactive compounds such as flavonoids, phenolics, and alkaloids in the extract, which are recognized for their strong antioxidant potential. The lower inhibition values observed for pure NaAlgNPs indicate that sodium alginate alone possesses limited antioxidant activity, potentially due to the absence of active functional groups necessary for effective radical scavenging. In contrast, the higher inhibition observed with NaAlgNPs_SI confirms the successful incorporation of bioactive compounds, thereby enhancing their antioxidant efficacy.
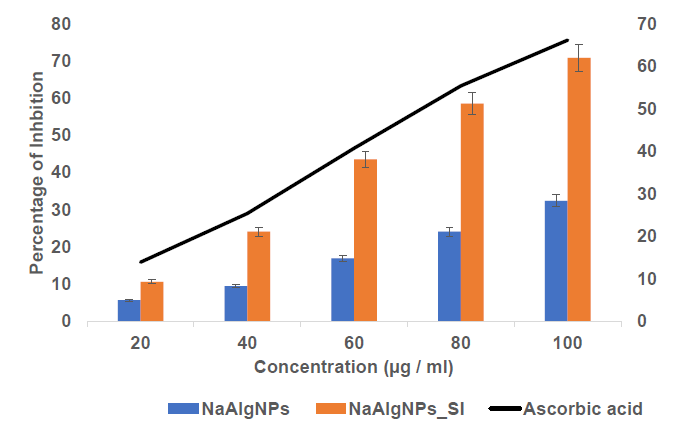
Figure 3. DPPH assay of NaAlgNPs and NaAlgNPs of Syringodium isoetifolium. The values expressed as mean ± SD values, analyzed by two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests.
The ABTS assay was employed to assess the antioxidant activity of sodium alginate nanoparticles (NaAlgNPs) and sodium alginate nanoparticles integrated with Syringodium isoetifolium (NaAlgNPs_SI) at varying concentrations ranging from 20 to 100 µg/mL (Figure 4). The results reveal a dose-dependent increase in the percentage of inhibition for both formulations.
The NaAlgNPs demonstrated relatively low antioxidant activity, with approximately 8% inhibition at 20 µg/mL, 12% at 40 µg/mL, 22% at 60 µg/mL, 30% at 80 µg/mL, and 38% at 100 µg/mL. In contrast, the NaAlgNPs_SI formulation exhibited significantly higher antioxidant activity across all tested concentrations, with inhibition values of approximately 15% at 20 µg/mL, 25% at 40 µg/mL, 42% at 60 µg/mL, 58% at 80 µg/mL, and 68% at 100 µg/mL.
The positive control, ascorbic acid, demonstrated the highest antioxidant activity, with inhibition increasing from approximately 18% at 20 µg/mL to nearly 75% at 100 µg/mL. These findings suggest that Syringodium isoetifolium extract enhances the antioxidant capacity of sodium alginate nanoparticles, likely due to the presence of bioactive compounds such as flavonoids, phenolics, and alkaloids, which are recognized for their robust radical-scavenging properties. The lower inhibition values observed for NaAlgNPs alone indicate limited inherent antioxidant potential, while the higher inhibition noted in NaAlgNPs_SI confirms the successful incorporation of seagrass-derived bioactives, thereby significantly improving their free radical scavenging efficiency.
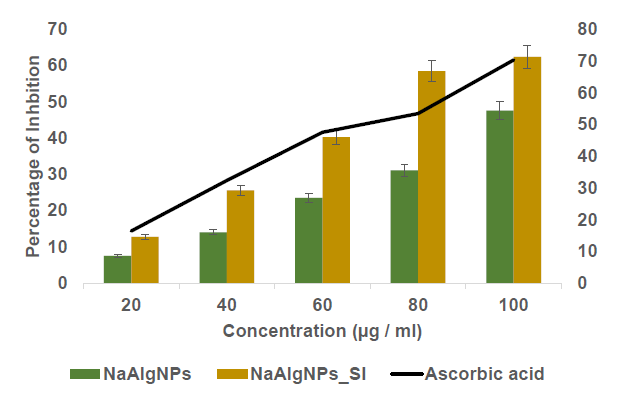
Figure 4. ABTS assay of NaAlgNPs and NaAlgNPs of Syringodium isoetifolium. The values expressed as mean ± SD values, analyzed by two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests.
Anti-inflammatory activity
The anti-inflammatory activity of NaAlgNPs and NaAlgNPs_SI (synthesized using Syringodium isoetifolium) was presented in Figure 5, with results quantified as the percentage of inhibition at varying concentrations (25, 50, 75, 100, and 125 μg/mL). The graph illustrates a comparative analysis of their efficacy against the standard anti-inflammatory drug Diclofenac Sodium. The findings indicate that NaAlgNPs_SI exhibited superior anti-inflammatory activity compared to NaAlgNPs alone across all tested concentrations. Specifically, at a concentration of 25 μg/mL, NaAlgNPs_SI demonstrated approximately 30% inhibition, whereas NaAlgNPs exhibited only around 10% inhibition. As the concentration increased, the inhibition efficiency of both formulations improved. Notably, at 125 μg/mL, NaAlgNPs_SI achieved nearly 85% inhibition, closely approximating the efficacy of Diclofenac Sodium, which consistently demonstrated over 90% inhibition at this concentration. This trend underscores the enhanced bioactivity of NaAlgNPs_SI, likely attributable to bioactive compounds derived from Syringodium isoetifolium. The gradual increase in activity with concentration suggests a dose-dependent efficacy.
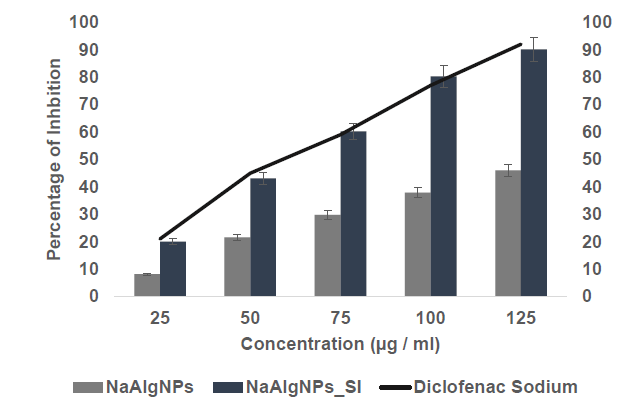
Figure 5. Anti-inflammatory activity of NaAlgNPs and NaAlgNPs of Syringodium isoetifolium. The values expressed as mean ± SD values, analyzed by two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests.
Anti-diabetic activity
The α-amylase inhibitory activity of sodium alginate nanoparticles (NaAlgNPs) and sodium alginate nanoparticles infused with Syringodium isoetifolium (NaAlgNPs_SI) was assessed at varying concentrations (25, 50, 75, 100, and 125 μg/mL) and presented as mean ± standard deviation (SD) (Figure 6). NaAlgNPs_SI consistently demonstrated maximum inhibitory activity across all concentrations in comparison to NaAlgNPs. At a concentration of 25 μg/mL, NaAlgNPs_SI achieved approximately 30% inhibition, whereas NaAlgNPs exhibited only 10% inhibition. This disparity became more pronounced at higher concentrations; at 125 μg/mL, NaAlgNPs_SI demonstrated nearly 85% inhibition, while NaAlgNPs reached approximately 50% inhibition. The consistent increase in inhibition with increasing concentration indicates a dose-dependent effect, underscoring the enhanced effectiveness of NaAlgNPs_SI, likely attributable to bioactive compounds that potentiate α-amylase inhibition.
The inhibitory activity of α-glucosidase by NaAlgNPs and NaAlgNPs_SI (Syringodium isoetifolium) was evaluated at concentrations ranging from 25 to 125 μg/mL (Figure 7). At all concentrations tested, NaAlgNPs_SI demonstrated significantly greater inhibitory effects compared to NaAlgNPs. Specifically, at 25 μg/mL, NaAlgNPs_SI achieved approximately 20% inhibition, while NaAlgNPs exhibited only 10% inhibition. This disparity became more pronounced at higher concentrations; at 125 μg/mL, NaAlgNPs_SI attained nearly 70% inhibition, whereas NaAlgNPs displayed around 50% inhibition. These findings suggest a dose-dependent increase in inhibitory activity, with NaAlgNPs_SI consistently outperforming NaAlgNPs, likely attributable to the bioactive compounds present in Syringodium isoetifolium that enhance enzyme inhibition.
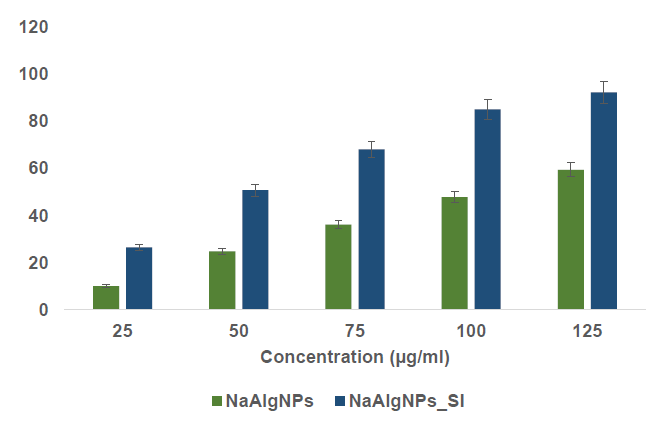
Figure 6. α-amylase inhibitory activity of NaAlgNPs and NaAlgNPs of Syringodium isoetifolium. The values expressed as mean ± SD values, analyzed by two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests.
DISCUSSION
Nanotechnology significantly improves drug pharmacokinetics, pharmacodynamics, biorecognition, and mitigates non-specific toxicity and immunogenicity. Nanoscale drug delivery systems exhibit considerable promise in the fields of imaging, diagnostics, and therapeutics due to their distinctive properties. In this study, sodium alginate nanoparticles encapsulated with seagrass extract were successfully synthesized. The physicochemical characteristics of these nanoparticles were evaluated, along with their bioactive properties, to determine their potential applications.
Fourier infrared spectroscopy (FTIR)
FTIR absorption spectroscopy is a potent technique for identifying chemical bonds and functional groups, as each molecule possesses a unique absorption spectrum akin to a molecular fingerprint. The resulting spectrum illustrates the absorption and transmission of infrared light by individual molecules, allowing for precise identification of functional groups based on their characteristic absorption wavelengths (Kwon et al., 2008; Herrero et al., 2009).
In this study, FTIR analysis of sodium alginate nanoparticles (NaAlgNPs) provided valuable insights into their functional groups and chemical composition (Figure 1). The spectra revealed distinct absorption bands associated with hydroxyl (O-H), ether (C-O-C), and carboxyl (C=O) groups. Broad stretching vibrations detected between 3,000 and 3,600 cm⁻¹ confirmed the presence of O-H bonds, while aliphatic C-H stretching vibrations were identified in the 2,920–2,850 cm⁻¹ range. The absorption bands at 1,649 cm⁻¹ and 1,460 cm⁻¹ were attributed to the asymmetric and symmetric stretching of carboxylate salt ions, respectively. A broad peak in the 3,200–3,500 cm⁻¹ range further indicated hydroxyl groups in the sodium alginate structure. Peaks in the 2,800–3,000 cm⁻¹ range suggested carbon-hydrogen bonds, while the 1,600–1,750 cm⁻¹ range indicated carbonyl (C=O) functional groups. Additional absorption bands in the 1,000–1,150 cm⁻¹ range corresponded to ether or ester linkages, and those in the 800–1,000 cm⁻¹ range confirmed the presence of carbon-carbon bonds in the polymer backbone (Yanaso et al., 2024). These results affirm the successful synthesis of sodium alginate nanoparticles, preserving their key functional groups essential for biocompatibility and bioactivity.
Scanning electron micrograph
The surface morphology of sodium alginate nanoparticles (NaAlgNPs) and seagrass extract-incorporated sodium alginate nanoparticles (NaAlgNPs_SI) was examined using scanning electron microscopy (SEM) (Figure 2). The SEM images indicated that NaAlgNPs_SI exhibited a more intact and interconnected porous structure compared to NaAlgNPs, which demonstrated a phase-separated morphology as a result of the lyophilization process. This enhanced structural integrity in NaAlgNPs_SI can be attributed to the incorporation of seagrass extract, which likely acted as a stabilizing agent, mitigating excessive phase separation and facilitating the formation of a more compact nanoparticle network. The porous nature of NaAlgNPs_SI is particularly advantageous for biomedical applications, as it promotes cell attachment, migration, and potential drug loading. To further validate the composition of the synthesized nanoparticles, energy-dispersive X-ray (EDX) spectroscopy was conducted in conjunction with SEM. The EDX spectra confirmed the presence of key elements such as sodium (Na), aluminum (Al), potassium (K), phosphorus (P), and nitrogen (N) in both NaAlgNPs SI and NaAlgNPs. The detection of these elements provides strong evidence for the successful integration of seagrass extract into the nanoparticle matrix. The presence of phosphorus and nitrogen in NaAlgNPs_SI suggests the incorporation of bioactive compounds from Syringodium isoetifolium, further supporting their enhanced stability and potential bioactivity.
The observed intact and porous morphology of NaAlgNPs_SI is crucial for their biocompatibility, drug encapsulation efficiency, and cellular interactions. The interconnected pore structure facilitates nutrient exchange and bioavailability, rendering them promising candidates for drug delivery and tissue engineering applications. Additionally, the presence of essential elements in the EDX analysis underscores the successful synthesis and modification of sodium alginate nanoparticles with seagrass-derived bioactives, contributing to their improved physicochemical and biological properties.
Antioxidant activity
The DPPH assay was performed to assess the efficacy of the nanoparticles in scavenging free radicals from the environment. All gel formulations effectively neutralized the DPPH radical, thereby demonstrating their antioxidant properties (Figure 3). The findings revealed that antioxidant activity was directly proportional to the concentration of NaAlg nanoparticles, indicating that NaAlg nanoparticles with a higher concentration of seagrass extract exhibited enhanced antioxidant capacity. NaAlgNp_SI functions as the primary antioxidant component within the formulations. According to Castaneda-Arriaga et al. (2021), the significant antioxidant potential of the modified polyphenols is attributed to the abundance of phenolic and flavonoid groups present in seagrasses (Pradheeba et al., 2011). Furthermore, variations in polymer concentration led to differences in antioxidant activity, as noted by Sivaperumal et al. (2013).
The ABTS antioxidant test was used to investigate the antioxidant capacity of NaAlg nanoparticles. This assay measures antioxidant capacity based on the ability of a sample to scavenge radical cations produced by ABTS (Figure 4). It was discovered that the level of antioxidant activity was proportional to the quantity of seagrass extract present in the nanoparticle. In comparison, the control NaAlg nanoparticle demonstrated a lower level of antioxidant activity of ABTS radical cation scavenging. The proportion of ABTS radical cations that were scavenged rose to 60.7 and 78.8%, respectively, when the ratio of NaAlgNp_SI was raised further. This demonstrates that seagrass extract may be used to modulate the activity of the NaAlg nanoparticle antioxidants and that the NaAlg themselves have antioxidant capabilities (Warinthip et al., 2023; Gangegoda et al., 2024).
Anti inflammatory (protein denaturation) activity
The protein denaturation activity of the Standard drug (Diclofenac Sodium) was found to be 77.17 percent. The NaAlgNp_SI nanoparticle had the most anti-inflammatory activity, with a value of 80.25 ± 1.77%. In contrast, the NaAlg nanoparticle alone held the least activity, with a value of 37.89 ± 1.21% (Figure 5). Both the standard concentration and the sample concentration were normalized to be equal to 50 µg/ml. To this day, no such anti-inflammatory solid response has been recorded from mice given subcutaneous injections of commercially available alginate nanoparticle (Vijayakumar et al., 2019). In addition, no evidence has been found that the extracted ALG had any anti-inflammatory action. Consequently, the assessment presented above has yet to have different outcomes to contrast.
Alpha amylase and alpha glucosidase assay
Figure 6 and 7 depict the inhibition of α-amylase and α-glucosidase activities by NaAlgNPs_SI of S. isoetifolium extract. The results of the experiment indicate that the NaAlgNPs displayed the most significant inhibition of α-amylase and α-glucosidase activities, with percentages ranging from 13.56% to 57.31% and 15.78% to 54.5%, respectively, compared to the other samples tested. The inhibitory effect of NaAlgNPs on α-glucosidase is significantly more significant than α-amylase. The findings of this study agreed with prior research that has demonstrated the potential α-glucosidase inhibitory activity of silver nanoparticles derived from Enhalus acoroides extract (Senthilkumar et al., 2016). The NaAlgNPs were found to have a suppressive effect on the enzymatic activity responsible for the hydrolysis of complex carbohydrates while concurrently enhancing the glucose consumption rate (Sengottaiyan et al., 2016). The inhibitory effects of NaAlgNPs extracts on α-glycosidase and α-amylase vary depending on the solvent's potential to extract the active principles. Overall, there is a limited amount of biological understanding regarding the significant mechanisms of action of phytocompounds in treating diabetes. However, numerous plants have been discovered to possess secondary metabolites such as glycosides, alkaloids, terpenoids, and flavonoids, which are commonly associated with exhibiting antidiabetic properties (Sengottaiyan et al., 2016). Concerning its mechanism of action, it is suggested that the extracts derived from seagrass may augment the enzymatic activity implicated in the synthesis and excretion of bile acid, thereby leading to a reduction in the levels of serum cholesterol and triglycerides (Dilipan et al., 2023). The significant reduction in alpha-glucosidase activity impedes alpha-glucosidase function in the small intestine, resulting in a reduction in carbohydrate consumption, which is advantageous. Alpha-glucosidase inhibitors are known to be efficacious in mitigating postprandial hyperglycemia in individuals with diabetes, thereby serving as a primary advantage of their usage (Thongra-ar et al., 2021). Moreover, prior research has demonstrated the inhibitory effect of metal nanoparticles on alpha-glucosidase (Thongra-ar et al., 2021).
CONCLUSION
This study successfully synthesized sodium alginate biopolymer nanoparticles (NaAlgNPs) encapsulating Syringodium isoetifolium extract (NaAlgNPs_SI) as a cost-effective and environmentally friendly alternative to traditional nanoparticle production methods. Fourier-transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FESEM) analyses confirmed the successful incorporation of the seagrass extract, with polyphenols playing a pivotal role in stabilizing the nanoparticles by mitigating agglomeration. The significant antioxidant activity of NaAlgNPs_SI contributed to its anti-inflammatory effects, reducing oxidative stress and inflammatory responses. Furthermore, its ability to inhibit α-amylase and α-glucosidase enzymes supports its hypoglycemic potential, thus positioning it as a promising candidate for diabetes management. The multifunctional bioactivity of NaAlgNPs_SI highlights its potential as a natural therapeutic agent for drug delivery applications, thereby paving the way for future investigations into its biomedical and pharmaceutical applications.
ACKNOWLEDGEMENTS
The authors thank the Department of Physiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 601205, Tamil Nadu, India for providing laboratory facility to carry out the work.
AUTHOR CONTRIBUTIONS
Mahashree S, B. Mahalakshmi, and KLG. Afeeza: Writing – review and editing, Writing – original draft, Visualization, Validation, Methodology Investigation, Formal analysis, S. Subriya, and M. Sridevi: Project administration, Formal analysis, Data curation. E. Dilipan: Writing – review and editing, Supervision, Resources, Project administration, Data curation, Conceptualization.
CONFLICT OF INTEREST
The authors are confirm that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
Bennet, D., and Kim, S. 2014. Polymer nanoparticles for smart drug delivery. Application of nanotechnology in drug delivery. IntechOpen. Chapter 8: 257-285.
Castañeda-Arriaga, R., Perez-Gonzalez, A., Marino, T., Russo, N., and Galano, A. 2021. Antioxidants into nopal (Opuntia ficus-indica), important inhibitors of free radicals’ formation. Antioxidants. 10(12): 2006.
Chaudhury, A., Duvoor, C., Reddy Dendi, V.S., Kraleti, S., Chada, A., Ravilla, R., Marco, A., Shekhawat, N.S., Montales, M.T., Kuriakose, K., et al. 2017. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Frontiers in Endocrinology. 8: 6.
Ching, S.H., Bansal, N., and Bhandari, B. 2017. Alginate gel particles–A review of production techniques and physical properties. Critical Reviews in Food Science and Nutrition. 57(6): 1133-1152.
Deepak, P., Amutha, V., Kamaraj, C., Balasubramani, G., Aiswarya, D., and Perumal, P. 2019. Chemical and green synthesis of nanoparticles and their efficacy on cancer cells. In: Green Synthesis, Characterization and Applications of Nanoparticles. 369-387.
Dharshini, B., Geetha, A., Vasugi, S., Balachandran, S. and Ilangovar, I.G.K., 2024. Optimizing titanium carbide-silver oxide nanostructures for targeted cancer therapy: Synthesis, functionalization, and in vitro evaluations. Cureus. 16(9): e69757.
Dilipan, E., Nobi, E.P., and Rajkumar, J., 2020. Manual for identification of seagrasses of India. Chandigarh: White Falcon Publishing.
Dilipan, E., Papenbrock, J., and Thangaradjou, T. 2017. Random amplified polymorphic DNA (RAPD) finger prints evidencing high genetic variability among marine angiosperms of India. Journal of the Marine Biological Association of the United Kingdom. 97(6): 1307-1315.
Dilipan, E., Sivaperumal, P., Kamala, K., Ramachandran, M., and Vivekanandhan, P. 2023. Green synthesis of silver nanoparticles using seagrass Cymodocea serrulata (R. Br.) Asch. & Magnus, characterization, and evaluation of anticancer, antioxidant, and antiglycemic index. Biotechnology and Applied Biochemistry. 70(3): 1346-1356.
Gangegoda, S., Abeywardhana, S., Sigera, S., Nirmani, A.A.E.B., and Peiris, D.C. 2024. Antioxidant and antimicrobial properties of Codium fragile (Suringar) methanol extract: Insights from molecular docking analysis. Algal Research. 82: 103619.
Ghosh, U., Subhashini, P., Dilipan, E., Raja, S., Thangaradjou, T., and Kannan, L. 2012. Isolation and characterization of phosphate-solubilizing bacteria from seagrass rhizosphere soil. Journal of Ocean University of China. 11: 86-92.
Gião, M.S., González‐Sanjosé, M.L., Rivero‐Pérez, M.D., Pereira, C.I., Pintado, M.E., and Malcata, F.X. 2007. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. Journal of the Science of Food and Agriculture. 87(14): 2638-2647.
Gigliobianco, M.R., Casadidio, C., Censi, R., and Di Martino, P. 2018. Nanocrystals of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics. 10(3): 134.
Gunathilake, K.D.P.P., Ranaweera, K.K.D.S., and Rupasinghe, H.V. 2018. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 6(4): 107.
Gupta, S., George, M., Singhal, M., Sharma, G.N., and Garg, V. 2010. Leaves extract of Murraya koenigii Linn for anti-inflammatory and analgesic activity in animal models. Journal of Advanced Pharmaceutical Technology & Research. 1(1): 68-77.
Hamidi, M., Azadi, A., and Rafiei, P. 2008. Hydrogel nanoparticles in drug delivery. Advanced Drug Delivery Reviews. 60(15): 1638-1649.
Herrero, M.A., Toma, F.M., Al-Jamal, K.T., Kostarelos, K., Bianco, A., Da Ros, T., Bano, F., Casalis, L., Scoles, G., and Prato, M. 2009. Synthesis and characterization of a carbon nanotube− dendron series for efficient siRNA delivery. Journal of the American Chemical Society. 131(28): 9843-9848.
Kwon, Y.I., Apostolidis, E., and Shetty, K. 2008. Inhibitory potential of wine and tea against α‐amylase and α‐glucosidase for management of hyperglycemia linked to type 2 diabetes. Journal of Food Biochemistry. 32(1): 15-31.
Lee, K.Y., and Mooney, D.J. 2012. Alginate: Properties and biomedical applications. Progress in Polymer Science. 37(1): 106-126.
Mealey, B.L., and Oates, T.W. 2006. Diabetes mellitus and periodontal diseases. Journal of Periodontology. 77(8): 1289-1303.
Miyazaki, K., and Islam, N. 2007. Nanotechnology systems of innovation—An analysis of industry and academia research activities. Technovation. 27(11): 661-675.
Mohammed, A.E., Abdalhalim, L.R., Atalla, K.M., Mohdaly, A.A.A., Ramadan, M.F., and Abdelaliem, Y.F. 2023. Chitosan and sodium alginate nanoparticles synthesis and its application in food preservation. Rendiconti Lincei. Scienze Fisiche e Naturali. 34(2): 415-425.
Pradheeba, M., Dilipan, E., Nobi, E.P., Thangaradjou, T., and Sivakumar, K. 2011. Evaluation of seagrasses for their nutritional value. Indian Journal of Geo-Marine Sciences. 40 (1): 105-111.
Paques, J.P., van der Linden, E., van Rijn, C.J., and Sagis, L.M. 2014. Preparation methods of alginate nanoparticles. Advances in Colloid and Interface Science. 209: 163-171.
Preshaw, P.M., Alba, A.L., Herrera, D., Jepsen, S., Konstantinidis, A., Makrilakis, K., and Taylor, R. 2012. Periodontitis and diabetes: A two-way relationship. Diabetologia. 55: 21-31.
Sengottaiyan, A., Aravinthan, A., Sudhakar, C., Selvam, K., Srinivasan, P., Govarthanan, M., Manoharan, K., and Selvankumar, T. 2016. Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. Journal of Nanostructure in Chemistry. 6: 41-48.
Senthilkumar, P., Santhosh Kumar, D.R., Sudhagar, B., Vanthana, M., Parveen, M.H., Sarathkumar, S., Thomas, J.C., Mary, A.S., and Kannan, C. 2016. Seagrass-mediated silver nanoparticles synthesis by Enhalus acoroides and its α-glucosidase inhibitory activity from the Gulf of Mannar. Journal of Nanostructure in Chemistry. 6: 275-280.
Selvamani, M., Elangovan, D., Alsalme, A., Kesavan, A.V., Ayyakannu Sundaram, G., and Santhana Krishna Kumar, A. 2025. Bi2W2O9 nanoflakes synthesized via a hydrothermal method: Antibacterial potency and cytotoxicity evaluation on human dermal fibroblasts. ACS Omega, 10(6): 5468–5477.
Shimada, K., Fujikawa, K., Yahara, K., and Nakamura, T. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry. 40(6): 945-948.
Sivaperumal, P., Kamala, K., Natarajan, E., and Dilipan, E. 2013. Antimicrobial peptide from crab haemolymph of Ocypoda macrocera (Maline Edwards 1852) with reference to antioxidant: A case study. International Journal of Pharmacy and Pharmaceutical Sciences. 5(2): 672-680.
Subhashini, P., Dilipan, E., Thangaradjou, T., and Papenbrock, J. 2013. Bioactive natural products from marine angiosperms: Abundance and functions. Natural Products and Bioprospecting. 3: 129-136.
Targhotra, M., and Chauhan, M.K. 2020. An overview on various approaches and recent patents on buccal drug delivery systems. Current Pharmaceutical Design. 26(39): 5030-5039.
Thongra-ar, K., Rojsanga, P., Chewchinda, S., Mangmool, S., and Sithisarn, P. 2021. Antioxidant, α-glucosidases and α-amylase inhibitory activities of Persicaria odorata. Chiang Mai University Journal of Natural Sciences. 20(3): e2021051.
Vijayakumar, S., Malaikozhundan, B., Saravanakumar, K., Durán-Lara, E.F., Wang, M.H., and Vaseeharan, B. 2019. Garlic clove extract assisted silver nanoparticle–antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. Journal of Photochemistry and Photobiology B: Biology. 198: 111558.
Warinthip N., Liawruangrath B., Natakankitkul S., Rannurags N., Pyne S.G., and Liawruangrath S. 2023. Chemical constituents antioxidant and antibacterial activities of the leaves and flowers from Gardenia carinata Wallich. Natural and Life Sciences Communications. 22(1): e2022004.
Yanaso, S., Jutiviboonsuk, A., Thanarangsarit, P., and Sawasdee, K. 2024. Development, validation, and greenness assessment of HPLC and ATR-FTIR for mangiferin quantitative analysis in raw material. Natural and Life Sciences Communications. 23(4): e2024061.
Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., Wu, S., Deng, Y., Zhang, J., and Shao, A., 2020. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Frontiers in Molecular Biosciences. 7: 193.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Mahashree Saravanan1, Balakrishnan Mahalakshmi1, Kudalavagothi Afeeza3, Senthilkumaran Subriya2, Muruhan Sridevi1, and Elangovan Dilipan3, *
1 Department of Biotechnology, VMKV Engineering College, Vinayaka Mission's Research Foundation, Vinayaka Mission University, Salem, Tamil Nadu 636308, India.
2 Department of Food Technology, Mahendra Engineering College, Mallasamudram, Namakkal 637503, Tamil Nadu, India.
3 Marine Material Chemistry Lab, Department of Physiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600077, Tamil Nadu, India.
Corresponding author: Elangovan Dilipan, E-mail: gerberadilip@gmail.com
ORCID: Elangovan Dilipan: https://orcid.org/0000-0002-7838-402X
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: December 10, 2024;
Revised: February 28, 2025;
Accepted: March 5, 2025;
Online First: March 13, 2025

