
Effect of Extraction and Detannification Methods on Cashew Apple Juice and Wine Quality in Vietnam
Thi Khanh Vinh Phan*, Thi Phuong Anh Tran, and Bao NguyenPublished Date : December 19, 2024
DOI : https://doi.org/10.12982/NLSC.2025.017
Journal Issues : Number 1, January-March 2025
Abstract The cashew apple (Anacardium occidentale L.), is a pseudofruit that grows alongside the cashew nut (true fruit) on the cashew tree. The cashew apple is a good source of nutrients and bioactive compounds. However, it is highly perishable and has an astringent, unpalatable taste, which has limited the utilisation of cashew apples in the food industry. The study focused on evaluating the impact of various extraction methods (hydraulic pressing, screw pressing, and blending) on the quality of cashew apple juice and wine and also aimed to compare the effects of using gelatin powder versus gelatin solution for astringent tannin removal. The results showed that the hydraulic pressing had the least impact on the fruit skin, resulting in the highest clarity for both the hydraulic pressing juice and the treated hydraulic pressing juice. A gelatin solution was more cost-effective than a gelatin powder to achieve the same desired level of tannin reduction. A strong correlation (r=0.992) was established between the gelatin dosage required and the initial total tannin content of the cashew apples to remove tannins effectively. The cashew apple wine, which was fermented from specific dry yeast Saccharomyces cerevisiae RV-002 in treated hydraulic pressing juice, is classified as a low-alcohol (ABV<10%), sweet wine (residual sugar 35-120 g/L), with antioxidant activities comparable to Vietnamese commercial red wine.
Keywords: Juice extraction, Cashew apple juice, Tannin removal/ Detannification, Cashew apple wine
Funding: This project is funded by Nha Trang University (NTU) under project number TR2023-13-34.
Citation: Phan, T. K. V., Tran, T. P. A., and Nguyen, B. 2025. Effect of extraction and detannification methods on cashew apple juice and wine quality in Vietnam. Natural and Life Sciences Communications. 24(1): e2025017.
INTRODUCTION
Anacardium occidentale L., commonly known as the cashew tree, is an evergreen species introduced to Vietnam in the 1980s as a multi-purpose industrial crop for afforestation and soil conservation. It is now widely cultivated across the southeastern, central highlands, and south-central coastal regions of the country, serving as a key economic crop. The cashew tree bears two food products: the cashew nut (the true fruit) and the cashew apple (pseudo fruit). Notably, the cashew apple is typically 6-7 times larger than the cashew nut itself (Gawankar et al., 2018). According to data from the Food and Agriculture Organization of the United Nations (2022), Vietnam is one of the world’s leading producers of cashew nuts, with an estimated annual production of 341680 tons. This substantial nut production implies a fresh cashew apple output of over 2.05-2.4 million tons annually in the country.
Previous studies have revealed considerable differences in the biochemical and nutritional profile of cashew apples, depending on the specific diverse cashew cultivars and growing regions (Marc et al., 2012; Cruz Reina et al., 2022; Akyereko et al., 2023). The cashew apple is rich in vitamin C (200-241 mg/100 g) and contains various antioxidative compounds such as flavonoids 29.86-47.98 mg/100 mL (anthocyanins, myricetin, quercetin, kaempferol) (Akyereko et al., 2023), tannins 84-380 mg tannic acid/100 mL (Emmanuelle et al., 2016), (Prommajak et al., 2018), and phenolic acids (caffeic acid 2.02-2.59 mg/100 mL, coumaric acid 0.25-0.79 mg/100 mL, ferulic acid 0.46-1.34 mg/100 mL, and gallic acid 0.19-1.54 mg/100 mL) (Marc et al., 2012; Cruz Reina et al., 2022). Moreover, cashew apple also contains about 2.9–136 mg/100 g carotenoids mainly composed of β-cryptoxanthin and β-carotene (De Abreu et al., 2013).
Cashew apples boast a rich array of bioactive compounds and have a high water content of 90.0-92.2%, along with a significant amount of total sugars 162.7-168.1g/L (Marc et al., 2012), protein 0.5-1.09%, vitamin C content of 200-241 mg/100 g (Aluko et al., 2023), copper 0.41-11.2 mg/L, zinc 0.21-2.1 mg/L, iron 0.26-1.82 mg/L (Cruz Reina et al., 2022). Consequently, their juices have emerged as a promising candidate for wine production through yeast fermentation. During this fermentation process, fruit-fermented wine (fruit wine) preserves the original nutrients like vitamins and minerals, and bioactive compounds present in the fruit, owing to the avoidance of heat processing (Joshi and Kumar, 2011). This processed cashew apple juice, in the form of wine, become a health-promoting beverage that could potentially aid in preventing cardiovascular diseases, and cancer and offer anti-inflammatory benefits (Fernandes et al., 2017; Shebeko et al., 2018).
Despite the valuable health benefits of cashew apples, they are often discarded as waste after harvesting the nuts due to several key challenges. Chief among these is the high tannin content of the cashew apple, which produces some disadvantages in its usage. Firstly, the tannins could bind to proline-rich proteins, a specific class of salivary proteins high in the amino acid proline, resulting in a dry, astringent sensation and irritating the tongue and oral tissues (Rossetti et al., 2009; Lee et al., 2012), making the cashew apple unpalatable for many consumers. Secondly, tannin exhibits antinutritional properties by forming complexes with various essential nutrients and compounds, such as ion elements, and proteins. They can bind to enzymes involved in carbohydrates and protein digestion, reducing the bioavailability of these important nutrients (Waghorn et al., 1994; Hagerman et al., 1998). Thirdly, the high tannin content in cashew apples also has the potential to inhibit yeast growth during fermentation. Tannin may interfere with membrane-bound reactions and chelate metal ions, reducing their availability to microorganisms (Mullins and NeSmith, 1988; Scalbert, 1991; Prommajak et al., 2019). Consequently, to capitalise on the plentiful and promising by-product resources, it is essential to eliminate tannins from cashew apples before incorporating them into various products, including wine.
Various methods have been explored to remove tannin from cashew apple juice, including chemical, physical and enzymatic approaches (Prommajak et al., 2020). However, the expense of eliminating tannin from cashew apple juice using tannase enzymes or polyvinyl pyrrolidone is very high due to the costly nature of these clarifying agents. Furthermore, treatment with tannase can successfully remove hydrolysable tannins (88%), but it was not effective at eliminating proanthocyanidins (2%) (Couri et al., 2002). In addition, techniques such as microfiltration can be difficult to implement effectively (Talasila et al., 2012). As a more accessible chemical approach, various types of proteins including gelatin, bovine serum albumin, and ovalbumin were used to remove tannins by leveraging their ability to interact with and precipitate these polyphenol compounds. Among them, gelatin has proven particularly effective for the tannin removal of cashew apple juice due to its highest proline content (high affinity for tannin) and largest molecular weight compared to bovine serum albumin and ovalbumin, which facilitates more tight binding with the tannins (Hemingway et al., 1989; Lee et al., 2012). Hence, some studies have been used gelatin as the tannin-removing agent for the cashew apple juice. For instance, the cashew apple juice was obtained either by hydraulic pressing (Prommajak et al., 2018), by blending/manual pressing (Aluko et al., 2023) or screw pressing (Talasila et al., 2012), then was treated with gelatin powder (Prommajak et al., 2018) or gelatin solution (Aluko et al., 2023).
This study aimed to explore the impact of different extraction methods, including hydraulic pressing, blending and screw pressing (slow pressing) on the quality of cashew apple juice and wine. Additionally, the study compared the effectiveness of using gelatin powder versus gelatin solution in removing tannins and reducing turbidity. The relationship between the initial total tannin content of juice and the amount of gelatin required has also been established.
MATERIAL AND METHODS
Materials
Cashew apple (Anacardium occidentale) fruits were harvested at the ripening stage (yellow color) and sized between 16-22 pieces per kilogram. They were sourced from a local farm in Van Gia District, Khanh Hoa Province, Vietnam. The intact cashew fruits were transported to the laboratory within 4-6 hours. Subsequently, the cashew apples were de-nutted and washed with potable water, dried in the open air, packaged in polyamide bags and immediately frozen at -18°C for further use. Gelatin powder (240 bloom, 30 mesh) was purchased from Ewald (Germany). The strain Saccharomyces cerevisiae RV-002 was supplied by the Angel Yeast Company. The commercial Vietnamese grape wines were used as control samples. The Classic White Wine and Classic Red Wine were purchased from Ladofoods Cooperation in Vietnam.
Folin Ciocalteu was obtained from Supelco-Sigma Aldrich, Germany; 2,2-diphenyl-1-picrylhydrazyl (DPPH) was sourced from TCI, Japan; gallic acid (GA) was provided from Asia Laboratory Instrument Company Limited; 2,6-Dichlorophenol indophenol sodium salt (DCPIP) was supplied from Oxford, India. NaOH, HCl, CuSO4.5H2O was purchased from Xilong Company, China. Indigo carmine was acquired from China; 0.1 N KMnO4 was attained from Cemaco HCMC, Vietnam.
Preparation of cashew apple juices using three distinct methods
Hydraulic pressing: the frozen cashew fruits were sliced to a thickness of 2-3 mm and then thawed completely. Next, the thawed slices were placed into a muslin bag and pressed. Finally, the hydraulic pressed juice (HPJ) was subjected to filtration through a 500-mesh nylon cloth.
Blending: the frozen cashew fruits were sliced into quarters and then thawed. In the next step, the thawed slices were blended with a blender (Phillips, model HR2222/00) for 3 minutes. The blended juice (BJ) was then filtered through the nylon cloth.
Slow pressing: the frozen cashew fruits were cut into quarters, and subsequently defrosted, following slowly pressed using a presser (Olivo slow juicer, model SJ 210). The slow-pressed juice (SPJ) was decanted using the nylon cloth.
The samples of HPJ, BJ, SPJ were analysed for physicochemical characteristics: total tannin content, total polyphenol content, vitamin C content, total titratable acidity, total soluble solid (Brix), turbidity, pH.
Detannification of cashew apple juices
Using gelatin solution. Tannins were removed from the juice following the method of Aluko et al., (2023), with some modifications. A 10% gelatin solution was prepared by dissolving 10 g of gelatin in 100 mL of distilled water. The mixture was then tightly covered and placed in a water bath maintained at 60-65°C until the gelatin completely dissolved. From this 10% gelatin solution, weigh 0.2 to 1.6 grams and add it to 100 grams of juice. This corresponds to gelatin mass percentage in juice sample with range of 0.02-0.16% w/w. After manual stirring for approximately 10-15 seconds, the juice was poured into a 500-mesh nylon cloth. The treated juice, along with untreated as a control, were then tested for total tannin content and turbidity.
Using gelatin powder. The juice was treated with gelatin powder following a method of Prommajak et al., (2018), with some modification. Each 100 g of the HPJ was mixed with gelatin powder to make a ratio of gelatin to juice ranging from 0.5-1.5% (w/w). After shaking for 10 minutes at 100 rpm in a KS 4000 I control incubator (IKA, Germany) at room temperature (32 ± 2 °C), the mixture was centrifugated at 2,000 rpm for 10 minutes using a Hettich EBA 21 centrifuge (Germany), followed by filtration. Total tannin content (TTC) and turbidity were then determined for both the treated juice and the untreated juice control.
Establishing the relationship between the initial TTC of the HPJ and the amount of gelatin for detannification
- The HPJ with initial TTC ranging from 83.14 to 209.93 mg/100 mL was prepared by diluting HPJ with initial TTC 442.72 mg/100 mL and 455.19 mg/100 mL.
- The HPJ with an initial TTC between 334.64 to 455.19 mg/100 mL came from various batches of cashew apples.
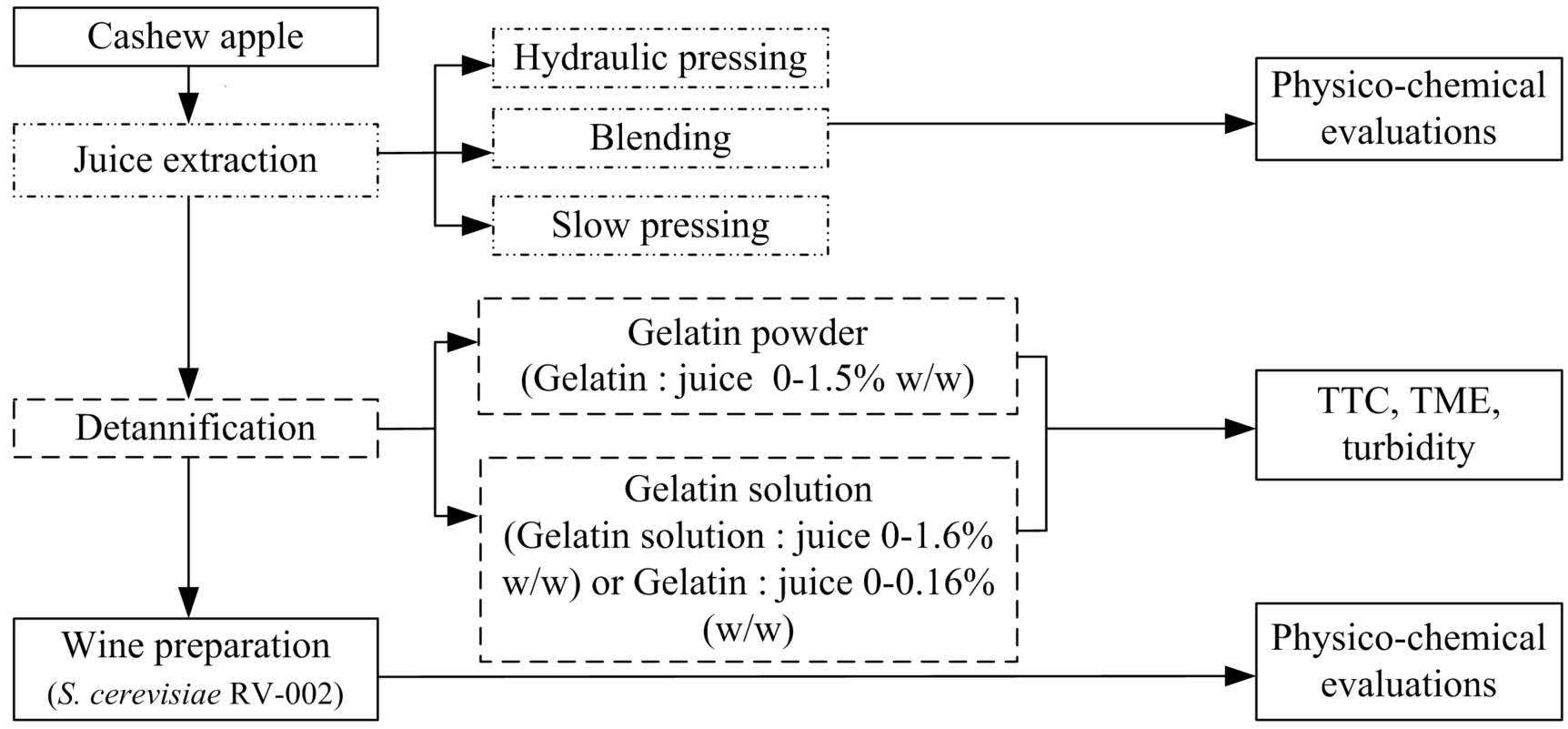
Figure 1. Diagram of the experimental methodology used.
Preparation of cashew apple wine
The total soluble solid (TSS) of the treated HPJ was first adjusted 25oBx by adding sucrose. Before fermenting the juice with a 0.05% yeast slurry, dried yeast was rehydrated in lukewarm water (30-35 °C) with a yeast/water ratio of 1:10 (w/v) following the producer’s instruction. The fermentation process then took place for 1 week at room temperature (32 ± 2 °C). Afterwards, the cashew apple wine was racked, decanted, corked and sealed with a bottle cap (Vinh et al., 2023).
Evaluation of cashew apple juice, cashew apple wines and commercial Vietnamese grapes wines
Determination of total tannin content. TTC of cashew apple juice, cashew apple wine, and grape wines was analysed following the AOAC 955.35 method (1955). Initially, 10 mL of juice or wine sample was measured into a 1 L conical flask. Then, 25 mL of indigo carmine solution and 750 mL of distilled deionized water were added. The titration was carried out using a 0.1 N aqueous solution of KMnO4 until the blue-colored solution changed to yellow. Blank tests were performed by titrating a mixture of 25 mL indigo carmine solution and 750 mL deionised water.
The TTC (T, mg tannic acid/100 mL) in the sample is calculated using the following equation (1):
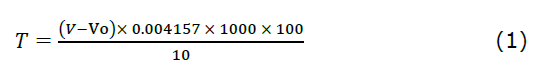
Where: V – volume of 0.1 N aqueous solution of KMnO4 for titration of the sample (mL);
Vo – volume of 0.1 N aqueous solution of KMnO4 for the titration of the blank sample (mL); 0.004157 – tannic acid equivalent in 1 mL of 0.1 N aqueous solution of KMnO4, g;
Determination of % tannin mitigation efficiency. Tannin mitigation efficiency (TME) (%) was measured by the following equation (2):
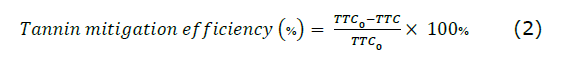
Where: TTC0 is the initial TTC of the sample; TTC is that of the treated sample with gelatin.
Determination of total polyphenol content. The total polyphenol content (TPC) of the juices and wines was determined using the Folin-Ciocalteu method, with slight modifications (Tran et al., 2024). Briefly, 0.05 mL of the sample (diluted 10 times in ethanol from the juice) was mixed with 1 mL of Folin-Ciocalteu reagent solution (diluted at a ratio of 1:10 v/v with distilled water). Then, 0.32 mL of 20% sodium carbonate solution was added to adjust the mixture at pH 10. The reaction mixture was then kept in the dark at room temperature for 30 minutes. The final volume was made up to 10 mL by distilled water, and the absorbance was measured at 750 nm using a UV-vis Spectrophotometer (Libra S50, USA). TPC was calculated using a calibration curve prepared with gallic acid in the concentration range from 0.5 to 3.0 µg/mL. The results were expressed as micrograms of gallic acid equivalent (GAE) per 100 mL of juice or wine.
In vitro antioxidant activity. DPPH scavenging activity was used to evaluate the antioxidant capacity of samples (either juice or wines), following the method of Pothitirat et al., (2009), with minor changes. Aliquot (3.0 mL) of a 0.2 mM DPPH solution in ethanol was added to different concentrations of the samples. The reaction mixtures were then kept in the dark for a period of 30 minutes. The absorbance of the samples was later measured at 519 nm using the UV-vis Spectrophotometer (Libra S50, USA). The antioxidant activity was evaluated by calculating the percentage of inhibition (I%) using the following equation (3), relative to the blank (ethanol):
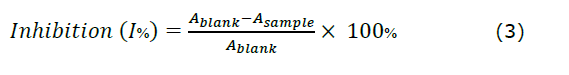
Where Ablank: is the absorbance of the control reaction, which contains reagents but excludes the test sample, and Asample: is the absorbance of the test sample. IC50 values were calculated in µg/mL.
Determination of Vitamin C content. To determine the ascorbic acid content of the juice and wine samples, the AOAC 967.21 method (2010) was adopted. Briefly, 1 mL of the sample was first acidified by adding 9 mL of a 2% HCl solution. 1 mL of the solution was taken into the volumetric flask, and distilled water was then added to make up the total volume to 15 mL. The resulting mixture was titrated with a DCPIP solution (0.2 g in 1000 mL of distilled water) until a persistent pink color was observed for over 30 seconds. The volume of DCPIP used for the titration was recorded. Additionally, 0.1 mg of ascorbic acid was used to standardize the DCPIP solution. A 2% HCl solution was used as the blank. The vitamin C content was then calculated and expressed as milligrams of ascorbic acid per milliliter of juice or wine, using the equation (4):

Where: Vsample: volume of DCPIP used to titrate the samples (mL); Vblank: volume of DCPIP used to titrate the blank (mL); 0.1: weight of ascorbic acid used to standardize the DCPIP solution (mg); F: dilution factor; 1.333: volume of DCPIP equivalent to 0.1 mg ascorbic acid when titrated (mL).
Determination of total titratable acidity. Total titratable acidity (TTA) was determined by the AOAC 942.15 method (1965), corresponding to the percentage of tartaric acid. 5 mL of juice was added into a flask, diluted with 50 mL of distilled water, followed by the addition of 0.15 mL phenolphthalein solution 1%. The flask was then titrated with 0.1 N NaOH until the solution changed into a pink color which lasted for 30 seconds. TTA was calculated and expressed as gram of tartaric acid per 100 mL juice or wine, using the equation (5):

Where: V1: volume of 0.1 N NaOH used to titrate the samples (mL); V2: volume of sample (mL); 0.0075: tartaric acid equivalent to 1 mL 0.1N NaOH (g).
Determination of alcohol by volume from specific gravity. The alcohol content by volume was measured by the AOAC 920.57 method (1920). A 100 mL sample was transferred into a 500 mL round-bottom flask, 50 mL of distilled water was then added to the flask, which was connected to the vertical condenser. The distillation was performed and approximately 100 mL of liquid was collected, then added distilled water to exactly 100 mL at the same temperature of the sample. The alcohol content was determined by measuring its specific gravity at 20°C using an alcohol hydrometer and expressed as percentage volume by volume (% v/v).
Determination of residual sugar content. Residual sugar content was determined by the AOAC 923.09 method (1923), corresponding to the g/L of inverted sugar. Briefly, an amount of sample was added 2.5 mL 37% HCl then was left overnight. Next, the mixture was neutralised with 20% NaOH to reach pH 8.2 and the volume was diluted with distilled water. An aliquot of this solution was titrated against a mixture of 5 mL Fehling A and 5 ml Fehling B using a 1% methylene blue indicator.

Where: V: the volume of the aliquot used to titrate with Fehling A and Fehling B, mL;
f: diluted factor; 0.0564 – grams of inverted sugar equivalent to 5 mL Fehling A and Fehling B.
Analysis of turbidity, pH and total soluble solids. The turbidity of the juices and clarified juices was conducted with the Turbidimeter (Aqualytic Model TS705, Germany).
pH of the samples was determined using a digital pH meter (BP 3001 Trans Instruments, Singapore) calibrated at three buffer solutions of pH 4, 7 and 10. Total soluble solids (TSS) as °Brix was determined using a digital hand-held pocket refractometer (0-85 Brix) (PAL-α, Atago Co., Ltd., Japan).
Statistical analysis
Each data point is a result of triplicate experiments and is expressed as the mean ± standard deviation. Microsoft Excel was used to process the experimental data. The data were subjected to analysis of variance (ANOVA), followed by Tukey's multiple range tests with a 95% confidence interval. Statistical analysis was performed using SPSS Statistics version 27.
RESULTS
Extraction of the obtained cashew apple juices using three different methods
Results of the physical-chemical properties: TSS, pH, turbidity, and biochemical composition: TTC, TPC, Vitamin C, TTA of the cashew apple juices HPJ, BJ, and SPJ were presented in Table 1. First, TTC values ranged from 414.66 ± 48.50 to 433.37 ± 16.17 (mg tannic acid/100 mL juices) for all three juices, indicating no significant difference (P > 0.05) between them. Similarly, there were no significant differences (P > 0.05) in total soluble solids (TSS) (12.15-13.7 °Brix), total titratable acidity (TTA) (0.45-0.48%), and pH (4.21-4.31) among HPJ, BJ, and SPJ. However, there was a variation in the vitamin C content, ranging from 216.3 ± 0.06 to 245.10 ± 0.04 mg/100 mL, showing a slight effect of the type of mechanical force on vitamin C content (P < 0.05) among the samples. The highest vitamin C content was found in HPJ (245.10 ± 0.04 mg/100 mL). Additionally, HPJ had the highest total phenolic content (545.99 ± 5.02 mg/100 mL) and minimum turbidity (275.33 ± 5.69 NTU).
Table 1. Characterisation of HPJ, BJ and SPJ.
|
Parameters |
HPJ |
BJ |
SPJ |
|
TTC, mg tannic acid/100 mL |
433.37 ± 16.17a |
415.70 ±17.64a |
414.66 ± 48.50a |
|
TPC, mg GAE/100 mL |
545.99 ± 5.02a |
494.53 ± 6.26b |
501.09 ± 4.71b |
|
Vitamin C, mg/100 mL |
245.10 ± 0.04a |
216.3 ± 0.06c |
230.1 ± 0.04b |
|
TSS, °Brix |
12.15 ± 0.78a |
13.70 ± 0.71a |
12.25 ± 0.07a |
|
TTA, % |
0.48 ± 0.00a |
0.46 ± 0.07a |
0.45 ± 0.02a |
|
pH |
4.26 ± 0.03a |
4.31 ± 0.08a |
4.21 ± 0.01a |
|
Turbidity, NTU |
275.33 ± 5.69c |
4,308.33 ± 142.16a |
2,791.67 ± 101.04b |
Note: Mean value (n=3) ± SD. The same superscript letter within the row has no significant difference (P >0.05).
Detannification of the obtained juices
The HPJ, BJ and SPJ were first treated with gelatin in solution form (0.08% w/w in juice) to examine tannin removal efficiency. The method of juice extraction would be then determined based on the results obtained, as shown in Table 2, presenting TTC and turbidity of the three treated juices.
Table 2. Characterization of the treated HPJ, BJ and SPJ using the gelatin solution.
|
Parameters |
Treated HPJ |
Treated BJ |
Treated SPJ |
|
TTC, mg tannic acid/100 mL |
229.67 ± 13.23a |
241.11 ± 20.58a |
231.75 ± 16.17a |
|
Turbidity, NTU |
15.65 ± 0.07c |
139.00 ± 1.41a |
49.50 ± 9.19b |
Note: Mean value (n=3) ± SD. The same superscript letter within the row has no significant difference (P >0.05).
The TTC of the three clarified juices (HPJ, BJ, SPJ) did not show any statistically significant difference (P >0.05) and ranged from 229.67 ± 13.23 to 241.11 ± 20.58 mg/100 mL, similar to the three unclarified juice samples. The clarified HPJ (hydraulic pressed juice) had a turbidity of 15.65 ± 0.07 NTU. The hydraulic pressing method produced the clearest juice with a minimum turbidity 275.33 ± 5.69 NTU, making it the chosen method for further clarification and detannification under different conditions.
In the next step, the HPJ was investigated in more detail to determine the effect of different ratios of juice to gelatin, in both solution and powder form, for tannin removal.
The changes in TTC and TME of the HPJ sample during treatment with the gelatin solution and the gelatin powder are illustrated in Figure 2 and Figure 3, respectively. Figure 2 shows that the TTC in HPJ declined sharply from 432.33 ± 2.94 to 221.36 ± 1.47 mg/100 mL as the percentage of gelatin increased from 0 to 0.08% (w/w) (P < 0.05). Notably, the white flocculent precipitates occurred when the gelatin concentration reached 0.08%, with a TME of 48.80 ± 1.47%. As the percentage of gelatin was further increased from 0.08 to 0.16% (w/w), the TME remained relatively stable (48.80 - 51.24%), with no significant difference (P > 0.05).
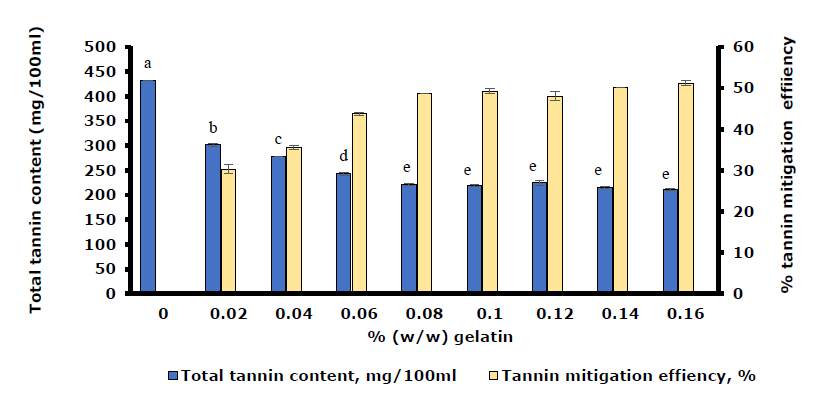
Figure 2. TTC and TME changes of the HPJ treated with the increasing amount of gelatin solution. Note: a-e: different letters show the significant differences between the values (P <0.05).
In Figure 3, it was observed that without gelatin powder (0%), the TTC of HPJ decreased slightly (4.8 ± 0.6%) due to centrifugation (2000 rpm, 10 minutes). The results showed that the most effective reduction in tannin content, reaching a maximum of 51.0 ± 0.3%, was achieved by increasing the percentage of gelatin to 1.0% and combining it with agitation and centrifugation.
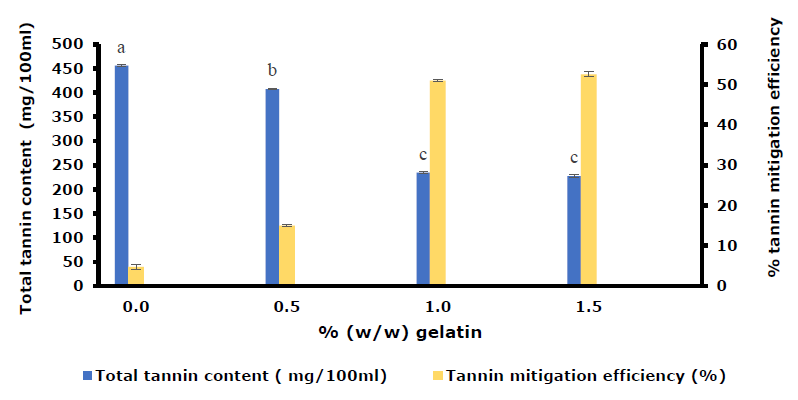
Figure 3. TTC and tannin mitigation efficiency changes of the HPJ treated with the increasing amount of gelatin powder. Note: a-c: different letters show the significant differences between the values (P <0.05).
Turbidity of the initial HPJ and clarified HPJ was measured after adding gelatin solution and gelatin powder, as shown in Figure 4a and Figure 4b, respectively. Adding a 0.1% (w/w) gelatin solution resulted in a 95.2% reduction in the turbidity of HPJ. However, the turbidity slightly increased after this point, as illustrated in Figure 4a. The greatest reduction in turbidity (81.4%) was achieved by adding 1.5% gelatin powder (Figure 4b). In summary, using gelatin solution for detannification and clarification proved to be a cost-effective method and enhanced the visual clarity of juice compared to using gelatin powder. Therefore, the gelatin solution could be chosen for the next step to investigate the relationship between the initial TTC of juice and the amount of gelatin.
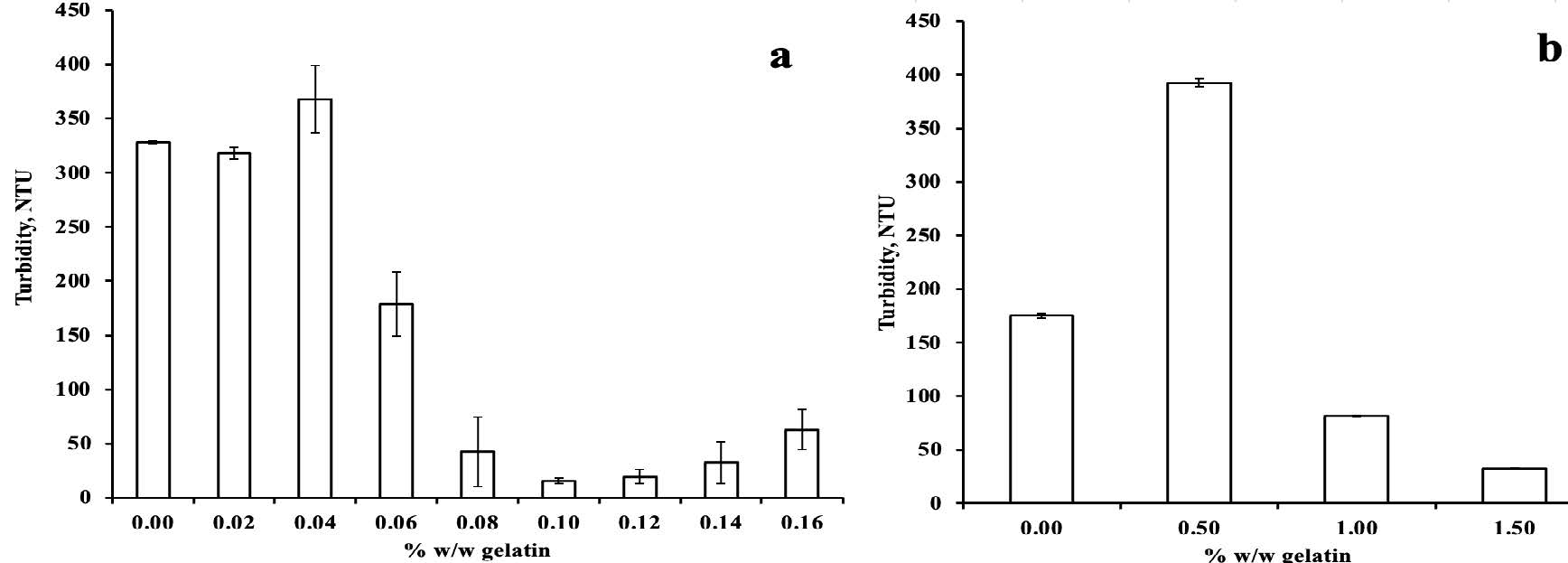
Figure 4. Turbidity of the HPJ and clarified HPJ after being treated with gelatin solution (a) and gelatin powder (b).
The relationship between the initial TTC of the HPJ and the amount of gelatin for detannification
The appropriate amount of gelatin needed to reach the maximum level of precipitation (48.0-49.0% tannin mitigation efficiency) varies for each initial TTC of the juice. Figure 5 showed a strong correlation (Pearson correlation coefficient r = 0.992) between the initial TTC of HPJ and the amount of gelatin to juice (w/w).
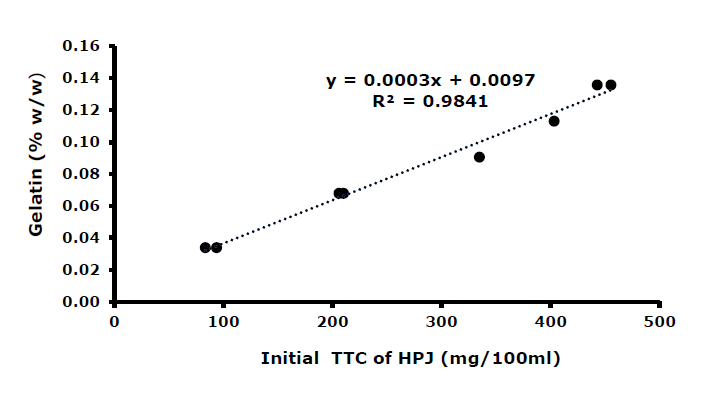
Figure 5. Correlation between initial TTC of the HPJ and gelatin added to obtain 48% TME.
Preparation of cashew apple wine from the treated HPJ
After being treated with the gelatin solution at 0.08% w/w, the cashew apple juice was fermented by yeast to produce wine. The wine was then characterized for its alcohol by volume, TTC, DPPH activity, residual sugar, and total acid, as presented in Table 3. Two commercial Vietnamese grape wines, a red wine and a white wine, were also analyzed for comparison.
Table 3. Physical and chemical properties of the cashew apple wine and the commercial grapes wines.
|
Parameters |
Cashew apple wine |
White wine |
Red wine |
|
Alcohol by volume, % (v/v) |
9.00 ± 0.00 |
10.50 ± 0.00 |
10.50 ± 0.00 |
|
TTC, mg tannic acid/100 mL |
142.38 ± 4.41a |
58.20 ± 0.00b |
148.62 ± 7.35a |
|
DPPH (IC50), µg/mL |
2.64 ± 0.06b |
42.13 ± 0.63a |
1.57 ± 0.35b |
|
Residual sugar, g/L |
80.83 ± 6.54a |
3.42 ± 0.74b |
4.43 ± 0.07b |
|
Total titratable acididy, g tartric acid/100 mL |
0.36 ± 0.02b |
0.40 ± 0.01ab |
0.45 ± 0.00a |
|
pH |
3.88 ± 0.03a |
3.40 ± 0.05ab |
3.32 ± 0.03b |
|
Turbidity, NTU |
11.70 ± 0.42a |
7.20 ± 0..14b |
Nd |
Note: Mean value (n=3) ± SD. The same superscript letter within the row has no significant difference (P >.05). Nd: not determined
The tannin contents are similar between the commercial red wine and the cashew wine, measuring at 142.38 ± 4.41 mg/100 mL and 148.62 ± 7.35 mg/100 mL, respectively. These values are higher compared to the tannin content of the commercial white wine, which is 58.20 ± 0.00 mg/100 mL. As a result, the DPPH IC50 values showed that the red and cashew wines have a stronger capacity to scavenge DPPH radicals compared to the white wine (Table 3). The results showed that the cashew wine has a much higher residual sugar content (80.83±6.54 g/L) than commercial wines.
DISCUSSION
In recent years, there has been a growing trend towards more efficient utilization of agro-industrial residues, aiming to reduce food waste and promote sustainable agricultural development while ensuring food security. Consequently, this study focuses on the potential use of cashew apples as a valuable and abundant by-product resource in Vietnam.
The cashew apple juices were obtained using three different extraction methods - hydraulic pressing, screw pressing (slow pressing) and blending. The TPC, TSS, TTA and pH of HPJ, BJ and SPL were not significantly affected by extraction methods. However, the turbidity of these juices was noticeably different. After extraction, this turbidity in fresh fruit juices results from suspended pectin particles from plant cell walls (Pinelo et al., 2010). The more cloudiness observed in the BJ and SPJ, which was caused by the high amount of pectin in the skin of the cashew apple, dissolving into the juice during the blending or screw pressing process, as the whole fruit, including the skin, is crushed. When tannin and gelatin form a complex, they can trap a part of pectin and other cell wall materials, causing a precipitate that can be filtered (Pinelo et al., 2010). This process results in higher clarity for all treated juices. Reducing the pectin content in cashew apple juice is important, as higher pectin levels not only make the juice appear opaque, but also increase the risk of methanol production during the ethanol fermentation process (Jackson, 2014).
Next, the treatment of HPJ in gelatin solution form with gelatin in powder form was compared in terms of tannin mitigation efficiency and turbidity. TME reached maximum 51% to 52% when using either gelatin powder or solution. A study by Geetha et al. (2020) found that a mixture of 1% pectinase and 0.5% tannase produced the highest TME of 52.54% after 24 hours. According to report of Aluko et al., (2023), detannification also increased the sweetness and reduced the astringency of pressed cashew apple juice.
Interestingly, the turbidity increases dramatically at a 0.5% gelatin powder dosage (see Figure 4b). A model by Siebert et al. (1999) suggests that polyphenols bind to multiple sites on proteins to explain this behavior. Polyphenols, on the other hand, are supposed to have a number of ends that can bind to proteins. When the concentration of polyphenol ends is equal to the number of protein binding sites, a large network is formed, corresponding to the largest complexes and resulting in maximum light scattering (maximum turbidity NTU).
Remarkably, the use of a 10% gelatin solution required less than 12.5 times the amount of gelatin compared to using gelatin powder to achieve a relatively stable tannin reduction efficiency (48.80-51.24%). It could be explained that when gelatin powder is mixed with HPJ, it swells, and the interactions between tannins and gelatin occur only on the surface of the swollen gelatin particles, necessitating a significantly greater quantity of gelatin compared to using it in a gelatin solution. Furthermore, there was no need for an extra centrifugation step as the precipitation occurred quickly and settled at the bottom. The decrease in TTC of the juice after the addition of gelatin is a result of the formation of insoluble gelatin-tannin complexes, which form through hydrogen bonding between the phenolic hydroxyl groups of the tannins and the carbonyl groups of the gelatin protein (Haslam, 1974). The insoluble complexes precipitate as flocculates and settle at the bottom of the container. This precipitation allows for easy separation from the clear juice through decantation and filtration processes. Interestingly, studies by Naka et al. (2016) observed a gradual increase in tannin content when higher concentrations of gelatin (beyond 2.4 g/L) were used, also a similar trend to Aluko et al. (2023). In our research, we observed a rise in turbidity after reaching the lowest level of 15.75 NTU when the gelatin dosage in the juice (beyond 0.1% w/w) was used. Similarly, Promajak et al. (2018) found that the turbidity slightly increased when gelatin powder was added within the range of 0.67-1.0% (w/v). This phenomenon may occur because higher levels of gelatin cause the tannins from the gelatin-tannin complexes to re-dissolve back into the juice (Haslam, 1974). Precipitation when tannin is in excess is due to the formation of hydrophobic tannin-coated proteins, rather than to the formation of cross-linked tannin-protein complexes. When protein is present in excess, the complexes remain soluble because each molecule of protein is bound by only a few phenolic ligands (McManus et al., 1981; Hagerman et al., 1998). In summary, removing tannins through gelatin-induced precipitation is an effective clarification method. However, it is essential to carefully consider the optimal gelatin concentration to balance the tannin reduction and potential re-solubilization of the complexes.
The tannin level in the juice is substantially influenced by several factors, including the variety of cashew apples, growing conditions, soil characteristics, and regional climate (Queiroz et al., 2011; Dabonne et al., 2015). Plants can activate the synthesis of polyphenols, including tannin compounds, in response to stress such as injury, pathogens, or nutrient deficiencies (Dixon’ & Paiva, 1995). Some studies recorded that the tannin content of cashew apples varied from 84-380 mg tannic acid/100 mL (Emmanuelle et al., 2016; Prommajak et al., 2018). In our study, the TTC in cashew apple juices grown in Van Gia town, Khanh Hoa province range from 248.2 to 478.9 mg/100 mL. Hence, it is important to establish the relationship between the initial TTC in the juice and the corresponding percentage of gelatin required for each production batch. This allows the appropriate gelatin dosage to be determined based on the initial tannin levels in the raw juice, to effectively remove the astringent tannins. The correlation between gelatin dosage (%w/w) (y) and initial TTC (x) can be expressed by: y = 0.003x + 0.00097 (Pearson correlation coefficient r = 0.992). These results indicated that this model effectively estimates the appropriate amount of gelatin needed to achieve a 48.0 % reduction in initial total tannin.
Finally, the cashew apple wine was prepared and compared to commercial grape wines. The astringency of wine is usually indicated by its TTC, while the sweetness of wine is directly related to its residual sugar levels. According to WineFolly, this cashew apple wine, fermented from dry yeast Saccharomyces cerevisiae RV-002, is categorized as a low-alcohol (ABV < 10%), sweet wine (residual sugar 35-120 g/L). The antioxidant activities of cashew apple wine are equivalent to Vietnamese commercial red wine. However, further research and testing are needed to fully assess the safety and stability of the cashew apple wine.
CONCLUSION
This study illustrated how different extraction methods - hydraulic pressing, slow pressing, and blending affect the quality of the cashew apple juices. It reveals that the hydraulic pressing had the least impact on the fruit skin, resulting in the lowest turbidity for the HPJ at 275.33 NTU. The treated HPJ exhibited the lowest turbidity of 15.65 NTU.
The study further demonstrated that using a gelatin solution required less than 12.5 times the amount of gelatin compared to using gelatin powder to achieve a relatively stable TME (48.80-51.24%). Furthermore, using gelatin solution also improved the overall visual clarity of the treated cashew apple juice.
The correlation between gelatin dosage (%w/w) (y) and initial total tannin content (x) was established as y = 0.003x + 0.00097. The strong Pearson correlation coefficient (r = 0.992) indicated this model can reliably estimate the appropriate gelatin dosage needed to achieve a 48.0 % reduction in the initial total tannin level.
The cashew apple wine fermented with Saccharomyces cerevisiae RV-002, is categorized as a low-alcohol, sweet wine and exhibits antioxidant properties similar to those of commercial red wines from Vietnam.
ACKNOWLEDGEMENTS
We sincerely acknowledge the valuable support provided by Nha Trang University for this study.
AUTHOR CONTRIBUTIONS
Thi Khanh Vinh Phan played a role in conceiving and designing the study, as well as in collecting data, analyzing it, interpreting the results, and preparing the manuscript. Thi Phuong Anh Tran participated in data collection, analysis and interpretation of results, editing and reviewing the manuscript. Nguyen Bao participated in analyzing and interpreting the results, editing and reviewing the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Akyereko, Y. G., Yeboah, G. B., Wireko-Manu, F. D., Alemawor, F., Mills-Robertson, F. C., and Odoom, W. 2023. Nutritional value and health benefits of cashew apple. JSFA Reports. 3(3): 110-118.
Aluko, A., Makule, E., and Kassim, N. 2023. Effect of clarification on physicochemical properties and nutrient retention of pressed and blended cashew apple juice. Food Science & Nutrition. 11(4): 1891–1903.
AOAC 920.57-1920, Alcohol in wines. By volume from specific gravity. The Association of Analytical Chemists (AOAC).
AOAC 923.09-1923, Invert sugar in sugars and syrups. Lane-eynon general volumetric method. The Association of Analytical Chemists (AOAC).
AOAC 942.15-1965, Acidity (Titratable) of Fruit Products. The Association of Analytical Chemists (AOAC).
AOAC 955.35-1955, Tannin in cloves and all spice. Titrimetric method. The Association of Analytical Chemists (AOAC).
AOAC 967.21-1968(2010), Ascorbic acid in vitamin preparations an juices. 2,6-dichloroindophenol titrimetric method. The Association of Analytical Chemists (AOAC).
Couri, S., Menezes, L. D., Pinto, G. S., Souza, M. D. L., and Freitas, S. P. 2002. Comparison of the cashew apple (Anacardium occidentale L.) juice clarification with tannase and gelatin. 2002. Boletim Do Centro de Pesquisa e Processamento de Alimentos. 20(1): 41–54.
Cruz Reina, L. J., Durán-Aranguren, D. D., Forero-Rojas, L. F., Tarapuez-Viveros, L. F., Durán-Sequeda, D., Carazzone, C., and Sierra, R. 2022. Chemical composition and bioactive compounds of cashew (Anacardium occidentale) apple juice and bagasse from Colombian varieties. Heliyon. 8(5): e09528.
Dabonne, S., Naka, T., Martin, D. K., and Patrice, K. L. 2015. Assessment of some biochemical parameters of apple juices from two cashew varieties as affected by three regions of Côte d'Ivoire. Journal of Advances in Agriculture. 5(2): 621–633.
De Abreu, F. P., Dornier, M., Dionisio, A. P., Carail, M., Caris-Veyrat, C., and Dhuique-Mayer, C. 2013. Cashew apple (Anacardium occidentale L.) extract from by-product of juice processing: A focus on carotenoids. Food Chemistry. 138(1): 25–31.
Dixon’, R. A. and Paiva, N. L. 1995. Stress-induced phenylpropanoid metabolism. The Plant Cell. 7(7): 1085.
Emmanuelle, D., Joseph, D., Victor, A., and Mohamed, M. S. 2016. A review of cashew (Anacardium occidentale L.) apple: Effects of processing techniques, properties and quality of juice. African Journal of Biotechnology. 15(47): 2637–2648.
Fernandes, I., Pérez-Gregorio, R., Soares, S., Mateus, N., and De Freitas, V. 2017. Wine flavonoids in health and disease prevention. Molecules. 22(2): 292.
Gawankar, M., Salvi, B., Pawar, C., Khanvilkar, M., Salvi, S., Dalvi, N., Malshe, K., Kadam, D., Saitwal, Y., and Haldankar, P. 2018. Technology development for cashew apple processing in Konkan region–a review. Advanced Agricultural Research & Technology Journal. 2(1): 40–47.
Geetha, P. 2020. Storage studies of enzyme clarified astringency free cashew apple juice and its value-added products. Journal of Pharmacognosy and Phytochemistry. 9(3): 574–576.
Hagerman, A. E., Riedl, K. M., Jones, G. A., Sovik, K. N., Ritchard, N. T., Hartzfeld, P. W., and Riechel, T. L. 1998. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry. 46(5): 1887–1892.
Haslam, E. 1974. Polyphenol-protein interactions. The Biochemical Journal. 139(1): 285–288.
Hemingway, R. W., Karchesy, J. J., and Branham, S. J. 1989. Chemistry and significance of condensed tannins. Springer. United States. https://doi.org/10.1007/978-1-4684-7511-1
Jackson, R.S. 2014. Wine science: Principles and applications. Elsevier Academic Press. Amsterdam.
Joshi, V. K. and Kumar, V. 2011. Importance, nutritive value, role, present status and future strategies in fruit wines in India.p.39-62. In Eds. P.S. Panesar et al. Bio-Processing of Foods.
Lee, C. A., Ismail, B., and Vickers, Z. M. 2012. The role of salivary proteins in the mechanism of astringency. Journal of Food Science. 77(4): C381-387.
Marc, A., Ange, K. D., Achille, T. F., and Georges, A. N. 2012. Phenolic profile of cashew apple juice (Anacardium occidentale L.) from Yamoussoukro and Korhogo (Côte d’Ivoire). Journal of Applied Biosciences. 49: 3331-3338.
McManus, J. P., Davis, K. G., Lilley, T. H., and Haslam, E. 1981. The association of proteins with polyphenols. Journal of the Chemical Society, Chemical Communications. 7: 309 – 311.
Mullins, J. T. and NeSmith, C. 1988. Nitrogen levels and yeast viability during ethanol fermentation of grain sorghum containing condensed tannins. Biomass. 16(2): 77–87.
Naka, T., Martin, D., Soumaila, D., Simplice, G. T., and Patrice, K. 2016. Some physico-chemical properties of cashew gum from cashew exudates and its use as clarifying agent of juice from cashew apple. Agriculture and Biology Journal of North America, 7(2): 107-115.
Pinelo, M., Zeuner, B., and Meyer, A. S. 2010. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food and Bioproducts Processing. 88(2–3): 259–265.
Pothitirat, W., Chomnawang, M. T., Supabphol, R., and Gritsanapan, W. 2009. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia, 80(7): 442–447.
Prommajak, T., Leksawasdi, N., and Rattanapanone, N. 2018. Optimizing tannin precipitation in cashew apple juice. Chiang Mai University Journal of Natural Sciences. 17(1): 13–23.
Prommajak, T., Leksawasdi, N., and Rattanapanone, N. 2019. Selection of microorganisms for ethanol production from cashew apple juice. Chiang Mai Journal of Science. 46(3): 469-480.
Prommajak, T., Leksawasdi, N., and Rattanapanone, N. 2020. Tannins in fruit juices and their removal. Chiang Mai University Journal of Natural Sciences. 19(1): 76–90.
Queiroz, C., Lopes, M. L. M., Fialho, E., and Valente-Mesquita, V. L. 2011. Changes in bioactive compounds and antioxidant capacity of fresh-cut cashew apple. Food Research International. 44(5): 1459–1462.
Rossetti, D., Bongaerts, J. H. H., Wantling, E., Stokes, J. R., and Williamson, A. M. 2009. Astringency of tea catechins: More than an oral lubrication tactile percept. Food Hydrocolloids. 23(7): 1984–1992.
Scalbert, A. 1991. Antimicrobial properties of tannins. Phytochemistry. 30(12): 3875–3883.
Siebert, K. J. 1999. Effects of protein-polyphenol interactions on beveerage haze, stabilization, and analysis. Journal of Argicultural and Food Chemistry. 47(2): 353-362.
Shebeko, S. K., Zupanets, I. A., Popov, O. S., Tarasenko, O. O., and Shalamay, A. S. 2018. Effects of quercetin and its combinations on health. p.373–394. In Ronald R. Watson, Victor R. Preedy, Sherma Zibadi (eds) Polyphenols: Mechanisms of Action in Human Health and Disease (Second Edition). Academic press, London. https://doi.org/10.1016/B978-0-12-813006-3.00027-1
Talasila, U., Vechalapu, R. R., and Shaik, K. B. 2012. Clarification, preservation, and shelf life evaluation of cashew apple juice. Food Science and Biotechnology. 21(3): 709–714.
Tran, T. P. A., Tran, T. T. V., Pham, T. L., and Phan, T. K. V. 2024. Potential use of polyphenol-enriched extract from Moringa oleifera leaves as an active ingredient in sunscreen. Natural and Life Sciences Communications. 23(2): e20240.
Vinh, P. T. K., Sáu, N. T., and Bảo, N. 2023. Khảo sát quá trình lên men nước mía ROC16 và chưng cất rượu mía sử dụng chế phẩm thương mại Saccharomyces cerevisia. Tạp Chí Phân Tích Hóa, Lý và Sinh Học. 29(2): 158–158.
Waghorn, G. C., Shelton, I. D., McNabb, W. C., and McCutcheon, S. N. 1994. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogenous aspects. The Journal of Agricultural Science. 123(1): 109–119.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Thi Khanh Vinh Phan1, *, Thi Phuong Anh Tran2, and Bao Nguyen3
1 Department of Food Technology, Nha Trang University, Khanh Hoa 650000, Vietnam.
2 Department of Chemical Engineering, Nha Trang University, Khanh Hoa 650000, Vietnam.
3 Department of Aquatic Processing, Nha Trang University, Khanh Hoa 650000, Vietnam.
Corresponding author: Thi Khanh Vinh Phan, E-mail: vinhptk@ntu.edu.vn
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 3, 2024;
Revised: November 27, 2024;
Accepted: November 29, 2024;
Online First: December 19, 2024

