
Combination of Ultrasonic Cavitation and Sanitizing Agents to Reduce Bacterial Biofilm on Stainless Steel
Nutthawut MeesilpPublished Date : December 16, 2024
DOI : https://doi.org/10.12982/NLSC.2025.016
Journal Issues : Number 1, January-March 2025
Abstract Pathogenic bacteria can contaminate in food products and line productions that causing foods spoilage and foodborne diseases. Biofilm is the important structure to support the survival and adherence of bacteria on materials from cleaning practices. Therefore, this research aimed to investigate the combination of sanitizing agents and ultrasonic cavitation for elimination of bacterial biofilm stained on stainless steels grade 316 L. Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli were proliferated by UHT milk, palm juice, and LB broth including the construction of their biofilm on stainless steel. Growth characteristics of all bacteria cultured with LB broth exhibited 9.60 log cfu/mL that greater than UHT milk, and palm juice after incubation at 37°C for 24 hours. Particularly, E.coli that cultured with every liquid culture medium has highly constructed biofilm on stainless steel, compared to other strains. The highest reducing biofilm value of P.aeruginosa, S.aureus, and E.coli after the elimination processes with sanitizing agents and ultrasonic cavitation have been discovered 89.34%, 88.46%, 81.13%, respectively.
Keywords: Biofilm, Sanitizing agent, Cavitation, Ultrasonic, Oxisan, Chlorine
Citation: Meesilp, N. 2025. Combination of ultrasonic cavitation and sanitizing agents to reduce bacterial biofilm on stainless steel. Natural and Life Sciences Communications. 24(1): e2025016.
INTRODUCTION
Milk and dairy products consist abundant source of essential nutrients for humans dietary such as proteins, fats, carbohydrates, vitamins, and minerals (Hooijdonk and Hettinga, 2015). The systems of dairy industry encounter the problems of hygiene process leading to cross contamination (Zastempowska et al. 2016). Raw milk can be contaminated with microorganisms from various sources such as feces in the dairy farms, including poor cleaning materials and equipment (Sanaa et al., 1993). The excellent practice of farm such as health and hygiene of cow, farm environment, cleaning and sanitizing procedures, and temperature to storage milk have the effect on the diversity of microorganisms to contaminate in raw milk (Zastempowska et al. 2016). In addition, sweet flavor drink with high sugar content such as palm (Arenga pinnata) juice containing sugar 100–144 g/kg (200−250 Liter of juice containing 12−15% sucrose) can be found as a source of microorganisms contamination and also support biofilm formation (Rana et al., 2009; Hemalatha et al., 2018).
Industries of diary, beverage, and food have the corrosion environment causing from the effect of chlorides ions and organic acid that aggressive damage on metals (Santamaria et al., 2020). Therefore, austenitic stainless steels, high corrosion resistant materials, are widely used in the line production and materials contact with foods. The corrosion resistance of stainless steels is due to the existence of a very thin chromium oxide rich passive film contains typically 1–3 nm thick (Santamaria et al., 2020). Stainless steel grade 316 L is a member of austenitic grades within main categories, alloy with a high chromium and nickel content, which resist to corrosion and high temperatures effect. It has been widely used in food industries, especially pipes, heat exchangers, and high temperature bolt (Li et al., 2012).
Several species of bacteria can grow in both forms as freedom single−cells in liquid and thickly assembled communities of viable cells attached to solid surfaces (Shen et al., 2021). P.aeruginosa, an opportunistic human pathogen, can be generally discovered this bacteria in soil, vegetation, and water, including clinical environments, surgical materials, and pharmaceutical products causing nosocomial infections. E.coli is a major disease agent infecting in intestinal tract, urinary tract, neonatal sepsis, meningitis, including animal mastitis (Sharma et al., 2016). Particularly, S.aureus is the cause of osteomyelitis and septic arthritis, bacteraemia or septicaemia, pneumonia and endocarditis, and it constructs staphylococcal biofilm containing a matrix of structure with environmental DNA, protein and polysaccharide (Howden et al., 2023; Otto, 2008). Therefore, the matrix structure of biofilm assists bacteria to survive under inappropriate environments such as dangerous chemical, desiccation, action of oxidizing biocides and antibiotics, including host immune defenses (Shen et al., 2021). The bio−structures contain many components of the residues of protein, carbohydrate, and fat played as the optimum environments for bacterial growth and formation of biofilm (Kütük and Temiz, 2022). Especially, production line of industries such as scratch, groove, and pipe joint are the critical point to accumulate biofilm (Kütük and Temiz, 2022). Therefore, piping systems and pasteurizers in the dairy and beverage industries have been damaged with abundant corrosion from bacterial biofilms due to post−pasteurization.
Food products and their equipment have to control microorganisms with appropriate and safe methods for consumers. Cleaning and sanitation programs are the important strategy to eliminate the accumulation of particulates, cells and biofilm formation in line production. However, the elimination of biofilm with mechanical treatments such as brushing, scrubbing, flushing is inefficient practices. Therefore, cleaning−in−place (CIP) is a process to apply chemical sanitizers to clean many equipment for reducing microbial contamination, but it cannot eliminates to complete, especially biofilm. The suitable elimination of biofilm depends on the condition of practices as follow temperature, time, type and concentration of chemical sanitizer (Furukawa et al., 2010; Gillham et al., 1999). Detergent and disinfectant properties have been used in the food industry as the conventional strategies based on the efficacy, safety, environmentally friendly, anti−foam, dregs reduce, and low cost. Likewise, chlorine and oxisan as bactericidal chemical agents are widely used in CIP systems of food industries due to the safety and without residue on materials (Furukawa et al., 2010). The concentrations of sanitizing agents are applied on the surface contact with food made by stainless steels, aluminums, rubber, and plastic depend on dirty stain levels, especially lipid and protein found in industries of dairy, beverage, and beer. In addition, oxisan is applied to terminal disinfectant and the sanitization of ultrafiltration and reverse osmosis membranes including other similar type membranes, and their associated piping systems (Tang et al., 2015). Particularly, Listeria monocytogenes, E. coli O157:H7, and Salmonella enterica are destroyed with lactic acid, and hydrogen peroxide–based sanitizer with a mild heat treatment (Robbins et al., 2005).
Cavitation is the mechanical technique used in dairy manufacturing to reduce thermoduric, spore formers, and microbial biofilms (Almalki and Anand, 2023). This technique generates the frequency sound wave above human hearing as ultrasound power such as 20 kHz leading to many bubbles with strong vibration in liquid conditions (Piyasena et al., 2003). At the level of 35 kHz can effectively eliminates L. monocytogenes biofilm formed on a polystyrene surface (Sanaa et al., 1993). The low−frequency of ultrasound consisting intensity levels either low or high are utilized in food processing and quality control for many kinds of material types such as polystyrene, stainless steel surface in the food industries (Bavisetty et al., 2024).
Bacterial growth under structure of biofilms can establish the resistant to chemical disinfectants more than planktonic cell. Thus, deficiency of the chemical disinfectants application has the cause of difficult to penetrate and diffuse into the biofilm structure (Yu et al., 2020; Bridier et al., 2021). High temperature and mechanical treatments have also utilized to reduce pathogens and microbial communities, but sometime may not successfully to remove exhaustive biofilms resulting hygiene problems (Branck et al., 2017; Meyer, 2003). The damage on surfaces of materials causing from some mechanical treatments can increase the accumulation of biofilms in scratch and recontamination in products from food processing (Hilbert et al., 2003). Therefore, the application of oxisan and chlorine sanitizer combined ultrasonic cavitation were investigated the efficiency to eliminate bacterial biofilm on stainless steels.
MATERIAL AND METHODS
Preparation of bacteria
Pathogenic bacteria as P. aeruginosa, S. aureus and E. coli were contaminated to UHT milk and palm juice. These bacteria were verified and re−checked the purity with their characteristics presenting on each selective medium. P. aeruginosa was cultured on King’s B medium (proteose peptone 20 g/L; dipotassium hydrogen phosphate (K2HPO4) 1.5 g/L; magnesium sulphate heptahydrate (MgSO4) 1.5 g/L; glycerol 15 mL; agar 15.0 g/L). S.aureus was grown on Mannitol salt agar (pancreatic digest of casein 5.0 g/L; peptone 5.0 g/L; beef extract 1.0 g/L sodium chloride (NaCl) 75 g/L; d−mannitol 10.0 g/L; phenol red 0.025 g/L; agar 15.0 g/L). E. coli was cultured on lauryl sulphate agar (casein peptone 40.0 g/L; yeast extract 6.0 g/L; sodium lauryl sulphate (NaC12H25SO4) 1.0 g/L; phenol red 0.2 g/L; agar 15.0 g/L). The growths of bacteria on medium were investigated colony morphology after incubation at 37°C for 24−48 hours. In addition, bacterial cell were examined Gram−stain with standard methods and observed under light microscopy.
Characteristics of bacterial growth
Inoculums of bacteria were prepared by using LB (Luria−Bertani) broth and incubation at 120 rpm, 37°C for 24 hours. Culture broths after incubation were harvested bacterial cell by using a centrifuge at 8,500 rpm, 4°C for 10 minutes, and then discarded supernatants. Cell pellets were thrice washed and re−suspended by using 0.85% normal saline solution. The turbidity of bacterial cell suspensions were adjusted and measured the value by spectrophotometer with an absorbance at 600 nm to 0.05 (108 cfu/mL). The prepared bacterial inoculums were inoculated as the starter of contamination to UHT milk, palm juice, and LB broth with incubation at 120 rpm, 37°C for 24 hours. Culture broth were collected the samples every 2 hours and then serially diluted in 0.85% normal saline solution. The appropriate bacterial diluents were detected the amount of bacteria on LB agar incubated at 37°C for 24 hours, and expressed the result as log cfu/mL unit.
Bacterial biofilm construction
Preparation of bacterial inoculum
All bacterial strains were prepared as inoculums by cultivating with LB broth and incubation at 120 rpm, 37°C for 24 hours. Culture broths after incubation were harvested bacterial cells by using a centrifuge at 8,500 rpm, 4°C for 10 minutes. The cell pellets were thrice washed and re−suspended with distilled water. The inoculums were measured a turbidity by using a spectrophotometer with an absorbance at 600 nm to 0.05 (108 cfu/mL).
Preparation of stainless steel
Stainless steel grade 316 L was utilized the surface for bacterial adherence and construction of biofilm. It was prepared as a single square piece consisting a size 10.0 × 15.0 mm. All stainless steel pieces were prepared by immersing in acetone solution for 1 hour, washed by distilled water, and finally soaked in 70% ethanol. Sterilization of stainless steel was done by autoclaving at 121°C, 15 psi for 15 minutes, and oven dried at 80°C for 2 hours (Meeslip and Meesil, 2018; Rossoni and Gaylarde, 2000).
Biofilm formation
Prepared stainless steels placed arrangement in the glass containers containing each liquid substrates were inoculated with 10% (v/v) bacterial inoculums and incubation at 50 rpm, 37°C for 24 hours. Pieces of stainless steels samples were collected every 4 hours until 24 hours, and then washed the free−cell with distilled water. Bacterial biofilm was assessed the stains on stainless steels surface by soaking crystal violet solution (crystal violet 2.0 g/L; 95% ethanol 20.0 mL; ammonia citrate 0.8%; distilled water 80.0 mL) for 5 minutes and then eluted with acetic acid solution 5.0 mL. Concentration of biofilm solubilized in acetic acid were measured by using a spectrophotometer with absorbance at 590 nm (Meeslip and Meesil, 2018).
Elimination of biofilm
Preparation of stainless steel
Bacterial biofilm constructed on stainless steels were prepared by following the above procedures.
Bacterial biofilm cleaning
Contaminated stainless steel samples were cleaned bacterial biofilm stains by using CIP sanitizing agents as follow 4% oxisan (hydrogen peroxide (H2O2) 25% v/v; peracetic acid (C2H4O3) 5% v/v; acetic acid 15% v/v; distilled water 55.0 mL), including 4% (v/v) chlorine (C5H14NO.Cl) solution (Rossoni and Gaylarde, 2000). Ultrasonic cavitation at the frequency 50−60 KHz were provided to eliminate bacterial biofilm with each sanitizing agents. The conditions to eliminate were 30°C, 55°C, and 80°C for 15 and 30 minutes. Biofilm on stainless steels without any cleaning practices were control treatments of each bacteria. Bacterial biofilm was estimated by using above methods. Remaining and reducing biofilm values on material surface were calculated by comparing to not−cleaning stainless steels.
Statistical analysis
Data were analyzed the statistic by using the One Way ANOVA.
RESULTS
Bacterial characteristic
All bacteria could grow on each medium. The purity and characteristic of bacterial colony, P.aeruginosa, S.aureus, and E.coli, have correctly exhibited on those mediums after incubation. The results of colony and cell morphology presented in Table 1.
Table 1. Bacterial characteristic on each selective medium.
|
Bacteria |
Morphology |
Mediums |
|
|
Colony* |
Cell and gram strain |
||
|
P.aeruginosa |
Yellow−green convex, circular, entire |
Gram negative Bacilli |
King’s B agar |
|
S.aureus |
Gold−yellow circular, convex, entire |
Gram positive Staphylococci |
Mannitol salt agar |
|
E.coli |
Yellow−green convex, circular, entire |
Gram negative Rod |
Lauryl sulphate−aniline blue agar |
Note: *Colony morphology contained the characteristics of size, color, texture, surface appearance, elevation, shape, and edge.
Characteristic of bacterial growth
rowth of each bacteria cultivated by UHT milk, palm juice, and LB broth has exhibited the different amount of population during incubation for 0−24 hours (Figure. 1). UHT milk cultivation has enhanced the growth of S.aureus rapidly at the initial time for 0−4 hours. In the other hand, bacterial strains P.aeruginosa and E. coli slowly increased in UHT milk at the same time, especially E.coli. Furthermore, the exponential growth phase of all strains in UHT milk has found after 4 hours expending until to 7 hours. The stationary phase of P.aeruginosa and E.coli immediately occurred after exponential phase ending at 8 hours, while, S.aureus lately exhibited this phase until at 10 hours. In case of cultivation in palm juice and LB broth, all bacteria similarly explored the lag phase characteristic at 0−4 and ending before 4 hours. Palm juice demonstrated that amount of all bacteria slowly increased in a short period time for 3−4 hours, especially E.coli. After 4 hours, the growth slightly and continuously increased for 4 hours extending until 24 hours, which P.aeruginosa amount did not decline in palm juice. Whereas, LB broth cultivation has not exhibited different characteristic of bacterial growths.
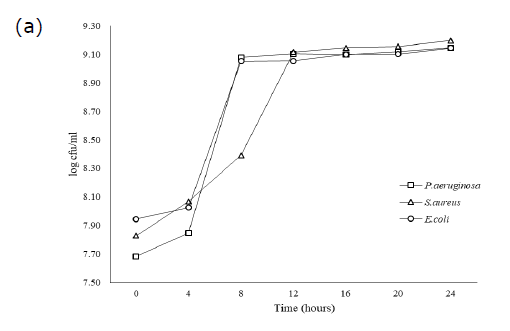
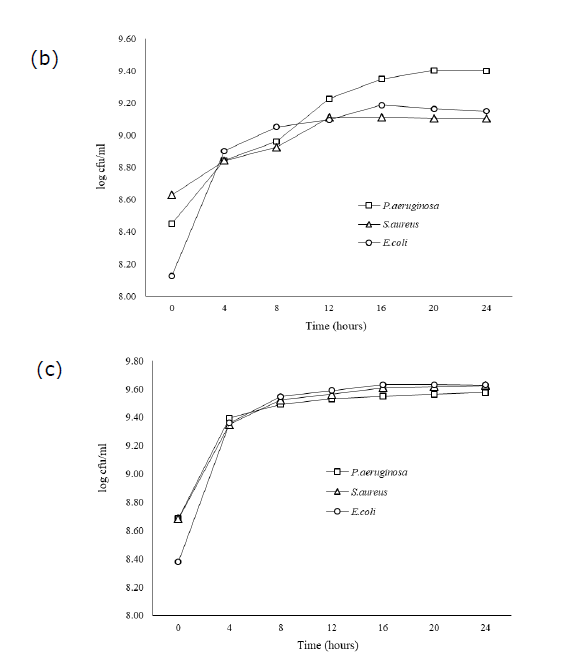
Figure 1. Growth of P.aeruginosa, S.aureaus, and E.coli in UHT milk (a) Palm juice (b) and LB broth (c) during 0−24 hours of incubation
Biofilm formation
During bacterial growth, the cultivation with different liquid medium has also affected on biofilm construction as shown in Figure 2. Particularly, P.aeruginosa and E.coli have rapidly produced a strong biofilm on stainless steel cultivated by UHT milk within 24 hours. In addition, LB broth could also highly enhance the formation of E.coli biofilm, compared to other liquid cultures. Nevertheless, S.aureus could construct biofilm on material surface, but the value lower than other bacteria grown in different culture substrates. Every liquid mediums have highly effected on the ability of P.aeruginosa to increase biofilm construction, whereas, S.aureus and E.coli have produced a constant amount of biofilm.
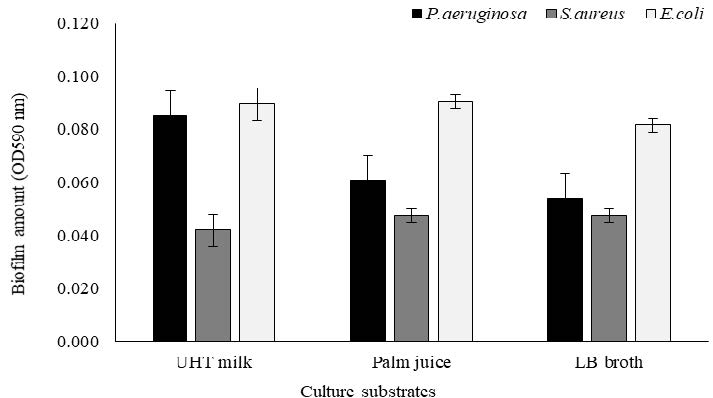
Figure 2. Biofilm formation of P.aeruginosa, S.aureus, and E.coli growing in UHT milk, Palm juice, and LB broth after incubation for 24 hours.
Bacterial biofilm on stainless steel after elimination
Stainless steel contaminated the stain of bacterial biofilm have been estimated the efficiency of various methods to eliminate. The result of remaining biofilm value after cleaning have presented data in Figure 3. Control treatments, without application of chemical agents and ultrasonic, have been discovered the highest remaining biofilm value on the stainless steel. Particularly, P.aeruginosa and E.coli cultured by UHT milk and palm juice have constructed a strong biofilm. The amount of bacterial biofilm stain on stainless steel after cleaning could reduce depended on the different conditions of cleaning practices.
Application of sanitizer and ultrasonic cavitation could significantly reduce P.aeruginosa biofilm cultured with various liquid mediums showing a little remaining stain on stainless steels, compared to control treatments. Increasing temperatures and times of ultrasonic providing could enhance the efficiency of oxisan and chlorine application. Elimination at 30°C for time 15 minutes, especially UHT milk, has exhibited the highest remaining biofilm value up to 86.47% when using distilled water with ultrasonic. On the other hand, utilization either oxisan or chlorine with ultrasonic at 80°C displayed the desirable result with the lowest remaining biofilm values on the material surface cultured by palm juice as 10.66%. However, application of distilled water with ultrasonic at 55°C and 80°C for 15 minutes, and 30−80 °C for 30 minutes, has also reduced bacterial biofilm that showing a low remaining value average as 12.41%. Therefore, at least the utilization only ultrasonic cavitation without any chemical reagents could reduce P.aeruginosa biofilm by demonstrating a low remaining stain on material surface.
Cultivation of S.aureus with UHT milk exhibited the highest remaining biofilm value up to 76.19%. Utilization either oxisan or chlorine with ultrasonic cavitation at 80°C exhibited the lowest remaining biofilm on stainless steels in palm juice as 11.54%. S.aureus grown with UHT milk displayed the difficult elimination of biofilm with every sanitizing agents at 30°C for 15 minutes, but the efficiency has increased at 80°C. Besides, ultrasonic cavitation and extension of time up to 30 minutes at 30−80 °C has exhibited the efficient outcomes showing a low remaining biofilm value, especially at 80°C with sanitizing agents. Therefore, application of ultrasonic with or without sanitizing agents demonstrated the reduction of S.aureus biofilm on material surface.
In addition, E.coli grown with LB broth has produced a strong biofilm on material surface that difficult elimination with distilled water and ultrasonic cavitation at 30°C for 15 minutes showing the highest remaining value by 85.71%. Particularly, cultivation of bacteria with UHT milk has displayed the difficult elimination of biofilm at 30°C and 55°C for 15 minutes with sanitizing agents and ultrasound cavitation, especially distilled water, compared to palm juice and LB broth. However, the rapid elimination of E.coli biofilm has been discovered in the appropriate condition to utilize ultrasonic cavitation at 80°C for 15 minutes that exhibiting a lower remaining biofilm value than other conditions. In addition, utilization either oxisan or chlorine with ultrasonic cavitation at 80°C for 30 minutes excellently eliminated E.coli biofilm stain on stainless steels in UHT milk, palm juice, and LB broth displaying the lowest remaining biofilm value by 20.56%, 18.87%, and 31.75%, respectively. However, only application of distilled water with ultrasonic cavitation at 80°C for 30 minutes also disposed bacterial biofilm, compared to without cleaning practices.
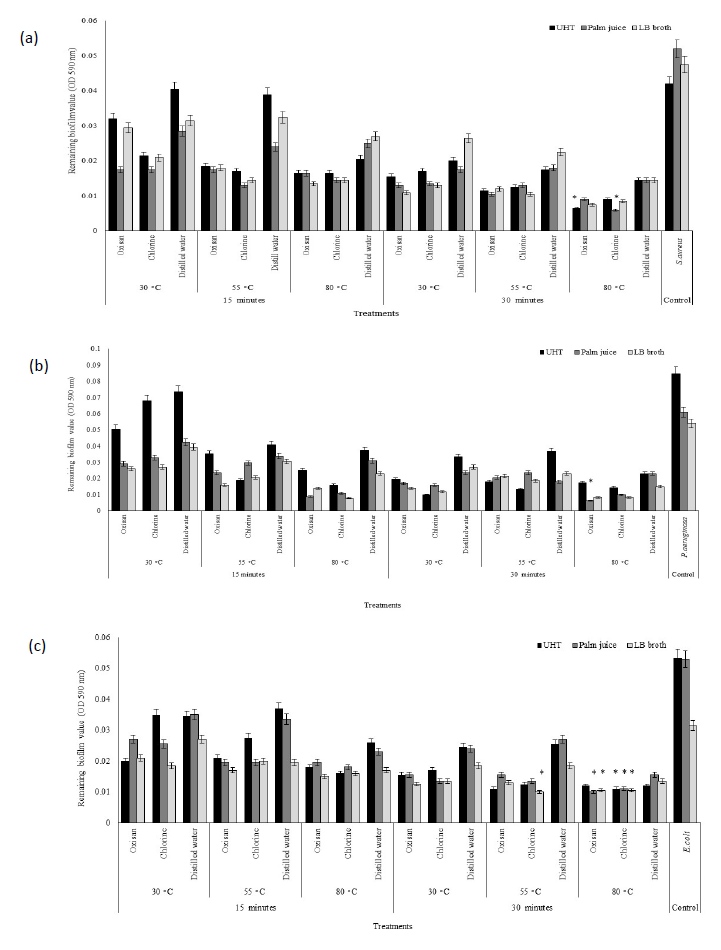
Figure 3. Remaining bacterial biofilm on stainless steel of P.aeruginosa (a), S.aureus (b), and E.coli (c) after elimination. * The lowest remaining biofilm value.
Efficiency of sanitizer
Potential elimination of biofilm on stainless steel depended on sanitizing agents, times, and temperatures, especially ultrasonic cavitation providing. Application of sanitizing agents with ultrasonic cavitation presented the results in Figure 4. The condition without the factors as follow sanitizing agents, temperature, and ultrasonic cavitation used as the control treatments with the reducing biofilm value by 0% have been applied as reference to compare the value of biofilm elimination. Biofilm of P.aeruginosa and S.aureus after cleaning have been discovered the highest reducing percentage as 89.34%.
Particularly, combination of oxisan sanitizer and ultrasonic cavitation at 80°C for 30 minutes to eliminate P.aeruginosa biofilm on stainless steels in palm juice demonstrated the highest reducing percentage as 89.34%. The application of chlorine with ultrasonic cavitation at 80°C for 30 minutes also exhibited the highest average reducing percentage (83.60%) from every liquid mediums, including at 80°C for 15 minutes rapidly reduced bacterial biofilm cultured with UHT milk average 82.78%, compared to oxisan (76.64%). In addition, utilization of distilled water with ultrasonic cavitation at 80°C for 15 minutes, and 30−80°C for 30 minutes could reduce P.aeruginosa biofilm more than 50%.
The elimination of biofilm, chlorine with ultrasonic cavitation at 80°C for 30 minutes exhibited the highest reducing percentage of S.aureus biofilm on stainless steels in palm juice as 88.46%. Biofilm of S.aureus in UHT milk has been difficultly eliminated the stain on material surface with every sanitizing agents and ultrasonic cavitation at 30°C for 15 minutes showing lower reducing percentage average 24.71% than palm juice (42.90%) and LB broth (42.90%). Application of oxisan and ultrasonic cavitation at 80°C for 30 minutes highly eliminated bacterial biofilm in UHT milk showing a high reducing percentage as 84.52%. On the other hand, distilled water with ultrasonic cavitation at 30°C for 15 minutes exhibited the lowest reducing percentage as 3.57%, whereas, replacing distilled water to oxisan or chlorine could increase the reducing percentage of biofilm up to 23.81% and 48.81%, respectively. Furthermore, increasing temperature and time of distilled water and ultrasonic cavitation up to 80°C for 15 minutes, or 30−80°C for 30 minutes enhanced the efficient the reduction of S.aureus biofilm more than 50%.
Almost conditions could greatly reduce E.coli biofilm stain on stainless steels. Application of both sanitizing agents clearly exhibited the efficiency to eliminate biofilm on the material surface average 45.46% in UHT milk and palm juice more than distilled water (27.92%) under providing ultrasonic cavitation at 30°C for 15 minutes. Either chlorine or oxisan with ultrasonic cavitation at 80°C for 30 minutes have been found the highest reducing percentage of biofilm in palm juice and UHT as 81.13% and 77.57%, respectively. E.coli biofilm on stainless steels in LB broth has been difficultly eliminated with ultrasonic cavitation in every conditions showing low reducing percentage, especially distilled water application. However, increasing temperature and time of distilled water with ultrasonic cavitation up to 80°C for 15 minutes, or 30−80°C for 30 minutes also enhanced the efficient to reduce E.coli biofilm more than 50%.
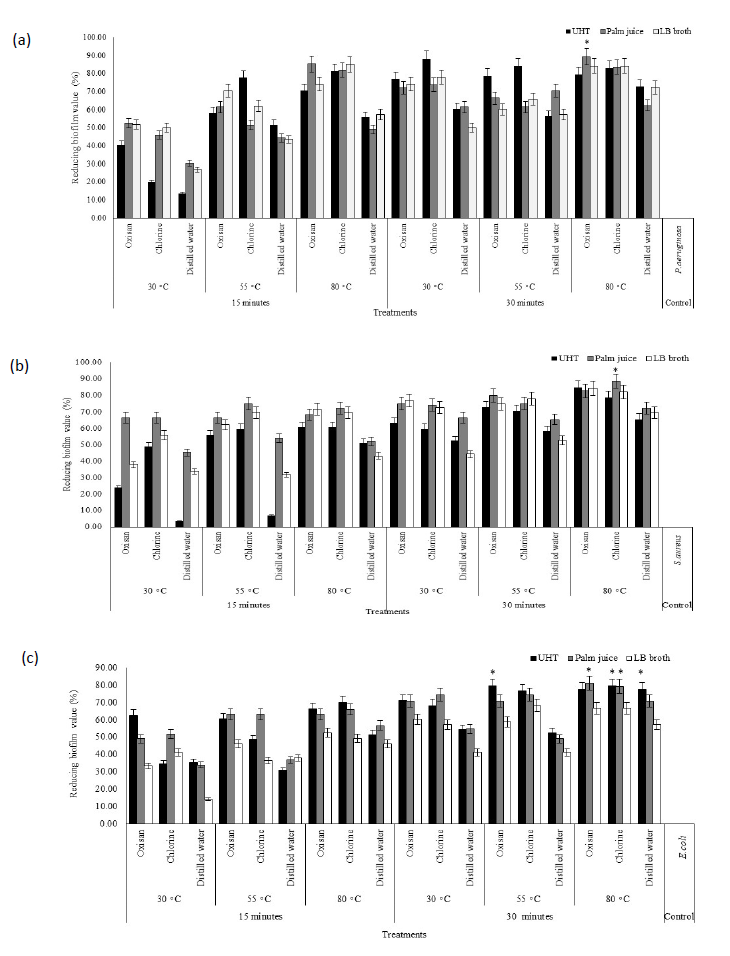
Figure 4. Reducing bacterial biofilm on stainless steel of P. aeruginosa (a), S. aureus (b), and E. coli (c) after elimination. * The highest reducing biofilm value.
DISCUSSION
Gram−negative bacteria, P.aeruginosa and E.coli, strongly exhibited the ability to construct biofilm on stainless steel surface. These strains contained the structural components of motile function such as flagella and fimbriae used to find out and adhesion on the surface navigated by many factors such as chemotactic, aerotactic, or phototactic responses. In case of non−motile cell, S.aureus has not consisted the motile structure, but it has also exhibited the capable to construct biofilm. Non−motile bacteria had the random motion carried by the flow of the suspending fluid beginning adhesion on the material surface or assemble as group (Haaber et al., 2012). Conditioning films (CFs) have been produced the structure in a natural aquatic environment discovered on solid surfaces due to the adsorption of naturally occurring dissolved exopolysaccharides, glycolipids, and other substances secreted by microorganisms (Bhagwat et al., 2021). In addition, conditioning films had the impact on initial adhesion of bacteria that changing the properties of solid surface physicochemical such as surface tension, charge density, and roughness. Hydrophobic and nonpolar surfaces have been often attached by many microorganisms more than hydrophilic materials (Kütük and Temiz, 2022; Raya et al., 2022).
Glucose concentration significantly increased the biofilm construction of P.aeruginosa during 8−24 hours of incubation compared to without glucose, which they have strongly affected to enhance cell attachment and increase the extracellular matrix as exopolysaccharide (EPS) of biofilm formation (She et al., 2019). P.aeruginosa secreted mucoid grouped in ESP that containing D−mannose, D−glucose, and D−rhamnose (Byrd et al., 2009). Moreover, protein GbaAB of S.aureus has been induced activity with glucose playing as intercellular polysaccharide to form biofilm (Lopezet al., 2011; She et al., 2019). Cell surface proteins of S.aureus consist of fibronectin and fibrinogen binding proteins, protein A, and poly−N-acetyl−1,6−glucosamine (PNAG) have contributed in biofilm formation at the early stage. Population of S.aureus grown under biofilm structures have been protected the damage of cell to survive in the conditions of pasteurization process that remaining bacterial amount under bio−structure more than free−living cell (Shen et al., 2021).
Planktonic as non−attached bacteria on surface would firstly assembly to small clusters and then increase the number of cell to large aggregates that high susceptible to antimicrobial and sanitizing agent more than mature biofilms. Bacteria in biofilm could return to planktonic cell in the liquid phase or irreversible adhesion depended on environmental factors such as pH, hydrodynamics, nutrients, temperatures, osmolality, ions, and oxygen (Aaron et al., 2002). Planktonic cell contained adhesin as surface proteins, such as sortase to protect cell washing out of the surface resulting a non−reversible attachment discovered in several Gram−positive bacteria including S.aureus, Streptococcus mutans, and Streptococcus pneumoniae (Marraffini et al., 2006; Mazmanian et al., 1999). Therefore, destroy adhesins of bacteria have been focused to increase the efficiency of sanitizing agent application to prevent biofilm development (Ming et al., 2017; Wang et al., 2018).\
Chlorine, the most common disinfectant, moderately reacted various components of bacterial cell, especially integrity of inner membrane of cells. E.coli biofilm resisted to chlorine more than planktonic cell. In addition, chlorine−treated cells have displayed the membrane integrity reduce (Kim et al., 2008). Oxisan consisted a high concentration of hydrogen peroxide including peracetic acid−based disinfectant in its compositions. Hydrogen peroxide contained a strong oxidizing property to damage bacterial components as follow proteins, DNA, and cellular membranes cytogenes, likewise, peracetic acid destroyed the outer cell membranes. Stronger oxidant had the effect on electrons transference much faster to deactivate rapidly on the microorganism. Application of sanitizing agents with a mild heat treatment significantly reduced microbial contamination exhibiting the bactericidal (Dominguez et al., 1987; Robbins et al., 2005). Attached bacterial cells in biofilm had more resistance to sanitizing agents than their planktonic (unattached) counterparts. However, survival of bacteria could be discovered the existence within biofilm withstood under cleaning process (Robbins et al., 2005).
The proportional of duration and intensity level of the mechanical oscillation generated by the ultrasound cavitation had no significant difference in the bactericidal effect between Gram−positive or Gram−negative bacteria (Scherba et al., 1991). The fragment of water and other molecules becoming as free radicals as a strong bactericide such as H and OH have been occurred the activities by effect of implosion after absorbing sufficient energy and the sudden reversal in the motion of bubble wall that produces a shock wave and high temperature (Joyce et al., 2003). Consequently, the ultrasonic treatment has affected on the destruction of EPS in the biofilms resulting change of permeability and cell membranes maturity. An ultrasonic treatment treating on evaluating stage of P.aeruginosa biofilm has discovered some perturbations and holes in outer membrane lipid bilayer resulting damage on inner metabolites and ions of cell wall (Runyan et al., 2006). In addition, E.coli and S.aureus biofilms on a stainless steel surface in milk contamination have been completely removed by using the flat ultrasonic (Oulahal et al., 2004; Yu et al., 2020). Thus, the bactericidal effect treated with ultrasound cavitation has significantly reduced biofilms adhesion. Likewise, the reaction of chemical to biofilm stain on the material surface had the effect on swelling, dissolve and change of the composition and structure of the deposit over time (Gillham et al., 1999).
CONCLUSION
The result stated that Gram−negative bacteria, P.aeruginosa and E.coli could construct biofilm on the surface of stainless steel greater than Gram−positive bacteria S.aureus. Oxisan or chlorine with ultrasonic cavitation at 80°C for 15−30 minutes had the efficiency to eliminate bacterial biofilm on stainless steel. The success of foodborne microbial biofilms removal has considered the important factors of surface conditions, food matrix nature, or microbial strains. Ultrasonic not only eliminated biofilm, but also decrease the adhesion of microbes, and inhibit their further attachments.
ACKNOWLEDGEMENTS
Department of Applied Biology, Faculty of Science and Liberal Art, Rajamangala University of Technology Isan Nakhon Ratchasima supported this work.
AUTHOR CONTRIBUTIONS
Nutthawut Meesilp (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing − original draft, Writing − review & editing)
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
Almalki, T. and Anand, S. 2023. Ultrasound−assisted cavitation effect on the biofilm−forming ability of common dairy sporeformers. Dairy. 4: 100–107.
Aaron, S. D., Ferris, W., Ramotar, K., Vandemheen, K., Chan, F., and Saginur, R. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm−grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. Journal of Clinical Microbiology. 40: 4172–4179.
Bavisetty, S.C.B., Karnjanapratum, S., Dave, J., Purba, D.T., Kudre, T., Maser, W.H., Nur Maiyah, N., Kingwascharapong, P., and Ali, A.M.M. 2024. Ultrasonication on collagen yield, physiochemical and structural properties from seabass (Lates Calcarifer) scales as affected by pretreatment and extraction conditions. Natural and Life Sciences Communications. 23(1): e2024003.
Bhagwat, G., O’Connor, W., Grainge, I., and Palanisami, T. 2021. Understanding the fundamental basis for biofilm formation on plastic surfaces: Role of conditioning films. Frontiers in Microbiology. 12: 687118.
Branck, T. A., Hurley, M. J., Prata, G. N., Crivello, C. A., and Marek, P. J. 2017. Efficacy of a sonicating swab for removal and capture of Listeria monocytogenes in biofilms on stainless steel. Applied and Environmental Microbiology. 83(1): e00109-17.
Bridier, A., Briandet, R., Thomas, V., and Dubois-Brissonnet, F. 2011. Resistance of bacterial biofilms to disinfectants: A review. Biofouling. 27: 1017−1032.
Byrd, M. S., Sadovskaya, I., Vinogradov, E., Lu, H., Sprinkle, A. B., Richardson, S. H., and Wozniak, D. J. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Molecular Microbiology. 73(4): 622−638.
Furukawa, S., Akiyoshi, Y., Komoriya, M., Ogihara, H., and Morinaga, Y. 2010. Removing Staphylococcus aureus and Escherichia coli biofilms on stainless steel by cleaning-in-place (CIP) cleaning agents. Food Control. 21: 669−672.
Dominguez, L., Garayazabal, J. F. F., Ferri, E. R., Vazquez, J. A., Gomez−Lucia, E., Ambrosio, C., and Suarez, G. 1987. Viability of Listeria monocytogenes on milk treated with hydrogen peroxide. Journal of Food Protection. 50: 636–639.
Gillham, C. R., Fryer, P. J., Hasting, A. P. M., and Wilson, D. I. 1999. Cleaning-in-Place of whey protein fouling deposits: Mechanisms controlling cleaning. Food and Bioproducts Processing. 77(2): 127−136.
Haaber, J., Cohn, M. T., Frees, D., Andersen, T. J., and Ingmer, H. 2012. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PLoS One. 7(7): 1−12.
Hemalatha, R., Kumar, A., Prakash, O., Supriya, A., Chauhan, A. S., and Kudachikar, V. B. 2018. Development and quality evaluation of ready to serve (RTS) beverage from cape gooseberry (Physalis peruviana L.). Beverages. 4(2): 42.
Hilbert, L. R., Bagge-Ravn, D., Kold, J., and Gram, L. 2003. Influence of surface roughness of stainless steel on microbial adhesion and corrosion resistance. International Biodeterioration & Biodegradation. 52: 175−185.
Hooijdonk, T. and Hettinga K. 2015. Dairy in a sustainable diet: A question of balance. Nutrition Reviews. 73(1): 48−54.
Howden, B.P., Giulieri, S.G., Lung, W.F., Baines, S.L., Sharkey, L.K., Lee, J.Y.H., Hachani, A., Monk, I.R., and Stinear, T.P. 2023. Staphylococcus aureus host interactions and adaptation. Nature Reviews Microbiology. 21: 380−395.
Joyce, E., Phull, S.S., Lorimer, J.P., and Mason, T.J. 2003. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrasonics Sonochemistry. 10: 315−318.
Kim, J., Pitts, B., Stewart, P. S., Camper, A., and Yoon, J. 2008. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrobial Agents and Chemotherapy. 40(11): 4172–4179.
Kütük, D. and Temiz, A. 2022. Biofilm formation potential of Bacillus toyonensis and Pseudomonas aeruginosa on the stainless steel test surfaces in a model dairy batch system. Folia Microbiologica. 67: 405−417.
Li, Y., Xiang, S., Zeng, H., Wang, J., and Wang, Q. 2015. The corrosion behavior of 304L and 316L stainless steel in food grade phosphoric acid solutions. Applied Mechanics and Materials. 109: 28−31.
Lopez, M. R., de Leon,L., and Moujir, L. 2011. Antibacterial properties of phenolic triterpenoids against Staphylococcus epidermidis. Planta Medica. 77(7): 726−729.
Marraffini, L. A. and Schneewind, O. 2006. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Molecular Microbiology. 62(5): 1402−1417.
Mazmanian, S. K., Liu, G., Ton-That, H., and Schneewind, O. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 285: 760−763.
Meesilp, N. and Mesil, N. 2018. Effect of microbial sanitizers for reducing biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa on stainless steel by cultivation with UHT milk. Food Science and Biotechnology. 28: 289−296.
Meyer, B. 2003. Approaches to prevention, removal and killing of biofilms. International Biodeterioration & Biodegradation. 51: 249−253.
Ming, D., Wang, D., Cao, F., Xiang, H., Mu, D., Cao, J., Li, B., Zhong, L., Dong, X., Zhong, X., et al. 2017. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Frontiers in Microbiology. 8: 2263.
Otto, M. 2008. Staphylococcal biofilms. Current Topics in Microbiology and Immunology. 322: 207−228.
Oulahal, N., Martial-Gros, A., Bonneau, M., and Blum, L.J. 2004. Combined effect of chelating agents and ultrasound on biofilm removal from stainless steel surfaces. Application to “Escherichia coli milk” and “Staphylococcus aureus milk” biofilms. Biofilms. 1: 65−73.
Piyasena, P., Mohareb, E., and McKellar, R. C. 2003. Inactivation of microbes using ultrasound: A review. International Journal of Food Microbiology. 87: 207−216.
Rana, P., Sohel, S., Islam, S., Akhter, S., Chowdhury, M. S., Alamgir, M., and Koike, M. 2009. Traditional practice of palm husbandry in the southeastern region of rural Bangladesh: Status and potentials. International Journal of Biodiversity Science & Management. 5: 155−161.
Raya, D., Shreya, A., Kumar, A., Giri, S. K., Salem, D. R., Gnimpieba, E. Z., Gadhamshetty, V., and Dhiman, S. S. 2022. Molecular regulation of conditioning film formation and quorum quenching in sulfate reducing bacteria. Frontiers in Microbiology. 13: 1008536.
Robbins, J. B., Fisher, C. W., Moltz, A. G., and Matrin, S. E. 2005. Elimination of Listeria monocytogenes biofilms by ozone, chlorine, and hydrogen peroxide. Journal of Food Protection. 68(3): 494–498.
Rossoni, E. M. M. and Gaylarde, C. C. 2000. Comparison of sodium hypochlorite and peracetic acid as sanitising agents for stainless steel food processing surfaces using epifluorescence microscopy. International Journal of Food Microbiology. 61(1): 81−85.
Runyan, C. M., Carmen, J. C., Beckstead, B. L., Nelson, J. L., Robison, R. A., and Pitt, W.G. 2006. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. Journal of General and Applied Microbiology. 52: 295−301.
Sanaa, M., Poutrel, B., Menard, J. L., and Serieys, F. 1993. Risk factors associated with contamination of raw milk by Listeria monocytogenes in dairy farms. Journal of Dairy Science. 76: 2891−2898.
Santamaria, M., Tranchida, G., and Franco, F. D. 2020. Corrosion resistance of passive films on different stainless steel grades in food and beverage industry. Corrosion Science. 173: 108778.
Scherba, G., Weigel, R. M., and Brien, W. D. 1991. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Applied and Environmental Microbiology. 57(7): 2079−2084.
Sharma, G., Sharm, S., Sharma, P., Chandola, D., Dang, S., Gupta, S., and Gabrani, R. 2016. Escherichia coli biofilm: Development and therapeutic strategies. Journal of Applied Microbiology. 121: 309−319.
She, P., Wang, Y., Liu, Y., Tan, F., Chen, L., Luo, Z., and Wu, Y. 2019. Effects of exogenous glucose on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. MicrobiologyOpen. 8: e933.
Shen, J., Wang, H., Zhu, C., Zhang, M., Shang, F., and Xue, T. 2021. Effect of biofilm on the survival of Staphylococcus aureus isolated from raw milk in high temperature and drying environment. Food Research International. 149: 110672.
Tang, X., Flint, S., Bennett, R., Brooks, J., and Zain, S.N.M. 2015. Biofilm contamination of ultrafiltration and reverse osmosis plants. In K.H. Teh, and S. Flint (Eds.). Biofilms in the dairy industry (pp. 138−153). John Wiley & Sons, Ltd.
Wang, W., Lin, X., Tao, J., Peng, Z., Xu, J., Yi, L., Li, F., Fanning, S., and Baloch, Z. 2018. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing China. Frontiers in Microbiology. 9: 1123.
Yu, H., Liu, Y., Li, L., Guo, Y., Xie, Y., Cheng, Y., and Yao, W. 2020. Ultrasound−involved emerging strategies for controlling foodborne microbial biofilms. Trends in Food Science & Technology. 96: 91−101.
Zastempowska, E., Grajewski, J., and Twarużek, M. 2016. Food-borne pathogens and contaminants in raw milk−A review. Annals of Animal Science. 16(3): 623−639.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Nutthawut Meesilp
Department of Applied Biology, Faculty of Science and Liberal Art, Rajamangala University of Technology Isan, Nakhon Ratchasima, 30000, Thailand.
Corresponding author: Nutthawut Meesilp, E-mail: nutthawutm@hotmail.com
ORCID: Nutthawut Meesilp: https://orcid.org/0009-0006-2589-8699
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 3, 2024;
Revised: November 27, 2024;
Accepted: November 29, 2024;
Online First: December 16, 2024

