
Potential Effects of Clitoria ternatea on Rotenone-Induced Rat Model of Parkinson Disease
Genevieve Simboh Anak Umbar, Hani Qistina Dayana, Hussin Muhammad, Mohamad Anuar Ahad, Azlina Zulkapli, Zolkapli Eshak, and Muhammad Danial Che Ramli*Published Date : December 3, 2024
DOI : https://doi.org/10.12982/NLSC.2025.015
Journal Issues : Number 1, January-March 2025
Abstract Parkinson's disease (PD) is a progressive neurodegenerative disorder characterised by motor dysfunction due to the loss of dopaminergic neurons in the substantia nigra. Clitoria ternatea (CT), a medicinal plant used in Ayurvedic medicine, has shown promising neuroprotective properties that may be beneficial in the context of PD. This study investigates the potential effects of CT on a rotenone-induced rat model of Parkinson's disease. Sixty adult male Sprague Dawley rats were divided into six groups: the normal control group, the positive control group treated with Levodopa, the negative control group, and three experimental groups treated with CT extract at doses of 150 mg/kg, 250 mg/kg, and 500 mg/kg, respectively, for 28 days. Behavioural analyses, including motor and cognitive assessments, and histological examinations were performed. The group treated with the highest dose (500 mg/kg) showed the most significant reduction in immobility time from day 1 to day 28. Anxiety-like behaviour was assessed using the open field test, and motor impairment was evaluated with the beam walking test. The highest dose group showed a significant increase in total distance travelled, from 72.3% to 95.4% over the treatment period, compared to the control groups. Histological analysis revealed that the 500 mg/kg treatment group had the most substantial reduction in Lewy bodies. Overall, the administration of CT extract at 500 mg/kg improved motor deficits, neurobehavioral performance, and reduced neurodegeneration. These findings suggest that CT could be a promising natural therapeutic agent for the treatment and management of PD.
Keywords: Parkinson’s disease, Clitoria ternatea, Behavioural study, Neurodegenerative disease
Funding: The authors are grateful for the research funding provided by the Management and Science University (MSU), Malaysia.
Citation: Umbar, G. S. A., Dayana, H. Q., Muhammad, H., Ahad, M. A., Zulkapli, A., Eshak, Z., and Ramli, M. D. C. 2025. The potential effects of Clitoria ternatea on the rotenone-induced rat model of Parkinson disease. Natural and Life Sciences Communications. 24(1): e2025015.
INTRODUCTION
Parkinson’s disease (PD) in an irreversible, progressive neurodegenerative disorder that predominantly arises from unknown causes, though it has some identifiable risk factors, such as aging, family history, pesticides exposure, and environmental chemicals (Chaiwut et al., 2020; Wimalasena et al., 2024). It primarily affects people over the age of 70, and as it progresses, patients often experience motor symptoms like rest tremors, rigidity, bradykinesia (slowness of movement), and postural instability, as well as non-motor symptoms (Khobkhun et al., 2014; Hou et al., 2019). PD occurs when nerve cells in the brain, especially those located in the substantia nigra, deteriorate and die. These cells produce dopamine, a neurotransmitter essential for controlling movement and coordination, so their loss results in dopamine deficiency and contributes to the typical motor symptoms of PD (Armstrong and Okun, 2020; Ramli et al., 2021).
Genetic predisposition and mitochondrial dysfunction are thought to play important roles, contributing to the disease through oxidative stress and inflammation (Belarbi et al., 2017). Neuroinflammation, a key factor in PD, leads to neuronal injury and cell death. This occurs through molecular mechanisms like increased glial cell activation and oxidative stress, which trigger the release of pro-inflammatory and neurotoxic substances that damage neurons and may contribute to dementia (Harry and Kraft, 2008; Simpson and Oliver, 2020). Neuronal death in PD is also influenced by genetic mutations that cause accumulation of alpha-synuclein in Lewy bodies, impair protein degradation systems, and increase oxidative stress and mitochondrial failure, all which contribute to neurodegeneration (Beitz, 2014).
The increase prevalence of neurodegenerative illnesses like PD poses significant challenges for the healthcare system and economy. If effective treatments are not developed soon, the impact of resources will be substantial. Clitoria ternatea (CT), a member of the Fabaceae family with a long history of use in traditional medicine, is being investigated as a promising treatment. Its primary benefit is its antioxidant property, which is largely attributed to the presence of ternatins, a key anthocyanin in the plant. This antioxidant property is vital for protecting cells from oxidative stress (Escher et al., 2020). Additionally, CT extract exhibit anti-inflammatory effects due to their flavonoid content, which can help manage conditions associated with inflammation. Studies have demonstrated that these extracts may ease symptoms of arthritis and other inflammatory disorders (Al-Snafi, 2016; Jeyaraj et al., 2021). The current study explores the neuroprotective potential of CT against PD induced by the neurotoxin rotenone in rats, suggesting that CT may offer new avenues for neuroprotection in PD.
MATERIALS AND METHODS
Animals
A total of 60 adult male Sprague Dawley rats, 8 weeks old, weighing 250 ± 50 grams were obtained from the Animal Research and Service Centre, Institute for Medical Research (IMR) breeding colony. The rats were housed in groups of three to four rats per cage and kept at a constant temperature under a 12-hour light/dark cycle. Food and water were given ad libitum. The experiments were conducted with the approval of the Research Management Centre (RMC), Animal Care and Use Committee at Management and Science University (MSU) (MSURMC02/FR01/02/L3/020). All surgical procedures and behavioral experiments occurred during the light phase.
Ethical considerations and sample size
Calculating the appropriate sample size for research is crucial to ensure that the study results are reliable and statistically significant, without including more animals than necessary to detect a significant effect. The principle of the 3Rs (Replacement, Reduction, Refinement) should always guide animal research. Institutional guidelines or ethical committees often require a detailed justification of the sample size calculation. To achieve the desired statistical power, sixty (60) Sprague Dawley rats were included in the study, with ten (10) animals allocated to the normal, positive, negative and three treatments groups respectively. The sample size was calculated using software, resulting in 10 animals per group (Naduvilath et al., 2020). This can be expressed using the following formula:
Corrected sample size = Sample size/ (1− [% attrition/100])
Toxicology study and mean body weight of rat throughout 28-days post treatment
Toxicology research examines the harmful effects that a medicine or chemical may have on animals over short or long periods (Saganuwan, 2017). In this study, CT was administered orally in three different dosages: 150 mg/kg, 250 mg/kg, and 500 mg/kg. Additionally, the cytotoxic effects on the liver and kidneys were analyzed to detect any cellular changes due to the varying dosages. Before administering CT, all rats in each group were weighed using a balance, and CT was given according to the selected dosage. The mean body weight was then calculated and recorded (Saganuwan, 2017).
Clitorea ternatea (CT) extraction
CT flowers were obtained from Forest Research Institute Malaysia (FRIM) in Kuala Lumpur. The flowers were cut, air-dried and ground into a fine powder by using mortar. All samples were stored at 20 °C until required for extraction (Caroline Paz Gonçalves et al., 2024). The process of aqueous extraction is summarized in Figure 1 below:
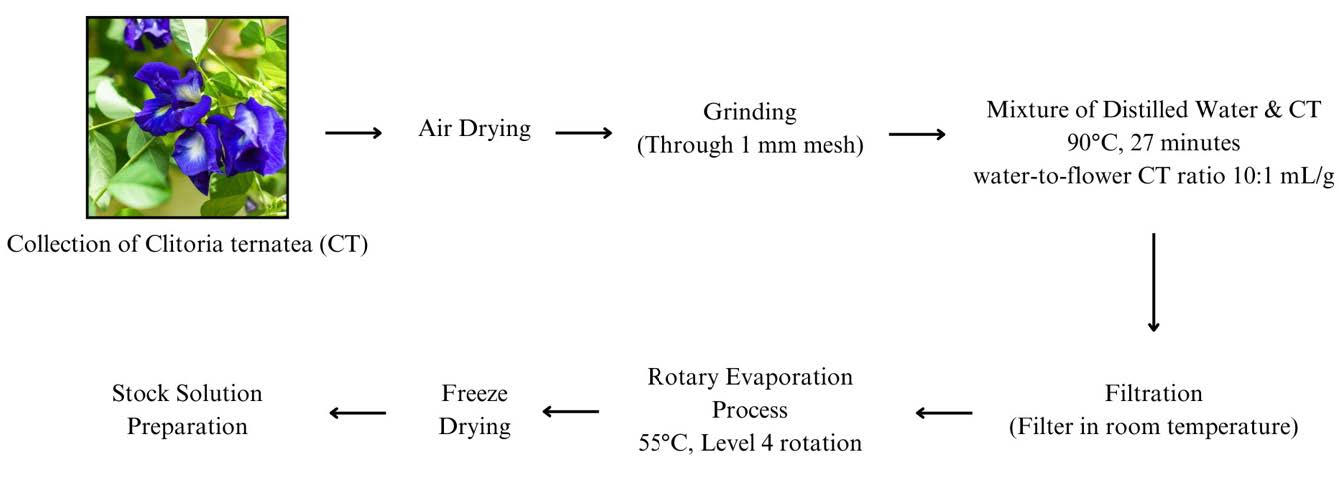
Figure 1. Extraction process for aqueous extracts of CT.
Study design
This research was an experimental study conducted over approximately 5 months, with a 58-day laboratory trial to assess neuronal functional recovery parameters following treatment in rats. Rotenone was administered via intraperitoneal injection to induce a PD-like effect, followed by treatment with CT after 28 days of rotenone/levodopa induction.
The rats were divided into six groups (n = 10 per group) as outlined below:
• Normal group: Rats were not induced with rotenone and served as a control. They were fed with a conventional diet.
• Positive control group: Rats were induced with rotenone (2.0 mg/kg/day) for 28 consecutive days to developed PD-like effect and received levodopa treatment (10 mg/kg, i.p.). They were fed a conventional diet.
• Negative control group: Rats were induced with rotenone (2.0 mg/kg/day) for 28 consecutive days to develop a PD-like effect but did not receive any treatment. They were fed a conventional diet.
• Experimental 1 group: Rats were induced with rotenone (2.0 mg/kg/day) for 28 consecutive days to develop a PD-like effect and received treatment with CT extract (150 mg/kg) (Taur and Patil, 2011) for the following 28 days. They were fed a conventional diet.
• Experimental 2 group: Rats were induced with rotenone (2.0 mg/kg/day) for 28 consecutive days to develop a PD-like effect and received treatment with CT extract (250 mg/kg) (Jiji and Muralidharan, 2022) for the following 28 days. They were fed a conventional diet.
• Experimental 3 group: Rats were induced with rotenone (2.0 mg/kg/day) for 28 consecutive days to develop a PD-like effect and received treatment with CT extract (500 mg/kg) (Jiji and Muralidharan, 2022) for the following 28 days. They were fed a conventional diet.
Behavioral analysis
Forced swim test
In the forced swimming test, each rat was placed in an open cylindrical container with a diameter of 10 cm, a height of 25 cm, and filled with water to a depth of 19 cm at a temperature 25°C. Behavioral responses were recorded and analyzed over a 5-minute test session, focusing on immobility time (the duration spent floating unassisted with only minimal movements to keep the head above water) and mobility time (climbing behavior), which was defined as upward-directed movements of the forepaw along the cylinder walls (Kraeuter et al., 2019).
Open field test
To assess general activity associated with Parkinson-like symptoms in rat models, the animals were subjected to open-field behavioral assessment. The rats received no prior training. Each rat was placed in 100 cm x 100 cm arena for 5 minutes, and their horizontal movement was monitored by an observer and recorded using SMART software. The experiment was repeated on the following day, and the average distance traveled across the two trials per rat was calculated for further analysis (Seibenhener and Wooten, 2015). The results are reported as the average travelled distance (cm) per 5 minutes.
Beam walking test
The beam walking test was performed on all groups of rats on days 1, 7, 14, 21 and 28 for both pre- and post-treatment (except the normal control group, which was assessed only pre-treatment). The rats were trained to traverse a beam (100 cm in length, 2.8 mm in diameter) to reach a cage at the other end. The completion time (the interval from the moment the rat was released to when it entered the cage) and the number of paw slips (defined as a forelimb descending more than 1.5 cm below the surface of the beam) were recorded by observers blinded to the treatment conditions. Each rat was allowed up to 3 minutes to complete a trial. If the rat fell or took more than 3 minutes, the trial was recorded as incomplete (Russell et al., 2011).
Histological analysis
Hematoxylin and eosin (H&E) staining
The midbrain region containing the substantia nigra tissue was fixed in 10% formaldehyde overnight at room temperature, then dehydrated in an ethanol gradient (70%, 95%, 100%, 100%, and 100%) for one hour each. The tissue was cleared twice in xylene for one hour each and then embedded in paraffin. The paraffin blocks were sectioned into 5 µm thick slices, which were air-dried for one day. The sections were then cleared in xylene, dehydrated with a standard ethanol gradient, and washed at room temperature.
Hematoxylin staining was applied for 4 minutes at room temperature, followed by a rinse with buffer to remove excess stain. The samples were then dehydrated through a graded ethanol series for 2 minutes at each concentration, counterstained with eosin for 1 minute at room temperature, and cleared three times with xylene for 3 minutes each. The sections were mounted with dibutyphthalate polystyrene xylene (DPX) mountant and examined under a light microscope to assess pathological characteristics. Photographs were taken to document the staining (Feldman and Wolfe, 2014).
Cresyl violet staining
Brain slices stained with Cresyl Violet were preserved in 10% formaldehyde. The sections were cleaned with xylene twice (3 minutes each) and then immersed in two ethanol washes. Next, the samples were stained in 0.5% cresyl violet acid solution for 2 minutes and rinsed in deionized water. Afterward, the sections were briefly cleaned in ethanol for 1 minute. The slices were then covered with DPX mountant. Once dried, histological images at the substantia nigra level were captured using a light microscope (Miko and Varga, 2015).
Statistical analysis
The data were statistically analysed and presented as mean ± S.E.M. Comparisons across different groups were performed using SPSS version 29, employing repeated measures ANOVA followed by Tukey's post hoc test. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Mean body weight of rat through 28-days post treatment
All rats in each group were weighed using a balance before being administered CT at the selected dosage. The mean body weight was calculated and is presented in Table 1 below.
Table 1. Mean body weight of rats after 28-days after post-treatment with CT flower extract.
|
Treatment group |
Dose (mg/kg) |
Weekly mean body weight (g) 28-days post treatment |
Mean weight gain (g) |
||||
|
DAY 1 |
DAY7 |
DAY 14 |
DAY 21 |
DAY 28 |
|||
|
Control |
N/A |
357.60 ± 0.24 |
361.10 ± 0.26 |
362.50 ± 0.31 |
370.30 ± 0.42 |
372.10 ± 0.20 |
14.50 |
|
Group 1 |
150 |
320.10 ± 0.32 |
323.00 ± 0.56 |
325.40 ± 0.72 |
330.90 ± 0.13 |
335.70 ± 0.21 |
15.60 |
|
Group 2 |
250 |
290.40 ± 0.50 |
295.10 ± 0.42 |
300.20 ± 0.24 |
302.60 ± 0.39 |
304.50 ± 0.30 |
14.10 |
|
Group 3 |
500 |
327.40 ± 0.87 |
328.30 ± 0.61 |
330.40 ± 0.32 |
335.70 ± 0.54 |
341.80 ± 0.10 |
14.40 |
Behavioral analysis
Each group evaluated rat behavior using the forced swim test, open field test, beam walking test. Following model establishment, behavioral changes in each group were observed at days 1, 7, 14, 21, and 28 during both pre- and post-treatment phases.
Forced swim test
Figures 2a and 2b present the results from the forced swim test, used to assess overall motor ability/deficits in the animals. At all-time points, the control group displayed the highest values compared to other groups. In the forced swim test, total swimming time significantly increased in all treatment groups from day 1 to day 28 compared to the negative control group. The negative control rats exhibited longer immobility times, reflecting the “giving up” behavior typically observed in depressive patients, compared to the other groups, particularly the treatment groups receiving different doses of CT (Figure 2b) (Fontoura et al., 2017).
Among the treatment groups, Group 3 demonstrated the greatest improvement in immobility from day 1 to day 28. In contrast, the treatment group receiving CT at 250 mg/kg showed an inconsistent reduction in immobility time compared to other groups. One-way ANOVA indicated a significant treatment effect in rotenone-induced rats treated with different doses of CT, with the highest CT dose showing the most substantial improvement in immobility.
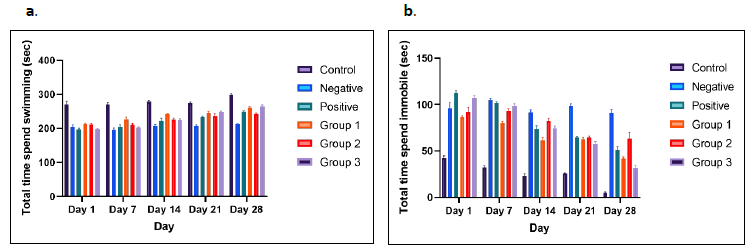
Figure 2. Forced swim test. Representative motor activity maps of rat movement from each treatment group and control groups (day 1 until day 28); total time spent swimming bar graph (a), and total time spent immobile bar graph (b). The results showed significant different (P <0.05).
Open field test
The anxiety-like behavior induced by the lesion was confirmed by testing the rat model in the open field. Representative motor activity maps showing the movement of each treatment group from day 1 to day 28 post-treatment are presented in Figure 3. Compared with the control group, treatment groups 1 and 2 showed minimal differences in total distance traveled. In contrast, treatment group 3 exhibited a significant increase in total distance, ranging from 72.3% on day 1 to 95.4% on day 28.
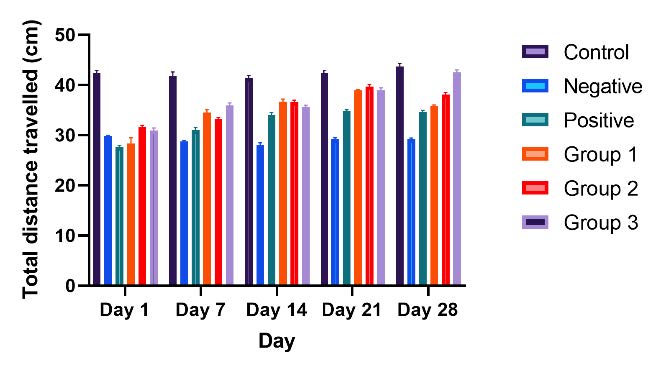
Figure 3. Open field test. Representative motor activity maps of rat movement from each treatment group and control groups (day 1 until day 28); in total distance travelled bar graph. The results showed significant different (P <0.05).
Beam walking test
In this study, rats were tested on 2.8 mm beams, 100 cm in length. Performance was analyzed based on the number of paw slips and the time take to complete each trial. While there were occasional slips and falls, these behaviors can become more frequent and measurable in genetically or pharmacologically manipulated animals. Impaired rats in the negative control group frequently clung to the side of the beam, which increased their crossing time. Activity levels also varied based on the rat strain.
As shown in Figure 4a, treatment group 1 displayed a consistent reduction in paw slips compared to treatment groups 2 and 3. In Figure 4b, the time required to complete the test per round was significantly reduced from day 1 to day 28 post-treatment.
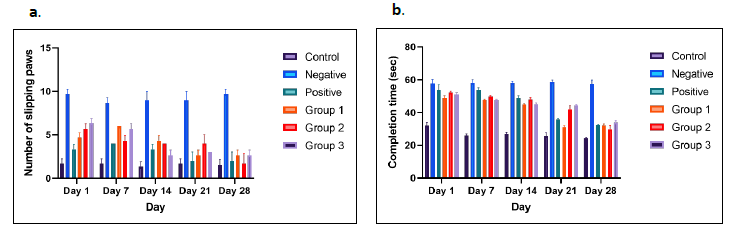
Figure 4. Beam walking test. Comparison of the number of slipping paws (a) and completion time (seconds) (b) of rat model from day 1 to day 28 in each group. The results showed significant different (P <0.05).
Histological analysis
Hematoxylin and eosin (H&E) staining
The H&E staining demonstrated that substantia nigra neurons in the normal group exhibited higher density, greater numbers, larger volumes, and clear, elliptical nuclear structures. In contrast, substantia nigra neurons in the PD group, which served as the negative control group, displayed fewer neurons, pyknosis, condensation, interstitial edema, and visible slender, darkly stained neurons. Figure 5(a) clearly shows the presence of Lewy bodies in the microscopic view of the negative control group.
The comparison between each treatment group was conducted by analyzing the number of Lewy bodies in a single microscopic field. Among these, the treatment group 3 exhibited the greatest reduction in Lewy bodies compared to other groups, though all treatment groups showed reductions in Lewy bodies relative to the negative control group.
Figure 5(b) illustrates histological differences in liver tissue. The histopathological examination of liver sections from the control revealed a normal histological structure, closely resembling that of other control and treatment groups. The liver parenchyma consisted of small, roughly hexagonal lobules with portal tracts at the apices. Within these tubules, hepatocytes were organized as cords of cells connecting peripheral portal tracts to central veins (the terminal branches of hepatic veins).
Figure 5(c) displays the histological differences in kidney tissue. Histopathological examination of kidney sections from the control group revealed a normal histological structure, like other control and treatment groups. Glomerular and pericapillary endothelial cells were separated, even in the initial whole-kidney single-cell sequencing analysis.
Cresyl violet staining
Cresyl violet staining is a histological method frequently used to analyze neuronal morphology, assess pathology, and study the brain’s cytoarchitecture. In our study, the results showed signs of neuronal damage in the substantia nigra of the treated group and the negative control group when compared to the normal group. Additionally, Figure 5(d) clearly shows a noticeable reduction in the number of neurons in these groups.
The comparison between treatment groups was assessed by counting the number of neurons within a single field of microscopic view, as shown in Figure 5(d). Among the treatment groups, treatment group 3 had the highest neuron count compared to the other groups, with an increase in neuron numbers relative to the negative control group.
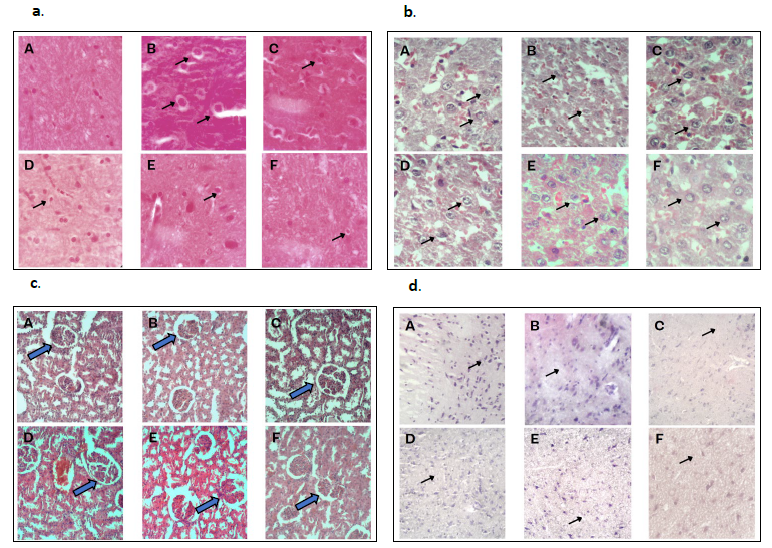
Figure 5. H&E staining of substantia nigra region (a), liver (b), kidney (c) and Cresyl violet stained (d) sections of brain from rats’ model. Control (A), negative (B), positive (C), treatment of CT at a dose of 150mg/kg body weight (D), 250mg/kg body weight (E) and 500mg/kg body weight (F). Original total magnification of ×400. The arrow (↗) symbol represents Substantia nigra (a), hepatocytes (b), glomerulus (c) and neurons (d).
DISCUSSION
Safety and toxicology effect of CT towards rat
In this study, we assessed the potential for mortality and toxic effects associated with long-term oral administration of CT flower extract. Rat models received doses of 150, 250 and 500 mg/kg body weight via oral gavage. The doses studies did not lead to any deaths or acute or chronic toxic effects over the extended study period of 28 days-post treatment. Throughout this period, the doses had no significant effect on water or food consumption. Additionally, there were no significant differences in the weekly mean body weights between the extract-treated and control groups, as shown in Table 1.
These findings suggest that CT flower is safe, as it did not cause lethality or behavioral changes in the test animals. This conclusion is consistent with previous research by Islam and Kabir in 2019, who found that the highest dose of CT (400 mg/kg body weight) in their study did not result in mortality in their mouse model (Islam and Kabir, 2019). Additionally, a 2019 study on the toxicity evaluation of methanol extract of CT L. leaf, which tested doses of 50, 300, and 2,000 mg/kg body weight on rat models, also reported no lethality at any dose level (Kamilla et al., 2012). The authors further noted that administering higher doses could be physiologically unsound and is not generally recommended.
The use of CT extract at a dose of 500 mg/kg for 28 days has been widely studied in animal models for various pharmacological activities, such as antioxidant, antidiabetic, and anti-inflammatory effects (Dewi et al., 2023). However, clinical studies translating these doses to humans are limited. An acute human trial involving CT demonstrated positive effects on postprandial glucose levels and antioxidant capacity when smaller quantities (1–2 g of extract) were consumed along with sucrose, indicating that the compounds in CT could offer health benefits (Chusak
et al., 2018).
Behavioral analysis
Our findings show that levodopa treatment does not effectively counteract the depressive and anxiety-like behaviours induced by rotenone. This lack of efficacy prompted the investigation of additional dopaminergic therapies. According to our findings, CT therapy successfully reversed the increased immobility time observed in lesioned rats during the forced swim test, as well as the anxiogenic-like effects induced by rotenone in the beam walking test. These results confirm the accuracy and predictive value of the current model researching viable therapies for mental conditions associated with PD and demonstrate the effectiveness of CT in reducing PD-related behavioural symptoms (Chayaratanasin et al., 2021).
The forced swim test is widely used to assess depressive-like behaviour in rodent models. Here, we observed that rats in treatment group 3 spent significantly more time swimming from day 1 to day 28 compared to other treatment groups. This increase in swimming time correlated with a reduction in total immobility time across the same period, suggesting an antidepressant effect.
However, all three treatment groups exhibited increased vulnerability to forced swimming, evidenced by elevated immobility and reduced swimming behaviour. Under these experimental conditions, learned helplessness appeared in the negative control group but was absent in the CT-treated groups. These results suggest that sensitivity to forced swimming positively correlated with drug sensitivity, as demonstrated by post-treatment analysis of CT administration.
Treatment group 2 showed notable immobility throughout the day, especially compared to treatment group 1. This could explain why treatment group 2 spent more time immobile than other treatment groups. Climbing behaviour was also significantly reduced across all treatment groups during the forced swim test. Our findings align with those Parvathi and Ravishankar in 2013, who observed increased locomotor activity and muscle coordination in rats administered 300 mg/kg of CT, with reductions in immobility time during forced swimming test. These authors suggested that CT possess significant antidepressant activity, potentially making it a valuable natural psychotherapeutic agent for treating depression and mood disorders (Rai et al., 2001; Parvathi and Ravishankar, 2013).
The open field test also revealed improvements in motor coordination in the treatment groups compared to the negative control group. In line with previous research showing that dopamine lesions lead to reduced coordination and motor performance, the negative control group in this study displayed significant declines in locomotor activity and motor coordination. In contrast, all treatment groups exhibited increased total distance travelled, indicating improved motivation and motor abilities. Treatment group 3 showed a remarkable increase in total distance travelled, from 72.3% on day 1 to 95.4% on day 28. Treatment groups 1 and 2 also demonstrated substantial improvements, with increases from 68.9% to 80.6% and 72.3% to 95.5%, respectively (Rai et al., 2001).
The beam walking test, used to assess depression and motor impairment, showed a significant reduction in crossing time and fewer paw slips errors in all treatment groups compared to the negative control group, suggesting improved motor function. Similar to humans, stress-exposed rats generally exhibit less activity and coordination. With CT administration, motor and non-motor symptoms significantly improved across treatment groups. Reduced paw slips and quicker beam traversal from days 1 to 28 reflected improvements in balance, vestibular function, and coordinated muscle movement, with treated rats displaying less motor impairment compared to the negative control group (Hortobágyi et al., 2019).
Histological analysis
The H&E staining results align with previous research indicating that rotenone induces neuronal damage in the midbrain and striata of rats (Azmy et al., 2018). This damage is evident in the negative control group compared to the normal control, with Lewy bodies abundantly visible in a single microscopic field in the negative control group. In contrast, rats treated with CT exhibited reduced neurotoxicity and histological alterations across all dosage levels, though some Lewy bodies were still present in the negative control group.
The number of Lewy bodies in the treatment was lower than in the negative control group. This finding is supported by a 2022 study, which found that Lewy bodies were more abundant in the rotenone-treated group compared to the treatment group (Fikry et al., 2022). However, this reduction was entirely reliable due to potential human error during manual counting of Lewy bodies in a microscopic field. Therefore, the H&E histological investigation alone could not conclusively determine the efficacy of CT treatment, as the variation in Lewy body count across treatment groups remained similar.
The histological findings are further supported by Cresyl violet staining, which revealed that the configuration and neuron count in the substantia nigra varied according to the CT dosage administered. The study demonstrated that CT administration could alleviate and potentially treated PD-like symptoms in a rat model. Notably, the negative control group showed a decrease in the substantia nigra’s viscosity and, to a lesser extent, elasticity. This finding aligns with a 2021 study by Alipour colleagues, in which treatment protected against 6-OHDA-induced neuronal loss in the substantia nigra by preserving neuron configuration and count compared to the 6-OHDA-only group (Alipour Nosrani et al., 2021).
Nevertheless, as with H&E staining, the Cresyl violet method alone could not reliably establish the effectiveness of a particular treatment group, as manual counting may introduce error. Furthermore, histological analysis of the liver and kidney in both the negative control and CT-treated groups showed no damage, indicating that the administered doses rotenone, levodopa, and CT did not induce cell toxicity or approach lethal levels.
CONCLUSIONS
This study demonstrated that CT shows potential for treating Parkinson-like symptoms in rats, exhibiting a therapeutic effect on PD-induced models. Based on behavioural assessments and histological observations, CT appears to have a protective role. Histological analyses indicated that CT increased the number of neurons in the substantia nigra and reduced the presence of Lewy bodies compared to the negative control group, highlighting its therapeutic impact on PD-related neuropathology in rats. Furthermore, behavioural tests revealed that CT-treated rats showed improvements in coordination and motor function compared to the negative control group. These findings suggest that CT may be a promising option for managing the neuropathological progression of PD. However, further research is needed to explore the specific cellular and molecular signalling pathways involved.
LIMITATION OF THE STUDY
While Clitoria ternatea has demonstrated potential neuroprotective effects in animals, there is limited clinical data available to confirm similar results in humans with Parkinson's disease. The safety profile for chronic use in rats or humans remains unclear. Long-term studies on potential adverse effects are necessary to fully evaluate its therapeutic index. More detailed histological analysis of brain tissue, such as through staining for neurodegeneration markers (alpha-synuclein and dopaminergic neurons), would provide more precise data on the cellular and molecular effects of CT treatment. This could help verify the neuroprotective mechanisms at play and determine the extent of neuronal damage.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Al-Snafi, A. E. 2016. Pharmacological importance of Clitoria ternatea–A review. IOSR Journal of Pharmacy. 6(3): 68–83.
Alipour Nosrani, E., Tamtaji, O. R., Alibolandi, Z., Sarkar, P., Ghazanfari, M., Azami Tameh, A., Taghizadeh, M., Banikazemi, Z., Hadavi, R., and Naderi Taheri, M. 2021. Neuroprotective effects of probiotics bacteria on animal model of Parkinson's disease induced by 6-hydroxydopamine: A behavioral, biochemical, and histological study. Journal of Immunoassay and Immunochemistry. 42(2): 106-120.
Armstrong, M. J. and Okun, M. S. 2020. Diagnosis and treatment of Parkinson disease: A review. JAMA. 323(6): 548–560.
Azmy, M. S., Menze, E. T., El-Naga, R. N., and Tadros, M. G. 2018. Neuroprotective effects of filgrastim in rotenone-induced Parkinson’s disease in rats: Insights into its anti–inflammatory, neurotrophic, and antiapoptotic effects. Molecular Neurobiology. 55: 6572–6588.
Beitz, J. M. 2014. Parkinson’s disease: A review. Frontiers in Bioscience (Scholar edition). 6(1): 65–74.
Belarbi, K., Cuvelier, E., Destée, A., Gressier, B., and Chartier-Harlin, M. C. 2017. NADPH oxidases in Parkinson’s disease: A systematic review. Molecular Neurodegeneration. 12: 84
Chaiwut, T., Thaweekhotr, P., Thongsopha, C., Phasukdee, N., Gomonchareonsiri, S., Boontim, N., and Quiggins, R. 2020. Thunbergia laurifolia lind. extract alleviates motor impairments in acute MPTP mouse model of Parkinson’s disease. Chiang Mai University Journal of Natural Sciences. 19 (3): 456–468.
Chayaratanasin, P., Adisakwattana, S., and Thilavech, T. 2021. Protective role of Clitoria ternatea L. flower extract on methylglyoxal-induced protein glycation and oxidative damage to DNA. BMC Complementary Medicine and Therapies. 21: 1–11.
Chusak, C., Thilavech, T., Henry, C. J., and Adisakwattana, S. 2018. Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: A randomized crossover trial. BMC Complementary and Alternative Medicine. 18(1): 6.
Dewi, I., Chodidjah, C., and Atina, H. 2023. Evaluation of Clitoria ternatea L. flower extract in preventing complications of diabetes mellitus. Tropical Journal of Natural Product Research. 7(10): 4908–4911.
Escher, G. B., Marques, M. B, do Carmo, M. A. V. , Azevedo, L., Furtado, M. M., Sant'Ana, A. S., da Silva, M. C., Genovese, M. I., Wen, M., Zhang, L., et al. 2020. Clitoria ternatea L. petal bioactive compounds display antioxidant, antihemolytic and antihypertensive effects, inhibit α–amylase and α–glucosidase activities and reduce human LDL cholesterol and DNA induced oxidation. Food Research International. 128: 108763.
Feldman, A. T. and Wolfe, D. 2014. Tissue processing and hematoxylin and eosin staining. Histopathology: Methods and Protocols. 1180: 31–43.
Fikry, H., Saleh, L. A., and Abdel Gawad, S. 2022. Neuroprotective effects of curcumin on the cerebellum in a rotenone-induced Parkinson’s disease model. CNS Neuroscience & Therapeutics. 28(5): 732–748.
Fontoura, J.L., Baptista, C., Pedroso, F. D.B., Pochapski, J.A., Miyoshi, E., and Ferro, M.M. 2017. Depression in Parkinson’s disease: The contribution from animal studies. Parkinson’s Disease. 2017(1): 9124160.
Gonçalves, G. C. P., Rosas, A. L. G., de Sousa, R. C., Vieira, T. R. R., de Albuquerque Sousa, T. C., Ramires, T., da Silveira, T. F. F., Barros, L., da Silva, W. P., Dias, Á. R. G., and da Rosa Zavareze, E. 2024. A green method for anthocyanin extraction from Clitoria ternatea flowers cultivated in southern Brazil: Characterization, in vivo toxicity, and biological activity. Food Chemistry. 435: 137575.
Harry, G. J. and Kraft, A. D. 2008. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert Opinion on Drug Metabolism & Toxicology. 4(10): 1265–1277.
Hortobágyi, T., Uematsu, A., Sanders, L., Kliegl, R., Tollár, J., Moraes, R., and Granacher, U. 2019. Beam walking to assess dynamic balance in health and disease: A protocol for the “BEAM” multicenter observational study. Gerontology. 65(4): 332–339.
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., and Bohr, V. A. 2019. Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology. 15(10): 565–581.
Islam, J. and Kabir, Y. 2019. Effects and mechanisms of antioxidant-rich functional beverages on disease prevention. p.157–198. In Functional and Medicinal Beverages. Woodhead Publishing Elsevier.
Jeyaraj, E. J., Lim, Y. Y., and Choo, W. S. 2021. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. Journal of Food Science and Technology. 58(6): 2054–2067.
Jiji, K. N. and Muralidharan, P. 2022. Evaluation of the protective effect of Clitoria ternatea L. against propionic acid induced autistic spectrum disorders in rat model. Bulletin of the National Research Centre. 46(1): 71.
Kamilla, L., Ramanathan, S., Sasidharan, S., and Mansor, S. M. 2012. Toxicity evaluation of methanol extract of Clitoria ternatea L. leaf. Malaysian Journal of Medicine Health Sciences. 8: 33–39.
Khobkhun, F., Bovonsunthonchai, S., Vachalathiti, R., and Pisarnpong, A. 2014. Effect of physical therapy training on gait initiation in patients with moderate Parkinson’s disease. Chiang Mai University Journal of Natural Sciences. 13(1): 43–50.
Kraeuter, A. K., Guest, P. C., and Sarnyai, Z. 2019. The forced swim test for depression-like behaviour in rodents. Pre-Clinical Models: Techniques and Protocols. 1916: 75–80.
Miko, M. and Varga, I. 2015. Histologic examination of peripheral nerves. p. 79–89. In Nerves and Nerve Injuries. Elsevier.
Naduvilath, T. J., John, R. K., and Dandona, L. 2000. Sample size for ophthalmology studies. Indian Journal of Ophthalmology. 48(3): 245–250.
Parvathi, M. and Ravishankar, K. 2013. Evaluation of antidepressant, motor coordination and locomotor activities of ethanolic root extract of Clitoria ternatea. Journal of Natural Remedies. 13(1): 19–24.
Rai, K. S., Murthy, K. D., Karantha, K. S., and Rao, M. S. 2001. Clitoria ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian Journal of Physiology and Pharmacology. 45(3): 305–313.
Ramli, M. D. B. C., Uzid, M. B. M., Muhammad, H., and Hasan, M. K. N. 2021. The therapeutic effect of Bacopa monnieri in treating Parkinson's disease. International Journal of Medical Toxicology & Legal Medicine. 24(3 and 4): 206–211.
Russell, K. L., Kutchko, K. M., Fowler, S. C., Berman, N. E., and Levant, B. 2011. Sensorimotor behavioural tests for use in a juvenile rat model of traumatic brain injury: Assessment of sex differences. Journal of Neuroscience Methods. 199(2): 214–222.
Saganuwan, S. A. 2017. Toxicity studies of drugs and chemicals in animals: An overview. Bulgarian Journal of Veterinary Medicine. 20(4):291-318.
Seibenhener, M. L. and Wooten, M. C. 2015. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments. 96: e52434.
Simpson, D. S. and Oliver, P. L. 2020. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 9(8): 743.
Taur, D. J. and Patil, R. Y. 2011. Antihistaminic activity of Clitoria ternatea L. roots. Journal of Basic and Clinical Pharmacy. 2(1): 41.
Wimalasena, K., Adetuyi, O., and Eldani, M. 2024. Metabolic energy decline coupled dysregulation of catecholamine metabolism in physiologically highly active neurons: Implications for selective neuronal death in Parkinson’s disease. Frontiers in Aging Neuroscience. 16: 1339295.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Genevieve Simboh Anak Umbar1, Hani Qistina Dayana1, Hussin Muhammad2, Mohamad Anuar Ahad3, Azlina Zulkapli4, Zolkapli Eshak5, and Muhammad Danial Che Ramli1, *
1 Department of Diagnostic and Allied Health Sciences, Faculty of Health and Life Sciences, Management and Science University, 40100 Shah Alam, Selangor, Malaysia.
2 Toxicology & Pharmacology Unit, Herbal Medicine Research Center, Institute for Medical Research, National Institute of Health, Setia Alam Selangor, Malaysia.
3 Department of Basic Health Sciences, Faculty of Pharmacy & Biomedical Sciences, MAHSA University, 42610 Jenjarom Selangor, Malaysia.
4 Laboratory Animal Resources Unit, Special Resource Center, Institute for Medical Research, National Institute of Health, Malaysia.
5 Department of Pharmacology and Chemistry, Faculty of Pharmacy, UiTM Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor.
Corresponding author: Muhammad Danial Che Ramli, E-mail: muhddanial_cheramli@msu.edu.my
Total Article Views
Editor: Waraporn Boonchieng
Chiang Mai University, Thailand
Article history:
Received: September 9, 2024;
Revised: November 26, 2024;
Accepted: November 27, 2024;
Online First: December 3, 2024

