
Inheritance of Sterile Traits in Fifth Generation Chinese Cabbage and Evaluation of Yield Components
Patiphan Boonlao, Nakarin Jeeatid, Kamon Thippachote, Tonapha Pusadee, and Jutamas Kumchai*Published Date : December 3, 2024
DOI : https://doi.org/10.12982/NLSC.2025.012
Journal Issues : Number 1, January-March 2025
Abstract Chinese cabbage is a popular vegetable in Thailand and internationally. In Thailand, the majority of seeds used by farmers for cultivation are imported, a trend driven by increasing demand from both farmers and consumers. To reduce reliance on imported Chinese cabbage seeds and develop varieties suited to Thailand's environment, experiments have focused on transferring the genetic traits of Chinese cabbage into regular varieties through backcrossing methods. A randomized complete block design (RCBD) with seventeen treatments, including five fertile varieties, six backcrossed varieties, and six commercial varieties, was used in our experiments. Results from the experiments indicated that, by the fifth generation, backcrossed Chinese cabbage varieties showed no pollen staining, distinguishing them from other varieties that exhibited normal pollen staining. In terms of yield components and desirable horticultural traits, two backcrossed (BC) varieties, BC5 142-6-5 and BC5 142-7-12, demonstrated statistically similar pre-trimming and post-trimming weights compared to commercial varieties, along with a shape index close to the desired market standard of 1.6. Additionally, other potential varieties, such as BC5 23-9-3 and BC5 27-8-7, alongside fertile varieties, 23-9-3, 23-8-7, 27-8-7, and 142-6-5, showed promise as male lines. These findings underscore several promising varieties for further breeding. These new backcross lines could serve as female lines for developing hybrids in Thailand, exhibiting high post-trimming yields and desirable head characteristics that align with consumer preferences.
Keywords: Chinese cabbage, Product, Trimming, Head shape index
Funding: This research was partially supported by the Teaching Assistants and Research Assistants Scholarship, with research funding provided by the Graduate School of Chiang Mai University and the Center of Excellence on Agricultural Biotechnology, Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (AG-BIO/MHESI).
Citation: Boonlao, P., Jeeatid, N., Thippachote, K., Pusadee, T., and Kumchai, J. 2025. Inheritance of sterile traits in fifth generation Chinese cabbage and evaluation of yield components. Natural and Life Sciences Communications. 24(1): e2025012.
INTRODUCTION
Chinese cabbage (Brassica rapa L. var. pekinensis), an economically important and popular vegetable, similar to regular cabbage. It is widely consumed due to its popularity and nutritional value, including fiber, calcium, and vitamins. The edible part is the leaf, which consistently exhibits wide and flat leaf stalks rich in nutrients such as calcium and vitamins (Tawatsinlapasorn et al., 2017; Okamoto et al., 2021). Besides being consumed fresh, Chinese cabbage is used in various dishes and processed foods, such as dried vegetables, ready-to-eat vegetables, crispy vegetables, and kimchi (Khiaoamphai, 2011). Chinese cabbage varieties are categorized into non-heading and heading types, each with distinct shapes requiring specific temperature requirements, particularly in low-temperature or temperate climates, similar to other cruciferous crops (Sun et al., 2018; Khan et al., 2019). Water deficiency affects growth, causing the wilting and non-closure of leaves (Nikornpun, 2002). Hsiao and Acevado (1974) highlighted roots' role in water and mineral absorption, aiding the plant's tolerance to water scarcity. Additionally, Kuo et al. (1988) noted that water deficiency inhibits Chinese cabbage growth, while long daylight hours promote flowering (16 hours/day/month). Conversely, short days and warm temperatures stimulate growth, making winter the preferred season for cultivation in warm and semi-warm regions (Khiaoamphai, 2011).
Currently, the majority of Chinese cabbage seeds used in cultivation are F1 hybrids due to their stable lines, high yields, robust growth, consistent quality, and resilience to diseases and pests. Some regions face challenges in seed production due to the requirement for low temperatures for flowering and high-quality seed yield, necessitating large-scale seed imports (Fujimoto et al., 2018; Kang et al., 2023). F1 hybrids exploit vegetative heterosis, employing mechanisms like self-incompatibility (SI) or cytoplasmic male sterility (CMS) for seed production (Fujimoto and Nishio, 2007; Yamagishi and Bhat, 2014; Okamoto et al., 2021).
The breeding method known as the self-incompatibility (SI) system is widely adopted in parental lines for hybrid seed production to streamline emasculation procedures and reduce labor costs (Li et al., 2019). Hybrid Chinese cabbage seeds typically utilize SI lines, ensuring that male flowers function normally and are capable of self-pollination (self-compatible: SC) (Kaothien-Nakayama et al., 2010; Muñoz-Sanz et al., 2020). SI plays a critical role in enabling successful pollination between pollen grains and female stigmas, facilitated by genes associated with S-locus cysteine-rich (SCR) and S-locus receptor kinase (SRK) in both male and female gametophyte cells, influencing the production of proteins in pollen and stigma (Goring et al., 2023; Kusaba et al., 2001).
Another breeding method is cytoplasmic male sterility (CMS), which causes a range of reproductive abnormalities through rearrangements in mitochondrial DNA, thereby impacting mitochondrial function and influencing adenosine triphosphate (ATP) levels as well as reactive oxygen species levels. Restorer of fertility (Rf) genes, frequently encoding PPR proteins, counteract the effects of CMS. Stable lines with CMS are developed using methods such as interspecific hybridization or somatic fusion (Chen and Liu, 2014; Toriyama, 2021). In Brassica crops, Ogura CMS, associated with the mitochondrial orf138 gene, is pivotal for hybrid seed production, initially discovered in Japanese radish and subsequently transferred to other Brassica species (Yamagishi and Terachi, 1996). Various male sterility types, including genic male sterility (GMS), CMS, Ogura CMS, Pol CMS, and Nig CMS, are utilized, with their traits controlled by nuclear genes in mitochondria. GMS traits are recessive but influenced by environmental factors such as temperature extremes, drought, and saline soils (Storme and Geelen, 2014; Sage et al., 2015; Zhang et al., 2016a; and Zhang et al., 2016b; Singh et al., 2019; Ji et al., 2020). Male-sterile characteristics are preferred in hybrid seed production for stability over self-incompatible traits (Nikornpun, 2002; Singh et al., 2019)
Numerous studies have explored the transfer of CMS genes through backcrossing methods as a breeding strategy with which to develop parental lines for future hybrid seed production. For instance, Na Jinda et al. (2023) successfully transferred the CMS system to normal chilli plants to establish a new A-line. Additionally, Nikornpun et al. (2023) enhanced the pungency of CMS chilli lines through backcrossing with the B cultivar. Furthermore, Ahmadikhah et al. (2015) pioneered the transfer of rice's CMS IR68897A into Yosen B using marker-assisted backcrossing (MABC) for detection.
This research focuses on improving and developing male-sterile Chinese cabbage parent lines through backcrossing and selfing methods to create authentic lines. Following the breeding process, these lines were cultivated to evaluate yield and yield components, including head wrapping, head shape, and the plant vigor index. The findings of this study will contribute to further advancements in hybrid Chinese cabbage varieties in Thailand.
MATERIALS AND METHODS
Chinese cabbage, comprising 17 varieties, includes 6 male sterile lines developed from 5 backcrossing cycles following the Figure 1 diagram, 5 male fertile lines, and 6 commercial varieties (Table 1). These were evaluated during the winter season from November 2022 to February 2023 in experimental plots at the Horticulture Department, Agricultural Innovation Research, Integration, Demonstration and Training Center, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand. The experiment was conducted using a completely randomized block design with 3 replications. Seeds were sown in tissue paper, and when the seedlings reached approximately 1 centimeter in length, they were transplanted into seedling trays filled with peat moss. Seedlings were watered thoroughly when they reached 20-25 days old. Then, they were transplanted into experimental plots measuring 1.2 × 20 m. A fertilizer (NPK) with a ratio of 15-15-15 was applied at a rate of 50 kilograms per hectare in the planting plots. Seedlings of Chinese cabbage were spaced at 50 × 50 cm intervals between plants and rows and were irrigated and fertilized using a drip irrigation system. Harvesting of Chinese cabbage occurs when it reaches 45 days after planting. Data on horticultural characteristics such as plant height, weight before trimming, weight after trimming, percentage of trimming, perimeter of the head, length of the head, and head shape index (HSI = head length / head width) and head solidity were recorded (Nikornpun, 2002).
When Chinese cabbage begins to flower (at 60 days after transplanting), the viability of pollen grains is examined. Pollen grains are stained with potassium iodide and then observed under an electron microscope at a magnification of 40 times. In the case of varieties where there is no growth in the pollen grains or where the pollen grains are present but not viable, no pollen grains will appear or they will not stain (Figure 2A). Conversely, in varieties where there is normal growth in the pollen grains or where the pollen grains are viable, they will stain properly (Figure 2B).
Table 1. The list includes Chinese cabbage lines, male fertile lines, male sterile lines and commercial lines.
|
Code |
Male fertile line |
Code |
Male sterile line |
Code |
Commercial line |
|
23-6 |
Inbred line |
BC5 23-6 |
Backcross line |
C-001 |
C1 |
|
23-9-3 |
Inbred line |
BC5 23-9-3 |
Backcross line |
C-003 |
C2 |
|
23-8-7 |
Inbred line |
BC5 23-8-7 |
Backcross line |
C-008 |
C3 |
|
27-8-7 |
Inbred line |
BC5 27-8-7 |
Backcross line |
C-009 |
C4 |
|
142-6-5 |
Inbred line |
BC5 142-6-5 |
Backcross line |
C-010 |
C5 |
|
|
|
BC5 142-7-12 |
Backcross line |
C-011 |
C6 |
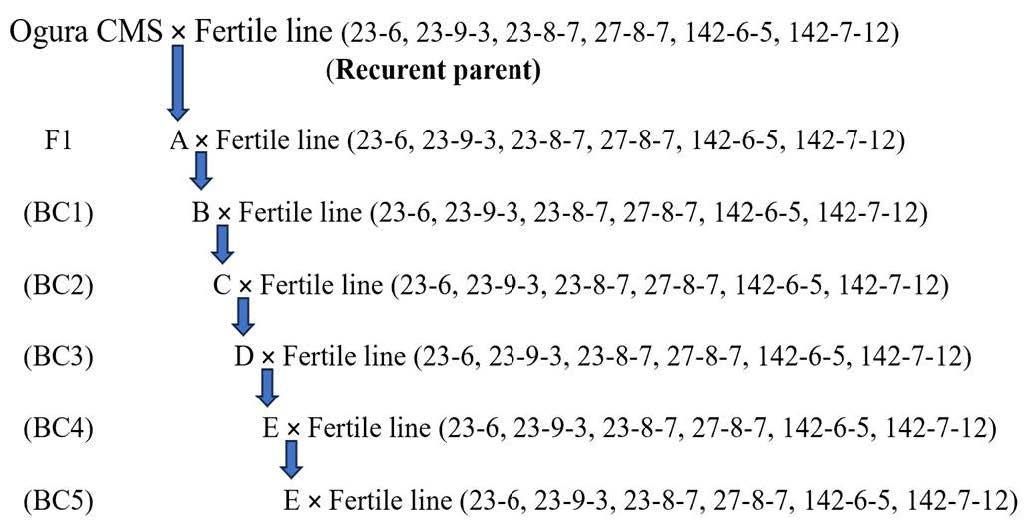
Figure 1. Backcross breeding with a recessive trait program for the development of Ogura cytoplasmic male sterility (CMS) (A-line program).
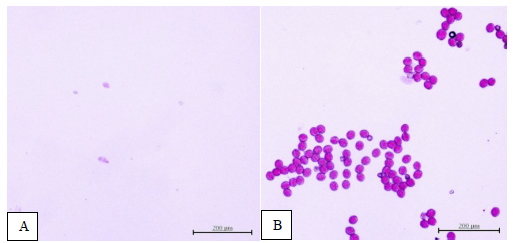
Figure 2. Pollen staining with potassium iodide was employed to assess pollen viability. The classification is as follows: A = no pollen grains or pollen grains are not living, B = pollen is alive (scale bar 0.2 mm).
RESULTS
The examination of pollen viability
Upon staining with potassium iodide, all five Chinese cabbage varieties (strains 23-6, 23-9-3, 23-8-7, 27-8-7, and 142-6-5) showed proper staining and exhibited pollen grains with a relatively round shape (Figure 3A-E). In contrast, strains BC5 23-6, BC5 23-9-3, BC5 23-8-7, BC5 27-8-7, BC5 142-6-5, and BC5 142-7-12 did not display any stained pollen grains (Figure 3F-K).
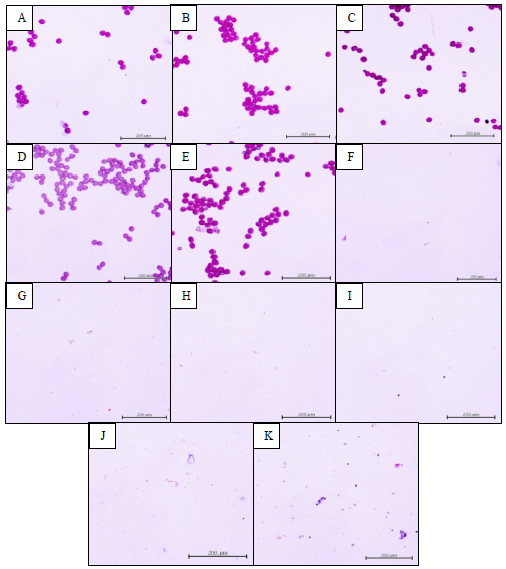
Figure 3. Pollen staining with potassium iodide was employed to assess pollen viability. The classification is as follows: A = 23-6, B = 23-9-3, C = 23-8-7, D = 27-8-7, E = 142-6-5, F = BC5 23-6, G = BC5 23-9-3, H = BC5 23-8-7, I = BC5 27-8-7, J = BC5 142-6-5, K = BC5 142-7-12 (scale bar 0.2 mm).
Weight before trimming, weight after trimming, and trimming percentage
When comparing the cultivation of different varieties of Chinese cabbage, including 5 lines of normal type, 6 lines of Ogura cytoplasmic male sterility (CMS), and 6 commercial lines (Figure 4), significant statistical differences were found in the studied characteristics, which include weight before trimming, weight after trimming, and trimming percentage. The lines with higher weights before trimming were 23-9-3, 23-8-7, 27-8-7, 142-6-5, BC5 23-9-3, BC5 27-8-7, BC5 142-6-5, BC5 142-7-12, C-001, C-003, C-008, and C-011, with values of 9.44, 10.35, 9.49, 8.27, 8.69, 8.75, 8.05, 9.12, 9.49, 8.37, 10.24, and 9.44 tons/ha, respectively. These values showed statistically significant differences compared to the commercial lines C-009 and C-010 (Table 2).
The varieties with higher weights after trimming are 23-9-3, 23-8-7, 27-8-7, 142-6-5, BC5 23-9-3, BC5 27-8-7, BC5 142-6-5, BC5 142-7-12, C-001, C-003, C-008, and C-011, with values of 6.68, 6.45, 6.24, 5.39, 6.03, 5.61, 6.03, 6.51, 4.96, 4.00, 6.21, and 5.56 tons/ha, respectively. These values showed statistically significant differences compared to all commercial strains C-009 and C-010. Regarding the quality characteristics of Chinese cabbage, it was found that the size of the heads was similar, both large and small. The varieties with trimming percentages below 35%, including 23-9-3, 142-6-5, BC5 23-9-3, BC5 142-6-5, and BC5 142-7-12, have values of 29.94, 34.70, 30.15, 31.6, and 27.37%, respectively, which significantly differ statistically from all commercial strains, with trimming percentages ranging from 39.76% to 51.47% (Table 2).
Commercial Chinese cabbage varieties such as C-003 and C-009 are lightweight with a short head-forming period and early bolting. Varieties C-001, C-008, and C-011 have longer head-forming periods.

Figure 4. Head shape of parental lines; A = 23-6, B = 23-9-3, C = 23-8-7, D = 27-8-7, E = 142-6-5, male sterile line; F = BC5 23-6, G = BC5 23-9-3, H = BC5 23-8-7, I = BC5 27-8-7, J = BC5 142-6-5, K = BC5 142-7-12, commercial line; L = C-001, M = C-003, N = C-008, O = C-009, P = C-010, Q = C-011.
Table 2. Weight before trimming, weight after trimming, and percentage of trimming of male fertile line, male sterile line and commercial variety.
|
Line/Varieties
|
Weight before trimming |
Weight after trimming |
Percentage of trimming |
|||
|
(tons/ha) |
(tons/ha) |
(%) |
||||
|
Male fertile line |
|
|
|
|
|
|
|
23-6 |
0.96 |
cd |
0.53 |
de |
44.94 |
a-d |
|
23-9-3 |
1.51 |
a |
1.07 |
a |
29.24 |
ef |
|
23-8-7 |
1.66 |
a |
1.03 |
ab |
37.68 |
b-f |
|
27-8-7 |
1.50 |
a |
1.00 |
a-c |
33.62 |
c-f |
|
142-6-5 |
1.32 |
a-c |
0.86 |
a-d |
34.82 |
b-f |
|
Male sterile line |
|
|
|
|
|
|
|
BC5 23-6 |
0.75 |
d |
0.41 |
e |
45.42 |
a-c |
|
BC5 23-9-3 |
1.39 |
ab |
0.96 |
a-d |
30.61 |
ef |
|
BC5 23-8-7 |
0.86 |
d |
0.54 |
de |
37.66 |
b-f |
|
BC5 27-8-7 |
1.40 |
ab |
0.90 |
a-d |
35.89 |
b-f |
|
BC5 142-6-5 |
1.29 |
a-c |
0.96 |
a-d |
25.09 |
d-f |
|
BC5 142-7-12 |
1.46 |
a |
1.04 |
a |
28.62 |
f |
|
Commercial variety |
|
|
|
|
|
|
|
C-001 |
1.52 |
a |
0.79 |
a-e |
47.73 |
ab |
|
C-003 |
1.34 |
a-c |
0.64 |
a-e |
52.21 |
a |
|
C-008 |
1.64 |
a |
0.99 |
a-c |
39.36 |
a-e |
|
C-009 |
0.96 |
cd |
0.60 |
b-e |
37.81 |
a-e |
|
C-010 |
0.99 |
b-d |
0.58 |
c-e |
41.03 |
a-f |
|
C-011 |
1.51 |
a |
0.89 |
a-d |
41.10 |
a-e |
|
Mean |
1.30 |
0.81 |
37.44 |
|||
|
F-test |
* |
* |
* |
|||
|
CV (%) |
17.00 |
17.7 |
16.6 |
|||
Note: * = There was a statistically significant difference at P ≤ 0.05.
Plant height, head circumference, and head length
The plant height ranged from 13.87 to 20.54 cm. Varieties with taller plant heights included BC5 142-6-5, with a height of 20.54 cm, followed by 23-9-3 and 23-8-7, with heights of 19.44 cm and 18.50 cm, respectively. These differences were statistically significant compared to commercial varieties C-009 and C-003, which had heights of 16.86 cm and 18.00 cm, respectively (Table 3).
The head circumference ranged from 29.93 to 52.58 cm. Varieties with larger head circumferences included 23-9-3, 23-8-7, and 27-8-7, with circumferences of 45.21 cm, 52.58 cm, and 42.67 cm, respectively. These differences were statistically significant compared to commercial varieties C-001, C-003, C-008, C-009, and C-010. Following them were BC5 23-9-3 and BC5 142-7-12, with circumferences of 40.84 cm and 45.13 cm, respectively.
The head length ranged from 13.87 to 20.54 cm. Varieties with longer head lengths included BC5 142-6-5, with a length of 20.54 cm, which was statistically significantly different from commercial variety C-009, with a length of 16.86 cm. Following them were 23-9-3, 23-8-7, and BC5 23-8-7, with lengths of 18.50 cm, 19.03 cm, and 18.22 cm, respectively (Table 3).
Table 3. Plant height, head diameter, and head length of male fertile line, male sterile line and commercial variety.
|
Line/Varieties
|
Plant height (cm) |
Head circumference (cm) |
Head length (cm) |
|||
|
Male fertile line |
|
|
|
|
|
|
|
23-6 |
17.44 |
b-e |
39.74 |
b-e |
17.44 |
b-d |
|
23-9-3 |
18.50 |
a-c |
45.21 |
b |
18.50 |
ab |
|
23-8-7 |
19.44 |
a-c |
52.58 |
a |
19.03 |
ab |
|
27-8-7 |
17.61 |
b-e |
42.67 |
bc |
17.61 |
b-d |
|
142-6-5 |
15.42 |
ef |
40.88 |
b-d |
15.42 |
de |
|
Male sterile line |
|
|
|
|
|
|
|
BC5 23-6 |
13.87 |
f |
29.93 |
f |
13.87 |
e |
|
BC5 23-9-3 |
17.57 |
b-e |
40.84 |
b-d |
17.57 |
b-d |
|
BC5 23-8-7 |
18.22 |
bc |
34.23 |
d-f |
18.22 |
a-c |
|
BC5 27-8-7 |
15.88 |
d-e |
36.74 |
c-f |
15.88 |
c-e |
|
BC5 142-6-5 |
20.54 |
a |
29.93 |
f |
20.54 |
a |
|
BC5 142-7-12 |
15.88 |
g-e |
45.13 |
b-d |
15.88 |
c-e |
|
Commercial variety |
|
|
|
|
|
|
|
C-001 |
18.50 |
a-c |
33.96 |
d-f |
18.50 |
ab |
|
C-003 |
18.09 |
b-d |
31.95 |
ef |
18.09 |
a-c |
|
C-008 |
18.75 |
a-c |
33.96 |
d-f |
18.75 |
ab |
|
C-009 |
16.86 |
c-e |
31.52 |
f |
16.86 |
b-d |
|
C-010 |
19.44 |
ab |
32.71 |
ef |
19.44 |
ab |
|
C-011 |
18.93 |
a-c |
35.89 |
c-f |
18.93 |
ab |
|
Mean |
17.07 |
38.21 |
17.68 |
|||
|
F-test |
* |
* |
* |
|||
|
CV (%) |
7.52 |
10.72 |
7.52 |
|||
Note: * = There was a statistically significant difference at P ≤ 0.05.
Head shape index and head solidity
The head shape index ranges from 1.44 to 1.88, with commercial varieties ranging from 1.68 to 1.88. Varieties with a head shape index do not significantly differ statistically from commercial varieties, including BC5 23-6, BC5 23-8-7 and BC5 142-6-5, with values of 1.45, 1.67, and 1.56, respectively. BC5 23-8-7 and BC5 142-6-5 exhibit head shape index values of 1.67 and 1.56, respectively, which closely match Thai consumer preferences and market demands (rounded head shape, slightly shorter, roundish or oval). The top three varieties with high head solidity are BC5 27-8-7, BC5 23-6, and 142-6-5, with values of 0.78, 0.65, and 0.61 g /cm3, respectively (Table 4).
Table 4. Head shape index (HSI), and solidity of male fertile line, male sterile line and commercial variety.
|
Line/Varieties |
HSI |
Solidity (g/cm3) |
||||||
|
Male fertile line |
|
|
|
|
||||
|
23-6 |
1.40 |
d-f |
0.36 |
d |
||||
|
23-9-3 |
1.28 |
ef |
0.52 |
cd |
||||
|
23-8-7 |
1.14 |
f |
0.36 |
d |
||||
|
27-8-7 |
1.30 |
d-f |
0.57 |
b-d |
||||
|
142-6-5 |
1.19 |
ef |
0.61 |
bc |
||||
|
Male sterile line |
|
|
|
|
||||
|
BC5 23-6 |
1.45 |
c-e |
0.65 |
a-c |
||||
|
BC5 23-9-3 |
1.36 |
d-f |
0.60 |
bc |
||||
|
BC5 23-8-7 |
1.67 |
a-c |
0.47 |
cd |
||||
|
BC5 27-8-7 |
1.37 |
d-f |
0.78 |
ab |
||||
|
BC5 142-6-5 |
1.56 |
b-d |
0.51 |
cd |
||||
|
BC5 142-7-12 |
1.15 |
f |
0.59 |
b-d |
||||
|
Commercial variety |
|
|
|
|
||||
|
C-001 |
1.72 |
ab |
0.67 |
a-c |
||||
|
C-003 |
1.78 |
ab |
0.64 |
bc |
||||
|
C-008 |
1.76 |
ab |
0.88 |
a |
||||
|
C-009 |
1.69 |
a-c |
0.66 |
a-c |
||||
|
C-010 |
1.88 |
a |
0.52 |
cd |
||||
|
C-011 |
1.68 |
a-c |
0.69 |
a-c |
||||
|
Mean |
1.49 |
0.59 |
||||||
|
F-test |
* |
* |
||||||
|
CV (%) |
9.56 |
20.88 |
||||||
Note: * = There was a statistically significant difference at P ≤ 0.05.
DISCUSSION
The examination of pollen viability
This indicates that male sterile traits from cytoplasmic plastid genes (Ogura cytoplasmic male sterility) can be transmitted through multiple generations via backcrossing, starting from the initial backcross and continuing through the fifth backcross (Srima, 2018). Additionally, Tisa (2021) observed morphological differences in fully bloomed flowers between normal and male sterile Chinese cabbage plants, particularly in the anatomical structure of their reproductive organs. Male sterile plants showed shortened stigmas and underdeveloped anthers but normal female organs, contrasting with normal plants that exhibited typical male and female reproductive structures. The transfer of Cytoplasmic Male Sterility (CMS) traits through backcrossing involves crossing pollen from vegetables like peppers and cruciferous vegetables, which possess sterility traits, with plants carrying normal pollen. This process aims to introduce the sterility trait into resulting vegetable plants. Differentiating between fertile and sterile pollen grains in crops inheriting CMS traits is critical for monitoring the backcrossing process and developing new genetic traits in peppers and cruciferous vegetables exhibiting male sterility traits in various research studies (Gulyas et al., 2006; Na Jinda et al., 2023).
Weight before trimming, weight after trimming, trimming percentage, plant height, head circumference, and head length
The studied characteristics of weight before trimming, weight after trimming, and trimming percentage provide important information for evaluating the cultivation performance and potential yields of different Chinese cabbage varieties. Higher weights before and after trimming are generally desirable for maximizing crop yields. The quality of Chinese cabbage encompasses commodity, flavor, and nutritional aspects. Commodity quality pertains to external factors like freshness and the tightness of the leaf ball, assessed primarily through sensory perception. Flavor quality relates to the distinct aroma and taste of Chinese cabbage, discerned through tasting and analysis of flavor-related components. Nutritional quality is determined by nutrient content and distribution, such as crude protein, soluble sugars, fiber, organic acids, amino acids, and minerals, impacting human health. Currently, both domestically and internationally, Chinese cabbage quality evaluation predominantly emphasizes sensory attributes and flavor characteristics (Gong et al., 2016; Li et al., 2008; Wu et al., 2012).
The post-trimming weight and trimming percentage are important quality and yield parameters for Chinese cabbage. Varieties with higher post-trim weights generally have greater marketable yields. The Thai consumer preference for 0.5-1.5 kg heads after trimming, as noted by Nikornpun (2002), provides a target range for breeders and growers. Lower trimming percentages are also desirable as they indicate less waste and higher edible yield from the total plant biomass. Selecting varieties that combine high post-trim weights with low trimming loss percentages can help optimize both yield and quality for cultivation and marketing of Chinese cabbage.
Chinese cabbage is a cool-season vegetable that requires cool temperatures for growth, heading, and flowering. The suitable temperature for growth and heading development ranges between 15-16 degrees Celsius. Planting in temperatures higher than 24-25 degrees Celsius can result in delayed heading or poor head quality, including loose heading, leaf tip burn, and bitterness (Motoki et al., 2019). Song et al. (2016) reported that a low temperature of 15 degrees Celsius for a 5-day treatment induces flowering in Chinese cabbage 27 days after transplantation. The timing of flowering and bolting in cruciferous vegetables is influenced by factors such as temperature, plant hormones, genetics, physiology, and biochemical substances (Yan and Hunt 1999; Yang et al. 1996). Low temperatures signal stem elongation and flowering in winter annuals and biennials, inducing physiological and biochemical changes in plants over extended periods of cold exposure (Hébrard et al. 2013; Liang et al. 2018).
The harvesting age of Chinese cabbage depends on the variety characteristics. Varieties with loose heading require approximately 40-50 days from seed sowing to harvesting, while long-heading and round-heading varieties need about 50-80 days from seedling transplanting to harvesting. Harvesting is usually done when the heads are fully mature and reach the desired size. Varieties grown in this experiment showed statistically significant differences in weight before pruning, weight after pruning, pruning percentage, head circumference, and head length.
Planting Chinese cabbage during the winter season, with temperatures ranging from 13-21 degrees Celsius, is suitable for growth (Yamaguchi, 1983). Large outer leaves contribute to higher trimming percentages because typically, pruning involves removing outer leaves and uncurled leaves. Bayogan et al. (2018) reported that Chinese cabbage grown in cooler climates had trimming percentages as high as 43.9% due to favorable conditions for growth, resulting in abundant foliage. Conversely, in warmer areas, foliage tends to be less abundant, leading to lower trimming percentages, around 28.5%.
Head shape index and head solidity
Consumer preferences for Chinese cabbage in Thailand indicate a preference for long-headed varieties, with a head shape index greater than 1.6. In this experiment. Additionally, long-headed Chinese cabbage, with a head shape index of 4.33 (head length 57.33 / head width 13.25 cm), with a higher proportion of leaves than leaf stems, is popularly processed into kimchi in Korea. Furthermore, head solidity ranges from 0.36 to 0.88 g /cm3, with all varieties having densities close to commercial varieties ranging from 0.52 to 0.88 g /cm3. The agricultural and consumer quality of Chinese cabbage hinges on its shape, particularly the head top shape (HTS) formed by the folding of heading leaves. Consumers favor the overlapping HTS, characterized by inward-curved leaves overlapping at the top (Sun et al., 2021).
CONCLUSIONS
From this experiment, it was found that Chinese cabbage obtained from the fifth backcross (BC5) in all lines exhibits male sterile characteristics with suitability for future development of female line. Chinese cabbage varieties 23-9-3, 23-8-7, 27-8-7, 142-6-5, BC5 23-9-3, BC5 27-8-7, BC5 142-6-5, and BC5 142-7-12 have higher weights before and after pruning but lower pruning percentages. Additionally, in the BC5 142-6-5 and BC5 142-7-12 varieties, the head shape index is as good as that of commercial varieties. Therefore, overall, the best Chinese cabbage plant characteristics with the most potential for development as breeding parents for producing the highest hybrid Chinese cabbage yield are BC5 142-6-5 and BC5 142-7-12, followed by BC5 23-9-3 and BC5 27-8-7.
ACKNOWLEDGEMENTS
The authors would like to thank the Faculty of Agriculture, Chiang Mai University, for providing the experimental area and equipment.
AUTHOR CONTRIBUTIONS
Conceptualization, Jutamas Kumchai; methodology, Patiphan Boonlao, Kamon Thippachot and Jutamas Kumchai; formal analysis, Patiphan Boonlao and Nakarin Jeeartid; data curation, Patiphan Boonlao, Kamon Thippachot and Nakarin Jeeartid; writing original draft preparation, Patiphan Boonlao; writing review and editing, Tonapha Pusadee and Jutamas Kumchai. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Ahmadikhah, A, Mirarab, M., Pahlevani, M. H., and Nayyeripasand, L. 2015. Marker-assisted backcrossing to develop an elite cytoplasmic male sterility line in rice. The Plant Genome. 8(2): 1-12.
Bayogan, E. R. V., Secretaria, L. B., Lubaton, C. D. S., Barbin, K. B., Luayon, M. S., and Ekman, J. H. 2018. Trimming losses in Chinese cabbage. Acta Horticulturae. 1205: 411-418.
Chen, L. T. and Liu, Y. G. 2014. Male sterility and fertility restoration in crops. Annual Review of Plant Biology. 65: 579-606.
Fujimoto, R. and Nishio, T. 2007. Self-incompatibility. Advances in Botanical Research. 45: 139-154.
Fujimoto, R., Uezono, K., Ishikura, S., Osabe, K., Peacock, W. J., and Dennis, E. S. 2018. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breeding Science. 68(2): 145-158.
Gong, Z. P., Yu, S. C., Zhang, F.,. Yu, Y. J., Zhao, X. Y., Zhang, D. S., Wang, W. H., and Su, T. B. 2016. Evaluation of Chinese cabbage sensory quality and its relationship with contents of main nutrient components. Journal of Agricultural Science and Technology. 17(7): 1592-1596.
Goring, D. R., Bosch, M., and Franklin-Tong, V. E. 2023. Contrasting self-recognition rejection systems for self-incompatibility in Brassica and Papaver. Current Biology. 33(11): 530-542.
Gulyas G., Pakozdi, K., Lee, J. S., and Hirata, Y. 2006. Analysis of fertility restoration by using cytoplasmic male sterile red pepper (Capsicum annuum L.) lines. Breeding Science. 56(3): 331-334.
Hébrard, C., Trap-Gentil, V., Lafon-Placette, C., Delaunay, A., Joseph, C. Lefèbvre, M., Barnes, S., and Maury, S. 2013. Identification of differentially methylated regions during vernalization revealed a role for RNA methyltransferases in bolting. Journal of Experimental Botany. 64(2): 651-663.
Hsiao, T. C. and Acevado, E. 1974. Plant responses to water deficits, water-use efficiency and drought resistance. Journal of Agricultural Meteorology. 14(1-2): 59-84.
Ji, J., Huang, J., Yang, L., Fang, Z., Zhang, Y., Zhuang, M., Lv, H., Wang, Y., Liu, Y., Li, Z., and Han, F. 2020. Advances in research and application of male sterility in Brassica oleracea. Horticulturae. 6(4): 101.
Kang, T., Ro, S., Sok, K., Buntong, B., Roeurn, S., Chun, N., Serey, M., and Roek, P. 2023. The household economic evaluation of Chinese cabbage production in the rainy season at lowland area, Cambodia. Journal of Emerging Technologies and Innovative Research. 10(11): 481-485.
Kaothien-Nakayama, P., Isogai, A., and Takayama, S. 2010. Self-incompatibility systems in flowering plants. p. 459-485. In E.C. Pua and M.R. Davey (eds.). Plant developmental biology-biotechnological perspectives. Springer Science+Business Media, Berlin.
Khan, B. M., Afrin A. H., and Hossain, M. Mo. 2019. Effects of phosphorus on in-vitro seed germination and seedling growth of Brassica rapa L. in arsenic condition. Chiang Mai University Journal of Natural Science. 8(4): -498-514.
Khiaoamphai, C. 2011. Vegetable gardening. Bangkok, Kasetsart Siam Books.
Kuo, C. G., Shen, B. J., Chen, H. M., Chen, H. C., and Opeña, R. T. 1988. Associations between heat tolerance, water consumption, and morphological characters in Chinese cabbage. Euphytica International Journal of Plant Breeding. 39: 65-73.
Kusaba, M., Dwyer, K., Hendershot, J., Vrebalov, J., Nasrallah, J. B., and Nasrallah, M. E. 2001. Self-Incompatibility in the genus Arabidopsis: Characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. The Plant Cell. 13(3): 627-643.
Liang, N., Cheng, D., Liu, Q., Cui, J., and Luo, C. 2018. Difference of proteomics vernalization-induced in bolting and flowering transitions of Beta vulgaris. Plant Physiology and Biochemistry. 123: 222-232.
Li, J. H., Xu, M., and Zhang, C. 2008. Identification of comprehensive flavor quality of cabbage and its correlation and diameter analysis. Journal of Henan Agricultural Sciences. 5: 93-97.
Li, Y., Mamonova, E., KÖhler, N., Kleunen, M. V., and Stift, M. 2019. Breakdown of self-incompatibility due to genetic interaction between a specific S-allele and an unlinkedmodifier. Nature Communications. 14: 3420.
Motoki, K., Kinoshita, Y., and Hosokawa, M. 2019. Non-vernalization flowering and seed set of cabbage induced by grafting onto radish rootstocks. Frontiers in Plant Science. 9: 1967.
Muñoz-Sanz, J. V., Zuriaga, E., Cruz-García, F., McClure, B., and Romero, C. 2020. Self-(in)compatibility systems: target traits for crop-production, plant breeding and biotechnology. Frontiers in Plant Science. 11: 195.
Na Jinda, A., Nikornpun, M., Jeeatid, N., Thumdee, S., Thippachote, K., Pusadee, T., and Kumchai, J. 2023. Marker-assisted selection of male-sterile and maintainer line in chili improvement by backcross breeding. Horticulturae. 9(3): 357.
Nikornpun, M. 2002. Cabbage. Bangkok, Odeonstore.
Nikornpun M., Sukwiwat, K., Wongsing, K., and Kumchai, J. 2023. Development of male sterile lines of CMS chilies (Capsicum annuum L.) from F1 hybrids. Breeding Science. 73(2): 158-167.
Okamoto T., Wei, X., Mehraj, H., Mohammad, R. H., Akter, A., Miyaji, N., Takada, Y., Park, J., Fujimoto, R., Nou, I., and Watanabe, M. 2021. Chinese cabbage (Brassica rapa L. var. pekinensis) breeding: Application of molecular technology. p. 59-94. In J.M. Al-Khayri, S. Mohan Jain, and D.V. Johnson (eds.). Advances in plant breeding strategies: Vegetable crops. Springer Cham, Switzerland.
Sage T. L., Bagha, S., Lundsgaard-Nielsen, V., Branch, H. A., Sultmanis, S., and Sage, R. F. 2015. The effect of high temperature stress on male and female reproduction in plants. Field Crops Research. 182: 30-42.
Singh, S., Dey, S. S., Bhatia, R., Kumar, R., and Behera, T. K. 2019. Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reproduction. 32: 231-256.
Song, X., Liu, G., Huang, Z., Weike, D., Huawei, T., Ying, L., and Xilin, H. 2016. Temperature expression patterns of genes and their coexpression with LncRNAs revealed by RNA-Seq in non-heading Chinese cabbage. BMC Genomics. 17: 297.
Srima, S. 2018. Self-incompatibioity test and inheritance of Ogura CMS in chinese cabbage. Master's thesis, Chiang Mai University, Thailand.
Storme, N. D. and Geelen, D. 2014. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant, Cell & Environment. 37(1): 1-18.
Sun, X., Luo, S., Luo, L., Wang, X., Chen, X., Lu, Y., Shen, S., Zhao, J., and Bonnema, G. 2018. Genetic analysis of Chinese cabbage reveals correlation between rosette leaf and leafy head variation. Frontiers in Plant Science. 9: 1455.
Sun, X., Gao, Y., Lu, Y., Xiaomeng, Z., Shuangxia, L., Xing, L., Mengyang, L., Daling, F., Aixia, G., Xueping, C., Shuxin, X., Yanhua, W., Shuxing, S., Guusje, B., and Jianjun, Z. 2021. Genetic analysis of the “head top shape” quality trait of Chinese cabbage and its association with rosette leaf variation. Journal of Horticultural Research. 8: 106.
Tawatsinlapasorn, N., Kuljarachanan, T., Chiewchan, N., and Devahastin, S. 2017. Effects of drying techniques on selected functional properties and bioactive compounds of dietary fiber from the outer leaves of Cabbage. Chiang Mai University Journal of Natural Science. 16(1): 19-30.
Tisa, S. 2021. Inheritance of Ogura CMS gene and heterosis in Chinese cabbage. Master's thesis, Chiang Mai University, Thailand.
Toriyama, K. 2021. Molecular basis of cytoplasmic male sterility and fertility restoration in rice. Plant Biotechnology Journal. 38(3): 285-295.
Wu, C. Y., He, Q. W., Song, T. W., Deng, Y. L., Wang, C. H., and Xu, W. L. 2012. Selection of evaluation indexes in flavor quality of Chinese cabbage. Journal of Northwest Sci-Tech University of Agriculture and Forestry. 40: 161-168.
Yan, W. and Hunt, L. A. 1999. Reanalysis of vernalization data of wheat and carrot. Annals of Botany. 84(5): 615-619.
Yang, T., Davies, P. J., and Reid, J. B. 1996. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light grown peas. Journal of Plant Physiology. 110(3): 1029-1034.
Yamagishi, H., and Bhat, S.R. 2014. Cytoplasmic male sterility in Brassicaceae crops. Breeding Science. 64(1): 38-47.
Yamagishi, H. and Terachi, T. 1996. Molecular and biological studies on male sterile cytoplasm in the Cruciferae. III. distribution of Ogura-type cytoplasm among Japanese wild radishes and Asian radish cultivars. Theoretical and Applied Genetics. 93(3): 325-332.
Yamaguchi, M. 1983. World vegetables: Principles, production and nutritive values. United States, Westport, CT AVI Publishing Company.
Zhang, J., Liu, Z., Liu, X., Dong, J., Pang, H., and Yu, C. 2016a. Proteomic alteration of a thermo-sensitive male sterility SP2S in rapeseed (Brassica napus) in response to mild temperature stress. Plant Breeding. 135(2): 191-199.
Zhang, W., Xie, Y., Xu, L., Wang, Y., Zhu, X. W., Wang, R. H., Zhang, Y., Muleke, E. M., and Liu, L. W. 2016b. Identification of microRNAs and their target genes explores miRNA-mediated regulatory network of cytoplasmic male sterility occurrence during anther development in Radish (Raphanus sativus L.). Frontiers in Plant Science. 7: 1054.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Patiphan Boonlao1, Nakarin Jeeatid1, Kamon Thippachote1, Tonapha Pusadee1, and Jutamas Kumchai1, 2, *
1 Department of Plant and Soil Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50100, Thailand.
2 Center of Excellence on Agricultural Biotechnology, Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation. (AG-BIO/MHESI), Bangkok 10900, Thailand.
Corresponding author: Jutamas Kumchai, E-mail: jutamas.k@cmu.ac.th
ORCID: Jutamas Kumchai: https://orcid.org/0000-0001-7580-2794
Total Article Views
Editor: Wasu Pathom-aree
Chiang Mai University, Thailand
Article history:
Received: October 15, 2024;
Revised: November 21, 2024;
Accepted: November 25, 2024;
Online First: December 3, 2024

