
Development of Herbal Ultrasound Gel Prototype from Zingiber cassumunar Roxb., Curcuma longa L. and Zingiber officinale Roscoe for Antioxidant and Anti-Inflammation
Paphaphat Thiraphatthanavong, Warin Ohn-on, Sasalux Kaewbutra, Kanchanaporn Tongthong, Petcharat Rattanachompu, Namplon Panmuesng, Bhattaranitch Khampaenjiraroch, and Krissada Phandech*Published Date : November 26, 2024
DOI : https://doi.org/10.12982/NLSC.2025.011
Journal Issues : Number 1, January-March 2025
Abstract This research aimed to determine the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity of phlai, turmeric, ginger and combined extract of phlai, turmeric, and ginger at recipe 1:2:2 (PTG). In addition, the formula of herbal ultrasound gel for antioxidant and anti-inflammatory properties was developed. The TPC, flavonoid, antioxidant and anti-inflammation were investigated by using the Folin-Ciocalteu method, flavonoid, DPPH, ABTS and nitric oxide (NO) scavenging assay. The PTG extract was tested for the phytochemicals by GC-MS technique. The microbial contamination standard of herbal ultrasound gel prototype was determined. The result show that the PTG extract has the TPC and flavonoid at 105.11 ± 16.93 mg GAE/g extract and 63.58 ± 2.29 mg QE/g extract, respectively. Moreover, the DPPH and ABTS assay show that PTG extract has the half maximal inhibitory concentration (IC50) at 0.82 ± 0.11 and 0.98 ± 1.56 mg/mL, respectively. In addition, PTG extract has a high percentage of NO inhibition at 53.38 ± 0.16. Furthermore, the GC-MS method found that PTG extract has 7 active ingredients. The highest amount of phytochemical is AR-turmerone. The safety assessment for external use found that the herbal ultrasound gel prototype product passed standard criteria for microbial contamination at the laboratory level. To conclude, the PTG extract has a synergistic effect and a high ability to act as an antioxidant and anti-inflammation. Moreover, the PTG herbal ultrasound gel has a microbial contamination safety to use. Therefore, the prototype herbal ultrasound gel product may be further developed into health products to treat pain and inflammation efficiently in clinical practice.
Keywords: Ultrasound gel, Herb, Antioxidant, Anti-inflammation
Funding: This research was supported by Udon Thani Rajabhat University (UDRU), Thailand Science Research and Innovation (TSRI) and Natural Science, Research and Innovation Fund (NSRF) 2022.
Citation: Thiraphatthanavong, P., Ohn-on, W., Kaewbutra, S., Tongthong, K., Rattanachompu, P., Panmuesng, N., Khampaenjiraroch, B., and Phandech, K. 2025. Development of herbal ultrasound gel prototype from Zingiber cassumunar Roxb., Curcuma longa L. and Zingiber officinale Roscoe for antioxidant and anti-inflammation. Natural and Life Sciences Communications. 24(1): e2025011.
INTRODUCTION
Myofascial pain syndrome (MPS) is common in patients with musculoskeletal pain problems. MPS is a pain condition originating from muscle and fascia. Patients usually present with localized pain or referred pain of various patterns. Myofascial trigger points (MTrPs) are characterized by regional pain originating from hyperirritable spots located within taut bands of skeletal muscle that can be found in MPS [Leite et al., 2009]. Therapeutic physical modalities are commonly used in the clinical setting for the patients with MPS [Meltem et al., 2000; Draper et al., 2010; Unalan et al., 2011; Acar and Yilmaz, 2012; Ilter et al., 2015; Kavadar et al., 2015]. Ultrasound therapy is widely used to improve connective tissue extensibility and pain relief in MPS. In addition, phonophoresis is the permeability of drug molecules through the skin while being exposed to ultrasound. These facilitate the transfer of many active ingredients through the skin and the points of inflammatory areas. Moreover, it can relieve muscle pain. Therefore, some herbal medicines may be substituted for analgesics or anti-inflammation in ultrasound therapy. At present, a natural ultrasound gel has been developed with 10 and 20 percent aloe vera. It was effective in reducing pain in the shoulders and legs in volunteers. The combination of natural herbs that have antioxidant activity and phytochemical should have a positive effect and increase the efficiency of the treatment [Lijuan et al., 2019].
Medicinal plants have been used for medicine especially in the group of chronic non-communicable diseases (NCDs), which are important health problems for people in society. The main cause is lifestyle behaviors such as eating not nutritionally food, lack of exercise, high stress from social and environmental conditions. These factors cause cells in the body to create more free radicals. These substances stimulate the oxidation process affecting the functioning of cells in the body and leading to various diseases. Medicinal plants are considered important sources of potential substances. These plants as a result exhibit biological property. Particularly among them, phenolic compounds having one or more hydroxyl groups are capable of displaying antioxidant properties against free radicals or transition metals in oxidation reactions. In addition, these compounds can act as inhibitors against some key enzymes, which are associated with global health problems [Ceylan et al., 2021]. Previous research found that phlai, turmeric and ginger has ability to be an antioxidant that reduce the risk of disease [Hecker, 2018; Chumpawadee et al., 2019; Harding, 2020; Iverson et al., 2020]. The Zingiber cassumunar Roxb or phlai is commonly used for medicinal purposes including anti-inflammatory, anticancer, neuroprotective, cosmeceutical and antifungal or antimicrobial bioactivities, especially the rhizome [Prakatthagomol et al., 2012]. Previous study shows that, the rhizome of phlai contains active ingredients, which are curcumin and phenylbutanoid substances such as (E)-1-(3,4-dimethoxyphenyl) butadiene (DMPBD) that effects to reduce inflammation. A study of the effect of phlai cream for muscle pain relief with concentrations of 7 and 14 percent in 75 healthy volunteers who exercised by stretching the front thigh muscles 25 times, phlai cream was applied every 8 hours for 7 days. The results found that phlai cream with a concentration of 14 percent can significantly reduce muscle soreness after exercise compared to placebo (P<0.05) [Chongmelaxme et al., 2017]. The Curcuma longa L. or turmeric is the group of oldest cultivated spice plants. Curcuminoids consist of the major components including curcumin, bisdemethoxycurcumin and demethoxycurcumin [Aggarwal et al., 2003]. It was not soluble in water and ether but soluble in ethanol and other organic solvents. The scavenging of reactive oxygen species (ROS) activity of curcumin has also been implicated in anti-inflammation. Curcumin inhibits metabolism of arachidonic acid, cyclooxygenase (COX) and lipoxygenase (LOX) activities and releases of steroid hormones. It reduces pro-inflammatory leukotriene synthesis via inhibition of LOX enzyme. Curcumin is also a potent inhibitor of pro-inflammatory cytokines [Kohli et al., 2005]. The Zingiber officinale Roscoe or ginger has been the focus of interest because it has a lot of polyphenol compounds. These compounds act as an active ingredient to prevent significant oxidation-linked diseases [Sindhi et al., 2013]. Important biologically active substances found in ginger rhizomes include gingerols and shogaols, which give the spicy taste and inhibit the secretion of various inflammatory substances including cyclooxygenase-2 (COX-2), nitric oxide syntheses (iNOS), and phospho-NF-kB [Shim et al., 2011]. In addition, they can relieve muscle pain caused by exercise [Black et al., 2010] and reduces inflammation in osteoarthritis patients [Naderi et al., 2016]. Therefore, this study was interested for screening the antioxidant and anti-inflammatory activity of medicinal herbs including phlai, turmeric and ginger to develop a natural herbal ultrasound gel prototype.
MATERIALS AND METHODS
Plant materials and preparation
The selected plants including Zingiber cassumunar Roxb. (Z. cassumunar) or phlai, Curcuma longa L. (C. longa) or turmeric were collected from Nam Som-Udon Thani province and Zingiber officinale Roscoe (Z. officinale) or ginger from Lom Sak-Phetchabun province on July 2022. The reference specimen numbers for research plants (voucher specimens) are 25652001, 25652002, 25652003, respectively, kept at the Faculty of Science, Udon Thani Rajabhat University. They were cleaned, cut into small pieces and dried at 60°C for 24 hrs. They were ground to fine powder by using a homogenizer. The samples were macerated with 50% ethanol for 7 days at room temperature (1:10 w/v) and filtered through Whatman filter paper number 4. The liquid extract was evaporated using a rotary evaporator. They should be kept in opaque bottle and stored at 4°C until used.
Total phenolic contents (TPC) assay
A total phenolic compound was measured using the Folin-Ciocalteu colorimetric method [Quettier-Deleu et al., 2020]. 20 µL of each plant extracts was mixed with 0.2 mL of Folin-Ciocalteu reagent, added 2 mL of distill water and incubated at room temperature for 5 min. Then, 1 mL of 20% sodium carbonate was added and incubated at room temperature for 2 hr. The total polyphenolic contents was determined by measuring the absorbance 765 nm with a spectrophotometer. Gallic acid was used as a standard and the total phenolics were expressed as gallic acid equivalents (mg GAE/g extract). All determinations were performed in triplicate.
Total flavonoid contents (TFC) assay
The aluminum chloride method was used for the determination of the total flavonoid content. 100 µL of each plant extracts was mixed with 100 µL of 2% AlCl3. Absorbance at 415 nm was recorded after 15 min of incubation. Quercetin was used as a standard and the total flavonoids were expressed as quercetin equivalent (mg QE/g extract). All determinations were performed in triplicate.
DPPH assay
The capacity to scavenge the stable free radical DPPH was determined by using DPPH assay [Tai et al., 2011]. The working solutions of each plant extract at 0.01, 0.1, 1, 5 and 10 mg/mL were prepared in methanol. Various concentrations of ascorbic acid similar to that of the plant extract were prepared and used as standard. The solution containing 1 mM DPPH solution in methanol was incubated at room temperature for 30 min in a dark condition. The absorbance at 517 nm was recorded. Equal amount of methanol and DPPH solution were mixed and processed as similar as that of the plant extract and the obtained solution was served as control. The DPPH radical scavenging activity was calculated using the following formula:
DPPH scavenging activity (%) = [(Ac-At) / Ac] x 100
Ac is the absorbance value of the control.
At is the absorbance value of the sample extracts or STD.
The half maximal inhibitory concentration (IC50) was calculated by plotting inhibition percentage against extract concentration using the linear regression analysis. Ascorbic acid was used as positive control.
ABTS assay
ABTS radical scavenging activity was done according to the procedure described by Marino BA, et al. [Marino et al., 2011] with some modifications. In brief, the working solutions of standard concentrations at 0.0125, 0.025, 0.05, 0.1 and 0.2 mg/mL and each plant extract concentrations at 0.01, 0.1, 1, 5 and 10 mg/mL were prepared in solvent. 20 µL of diluted sample was mixed with 150 µL of ABTS working solution and left at room temperature in the dark for 5 min. Subsequently, absorbance of the mixture was measured at 750 nm. ABTS radical scavenging activity was calculated using the following formula:
ABTS radical scavenging activity (%) = [(Ac- At)/Ac] x100
Ac is the absorbance value of the control.
At is the absorbance value of the sample extracts or STD.
The half maximal inhibitory concentration (IC50) was calculated by plotting inhibition percentage against extract concentration using the linear regression analysis. Trolox was used as a positive control.
Nitric oxide (NO) assay
Nitric oxide (NO) was produced from sodium nitroprusside (SNP) which modify measured as described by Nihal Aktas, et al. [Aktas et al., 2013] and by using the Griess reagent. The mixture containing 10 mM of SNP in PBS (pH 7.4), with the herbal extracts were prepared in PBS and incubated for 150 min at room temperature. Finally, an equal volume of the Griess reagent (1% sulfanilamide, 0.1% napthylethylenediamine dihydrochloride, 2.5% H3PO4) was added to each well in order to measure the nitrite content. Quercetin concentration at 10 mg/mL was used as a reference standard. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions. The NO scavenging activity was calculated using the following formula:
NO scavenging activity (%) = [(Ac-At) / Ac] x 100
Ac is the absorbance value of the control
At is the absorbance value of the sample extracts or STD.
Gas chromatography-mass spectrometry (GC-MS) analysis
The GC-MS was performed using a PerkinElmer Headspace Therbo matrix 40 auto-sampler (Clarus 690, Perkin Elmer, Waltham, MA USA). The condition parameters were set for the method described by Boonyanugomol, et al. [Boonyanugomol et al., 2022] with some modifications. A 30 m x 0.25 mm i.d. Elite-5MS capillary column with 0.25 µm film thickness (Perkin Elmer Elmer, Waltham, MA USA) was used for the GC-MS analysis. High purity helium was used as carrier gas and a set flow rate of 1 mL/min constantly. The injector was set at 200°C and performed in split ratio 10:1. The temperature of column oven was set at 60°C for 5 min and then increased by 4°C per min until 280°C, where it was held at this temperature for 13 min. The mass spectrometry (MS) detection was carried out at 200°C, and the electron impact (EI) mode was required, using the full scan mode from m/z 30 to 600 with a scanning speed at low level. The chemicals of extracts were identified based on a comparison of corresponding GC retention times, peak area and mass spectra to a reference from the US National Institute of Standard and Technology (NIST 2017X, with more than 75 percentage similarity being required for a match.
Development of the herbal ultrasound gel prototype
The combination extract of phlai, turmeric, and ginger at recipe 1:2:2 was used to dissolve in 24.81 percent deionized (DI) water by weight, stirred until all the ingredients are mixed together and filtered through Whatman filter paper number 4 (clear, light-yellow liquid). For gel formulation, 0.30 percent of Carbopol 940 were dissolved in 74.44 percent DI water by weight and used a gel agitator to mix. Then, 0.10 percent triethanolamine by weight were added to form a pH balance. The prepared herbal extract combination and 0.25 percent sodium benzoate by weight were added to gel. The herbal ultrasound gel prototype was packed in the opaque pump bottle that has been sterilized by autoclave and stored at room temperature.
Microbial contamination standard test
Microbial contamination standard test of herbal ultrasound gel prototype was sent to testing at the central laboratories (Thailand) include 6 types following:
1) Aerobic Plate Count by FDA BAM online, 2011 method
2) Clostridium spp. by INH-USP38/33:2015 method
3) Pseudomonas aeruginosa by USP38/33:2015 method
4) Salmonella spp.by INH-ISO6579-1:2017(E) method
5) Staphylococcus aureus by USP38/33:2015 method
6) Yeasts and Molds by In-house based on FDA BAM method
Statistical analysis
All data were presented as mean ± standard error means (SEM). Comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Bonferroni tests using SPSS statistical software version 26. P-value ≤ 0.05 was considered significant.
RESULTS
Yield of the extracts
The percentage yields of the selected plants are shown in Table 1. The rhizome of Z. cassumunar., C. longa. and Z. officinale have the percentage yields (dried sample) at 25.57, 12.81 and 11.70, respectively. However, the percentage of yield (crude extract) in all groups (P), (T) and (G) of maceration was shown.
Table 1. Percentage of yield.
|
Sample |
% yield |
|
Zingiber cassumunar Roxb. (Phlai) |
25.57 |
|
Curcuma longa L. (Turmeric) |
12.81 |
|
Zingiber officinale Roscoe (Ginger) |
11.70 |
|
50 percent of ethanol extract : Phlai (P) |
3.29 |
|
50 percent of ethanol extract: Turmeric (T) |
2.60 |
|
50 percent of ethanol extract: Ginger (G) |
3.06 |
The TPC, TFC, and antioxidative activity of phlai, turmeric, and ginger and combination PTG extracts
The total phenolic content (TPC) was calculated from the gallic acid standard curve (y=0.005x + 0.0872, R2=0.9916). It was found that a percent of the gallic acid as STD has the highest content of phenolic compounds at 1,076.70 ± 6.90 milligram equivalents of gallic acid per gram of sample extract. Furthermore, the percent of ethanol extract of ginger (G), 50 percent of ethanol extract of phlai (P) and 50 percent of ethanol extract of turmeric (T) have high content of phenolic compounds at 75.93 ± 17.71, 61.73 ± 10.60 and 27.97 ± 8.66 milligram equivalents of gallic acid per gram of sample extract, respectively. In addition, the combination PTG extracts had a higher amount of TPC than all groups at 105.11 ± 16.93 milligram equivalents of gallic acid per gram of sample extract. The TPC of PTG significantly increased at P-value ≤0.05 and 0.01 when compared to the (P) and (T) groups, respectively, as shown in Table 2.
The total flavonoid content (TFC) was calculated from the quercetin standard curve (y=0.0035x + 0.1436, R2=0.9905). It was found that a percent of the quercetin as STD has the highest content of total flavonoid content at 1,003.72 ± 15.36 milligram equivalents of quercetin per gram of sample extract. Furthermore, the 50 percent of ethanol extract of ginger (G), 50 percent of ethanol extract of phlai (P) and 50 percent of ethanol extract of turmeric (T) have the high content of total flavonoid at 33.67 ± 1.82, 19.11 ± 2.68 and 6.76 ± 0.96 milligram equivalents of quercetin per gram of sample extract, respectively. In addition, the combination PTG extracts had a higher amount of total flavonoid content than all groups at 63.58 ± 2.29 milligram equivalents of quercetin per gram of sample extract. The total flavonoid content of PTG significantly increased at P-value ≤ 0.001 when compared to all groups, as shown in Table 2.
Table 2. Total phenolic compound (TPC) and total flavonoid content (TFC) (Mean ± SEM).
|
Sample (crude extract) |
TPC (mg GAE/g extract) |
TFC (mg QE/g extract) |
|
STD |
Gallic acid = 1076.70 ± 6.90 |
Quercetin = 1003.72 ± 15.36 |
|
Phlai (P) |
61.73 ± 10.60 |
19.11 ± 2.68 |
|
Turmeric (T) |
27.97 ± 8.66 |
6.76 ± 0.96 |
|
Ginger (G) |
75.93 ± 17.71 |
33.67 ± 1.82 |
|
Combination PTG |
105.11 ± 16.93a, bb |
63.58 ± 2.29aaa, bbb, ccc |
Note: a = Statistically significant difference at P-value ≤0.05
aaa = Statistically significant difference at P-value ≤0.001
when compared with phlai (P) group
bb = Statistically significant difference at P-value ≤0.01
bbb = Statistically significant difference at P-value ≤0.001
when compared with turmeric (T) group
ccc = Statistically significant difference at P-value ≤0.001
when compared with ginger (G) group
The analysis of antioxidant activity by DPPH method found that the 50 percent of ethanol extract of ginger (G), 50 percent of ethanol extract of phlai (P) and 50 percent of ethanol extract of turmeric (T) has the half maximal inhibitory concentration (IC50) at 0.61 ± 0.01, 3.56 ± 0.30 and 4.19 ± 0.56 mg/mL, respectively. In addition, the combination PTG extracts had significantly IC50 better than (P) and (T) at P-value. ≤ 0.001. However, it was found that the combination PTG extracts had IC50 better than (G) but no statistically significant difference was found. The antioxidant activity of ascorbic acid, which is the standard substance (STD) of the DPPH test has IC50 better than all groups at 0.01 ± 0.00 mg/mL as shown in Table 3.
The analysis of antioxidant activity by ATBS method found that the 50 percent of ethanol extract of ginger (G), 50 percent of ethanol extract of turmeric (T) and 50 percent of ethanol extract of phlai (P) has the half maximal inhibitory concentration (IC50) at 5.57 ± 0.25, 8.29 ± 0.24 and 10.66 ± 0.21 mg/mL, respectively. Moreover, the combination PTG extracts had significantly IC50 better than all groups at P-value ≤ 0.001. The Trolox, which is the standard substance (STD) of the ATBS test has IC50 better than all groups at 0.06 ± 0.01 mg/mL as shown in Table 3.
Table 3. The half-maximal inhibitory concentration (IC50) of DPPH and ABTS (Mean ± SEM).
|
Sample (crude extract) |
DPPH IC50 (mg/mL) |
ABTS IC50 (mg/mL) |
|
STD |
Ascorbic acid = 0.01 ± 0.00 |
Trolox = 0.06 ± 0.01 |
|
Phlai (P) |
3.56 ± 0.30 |
10.66 ± 0.21 |
|
Turmeric (T) |
4.19 ± 0.56 |
8.29 ± 0.24 |
|
Ginger (G) |
0.61 ± 0.01 |
5.57 ± 0.25 |
|
Combination PTG |
0.82 ± 0.11aaa, bbb |
0.98 ± 1.56 aaa, bbb, ccc |
Note: aaa = Statistically significant difference at P-value ≤0.001
when compared with phlai (P) group
bbb = Statistically significant difference at P-value ≤0.001
when compared with turmeric (T) group
ccc = Statistically significant difference at P-value ≤0.001
when compared with ginger (G) group
The inhibitory effect of nitric oxide (NO) was expressed as the percentage of maximum inhibition. It was found that 50 percent of ethanol extract of ginger (G), 50 percent of ethanol extract of phlai (P) and 50 percent of ethanol extract of turmeric (T) at the concentration of 10 mg/ml have the percentage inhibition of nitric oxide at 47.09 ± 0.95, 44.63 ± 0.53 and 38.75 ± 2.55, respectively. In addition, the combination PTG extracts had a higher nitric oxide inhibition percentage than all groups. Moreover, the combination PTG extracts had significantly higher than 50 percent of ethanol extract of turmeric (T) at P-value ≤ 0.05. The quercetin, which is the standard substance (STD) of the test at concentration of 0.1 mg/mL has the percentage of nitric oxide inhibition at 71.58 ± 2.21 as shown in Table 4.
Table 4. The percentage of NO inhibition (Mean ± SEM).
|
Sample (crude extract) |
NO % inhibition |
|
Ascorbic acid (STD) |
71.58 ± 2.21 |
|
Phlai (P) |
44.63 ± 0.53 |
|
Turmeric (T) |
38.75 ± 2.55 |
|
Ginger (G) |
47.09 ± 0.95 |
|
Combination PTG |
53.38 ± 0.16b |
Note: b = Statistically significant difference at P-value ≤0.05
when compared with turmeric (T) group
Gas chromatograph mass spectrometer (GC-MS) of combination PTG extracts
The combination PTG extracts were examined for important phytochemicals by using GC-MS. It was found that the chemical composition of the combination PTG extracts have time to move through the column in the range of 8.99-19.13 minutes. There are seven chemical compositions of the substances. The descending order highest amounts of phytochemical is ar-Turmerone, Tumerone and 6-(p-Tolyl)-2-methyl-2-heptenol, trans- as shown in Table 5 and Figure 1.
Table 5. Chemical composition of combination PTG extract from analysis by using GC-MS technique.
|
Peak No. |
Retention time |
Assignment compounds |
Formula |
% Match |
% Area |
|
1 |
8.99 |
Cyclohexene, 1-methyl-4-(1-methylethylid |
C10H16 |
90.30 |
1.26 |
|
2 |
9.87 |
Gamma-Terpinene |
C10H16 |
91.30 |
4.46 |
|
3 |
17.98 |
(S,Z)-2-Methyl-6-(p-tolyl)hept-2-en-1-ol or cis-Nuciferol |
C15H22O |
83.50 |
2.98 |
|
4 |
18.72 |
ar-Turmerone |
C15H22O |
97.90 |
43.73 |
|
5 |
18.78 |
Turmerone |
C15H22O |
92.70 |
25.27 |
|
6 |
19.15 |
6-(p-Tolyl)-2-methyl-2-heptenol, trans-. |
C15H22O |
75.10 |
20.65 |
|
7 |
19.93 |
(E)-Gamma-atlantone |
C15H22O |
77.80 |
1.65 |
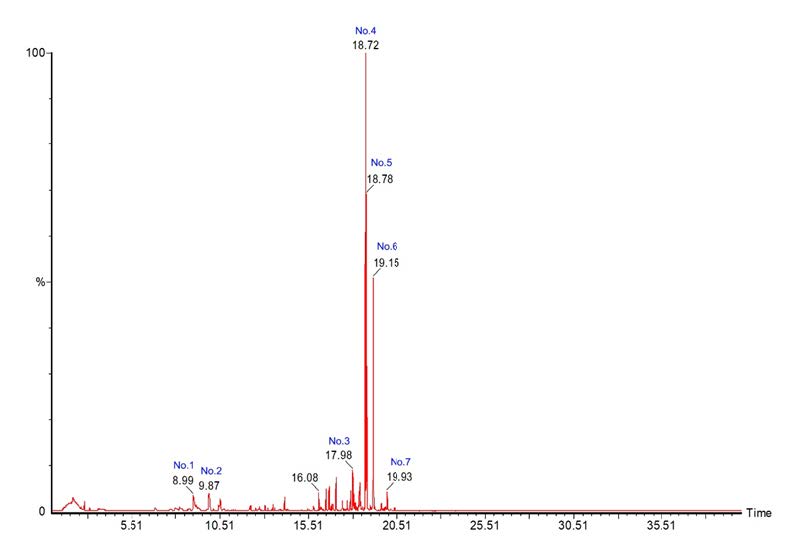
Figure 1. Chromatograms of combination PTG extract from analysis by using GC-MS technique.
Development of the natural herbal ultrasound gel prototype
The combination PTG extract herbal ultrasound gel has a viscosity value of 1,636.00 ± 14.70 Pa.s and pH-value of 5.36 ± 0.01, which is an approximate value to the standard ultrasound gel that manufactured by A.N.B Laboratories. Co. LTD, Thailand and were used in clinical practice. It has a viscosity value of 1,708.67 ± 13.69 Pa.s and pH-value of 6.52 ± 0.03. The characteristics of the ultrasound gel prototype as shown in figure 2.

Figure 2. The characteristics of the ultrasound base gel prototype (A) and the ultrasound PTG gel prototype (B)
Microbial contamination standard safety test of the herbal ultrasound gel prototype
Microbial contamination standard test is an important characteristic for evaluating the quality criteria of the natural herbal product for external usage. It was found that the Aerobic Plate Count test had a value <2.5x102 EAPC cfu/g. The Clostridium spp., Pseudomonas aeruginosa, Salmonella spp. and Staphylococcus aureus test were not found. In addition, the number of Yeasts and Molds test has 1.7x102 cfu/g. All data were shown in Table 6.
Table 6. The microbial contamination standard test of combination PTG extract herbal ultrasound gel.
|
Test |
Results |
Unit |
|
Aerobic Plate Count |
<2.5x102 EAPC |
cfu/g |
|
Clostridium spp. |
Not detected |
per 1 g |
|
Pseudomonas aeruginosa |
Not detected |
per 1 g |
|
Salmonella spp. |
Not detected |
per 25 g |
|
Staphylococcus aureus |
Not detected |
per 1 g |
|
Yeasts and Molds |
1.7x102 |
cfu/g |
DISCUSSION
Ultrasound therapy is widely used to improve extensibility of connective tissue and pain relief in MPS. In addition, phonophoresis is a permeability of drug molecules through the skin while being exposed to ultrasound. These facilitate the transfer of many active ingredient through the skin and the points of inflammatory areas. Moreover, it can relieve muscle pain. Therefore, some herbal medicines may be substituted for analgesic or anti-inflammation in ultrasound therapy. Herbal medicine is long time used for treating numerous diseases. It has been believed that it safer than synthetic drugs. Therefore, herbal medicine is very popular and gains much attention nowadays. Phlai has been used for antioxidant and anti-inflammatory properties by therapeutic ultrasound in a rat model [Leelarungrayub et al., 2017] and applied Thai herbal poultice in a clinical study [Srikaew et al., 2023]. The natural ultrasound gel has been developed with 10 and 20 percent aloe vera. It was effective in reducing pain in the shoulders and legs in volunteers [Mahendran et al., 2017]. Therefore, this research focus on the antioxidant and anti-inflammation activity of
Z. cassumunar or phlai, C. longa or turmeric and Z. officinale or ginger. The results shown that the 50 percent ethanol extract of them have the phenolic compounds content, total flavonoid content, antioxidant capacity by the DPPH and ATBS methods and ability to inhibit of nitric oxide (NO), which plays a role in the inflammatory process as shown in previous study [Thiraphatthanavong et al., 2023]. Moreover, the 50 percent ethanol extract of phlai (P), turmeric (T), and ginger (G) combination or PTG were combined at recipe 1:2:2 that has synergistic effect. It was found that PTG have high antioxidant and anti-inflammation properties than single plants. The possible mechanism that makes the PTG extract better than each single extract may be 1) protection each other because the combined phytochemicals act in different ways of antioxidation that enable them to protect each other from oxidative agents; 2) one antioxidant reacts with free radicals or singlet oxygen first to protect the partner from oxidants; 3) one antioxidant is oxidized and becomes a free radical, and this free radical then receive electrons or hydrogen atoms from the other antioxidant to regenerate itself [Vijayalakshmi et al., 2014; Lijuan et al., 2019]. The combined herbal extracts (two or more) offer great potential for exhibiting synergistic actions. The mechanisms underlying synergistic actions are 1) different agents may regulate either the same or different target in various pathways, and therefore cooperate in an agonistic, synergistic way; 2) regulate the enzymes and transporters; 3) overcome the drug resistance mechanisms of microbial cells; and 4) eliminate the adverse effect and enhance pharmacological potency of agents by “processing” or by drug-drug interaction. [Yang et al., 2014; Sharma et al., 2020]. The analysis phytochemical by using the GC-MS technique, 7 types of phytochemicals were found. The main substance was turmerone group that the previous report shown the anti-inflammation properties in several disease [Ashish et al., 2013, Bibi et al., 2021]. The data from HPLC may still be needed for high molecular weight compounds present in herbal extracts that specific compounds in each herb such as gingerol, shogaols in ginger; curcuminoid in phlai, and turmeric. These compounds are highly pleiotropic molecules that anti-inflammatory, antioxidant and antimicrobial activities [Kunnumakkara et al., 2023]. For long-term treatment efficiency, the stability test of the PTG prototype gel was the focus to provide evidence on how the quality of an active substance product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light. The data from In vitro study, the PTG extract was used to develop a prototype product of herbal ultrasound gel for relief pain and inflammation. The prototype PTG herbal ultrasound gel was formulated with Carbopol 940 as a gel agent and triethanolamine as neutralize agent. The pH values of gel formulations at 5.36 ± 0.01 were in the range of skin pH [Chaitra et al., 2017] so it indicated that might not cause skin irritation. Moreover, the PTG prototype gel has a viscosity value of 1,636.00 ± 14.70 Pa.s. This viscosity range is suitable for contact and stays on the skin without slipping off. The texture is similar to the gel available in the commercial product. The safety assessment followed the principles for developing herbal products for external use on the skin were analyzed. The prototype PTG herbal ultrasound gel product passed standard criteria in terms of microbial contamination including Aerobic Plate Count, Clostridium spp., Pseudomonas aeruginosa, Salmonella spp. and Staphylococcus aureus test.
CONCLUSION
The prototype product of PTG herbal ultrasound gel is the potential candidate to serve as antioxidant and anti-inflammation media gel for ultrasound therapy. However, the data from HPLC may still be needed for high molecular weight compounds present in herbal extracts. In addition, the further study should focus on amount of active compound after forming the ultrasound gel, the stability test and skin permeation study. These is importance information to ensure their therapeutic effect for use to develop the most effective health products for further clinical treatment.
ACKNOWLEDGEMENTS
This research was supported by Udon Thani Rajabhat University (UDRU), Thailand Science Research and Innovation (TSRI) and Natural Science, Research and Innovation Fund (NSRF) 2022.
AUTHOR CONTRIBUTIONS
Paphaphat Thiraphatthanavong and Krissada Phandech assisted in conducting the experiments, performed the herbal ultrasound gel prototype, data analysis and wrote the manuscript. Warin Ohn-on and Sasalux Kaewbutra assisted in the antioxidant and active ingredient test and data analysis. Kanchanaporn Tongthong, Petcharat Rattanachompu, Namplon Panmuesng and Bhattaranitch Khampaenjiraroch assisted in data support and provide instrument that used in research. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
REFERENCES
Acar, B. and Yilmaz, O. T. 2012. Effects of different physiotherapy applications on pain and mobility of connective tissue in patients with myofascial pain syndrome. Journal of Back and Musculoskeletal Rehabilitation. 25(4): 261-267.
Aggarwal, B. B, Kumar, A., and Bharti, A. C. 2023. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Research. 23: 363-398.
Aktas, N., Genc, Y., Gozcelioglu, B., Konuklugil, B., and Harput, U. S. 2013. Radical scavenging effect of different marine sponges from Mediterranean coasts. Records of Natural Products. 7(2): 96-104.
Ashish, S. B., Joshua, A.J., Natarajan, B., and Amit, A. 2013. Comparative evaluation of anti-inflammatory activity of Curcuminoids, Turmerones, and Aqueous extract of Curcuma longa. Advances in Pharmacological Sciences. 23: 1-7.
Bibi, M. R., Mahboobeh, G. R., and Hossein, H. 2021. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytotherapy Research. 35 (12): 6487-7064.
Black, C. D., Herring, M. P., Hurley, D. J., and O'Connor, P. J. 2010. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. The Journal of Pain. 11(9): 894-903.
Boonyanugomol, W., Rukseree, K., Prapatpong, P., Reamtong, O., Baik, S. C., Jung, M., Shin, M. K., Kang, H. L., and Lee, W. K.2022. Endoplasmic reticulum stress and impairment of ribosome biogenesis mediate the apoptosis induced by ocimum x africanum essential oil in a human gastric cancer cell line. Medicina. 58 (6): 799.
Ceylan. R., Zengin, G., Mahomoodally, M. F., Sinan, K. I., Jugreet, G. A. S., Cakır, O., Ouelbani, R., Paksoy, M. Y., and Yılmaz, M. A. 2021. Enzyme inhibition and antioxidant functionality of eleven Inula species based on chemical components and chemometric insights. Biochemical Systematics and Ecology. 95: 104225.
Chaitra, P., Puneet, B., Siddhi, T., Banashree, M., and Rishi, K. B. 2017. Skin surface pH in acne vulgaris: Insights from an observational study and review of the literature. The Journal of Clinical and Aesthetic Dermatology. 10(7): 33-39.
Chongmelaxme, B., Sruamsiri, R., Dilokthornsakul, P., Dhippayom, T., Kongkaew, C., Saokaew, S., Chuthaputti, A., and Chaiyakunapruk, N. 2017. Clinical effects of Zingiber cassumunar (Plai): A systematic review. Complementary Therapies in Medicine. 35: 70-77.
Chumpawadee, S., Sommart, K., Vongpralub, T., and Paatarajinda, V. 2006. Effect of synchronizing the rate of dietary energy and nitrogen release on ruminal fermentation, microba Al protein synthesis and blood urea nitrogen in beef cattle. Asian-Australasian Journal of Animal Sciences .19(2): 364-366.
Draper, D. O., Mahaffey, C., Kaiser, D., Eggett, D., and Jarmin, J. 2010. Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiotherapy Theory and Practice. 26(3): 167-172.
Harding, D. J. 2020. Redox-active group 6 transition metal alkyne complexes. Ph. D. Dissertation. University of Bristol, Bristol, England.
Hecker, A.L. 2018. Nutrition and physical performance. p.23-40 In: R.H. Strauss (ed.). Drugs & performance in sport. WB Saunders, Philadelphia.
Ilter, L., Dilek, B., Batmaz, I., Ulu, M. A., Sariyildiz, M. A., Nas, K., and Cevik, R. 2015. Efficacy of pulsed and continuous therapeutic ultrasound in myofascial pain syndrome: A randomized controlled study. American Journal of Physical Medicine & Rehabilitation. 94(7): 547-554.
Iverson, L.I., Iverson, S.D., and Snyder, S.H. 2020. Handbook of psychopharmacology. p.99-115. Plenum Press, New York.
Kavadar, G., Caglar, N., Ozen, S., Tutun, S., and Demircioglu, D. 2015. Efficacy of conventional ultrasound therapy on myofascial pain syndrome: A placebo-controlled study. Agriculture Journals. 27(4): 190-196.
Kohli, K., Ali, J., Ansari, M. J., and Raheman, Z. 2005. Curcumin: A natural anti-inflammatory agent. Indian Journal of Pharmacology. 37(3):141-147.
Kunnumakkara, A. B., Hegde, M., Parama, D., Girisa, S., Kumar, A., Daimary, U. D., Garodia, P., Yenisetti, S. C., Oommen, O. V., and Aggarwal, B. B.2023. Role of turmeric and curcumin in prevention and treatment of chronic diseases: Lessons learned from clinical trials. ACS Pharmacology and Translational Science. 6(4): 447-518.
Leelarungrayub, J., Manorsoi, J., and Manorsoi, A. 2017. Anti-inflammatory activity of niosomes entrapped with Plai oil (Zingiber cassumunar Roxb.) by therapeutic ultrasound in a rat model. International Journal of Nanomedicine. 12: 2469–2476.
Leite, F., Atallah, A., El Dib, R., Grossmann, E., Januzzi, E., Andriolo, R. B., and da Silva, E. M. 2009. Cyclobenzaprine for the treatment of myofascial pain in adults. Cochrane Database of Systematic Reviews. 3: 1-21.
Lijuan, Z., Carlos, V., and Hongwei, S. 2019. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. Journal of Nutritional Biochemistry. 69: 19-30.
Mahendran, S., Afikaah Sha, A. A., Ganesh, S. S., Vengata, S. M., Isaac, J. J., and Phan, A. Y. 2017. Formulation and evaluation of natural ultrasound gel for physiotherapy treatment. Indo American Journal of Pharmaceutical Sciences. 4 (08): 2548-2554.
Marino, B. A., Antonio, C., and Manuel, A. 2011. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry. 73: 239-244.
Meltem, E., Nil, C., and Tayfun, A. 2000. Treatment of myofascial pain. American Journal of Physical Medicine & Rehabilitation. 79(1): 48-52.
Naderi, Z., Mozaffari-Khosravi, H., Dehghan, A., Nadjarzadeh, A., and Husein, H.F. 2016. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. Journal of Traditional and Complementary Medicine. 6: 199-203.
Prakatthagomol, W., Sirithunyalug, J., and Okonogi, S. 2012. Comparison of antibacterial activity against food-borne bacteria of Alpinia galanga, Curcuma longa, and Zingiber cassumunar. Chiang Mai University Journal of Natural Sciences. 11(2): 177-186
Quettier-Deleu, C., Gressier, B., Vasseur, J., Dine, T., Brunet, C., Luyckx, M., Cazin, M., Cazin, J. C., Bailleul, F., and Trotin, F. 2020. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. Journal of Ethnopharmacology. 72: 35-42.
Sharma, K., Guleria, S., Razdan, V.K., and Babu, V. 2020. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Industrial Crops and Products. 154: 112569.
Shim, S., Kim, S., Choi, D.S., Kwon, Y.B., and Kwon, J. 2011. Anti-inflammatory effects of [6]-shogaol: Potential roles of HDAC inhibition and HSP70 induction. Food and Chemical Toxicology. 49: 2734-2740.
Sindhi, V., Gupta, V., Sharma, K., Bhatnagar, S., Kumari, R., and Dhaka, N. 2013. Potential applications of antioxidants-A review. Journal of Research in Pharmacy. 7: 828-835.
Srikaew, N., Phewkham, N., Tungsukruthai, S., Sriyakul, K., Tungsukruthai, P., Phetkate, P., Kamalashiran, C., and Jumpa-ngern, P. 2024. The effectiveness of herbal poultice in relieving pain and flexibility in osteoarthritis patients. Natural and Life Sciences Communications. 23(3): e2024031.
Tai, A., Sawano, T., Yazama, F., and Ito, H. 2011. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochimica et Biophysica Acta. 1810: 170-177.
Thiraphatthanavong, P., Ohn-on, W., and Kaewbutra, S. 2023. Evaluation of phytochemical compositions, total phenolic contents and antioxidant activities in different solvent maceration techniques of Zingiber cassumunar Roxb., Curcuma longa L. and Zingiber officinale Roscoe. Health Science, Science and Technology Review. 16 (3): 53-68.
Unalan, H., Majlesi, J., Aydin, F.Y., and Palamar, D. 2011. Comparison of high-power pain threshold ultrasound therapy with local injection in the treatment of active myofascial trigger points of the upper trapezius muscle. Archives of Physical Medicine and Rehabilitation. 92(4): 657-662.
Vijayalakshmi, G., Adinarayan, M., and Rao, P.J. 2014. A synergistic approach to kinetic and mechanistic studies of regeneration of beta carotene from tert-butoxyl radical induced beta-carotene radical cation by chlorogenic acid. International Journal of Pharmaceutical Sciences and Research. 5: 942-950.
Yang, Y., Zhang, Z., Li., S., Ye, X., Li, X., and He, K. 2014. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia. 92: 133-147.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Paphaphat Thiraphatthanavong1, Warin Ohn-on2, Sasalux Kaewbutra3, Kanchanaporn Tongthong1, Petcharat Rattanachompu1, Namplon Panmuesng1, Bhattaranitch Khampaenjiraroch1, and Krissada Phandech4, *
1 Department of Thai Traditional Medicine, Faculty of Science, Udon Thani Rajabhat University, Udon Thani, 41000, Thailand.
2 Department of Health and Beauty Science, Faculty of Natural Resources, Sakon Nakhon Campus, Rajamangala University of Technology Isan, 47160, Thailand.
3 Department of Medical Science, Amnatcharoen Campus, Mahidol University, Amnatcharoen, 37000, Thailand.
4 Department of Rehabilitation, Faculty of Medicine, Khon Kaen University, Khon Kaen, 40002, Thailand
Corresponding author: Krissada Phandech, E-mail: pkriss@kku.ac.th, krisslfc@gmail.com
ORCID: Paphaphat Thiraphatthanavong: https://orcid.org/0000-0002-6081-1935
Total Article Views
Editor: Wipawadee Yooin
Chiang Mai University, Thailand
Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: September 12, 2024;
Revised: November 12, 2024;
Accepted: November 13, 2024;
Online First: November 26, 2024

