
Synthesis and Characterization of Inulin Polymer and Study Its Role on Wound Healing
Raisan Kadhim Taresh*, Ahmed Abbas Sahib, Firas A. Nawar, Taleb Flieh Hassen, Zainab Jamal Hamoodah, and Raed Madhi*Published Date : November 22, 2024
DOI : https://doi.org/10.12982/NLSC.2025.008
Journal Issues : Number 1, January-March 2025
Abstract Inulin is a particularly effective drug delivery vehicle as compared to other biodegradable polysaccharides because of its unique and flexible structure, protective and stabilizing properties, and organ targeting abilities. This strategy may result in targeted, delayed, and regulated release of medications and biomolecules as well as increased bioavailability and improved cellular absorption. In the present study, thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), 1H-NMR, and ultraviolet spectroscopy were among the techniques used to further characterize the physicochemical properties of inuline. The inulin-g-[N-sulfamethoxazole maleic amic acid] (INMS) was prepared and used. INMS efficacy against bacterial activity was also examined. In order to examine the activity of INMS in vivo, mice were underwent to second-degree burn. The results showed, after three days, the basic media produced better regulated drug release than the acidic one. This was discovered by contrasting the pace at which the amide drug polymer hydrolyzed in each media. It was discovered that a drug polymer with altered drug release and a prolonged duration of action has as an adhesive probity. The present results found that INMS has a significant antibacterial effect on burned wound isolated bacteria. The in vivo performance using a natural polymer as a drug carrier results a great healing of skin wound of mice. Thus, the present study is aimed to modify the inulin to serve as a scaffold for various drug delivery systems.
Keywords: Inulin, INMS, Antibacterial activity, Wound healing
Citation: Taresh, R. K., Sahib, A. A., Nawar, F.A. , Hassen, T. F., Hamoodah, Z. J., and Madhi, R. 2025. Synthesis and characterization of inulin polymer and study its role on wound healing. Natural and Life Sciences Communications. 24(1): e2025008.
INTRODUCTION
Because synthetic polymers employed in controlled drug delivery systems have issues such brittleness, the necessity for expensive chemical modification (Liechty et al., 2010; Yan et al., 2020), poor mechanical properties, weak receptor targeting capabilities (S et al., 2023), excretion and biodegradation (Liechty et al., 2010), there is a lot of interest in using natural polysaccharides. Natural polysaccharides have numerous benefits, including targeting and stealth properties (Chatterjee et al., 2023), which makes them a viable option for application in enhanced drug delivery systems (Barclay et al., 2019). Unlike artificial polymers, natural polysaccharides are safe and biodegradable (Benalaya et al., 2024). Inulin (INU) is a naturally occurring, very abundant storage fructan polysaccharide that is found in a variety of fruits, vegetables, and grains, including wheat, garlic, leeks, bananas, and onions (Afinjuomo et al., 2021; Jaikham et al., 2024), as well as plants like chicory, dahlia, and Jerusalem artichoke (Shoaib et al., 2016). The β(2-1) glycosidic linkages and lack of cyclic sugar units in inulin's chemical structure give it its great flexibility, chemical stability in the gastrointestinal tract, and resistance to enzymatic breakdown until it reaches the colon (Liang et al., 2024). Highlighted are the special creteria of inulin-based hydrogels, including their capacity to replace fat, their antibacterial activity, and their stimuli-responsiveness. Particular attention is paid to their possible uses in medication delivery systems.
Instead of an invasive infection, burn wound infection is typically the result of bacterial colonization (Norbury et al., 2016). The end effect is a re-epithelialized area losing epithelium. Because of the high bacterial concentrations in the wound and surrounding area, as well as the new necrosis areas in the unburned tissues, invasive infection of burn wounds poses a surgical urgency (Ji et al., 2024). Sepsis symptoms and eschar discolorations (black, blue, or brown) are frequently present in conjunction with this condition. Surgical debridement of the afflicted area, antifungals, and broad-spectrum antibacterial medicines are needed, as well as immediate resuscitation treatments (Agarwal and Yasin, 2018; Hawez et al., 2022). To help identify the causal organism or organisms, specimens of this tissue need to be subjected to histopathologic and microbiologic investigation (Bowler et al., 2001). Patients who experience a delay in the presentation or excision of burnt tissues are more vulnerable to this illness. INU has been modified to serve as a drug carrier. t has been found that the drugs carried by INU show a great release and are more effective as compared to drugs administered alone. In addition, new findings observed that INU shows a sustained antibacterial activity (Afinjuomo et al., 2021).
Since it is the largest organ in the human body, the skin serves as a vital physical barrier against damage and external pressure in addition to its ability to fend off microbe invasion, maintain bodily fluid and water balance, and control body temperature (Chuong et al., 2002). Inflammation, epithelial remodeling, and tissue remodeling are all part of the wound-healing process following skin damage (Guo and Dipietro, 2010). A typical wound heals mostly during the inflammatory stage of skin tissue renewal (Canedo-Dorantes and Canedo-Ayala, 2019). It has been shown that leukocytes such as neutrophils can be infiltrated to the inflamed area and make the inflammation even worse (Linders et al., 2020; Madhi et al., 2024). Hyper-production of reactive oxygen species (ROS) from recruited cells can result in cell and tissue damage (Mittal et al., 2014; Madhi et al., 2019), and subsequently it has been linked to a number of illnesses, including cancer (Zhou et al., 2014), chronic illnesses, delayed wound healing, and infections (Paiva and Bozza, 2014; Polaka et al., 2022). INU has been shown to have a great antimicrobial effect against different bacteria (Boucher et al., 2023). Thus, the present study was aimed to use the INU as drug carrier to investigate its role in wound healing.
MATERIALS AND METHODS
Inulin purchased from (Fluka, Sigma-Aldrich, USA) and dried it for around two hours at 110 degrees Celsius to remove any moisture that it may have absorbed. All of the chemical reagents were purchased in their unpurified forms from Sigma Chemicals. Among them were sulfamethoxazole, maleic anhydride (MA), and cerium ammonium nitrate (APS).
Preparation of IN-g-M (INM)
The Kofler technique was applied in order to ascertain the melting point. A Bruker spectrophotometer was utilized to obtain 1H-NMR spectra, and a Shimadzu 8400 (Shimadzu, Japan) was employed to obtain FT-IR infrared spectra. Utilizing the VARIAN type NET Z U. (UV-Vis) spectra photometer, the DSC thermogram was obtained.
A solution of twenty milliliters of distilled water was combined with three grams of (IN). A polymerization flask containing 0.5 ml of maleic anhydride and 1 g of ammonium persulphate was heated for 45 minutes at 50 degrees Celsius in an inert environment. The polymer was produced at 140–160 degrees Celsius with a conversion ratio of 85%. The carboxylic acid group was changed into the corresponding acid chloride by thionyl chloride.
Substitution of INM with amino drugs (INMS)
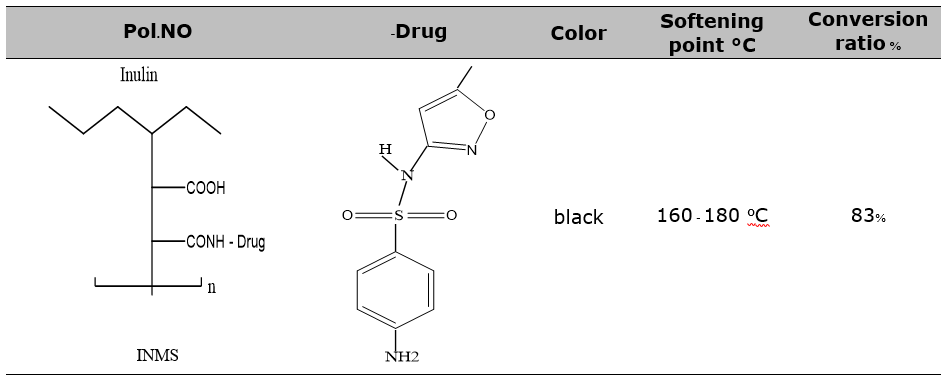
5 ml of dioxin, 0.98 grams of inulin g-maleic anhydride, 0.99 grams of sulfamethoxazole, and 0.3 ml of dimethylformamide (DMF) were combined. After raising the combination's temperature to 90 degrees Celsius and simmering it for an hour while stirring, the color was removed by filtering the mixture, separating the filtrate, and evaporating the solvent. After two ether washes and a 50 degree Celsius drying temperature, the inulin-g-[N-sulfamethoxazole maleic amic acid] black product (A) produced an 83% conversion ratio and a S. p. of 160–180 degrees Celsius, as shown in the figure 1 and table 1.
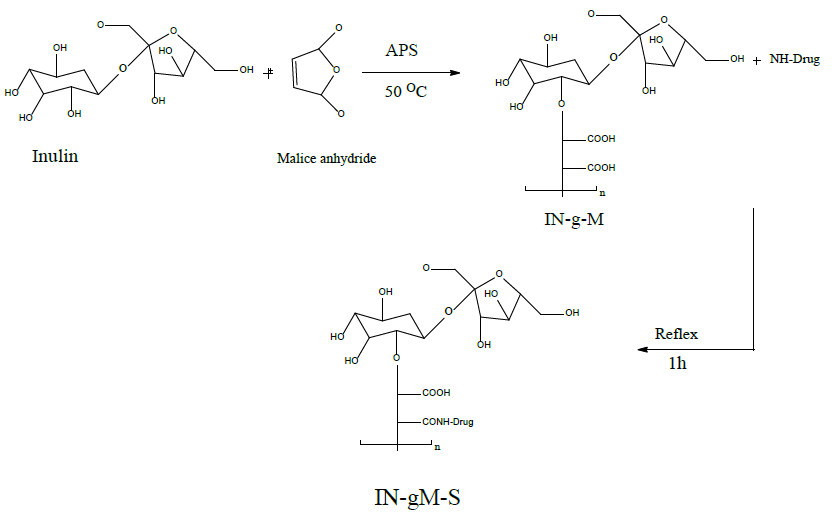
Scheme 1. Synthesis of INMS.
Table 1. All physical properties prepared polymer (INMS) were listed.
Measurement the bactericidal effect of INMS
The Kirby-Bauer method was utilized to test the susceptibility and resistance of several antibiotics in vitro (Schiller et al., 2022). An overnight culture was conducted for 24 hours at 37°C and 7.0 pH to create a suspension of the test organism in nutritional broth. A sterilized glass spreader was used to evenly streak the broth throughout the medium. Using sterile forcepson Mueller-Hinton agar plates, an antibiotic disc was put aseptically to the surface of the infected plates at a suitable specific arrangement. Following that, the plates were inverted and incubated for 24 hours at 37°C. After placing the diffusion discs containing antimicrobial medications on the plates, they were incubated at 37°C for a whole day. We utilized eighteen antibiotic discs. The culture was uniformly distributed across the medium using a sterile glass spreader. Using sterile forceps, an antibiotic disc was aseptically applied to the inoculation plates' surface in a specific pattern. After that, the plates were turned over and incubated for a full day at 37°C. The plates were inspected and the diameters of the zone of complete inhibition were measured after incubation.
Animal model
Eight to nine week old male SWISS mice were purchased from University of Sumer. Mice weighing 20–26 g were kept in an animal facility with a 12-hour light–dark cycle and a temperature of 22°C. The animals were given free-choice water and a typical laboratory meal. The Research Ethics Committee of University of Sumer, has approved the present study with certificate No. 340-THI-NA.
Each mouse had a minimum of 2 cm2 of skin shaved off its back prior to the start of the experiment. After that, animals were given isoflurane to induce unconsciousness, and the area was cleaned with a 70% alcohol solution. After that, the mice's rear skin was burned in the second degree by applying a hot, 120°C stainless steel rod. During the course of 21 days of therapy, topical treatment was administered in the following ways, twice daily. The administration of drugs immediately followed the burning process and as follows: control group (untreated), and inulin-g-[N-sulfamethoxazole maleic amic acid] (INMS) group.
INMS and drug release
A pH (1.1-7.4) buffer solution containing 0.1 gramme was added and tested at 37 degrees Celsius using a UV spectrometer. UV spectroscopes periodically reserved the persistent discharge. The mole fraction was determined by using UV spectra and measurements of the wavelength of max obtained at different times.
Determination of wound area characteristics
On days 0, 3, 7, 14, and 21, pictures of the wounds were captured with a camera (USA). As previously mentioned (Moeini et al., 2020), the following formula was used to determine the percentage of wound contraction (WC):
WC %= (wound area on day 0 - wound area on day n/ wound area on day 0)* 100
Statistical Analysis
The statistical analysis of the current data was carried by applying GrapgPad Pism 8.02. The present study included 6 mice per group and data were presented as mean and standard error mean (mean ± SE). Sample size was designed in line with a recent study (Safoine et al., 2024). T-test was used to analyze the statistical difference between two groups. P value less than 0.05 was considered a significant.
RESULTS
FTIR Spectrum
In the present study, we could show that the (OH) group stretching vibration (at 3412 cm1) in the inuline FTIR spectrum, as well as the (-CH) aromatic (at 3059 cm1) and the (-CH) aliphatic (at 2938, 2660, 2606) vibrations. In addition, another band manifested itself at 1028, 1078, and 1220 cm1 owing to the (COC) ether, while (C=O) absorption emerged at 1724 cm1 (Figure 1A). In addition, the present results also showed FTIR spectrum of copolymer (IN-g-N[sulfamethoxazole]) absorption bands at (3410 cm-1) from (-OH) and (3082 cm-1) from (-NH) stretching, followed by bands at (1622, 1674 cm-1) from (C=O) stretching, and finally carboxamide absorption at (1413 cm-1) from (S=O) sulfaton stretching (Figure 1B).
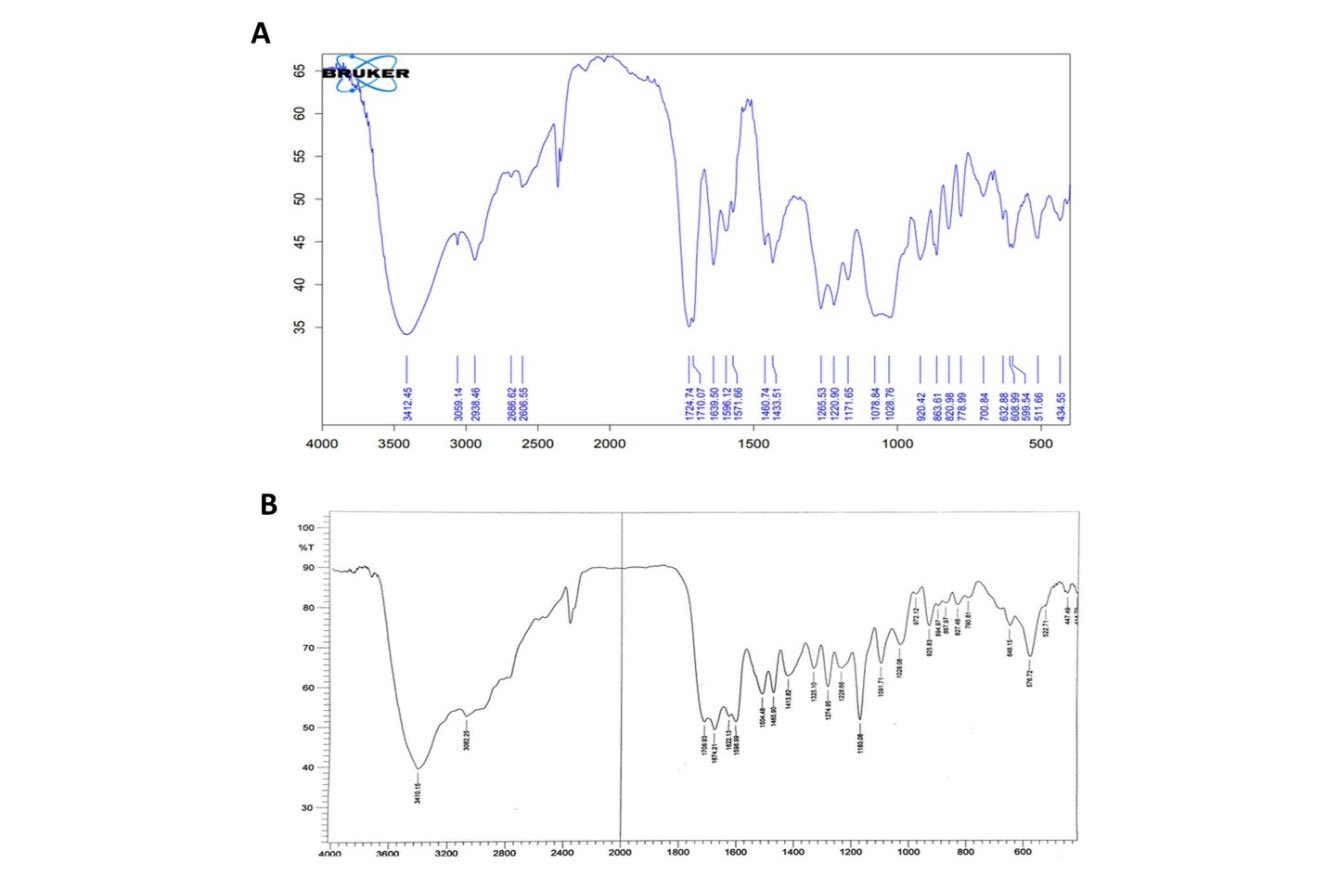
Figure 1. Inulin FTIR spectrum. A) FTIR spectrum of polymer (INM) and B) FTIR Spectrum of polymer (INMS).
1H-NMR Spectrum
Next, we found that the 1H-NMR spectrum of prepared INM shows the following signals: 1.2 ppm (doublet, 1H, CH2-CH) for inuline, 3.5 ppm (quartet, 1H, CH-CH-CH2) for inuline, 3.8 ppm (triplet, 1H, CH-CH2), and 4 ppm (triplet, 1H.CH-CH2) for inuline , 5 ppm (singlet. 1H, OH), 6.5 ppm (Multiple, 5H, Ar-H ), 10 ppm (singlet,1H,COOH) for carboxylic (Figure 2A). Moreover, the results also found the 1 HNMR spectrum of the prepared INMS the following signals: 4.5 ppm (singlet, 1H,OH), 6.5 ppm (multiple, 5H, Ar-H), 8.5 ppm (singlet, 1H, Ar-NH2), 9 ppm (singlet, 1H,CONH), for amide, and 11 ppm (singlet, 1H,COOH) for carboxylic. HNMR Spectrum (Figure 2B).
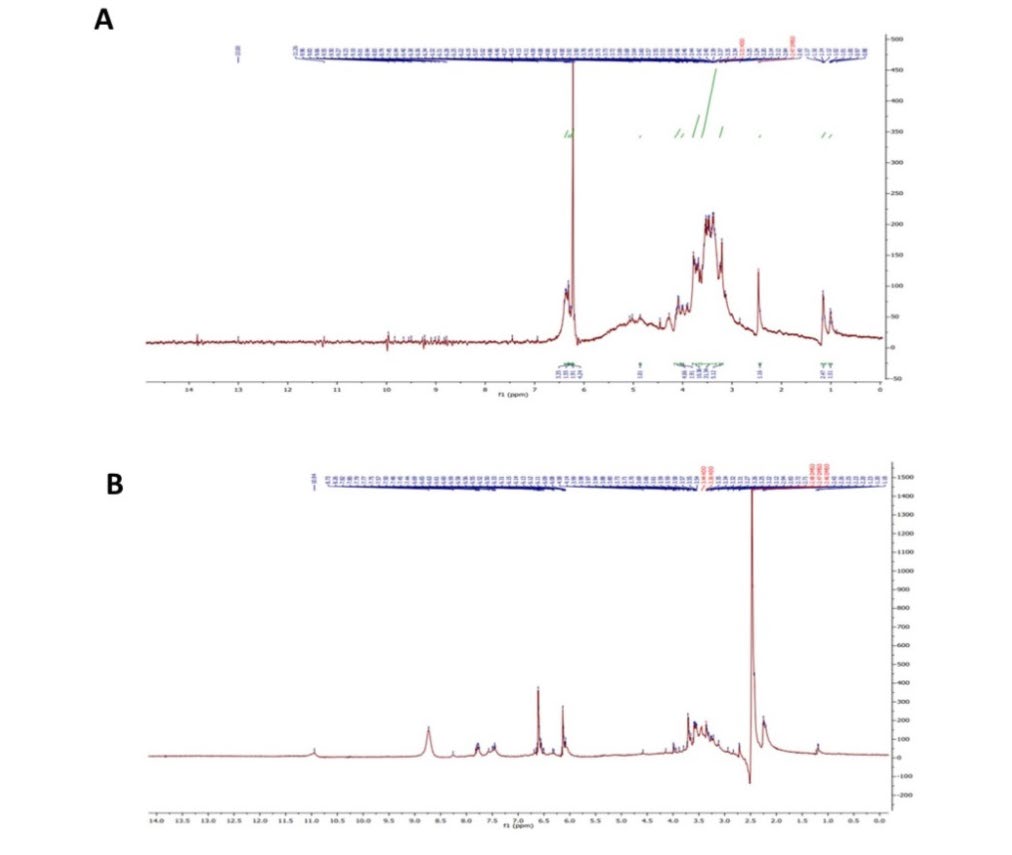
Figure 2. 1H-NMR spectrum. A) 1H-NMR Spectrum of prepared polymer (INM) and B) 1HNMR Spectrum of prepared polymer (INMS).
The results of the UV spectrometer analysis, as shown in (Figure 3A), indicated that the drug remained in the basic medium for a longer duration than in the acidic one. We could calculate the molar fractions using this data Controlled drug release. A 100 ml buffer solution was continuously supplemented with 100 mg at 37°C. We measured the maximum wavelength at various periods and pH levels (ranging from 1.1 to 7.3) using a UV spectrometer. Periodic samples were taken for examination using UV spectroscopes, and the rate of sustained release was determined using the mole fraction computed from those spectra. The results of the UV spectrometer analysis, (Figure 3A), indicate that the drug remained in the basic medium for a longer duration than in the acidic one. We could calculate the molar fractions using this data Controlled drug release.
Furthermore, thermal analysis showed an endothermic peak appeared at 100°C (Figure 3B). Our results also found that two peaks were observed at 200°C and 250°C, which indicates a heat peak that may result from the decomposition of the amide bond. TGA in (two-stages weight loss. Amount of weight: 25°C and 150°C showed about 10% of the weight loss. This loss water weight. The second phase of weight loss begins at 150°C and continues up to 500°C, during which there was a 60% weight loss than copolymer.
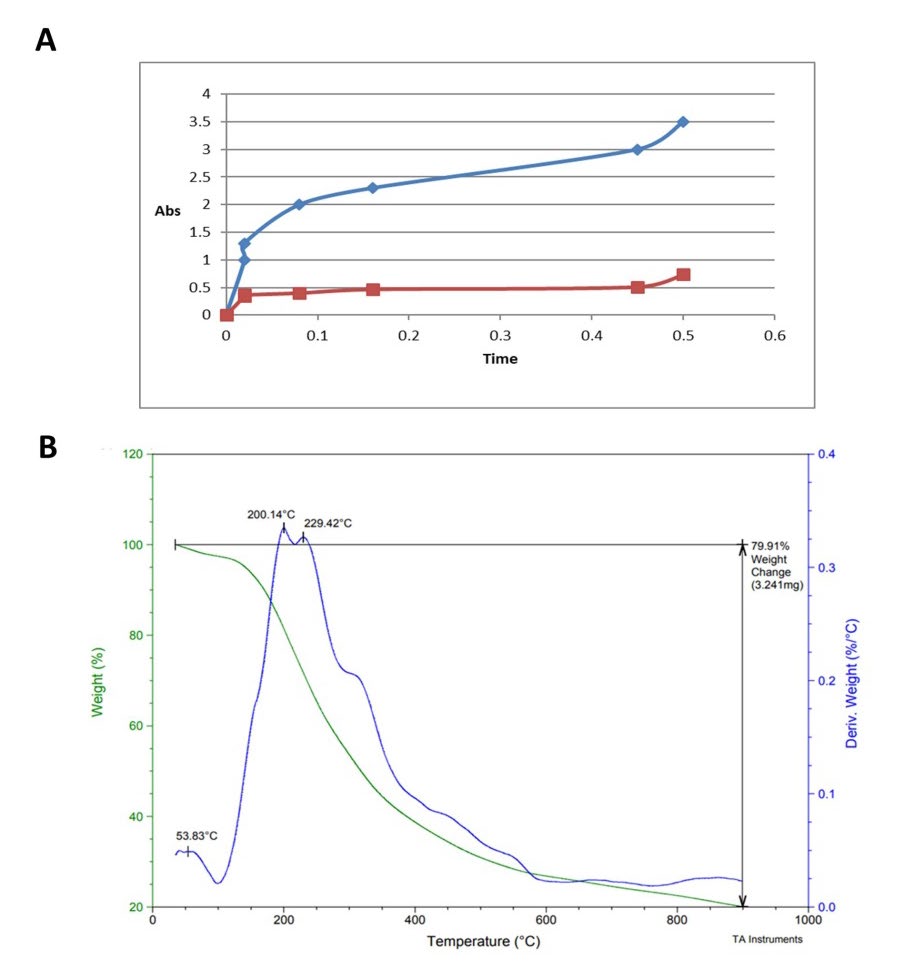
Figure 3. UV spectrometer and thermal analysis. A) UV Spectra of hydrolysis of [INMS] in pH 7.4 and B) TGA Thermal of (IN-g-MS).
INMS efficacy against bacterial activity
Using gentamicin as a reference with different concentrations 100µg, 50µg, 25µg, and 12µg, the antibacterial activity of drug polymers was evaluated using the agar diffusion method. In order to examine the antibacterial activity, the polymer was incubated with bacterial cultured plates at 37°C for a day. By measuring the inhibitory zone in mm, the efficacy against several bacterial species, such as Serratia marcescens, Staphylococcus aureus, and Streptococcus pneumonia, was significantly evaluated (P<0.05) at concentrations 100µg, 50µg, 25µg, and 12µg of Serratia marcescens, 100µg and 50µg of Staphylococcus aureus, and 100µg, 50µg, and 12µg of Streptococcus pneumonia. The present results found that polymer has good diffusibility at different drug concentrations as compared to the control (Figure 4).

Figure 4. Antibacterial activity of polymer drug at 100µg, 50µg, 25µg, and 12µg. A) Plates show the ininhibition zones of Serratia marcescens, Staphylococcus aureus, and Streptococcus pneumonia in INMS and control group. The inhibition zones (mm) in B) Serratia marcescens, C) Staphylococcus aureus, and E) Streptococcus pneumonia in INMS and control group. Control group (white box). INMS group (gray box). Data presented as means ± SEM. *P-value less than 0.05 considered as a significant different.
INMS efficacy on wound healing
It was interesting to explore the role of INMS on wound healing. Mice were exposed to second burn injury and INMS was administrated for injured mice (Figure 5). We could recognize that the wound begin to be healed after treatment with INMS at day 7 comparing to control group (Figure 5A and C). Notably, the wound was completely healed after treatment with INMS at day 21 and as compared to control group (Figure 5A and E).
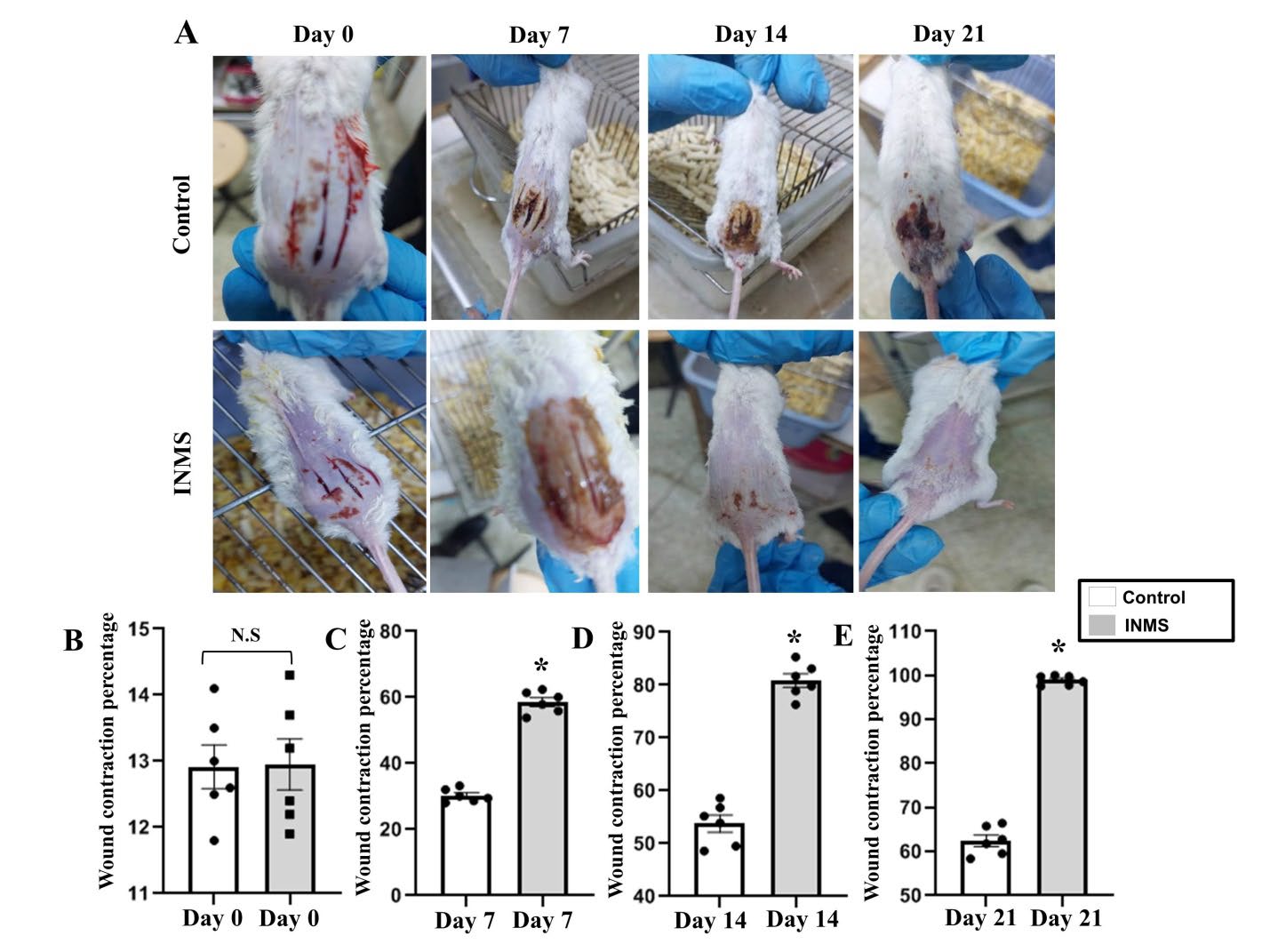
Figure 5. Role of INMS in wound healing in burned mice. A) Mice images show the period of wound healing at day 0, 7, 14, and 21. Wound contraction percentage at B) day 0, C) day 7, D) day 14, and E) day 21 in INMS and control group. Control group (white box). INMS group (gray box). Data presented as means ± SEM. *P-value less than 0.05 considered as a significant difference.
DISCUSSION
Highlighted are the special qualities of inulin-based carriers, such as their capacity to replace fat and their sensitivity to stimuli and antimicrobial activity (Liang et al., 2024). To manufacture inulin drug carrier, many synthesis procedures are employed, such as physical methods (thermal induction and non-thermal induction), chemical methods (free-radical polymerization and chemical crosslinking), and enzymatic approaches (Liang et al., 2024). In the present study, INMS was prepared using thermal induction. The following were combined: 0.3 ml of dimethylformamide (DMF), 0.98 grams of inulin g-maleic anhydride, 0.99 grams of sulfamethoxazole, and 5 ml of dioxin in order to prepared INMS. Moreover, The graft copolymerization process was made possible by maleic anhydride on the backbone of IN (Zhang et al., 2023), which led to the acquisition of new properties and the modification of IN with drugs. Amine-group-modified and enhanced solubility. New or improved properties were added while maintaining the unique structure of IN during the chemical change. The composition of the chemicals was deliberate. The grafted inulin was attached to the amino group of sulfamethoxazole by reacting maleic anhydride IN-g-maleic anhydride with ammonium per sulphate (APS) as an initiator to change the (IN) structure, resulting in the formation of the polymer.
Medication delivery using modified inulin has also been utilized to target the colon (Pitarresi et al., 2008; Antunes et al., 2021; Sun et al., 2021) and pulmonary medication delivery (Zijlstra et al., 2009). Previous studies have examined the specific physicochemical characteristics and medicinal uses of inulin (Cooper et al., 2013; Mensink et al., 2015). Additionally, Giri et al. showed how to target the colon using inulin derivatives (Giri et al., 2021). A previous study has examined the antibacterial activity of inulin as drug carrier against Mycobacterium smegmatis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Streptococcus pyogenes in the lung. The authors found that inulin carried rifampicin shows a great antibacterial activity. Moreover, in vitro study showed less uptake of inulin carried rifampicin by macrophage (Tripodo et al., 2019). Therefore, it was interesting to examine the antibacterial activity of inulin as drug carrier against Serratia marcescens, Staphylococcus aureus, and Streptococcus pneumonia. Our results were in line with previous study found that inulin –based carrier play a substantial role in antibacterial activity against bacteria. A recent study has created a medication delivery technology using vitamin E-functionalized inulin. Given that vitamin E is the hydrophobic component of the bioconjugate and inulin is its hydrophilic component, the end product is an amphiphile that, when dissolved in water, forms polymeric micelles. Thus using inulin as drug carrier could be a useful strategy to target the pathogenic bacteria (Usman et al., 2021).
The human skin, being the largest organ, possesses the ability to fend off microbial invasion, preserve bodily fluid and water balance, control body temperature, and serve as a crucial physical barrier that guards against external pressure and harm (Wells et al., 2016). The healing process of a wound on the skin includes tissue remodeling, epithelial remodeling, and inflammation (Mottola et al., 2023). Normal wound healing relies heavily on the inflammatory stage of skin tissue renewal (Ross and Benditt, 1962). But this is also the time when the wound releases reactive oxygen species (ROS) and free radicals (Yan et al., 2020). The build-up of these products damages cells and tissues, disrupts the equilibrium between pro- and antioxidant-oxidants, raises the level of oxidative stress in the cells, interferes with redox signal transduction, and results in a host of illnesses, including cancer, chronic illnesses, delayed wound healing, infection, and inflammation (Rashaan et al., 2019). Polysaccharide addition is a popular strategy used to stabilize proteins in vaccines and other medicinal delivery systems. As proteins dry, the polysaccharide takes over the connections between water and proteins due to the numerous hydroxyl groups found in inulin. Due to its ability to be mostly excreted by the kidneys quickly, soluble inulin has been shown in numerous studies to be the "gold standard" for evaluating kidney function. As such, it is a valuable chemical for determining the glomerular blood filtration rate (Den Hond et al., 2000). In the present study, we examined inulin as drug carrier in wound healing. Mice were exposed to second degree of burn, then treated with INMS. Our results showed a substantial wound healing as compared to control group. Because of its flexible and linear backbone, inulin has shown to be more effective than many other polysaccharides at stabilizing proteins and lipid-based delivery systems (Van den Mooter et al., 2003). This could explain why INMS results in a great wound healing of burned mice.
CONCLUSION
This study emphasised inulin's potential as a medication carrier. Because of its hydroxyl group, inulin is easily chemically modified and has excellent biocompatibility, biodegradability, molecular flexibility, and biodegradability. Our findings could reveal that prepared polymer INMS has a great antibacterial activity. Moreover, INMS could also show a substantial wound healing in back burned mice. Thus, a drug polymer with longer drug action and modified drug release might be employed as an adhesive.
ACKNOWLEDGEMENTS
We thank University of Sumer in Thi – Qar for authorizing the present study. We are also thankful to Department of Medical Laboratory Technologies for their unlimited support of the present study.
AUTHOR CONTRIBUTIONS
The manuscript writing and interpretation of the results were done by Raisan Kadhim Taresh, Ahmed Abbas Sahib, Firas A. Nawar, Taleb Flieh Hassen, Zainab Jamal Hamoodah and Raed Madhi. The experiments were conducted by Raisan Kadhim Taresh, Ahmed Abbas Sahib, Firas A. Nawar, and Taleb Flieh Hassen. Statistical analysis and data visualization were done by Zainab Jamal Hamoodah and Raed Madhi. After reading the completed manuscript, each author gave their approval.
CONFLICTS OF INTEREST
No conflicts of interest, as the authors declared.
REFERENCES
Afinjuomo, F., Abdella, S., Youssef, S. H., Song, Y., and Garg, S. 2021. Inulin and its application in drug delivery. Pharmaceuticals (Basel). 14: 855.
Agarwal, L. and Yasin, A. 2018. Necrotising fasciitis: A fatal case of sepsis and a diagnostic challenge - case report and review of literature. International Journal of Emergency Medicine. 11: 23.
Antunes, J. C., Seabra, C. L., Domingues, J. M., Teixeira, M. O., Nunes, C., Costa-Lima, S. A., Homem, N. C., Reis, S., Amorim, M. T. P., and Felgueiras, H. P. 2021. Drug targeting of inflammatory bowel diseases by biomolecules. Nanomaterials (Basel). 11: 2035.
Barclay, T. G., Day, C. M., Petrovsky, N., and Garg, S. 2019. Review of polysaccharide particle-based functional drug delivery. Carbohydrate Polymers. 221: 94-112.
Benalaya, I., Alves, G., Lopes, J., and Silva, L. R. 2024. A review of natural polysaccharides: Sources, characteristics, properties, food, and pharmaceutical applications. International Journal of Molecular Sciences. 25(2): 1322.
Boucher, E., Plazy, C., Le Gouellec, A., Toussaint, B., and Hannani, D. 2023. Inulin prebiotic protects against lethal Pseudomonas aeruginosa acute infection via gammadelta T cell activation. Nutrients. 15: 3037.
Bowler, P. G., Duerden, B. I., and Armstrong, D. G. 2001. Wound microbiology and associated approaches to wound management. Clinical Microbiology Reviews. 14: 244-269.
Canedo-Dorantes, L. and Canedo-Ayala, M. 2019. Skin acute wound healing: A comprehensive review. International Journal of Inflammation. 2: 3706315.
Chatterjee, S., Mahmood, S., Hilles, A. R., Thomas, S., Roy, S., Provaznik, V., Romero, E. L., and Ghosal, K. 2023. Cationic starch: A functionalized polysaccharide based polymer for advancement of drug delivery and health care system - A review. International Journal of Biological Macromolecules. 248: 125757.
Chuong, C. M., Nickoloff, B. J., Elias, P. M., Goldsmith, L. A., Macher, E., Maderson, P. A., Sundberg, J. P., Tagami, H., Plonka, P. M., Thestrup-Pederson, K., et al. 2002. What is the 'true' function of skin? Experimental Dermatology. 11: 159-187.
Cooper, P. D., Barclay, T. G., Ginic-Markovic, M., and Petrovsky, N. 2013. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 23, 1164-74.
Den Hond, E., Geypens, B., and Ghoos, Y. 2000. Effect of high performance chicory inulin on constipation. Nutrition Research. 20: 731-736.
Giri, S., Dutta, P., and Giri, T. K. 2021. Inulin-based carriers for colon drug targeting. Journal of Drug Delivery Science and Technology. 64: 102595.
Guo, S. and Dipietro, L. A. 2010. Factors affecting wound healing. Journal of Dental Research. 89: 219-229.
Hawez, A., Ding, Z., Taha, D., Madhi, R., Rahman, M., and Thorlacius, H. 2022. c-Abl kinase regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. Laboratory Investigation. 102: 263-271.
Jaikham, P., Leelapornpisid, P., and Poomanee, W. 2024. Development of transethosomes delivery system loaded with Bouea macrophylla griffith seed kernel extract for cosmeceutical application. Natural and Life Sciences Communications. 23(1): e2024002.
Ji, S., Xiao, S., Xia, Z., Chinese Burn Association Tissue Repair of, B., and Trauma Committee, C.-S. M. E. A. o. C. 2024. Consensus on the treatment of second-degree burn wounds (2024 edition). Burns Trauma. 12: tkad061.
Liang, X., Lin, D., Zhang, W., Chen, S., Ding, H., and Zhong, H. J. 2024. Progress in the preparation and application of inulin-based hydrogels. Polymers (Basel). 16(11): 1492.
Liechty, W. B., Kryscio, D. R., Slaughter, B. V., and Peppas, N. A. 2010. Polymers for drug delivery systems. Annual Review of Chemical and Biomolecular Engineering. 1: 149-173.
Linders, J., Madhi, R., Morgelin, M., King, B. C., Blom, A. M., and Rahman, M. 2020. Complement component 3 is required for tissue damage, neutrophil infiltration, and ensuring NET formation in acute pancreatitis. European Surgical Research. 61: 163-176.
Madhi, R., Al Gber, A., Munshid, M.C, and Jassim, K. I. 2024. Association between neutrophil recruitment and lung inflammation in type I hypersensitivity reaction. World Academy of Sciences Journal. 6: 6.
Madhi, R., Rahman, M., Morgelin, M., and Thorlacius, H. 2019. c-Abl kinase regulates neutrophil extracellular trap formation, inflammation, and tissue damage in severe acute pancreatitis. Journal of Leukocyte Biology. 106: 455-466.
Mensink, M. A., Frijlink, H. W., van der Voort Maarschalk, K., and Hinrichs, W. L. 2015. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydrate Polymers. 134: 418-28.
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. 2014. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling. 20: 1126-1167.
Moeini, A., Pedram, P., Makvandi, P., Malinconico, M., and Gomez d'Ayala, G. 2020. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydrate Polymers. 233: 115839.
Mottola, S., Viscusi, G., Iannone, G., Belvedere, R., Petrella, A., De Marco, I., and Gorrasi, G. 2023. Supercritical impregnation of mesoglycan and lactoferrin on polyurethane electrospun fibers for wound healing applications. International Journal of Molecular Sciences. 24(11): 9269.
Norbury, W., Herndon, D. N., Tanksley, J., Jeschke, M. G., and Finnerty, C. C. 2016. Infection in burns. Surgical Infections (Larchmt). 17: 250-255.
Paiva, C. N. and Bozza, M. T. 2014. Are reactive oxygen species always detrimental to pathogens? Antioxidants & Redox Signaling. 20: 1000-1037.
Pitarresi, G., Tripodo, G., Calabrese, R., Craparo, E. F., Licciardi, M., and Giammona, G. 2008. Hydrogels for potential colon drug release by thiol-ene conjugate addition of a new inulin derivative. Macromolecular Bioscience. 8: 891-902.
Polaka, S., Katare, P., Pawar, B., Vasdev, N., Gupta, T., Rajpoot, K., Sengupta, P., and Tekade, R. K. 2022. Emerging ROS-modulating technologies for augmentation of the wound healing process. ACS Omega Journal. 7: 30657-30672.
Rashaan, Z. M., Krijnen, P., Kwa, K. A. A., van der Vlies, C. H., Schipper, I. B., and Breederveld, R. S. 2019. Flaminal(R) versus Flamazine(R) in the treatment of partial thickness burns: A randomized controlled trial on clinical effectiveness and scar quality (FLAM study). Wound Repair Regen. 27: 257-267.
Ross, R. and Benditt, E. P. 1962. Wound healing and collagen formation. II. Fine structure in experimental scurvy. Journal of Cell Biology. 12: 533-551.
S, S., R, G. A., Bajaj, G., John, A. E., Chandran, S., Kumar, V. V., and Ramakrishna, S. 2023. A review on the recent applications of synthetic biopolymers in 3D printing for biomedical applications. Journal of Materials Science: Materials in Medicine. 34: 62.
Safoine, M., Paquette, C., Gingras, G. M., and Fradette, J. 2024. Improving cutaneous wound healing in diabetic mice using naturally derived tissue-engineered biological dressings produced under serum-free conditions. Stem Cells International. 2024: 3601101.
Schiller, H., Young, C., Schulze, S., Tripepi, M., and Pohlschroder, M. 2022. A Twist to the Kirby-Bauer disk diffusion susceptibility test: An accessible laboratory experiment comparing Haloferax volcanii and Escherichia coli antibiotic susceptibility to highlight the unique cell biology of archaea. Journal of Microbiology and Biology Education. 23(1): e00234-21.
Shoaib, M., Shehzad, A., Omar, M., Rakha, A., Raza, H., Sharif, H. R., Shakeel, A., Ansari, A., and Niazi, S. 2016. Inulin: Properties, health benefits and food applications. Carbohydrate Polymers. 147: 444-454.
Sun, Q., Arif, M., Chi, Z., Li, G., and Liu, C. G. 2021. Macrophages-targeting mannosylated nanoparticles based on inulin for the treatment of inflammatory bowel disease (IBD). International Journal of Biological Macromolecules 169, 206-215.
Tripodo, G., Perteghella, S., Grisoli, P., Trapani, A., Torre, M. L., and Mandracchia, D. 2019. Drug delivery of rifampicin by natural micelles based on inulin: Physicochemical properties, antibacterial activity and human macrophages uptake. European Journal of Pharmaceutics and Biopharmaceutics. 136: 250-258.
Usman, M., Zhang, C., Patil, P. J., Mehmood, A., Li, X., Bilal, M., Haider, J., and Ahmad, S. 2021. Potential applications of hydrophobically modified inulin as an active ingredient in functional foods and drugs - A review. Carbohydrate Polymers. 252: 117176.
Van den Mooter, G., Vervoort, L., and Kinget, R. 2003. Characterization of methacrylated inulin hydrogels designed for colon targeting: In vitro release of BSA. Pharmaceutical Research. 20: 303-307.
Wells, A., Nuschke, A., and Yates, C. C. 2016. Skin tissue repair: Matrix microenvironmental influences. Matrix Biology. 49: 25-36.
Yan, T., Kong, S., Ouyang, Q., Li, C., Hou, T., Chen, Y., and Li, S. 2020. Chitosan-gentamicin conjugate hydrogel promoting skin scald repair. Marine Drugs. 18(5): 233.
Zhang, C., Lu, Z., Wu, B., Jiang, S. D., and Qian, J. 2023. Grafting of maleic anhydride onto poly(vinylidene fluoride) using reactive extrusion. Molecules. 28(5): 2246.
Zhou, D., Shao, L., and Spitz, D. R. 2014. Reactive oxygen species in normal and tumor stem cells. Advances in Cancer Research. 122: 1-67.
Zijlstra, G. S., Ponsioen, B. J., Hummel, S. A., Sanders, N., Hinrichs, W. L., de Boer, A. H., and Frijlink, H. W. 2009. Formulation and process development of (recombinant human) deoxyribonuclease I as a powder for inhalation. Pharmaceutical Development and Technology. 14: 358-368.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Raisan Kadhim Taresh1, *, Ahmed Abbas Sahib1, Firas A. Nawar1, Taleb Flieh Hassen1, Zainab Jamal Hamoodah2, and Raed Madhi3, *
1 Department of Chemistry, College of Education, University of Sumer Al- Refaee, Thi – Qar 64005, Iraq.
2 Department of Medical Laboratory Technologies, Mazaya university college Iraq.
3 Department of Biology, Collage of Science, University of Misan, 62001 Maysan, Iraq.
Corresponding author: Raisan Kadhim Taresh, E-mail: raisankadim@gmail.com
Raed Madhi, E-mail: raedsaddam@uomisan.edu.iq
Total Article Views
Editor: Wipawadee Yooin
Chiang Mai University, Thailand
Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: September 17, 2024;
Revised: November 5, 2024;
Accepted: November 11, 2024;
Online First: November 22, 2024

