
Comparison of Safety Outcomes of Topical Capsaicin-Loaded Niosome and Capsaicin Gel in Healthy Volunteers
Tipada Samseethong, Sureewan Duangjit and Phaijit Sritananuwat*Published Date : November 21, 2024
DOI : https://doi.org/10.12982/NLSC.2025.007
Journal Issues : Number 1, January-March 2025
Abstract Capsaicin is a crucial molecule from chili that is approved by the Thai FDA for relieving joint and muscle pain. However, skin irritation causes 40% of patients to withdraw from treatment. To assess the effect of the niosome formulation on reducing side effects, this prospective study compared the safety outcomes of 0.025% capsaicin-loaded niosome (CLN), capsaicin-gel (CG), and placebo in 75 healthy volunteers. The physicochemical characteristics of CLN formulation were evaluated before applying to volunteers. CLN exhibits an average vesicle size of 246.70 nm with 238.06 ± 11.26 µg/mL of capsaicin. CG is a commercial product. Capsaicin formulations were blindly administered to the upper arm of the volunteers three times a day at 4-hour intervals. The safety outcomes were measured by skin erythema and melanin content using a colorimeter and pain score before and after application at times 0, 4, 8, and 24 hours after the first administration for the skin before application (baseline), and placebo.
CLN significantly increased skin erythema with scores of 8.39 ± 1.88 and 6.46 ± 2.15 when applied at 0 and 4 hours. In contrast, CG achieved significant increases for all 3 times of applications, with scores of 9.87 ± 2.08, 7.99 ± 2.32, and 6.78 ± 1.47 when applied at 0, 4, and 8 hours respectively. CG treatment significantly increased erythema scores after 24 hours compared to the baseline and placebo (P<0.01), while CLN had no effect. CG and CLN showed a significant increase in pain after 24 hours when compared to the baseline (P<0.05). The study suggests that CLN could be safer for the capsaicin-loaded formulation, causing less irritation and potentially reducing melanin content.
Keywords: Capsaicin, Chili extract, Capsicum, Cancer pain relief, Transdermal delivery
Funding: The Thailand Research Fund (Grant no. RGNS 64–237). The Faculty of Pharmaceutical Sciences, Ubon Ratchathani, Thailand, (Grant no. 0604.11-6/2566).
Citation: Samseethong, T., Duangjit, S., and Sritananuwat, P. 2025. Comparison of safety outcomes of topical capsaicin-loaded niosome and capsaicin gel in healthy volunteers. Natural and Life Sciences Communications. 24(1): e2025007.
INTRODUCTION
Chili (Capsicum annum L. and Capsicum frutescens L.) is a plant in the Solanaceae family. Chili is commonly used in Thailand as a seasoning and cooking ingredient. The chili from Ubon Ratchathani and Sisaket provinces is an economically important and famous variety called Hua Ruea (Surapat et al., 2013). Chili has also been used for a long time as a topical medicine to treat sprains and to reduce joint and muscle pain. The primary substance in chili peppers that relieves pain is the capsaicinoids group. Capsaicinoids primarily comprise capsaicin (70%), followed by dihydrocapsaicin (22%) and carotenoids (8%) (Antonio et al., 2018).
The pharmacological effects of capsaicin reveal that it first produces a pain sensation, followed by the gradual onset of pain reduction, and has an analgesic effect. Initially, capsaicin-induced pain occurs during the first use due to the stimulated transient receptor potential vanilloid 1 (TRPV1), a nociceptive receptor activated by heat, protons, voltage, lipids, and vanilloid ligands such as capsaicin (Zhang et al., 2024). This activation is reinforced in nociceptive primary afferent C and Ab fibers, resulting in burning pain. When used at a high or low doses with multiple applications, capsaicin activates more Ca2+ influx, which inhibits voltage Na+ and Ca2+ and activates glutamate release. This affects catalytic enzymes and ablates axonal neurons, leading to long-acting analgesia. Thus, continuous application of capsaicin for 4-6 weeks gradually reduces pain, with the reduction in pain typically beginning after about two weeks (Harnphadungkit et al., 2018).
The Thai Food and Drug Administration has approved 0.0125-0.025% capsaicin for topical application to relieve musculoskeletal and arthritis pain (Kosuwon et al., 2010; Altman et al., 1994). In clinical practice, topical capsaicin is used to relieve various chronic pain conditions such as diabetic neuropathic pain, post-herpetic neuralgia, osteoarthritis, chronic musculoskeletal pain, and post-mastectomy pain syndrome (Hayman and Kam, 2008). Capsaicin for topical use is available in the form of creams, gels, patches, and ointment formulations. Nevertheless, patients using topical capsaicin have reported experiencing side effects. In particular, moderate-severe skin irritation can cause patients to withdraw from treatment. High capsaicin concentrations cause more severe side effects than lower concentrations (Alalami et al., 2024). Common side effects include skin irritation at the application area, burning, stinging, and redness (erythema). When the topical capsaicin is applied frequently, these side effects can be reduced. Other adverse symptoms were found, including coughing, sneezing, and dry skin. It is recommended that the drug be administered at most four times per day (Kosuwon et al., 2010).
Transdermal drug delivery systems present a promising alternative to the topical administration of various capsaicin compounds (Duangjit et al., 2023; Manosroi et al., 2009; Manosroi et al., 2011; Manosroi et al., 2013), including microemulsion (Duangjit et al., 2016), transinvasomes (Duangjit et al., 2017), solid lipid nanoparticles (Duangjit et al., 2016), nanostructured lipid carriers (Anantaworasakul et al., 2020), and transdermal patches (Arunprasert et al., 2022) which have been developed to improve the transdermal delivery of capsaicin.
Niosomes, vesicles composed of non-ionic surfactants, are formed through the self-assembly of non-ionic amphiphiles and cholesterol molecules in an aqueous medium, resulting in closed bilayer structures. These vesicles share similarities with liposomes, making them ideal for drug delivery applications. Niosomes offer several advantages over traditional phospholipid-based systems, including lower production costs, enhanced stability, and ease of storage. Various botanical and active pharmaceutical ingredients, such as oryzanol (Duangjit et al., 2023), purple glutinous rice extract (Manosroi et al., 2020), Centella asiatica extract (Wichayapreechar et al., 2020), aromatic plants (Manosroi et al., 2013), and capsaicin (Sritananuwat et al., 2024), have shown significant potential in transdermal drug delivery systems. These compounds, with their diverse therapeutic properties, have been extensively explored for enhancing skin permeation and providing controlled, effective delivery through the skin. While niosomes have been proposed for the transdermal delivery of capsaicin in several studies (Tavano et al., 2011; Agrawal et al., 2015; Gupta et al., 2016), limited research has focused on the skin irritation in humans caused by capsaicin-loaded niosomes.
Therefore, this study utilized capsaicin-loaded niosomes to further investigate their potential to improve transdermal drug delivery and reduce skin irritation. To assess the niosome formulation's effect on reducing side effects, a prospective study compared the safety outcomes of 0.025% capsaicin-loaded niosome (CLN), capsaicin-gel (CG), and a placebo in 75 healthy volunteers. The safety outcomes, including erythema, melanin content, and pain scores, were evaluated in this study.
MATERIAL AND METHODS
Materials
Capsaicin resin (CAP) was procured from a specialized natural product company, Specialty Natural Products Co., Ltd. The CAP has a capsaicinoid content of 6 g/Kg. The capsaicin standard (95%) was acquired from Sigma-Aldrich (Missouri, USA). Polysorbate 60 (Tween® 60; T60) was purchased from Merck KGaA (Darmstadt, Germany). Cholesterol was sourced from Wako Pure Chemical Industries (Osaka, Japan). All other chemicals used were of reagent grade and sourced from Chemical Express Co., Ltd. (Bangkok, Thailand).
Preparation of capsaicin-loaded niosomes (CLN)
The 0.025% capsaicin-loaded niosome gel was prepared in 4 steps. Firstly, the stock 0.15% capsaicin-loaded niosome was prepared using the thin film hydration method with slight modifications (Duangjit et al., 2017; Duangjit et al., 2023), comprising a constant percentage of 9.2 g of polysorbate 60, 2.7 g of cholesterol, 1.3 g of cocamide diethanolamine, and 25 g of capsaicin resin. Next, a 2% (w/w) stock solution of carbomer (carbopol 940) in deionized water was prepared to form an aqueous dispersion. Subsequently, a mixture comprising 2 g of glycerin, 2 g of propylene glycol, 1 g of paraben concentrate, and 16.7 g of stock 0.15% capsaicin-loaded niosome was formulated to achieve a 0.025% capsaicin-loaded niosome dispersion. Finally, the dispersion was neutralized using triethanolamine for pH adjustment and viscosity enhancement.
Preparation of capsaicin gel (CG) and blank gel
The capsaicin gel (CG) was prepared by first creating a 2% (w/w) carbomer solution in deionized water as the gel base, followed by the addition of glycerin, propylene glycol, paraben concentrate, and 0.025% capsaicin. The pH and viscosity were then adjusted using triethanolamine to achieve a stable formulation. For the placebo (blank gel), the same process was followed, excluding the addition of capsaicin. An orange colorant was added to the blank gel to match the appearance of the 0.025% capsaicin gel, and the pH and viscosity were similarly adjusted with triethanolamine to ensure stability.
Physicochemical characterization
The average vesicle size, size distribution and zeta potential of the capsaicin-loaded niosome were determined by photon correlation spectroscopy (PCS) (Zetasizer Nano series, Malvern Instruments, UK). All measurements were taken at room temperature (25°C). Twenty microliters of the nanoscale vesicles were diluted with 1480 µL of deionized water. At least three independent measurements were recorded.
The capsaicin concentration in the niosome formulation was determined following niosome disruption with methanol at a 1:1 volume ratio. The niosome/methanol mixture underwent centrifugation at 10,000 rpm at 25°C for 10 minutes. Subsequently, the supernatant was filtered through a 0.45 µm nylon syringe filter before analysis using High-Performance Liquid Chromatography (HPLC). The capsaicin HPLC analysis method was adopted by Duangjit et al. (2017). Samples were stored at 4°C prior to analysis. The HPLC system used was a Thermo Fisher HPLC system (Thermo Ultimate 3000 HPLC, MA, USA) with a reversed-phase column (ODS C18, 4.6 mm × 150 mm, 5 µm particle size VertiSepTM, Ligand Co., Ltd., Thailand). A mobile phase consisting of acetonitrile and diluted phosphoric acid in reverse osmosis water (50:50, v/v) was employed at a flow rate of 1 mL/min. Detection was carried out using a UV detector set at 280 nm. The calibration curve for CAP ranged from 1 to 100 µg/mL, achieving a correlation coefficient of 0.999.
Method
This research is a prospective, single-blind study. This study was approved for ethical considerations as no.2C/607990 by Ubon Ratchathani University. Participants were required to pass the inclusion criteria in this study: healthy volunteers aged 18 years and over who are willing to participate, can read and write the Thai language, and communicate well. Participants who had a history of capsaicin allergies or allergies to the ingredients in the formula, a history of skin disease, asthma, or any allergy that used oral or inhaled medicine were excluded. They were assigned a consent form after passing the inclusion/exclusion criteria.
Research assistants and healthy volunteers were blinded to the type of medication (placebo; 0.025% CG; 0.025% CLN). Due to the intrinsic properties of capsaicin in terms of odor and color, to ensure proper blinding, an orange colorant was added to the placebo gel to match the appearance of the capsaicin-containing gels. While capsaicin has a characteristic pungent odor, measures were taken to minimize visual cues and ensure the experiment remained blinded.
The three areas for the skin irritation test were separated using stickers with a 1 x 1 cm board per formulation on the healthy volunteers’ upper arm and left shoulder (Figure 1). In the following positions, capsaicin and placebo were applied using a cotton swab in the specified frame (1 cm2). At day 0, capsaicin and placebo were applied at the same area 3 times at 4-hour intervals (time 0 hours = 8.00 a.m., time 4 hours = 12.00 p.m., and time 8 hours = 4.00 p.m.). Safety outcomes were evaluated after three applications and completed 24 hours as day 1. After thirty minutes of application each time, safety outcomes indicating skin irritation symptoms were evaluated with a colorimeter and Mexameter. The Mexameter® MX 18 probe of multiprobe adapter (MPA) 9 device (Courage-Khazaka Electronic GmbH, Germany) was used to evaluate the skin in terms of erythema and melanin content and to report the percentage change of treatment compared to the status before treatment. The percentage change was calculated from the equations below.
Erythema assessment (Colorimeter)
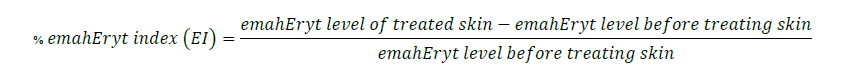
Melanin assessment
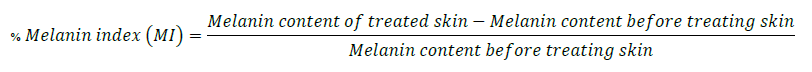
Skin irritation was evaluated by measuring pain sensation using a visual analog score. Healthy volunteers were interviewed before and after applying formulations for 30 minutes using a visual analog scale (VAS) (0-10 score) 0 = no pain and 10 = severe pain. Other symptoms were recorded as common side effects, such as coughing, red-eye irritation, and watery eyes. The participants were tested using a cotton swab to apply topical capsaicin for the smell. For this test to start, it must first be done once for each formula at intervals of one minute. After completion, topical capsaicin is applied to the upper arm to reduce the interference of capsaicin odor.
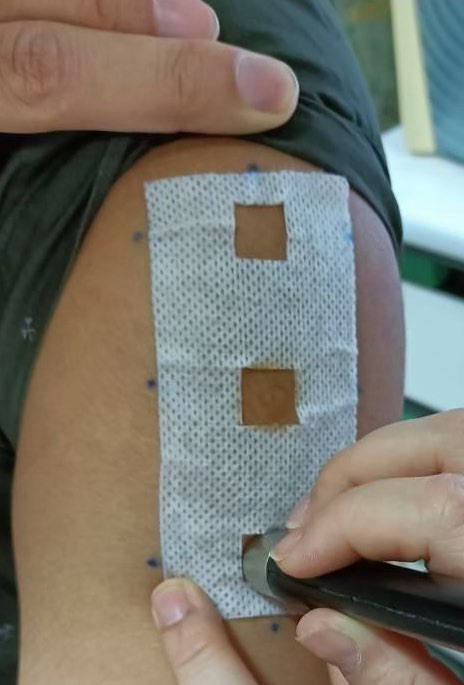
Figure 1. Erythema assessment by colorimeter.
Sample size calculation
This sample size was calculated, and the required number of participants was 72 persons, basing the calculation upon the work of Kulkantrakorn et al. (2013). This used the percentage of any skin events calculated by using the following equation.
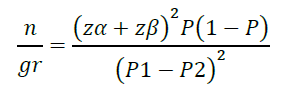
n/gr = The number of healthy volunteers/group treatment (24 person/group)
P1 = Proportion of any skin events in the group treated with 0.025% capsaicin as identified from the study of Kulkantrakorn et al. (2013), which was 13.6%.
P2 = Proportion of any skin events in the group treated with the placebo as identified from the study of Kulkantrakorn et al. (2013), which was 0%.
P = (P1+P2)/2
Zα = The normal deviance at the level of significance (Zα is 1.96 for 5%)
Zβ = The normal deviance at β% of a type II error (0.84 at 80% power)
Statistical analysis
Baseline characteristic data consisted of descriptive analyses, including frequencies, means, and standard deviations (SD). Binary data were presented as absolute by percentage, including gender, number of diseases, and occurrence of side effects. Continuous data were presented as mean ± SD. Continuous variables included age, weight, height, percentage change before and after applying an erythema index (EI), percentage change before and after applying a melanin index (MI), and pain scores. Erythema assessment, melanin index, and pain score were the test statistics investigated between groups using the Kruskal-Wallis H test (nonparametric data) and ANOVA (parametric data). The predetermined threshold for significance was set at a P-value less than 0.05 using SPSS version 11.0.
RESULTS
The 0.025% capsaicin-loaded niosome gel (CLN), 0.025% capsaicin gel (CG), and the placebo were orange-colored. The average vesicle size of the capsaicin-loaded niosome was 246.70 ± 4.29 nm with PDI 0.25 ± 0.01. The zeta potential was -41.77 ± 0.76 mV. The formulation had a capsaicin content of 238.06 ± 11.26 µg/mL or 0.024 ± 0.001% (w/w). CG is a commercially available prescription.
For safety outcomes, 75 healthy volunteers passed the inclusion/exclusion criteria in this study. Most participants were female, and the mean age was 29.21 years (Table 1). All the participants underwent application of the placebo, CG, and CLN formulations and then the safety outcomes were assessed by division into two types: 1) skin irritation using erythema assessment by colorimeter and melanin index, and 2) pain score assessment by visual analog score.
Table 1. Baseline characteristics.
|
Variable |
Results (n=75) |
|
Age (years, mean ± SD) |
29.21 ± 11.63 |
|
Female (n, (%)) |
61.00 (83.60) |
|
Weight (Kg, mean ± SD) |
57.39 ± 10.70 |
|
Height (Cm, mean ± SD) |
160.07 ± 7.98 |
|
No Disease |
62.00 (84.90) |
Erythema assessment
As Table 2 shows, the skin irritation was measured for erythema via a comparison of the percentage difference before and after applying formulations. The placebo did not show any erythema change at any time points. CG showed significant increases in the erythema index (EI) percentage after application at times 0, 4, 8, and 24 hours after treatment, which were 9.87 ± 2.08 (P <0.001), 7.99 ± 2.32 (P=0.002), 6.78 ± 1.47 (P=0.001), and 7.04 ± 1.42 (P=0.001). CLN showed a significant increase in the EI percentage after application at times 0 hours and 4 hours, which were 8.39 ± 1.88 (P <0.001) and 6.46 ± 2.15 (P=0.02). The increased EI percentage in CG treatment was significant at the times of 0 hours (P <0.001), 4 hours (P =0.001), and 8 hours (P =0.01), while CLN was significant at times of 0 hours (P <0.001) and 4 hours (P=0.008) when compared with the placebo. Between the two capsaicin formulations, CG treatment showed significantly higher skin erythema than CLN treatment at time 8 hours (P=0.03).
Table 2. Percentage difference before and after when comparing the erythema assessment by colorimeter.
|
Formulation/Time |
Before and after at time 0 hours |
P- valuea |
Before and after at time 4 hours |
P-valuea |
Before and after at time 8 hours |
P-valuea |
24 hours compared to baseline |
P- valuea |
|
Placebo |
-1.02 ± 1.30 |
0.30 |
-1.19 ± 1.62 |
0.180 |
-0.29 ± 1.93 |
0.22 |
0.76 ± 1.54 |
0.880 |
|
0.025% CG |
9.87 ± 2.08 |
<0.001 |
7.99 ± 2.32 |
0.002 |
6.78 ± 1.47 |
0.001 |
7.04 ± 1.42 |
0.001 |
|
0.025% CLN |
8.39 ± 1.88 |
<0.001 |
6.46 ± 2.15 |
0.020 |
-1.15 ± 1.29 |
0.21 |
1.66 ± 1.55 |
0.620 |
|
Note: Comparison of all groups and between groups using the Kruskal-Wallis test; time 0 hours (P<0.001), time 4 hours (P=0.002), time 8 hours (P=0.03), time 24 hours (p=0.08) - Placebo VS CG; time 0 hours (P<0.001), time 4 hours (P=0.001), time 8 hours (P=0.01), time 24 hours (NS) - Placebo VS CLN; time 0 hours (P<0.001), time 4 hours (P=0.008), time 8 hours (P=0.79), time 24 hours (NS) - CG VS CLN; time 0 hours (P=0.76), time 4 hours (P=0.60), time 8 hours (P=0.03), time 24 hours (NS) aCompared before and after application using Paired T-test. CG: Capsaicin gel; CLN: Capsaicin loaded niosome; NS: not significant |
||||||||
Melanin assessment
Acute or chronic inflammation can cause hyperpigmentation. Thus, this study measured the melanin index (MI) to determine the effect of skin inflammation after using capsaicin formulations. The placebo did not show a difference in MI at any treatment times.
The CG formulation showed a significant increase in MI percentage at times of 4, 8, and 24 hours, which were 4.04 ± 1.63 (P=0.01), 3.55 ± 1.25 (P=0.01), and 5.68 ± 1.28 (P=0.001). In contrast, the CLN formulation reduced the percentage of MI significantly when comparing before and after application only at time 0 hours, which was -4.50 ± 1.39 (P<0.01), while no significant changes were found at times of 4, 8, and 24 hours. When compared with the placebo, CG had different statistics at 24 hours (P=0.001), but CLN had different statistics at time 0 hours (P=0.009) (Table 3).
Table 3. Percentage difference in before and after comparisons of the melanin index by Mexameter.
|
Formulation |
Before and after at time 0 hours |
P-valuea |
Before and after at time 4 hours |
P-valuea
|
Before and after time |
P-valuea |
24 hours compare baseline |
P-valuea |
|
Placebo |
-1.85 ± 0.76 |
0.070 |
1.17 ± 1.37 |
0.78 |
0.79 ± 1.57 |
0.92 |
-0.88 ± 1.13 |
0.210 |
|
0.025% CG |
-1.24 ± 0.84 |
0.250 |
4.04 ± 1.63 |
0.01 |
3.55 ± 1.25 |
0.01 |
5.68 ± 1.28 |
0.001 |
|
0.025% CLN |
-4.50 ± 1.39 |
0.001 |
-1.29 ± 1.02 |
0.19 |
0.40 ± 1.35 |
0.51 |
-0.35 ± 0.90 |
0.680 |
|
Note: Comparison of all groups and between groups using the Kruskal-Wallis H test: time 0 hours (P=0.001), time 4 hours (P=0.015), time 8 hours (P=0.064), time 24 hours (p=0.001) - Placebo VS CG; time 0 hours (P=0.30), time 4 hours (P=0.30), time 8 hours (NS), time 24 hours (P=0.001) - Placebo VS CLN; time 0 hours (P=0.009), time 4 hours (P=0.06), time 8 hours (NS), time 24 hours (P=1.00) - CG vs CLN; time 0 hours (P<0.001), time 4 hours (P=0.04), time 8 hours (NS), time 24 hours (P=0.001) aCompared before and after application using Paired T-test. CG: Capsaicin gel; CLN: Capsaicin loaded niosome; NS: not significant |
||||||||
Pain score assessment
After applying all formulations, the pain scores of all volunteers were reported to be less than 3. Table 4 shows the average pain score before and after treatment. At initial time of treatment (time 0 hours), all formulations significantly increased the mean pain score from the baseline, which was 0.79 ± 0.21 (P=0.001), 1.39 ± 0.29 (P=0.001), and 1.36 ± 0.29 (P=0.001) for placebo, CG, and CLN, respectively. There were no significant changes at times of 4 and 8 hours after application. The CG and CLN treatments showed a higher difference in average pain scores at times of 4 and 8 hours than the placebo. At 24 hours after treatment, both CG and CLN treatments still had a pain sensation when compared with the baseline (P<0.05). The average pain score of CLN at every time point showed a tendency to be higher than that of CG. However, there was no difference in pain scores between the CG and CLN formulations at any of the treatment times.
Table 4. Comparison of pre- and post- mean pain scores.
|
Formulation |
Day 0 |
Day 1 |
||||||||||||||
|
Time 0 hours |
Time 4 hours |
Time 8 hours |
Time 24 hours |
|||||||||||||
|
Baseline |
after 30 min |
P-value a |
before |
after 30 min |
P-valuea |
before |
after 30 min |
P-valuea |
Baseline |
complete 24 hours |
P-valuea |
|||||
|
Placebo |
0.00 |
0.79 ± 0.21 |
0.001 |
0.595 ± 0.220 |
0.85 ± 0.22 |
0.09 |
0.55 ± 0.18 |
0.85 ± 0.21 |
0.16 |
0.00 |
0.12 ± 0.09 |
0.18 |
||||
|
0.025%CG |
0.00 |
1.39 ± 0.29 |
0.001 |
1.200 ± 0.260 |
1.61 ± 0.27 |
0.08 |
1.03 ± 0.22 |
1.44 ± 0.29 |
0.27 |
0.00 |
0.34 ± 0.15 |
0.01 |
||||
|
0.025% CLN |
0.00 |
1.36 ± 0.29 |
0.001 |
1.650 ± 0.340 |
2.18 ± 0.33 |
0.09 |
1.68 ± 0.30 |
2.27 ± 0.34 |
0.12 |
0.00 |
0.51 ± 0.20 |
0.04 |
||||
|
Note: Comparison of all groups and between groups using the Kruskal-Wallis H test: time 0 hours (P=0.14), time 4 hours (P<0.001), time 8 hours (P=0.001), time 24 hours (P=0.49) - Placebo VS CG; time 0 hours (NS), time 4 hours (P<0.001), time 8 hours (P<0.001), time 24 hours (NS) - Placebo VS CLN; time 0 hours (NS), time 4 hours (P=0.001), time 8 hours (P=0.004), time 24 hours (NS) - CG vs CLN; time 0 hours (NS), time 4 hours (P=0.59), time 8 hours (P=0.61), time 24 hours (NS) aCompared before and after application using Wilcoxon Signed Rank test. |
|
|||||||||||||||
Side effects of other systems
For other side effects, this study found coughing, eye irritation, and eye watering. The CG formulation presented one case of cough and two cases of red eyes. The CLN formulation presented two cases with cough and two cases with red eyes, as shown in Table 5.
Table 5. Other side effects.
|
Symptoms |
Formulations |
||
|
Placebo |
0.025%CG |
0.025%CLN |
|
|
Cough |
0 |
1 (1.3%) |
2 (2.6%) |
|
Red eye |
1(1.3%) |
2 (2.6%) |
2 (2.6%) |
|
Eye watering |
1 (1.3%) |
0 |
0 |
DISCUSSION
This comprehensive study is the first to compare capsaicin niosomes with capsaicin gel and a placebo in a large group of healthy volunteers. The previous human study of the nanotechnology of capsaicin products, which compared 0.075% capsaicin nanoparticles with a placebo to treat painful diabetic neuropathy in 38 diabetic patients, found that capsaicin nanoparticles effectively relieve pain, but safety concerns were raised with 44% of patients experiencing burning sensations compared to 8% in the placebo group (Suthisisang et al., 2013). In the capsaicin niosomes, only an in vitro study found better penetration than capsaicin gel, and in vivo studies were done in animals only (Gupta et al., 2016). Thus, the study is prospective to evaluate safety outcomes. Seventy-five healthy volunteers were included; 72.41% of volunteers were less than 40 years old (mean age 29.63 ± 11.81 years), and 83.60% were female (Table 1). This study was mainly conducted among young adult age groups. This population in these studies has a trend occurring with skin side effects. Inamadar and Palit's study showed factors relating sensitive skin to female sex in young adults between 18 and 50 years. These populations were sensitive to skin irritation because of the ability to cause fluctuations in female hormone levels, resulting in sensitive skin, including dryness, itchiness, and redness (Inamadar and Palit, 2013). Age-specific skin blood flow response to acute capsaicin found capsaicin concentrations of 0.1% in middle-aged (40-55 years) and younger skin (18-30 years). However, there was a significant elevation in local blood flow with 10% capsaicin in older skin (65-80 years) (Munce and Kenney, 2003).
The pathophysiology of skin erythema from capsaicin occurs in the blood flow response by vasoactive neurotransmitters released from sensory nerves, resulting in modifying cutaneous blood flow and vascular permeability that is characterized by a sensation of warmth, burning pain, cutaneous hypersensitivity, as well as local vasodilation and flare (Inamadar and Palit, 2013). Capsaicin-induced skin erythema, which is a common side effect of the treatment, is usually attenuated by repeated treatment (Derry and Moore, 2012). The study of Kulkantrakorn et al. (2013) revealed that when using 0.025% capsaicin to treat diabetic neuropathic pain, skin reactions were reported by 14.7%, a burning sensation at 3%, and erythema at 3%. A physician evaluated the patients with skin irritation by physical examination, but no scientific instrumental analysis was performed. This study collected scientific instrumental analysis and quantitative measurements using colorimeters. The instrument emits light of three wavelengths and receives the light reflected by the skin which corresponds to the absorbance of melanin and hemoglobin (erythema) (Baquie and Kasraee, 2014). This device is widely used to evaluate the shade of melanin and erythema, for example by testing erythematous skin with dried brewer’s yeast (Hibino et al., 2010), testing for irritation with sodium lauryl sulfate, tretinoin, and UV exposure (Fluhr et al., 2001), and determination of UVB induced pigmentation (Shin et al., 2014). The safety outcome for skin irritation when applying capsaicin was determined by the erythema and melanin content level. Erythema is one of the clinical features of allergic contact dermatitis (Tramontana et al., 2023). Skin hyperpigmentation (increased melanin content) is found in acute and chronic irritation and can cause the inflammation response and stimulate melanocytes, resulting in skin hyperpigmentation (Hossain et al., 2021). We simulated applying capsaicin thrice daily in the real world (8.00 a.m., 12.00 p.m., 4.00 p.m., for intervals of 4 hours), and the effect was determined 30 minutes after each treatment and further measured for 24 hours. This study showed that the different formulations may alter safety effects. Niosomes, similar to liposomes, are colloidal carriers made with nonionic surfactants and often cholesterol, enhancing drug effectiveness and targeted delivery (Abdel Samie and Nasr, 2020). When repetitive applications are used, the CLN presented a faster-reduced skin erythema than CG as shown in the low percentage of EI at times of 8 and 24 hours after treatment. This faster reduction in skin erythema may be attributed to the smaller size, controlled deposition, and gradual release of capsaicin from the niosomal system, which helps mitigate irritation. However, while Gupta et al. (2016) reported minimal irritation after 48 hours, our study demonstrates that 0.025% capsaicin-loaded niosome gel formulations significantly reduce irritation within 24 hours. This suggests that the niosomal system enhances capsaicin retention and minimizes irritation caused by rapid absorption or accumulation on the skin surface due to its small vesicle size. These findings highlight the critical role of nanovesicular systems in modulating capsaicin’s interaction with the skin, promoting localized release and reducing irritation. Specifically, in our study, the CLN (niosomal) formulation exhibited a faster reduction in skin erythema compared to the capsaicin gel (CG), with a lower erythema index (EI) recorded at times 8 and 24 hours after treatment (Gupta et al., 2016).
This suggests that CLN might derive capsaicin from the targeted nerve cell without irritating other skin cells. Moreover, skin pain in CLN was shown to reach a higher level than for CG at all measurement times, which could be the effect of capsaicin on activating transient receptor potential vanilloid 1 (TRPV1) and releasing substance P at the end of the neuron (Zhang et al., 2024). This study has shown that capsaicin can reduce the number of melanin cells in the acute period. A study by Wu et al. (2020) found that capsaicin can lower melatonin levels, with the reduction being dependent on the dose and duration of exposure. Melatonin cells decreased 24 hours after capsaicin treatment, and the reduction was even observed at 72 hours compared to a control treatment with the same dose. This effect is related to the dose of capsaicin. Capsaicin’s potential to reduce the melanin count may be due to its inhibition of the TRPV1 receptor, which triggers the phosphorylation of extracellular signal-regulated kinase. This process results in the degradation of the transcription factor, thereby reducing melanogenic enzymes and melanin (Wu et al., 2020). These findings suggest possible cosmetic uses for capsaicin. Similar results were shown in CLN treatment which reduced melanin content at times of 0, 4, and 24 hours of treatment. In contrast, CG can decrease melatonin content at the first time of treatment, but the later applications increase the melatonin content. This result in CG might be related to the irritation of the skin and induced melanogenesis.
The study was limited to a one-day application. In practical settings, it would require more prolonged monitoring for side effects. Using capsaicin gel, a scented medication, could influence the volunteers’ perceptions of the tested formula. However, this potential was minimized by masking the gel with color, thereby enhancing the study's reliability. Further studies should be conducted in patients with different types of neuropathic pain. This study is the first to evaluate a new formulation of niosome in healthy volunteers from previous studies, showing higher bioavailability than the conventional formulation. Thus, drug initiation may be evaluated in terms of efficacy and patient tolerance.
CONCLUSION
This study indicates that while both Capsaicin-Loaded Niosome (CLN) and Capsaicin-Gel (CG) formulations can cause initial skin erythema, CLN may have a lower risk of sustained irritation compared to CG. CLN was found to have the potential to reduce melanin content. The CLN formulation seems to be a promising alternative to CG, with a potentially better safety profile for treating pain.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Office of the Higher Education Commission and the Thailand Research Fund (Grant no. RGNS 64–237) for their financial support. Sincere thanks also go to the Faculty of Pharmaceutical Sciences, Ubon Ratchathani, Thailand, for additional support through finance (Grant no. 0604.11-6/2566), facilities, and equipment. In addition, the authors would like to thank the Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand, for kindly providing the shed snake skin used in the pre-formulation study.
AUTHOR CONTRIBUTIONS
Tipada Samseethong: conceptualization, methodology, visualization, writing—original draft preparation, funding acquisition; Sureewan Duangjit: formulation preparation, methodology, intellectual contribution in nanovesicle research, funding acquisition, writing—review and editing; Phaijit Sritananuwat: supervision, methodology, writing—review and editing.
CONFLICTS OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdel Samie, S. M. and Nasr, M. 2020. Food to medicine transformation of stilbenoid vesicular and lipid-based nanocarriers: Technological advances. p. 227-245. In Shegokar R. (ed.), Drug Delivery Aspects. Elsevier.
Agrawal, U., Gupta, M., and Vyas, S.P. 2015. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis, Artificial Cells, Nanomedicine, and Biotechnology. 43: 33-39.
Alalami, K., Goff, J., Grimson, H., Martin, O., McDonald, E., Mirza, T., Mistry, D., Ofodile, A., Raja, S., Shaker, T., et al. 2024. Does topical capsaicin affect the central nervous system in neuropathic pain? a narrative review. Pharmaceuticals (Basel). 7: 1-14.
Altman, R.D., Aven, A., Holmburg, C. E., Pfeifer, L. M., Sack, M., and Young, G. T. 1994. Capsaicin cream 0.025% as monotherapy for osteoarthritis: A double-blind study. Seminars in Arthritis and Rheumatism. 6: 25-33.
Anantaworasakul, P., Chaiyana, W., Michaniak-Kohn, B. B., Rungseevijitprapa, W., and Ampasavate C. 2020. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics. 5: 463.
Antonio, A. S., Wiedemann, L. S. M., and Veiga Junior, V. F. 2018. The genus Capsicum: A phytochemical review of bioactive secondary metabolites. RSC Advances. 45: 25767-25784.
Arunprasert, K., Pornpitchanarong, C., Piemvuthi, C., Siraprapapornsakul, S., Sripeangchan, S., Lertsrimongkol, O., Opanasopit, P., and Patrojanasophon, P. 2022. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. European Journal of Pharmaceutical Sciences. 173: 106169.
Baquié, M. and Kasraee, B. 2014. Discrimination between cutaneous pigmentation and erythema: Comparison of the skin colorimeters Dermacatch and Mexameter. Skin Research and Technology. 2: 218-227.
Derry, S. and Moore, R. A. 2012. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systamatic Reviews. 9: CD010111.
Duangjit, S., Akarachinwanit, C., Sila-on, W., Bumrungthai, S., Opanasopit, P., and Ngawhirunpat, T. 2023. Comparative and stability study of rice bran oil in nanovesicle: Conventional niosomes and pH-sensitive niosomes. Natural and Life Sciences Communications. 22(4): e2023070.
Duangjit, S., Chairat, W., Opanasopopit, P., Rojanarata, T., Poanomsuk, S., and Ngawhirunpat, T. 2016. Development, characterization and skin interaction of capsaicin-loaded microemulsion-based nonionic surfactant. Biological and Pharmaceutical Bulletin. 4: 601-610.
Duangjit, S., Nimcharoenwan, T., Chomya, N., Locharoenrat, N., and Ngawhirunpat, T. 2017 Computational design strategy: An approach to enhancing the transdermal delivery of optimal capsaicin-loaded transinvasomes. Drug Development and Industrial Pharmacy. 43(1) 98-107.
Duangjit, S., Pucharean, C., Yoddee, P., Kanlayawuttipong, K., and Ngawhirunpat, T. 2016. Effect of terpene on physicochemical properties and skin permeability of capsaicin loaded solid lipid nanoparticles. Isan Journal of Pharmaceutical Sciences. Supplement. 11(2016): 124-134.
Gupta, R., Gupta, M., Mangal, S., U. Agrawal, U., and Vyas, S. P. 2016. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy, Artificial Cells, Nanomedicine, and Biotechnology. 44: 825-834.
Fluhr, J. W., Kuss, O., Diepgen, T., Lazzerini, S., Pelosi, A., Gloor, M., and Berardesca, E. 2001. Testing for irritation with a multifactorial approach: Comparison of eight non‐invasive measuring techniques on five different irritation types. British Journal of Dermatology. 5: 696-703.
Harnphadungkit, K., Akarasereenont, P., Chadvongvan P., and Chotiyarnwong, C. 2018. Efficacy of 0.0125% capsaicin patch at acupuncture point for pain relief in knee osteoarthritis: A randomized controlled trial. Siriraj Medical Journal. 5: 382-390.
Hayman, M., and Kam, P. C. A. 2008. Capsaicin: A review of its pharmacology and clinical applications. Current Anaesthesia and Critical Care. 5-6: 338-343.
Hibino, S., Hamada, U., Takahashi, H., Watanabe, M., Nozato, N., and Yonei Y. 2010. Effects of dried brewer’s yeast on skin and QOL: A single-blind placebo-controlled clinical study of 8-week treatment. Anti-Aging Medicine. 4: 18-25.
Hossain, M. R., Ansary, T. M., Komine, M., and Ohtsuki, M. 2021. Diversified stimuli-induced inflammatory pathways cause skin pigmentation. International Journal of Molecular Sciences. 8: 3970.
Inamadar, A. C. and Palit, A. 2013. Sensitive skin: An overview. Indian Journal Dermatology Venereology and Leprology. 79(1): 9-16.
Kosuwon, W., Sirichatiwapee, W., Wisanuyotin, T., Jeeravipoolvarn P., and Laupattarakasem, W. 2010. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. Journal of the Medical Association of Thailand. 10: 1188-1195.
Kulkantrakorn, K., Lorsuwansiri C., and Meesawatsom, P. 2013. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: A randomized, double-blind, crossover, placebo-controlled trial. Pain Practice. 6: 497-503.
Manosroi, A., Jantrawut, P., Khositsuntiwong, N., Manosroi, W., and Manosroi, J. 2009. Novel elastic nanovesicles for cosmeceutical and pharmaceutical applications, Chiang Mai Journal of Science. 36(2): 168-178.
Manosroi, A., Kietthanakorn, B. O., Chankhampan, C., Khositsuntiwong, K., Manosroi, W., Abe, M., and Manosroi, J. 2013. Physical characteristics and biological activities of Thai flower extracts loaded in niosomes. Chiang Mai Journal of Science. 40(4): 603 - 617.
Manosroi, A., Witkittilak, W., Chutoprapat, R., Todo, H., Sugibayashi, K., Manosroi, W., and Manosroi, J. 2011. Moisture enhancement of niosomes entrapped with mineral water in pig ear skin. Chiang Mai Journal of Science. 38(1): 126-138.
Manosroi, J., Chankhampan, C., Kitdamrongtham, W., Zhang, J., Abe, M., Akihisa, T., Manosroi, W., and Manosroi, A., 2020. In vivo anti-ageing activity of cream containing niosomes loaded with purple glutinous rice (Oryza sativa Linn.) extract. International Journal of Cosmetic Science. 42: 622-631.
Munce, T. A. and Kenney, W. L. 2003. Age-specific skin blood flow responses to acute capsaicin. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 4: 304-310.
Shin, J. W., Yoon, S. W., Jeong, J. B., and Park, K. C. 2014. Different responses of the melanin index to ultraviolet irradiation in relation to skin color and body site. Photodermatology Photoimmunology and Photomedicine 6: 308-315.
Sritananuwat, P., Samseethong, T., Jitsaeng, K., Duangjit, S., Opanasopit, P., and Rangsimawong, W., 2024. Effectiveness and safety of Boesenbergia rotunda extract on 3T3-L1 preadipocytes and its use in capsaicin-loaded body-firming formulation: In vitro biological study and in vivo human study, Cosmetics.11: 24.
Surapat, W., Pukahuta, C., Rattanachaikunsopon, P., Aimi, T., and Boonlue, S. 2013. Characteristics of phosphate solubilization by phosphate-solubilizing bacteria isolated from agricultural chili soil and their efficiency on the growth of chili (Capsicum frutescens L. cv. Hua Rua). Chiang Mai Journal of Science. 40(1): 11-25.
Suthisisang, C., Kulkantrakorn, K., Dejthevaporn, J., and Meesawatsom, P., 2013. The study of the effects of external medicines prepared from Capsaicin Nanoparticle in Patients with Painful Diabetic Neuropathy. Research report RDG5120053. Mahidol University, Bangkok,Thailand.
Tramontana, M., Hansel, K., Bianchi, L., Sensini, C., Malatesta, N., and Stingeni, L. 2023. Advancing the understanding of allergic contact dermatitis: From pathophysiology to novel therapeutic approaches. Frontier in Medicine (Lausanne). 10: 1184289.
Tavano, L., Alfano, P., Muzzalupo, R., and de Cindio, B. 2011. Niosomes vs microemulsions: New carriers for topical delivery of capsaicin. Colloids and Surfaces B: Biointerfaces, 87: 333-339.
Wichayapreechar, P., Anuchapreeda, S., Phongpradist, R., Rungseevijitprapa, W., and Ampasavate, C., 2020. Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes. Journal of Liposome Research. 30: 197-207.
Wu, Q., Bai, P., Xia, Y., Xia, Y., Xu, B., Dai, K., Zheng, Z., Guo, M. S. S., Fung, K. W. C., Dong, T.T.X., et al. 2020. Capsaicin inhibits the expression of melanogenic proteins in melanocyte via activation of trpv1 channel: Identifying an inhibitor of skin melanogenesis. Journal of Agricultural and Food Chemistry. 50: 14863-14873.
Zhang, W., Zhang, Y., Fan, J., Feng, Z., and Song, X. 2024. Pharmacological activity of capsaicin: Mechanisms and controversies (Review). Molecular Medicine Reports. 3: 1-7.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tipada Samseethong1, 2, Sureewan Duangjit1, 2 and Phaijit Sritananuwat1, 2, *
1 Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand
2 Innovation in Drug and Extract of Agriculture (IDEA) Research Group, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand
Corresponding author: Phaijit Sritananuwat, E-mail: phaijit.s@ubu.ac.th
.ORCID:
Tipada Samseethong: https://orcid.org/0000-0003-1613-1075
Sureewan Duangjit: https://orcid.org/0000-0002-6187-9368
Phaijit Sritananuwat: https://orcid.org/0000-0002-7462-7354
Total Article Views
Editor: Wipawadee Yooin
Chiang Mai University, Thailand
Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: April 18, 2024;
Revised: October 29, 2024;
Accepted: October 31, 2024;
Online First: November 21, 2024

