
Dynamic Changes in Maternal Treg, CD4+, and CD8+ T Cells After Administration of Candle Bush Leaves (Cassia alata) Extract in Gestational Diabetes Mellitus
Rizky Senna Samoedra, Setyaki Kevin Pratama, Fikriya Novita Sari, Sri Rahayu, Aris Soewondo, and Muhaimin Rifa’I*Published Date : November 21, 2024
DOI : https://doi.org/10.12982/NLSC.2025.003
Journal Issues : Number 1, January-March 2025
Abstract Gestational diabetes mellitus is characterized by glucose intolerance during pregnancy, leading to elevated glucose levels, oxidative stress, and inflammation. Candle bush (Cassia alata) extract (CBE) is an alternative potential therapeutic approach due to its anti-diabetic and anti-inflammatory properties. This study aims to evaluate the efficacy of Candle bush leaf extract during GDM by dynamic changes of CD4+, CD8+, and regulatory T cells (Tregs). Simplicia of Candle bush leaf was extracted by maceration with distilled water (1:10; w:v) for 42 hours at 40°C. Twenty-five pregnant mice were spread randomly into five groups (n=5). Mice were induced to develop GDM using 300 mg/kg BW niacinamide (NA) and 145 mg/kg BW streptozotocin (STZ) via intraperitoneal injection. The treatment groups received metformin (307 mg/kg BW) and CBE (Dose 1 with 500 mg/kg BW and Dose 2 with 1,500 mg/kg BW) orally once a day from Gestational-day/GD6 to GD18. The pancreas and spleen organs were isolated for immunostaining. The antioxidant activity was analyzed using 0.4mM 2,2-diphenyl-1-picrylhydrazyl (DPPH). The results demonstrated that CBE significantly reduced blood glucose levels, increased insulin secretion, and modulated immune cell populations by decreasing cytotoxic T cells (CD8+) as factor that induce inflammation and increasing helper T cells (CD4+) and Tregs activities as modulator to induce anti-inflammatory (P < 0.05). Furthermore, CBE has antioxidant activity with IC50 475 μg/ml. In conclusion, the Dose 1 appears as a promising potential treatment for GDM by greater improvements in glucose regulation, insulin secretion, and immune modulation compared to metformin and the higher dose of CBE.
Keywords: Diabetes, Gestational, Immunomodulator, Inflammation
Funding: The authors are grateful for the research funding provided by LPPM UB through the HGB grant.
Citation: Samoedra, R.S., Pratama, S.K., Sari, F.N., Rahayu, S., Soewondo, A., and Rifa’I, M. 2025. Dynamic changes in maternal treg, CD4+, and CD8+ T cells after administration of candle bush leaves (Cassia alata) extract in gestational diabetes mellitus. Natural and Life Sciences Communications. 24(1): e2025003.
INTRODUCTION
Hyperglycemia in pregnancy is a type of physiological adaptation of metabolism and normally occurs during pregnancy (Modzeleweski et al., 2022). The purpose of metabolic adaptations during pregnancy was to assist fetal development, especially in terms of nutrition (Lizárraga and Gasca, 2021). The inability to respond to glucose demand during pregnancy can lead to gestational diabetes mellitus (Plows et al., 2018).
Gestational diabetes mellitus (GDM) is a reference to high blood glucose levels (>200 mg/dL) during pregnancy. The International Diabetes Federation (IDF) estimated the prevalence of GDM reached 16.7% of births annually in 2021 and will keep on increasing (IDF, 2021). The study conducted by Jatavan et al. (2023) reported that the prevalence of gestational diabetes mellitus had increased to 22% in 2022 based on research conducted in northern Thailand (Jatavan et al., 2023). The increase in GDM cases is influenced by many factors which include ethnicity, lifestyle and history, and other factors (Plows et al., 2018; Wang et al., 2022). Women with GDM are defined as pregnant women who are diagnosed with diabetes or hyperglycemia diagnosis in the second or third trimester which also includes women with and without a diabetes history (ADA, 2021).
The effects of hyperglycemia cause failure of the immune cells to reduce inflammation in GDM. Disruptions in the immune response cause an imbalance in the body's condition. It leads to excessive inflammatory conditions caused by increased pro-inflammatory immune responses and disruptions in the production of anti-inflammatory immune cells (McElwain et al., 2021). Effective medication is required to avoid the imbalance of immune response. One of the alternative approaches for decreasing blood glucose levels is by using leaf extract from Candle bush (C. alata) (Oladeji et al., 2020).
Candle bush has previously been thoroughly researched in terms of numerous bioactivities derived from all sections of the plant, including leaves, seeds, stems, twigs, and flowers. Candle bush has antimicrobial, antifungal, anticryptococcal, antibacterial, antitumor, anti-inflammatory, antidiabetic, antioxidant, wound healing, and anti-helminthic properties (Oladeji et al., 2020). Its ability is supported by the secondary metabolite composition of Candle bush, which includes alkaloids, tannins, saponins, phenols, flavonoids, anthraquinones, and cardiac glycosides (Chew et al., 2022).
Candle bush leaf extract has great potential as an anti-diabetic drug in GDM as shown by previous studies (Yakubu et al., 2010; Kazeem et al., 2015; Naowaboot and Piyabhan, 2016; Onyegeme-Okerenta and Anacletus, 2017). Candle bush leaf has also been used in traditional medicine by boiling it in water (Chew et al., 2022). Oral therapy, such as taking medicines and herbs, is very successful and safe in treating GDM. The treatment focuses on increasing insulin sensitivity to manage glucose levels during pregnancy (Plows et al., 2018).
There are limited studies regarding the administration of herbal extracts in pregnant conditions. Effective medication is required to avoid the harmful effects of GDM. Based on previous research, it has been determined that a dosage of 500 mg/kg BW is the most effective to decrease blood glucose levels. Lower doses than this threshold have shown no meaningful effect on blood glucose reduction while staying safe for pregnant mice (Yakubu et al., 2010; Naowaboot and Piyabhan, 2016). However, a full evaluation of this dosage's effects and efficacy on pregnant women with GDM is unclear. This study aims to evaluate the efficacy of Candle bush leaf extract during GDM by dynamic changes of CD4+, CD8+, and regulatory T cells (Tregs). This study addresses critical gaps in the literature by comparing the effects of C. alata extract at optimal (500 mg/kg BW) and higher (1,500 mg/kg BW) dosages on pregnant mice, evaluating both toxicity and efficacy through dynamic changes in CD4+, CD8+, and regulatory T cells (Tregs), thereby providing valuable insights into the immunomodulatory effects of Candle bush Extract (CBE) in managing hyperglycemia during pregnancy and advancing potential therapeutic strategies for gestational diabetes mellitus.
MATERIAL AND METHODS
Materials
The leaves of Candle bush (C. alata) were obtained from UPT Materia Medica Batu during the dry season, with sample number 220110.KPC.F.KJY.001. The plant was identified by Materia Medica Batu with the identification number 067/892/102.20/2023. A total of 8 kg of fresh leaf samples were processed into 2 kg of simplicia samples for further extraction and analysis. Twenty-five 8-week-old strain BALB/c female mice were obtained from the Department of Pharmacy, UGM (Yogyakarta, Indonesia). The sample size was calculated using the Federer’s formula for five groups. The calculation using the Federer formula resulted in a value of 4.75, indicating that the sample size we have employed is adequate for this study. Considering 5 replications per group is sufficient to yield statistically valid and significant results in the analysis of the sample. Mice were induced for GDM using Niacinamide (NA) in water for injection and streptozotocin (STZ) (Bioworld, USA) in citrate buffer with a pH of 4.5. Immunostaining for flow cytometry was done using wash and permeable buffer, cytofix, and anti-CD4-APC, anti-CD25-PE, anti-CD8-PE, and anti-insulin-APC (Biolegend, USA) antibodies. The antioxidant activity was done using 0.4 mM DPPH in ethanol and distilled as a solvent in this assay.
Extraction
Leaves of Candle bush that had been dried and processed then were turned into simplicia or powder form (8 kg fresh leaf sample into 2 kg simplicial sample). Simplicia was extracted by maceration with distilled water as a solvent. Simplicia with the amount of 100 grams was added to a beaker glass and dissolved using 1,000 mL distilled water (1:10; w:v). The sample was stirred using a magnetic stirrer for 42 hours at 40°C. Afterwards, the sample is filtered with a filter cloth of Whatman filter paper in addition to a vacuum pump to remove any remaining residue. Then, a rotary evaporator at 40°C was used to turn it into a paste form, and freeze-dry was used for optimization to remove the solvent.
GDM mice model
This experiment was conducted in the Laboratory of Animal Physiology (Malang, Indonesia) from August until September 2023. The ethical approval was obtained from Brawijaya University research ethics commission for animal care and use committee (No:108-KEP-UB-2023). Animals were housed in a pathogen-free room with enough food and drink. Twenty-five 8-week-old female BALB/c mice in the estrous cycle were mated with male BALB/c mice. The vaginal plug was checked to ensure the mating process is complete. Mated female mice were then separated from male mice and induced to develop a GDM model using the method described by Wu et al. (2016), with a few modifications. Mice were fasted for 6 hours before injection. Mice were administered with 300 mg/kg NA in water for injection, followed by the administration of 145 mg/kg STZ in citrate buffer with a pH of 4.5 via intraperitoneal injection 15 minutes later. The blood glucose levels of the mice were assessed by milking and measuring blood from the tips of their tails using a glucometer consistently at 8 a.m. The mice were successfully modeled as GDM mice as shown by the vaginal plug and hyperglycemia after six days of evaluation (blood glucose level >200mg/dL).
GDM treatment
Pregnant mice were randomly assigned to various groups: normal pregnant, GDM, Metformin (GDM mice treated with metformin 307 mg/kg BW), Dose 1 (GDM mice treated with CBE 500 mg/kg BW), and Dose 2 (GDM mice treated with CBE 1500 mg/kg BW) with 5 mice for each group (n=5). Metformin dose is based on a human-to-mouse dose conversion that was adjusted in previous research (Nair and Jacob, 2016). GDM treatment with metformin and extract was started on gestational day 6 (GD6) orally once per day. Blood glucose level was measured before pregnancy (GD0), after pregnancy (after six days of pregnancy in GD6), and after treatment (after 12 days of treatment in GD18) using EasyTouch glucometer (Bioptik Technology Inc., Taiwan).
Immunostaining and flow cytometry
Twenty-five pregnant mice were sacrificed by neck dislocation method on GD18 to examine CD4+CD25+, CD4+, CD8+, and insulin-expressing cells alongside tissue histology. The pancreas and spleen were isolated and immunostained. Extracellular staining of antibodies was done using the spleen organ, while intracellular staining of antibodies was done using the pancreas organ. Organs were crushed gently in phosphate buffer saline (PBS) to isolate the cells. Samples were then placed in a propylene tube and centrifuged at 2400 rpm for 5 minutes at 10°C. The supernatant was discarded and 1 ml of PBS was added to the pellet.
Extracellular staining
Fifty microliters of isolated spleen samples were placed in a 1.5 mL microtube in preparation for extracellular staining of antibodies. Samples were added with 50 μL of extracellular antibodies (anti-CD4-FITC, anti-CD8-PE, and CD25-PE) (BioLegend, USA). Samples were mixed using a vortex and incubated in low light conditions at 4°C for 20 minutes. After incubation, samples were added with 400 μL PBS and transferred to a FACS tube. Next, the samples were run on a flow cytometry machine (BDFACSCalibur). Afterwards, the data was analyzed using the BD CellQuest Pro software.
Intracellular staining
Fifty microliters of isolated pancreas samples were placed in a 1.5 mL microtube in preparation for intracellular staining of antibodies. Samples were added with 50 μL of cytofix and incubated in low light conditions at 4°C for 20 minutes. Samples were then added with 500 μL of wash and permeabilization buffer and centrifuged for 10 minutes at 2500 rpm and 4°C. The supernatant was discarded and the pellet was added with 50 μL intracellular antibodies (anti-insulin-APC) (BioLegend). The samples were mixed using a vortex and continued with incubation at 4°C for 20 minutes in low light conditions. The sample was then added with 400 μL PBS and transferred to a FACS tube. Next, the samples were run on a flow cytometry machine (BDFACSCalibur) and the data were analyzed using the BD CellQuest Pro software.
Histology analysis
The pancreas was isolated to make histology slides. It was immersed in 10% formalin and the paraffin block method was performed. Paraffin blocks were sliced using microtome with 6-8 µm thickness and placed on a glass slide. Samples were then deparaffinized using xylene and alcohol. Afterwards, samples were dyed with hematoxylin and eosin to stain cells in the pancreas tissue. The samples were examined to analyze the histology of the pancreas under a light microscope equipped with a digital camera under a magnification of X200.
DPPH assay
The antioxidant activity of CBE was assessed using the DPPH compound as a free radical to evaluate the extract's capability to scavenge free radicals. The DPPH assay followed the methodology outlined by Sari et al. (2023) with a few modifications. In a 96-well plate, 100 μl of CBE at different concentrations (31.25-500 μg/ml) was added. The extract was tested with triplicates of 3 wells for each concentration. Subsequently, 100 μl of DPPH at a concentration of 0.4 mM was added to each well containing CBE. The samples were then incubated for 30 minutes at room temperature and the readings were taken using a microplate reader at a wavelength of 517 nm.
Statistical analysis
All data obtained from this research were statistically analyzed using One-Way ANOVA and Tukey HSD follow-up test to assess the differences between groups with n=5 for each group (five mice for each group). Statistical analysis was conducted using GraphPad Prism 8. The data was expressed as mean ± SD and P<0.05 was regarded as a statistically significant difference.
RESULTS
Blood glucose level on gestational diabetes mellitus
Pregnancy has an effect on rising blood glucose levels (Table 1). The increase in blood glucose in the Normal mice group has an increasing trendline during the study period. Blood glucose levels in all mice started at 126.4 ± 19.07 mg/dL without treatment and pregnancy factor. However, the blood glucose levels were still below the hyperglycemia condition (<200 mg/dL) with 140 ± 17.98 mg/dL at GD18. In the GDM mice group, GDM condition caused a significant increase in blood glucose. Blood glucose levels in all groups of mice with GDM condition were greater than 200 mg/dL. In the groups of GDM mice without treatment, the increase in blood glucose became higher until it reached 336 ± 37.06 mg/dL and was significantly different from the Normal group.
GDM mice in the treatment groups which include CBE and metformin showed a decrease in blood glucose levels (Table 1). Metformin administration reduced blood glucose levels by 190 ± 25.96 mg/dL with no significant difference from the Normal group. In the extract groups, both extract groups reduced blood glucose levels in GDM conditions. Blood glucose levels in the Dose 1 and Dose 2 groups were 251.4 ± 38.30 mg/dL and 259.6 ± 45.46 mg/dL, respectively (Table 1). The decrease in blood glucose levels in the two extract groups was still not comparable to the glucose levels in the Normal group, which showed significant differences between groups. The decrease in the extract groups was only similar to that in the Metformin group and the extract groups did not significantly reduce blood glucose levels compared to the GDM group. This decrease in blood glucose levels correlated with insulin cell expression (Figure 1).
Table 1. Blood glucose level on GDM.
|
Group |
Before Pregnancy (mg/dL) |
Before Treatment in GD6 (mg/dL) |
After Treatment in GD18 (mg/dL) |
Sig |
|
Normal |
126.4 ± 19.07 |
122.4 ± 20.57 |
140 ± 17.98 |
a |
|
GDM |
126.4 ± 19.07 |
230 ± 59.67 |
336 ± 37.06 |
c |
|
Metformin |
126.4 ± 19.07 |
269.6 ± 66.91 |
190 ± 25.96 |
ab |
|
Dose 1 |
126.4 ± 19.07 |
277.6 ± 67.23 |
251.4 ± 38.30 |
bc |
|
Dose 2 |
126.4 ± 19.07 |
229.2 ± 40.27 |
259.6 ± 45.46 |
bc |
Note: one-way ANOVA and Tukey HSD analysis p<0.05 is considered statistically significant. Different letter annotation means it is significantly different between groups. Normal = Normal mice group (n = 5); GDM = Gestational diabetes mellitus mice group (n = 5); Metformin= GDM mice treated with Metformin 307.5 mg/kg BW; Dose 1 = GDM mice treated with CBE 500 mg/kg BW (n = 5); Dose 2 = GDM mice treated with CBE 1500 mg/kg BW (n = 5).
Pancreatic cell condition after extract administration during gestational diabetes mellitus
The pancreas has a vital role during GDM. The number of cells expressing insulin in the Normal pregnant group of mice was elevated and this increase correlated with glucose levels in the Normal pregnant mice (Figure 1). The increase of cells that expressed insulin was shown to be in the value 58.27 ± 6.40% and this was supported by the normal condition of the pancreas histology. In the GDM condition, cells that expressed insulin showed a significant reduction at 14.69 ± 2.09% and this was supported by the abnormality of pancreas histology. Microscopic examinations of pancreas islets showed untreated GDM condition will lead to abnormality of structure such as hyperplasia and loss of structure border (black arrow). Pancreas structures were improved during treatment with metformin and extract. It showed that there is no indication of loss of structure or hyperplasia. The pancreas histology of treated mice with extract showed an increase in the compactness and density of pancreatic cells (Figure 1).
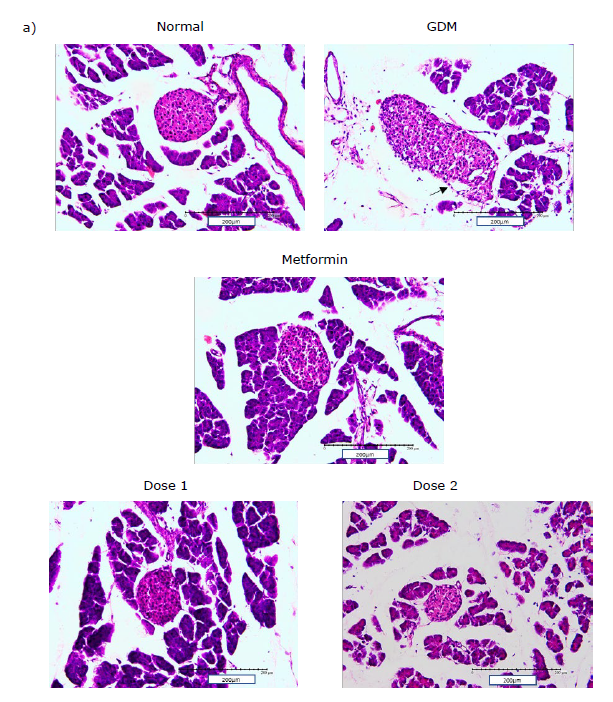
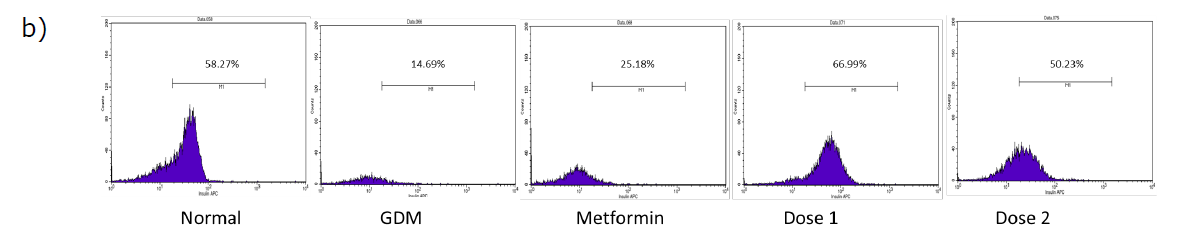
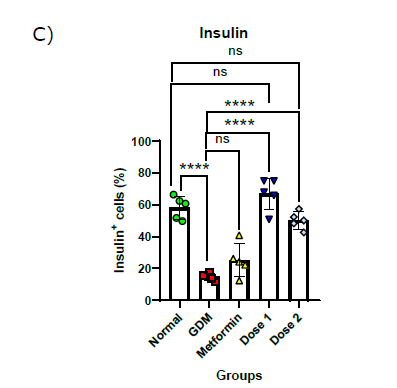
Figure 1. Pancreas histology and insulin-expressing cells in the pancreas: histology of pancreatic islet (200x magnificent) a); flow cytometry plot of insulin-expressing cells in the pancreas b); Statistical comparison of insulin-expressing cells in the pancreas of all group in the study after flow cytometry analysis c). The black arrow shows there is a loss of structure in pancreas histology. Normal = Normal mice group; GDM = Gestational diabetes mellitus mice group (n = 5); Metformin= GDM mice treated with Metformin 307.5 mg/kg BW; Dose 1 = GDM mice treated with CBE 500 mg/kg BW (n = 5); Dose 2 = GDM mice treated with CBE 1500 mg/kg BW (n = 5). Statistical note: *= P <0.05, **= P< 0.01 ***= P< 0.001, ****= P< 0.0001, ns = not significant.
Treatment groups (metformin and CBE) have an effect on insulin-expressing cells and pancreas histology (Figure 1). Metformin treatment was shown to be not effective in increasing cells that express insulin. It was shown by the value of 25.18 ± 0.18%, which was not significant when compared to the GDM group and it was also significantly lower when compared to the Normal group. Both groups of extracts were shown to be able to increase cells that express insulin in the pancreas. The rise in insulin-expressing cells for both extract doses was significantly different when compared to the GDM group. The increase of insulin-expressing cells was shown to be at the value of 66.99 ± 8.87% in Dose 1 and 50.23 ± 4.83% in Dose 2. Dose 1 was shown to be the most effective in boosting insulin-expressing cells between all groups and was close to the normal condition.
Inflammatory response in CD4+ and CD8+ cells were suppressed after treatment intervention
Immune cells known as CD4+ and CD8+ cells serve as markers of inflammation in this study. Normal pregnant mice showed relatively low numbers of cells at 13.55 ± 1.30% in CD4+ and 4.91 ± 0.85% in CD8+ (Figure 2). In GDM condition, CD4+ and CD8+ cells increased significantly from the Normal group with the value of 17.35 ± 0.74 and 6.67 ± 0.12% respectively. The Metformin and CBE groups were able to reduce inflammation to close to the Normal group levels in CD8+ cells. CD8+ cells in the Metformin, Dose 1, and Dose 2 groups were 4.45 ± 0.47, 4.27 ± 0.49 and 5.32 ± 0.92% respectively. In CD4+ cells, only the Metformin group has their number of cells decreased significantly when compared to the GDM group. The CD4+ cells in the treatment groups were 14.84 ± 0.91% in Metformin, 17.06 ± 0.65% in Dose 1, and 19.32 ± 1.66% in Dose 2 groups.
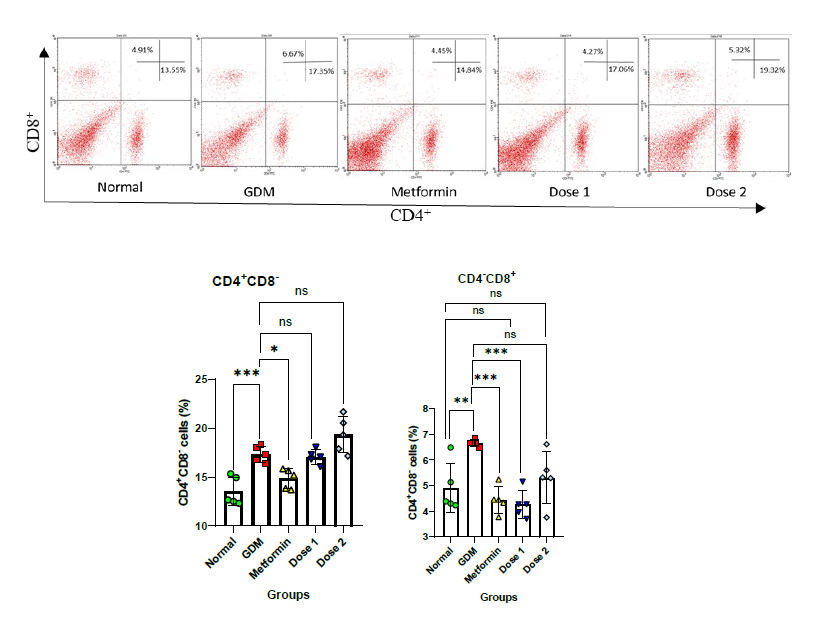
Figure 2. Relative number of CD4+ and CD8+ cells in the study: Flow cytometry plot of CD4+ and CD8+ cells a); Statistical comparison of CD4+ cells after flow cytometry analysis of all group in the study b); Statistical comparison of CD8+ cells after flow cytometry analysis of all group in the study c). Normal = Normal mice group (n = 5); GDM = Gestational diabetes mellitus mice group (n = 5); Metformin= GDM mice treated with Metformin 307.5 mg/kg BW; Dose 1 = GDM mice treated with CBE 500 mg/kg BW (n = 5); Dose 2 = GDM mice treated with CBE 1,500 mg/kg BW (n = 5). Statistical note: *= P <0.05, **= P< 0.01 ***= P< 0.001, ****= P< 0.0001, ns = not significant.
Counterbalance response in anti-inflammatory cell expression (CD4+ CD25+) were boosted after extract treatment
Tregs or CD4+CD25+ cells serve as markers of anti-inflammation or immunosuppressive cells in this study. The Normal group has shown a low number of CD4+CD25+ cells at 0.88 ± 0.09% (Figure 3a). The GDM and metformin groups showed that there was no increase of Tregs and there was no significant difference between the Normal group with 1.00 ± 0.04% in GDM and 0.99 ± 0.05% in metformin group mice. The administration of the extract enhanced the number of CD4+CD25+ cells. Dose 1 and Dose 2 were shown to be significantly different than the Normal group at 1.62 ± 0.12% and 1.60 ± 0.15%, respectively (Figure 3).
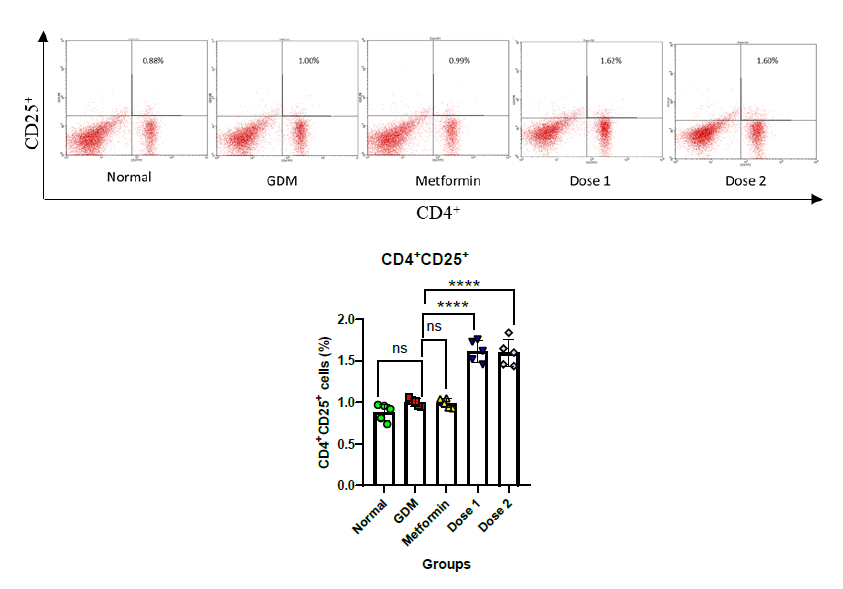
Figure 3. Relative number of CD4+CD25+ cells on spleen during GDM condition: Flow cytometry plot of CD4+CD25+ cells a); Statistical comparison of CD4+CD25+ cells after flow cytometry for all groups in the study b). Normal = Normal mice group (n = 5); GDM = Gestational diabetes mellitus mice group (n = 5); Metformin= GDM mice treated with Metformin 307.5 mg/kg BW; Dose 1 = GDM mice treated with CBE 500 mg/kg BW (n = 5); Dose 2 = GDM mice treated with CBE 1,500 mg/kg BW (n = 5). Statistic note: *= P <0.05, **= P< 0.01 ***= P< 0.001, ****= P< 0.0001.
Antioxidant activity of candle bush extract
The CBE extract exhibited bioactivity as an antioxidant agent, as demonstrated by the DPPH assay (Figure 4). CBE, at concentrations of 31.25, 62.5, 125, 250, and 500 μg/ml, demonstrated scavenging activity against the DPPH compound. The IC50 for CBE was determined to be 475 μg/ml, indicating that CBE requires a higher concentration to exhibit bioactivity as an antioxidant.
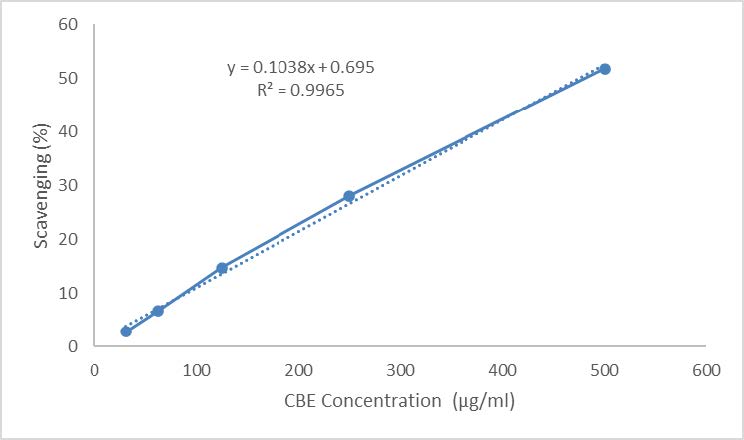
Figure 4. Antioxidant activity of CBE using DPPH assay to test free radical scavenging ability of CBE.
DISCUSSION
Pregnancy has an effect on rising blood glucose levels (Table 1). The increase in all groups is a result of the body response to increased glucose demand and insulin resistance during pregnancy (Sharma et al., 2022). Before entering the second and third trimesters, blood glucose levels must rise in order to develop lipid storage (Plows et al., 2018; Lizárraga and Gasca, 2021). The elevation in blood glucose levels throughout the second and third trimesters is driven by insulin resistance to meet the nutritional transportation needs of the fetus (Plows et al., 2018).
The increase of blood glucose in the Normal mice group has an increasing trendline during the study period (Table 1). However, the blood glucose level remained below hyperglycemia condition (<200 mg/dL) at GD18, with an average of 140 ± 17.98 mg/dL. This result is related to the body adaptability, such as balancing insulin expression (Plows et al., 2018). Insulin is required to keep blood glucose levels stable. The increase of insulin was shown in the Normal pregnant group of mice with a value of 58.27 ± 6.40% (Figure 1b and 1c).
In this study, GDM models were induced using i.p injection of STZ and NA. NA will provide protection to the pancreas while STZ will increase blood sugar levels by disrupting the pancreas and keeping blood glucose levels high which will lead to failure of blood glucose control. When the body fails to adapt to hyperglycemia, blood glucose is greatly elevated and this will lead to GDM (Wu et al., 2016).
The GDM disease caused a significant increase in blood glucose in this study (Table 1). Blood glucose levels in all groups of mice with GDM were greater than 200 mg/dL. In the group of GDM mice without treatment, the increase in blood glucose became higher until it reached 336 ± 37.06 mg/dL and was significantly different from the Normal group. The increase of blood glucose in the GDM condition is caused by failure of adaptation during pregnancy, such as decreased insulin secretion, chronic insulin resistance, oxidative stress, ER stress, inflammation, and others (Plows et al., 2018; Burgos-Morón et al., 2019). The inability of the GDM mice group to control blood glucose levels is shown by the decrease of insulin-expressing cells at 14.69 ± 2.09% which was significantly lower than the Normal group and this was further evidenced by the abnormality of the pancreatic islets in the GDM group without treatment (Figure 1).
Treatments of CBE and metformin will help to manage GDM adverse effects besides lifestyle interventions (exercises and diet) (Plows et al., 2018). Both treatments (metformin and extract) has an effect on blood glucose levels and insulin-expressing cells. Metformin administration reduced blood glucose levels to the value of 190 ± 25.96 mg/dL with no significant difference from the Normal group (Table 1). However, metformin was not effective in increasing insulin-expressing cells in the pancreas. It was shown by the number of insulin-expressing cells at 25.18 ± 0.18%, which was not significant when compared to the GDM group (Figure 1b and 1c). This happened because metformin works by raising insulin sensitivity. Metformin enhances insulin sensitivity by boosting insulin-mediated insulin receptor tyrosine kinase activity, which activates post-receptor insulin signaling pathways such as the translocation of GLUT4. It resulted in greater blood glucose absorption and reduced blood glucose levels (Herman et al., 2022).
Both CBE groups reduced blood glucose levels in GDM conditions, albeit not significantly. Blood glucose levels in the Dose 1 and Dose 2 groups were 251.4 ± 38.30 mg/dL and 259.6 ± 45.46 mg/dL, respectively (Table 1). Additionally, the decrease in blood glucose levels in the CBE groups was still significantly different when compared to the Normal group. Both groups of CBEs also did not show any significance when compared to the Metformin group. The efficacy of Candle bush leaf extract to reduce blood glucose levels was not as effective as metformin, but the extract groups were able to boost insulin-expressing cells in the pancreas and repair the structure of the pancreas islet (Figure 1). Herbal medicines offer an effective approach to improving β-cell function, with potential benefits including anti-apoptotic action, β-cell neogenesis/proliferation, enhanced glucose-stimulated insulin secretion via activation of glucose transporter-2 (GLUT-2) receptors, increased intracellular Ca2+ levels, modulation of various insulin signaling pathways and protective effects on β-cells by reducing oxidative stress and inflammation (Wickramasighe et al., 2021). These effects are attributed to the presence of secondary metabolites in CBE, such as alkaloids, tannins, saponins, phenols, flavonoids, anthraquinones, and cardiac glycosides (Wickramasighe et al., 2021; Chew et al., 2022).
Pancreas structure and function were improved during treatment with CBE. The repair process showed the structure of the pancreas was maintained to keep the cell cohesion and it returned to normal size and function (Figure 1). During GDM conditions, the pancreas islet showed hyperplasia and loss of structure border compared to the Normal pregnant group. Hyperglycemia condition disturbs the pancreas environment and structure due to the effect of oxidative stress and cell deaths lead to abnormal structure of pancreas islets such as hemochromatosis, lymphocyte infiltration, and loss of cell border in the pancreas islet (Yasmin et al., 2021).
Pancreas function is related to the ability to produce insulin to maintain blood glucose in the body. Both CBE groups showed improvement in pancreas function by increasing insulin-expressing cells significantly when compared to the GDM group (Figure 1), with insulin-expressing cells a relative number of 66.99 ± 8.87% and 50.23 ± 4.83%, respectively. Dose 1 was shown to be the most effective in boosting insulin-expressing cells between all groups.
The effectiveness of CBE in reducing diabetes was proven by increasing insulin-expressing cells in the pancreas in this study. The secondary metabolic component of Candle bush leaf plays an important role in its anti-diabetic properties. Previous studies showed that CBE can reduce glucose absorption in the gut while increasing insulin secretion and sensitivity and also improving insulin sensitivity (Kazeem et al., 2015; Naowaboot and Piyabhan, 2016).
The immune response is crucial for maintaining homeostasis. Immune cells known as CD4+, CD8+, and Tregs serve as markers of inflammation. Increased CD4+ and CD8+ cells are associated with pro-inflammatory responses, while increased Tregs expression is associated with anti-inflammatory responses (Wagers et al., 2022). In GDM conditions, hyperglycemia enhances inflammation and can lead to exacerbating GDM to give conditions such as chronic inflammation, damaged pancreatic β-cells, and chronic insulin resistance (Giri et al., 2018). In this study, CD4+ and CD8+ cells in the GDM mice group were increased significantly from the Normal group with the values of 17.35 ± 0.74% and 6.67 ± 0.12%, respectively (Figure 2). The number of CD4+ and CD8+ lymphocytes during pregnancy indicates the amount of inflammation. The presence of these two cells in large numbers shows that the body is inflamed (Barinov et al., 2017).
The Candle bush leaf extract possesses anti-diabetic and also anti-inflammatory properties (Oladeji et al., 2020). The Metformin and CBE groups in this study were able to reduce inflammation close to the Normal group levels in CD8+ cells (Figure 2). The number of CD8+ cells in Metformin, Dose 1, and Dose 2 groups were 4.45 ± 0.47, 4.27 ± 0.49, and 5.32 ± 0.92%, respectively. This decrease in inflammation levels was a result of numerous consequences from the treatment processes. Metformin can reduce inflammation by lowering blood glucose levels and boosting insulin sensitivity (Herman et al., 2022). Metformin also has a role in the modulation of plasma pro-inflammation cytokine to improve the condition in diabetic mice (Janthakhin et al., 2023). Meanwhile, the extract decreased inflammation by lowering blood glucose levels and modulation of inflammatory cells, and this was also enhanced by the effects of anti-inflammatory or immunosuppressive immune cells (Figure 3).
The inflammatory response during pregnancy must be controlled immediately to prevent fetal miscarriage due to cytotoxic cell activity. As a form of adaptation, the mother's immune cells will enhance immunosuppressive activity to decrease inflammation before the cytolytic pathway begins. Immune cells are going to encounter a balance of activation and repression under normal conditions (McElwain et al., 2021). The normal group has shown low expression of Tregs at 0.88% because the mice were not in pro-inflammatory condition (Figure 3). GDM and Metformin groups have shown no elevation of Tregs and thus, there was no significance when compared to the Normal group in this study. No enhancement of anti-inflammatory responses during inflammation conditions will lead to chronic inflammation (Plows et al., 2018; Burgos-Morón et al., 2019).
The Candle bush leaf extract was able to boost the anti-inflammatory response. The administration of CBE enhanced the number of CD4+CD25+ cells. Dose 1 and dose 2 were shown to be significantly higher than the Normal group at 1.62 ± 0.12% and 1.60 ± 0.15%, respectively (Figure 3). This showed that secondary metabolites from CBE have the ability to stimulate the production of regulatory T cells as an anti-inflammatory agent. Increased Tregs can suppress CD4+ and CD8+ cell activity in generating inflammation (Penaloza-MacMaster, 2017). Inflammation and blood glucose levels that are not controlled will worsen GDM symptoms. The administration of CBE extract in this study has shown to be a potential alternative to GDM treatment since it can boost anti-inflammatory responses and insulin secretion. Herbal administration has been studied to control and suppress inflammation effects such as cytokines and pro-inflammatory cells with counterbalance response by enhancing anti-inflammatory cells and cytokines (Sari et al., 2023).
The CBE also functions as an antioxidant agent with an IC50 of 475 μg/ml (Figure 4). It exhibits activity in scavenging DPPH as a free radical. Antioxidant activity from natural compounds such as CBE is known to provide protective effects against tissue and cell damage caused by free radicals (Lobo et al., 2010). The protective role of CBE was observed in pancreatic islets, where CBE administration prevented hyperplasia and loss of structural integrity compared to the Normal pregnant group after treatment (Figure 1a). Administration of herbal extract as an antioxidant does not only protect the pancreas organ. It also gives protection to other organs, such as the kidney and liver to prevent inflammation during hyperglycemia conditions (Nindita et al., 2023). Administration of herbal extracts in hyperglycemic conditions could enhance endogenous antioxidants. CBE, acting as an exogenous antioxidant, along with the enhancement of endogenous antioxidants, could reduce free radicals and provide a more pronounced protective effect (Sari et al., 2023).
The effectiveness of a lower dose (Dose 1) compared to a higher dose (Dose 2) of CBE can be attributed to several factors. One key aspect is the concept of an optimal dose-response relationship, where a specific dosage range elicits maximum therapeutic effects while minimizing adverse effects (Bommakanti et al., 2023). Dose 1 (500 mg/kg BW) may align with this optimal range, facilitating effective interactions with biological pathways involved in glucose metabolism and immune modulation. Additionally, many herbal extracts exhibit hormetic dose responses, where low doses may produce beneficial effects while high doses could be detrimental. This biphasic response emphasizes the need for careful dosage optimization to harness the positive effects while avoiding negative outcomes. Given that a large number of herbal extracts have demonstrated hormetic (biphasic) dose-response relationships across different immune cell models, as seen in prior studies, the dose-response data should also be evaluated for the occurrence, frequency, and quantitative features of these effects, including intensity and dosing range (Calabrese et al., 2019).
Biological saturation can occur at higher doses, where the body systems reach a point where extra quantities of the extract do not enhance efficacy due to receptor desensitization or limited capacity to metabolize the active compounds (Calabrese et al., 2019). Furthermore, the significance of herbal nutraceuticals in the prevention and treatment of diseases such as diabetes, obesity, dementia, hypertension, and hypercholesterolemia has been increasingly recognized. These compounds are also noted for their immunomodulatory and antimicrobial properties. However, inappropriate use of herbal nutraceuticals can lead to adverse effects, such as hepatotoxicity, pulmonary toxicity, cytotoxicity, and other toxicological impacts on various organs, including the heart and kidneys (Bommakanti et al., 2023). This underscores the importance of ensuring safe dosages, particularly in vulnerable populations such as pregnant women with GDM. Moreover, the pharmacokinetics of the compounds in C. alata may result in better absorption and utilization at lower doses, while higher doses could lead to suboptimal bioavailability due to physiological barriers.
CONCLUSION
This study highlights the therapeutic potential of Candle bush (C. alata) leaf extract in managing gestational diabetes mellitus (GDM) by effectively reducing blood glucose levels through the enhancement of insulin-expressing cells in the pancreas and demonstrating strong anti-inflammatory effects by decreasing pro-inflammatory CD8+ cells while increasing CD4+ and regulatory T cells (CD4+CD25+), as observed with Dose 1 (500 mg/kg BW). In contrast, a higher dosage of Dose 2 (1,500 mg/kg BW) did not yield significantly better results in terms of glucose reduction or immune modulation, indicating that increasing the dose does not improve efficacy. Therefore, the recommended dosage for therapeutic use is 500 mg/kg BW, as it provides effective metabolic and immune benefits without the need for higher doses. Future studies should aim to validate these findings in clinical trials and assess the long-term safety of C. alata extract in GDM management.
ACKNOWLEDGEMENTS
We acknowledge LPPM UB for funding this research through the HGB grant. We also grateful to Mr. Bambang Pristiwanto for assisting and giving us insight into this research.
AUTHOR CONTRIBUTIONS
Rizky Senna Samoedra assisted in designing and conducting all the experiments, performed data analysis and wrote the manuscript. Setyaki Kevin Pratama assisted in performing the statistical analysis, data visualization and wrote the manuscript. Fikriya Novita Sari performed the statistical analysis, data visualization and wrote the manuscript. Sri Rahayu designed and conducted the experiment and wrote the manuscript. Aris Soewondo designed and conducted the experiment and data analysis. Muhaimin Rifa’i assisted in designing and conducting all the experiments, performed data analysis, and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
American Diabetes Association (ADA). 2021. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 44(Suppl. 1): S15–S33.
Barinov, A., Galgano, A., Krenn, G., Tanchot, C., Vasseur, F., and Rocha, B. 2017. CD4/CD8/Dendritic cell complexes in the spleen: CD8+ T cells can directly bind CD4+ T cells and modulate their response. PLoS ONE. 12(7): e0180644
Bommakanti, V., Ajikumar, A.P., Sivi, C.M., Prakash, G., Mundanat, A.S., Ahmad, F., Haque, S., Prieto, M.A., and Rana, S.S. 2023. An overview of herbal nutraceuticals, their extraction, formulation, therapeutic effects and potential toxicity. Separations. 10(3): 177.
Burgos-Morón, E., Abad-Jiménez, Z., Martinez de Maranon, A., Iannantuoni, F., Escribano-López, I., López-Domènech, S., Salom, C., Jover, A., Mora, V., Roldan, I., et al. 2019. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. Journal of Clinical Medicine. 8(9): 1385.
Calabrese, E.J., Agathokleous, E., Kapoor, R., Kozumbo, W.J., and Rattan, S.I. 2019. Re-analysis of herbal extract data reveals that inflammatory processes are mediated by hormetic mechanisms. Chemico-Biological Interactions. 1(314): 108844.
Chew, Y.L., Khor, M.A., Xu, Z., Lee, S.K., Keng, J.W., Sang, S.H., and Ming, L.C. 2022. Cassia alata, Coriandrum sativum, Curcuma longa and Azadirachta indica: Food Ingredients as Complementary and Alternative Therapies for Atopic Dermatitis-A Comprehensive Review. Molecules. 27(17): 5475.
Giri, B., Dey, S., Das, T., Sarkar, M., Banerjee, J., and Dash, S.K. 2018. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomedicine & Pharmacotherapy. 107: 306-328.
Herman, R., Kravos, N.A., Jensterle, M., Janež, A., and Dolžan, V. 2022. Metformin and Insulin Resistance: A review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. International Journal of Molecular Sciences. 23(3):1264.
International Diabetes Federation (IDF). 2021. IDF Atlas 10th Edition. Brussels, Belgium.
Janthakhin, Y., Kingtong, S., Aphibanthammakit, C., and Juntapremjit, S. 2023. Metformin mitigates memory impairment of diabetic mice through modulation of plasma pro-inflammatory cytokines and Aβ1-42 levels. Natural and Life Sciences Communications. 22(1): e2023001.
Jatavan, P., Luewan, S., Sirilert, S., and Tongsong, T. 2023. Trends in the prevalence of Diabetes Mellitus in pregnancy during the past two decades in Northern Thailand. Healthcare. 11(9): 1315.
Kazeem, M.I., Azeez, G.A., and Ashafa, A.O. 2015. Effect of Senna alata (L) roxb (fabaceae) leaf extracts on alpha-amylase, alpha-glucosidase and postprandial hyperglycemia in rats. Tropical Journal of Pharmaceutical Research. 14(10): 1843-1848.
Lizárraga and Gasca, A.G. 2021. The placenta as a target of epigenetic alterations in women with gestational diabetes mellitus and potential implications for the offspring. Epigenomes. 5(2): 12.
Lobo, V., Patil, A., Phatak, A., and Chandra, N. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4(8): 118-126.
McElwain, C.J., McCarthy, F.P., and McCarthy, C.M. 2021. Diabetes melitus gestasional and maternal immune dysregulation: What we know so far. International Journal of Molecular Sciences. 22(8): 4261.
Modzelewski, R., Stefanowicz-Rutkowska, M.M., Matuszewski, W., and Bandurska-Stankiewicz, E.M. 2022. Gestational diabetes mellitus—recent literature review. Journal of Clinical Medicine. 11(19): 5736.
Naowaboot, J., and Piyabhan, P. 2016. Senna alata leaf extract restores insulin sensitivity in high-fat diet-induced obese mice. Clinical Phytoscience. 2(18): 1-7.
Nair, A.B., and Jacob, S. 2016. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy, 7(2): 27-31.
Nindita, Y., Utomo, A.W., Maharani, N., Mahati, E., Kristiandi, I.F., Kesumayadi, I., Kurniawati, E.D., Sobirin, M.A., and Wijayahadi, N. 2023. Protective effect of Curcuma domestica and Curcuma xanthorrhiza extracts toward kidney, liver, and pancreatic organ dysfunction in streptozotocin-induced diabetes mellitus mice. Natural Life Science Community. 22(2): 1-14.
Oladeji, O.S., Adelowo, F.E., Oluyori, A.P., and Bankole, D.T. 2020. Ethnobotanical description and biological activities of Senna alata. Evidence-Based Complementary and Alternative Medicine. 2020(13): 2580259.
Onyegeme-Okerenta, B.M., and Anacletus, F.C. 2017. Hypoglycaemic and hypolipidaemic potentials of Senna alata and its effect on the pancreas of alloxan-diabetic induced albino rats. Journal of Applied Life Sciences International. 11(1): 1-10.
Penaloza-MacMaster, P. 2017. CD8 T-cell regulation by T regulatory cells and the programmed cell death protein 1 pathway. Immunology. 151(2): 146-153.
Plows, J.F., Stanley, J.L., Baker, P.N., Reynolds, C.M., and Vickers, M.H. 2018. The pathophysiology of diabetes melitus gestasional. International Journal of Molecular Sciences. 19(11): 3342.
Sari, F.N., Samoedra, R.S., Pratama, S.K., Rahayu, S., Soewondo, A., Jatmiko, Y.D., Natsir, M. H., Tsuboi, H., and Rifa’i, M. 2023. Immunomodulatory effects of Unripe Sapodilla (Manilkara zapota) fruit extract through inflammatory cytokine regulation in type 1 diabetic mice. Jordan Journal of Biological Sciences. 16(2): 345-352.
Sharma, A. K., Singh, S., Singh, H., Mahajan, D., Kolli, P., Mandadapu, G., Kumar, B., Kumar, D., Kumar, S., Manoj Kumar Jena, M. K., et al. 2022. Deep insight of the pathophysiology of gestational diabetes Mellitus. Cells. 11(17): 2672.
Wang, H., Li, N., Chivese, T., Werfalli, M., Sun, H., Yuen, L., Hoegfeldt, C.A., Powe, C. E., Immanuel, J., Karuranga, S., et al. 2022. IDF diabetes atlas: Estimation of global and regional diabetes melitus gestasional prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s criteria. Diabetes Research and Clinical Practice. 183: 109050.
Wagers, B.S., Larson, J.H., Jin, S., and Blazar, B.R. 2022. Cytolytic CD4+ and CD8+ regulatory T-cells and implications for developing immunotherapies to combat graft-versus-host disease. Frontiers in Immunology. 13: 864748.
Wickramasinghe, A.S.D., Kalansuriya, P., and Attanayake, A.P. 2021. Herbal medicines targeting the improved β-cell functions and β-cell regeneration for the management of diabetes mellitus. Evidence-based Complementary and Alternative Medicine. 2021 (2920530): 1-32.
Wu, C.C., Hung, C.N., Shin, Y.C., Wang, C.J., and Huang, H.P. 2016. Myrciaria cauliflora extracts attenuate diabetic nephropathy involving the Ras signaling pathway in streptozotocin/nicotinamide mice on a high fat diet. Journal of Food and Drug Analysis. 24(1): 136-146.
Yakubu, M.T., Adeshina, A.O., Oladiji, A.T., Akanji, M.A., Oloyede, O., Jimoh, G.A., AWO b, O., and Afolayan, A.J. 2010. Abortifacient potential of aqueous extract of Senna alata leaves in rats. Journal of Reproduction and Contraception. 21(3): 163-177.
Yasmin, S., Cerchia, C., Badavath, V.N., Laghezza, A., Dal Piaz, F., Mondal, S.K., and Lavecchia, A. 2021. A series of ferulic acid amides reveals unexpected peroxiredoxin 1 inhibitory activity with in vivo antidiabetic and hypolipidemic effects. ChemMedChem. 16(3): 484-498.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Rizky Senna Samoedra1, Setyaki Kevin Pratama2, Fikriya Novita Sari1, Sri Rahayu1, Aris Soewondo1, and Muhaimin Rifa’I1, *
1 Biology Department, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia.
2 School of Biological Sciences, Faculty of Biology, Medicine, and Health, The University of Manchester, United Kingdom.
Corresponding author: Muhaimin Rifa’i, E-mail: immunobiology@ub.ac.id
ORCID:
Rizky Senna Samoedra: https://orcid.org/0000-0003-3789-8099
Muhaimin Rifa’i: https://orcid.org/0000-0001-5731-2951
Total Article Views
Editor: Veerasak Punyapornwithaya
Chiang Mai University, Thailand
Article history:
Received: November 21, 2023;
Revised: October 1, 2024;
Accepted: October 11, 2024;
Online First: November 21, 2024

