
Effects of Fermentation Temperatures on the Qualities of Monascus sp. PSRU03 Fermented Soybeans
Utaiwan Chattong*, Khongsak Srikaeo, Katekan Dajanta, Sirikarn Thanaboonrongkom, and Chutima LertluksameePublished Date : November 15, 2024
DOI : https://doi.org/10.12982/NLSC.2025.001
Journal Issues : Number 1, January-March 2025
Abstract This research evaluated the effects of fermentation temperatures on the qualities of soybeans fermented with Monascus sp. PSRU03. The conditions included consistent temperatures of 25°C for 20 days, 30°C for 20 days, and a cyclic pattern of 30°C for 4 days followed by 25°C for 16 days. The pigments and antioxidant properties of the fermented soybeans were evaluated. After fermentation, it was observed that the total pigment contents exhibited similarity across all conditions, with values ranging from 31.90 to 35.67 unit/g dry basis. Notably, Monascus sp. PSRU03 produced more yellow pigment than other colors, accounting for approximately 50-53%, which corresponded to a range of 6.94-14.31 unit/g dry basis. This phenomenon is distinctive in that the majority of industrially manufactured Monascus pigments are rich in red pigments. In terms of antioxidant properties, fermentation at 30°C produced the highest total phenolic compounds (6.10 mg GAE/g dry basis). However, DPPH radical-scavenging activity and ferric reducing antioxidant power were found to be not significantly different (P≥0.05) in all fermentation temperatures. These findings provide a foundation for optimizing industrial fermentation processes and exploring the health benefits of Monascus-fermented soybeans.
Keywords: Monascus, soybean, fermented soybean, pigment, fermentation temperature
Funding: The Office of the Higher Education Commission of Thailand supported this work.
Citation: Chattong, U., Srikaeo, K., Dajanta, K., Thanaboonrongkom, S., and Lertluksamee, C. 2025. Effects of fermentation temperatures on the qualities of Monascus sp. PSRU03 fermented soybeans. Natural and Life Sciences Communications. 24(1): e2025001.
INTRODUCTION
The Monascus fermentation industry has garnered global attention for its production of pigments, functional food ingredients, supplements, and medicinal products. Its key products-pigments, functional food ingredients, supplements, and medicinal compounds-are increasingly in demand globally (Srianta et al., 2021). The fermentation process is usually carried out through solid state fermentation or submerged fermentation methods. The Monascus species which are usually used for fermentation are Monascus purpureus, Monascus pilosus, Monascus anka and Monascus ruber. The first product which has been consumed over the centuries in Asian countries is Monascus-fermented rice, so called angkak, anka, beni koji, and red yeast rice. It has been used traditionally as food colorant and preservative, food supplement and in traditional medicine (Srianta et al., 2014; Agboyibor et al., 2018).
During the fermentation process, Monascus can produce various secondary metabolites, especially pigments and monacolin K. Monascus pigments are categorized into three (3) groups based on their color: red, orange, and yellow. There are as many as 111 compounds of Monascus pigments that have been identified recently (Chen et al., 2019). Many researchers reported Monascus pigment compounds to have bioactivity, for example, anti-inflammatory (Choe, Song, et al., 2020), anticancer (Yang et al., 2014), antimicrobial (Vendruscolo et al., 2014), antiobesity (Choe, Jung, et al., 2020), antidiabetic (Shi and Pan, 2010), and antioxidant (Chairote et al., 2009). Meanwhile, monacolin K which is a secondary metabolite of Monascus has been known to have antihypercholesterolemia activity, through inhibition of hydroxymethylglutaryl-CoA (HMG-CoA) reductase activity [a key enzyme in the cholesterol biosynthesis pathway] (Lachenmeier et al., 2012).
Pigments and monacolin K production are influenced by the strain of Monascus fungi, the fermentation substrate, and the conditions during fermentation (Subsaendee et al., 2014; Kraboun et al., 2019). Various incubation temperatures have been reported for the favorable formation of secondary metabolites during Monascus fermentation. Subsaendee et al. (2014) indicated that all pigment and monacolin K were examined in the peak of the Monascus sp. KB9 fermented rice with a constant temperature of 30°C for 15 days. Whereas, the highest contents of monacolin K or pigments were also reported in Monascus sp. fermented rice with a constant temperature of 25°C for 16 days (Chen and Hu, 2005) and 30°C for 20 days (Suraiya et al., 2017). The changing temperature during fermentation, 30°C for the first 4 days extended at 25°C until 20 days, was also suggested as the optimal condition for producing monacolin K and pigment in red yeast rice with nearly 20 times over temperature-constant cultivation (Tsukahara et al., 2009).
Our previous research isolated 13 different morphological characteristics of Monascus strains from red yeast rice, with Monascus sp. PSRU03 appears to be the most effective strain, as indicated by the presence of more pigments (Dajanta and Rongkam, 2016). In addition, high monacolin K contents were also examined in noodle fermented with this strain (Chattong and Dajanta, 2014). Innovative efforts to find substrates other than rice for the Monascus fermentation have continued to evolve. Various agricultural products such as cereals, tubers, legumes, and
agro-industrial products have been used as a fermentation substrate for the fermentation process (Nimnoi et al., 2015; Srianta et al., 2016, 2021). Compared to the well-known red yeast rice, Monascus fermented soybean is a novel product with enhanced qualities (Lim et al., 2010). It has been reported to exhibit increased antioxidant activity and angiotensin I-converting enzyme (ACE) inhibitory activity (Pyo and Lee, 2007). Given the significance of Monascus fermentation products and the promise of soybean as a functional substrate, this study aimed to evaluate the effects of fermentation temperatures on pigments and antioxidant qualities of soybeans fermented with Monascus sp. PSRU03.
MATERIALS AND METHODS
Materials
All chemicals are analytical grade. The catechin and trolox were purchased from Sigma-Aldrich Co. The 2,2-Diphenyl-1-picrylhydrazyl (DPPH), gallic acid, linoleic acid, polyoxyethylene sorbitan monopalmitate (Tween 20) and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Fluka Chemical Co. Folin-Ciocalteu reagent, ethanol, sodium carbonate, di-sodium hydrogen phosphate, sodium dihydrogen phosphate, potassium dihydrogen phosphate and dipotassium hydrogen phosphate were all obtained from Merck Ltd. Sodium nitrite and aluminium chloride were purchased from Ajax Finechem Pty Ltd. Methanol and sodium hydroxide were purchased from Labscan Ltd. Ammonium thiocyanate was obtained from Asia Chemie (Lao) Co., Ltd. Ferrous chloride was obtained from QREC Chemical Co., Ltd. All culture media were purchased from Merck.
Production of Monascus fermented soybeans
The soybean cultivar (Chiang Mai 60) was obtained from the Field Crops Research Institute (Chaing Mai, Thailand). The proximate compositions of Chiang Mai 60 were reported to be protein (36.38 g/100 g DW), fat (22.45 g/100 g DW), carbohydrate (35.24 g/100 g DW), dietary fiber (17.06 g/100 g DW), and ash (5.62 g/100 g DW) (Sahasakul et al., 2022). Soybeans were sorted, cleaned, and then soaked in tap water for 16 h. After discarding the water, the soybeans (50 g) were transferred to 250 mL Erlenmeyer flask. The pH of soaked soybeans was adjusted to 4.0 using a commercial vinegar and sterilized by autoclaving for 40 min.
Monascus sp. PSRU03 was isolated from red yeast rice as described previously (Dajanta and Rongkam, 2016). Briefly, the fungus was inoculated onto potato dextrose agar and incubated for 7 days at 30°C. After cultivation, the pieces of fungal colony were collected using the cork borer (5 mm diameter) and inoculated into potato dextrose broth (10 pieces per 100 mL broth), incubation in a 30°C incubator for 7 days. After completion, the broth was transferred to a stomacher bag, homogenized using a stomacher for 30 sec, and filtered. The filtered spore solutions were inoculated into sterilized soybeans at 5 mL/100 g soybeans. The fermentation conditions were (1) incubation at 25°C for 20 days, (2) incubation at 30°C for 20 days, and (3) incubation at 30°C for 4 days followed by incubation at 25°C for 16 days (total 20 days). In this study, those conditions were expressed as 25°C, 30°C, and 30°C/25°C. The fermented soybeans were sampled every 5 days during fermentation, dried using a tray dryer at 60°C until the water activity reached 0.5, then ground using a blender. Powdered fermented soybeans were kept in aluminum foil bags and stored at -20°C prior to analysis.
Monascus fermented soybean extractions
Powdered fermented soybeans (1.5 g) were extracted using 15 mL of 80% methanol under sonication for 1 h before centrifuged (3,000 rpm) for 30 min. The supernatants were subjected to pigment and antioxidation analysis.
Pigment analysis
Pigment content analysis was conducted by using spectrophotometry. The extract was measured for the absorbance at 400 nm (yellow pigment); 470 nm (orange pigment) and 500 nm (red pigment) (Johns and Stuart, 1991). The pigment content (unit) was calculated based on the corresponding absorbance unit multiplied by dilution factors.
Total phenolic contents
The total phenolic contents of the fermented soybean extracts were measured according to the method described by Chaisuwan et al. (2022). An aliquot of 0.4 mL methanolic soybean extract was added to 2 mL of 0.25N Folin-Ciocalteu phenol reagents in water. After that, 1.6 mL of 7.5% (w/v) sodium carbonate was added to the mixture and heated in a water bath at 50°C for 5 min. The absorbance was measured at 760 nm by a spectrophotometer (Spectronic Evolution 200 Series) after incubation for 30 min in darkness. The calibration curve was established using gallic acid (10-100 μg/mL) as the standard sample, with the correlation coefficient r = 0.9986. The gallic acid content was used as the total phenolic contents in the fermented soybean sample, and was expressed as milligram of gallic acid equivalent (mg GAE)/g dried basis (dbs) of fermented soybean.
DPPH radicals-scavenging assay
The antioxidant activity of the soybean extract was assessed on the basis of the radical scavenging effect of the stable DPPH-free radical activity by the method according Chaisuwan et al. (2022) with slight modification. Briefly, 0.2 mM methanolic DPPH solution was prepared and 150 μL of this solution were added to 75 µL sample extracts in each well of 96 well plate. After incubation in the dark at ambient temperature for 30 min, the absorbance was measured at 515 nm using microplate reader (Spectro Star Nano, BMG Labtech). The scavenging percentage of DPPH was calculated according to the following equation:
% scavenging effect = [1- (Absorbance of sample/Absorbance of control)] x 100
Ferric reducing antioxidant power (FRAP)
The FRAP assay was performed as previously described by Maier et al. (2009). The FRAP reagent was prepared from 2.5 mL of a TPTZ solution (10 mM) in hydrochloric acid (40 mM) and 2.5 mL of a ferric chloride solution (20 mM) mixed with 25 mL of an acetate buffer (0.3 M, pH 3.6). For the determination of the antioxidant capacity, the FRAP reagent (3 mL) was mixed with 400 μL of methanolic soybean extract. The mixture was allowed to incubate for 4 min at 37°C in water bath before the absorption was measured at 593 nm. The calibration curve was established using trolox (1 – 50 µg/mL) as the standard sample, with the correlation coefficient r = 0.9984. The results were expressed as milligram of trolox equivalent/g dbs of fermented soybean.
Statistical analysis
The experimental results were expressed as mean ± SD of triplicate observations made for three parallel extractions and determinations. Data were analyzed statistically by ANOVA and Duncan’s multiple range tests and a P-value of less than 0.05 was considered significant (SPSS Version 22).
RESULTS
The pigments of Monascus-fermented soybeans
The appearances of the Monascus-fermented soybeans obtained from different fermentation conditions are illustrated in Figure 1. In general, the products from all fermentation conditions were found to be visually similar in red color.
Figure 1. Appearances of the fermented soybeans from different fermentation conditions 25°C (a), 30°C (b), and 30°C/25°C (c).
The quantitative analysis of each pigment developed during the fermentation period is shown in Figure 2. The results clearly show that all pigment contents increased over the fermentation periods. The rates increased variably over the first 10 days depending on the conditions. Fermentation at 30°C showed the highest rate compared to the other conditions.
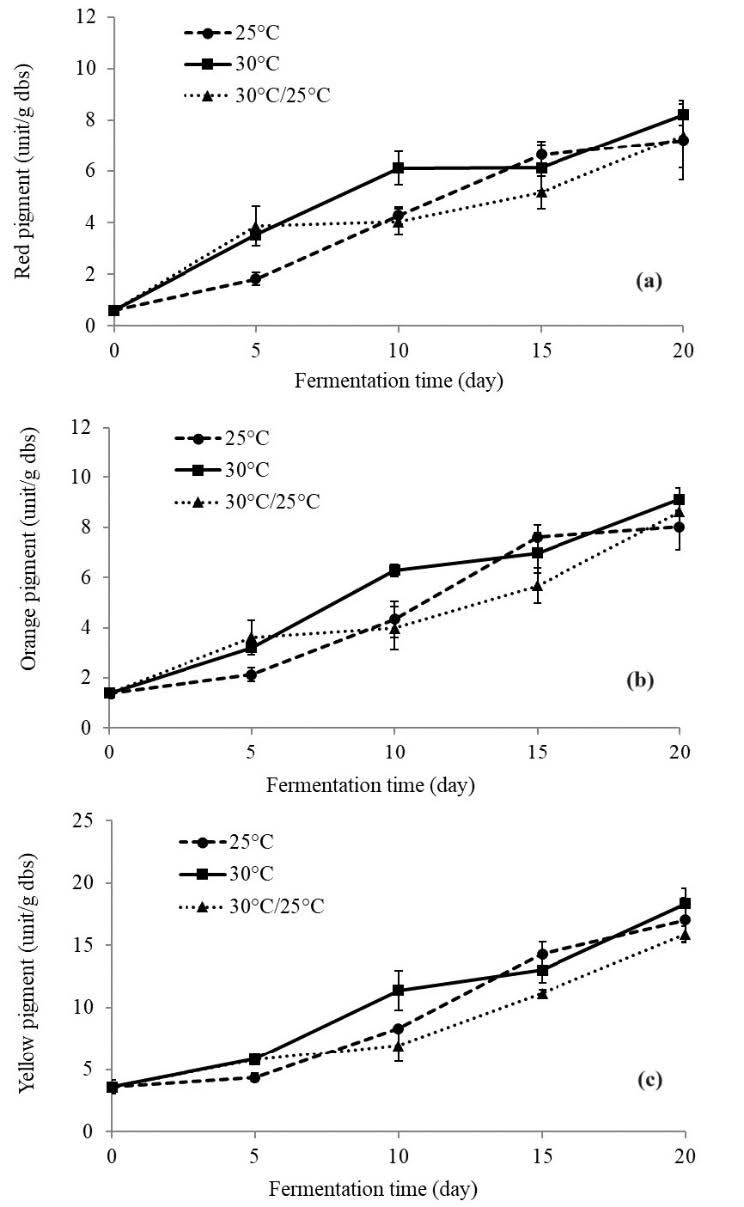
Figure 2. Pigments (red (a), orange (b), and yellow(c)) developed during the fermentation period obtained from different fermentation conditions. Data are expressed as the mean ± SD (n = 3).
In general, from Figure 2, it can be seen that pigments produced from the fermentation at all temperature conditions continuously increased throughout the fermentation periods. At 10 days of fermentation, the pigments were significantly different (P <0.05), fermentation at 30°C being the highest. Pigments produced from the fermentation at 25°C steadily increased during the first 5 days before dramatically increasing thereafter. Changing temperatures from 30°C to 25°C did not seem to produce significantly better results. After completed fermentation, the total pigment contents were found to be 32.27, 35.67, and 31.90 unit/g dry basis for the fermentation condition at 25°C, 30°C, and 30°C /25°C respectively. Monascus sp. PSRU03 produced more yellow pigment than the other colors, 50-53% proportion, corresponding to 6.94-14.31 unit/g dry basis. While orange color accounted for 25-27% proportion (8.02-9.11 unit/g dry basis) and red color accounted for 22-23% proportion (7.21-8.21 unit/g dry basis).
Antioxidant qualities of fermented soybeans
The total phenolic compounds of Monascus-fermented soybeans obtained from different fermentation conditions are shown in Table 1. Fermentation at 30°C for 20 days produced the highest total phenolic compounds (6.10 mg GAE/g dry basis) when compared to the other conditions. Fermentation time and temperature significantly affected total phenolic compounds after 15 days. In addition, after fermentation for 10 days, fermentation at 30°C significantly increased total phenolic compounds to 4.49 mg GAE/g dbs., which was higher than the other fermentation conditions.
Table 1. Total phenolic compounds of Monascus sp. PSRU03 fermented soybeans obtained from different fermentation conditions.
|
Fermentation time (days) |
Total phenolic compounds (mg GAE/g dbs.) |
||
|
25°C |
30°C |
30/25°C |
|
|
0 |
2.48 ± 0.29cA |
2.48 ± 0.29dA |
2.48 ± 0.29cA |
|
5 |
2.51 ± 0.26cA |
2.59 ± 0.33dA |
2.58 ± 0.27cA |
|
10 |
2.56 ± 0.31cB |
4.49 ± 0.39cA |
2.61 ± 0.21cB |
|
15 |
4.23 ± 0.05bB |
5.25 ± 0.06bA |
3.48 ± 0.07bC |
|
20 |
5.33 ± 0.25aB |
6.10 ± 0.36aA |
5.13 ± 0.07aB |
Note: Values are expressed as mean ± SD (n = 3). Means with letters (a-b) in a column without a common superscript letter differ (P<0.05). Means with letters (A-B) in a row without a common superscript letter differ (P<0.05). GAE, gallic acid equivalent; dbs, dry basis.
In terms of antioxidant capacity, as evidenced by DPPH radical-scavenging activity and ferric reducing antioxidant power (Tables 2-3), fermentation at 30°C generally exhibited better antioxidant capacity than those of the other conditions during the first 15 days. However, after completed fermentation, both DPPH radical-scavenging activity and ferric reducing antioxidant power were not significantly different (P≥0.05). Thus, the antioxidant capacities of Monascus sp. PSRU03 fermented soybeans were independent of the fermentation conditions.
Table 2. DPPH radical-scavenging activity of Monascus sp. PSRU03 fermented soybeans obtained from different fermentation conditions.
|
Fermentation time (days) |
DPPH radical-scavenging activity (%) |
||
|
25°C |
30°C |
30/25°C |
|
|
0 |
42.81 ± 1.39bcA |
42.81 ± 1.39dA |
42.81 ± 1.39cA |
|
5 |
41.39 ± 3.61cA |
44.01 ± 4.13cdA |
43.31 ± 3.02bcA |
|
10 |
45.14 ± 2.88bcB |
57.08 ± 3.75abA |
46.32 ± 0.20abcB |
|
15 |
47.80 ± 2.77bB |
58.78 ± 1.77aA |
47.63 ± 2.00abB |
|
20 |
50.59 ± 0.23aA |
50.76 ± 5.63bcA |
49.28 ± 3.14aA |
Note: Values are mean ± SD (n = 3). Means with letters (a-b) in a column without a common superscript letter differ (P<0.05). Means with letters (A-B) in a row without a common superscript letter differ (P<0.05).
Table 3. Ferric reducing antioxidant power of Monascus sp. PSRU03 fermented soybeans obtained from different fermentation conditions.
|
Fermentation time (days) |
Ferric reducing antioxidant power (mg trolox/g dbs.) |
||
|
25°C |
30°C |
30/25°C |
|
|
0 |
2.34 ± 0.37cA |
2.34 ± 0.37cA |
2.34 ± 0.37bA |
|
5 |
2.29 ± 0.28cA |
2.23 ± 0.14cA |
2.50 ± 0.32bA |
|
10 |
2.45 ± 0.19bcB |
3.70 ± 0.24abA |
2.49 ± 0.43bB |
|
15 |
2.86 ± 0.33abB |
3.91 ± 0.29aA |
2.58 ± 0.05bB |
|
20 |
3.22 ± 0.10aA |
3.36 ± 0.16bA |
3.21 ± 0.15aA |
Note: Values are mean ± SD (triplicate). Means with letters (a-b) in a column without a common superscript letter differ (P<0.05). Means with letters (A-B) in a row without a common superscript letter differ (P<0.05). dbs, dry basis.
In terms of DPPH radical-scavenging activity and ferric reducing antioxidant power, fermentation time and temperature significantly affected both antioxidant activities after 15 days. Similar to total phenolic compounds, after fermentation for 10 days, fermentation at 30°C significantly increased both antioxidant activities to 57.08% and 3.70 mg trolox/g dbs., respectively, which were higher than the other fermentation conditions.
DISCUSSION
It has been well accepted that most of the Monascus pigment products are rich in red pigment, especially in China. They are called “Monascus red rice” and “Monascus red pigment”. “Monascus red rice” is the product from solid-state fermentation in which rice is used as raw material and then inoculated with Monascus seed culture (Chairote et al., 2009; Srianta et al., 2014). Monascus red pigment produced by industrial scale fermentation is one of the most successful applications in China (Lian et al., 2015). In addition, the industrial scale production of Monascus will succeed with the help of Monascus improvement and intelligent fermentation control, promoting Monascus applications in food, cosmetic, agriculture, medicine, and environmental protection industries (Zhou et al., 2024).
Because of the good solubility of Monascus red pigment products in water or ethanol, it can be added in most foods, especially in meat, cakes, and drinks (Agboyibor et al., 2018). Among pigments, the red is of specific interest. This is because of its potential for therapeutic use particularly when produced in red rice, possessing antioxidant properties, immunosuppressive properties, teratogenicity, and antimicrobial, cytotoxic and antitumor activities (Lin et al., 2008). Orange azaphylones compounds which inhibit against human laryngeal carcinoma and human colon adenocarinoma, they again have antioxidant activity (Agboyibor et al., 2018).
It is highlighted here that although most of the Monascus pigments are rich in red pigments, which in fact are a mixture of red, yellow, and orange pigments with different proportions and the red one is the major composition in the existing Monascus pigments. Thailand scientists gained the Monascus strain which produced primarily yellow pigment by carrying out a study on the breeding of Monascus for producing yellow pigment (Srianta et al., 2014). China is also making progress in obtaining a high yield yellow pigment using conventional mutation techniques to obtain a Monascus mutant (Zhou et al., 2008).
It is anticipated that yellow pigment may have a wider application range than red pigment (Srianta et al., 2014). Water-soluble yellow pigments have been a popular area of research due to their high stability and convenient utilization (Qingqing et al., 2015). Notably that citrinin is also a yellow pigment. Hence, the specific components of yellow pigment should be carefully analyzed in the production of Monascus yellow pigment. This could be prevented by pH environment, whereas citrinin production was prevented by low pH below 3 (Kang et al., 2014). Monascus strains in Thailand i.e. Monascus sp. PSRU03 could be the promising one for use in the production of yellow pigments in which more research is required.
In this study, Monascus sp. PSRU03 fermented soybean for 20 days contained total phenolic compounds were in the rang 5.13 - 6.10 mg GAE/g dry basis. These results were found to be close to those fermented using M. pilosus BCRC31527 (6.05 mg/g) but lower than those fermented by M. purpureus BCRC31499 (8.50 mg/g) (Lee et al., 2008). Soybean cultivars could influence the production of phenolic compounds as a result of substrate compositions. Nevertheless, both Monascus species and soybean contain functional components, fermentation of a soybean matrix by Monascus species is a promising area of investigation (Lee et al., 2008).
Soybean fermentation has been used to develop specific foods and as a strategy for enriching soy-based products containing phenolic antioxidants, which are associated with consumer good health and well-being. The fermentation of soybeans by Monascus is a biotechnological strategy to produce fermented soybeans with more beneficial components (Lee et al., 2008; Lim et al., 2010). In the fermentation process by Monascus sp., antioxidant phenolic compounds are either produced via secondary metabolic pathways or released from the substrate by enzymes produced by the microorganisms, thus improving the phenolic content and antioxidant activity (Chairote et al., 2009; Dey et al., 2016). Many reports showed that soy isoflavone aglycones and Monascus sp. metabolite exhibited physiological activities (Lee et al., 2009). Promoting the use of soybeans as a functional substrate for Monascus sp. fermentation should be encouraged. In addition to the well-known red yeast rice, soybeans can be used as a functional substrate for Monascus fermentation. Future research should include exploring different strains of Monascus, testing additional fermentation conditions, using advanced analytical techniques to further investigate the mechanisms, and citrinin toxin production during fermentation.
CONCLUSION
These results reveal that Monascus sp. PSRU03 isolated from our study could be used to ferment soybeans that exhibited a higher production of yellow pigment than other colors. Yellow pigments may have a wider application range than red pigments due to their high stability and convenient utilization. The fermentation conditions employed in this study, which involved both consistent and cyclic temperatures, affected the total phenolic compounds but had no significant effect on antioxidant activities as evidenced by FRAP and DPPH assay. The fermentation at 30°C for 20 days is the potential condition that can be used to produce Monascus fermented soybeans with a high concentration of pigments and antioxidant quality. These findings could help optimize Monascus sp. fermentation conditions using soybeans as a substrate. However, red fermented substances can be contaminated with citrinin toxin during fermentation and this needs to be concerned.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Food and Agricultural Technology, Pibulsongkram Rajabhat University for providing instruments.
AUTHOR CONTRIBUTIONS
Concept and design: Utaiwan Chattong. Analysis and interpretation: Katekan Dajanta,Khongsak Srikaeo. Data collection: Sirikarn Thanaboonrongkom,Chutima Lertluksamee.Writing the article: Utaiwan Chattong, Khongsak Srikaeo, Katekan Dajanta. Critical revisionof the article: Utaiwan Chattong. Final approval of the article: all authors. Statistical analysis:Sirikarn Thanaboonrongkom, Katekan Dajanta, Khongsak Srikaeo. Obtained funding: UtaiwanChattong, Katekan Dajanta. Overall responsibility: Utaiwan Chattong.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Agboyibor, C., Kong, W.B., Chen, D., Zhang, A.M., and Niu, S.Q. 2018. Monascus pigments production, composition, bioactivity and its application: A review. Biocatalysis and Agricultural Biotechnology. 16: 433–447.
Chairote, E., Chairote, G., and Lumyong, S. 2009. Red yeast rice prepared from Thai glutinous rice and the antioxidant activities. Chiang Mai Journal of Science. 36(1): 42-49.
Chaisuwan, V., Dajanta, K., and Srikaeo, K. 2022. Effects of extraction methods on antioxidants and methoxyflavones of Kaempferia parviflora. Food Research. 6(3): 374-81.
Chattong, C., and Dajanta, K. 2014. The production of monacolin K, citrinin and pigments in noodle waste Angkak fermented by various Monascus strains. KKU Research Journal. 19(2): 215-222.
Chen, F., and Hu, X. 2005. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. International Journal of Food Microbiology. 103: 331-336.
Chen, W., Feng, Y., Molnár, I., and Chen, F. 2019. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Natural Product Reports. 36(4): 561–572.
Choe, D., Jung, H.H., Kim, D., Shin, C.S., Johnston, T.V., and Ku, S. 2020. In vivo evaluation of the anti-obesity effects of combinations of Monascus pigment derivatives. RSC Advances. 10(3): 1456–1462.
Choe, D., Song, S.M., Shin, C.S., Johnston, T.V., Ahn, H.J., Kim, D., and Ku, S. 2020. Production and characterization of anti-inflammatory Monascus pigment derivatives. Foods. 9(7): 858.
Dajanta, K., and Rongkam, H. 2016. Morphological characteristic, mycotoxin and pigment productions of Monascus sp. isolated from Angkak (red yeast rice). Srinakharinwirot University Journal of Science and Technology. 8(16): 13–26.
Dey, T.B., Chakraborty, S., Jain, K.K., Sharma, A., and Kuhad, R.C. 2016. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends in Food Science & Technology. 53: 60–74.
Johns, M.R., and Stuart, D.M. 1991. Production of pigments by Monascus purpureus in solid culture. Journal of Industrial Microbiology and Biotechnology. 8(1): 23–28.
Kang, B., Zhang, X., Wu, Z., Wang, Z., and Park, S. 2014. Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzyme and Microbial Technology. 55: 50–57.
Kraboun, K., Kongbangkerd, T., Rojsuntornkitti, K., and Phanumong, P. 2019. Factors and advances on fermentation of Monascus sp. for pigments and monacolin K production: A review. International Food Research Journal. 26(3): 751–761.
Lachenmeier, D.W., Monakhova, Y.B., Kuballa, T., Löbell-Behrends, S., Maixner, S., Kohl-Himmelseher, M., Waldner, A., and Steffen, C. 2012. NMR evaluation of total statin content and HMG-CoA reductase inhibition in red yeast rice (Monascus spp.) food supplements. Chinese Medicine. 7: 8.
Lee, Y.L., Yang, J.H., and Mau, J.L. 2008. Antioxidant properties of water extracts from Monascus fermented soybeans. Food Chemistry. 106(3): 1128–1137.
Lee, Y.L., Yang, J.H., and Mau, J.L. 2009. Antioxidant properties of ethanolic and methanolic extracts from Monascus-fermented soybeans. Journal of Food Biochemistry. 33(5): 707–727.
Lian, X., Liu, L., Dong, S., Wu, H., Zhao, J., and Han, Y. 2015. Two new Monascus red pigments produced by Shandong Zhonghui Food Company in China. European Food Research and Technology. 240(4): 719–724.
Lin, Y.L., Wang, T.H., Lee, M.H., and Su, N.W. 2008. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Applied Microbiology and Biotechnology. 77: 965-973.
Lim, J.Y., Kim, J.J., Lee, D.S., Kim, G.H., Shim, J.Y., Lee. I., and Imm, J.Y. 2010. Physicochemical characteristics and production of whole soymilk from Monascus fermented soybeans. Food Chemistry. 120(1): 255–260.
Maier, T., Schieber, A., Kammerer, D.R., and Carle, R. 2009. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chemistry. 112(3): 551-559.
Nimnoi, P., Pongsilp, N., and Lumyong, S. 2015. Utilization of agro-industrial products for increasing red pigment production of Monascus purpureus AHK12. Chiang Mai Journal of Science. 42(2): 331-338.
Pyo, Y., and Lee, T. 2007. The potential antioxidant capacity and angiotensin I‐converting enzyme inhibitory activity of Monascus‐fermented soybean extracts: evaluation of Monascus‐fermented soybean extracts as multifunctional food additives. Journal of Food Science. 72: S218–S223.
Qingqing, Z., Di, Z., Wenjing, T., and Chao, Y. 2015. Photostability of water-soluble and alcohol-soluble Monascus pigments. Food Science. 36: 94–98.
Sahasakul, Y., Aursalung, A., Thangsiri, S., Wongchang, P., Sangkasa-Ad, P., Wongpia, A., Polpanit, A., Inthachat, W., Temviriyanukul, P., and Suttisansanee, U. 2022. Nutritional compositions, phenolic contents, and antioxidant potentials of ten original lineage beans in Thailand. Foods. 11(14): 2062.
Shi, Y.C., and Pan, T.M. 2010. Anti-diabetic effects of Monascus purpureus NTU 568 fermented products on streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 58: 7634–7640.
Srianta, I., Kusdiyantini, E., Zubaidah, E., Ristiarini, S., Nugerahani, I., Alvin, A., Iswanto, N., and Zhang, B.B. 2021. Utilization of agro-industrial by-products in Monascus fermentation: A review. Bioresources and Bioprocessing. 8(1): 129.
Srianta, I., Ristiarini, S., Nugerahani, I., Sen, S.K., Zhang, B.B., Xu, G.R., and Blanc, P.J. 2014. Recent research and development of Monascus fermentation products. International Food Research Journal. 21: 1–12.
Srianta, I., Zubaidah, E., Estiasih, T., and Yamada, M. 2016. Comparison of Monascus purpureus growth, pigment production and composition on different cereal substrates with solid state fermentation. Biocatalysis and Agricultural Biotechnology. 7: 181-186.
Subsaendee, T., Kitpreechavanich, V., and Yongsmith, B. 2014. Growth, glucoamylase, pigments and monacolin K production on rice solid culture in flask and koji chamber using Monascus sp. KB9. Chiang Mai Journal of Science. 41(5.1): 1044-1057.
Suraiya, S., Siddique, M.P., Lee, J.M., Kim, E.Y., Kim, J.M., and Kong, I.S. 2017. Enhancement and characterization of natural pigments produced by Monascus spp. using Saccharina japonica as fermentation substrate. Journal of Applied Phycology. 30: 729-742.
Tsukahara, M., Shinzato, N., Tamaki, Y., Namihira, T., and Matsui, T. 2009. Red yeast rice fermentation by selected Monascus sp. with deep-red color, lovastatin production but no citrinin, and effect of temperature shift cultivation on lovastatin production. Applied Biochemistry and Biotechnology. 158(2): 476-482.
Vendruscolo, F., Tosin, I., Giachini, A.J., Schmidell, W., and Ninow, J.L. 2014. Antimicrobial activity of Monascus pigments produced in submerged fermentation. Journal of Food Processing and Preservation. 38(4): 1860-1865.
Yang, T., Liu, J., Luo, F., Lin, Q., Rosol, T.J., and Deng, X. 2014. Anticancer properties of Monascus metabolites. Anti-Cancer Drugs. 25(7): 735-744.
Zhou, B., Pu, Y.W., Zhu, M.J., and Liang, S.Z. 2008. Effects of nitrogen sources on Monascus yellow pigment production by Monascus mutant. Modern Food Science & Technology. 24(2): 123–127.
Zhou, J., Pan, Q., Xue, Y., Dong, Y., Chen, Y., Huang, L., Zhang, B., Liu, Z.Q., and Zheng, Y. 2024. Synthetic biology for Monascus: From strain breeding to industrial production. Biotechnology Journal. 19(7): 2400180.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Utaiwan Chattong*, Khongsak Srikaeo, Katekan Dajanta, Sirikarn Thanaboonrongkom, and Chutima Lertluksamee
Division of Food Science and Technology, Faculty of Food and Agricultural Technology, Pibulsongkram Rajabhat University, Phitsanulok 65000, Thailand.
Corresponding author: Utaiwan Chattong, E-mail: Utaiwan.c@psru.ac.th
ORCID:
Utaiwan Chattong: https://orcid.org/0009-0009-7826-0375
Khongsak Srikaeo: https://orcid.org/0000-0003-4621-1690
Katekan Dajanta: https://orcid.org/0009-0006-9368-1256
Sirikarn Thanaboonrongkom: https://orcid.org/0009-0005-5391-2665
Chutima Lertluksamee: https://orcid.org/0009-0006-0657-0815
Total Article Views
Editor: Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: May 27, 2024;
Revised: September 25, 2024;
Accepted: October 1, 2024;
Online First: November 15, 2024

