
Cytotoxic, Cell Apoptosis, Colony Formation and Anti-Migratory Activity of Three Herbal Plant Extracts in MCF-7 Breast Cancer Cells
Supavadee Boontha* , Benjaporn Buranrat, Kamchai Saepang, Prapapan Temkitthawon and Tasana PitaksuteepongPublished Date : November 14, 2024
DOI : https://doi.org/10.12982/NLSC.2025.004
Journal Issues : Number 1, January-March 2025
Abstract Herbal medicine has emerged as a safe and readily accessible reservoir of compounds for the treatment of cancer. This work focused on the anticancer activity of three herbal plant extracts in MCF-7 breast cancer cells. Andrographis paniculata (Burm. F.) Wall. ex Nees (AP), Momordica charantia L (MC) and Peperomia pellucida (L.) Kunth (PP) were extracted with 95% ethanol using the maceration method. Their impacts on biological processes, including cytotoxicity, cell apoptosis, colony formation, the generation of reactive oxygen species (ROS) and cell migration, were assessed using sulforhodamine B (SRB), dye staining with mixtures of acridine orange/ethidium bromide (AO/EB), a clonogenic method, flow cytometry using a DCF-DA fluorescent probe and a scratch wound healing method, respectively. The study revealed that the AP, MC and PP extracts exerted anticancer activity in MCF-7 cells through the induction of cytotoxicity, cell apoptosis and ROS levels and the suppression of cell growth and migration. All extracts displayed cytotoxic effects and triggered dose-dependent apoptosis in MCF-7 cells at concentrations ranging from 0 to 500 μg/mL and 0 to 250 μg/mL, respectively. Moreover, they showed dose-dependent anti-migratory activity in MCF-7 cells at concentrations ranging from 0 to 100 μg/mL. Compared to the MC and PP extracts, the extract of AP exhibited the strongest anticancer properties against MCF-7 breast cancer cells, suggesting its potential utility as an anticancer agent to both prevent and treat breast cancer.
Keywords: Andrographis paniculata (Burm. F.) Wall. ex Nees, Cell apoptosis, Cell migration, Colony formation, Momordica charantia L, Peperomia pellucida (L.) Kunth
Funding: This research was supported by the University of Phayao and the Thailand Science Research and Innovation Fund (Fundamental Fund 2024).
Citation: Boontha, S., Buranrat, B., Saepang, K., Temkitthawon, P., and Pitaksuteepong, T. 2025. Cytotoxic, cell apoptosis, colony formation and anti-migratory activity of three herbal plant extracts in MCF-7 breast cancer cells. Natural and Life Sciences Communications. 24(1): e2025004.
INTRODUCTION
Breast cancer is the most frequently diagnosed cancer worldwide and the leading cause of cancer-related deaths among women (Wilkinson and Gathani, 2022; Xu et al., 2023). As of 2023, the American Cancer Society has reported that approximately one in eight women (13%) will receive a diagnosis of breast cancer at some point in their lives. Globally, it is projected that there will be approximately 2.9 million new cases of female breast cancer by 2040, reflecting a 31% increase from the corresponding 2.2 million cases reported in 2020 (Arnold et al., 2022; Siegel et al., 2024). The predominant cause of mortality in breast cancer cases is often the metastasis of the primary cancer to various organ sites, including the brain, bones, liver, lungs and lymph nodes (Jin et al., 2018; Ibragimova et al., 2023). The emergence of medication resistance in cancer cells and the adverse side events associated with chemotherapy are the primary factors contributing to treatment failure in cancer management (Jin et al., 2018; Ibragimova et al., 2023). In response to the shortcomings of conventional chemotherapy, certain cancer patients turn to complementary or alternative treatments derived from various plants or nutrients (Jin et al., 2018; Wode et al., 2019). Medicinal-plant-based cancer therapies are known for their effectiveness, cost-effectiveness and minimal adverse effects. The exploration of potential anticancer agents among fruits, vegetables, herbs and spices has been prompted by the identification of medicinal properties in food plants and the demand for innovative approaches to cancer therapy (Jin et al., 2018; Tungmunnithum et al., 2018; Wode et al., 2019; Kopustinskiene et al., 2020). Herbal plants that are rich in antioxidants have garnered significant attention as promising resources for a wide range of complementary cancer treatments. Phenolic compounds, along with flavonoids, are widely recognized for their antioxidant properties and numerous other essential bioactive effects (Tungmunnithum et al., 2018; Kopustinskiene et al., 2020). They have garnered significant interest over time due to their potential health benefits in curing and preventing various diseases. Phenolic compounds, particularly flavonoids, have a well-established history as chemopreventive agents in cancer treatment (Pandey and Rizvi, 2009; Amawi et al., 2017). In general, the chemotherapeutic effects of herbal plant extracts are evaluated based on various parameters, including cell toxicity, the inhibition of colony formation, the production of reactive oxygen species (ROS) and the suppression of cell migration in MCF-7 cells. Commonly employed methods to assess these effects include the SRB method for cytotoxicity, the clonogenic assay for replicative ability and the wound scratch healing assay for metastasis evaluation (Menyhárt et al., 2016; Orellana et al., 2016; Wang et al., 2019). These assays provide valuable insights into the potential anticancer properties of herbal extracts.
Andrographis paniculata (Burm. F.) Wall. ex Nees (AP) belongs to the family of Acanthaceae. AP is a highly valued and extensively researched medicinal plant. For several centuries, the inhabitants of Southeast Asian nations such as India, China and Thailand have employed it as a traditional remedy to address a diverse range of health conditions, including boils, skin ailments, gonorrhea, wounds, malaria and jaundice. Numerous pharmacological investigations have showcased the medicinal potential of AP in managing conditions such as ulcers, diabetes, influenza, inflammation, cancer and hypertension (Hossain et al., 2021; Tohkayomatee et al., 2022). Momordica charantia L (MC), or bitter gourd, is a member of the Cucurbitaceae family and is used in traditional medicine and as a vegetable. The potential of the phytochemical fractions and chemicals isolated from MC to cure common conditions such as diabetes mellitus, cancer, heart disease, hypoglycemia, bacterial infections and viral infections has been investigated (Jia et al., 2017; Kim et al., 2018). Peperomia pellucida (L.) Kunth (PP) belongs to the family of Piperaceae. The leaves and stems are consumed as a vegetable. Serving as an ethnomedicinal plant, it has been employed to alleviate various health conditions, including abdominal pain, abscesses, acne, boils, colic, fatigue, gout, headache, renal disorders and rheumatic joint pain. Additionally, it has been utilized in the treatment of breast cancer, smallpox, mental disorders, measles and impotence (Teoh et al., 2021; Gomes et al., 2022; Ahmad et al., 2023).
While AP and MC are popularly utilized herbal remedies in Thailand (Jia et al., 2017; Kim et al., 2018; Siridechakorn et al., 2023), PP is frequently encountered across various Asian countries. Local communities claim that decoctions of this plant are beneficial in treating bone aches and pain (Ahmad et al., 2023). PP enjoys significant popularity as a local Thai vegetable, particularly in northeastern Thailand (Thanh Men et al., 2022). The cytotoxicity of the extracts of AP, MC and PP has been investigated in various cancer cells. Nevertheless, the scientific rigor of these methodologies is frequently constrained, emphasizing the necessity of further investigation for the robust scientific validation of traditional medicinal approaches, including the utilization of AP, MC and PP extracts. Thus, this investigation aimed to assess the cytotoxicity, cell death induction, colony formation, ROS production and cell migration suppression of extracts obtained from three Thai herbal plants, namely Andrographis paniculata (Burm. F) Wall. ex Nees, Momordica charantia L and Peperomia pellucida (L.) Kunth, in breast MCF-7 cancer cells.
MATERIALS AND METHODS
Materials
The chemicals and solvents were obtained from TTK Science (Bangkok, Thailand). Analytical-grade solvents were used for plant extraction; total phenolics and total flavonoids analysis; and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test. The components of the Gibco® cell culture medium were procured from Thermo Fisher Scientific, Inc. (Waltham, MA, USA), while all chemical compounds and solvents were sourced from Sigma Aldrich (Merck KGaA, Darmstadt, Germany).
Preparation of plant extracts
The specimens of Andrographis paniculata (Burm. F.) Wall. ex Nees (AP), Momordica charantia L (MC) and Peperomia pellucida (L.) Kunth (PP) were identified and stored at the Applied Thai Traditional Medicine Department, Faculty of Medicine, Mahasarakham University (specimen voucher no. 7391, 7392 and 7393), as well as at the Faculty of Science, Mahasarakham University, Thailand. The extraction process utilized the fresh leaves of AP and MC and the fresh leaves and stems of PP. The plant samples were cleaned with tap water, chopped and air-dried. Subsequently, they underwent further drying at 50°C in an oven (Memmert, Schwabach, Germany) for two days. The obtained plant material was pulverized into a powder. Subsequently, the dried plant powders underwent maceration with 95% ethanol. Following the filtration process, the filtrate was subjected to concentration using a rotary evaporator (Heidolph, Germany) at a regulated temperature of 50°C. By comparing the dried weight (g) of the crude extract with that of the plant powder, the percentage yield of the extract was computed.
Phytochemical content
The phytochemical compositions of the three herbal extracts were analyzed, evaluating particular chemical reactions indicative of secondary metabolites such as alkaloids, anthraquinones, cyanogenic glycosides, flavonoids, saponins, and tannins, as previously described (Om, et al., 2012; Dubale, et al., 2023). Positive outcomes in the screening assays were discerned through the color intensity or the formation of precipitates.
Quantification of phenolic and flavonoid content
Phenolic content
To assess the extracts’ phenolic content, a solution containing 80 µL of Folin–Ciocalteu reagent was combined with 40 µL of the extract solution (200 µg/mL). Following a 5-minute incubation period, 80 µL of 7% sodium carbonate (Na2CO3) was introduced to the mixture, followed by an additional 30-minute incubation period. Subsequently, a microplate reader (Synergy H1, Biotek Instruments, Friedrichshall, Germany) was utilized to measure the absorbance at 750 nm. The phenolic content was expressed as the gallic acid equivalent (GAE) in grams of the crude extract and was calculated using the gallic acid standard curve (20–100 µg/mL).
Flavonoid content
A mixture comprising 100 µL of a 2% AlCl3 solution and the extract solution (100 µL of 100 µg/mL) was employed to assess the flavonoid concentrations of the extracts. After a 10-minute incubation period, the absorbance was determined at 415 nm. The flavonoid concentration was quantified using the rutin (RE) equivalent in grams of the crude extract, determined by the rutin standard curve (20–100 µg/mL).
DPPH assay
In a 96-well plate, 100 µL of 5.0 mM DPPH was mixed separately with each extract solution (100 µL of 0.05–2.0 mg/mL). After a 20-minute incubation period, the absorbance of both the sample (A540sample) and the blank (A540blank, without the test sample) was recorded at 540 nm. The positive control used was gallic acid. This method facilitated the evaluation of the DPPH radical scavenging activity (S).
S (%) = ((A540blank – A540sample))/A540blank) x 100
Cytotoxic method
The extracts’ effects on cancer cell viability were evaluated using the sulforhodamine B (SRB) method, as previously described (Boontha et al., 2018). MCF-7 cells were exposed to different concentrations of the extract (ranging from 0 to 500 µg/mL) for 24 to 48 hours. Subsequently, the dose–response curve was utilized to determine the 50% inhibitory concentration (IC50), and the viability of MCF-7 cells was expressed as a percentage relative to the untreated control group.
Cell apoptosis assay
The impact of the extracts derived from AP, MC and PP on MCF-7 cells’ apoptosis were analyzed through AO/EB staining. Initially, the cells were treated with different concentrations of the extract (ranging from 0 to 250 μg/mL) for one complete day. Subsequently, staining was conducted using a dye solution comprising AO and EB, separately, at a concentration of 1 μg/mL, for 15 minutes at room temperature. Subsequently, photographic images were acquired with fluorescent inverted microscopy.
Clonogenic assay
Initially, 500 viable cells were seeded into a 6-well plate and allowed to culture for 24 hours to assess the extracts’ inhibitory effects on colony formation. Subsequently, various concentrations of the extract (100 µL) were added, and the mixture was further incubated for 24 hours. Following this, a fresh culture medium was introduced, and the cells were washed. The cells were then maintained in the culture for a total of ten days, with a two-day interval between the changes in the cell culture media. Ultimately, following the staining of proliferating cells with methanol containing 0.5% crystal violet, the number of colonies was determined through direct enumeration.
ROS formation assay
The extracts’ effects on ROS formation were measured utilizing the fluorescent probe 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA). The cells were treated with the extract at concentrations ranging from 50 to 250 μg/mL, in addition to 25 μM DCF-DA, and incubated in the dark for 30 minutes. Afterward, the cells were washed with PBS. The ROS levels were subsequently assessed via flow cytometry (BD Biosciences, CA, USA). An increase in ROS levels is indicated by a shift to the right in the fluorescence spectrum.
Anti-migratory assay
Initially, the cells were seeded into a 24-well culture plate and left to incubate for 24 hours at 37°C. Subsequently, a straight scratch was created in each well by gently scraping the cells with a sterile pipette tip. Next, the extract solution (0–100 µg/mL, 100 µL) was applied and the mixture was allowed to incubate for 48 hours. After the incubation period, the area of the exposed region of the scratch was measured using an inverted microscope (TS100, Nikon, Japan) at a magnification level of x10. The relative closure percentage of the scratch, which indicated the anti-migratory effect of the extract, was determined using area data calculations.
Data analysis
The data were presented as the mean ± standard deviation (SD). Statistical analysis was conducted using the Sigma Stat software version 3.5 (Systat Software Inc., San Jose, CA, USA), with a post hoc LSD test performed following a one-way Analysis of Variance (ANOVA). A P-value less than 0.05 was considered statistically significant.
RESULTS
Phytochemical profiles of the extracts
Table 1 presents the phytochemical constituents of the extracts from the three herbal plants. The findings revealed that flavonoids were present in all extracts, while alkaloids were detected in the extracts of AP and PP.
Table 1. Phytochemical screening results for extracts of three herbal plants.
|
Constituents |
Test |
AP |
MC |
PP |
|
Alkaloids |
Dragendorff |
+ |
- |
+ |
|
Anthraquinones |
Brontragen |
- |
- |
- |
|
Cyanogenic glycosides |
Grignard |
- |
- |
- |
|
Flavonoids |
Shinoda |
+ |
+ |
+ |
|
Saponins |
Frothing |
- |
- |
- |
|
Tannins |
Gelatin Ferric chloride |
- |
- |
- |
Note: + = Positive; - = Negative AP: Andrographis paniculata (Burm. F.) Wall. ex Nees; MC: Momordica charantia L; PP: Peperomia pellucida (L.) Kunth.
Yields, phenolic and flavonoid content and radical scavenging ability of extracts
The yields, phenolic and flavonoid content and radical scavenging ability of the extracts of AP, MC and PP are illustrated in Table 2.The AP extract exhibited the highest percentage yield, followed by the extracts from MC and PP. Regarding the determination of the phenolic content, the extracts of the three herbal plants yielded values within the range of 80 -100 mg GAE/g. There was no significant difference in the total phenolic content among the extracts obtained from AP, MC and PP. Regarding the determination of the flavonoid content, the extracts of the three herbal plants yielded values within the range of 140- 340 mg RE/g. Compared to the extracts of AP and MC, the PP extract showed the highest flavonoid content and the most potent antioxidant activity as it had the lowest IC50 value.
Table 2. Percentage yield, phenolic content, flavonoid content and radical scavenging activity of extracts.
|
Sample |
Yield (%w/w) |
Phenolic content (mg GAE/g) |
Flavonoids content (mg RE/g) |
IC50 of DPPH radical scavenging activity |
|
AP |
8.55 |
98.20 ± 19.50 |
141.40 ± 42.60 |
1.20 ± 0.10 mg/mL |
|
MC |
5.03 |
78.50 ± 12.10 |
205.60 ± 16.70 |
1.20 ± 0.30 mg/mL |
|
PP |
3.56 |
97.30 ± 28.20 |
335.20 ± 49.40 |
570.40 ± 53.30 µg/mL |
|
Gallic acid |
- |
- |
- |
8.10 ± 0.40 µg/mL |
Note: AP: Andrographis paniculata (Burm. F.) Wall. ex Nees; MC: Momordica charantia L; PP: Peperomia pellucida (L.) Kunth. The abbreviations "GAE/g" and "RE/g" denote the amount of gallic acid (GAE) and rutin (RE) equivalents, respectively, per gram of crude extract.
Cytotoxicity of extracts in MCF-7 cells
The cytotoxic effect of the extracts on MCF-7 cancer cells was assessed using the SRB method. The results indicated a rise in the level of MCF-7 cell death when treated with a larger extract concentration and an elevated incubation period (Figure 1A). At 24 h, the IC50 values of the extracts from AP, MC and PP were 119.5 ± 7.3, 59.2 ± 4.5 and 139.3 ± 11.3 µg/mL. At 48 h, the IC50 values of the extracts from AP, MC and PP were 27.2 ± 0.7, 28.8 ± 2.8 and 46.0 ± 2.1 µg/mL. The extracts from AP and MC were much more cytotoxic than the extracts from PP.
The apoptosis-inducing potential of the three extracts in MCF-7 cells was assessed via staining with dye mixtures containing AO/EB. AO produces bright green dots, indicating early apoptosis characterized by nuclear fragmentation and chromatin condensation. Conversely, an orange–red color signifies late-stage apoptosis or necrosis, indicative of cell membrane damage (Atale et al., 2014; Chittasupho et al., 2023). As depicted in Figure 1B, at concentrations ranging from 50 to 100 µg/mL, all extracts elicited cell death in a dose-dependent manner. Nevertheless, when administered at high concentrations (250 µg/mL), the AP, MC and PP extracts resulted in a reduction in the observed cell count, attributed to an abnormal cell population. In summary, this experiment revealed the cytotoxic effects of all extracts, resulting in the apoptosis of MCF-7 cells.
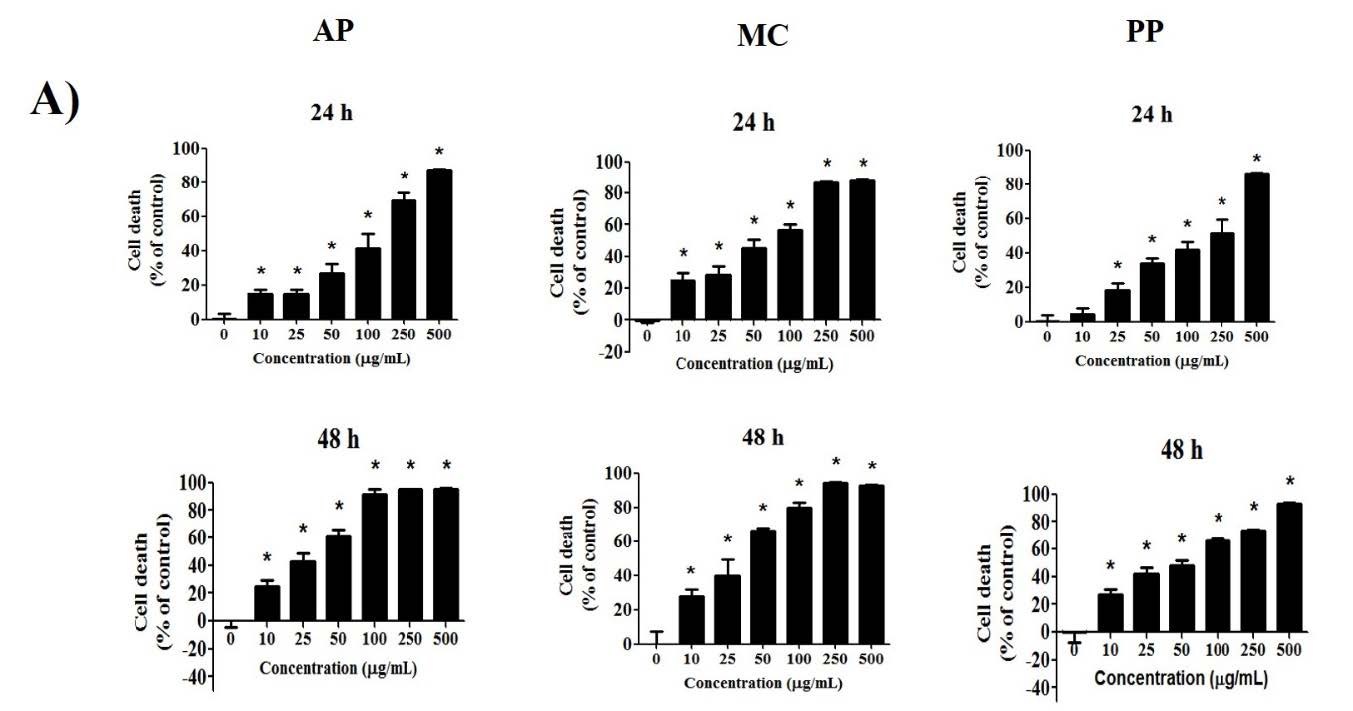
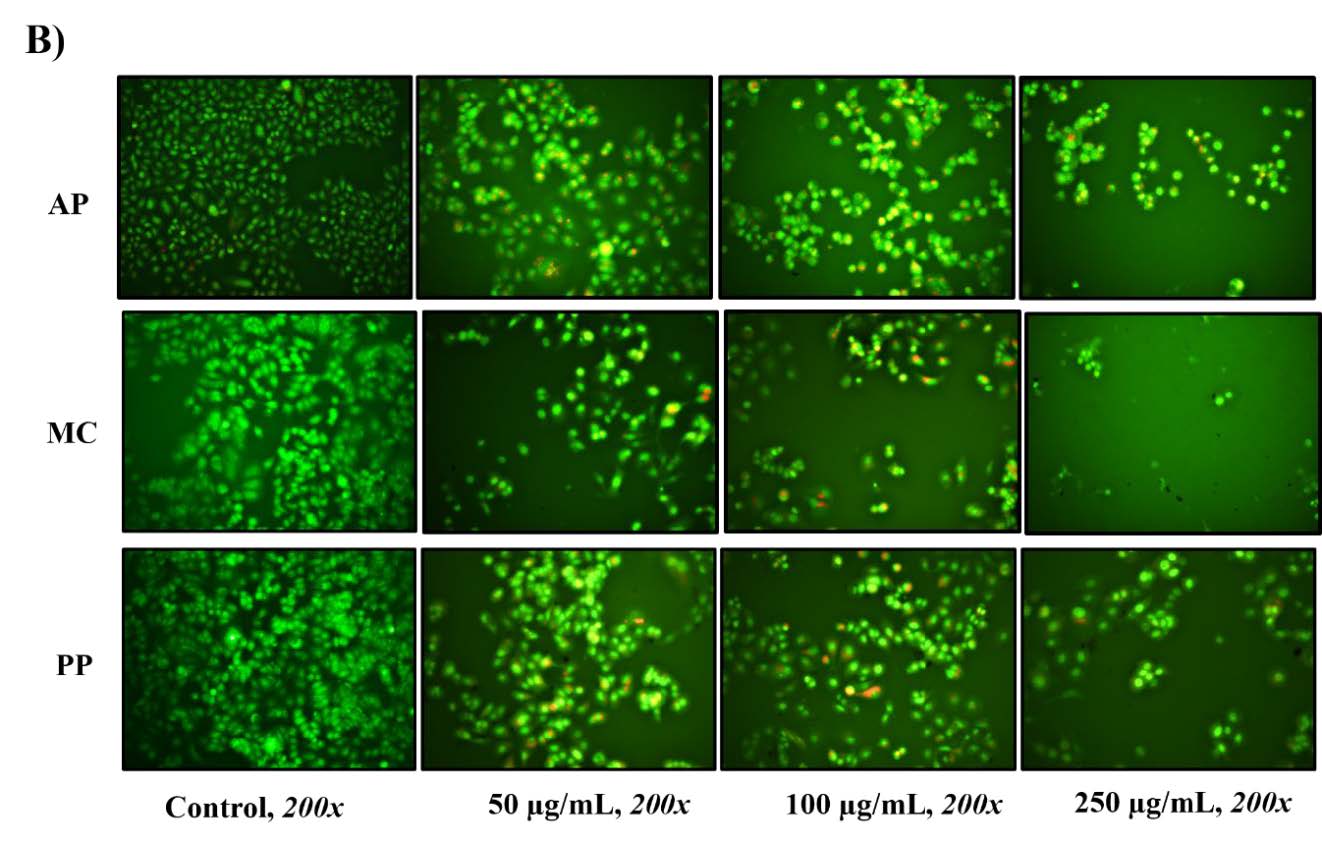
Figure 1. Cytotoxicity of Andrographis paniculata (Burm. F.) Wall. ex Nees (AP), Momordica charantia L (MC) and Peperomia pellucida (L.) Kunth (PP) extracts in terms of cell death was evaluated at 24 h and 48 h using the SRB method (A), and cell apoptosis was assessed using a mixture of AO/EB dye staining (B) in MCF-7 breast cancer cells. Differences from the control are marked as * (P < 0.05) to denote significance.
Clonogenic inhibition effects of extracts
The clonogenic inhibition effects of the extracts in MCF-7 cells were evaluated using the clonogenic assay. The three extracts could inhibit colony formation at low concentrations. The findings demonstrated that treatment with the extracts inhibited the colony formation of MCF-7 cells in a dose-dependent manner (Figure 2). All extracts exhibited the significant inhibition of MCF-7 cell colony formation at a concentration of 50 µg/mL. The AP, MC and PP extracts exhibited IC50 values of 78.1 ± 0.3, 48.0 ± 1.5 and 124.4 ± 9.0 µg/mL, respectively. The findings revealed that the extract derived from MC had the greatest potential to inhibit MCF-7 cell colony formation, followed by the extracts from AP and PP.
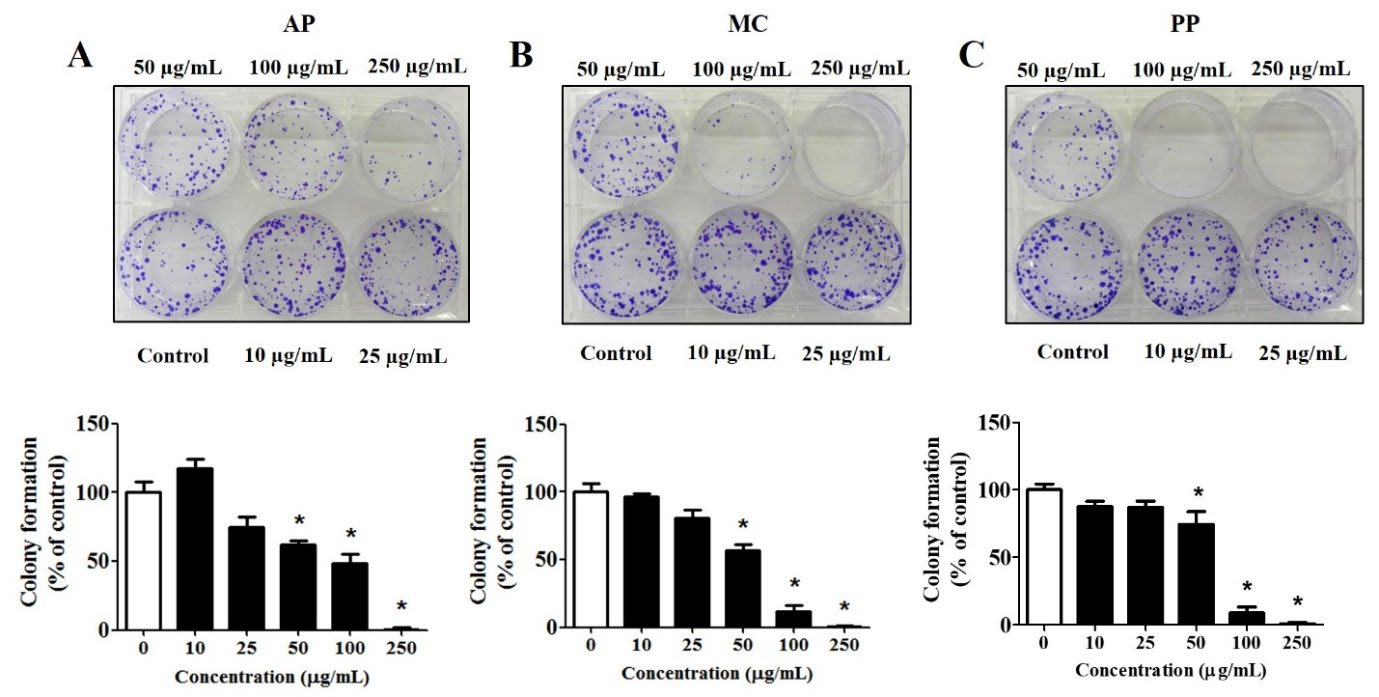
Figure 2. Clonogenic inhibition effects of the extracts from (A) Andrographis paniculata (Burm. F.) Wall. ex Nees (AP), (B) Momordica charantia L (MC) and (C) Peperomia pellucida (L.) Kunth (PP) on MCF-7 breast cancer cells. The cells were exposed to extract concentrations ranging from 0 to 250 µg/mL for 24 hours, cultured for 10 days and subsequently stained with crystal violet before being photographed. The results are presented as the percentage of colony formation relative to the non-treated cells (control); *P < 0.05 indicates statistical significance compared with the non-treated cells (control).
ROS production effects of extracts
In the ROS production evaluation, the fluorescence of DCF-DA was tracked, with a rightward shift indicating heightened levels of ROS. As depicted in Figure 3, only the MC extract exhibited ROS production when compared to the AP and PP extracts. Moreover, the MC extract exhibited dose-dependent ROS production at concentrations ranging from 50 to 100 μg/mL. After calculating the percentage of ROS production, it was determined that the levels induced by the MC extract were 7.8 % and 8.1 %, respectively, at concentrations of 50 and 100 µg/mL. Nevertheless, at a higher concentration (250 µg/mL), the ROS levels were not detectable in MCF-7 cells treated with the MC extract. This anomaly could be attributed to the abnormal cell population obtained, which might have been incapable of generating ROS.
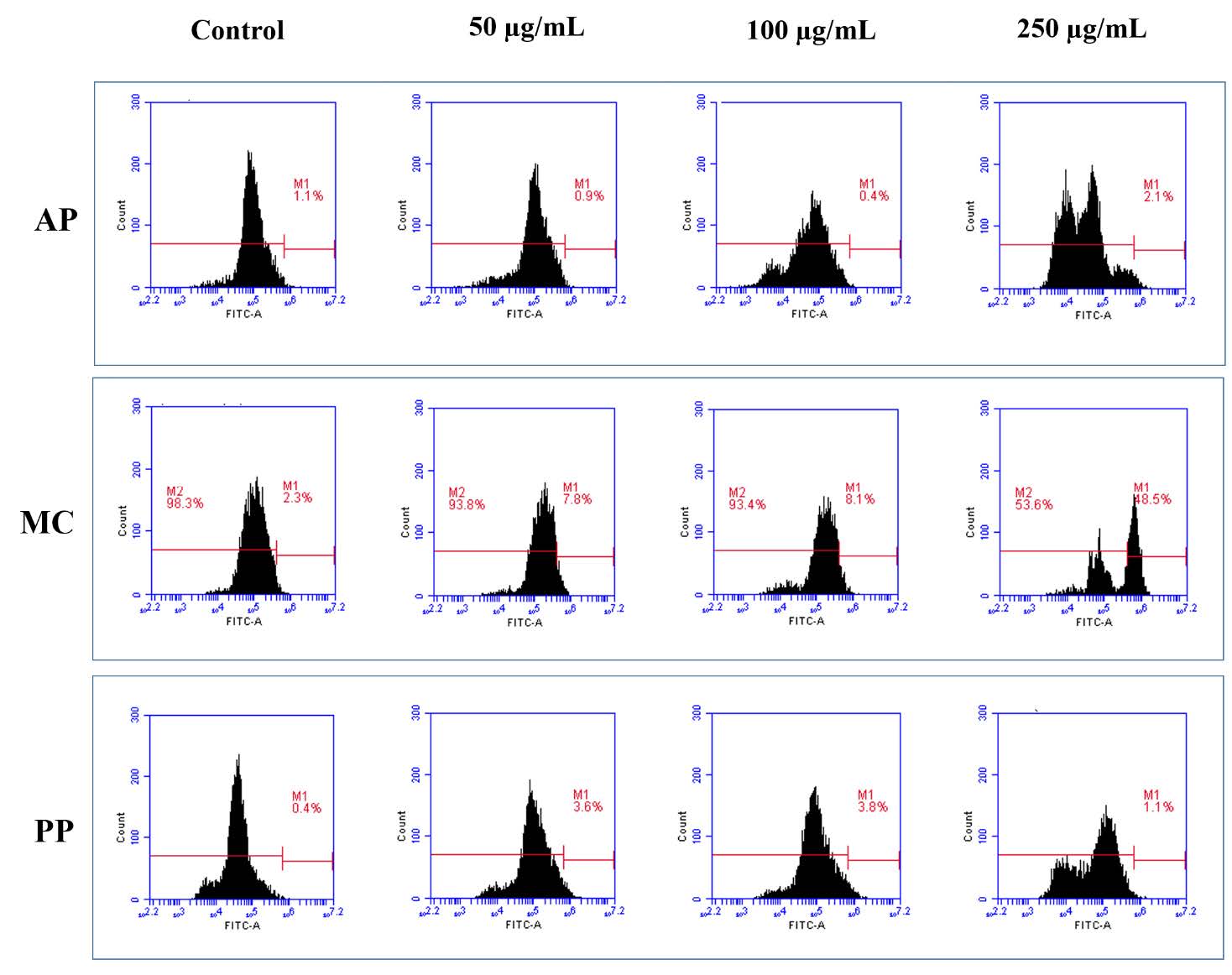
Figure 3. Reactive oxygen species (ROS) generation following the treatment of MCF-7 breast cancer cells with extracts derived from Andrographis paniculata (Burm. F.) Wall. ex Nees (AP), Momordica charantia L (MC) and Peperomia pellucida (L.) Kunth (PP).
Anti-migratory effects of extracts
The wound healing assay was employed to quantify the extent of MCF-7 cell migration inhibition caused by the extracts. A notable reduction in MCF-7 cell migration was noted with the AP extract at a concentration of 25 µg/mL (Figure 4A) and with the MC and PP extracts at a concentration of 100 µg/mL (Figure 4B and 4C). The IC50 values of the extracts from AP, MC and PP were 16.4 ± 0.8, 98.6 ± 7.5 and 129.2 ± 4.0 µg/mL, respectively. The results indicated that the AP extract exhibited the highest efficacy in suppressing MCF-7 cell migration, followed by the extracts from MC and PP.
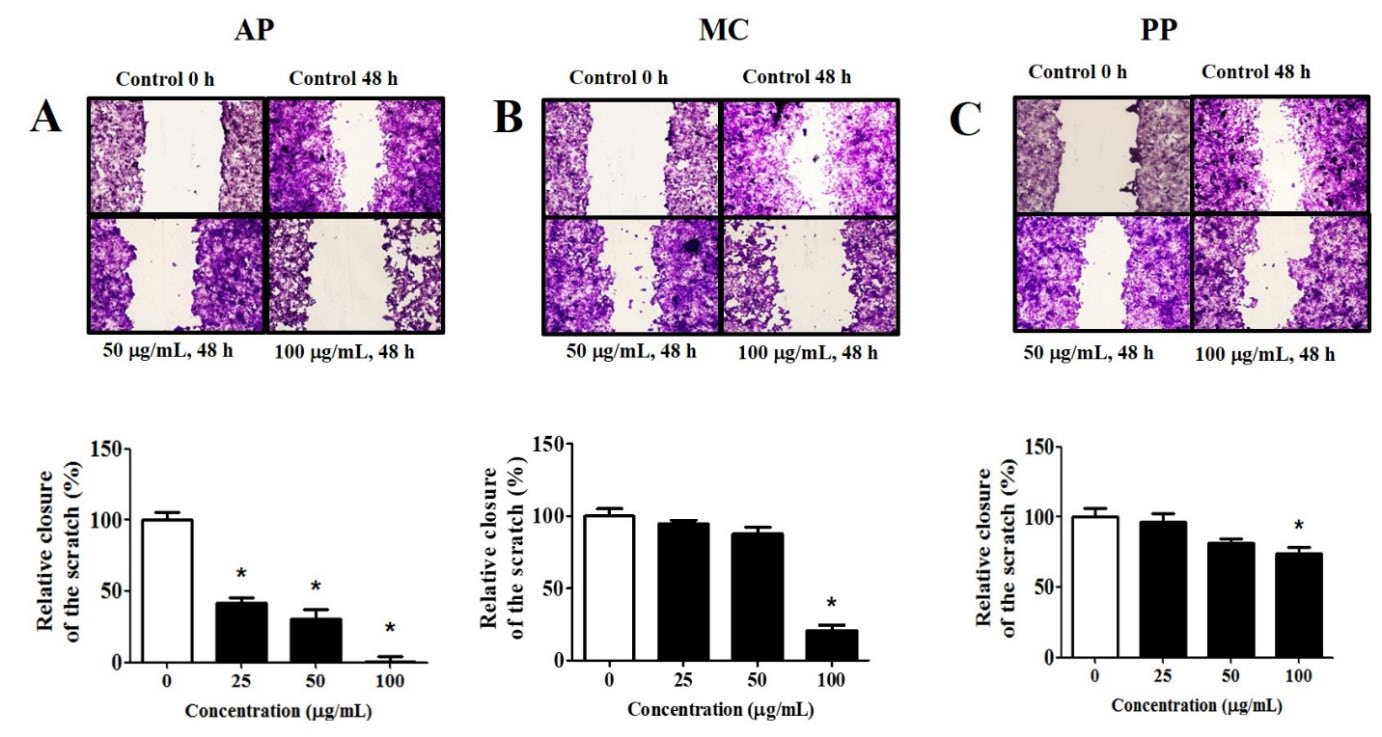
Figure 4. Anti-migratory effects of the extracts on MCF-7 cancer cells. The cells were treated with the extracts (0 – 100 µg/mL) for 48 hours and migration was observed using inverted microscopy (magnification x10). All findings are presented as percentages relative to the control groups, derived from three independent experiments and expressed as mean ± SEM values; * indicates statistical significance at P < 0.05 compared to the control.
DISCUSSION
The growing interest in exploring natural remedies for cancer has spurred investigations into various plant sources. In this study, extracts from AP, MC and PP were assessed to determine their potential as antioxidants and their anticancer effects. To this end, we conducted phytochemical screening and examined the levels of total phenolics, total flavonoids and antioxidant capacity using ethanol extracts derived from the aforementioned plants. In addition, this work studied the anticancer activity of the extracts derived from AP, MC and PP via cell toxicity, cell apoptosis, cell proliferation, ROS production and cell migration experiments on MCF-7 cells. In Thailand, AP and MC are commonly used herbal remedies, whereas PP is highly favored as a local Thai vegetable, especially in the northeastern region of the country. This was the first study examining the relative anticancer effects of extracts obtained from AP, MC and PP. Moreover, the impacts of all extracts on cell apoptosis, colony formation and ROS formation in MCF-7 breast cancer cells were explored. An essential element of anticancer activity is the inhibition of cell migration or the minimization of metastasis. Consequently, it is imperative to assess extracts' anti-migratory effects in terms of inhibiting cancer cell metastasis and evaluate their anti-colony formation effects in terms of preventing the recurrence of cancer cell growth.
The findings align with previous studies, indicating that the extracts from AP, MC and PP contained phenolic and flavonoid compounds and demonstrated antioxidant capabilities (Naqvi et al., 2020; Thanh Men et al., 2022; Adiguna et al., 2023). There was no significant difference in the total phenolic content among the extracts obtained from AP, MC and PP. When compared to the extracts from AP and MC, the PP extract exhibited the highest flavonoid content (335.2 ± 49.4 mg RE/g) and demonstrated the most potent antioxidant activity, as evidenced by IC50 value (570.4 ± 53.3 µg/mL), which was the lowest, as shown in Table 2. Flavonoids have demonstrated high efficacy in scavenging various free radicals due to their redox potential. The research interest in flavonoids derived from plants has been steadily increasing, as their pharmacological properties are directly correlated with their antioxidant potential (Nijveldt et al., 2001). These findings corroborate previous results demonstrating the anticancer activity of AP, MC and PP extracts on various cell lines (Li et al., 2012; Richmond et., 2017: Ala et al., 2018; Kim et al., 2018; Psilopatis et al., 2023;). Our results showed that the extracts of AP, MC and PP had cytotoxicity in MCF-7 cells. According to the National Cancer Institute (NCI) criteria, all extracts demonstrated moderate anticancer activity, with IC50 values falling within the range of 27-46 μg/mL. The NCI categorizes cytotoxic compounds as follows: they are highly active if the IC50 ≤ 20 μg/mL, moderately active if the IC50 ranges from 21 to 200 μg/mL, weakly active if the IC50 ranges from 201 to 500 μg/mL and inactive if the IC50 exceeds 501 μg/mL (Anywar et al., 2022). Based on its cytotoxic activity, the present study demonstrated that 10 µg/mL of the AP, MC, and PP extracts significantly induced cell death in MCF-7 cells. The cytotoxicity of the three extracts on normal fibroblast cells was also assessed using the MTT assay. The results indicated significant cytotoxic effects on fibroblast cells at concentrations of 100 µg/mL, 50 µg/mL, and 50 µg/mL for the AP, MC, and PP extracts, respectively. Compared to their effects on MCF-7 cancer cells, all three plant extracts exhibited lower cytotoxicity towards normal fibroblast cells, suggesting a level of safety. To evaluate the cytotoxic selectivity (i.e., safety) of the extracts in targeting cancer cells relative to normal cells, the selectivity index (SI) was determined as the ratio of the IC50 values of the extracts in normal cells compared to cancer cells. Extracts with an SI value greater than 3 are considered to possess excellent selectivity (Chothiphirat et al., 2019; Mahavorasirikul et al., 2010). Previous studies reported that the MTT and SRB assays demonstrated comparable performance, showing a moderate to excellent correlation in the assessment of the cytotoxicity of a Thai herbal extract (Vajrabhaya and Korsuwannawong, 2018). It has also been reported that the methanolic extract of Artocarpus heterophyllus exhibited IC50 values of 35.26 μg/mL in the MTT assay and 35.27 μg/mL in the SRB assay when tested against A549 lung cancer cells (Patel and Petal’s, 2011). Thus, in this work, the SI was calculated as the ratio of the IC50 value on normal fibroblast cells to that on MCF-7 cells. At 48 h, the IC50 values for AP, MC, and PP on fibroblast cells were 310 µg/mL (IC50 ≈ 27 µg/mL for MCF-7), 273 µg/mL (IC50 ≈ 29 µg/mL for MCF-7), and 408 µg/mL (IC50 ≈ 46 µg/mL for MCF-7), respectively. Consequently, the SI values for the AP, MC, and PP extracts were calculated as 11.42, 9.48, and 8.87, respectively. Among the three, the AP extract demonstrated the highest selectivity for MCF-7 cells compared to the MC and PP extracts. These findings suggest that all three plant extracts exhibit high selectivity for MCF-7 cancer cells.
Our findings confirmed the results obtained by Sholihah et al., (2019) who reported that an ethanol-based extract of AP could effectively inhibit the proliferation of MCF-7 cells. Moreover, this study revealed the higher cell toxicity of the AP extract against MCF-7 cells (IC50 = 27.2 µg/mL) in comparison to the study conducted by Sagadevan et al. (2013), wherein they reported IC50 values of 57.33 μg/mL for a methanol AP leaf extract. Our findings revealed that the AP extract induced cell apoptosis (Figure 1B) and significantly impeded MCF-7 cell growth (Figure 2A) at a concentration of 50 μg/mL. Conversely, at a lower concentration of 25 μg/mL, it effectively inhibited cell migration (Figure 4A). Nevertheless, the AP extract (50 -250 μg/mL) did not induce ROS production (Figure. 3). Many studies have reported the anticancer activity of andrographolide, which is an active compound in AP, predominantly located in AP leaves rather than other parts of the plant. It exhibits potential as a therapeutic agent against human cancer (Tohkayomatee et al., 2022; Tundis et al., 2023). However, it has been reported that individuals who consume andrographolide are more likely to exhibit elevated alanine aminotransferase (ALT) levels compared to those who do not (Kaewdech et al. 2022). The further evaluation of the potential risk of andrographolide consumption for liver function is warranted. It can be inferred that the use of AP in extract form may be safer compared to the use of andrographolide alone.
Regarding the anticancer activity of MC, studies have found that extracts from MC can exhibit in vitro anticancer activity in HeLa cervical cancer cells and MDBK kidney cancer cells (Naqvi et al., 2020), MiaPaCa2 pancreatic cancer cells (Richmond et al., 2017), MCF-7 breast cancer cells and MDA-MB-231 breast cancer cells (Feng et al., 2023; Psilopatis et al., 2023). However, only data on the anticancer activity of MC fruit extracts are available. Limited information exists regarding the anticancer activity of MC leaf extracts. Our results indicate the greater cell toxicity of the MC leaf extract against MCF-7 cells (IC50 = 28.8 µg/mL) compared to the findings reported by Ansari et al. (2019), where they observed IC50 values of approximately 800 μg/mL for an ethanol MC leaf extract. Our results indicated that, at a concentration of 50 μg/mL, the MC extract triggered cell apoptosis (Figure 1B) and ROS production (Figure 3), while also significantly hindering MCF-7 cell growth (Figure 2B). Conversely, at a higher concentration of 100 μg/mL, it effectively restrained cell migration (Figure 4B).
In regard to anticancer activity, studies have shown that PP extracts can exert cytotoxic effects in MCF-7 breast cancer cells (Xu et al., 2006; Wei et al., 2011; Teoh et al., 2021), A549 lung cancer cells (Teoh et al., 2021) and HT-29 colon cancer cells (Narayana moorthi et al., 2018). However, there remains a dearth of information regarding the influence of PP extracts on apoptosis, the inhibition of colony formation, the induction of ROS and cell migration in MCF-7 cells. In our study, the ethanol extract of PP displayed only moderate anticancer activity, with an IC50 of 46 μg/mL. This contrasts the findings of Wei et al. (2011) and Xu et al. (2006), where a methanol leaf extract of PP demonstrated high anticancer activity, with an approximate IC50 of 11 μg/mL. Conversely, Teoh et al. (2021) reported that an ethanol PP extract showed higher cytotoxicity in MCF-7 cells with lower IC50 values (IC50= 22.88 μg/mL) when compared to a methanol PP extract (IC50= 52.56 μg/mL). The variation in the results may be attributed to differences in both the extraction solvent and the plant part used, leading to discrepancies in the amounts of active compounds extracted. An ethanol extract of PP showed higher cytotoxicity in A549 lung cancer cells with an IC50 value of 13.02 μg/mL (Teoh et al., 2021), but lower cytotoxicity in HT-29 colon cancer cells with an IC50 value of 129.16 μg/mL (Narayana Moorthi et al., 2018), compared to the IC50 value of 46 μg/mL observed in our study for the ethanol PP extract. Our findings revealed that the PP extract induced cell apoptosis (Figure 1B) and significantly impeded MCF-7 cell growth (Figure 2C) at a concentration of 50 μg/mL. Conversely, at a higher concentration of 100 μg/mL, it effectively inhibited cell migration (Figure 4C). Nevertheless, the PP extract at concentrations ranging from 50 to 250 μg/mL did not stimulate ROS generation (Figure 3).
An essential element of anticancer activity is the inhibition of cell migration or the minimization of metastasis. Consequently, it is imperative to assess extracts' anti-migratory effects in terms of preventing cancer cell metastasis and evaluate their anti-colony formation effects in terms of inhibiting the recurrence of cancer cell growth. The extracts derived from AP, MC and PP inhibited cell growth (Figure 2) and suppressed the migration of MCF-7 cells (Figure 4). It is possible to conclude that all extracts possessed anticancer properties, capable of both preventing and treating breast cancer. This is supported by their ability to trigger apoptosis in cancer cells, inhibit metastasis and prevent the recurrence of cancer cell growth. A strategy for cancer treatment involves inducing oxidative stress to stimulate the production of ROS, as maintaining the redox balance of intracellular ROS levels is crucial for tumor initiation and progression. Increased ROS generation has been linked to apoptosis and cell cycle arrest in cancer cells. Our findings revealed that only the MC leaf extract (at concentrations of 50 and 100 µg/mL) led to a significant increase in ROS formation compared to non-treated cells (Figure 3). Elevated levels of cellular ROS can cause damage to proteins, nucleic acids, lipids, membranes and organelles, ultimately triggering processes associated with cell death, such as apoptosis (Redza-Dutordoir et al., 2016). The results of this study are in line with the findings of Kim et al. (2018), who observed that an ethanol extract from MC induced ROS formation in cancer cells, ultimately leading to cancer cell apoptosis. Andrographolide is the main bioactive part of AP extract that has been shown to increase ROS levels in HT-29 cells by about 20% at a dose of 16 mM (Khan et al., 2018). In this work, AP extract concentrations ranging from 50 to 250 µg/mL did not produce ROS in MCF-7 cells. It's possible that this lack of action is due to the lower andrographolide concentration in the AP extract used in this study than the 16 mM concentration found in other studies. To evaluate the anti-migratory effect, the findings from this study demonstrated that AP extract, at a concentration of 25 µg/mL, significantly inhibited the migration of MCF-7 breast cancer cells. These findings are consistent with those of Anoor et al. (2022), who reported that AP extract at the same concentration significantly suppressed the migration of HeLa cells. At a concentration of 150 µg/mL, MC extract has been shown to significantly suppress the migration of CL1 lung cancer cells (Hsu et al., 2012). Our study showed that MC extract can inhibit cancer cells from migrating even at a lower concentration of 50 µg/mL. This was confirmed by the fact that the extract inhibited MCF-7 cancer cells from migrating significantly. Compared to the MC and PP extracts, the extract of AP exhibited the strongest anticancer properties against MCF-7 breast cancer cells, suggesting its potential utility as an anticancer agent for both the prevention and treatment of breast cancer. However, the identification of herbs with anticancer properties, and their combination to achieve complementary effects, represents a highly advantageous option for individuals with cancer.
CONCLUSION
This study suggests that extracts from AP, MC and PP contain phenolic and flavonoid compounds, along with antioxidant and anticancer properties against breast cancer cells. Notably, the AP extract demonstrated the most potent anticancer effects in MCF-7 breast cancer cells compared to the MC and PP extracts. Further investigation into the anticancer activity, biological properties, active compounds and toxicity of AP, MC and PP would be valuable in confirming their utility as effective agents for cancer prevention.
ACKNOWLEDGEMENTS
This research was supported by University of Phayao and Thailand Science Research and Innovation Fund (Fundamental Fund 2024). The authors thank the Faculty of Medicine, Mahasarakham University, and the School of Pharmaceutical Sciences, University of Phayao for providing instruments.
AUTHOR CONTRIBUTIONS
Supavadee Boontha designed the study and the experiments, characterized the extract, conducted the project, and prepared the manuscript. Prapapan Temkitthawon prepared the extract. Benjaporn Buranrat was responsible for the MCF-7 cell study. Tasana Pitaksuteepong and Kamchai Saepang assisted in conducting the experiments. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adiguna, S.P., Panggabean, J.A., Swasono, R.T., Rahmawati, S.I., Izzati, F., Bayu, A., et al. 2023. Evaluations of andrographolide-rich fractions of Andrographis paniculata with enhanced potential antioxidant, anticancer, antihypertensive, and anti-Inflammatory activities. Plants. 12(6): 1220.
Ahmad, I., Hikmawan, B.D., Sulistyarini, R., and Mun’im, A. 2023. Peperomia pellucida (L.) Kunth herbs: A comprehensive review on phytochemical, pharmacological, extraction engineering development, and economic promising perspectives. Journal of Applied Pharmaceutical Science. 13(1): 1–9.
Ala, A.A., Olotu, B.B., and Ohia, C.M.D. 2018. Assessment of cytotoxicity of leaf extracts of Andrographis paniculata and Aspilia africana on murine cells in vitro. Archives of Basic and Applied Medicine. 6(1): 61–65.
Amawi, H., Ashby, C.R., and Tiwari, A.K. 2017. Cancer chemoprevention through dietary flavonoids: What’s limiting?. Chinese Journal of Cancer. 36(1): 50.
Anoor, P.K., Yadav, A.N., Rajkumar, K., Kande, R., Tripura, C., Naik, K.S., and Burgula, S. 2022. Methanol extraction revealed anticancer compounds quinic acid, 2(5H)-furanone and phytol in Andrographis paniculata. Molecular and Clinical Oncology. 17(5): 151.
Ansari, A.A, Singh, J., and Aminuddin, M. 2019. Biochemical characterization of Momordica charantia (leaf and fruit) and effect of soluble extract on MCF-7 breast cancer cell lines. Cell Biology & Development. 3(1): 1-5.
Anywar, G.U., Kakudidi, E., Oryem-Origa, H., Schubert, A., and Jassoy, C. 2022. Cytotoxicity of medicinal plant species used by traditional healers in treating people suffering from HIV/AIDS in Uganda. Frontiers in Toxicology. 4: 832780.
Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M., et al. 2022. Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast, 66: 15–23.
Atale N., Gupta S., Yadav U.C., and Rani V. 2014. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. Journal of Microscopy. 255(1): 7-19.
Boontha, S., Kaewjaiboon, N., Rattanatanyapat, P., Nanto, W, Taolam, S., Buranrat, B., et al. 2018. Cytotoxicity and cell migration suppression by noni fruit extract on Michigan Cancer Foundation-7 human breast cancer cells and development of topical microemulsions. Pharmacognosy Magazine. 14(1): 499–506.
Chittasupho, C., Samee, W., Tadtong, S., Jittachai, W., Managit, C., and Athikomkulchai, S. 2023. Cytotoxicity, apoptosis induction, oxidative stress, and cell cycle arrest of Clerodendrum chinense flower extract nanoparticles in HeLa cells. Natural and Life Sciences Communications. 22(4): e2023057.
Chothiphirat, A., Nittayaboon, K., Kanokwiroon, K., Srisawat, T., and Navakanitworakul, R. 2019. Anticancer potential of fruit extracts from Vatica diospyroides symington type SS and their effect on program cell death of cervical cancer cell lines. The Scientific World Journal. 2019: 5491904.
Dubale, S., Kebebe, D., Zeynudin, A., Abdissa, N., and Suleman, S. 2023. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. Journal of Experimental Pharmacology. 15: 51–62.
Feng, T., Wan, Y., Dai, B., and Liu, Y. 2023. Anticancer activity of bitter melon-derived vesicles extract against breast cancer. Cells. 12(6): 824.
Gomes, P.W.P., Barretto, H., Reis, J.D.E., Muribeca, A., Veloso, A., Albuquerque, C., et al. 2022. Chemical composition of leaves, stem, and roots of Peperomia pellucida (L.) Kunth. Molecules. 27(6): 1847.
Hossain, S., Urbi, Z., Karuniawati, H., Mohiuddin, R.B., Moh Qrimida, A., Allzrag, A.M.M., et al. 2021. Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life. 11: 348.
Hsu, H.Y., Lin, J.H., Li, C.J., Tsang, S.F., Tsai, C.H., Chyuan, J.H., Chiu, S.J., and Chuang, S.E. 2012. Antimigratory effects of the methanol extract from Momordica charantia on human lung adenocarcinoma CL1 cells. Evidence-Based Complementary and Alternative Medicine. 2012(12): 819632.
Ibragimova, M.K., Tsyganov, M.M., Kravtsova, E.A., Tsydenova, I.A., and Litviakov, N.V. 2023. Organ-specificity of breast cancer metastasis. International Journal of Molecular Sciences. 24(21): 15625.
Jia, S., Shen, M., Zhang, F., and Xie, J. 2017. Recent advances in Momordica charantia: Functional components and biological activities. International Journal of Molecular Sciences. 18(12): 2555.
Jin, L., Han, B., Siegel, E., Cui, Y., Giuliano, A., and Cui, X. 2018. Breast cancer lung metastasis: Molecular biology and therapeutic implications. Cancer Biology & Therapy. 19(10): 858–868.
Kaewdech, A., Nawalerspanya, S., Assawasuwannakit, S., Chamroonkul, N., Jandee, S., and Sripongpun, P. 2022. The use of Andrographis paniculata and its effects on liver biochemistry of patients with gastrointestinal problems in Thailand during the COVID-19 pandemic: A cross sectional study. Science Reports. 12: 18213.
Khan, I., Khan, F., Farooqui, A., and Ansari, I. A. 2018. Andrographolide exhibits anticancer potential against human colon cancer cells by inducing cell cycle arrest and programmed cell death via augmentation of intracellular reactive oxygen species level. Nutrition and Cancer. 70(5): 787–803.
Kim, K.B., Lee, S., Kang, I., and Kim, J.H. 2018. Momordica charantia ethanol extract attenuates H₂O₂-induced cell death by its antioxidant and anti-apoptotic properties in human neuroblastoma SK-N-MC cells. Nutrients. 10(10): 1368.
Kopustinskiene, D.M., Jakstas, V., Savickas, A., and Bernatoniene, J. 2020. Flavonoids as anticancer agents. Nutrients. 12(2): 457.
Li, C.J., Tsang, S.F., Tsai, C.H., Tsai, H.Y., Chyuan, J.H., and Hsu, H.Y. 2012. Momordica charantia extract induces apoptosis in human cancer cells through caspase- and mitochondria-dependent pathways. Evidence-Based Complementary and Alternative Medicine. 2012(1-2): 261971.
Mahavorasirikul, W., Viyanant, V., Chaijaroenkul, W., Itharat, A., and Na-Bangchang K. 2010. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complementary and Alternative Medicine. 10(55):1–8.
Menyhárt, O., Harami-Papp, H., Sukumar, S., Schäfer, R., Magnani, L., de Barrios, O., et al. 2016. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer, Biochimica et Biophysica Acta. 1866 (2): 300-319.
Naqvi, S.A.R., Ali, S., Sherazi, T.A., Haq, A.-U., Saeed, M., Sulman, M., et al. 2020. Antioxidant, antibacterial, and anticancer activities of bitter gourd fruit extracts at three different cultivation stages. Journal of Chemistry. 2020: 7394751.
Narayana moorthi, V., Vasantha, K., and Maruthasalam, A. 2018. In vitro evaluation of cytotoxic properties of Peperomia pellucida (L.) H.B.K. against human cancer cell lines. Bioscience Discovery. 9(3): 344-355.
Nijveldt, R.J., van Nood, E., van Hoorn, D.E., Boelens, P.G., van Norren, K., and van Leeuwen, P.A. 2001. Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition. 74(4): 418–425.
Om, S.C., Amit, J., and Bal, K.D. 2012. Pharmacognostical, phytochemical evaluation and antiinflammatory activity of stem of Kalanchoe pinnata pers. International Journal of Pharm Science and Research. 3(4): 1133-1140.
Orellana, E.A., and Kasinski, A.L. 2016. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio-protocol. 6(21): e1984.
Pandey, K.B., and Rizvi, S.I. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2(5): 270–278.
Patel, R.M., and Patel, S.K. 2011. Cytotoxic activity of methanolic extract of Artocarpus heterophyllus against A549, Hela and MCF-7 cell lines. Journal of Applied Pharmaceutical Science. 1(7):167–171.
Psilopatis, I., Vrettou, K., Giaginis, C., and Theocharis, S. 2023. The role of bitter melon in breast and gynecological cancer prevention and therapy. International Journal of Molecular Sciences. 24(10): 8918.
Redza-Dutordoir, M., and Averill-Bates, D.A. 2016. Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta. 1863(12): 2977–2992.
Richmond, R.A., Vuong, Q.V., and Scarlett, C.J. 2017. Cytotoxic effect of bitter melon (Momordica charantia L.) ethanol extract and its fractions on pancreatic cancer cells in vitro. Exploratory Research and Hypothesis in Medicine. 2(4):139-149.
Sagadevan, P., Suresh, S., Rathishkumar, S., Gayathri, S., and Eswari, D.V. 2013. Anticancer activity of methanolic leaf extracts of Andrographis paniculata (Nees) and Cardiospermum halicacabum (Linn) against human breast cancer cell line (MCF-7). International Journal of Pharmacy and Life Science. 4(9): 2983-2986.
Sholihah, M.M., Indarto, D., and Pramana, T.Y. 2019. The inhibitory effect of Andrographis paniculata extract on proliferation of breast cancer cell line. IOP Conference Series: Materials Science and Engineering. 546: 1-8.
Siegel, R. L., Giaquinto, A. N., and Jemal, A. 2024. Cancer statistics, 2024. CA: A Cancer Journal for Clinicians. 74(1): 12–49.
Siridechakorn, I., Bhattarakosol, P., Sasivimolrattana, T., Anoma, S., Wongwad, E., Nuengchamnong, N., et al. 2023. Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production. Scientific Reports. 13: 19738.
Teoh, L., Gnanasegaran, N., Adnan, A.F.M., and Taha, R.M. 2021. The comparative antimicrobial and anticancer of chemical extract from in vitro and in vivo Peperomia pellucida plantlet. Journal of Applied Biology & Biotechnology. 9(2): 115-123.
Thanh Men, T., Thi Kim Tu, L., Thi Kim Anh, N., Hong Phien, H., Thi Bich Nhu, N., Thi To Uyen, N., et al. 2022. Antioxidant and in vitro antidiabetic activities of Peperomia pellucida (L.) Kunth extract. Veterinary Integrative Sciences. 20(3): 683–693.
Tohkayomatee, R., Reabroi, S., Tungmunnithum, D., Parichatikanond, W., and Pinthong, D. 2022. Andrographolide exhibits anticancer activity against breast cancer cells (MCF-7 and MDA-MB-231 cells) through suppressing cell proliferation and inducing cell apoptosis via inactivation of ER-α receptor and PI3K/AKT/mTOR signaling. Molecules. 27: 3544.
Tundis, R., Patra, J.K., Bonesi, M., Das, S., Nath, R., Das Talukdar, A., et al. 2023. Anti-cancer agent: The labdane diterpenoid-andrographolide. Plants. 12(10): 1969.
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., and Yangsabai, A. 2018. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 5(3): 93.
Vajrabhaya, Lo., and Korsuwannawong, S. 2018. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical Science and Technology. 9(1): 1-6.
Wang, X., Decker, C.C., Zechner, L. Krstin, S., and Wink, M. 2019. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacology and Toxicology. 20 (1): 4.
Wei, L.S., Wee, W., Siong, J.Y., and Syamsumir, D.F. 2011. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Medica Iranica. 49(10): 670-674.
Wilkinson, L., and Gathani, T. 2022. Understanding breast cancer as a global health concern. The British Journal of Radiology. 95(1130): 20211033.
Wode, K., Henriksson, R., Sharp, L., Anna Stoltenberg, A., and Nordberg, J.H. 2019. Cancer patients’ use of complementary and alternative medicine in Sweden: A cross-sectional study. BMC Complementary and Alternative Medicine. 19 (1): 62.
Xu, S., Li, N., Ning, M.M., Zhou, C.H., Yang, Q.R., and Wang, M.W. 2006. Bioactive compounds from Peperomia pellucida. Journal of Natural Products. 69(2): 247-250.
Xu, Y., Gong, M., Wang, Y., Shu Liu, Y., and Zeng, Q. 2023. Global trends and forecasts of breast cancer incidence and deaths. Scientific Data. 10: 334.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supavadee Boontha1, 2,* , Benjaporn Buranrat2, 3, Kamchai Saepang1, 2, Prapapan Temkitthawon4 and Tasana Pitaksuteepong5
1 Division of Pharmacy and Technology, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
2 Research Group in Herbal and Development of Formulation and Delivery Systems for Elderly Adults and Cancer Treatment, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
3 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand.
4 Bioscreening Unit, Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
5 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Supavadee Boontha, E-mail: supavadee.bo@up.ac.th
ORCID: Supavadee Boontha: https://orcid.org/0000-0003-3335-6081
Total Article Views
Editor: Wipawadee Yooin
Chiang Mai University, Thailand
Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: April 11, 2024;
Revised: October 1, 2024;
Accepted: October 16, 2024;
Online First: November 14, 2024

