
Physicochemical Characteristics and Stability Assessment of A Topical Formulation Comprising Allium cepa and Quercetin for Cutaneous Scar Management
Sureewan Duangjit*, Suphaphon Jandum, Pakapol Palakun, Worranan Rangsimawong, Phaijit Sritananuwat, Tipada Samseethong, Kusuma Jitsaeng, and Sureewan BumrungthaiPublished Date : October 1, 2024
DOI : https://doi.org/10.12982/NLSC.2024.065
Journal Issues : Number 4, October-December 2024
Abstract The 12% Allium cepa extract topical formulation, with proven clinical benefits, targets improved surgical scar appearance. This study aims to comprehensively assess the physicochemical characteristics and stability of a scar-reducing formulation (15% Allium cepa-micellar extract, 0.01% quercetin). In vitro-release and skin permeation studies were conducted, alongside FTIR and DSC analyses to elucidate the skin permeation mechanism. Quercetin remaining at 25 and 40 °C ranged from 99 to 100% (day 60) and 101 to 104% (day 90), while antioxidant activity (53-65%, day 90) remained comparable to the standard quercetin (84.70% at 40 μg/mL). In the context of in vitro quercetin release, the performances were ranked as shallot-micellar extract > scar-reducing formulation plus shallot-micellar extract > scar-reducing formulation, with no significant differences observed (P < 0.05). Importantly, scar-reducing formulation plus shallot-micellar extract and shallot-micellar extract showed a significant difference (P < 0.05) compared to shallot-water extract and the commercial product. The treated skin showed minor changes in the microstructure compared to untreated skin, indicating a gentle formulation, as observed in the FT-IR spectra and DSC thermograms. The findings from skin permeation mechanism studies imply that the scar-reducing formulation may deposit on the skin surface with minor alterations to the skin microstructure. A crucial consideration for future investigations is a long-term stability assessment of at least 12 months at 30°C, following ASEAN guidelines. The scar-reducing formulation was stable at 25°C, without exceeding 40°C for 90 days. Storage at 4°C is not recommended due to the potential risk of decreased solubility and quercetin precipitation.
Keywords: Micelle, Scar treatment, Stability, Antioxidant activity, Drug delivery
Funding: The authors are grateful for the research funding provided by the Office of the Higher Education Commission and the Thailand Research Fund (Grant no. RGNS 64–237).
Citation: Duangjit, S., Jandum, S., Palakun, P., Rangsimawong, W., Sritananuwat, P., Samseethong, T., Jitsaeng, K., and Bumrungthai, S. 2024. Physicochemical characteristics and stability assessment of a topical formulation comprising Allium cepa and quercetin for cutaneous scar management. Natural and Life Sciences Communications. 23(4): e2024065.
INTRODUCTION
Wound healing, an inherent biological process in the human body, follows a meticulously orchestrated sequence of four phases: hemostasis, inflammation, proliferation, and remodeling (Mounir et al., 2023). The successful healing of a wound relies on the precise occurrence of all these phases in the correct sequence and timeframe (4 to 6 weeks) (Wallace et al., 2023). Consequently, the prevention and early identification of hypertrophic scars and keloids are imperative for effective management. Numerous well-established interventions are available for scar management, including intralesional steroid injection, cryotherapy, radiotherapy, laser therapy, dermabrasion, pulse dye, and surgical excision. Scars, often perceived as benign, can evoke distress in individuals, manifesting symptoms like discomfort, itching, pain, and functional limitations. Certain scars, extending beyond cosmetic considerations, may impede movement, thereby bearing implications for physical and psychological well-being. (Chanprapaph et al., 2012) A combination therapy that involves multiple intervention modalities has shown greater effectiveness than monotherapy (Hosnuter et al., 2007). Moreover, non-invasive approaches can potentially enhance patient adherence in managing hypertrophic scars and keloids. (Shin et al., 2019)
For over six decades, a topical gel incorporating onion (Allium cepa) extract and allantoin has been in existence, demonstrating efficacy in the treatment, prevention, and reduction of dermatologic scars and keloids. Onion extract is recognized for its anti-inflammatory, anti-microbial, anti-proliferative, and regenerative properties, while allantoin is known for its keratolytic, hydrating, epithelizing, and anti-irritant attributes (Chakraborty et al., 2022; Mounir et al., 2023; Srimai and Akarapisan, 2023). Recent studies have highlighted quercetin's role in scar treatment through its antioxidant activity. Keloid fibroblasts treated with quercetin show significantly reduced extracellular matrix (ECM) production and deposition compared to untreated cells. Phan et al. found that quercetin inhibits the overactive IGF-1 and TGF-β signaling pathways, which are responsible for fibroblast proliferation and collagen production in keloids (Poetschke and Gauglitz, 2020). Thus, quercetin's ability to modulate these pathways suggests its potential in cutaneous scar management by reducing excessive ECM and scar formation. Numerous clinical trials substantiate the well-tolerated nature of this gel, emphasizing its role in preventing pathological scarring and enhancing preexisting scars (Prager and Gauglitz, 2018). In a 12-week study, the topical application of 12% onion extract gel on Pfannenstiel's cesarean section scars in Asians demonstrated a statistically significant improvement in scar height and symptoms (Chanprapaph et al., 2012). In a 24-week study involving 30 children with an average age of 4.3 years (6 months – 15 years), the silicone derivative gel incorporated 10% onion extract demonstrated efficacy in preventing or reducing the severity of hypertrophic scars. However, it does not appear to prevent the formation of keloids, which are typically more severe in nature. (Wananukul et al., 2013) In a 12-week study involving 125 subjects, the topical gel containing Allium cepa extract and allantoin demonstrated a significant reduction in scar scores for treating scars at weeks 12 and 24. This investigation provides confirmation of the safe promotion of scar healing following minor dermatologic surgery. (Prager and Gauglitz, 2018) Based on this meta-analysis of randomized controlled trials, the efficacy of onion extract gel appears comparable to commonly used topical treatments, and its potential for increasing adverse effects on scar management warrants consideration. Onion extract in silicone gel appears to be a promising topical choice for scar treatment; however, further evidence is required to substantiate these conclusions. (Yuan et al., 2021) Comprehending the dynamics of factors influencing scar-reducing efficacy, skin safety, and formulation stability is crucial for evaluating the effectiveness of topical scar-reducing formulations and ensuring optimal desirability. The objective of this study is to conduct a comprehensive assessment of the physicochemical characteristics and stability of a scar-reducing formulation comprising 15% Allium cepa-micellar extract and 0.01% quercetin over a three-month period at varying temperatures (4, 25, and 40°C). Additionally, in vitro-release and skin permeation studies were performed, accompanied by Fourier transform infrared (FTIR) and differential scanning calorimetry (DSC) analyses to elucidate the skin permeation mechanism.
MATERIALS AND METHODS
Materials
The Allium cepa extract, shallot extract in water, shallot extract in micelle and quercetin were generously provided by Global Medical (Thailand), while the standard quercetin and polysorbate 20 was procured from Sigma-Aldrich (Merck Thailand). The chemicals employed in the formulation were of cosmetic grade, and those used for analysis were of HPLC grade.
Physical appearance observation
The scar-reducing formulation (Scar Q Plus), comprising 15% Allium cepa-micellar extract and 0.01% quercetin, was manufactured by Global Medical (Thailand) Co., Ltd., a local GMP-cosmetic company in Thailand, in batches of 40 kg. This paraben-free, alcohol-free, and silicone-free product was evaluated for non-irritation in 20 healthy volunteers by DermScan Asia Co., Ltd. The control for this study was a patch without the product. The safety test lasted 48 hours. Approximately 25 µL of Scar Q Plus was applied to the skin and covered with an 8 mm Finn Chamber® patch for 48 hours. Physical appearance assessments, encompassing color, odor, phase separation, and homogeneity, were conducted through visual observation. Storage utilized plastic containers (15 g) as primary packaging and paper boxes as secondary packaging. Skin feel assessments for stiffness and spreadability were performed on day 1.
pH values
The determination of the pH for 15 grams of the scar-reducing formulation was executed using the SevenDirect™ SD50 pH meter (Mettler Toledo, Thailand). The pH of the formulation was directly measured at ambient temperature (25°C).
Viscosity measurement
The viscosity of the scar-reducing formulation, employing 1 gram, was determined using the HAAKE™ MARS™ Rheometers (Thermo Scientific™, USA) at 25°C. All measurements were conducted in triplicate.
Drug remaining
Quercetin extraction from the formulation involved a precise procedure utilizing a methanol ratio of 5:10 (w/v). Five grams of the formulation were accurately weighed and mixed with 5 mL of methanol, followed by 10 minutes of sonication. The supernatant, obtained after centrifugation at 4,000 rpm for 10 minutes, underwent subsequent rounds of extraction with 4 mL of methanol. The combined extracts were then filtered through a 0.45 mm nylon filter for analysis via high-performance liquid chromatography (HPLC) to determine quercetin content and antioxidant activity.
Antioxidant activity
The sample (40 µL) was combined with a 0.004% w/v 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (160 µL), and the resulting solution's absorbance was measured at 517 nm after a 30-minute incubation at room temperature in the dark. The radical scavenging activity values are presented as IC50 values (μg/mL), representing the quercetin concentration needed to inhibit 50% of the free radicals. The quercetin standard employed in this study was at a concentration of 40 µg/mL. All determinations were conducted in five replicates.
Stability study
The physical appearance and physicochemical characteristics (e.g., pH, viscosity, quercetin remaining, and antioxidant activity) of the scar-reducing formulation (Scar Q Plus) were compared among samples stored in the refrigerator (4°C), at room temperature (25°C), and accelerated conditions (40°C). Furthermore, the influence of commercial packaging containers on these characteristics was investigated over a 3-month period using the described methods.
HPLC analysis
The concentration of quercetin in all formulations was determined using HPLC (UltiMate 3000 UHPLC System, Thermo Scientific™, USA). A C18 reversed-phase column (Symmetry®, VertiSepTM, Vertical, Thailand) with dimensions of 5 µm, 4.6×250 mm, was employed. The mobile phase for quercetin determination consisted of a mixture of acetonitrile and 2% acetic acid (40:60) (Ang et al., 2014)). The detector, set at UV 370 nm, operated with a flow rate of 1.0 ml/min under conditions of 35°C, and an injection volume of 20 µL. The retention time was 5.5 min, and the calibration curves for quercetin were established in the range of 1-100 µg/ml with a correlation coefficient of 0.99.
In vitro release and skin permeation
In vitro release studies utilized cellulose acetate membrane (Spectra-por® dialysis membrane MWCO 6-8 kDa, Thermo Fisher Scientific Inc., Sweden), while skin permeation studies employed shed snake skin through Franz-type cells (n=3) at 37°C. Circular sections (2.5 cm x 2.5 cm) of each membrane, cut into three pieces, were positioned facing the donor chamber. The test formulations were serums (Scar Q Plus and Scar Q Plus with shallot extract in micelle), micelles extraction of shallot and water extraction of shallot. Formulations (1 g) were added to each. The receiving chamber, containing 12.5 mL of receiver medium (acetate buffer pH 5.5: EtOH; 1:1 v/v) under sink conditions, was stirred at 600 rpm by a magnetic stirrer (Diffusion Cell Drive System FDC-6, LOGAN Instruments Corp, USA). At specified intervals, 0.5 mL aliquots of the receiver medium were withdrawn and replaced with an equal volume of fresh buffer. HPLC analysis determined quercetin concentration in the receiver.
Mechanism of skin permeation
Subsequent to skin permeation, the shed snake skin underwent meticulous investigation to elucidate skin permeability mechanisms using Fourier transform infrared (FTIR) spectrophotometry (Nicolet 4700, Thermo Scientific, Waltham, MA, USA) and differential scanning calorimetry (DSC) (Pyris Sapphire DSC, PerkinElmer instrument, Waltham, MA, USA). The treated skin, subjected to washing and drying, was recorded over a range of 500–4,000 cm-1 using the absorption mode and securely affixed to the sample holder. Simultaneously, 2 mg portions of the same skin were accurately weighed, cut into small pieces, and placed in aluminum seal pans (40 μL). The heat flow, ranging from 25 to 300 °C at a heat rate of 10°C/min, was then measured. The untreated snake skin served as a control in this comprehensive study.
Data analysis Means ± standard deviation (SD) (n=3) were used to report the data, and a p-value below 0.05, as determined by LSD, was regarded as statistically significant.
RESULTS
The physical appearance of the scar-reducing formulation was a white serum with a mild aroma, not sticky and easy to apply and spread. On the initial day of evaluation, the physicochemical characteristics were shown in Figure 1. The pH and viscosity of the formulation were 5.10 and 91 Pa, respectively. The total quercetin content in the scar-reducing formulation was 0.1188 mg/g or 0.0118% (w/w). The antioxidant activity at the initial day was 53%, while the antioxidant activity of the standard quercetin of 40 µg/mL was 77%.
The physicochemical stability of the scar-reducing formulation is shown in Figure 1. The pH of the formulation was stable in the range of 5.0 to 5.5. The viscosity of the formulation on day 1 significantly increased, different from other stability conditions. The quercetin remaining met the criteria ±10% through 90 days at 25 and 40 °C. The antioxidant activity of the formulation remained through 90 days in all stability conditions.
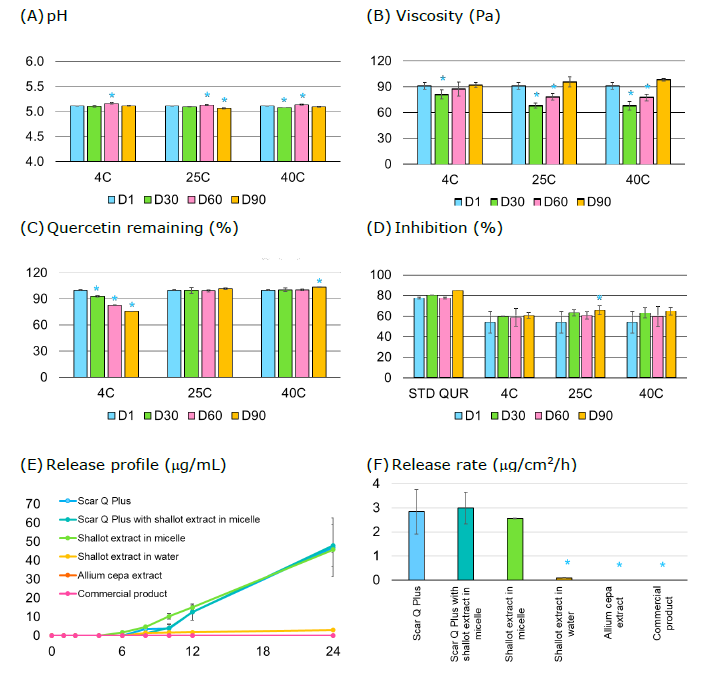
Figure 1. Physicochemical stability and drug release of the scar-reducing formulation.
Although the pH of days 60 and 90 was significantly different from the initial day, the pH of all stability conditions fell in the range of 5.0 to 5.5 (Figure 1A). The viscosity of the formulation during days 30, 60, and 90 significantly increased (Figure 1B), with a viscosity of 68.47 ± 2.36 to 98.53 ± 1.15 Pa was classified as a cream formulation, with quite low viscosity compared to day 1, which characteristics represent a serum. The quercetin remaining was at 25 and 40 °C day 90 was 101.7 ± 0.9 to 103.6 ± 0.4%, which fell in the criterion of the topical formulation in the United States Pharmacopeia (USP 46, 2023), While the temperature of 4°C for 60 days, the quercetin remaining was 82.9 ± 0.4% (Figure 1C). These revealed that the quercetin may re-crystal at low temperatures, as the solubility of quercetin was 2.15 and 665 µg/mL at 25 and 140 °C (Srinivas et al., 2010). However, the antioxidant activity at 4, 25, and 40°C for 90 days remained comparable to the standard quercetin (84.70% at 40 μg/mL), and no significant difference from the initial day (except at 25°C, day 90) (Figure 1D).
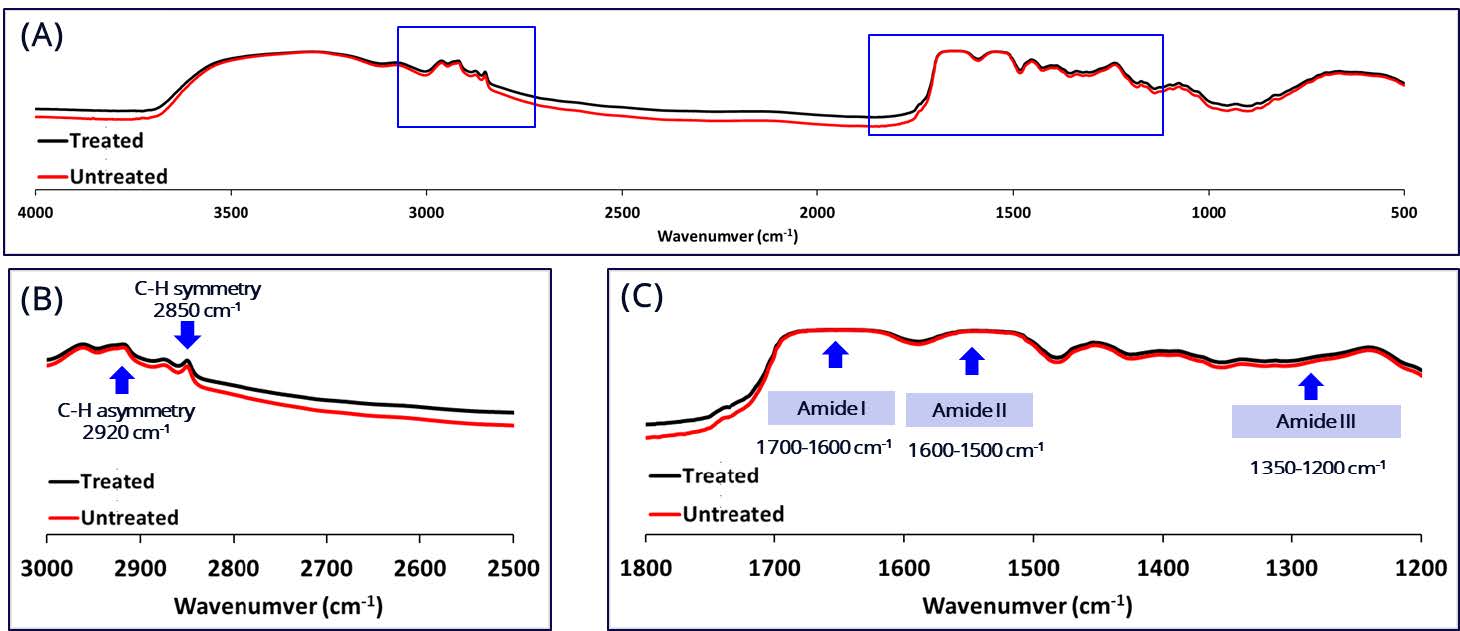
Figure 2. FTIR spectra of the shed snake skin after the skin permeation study.
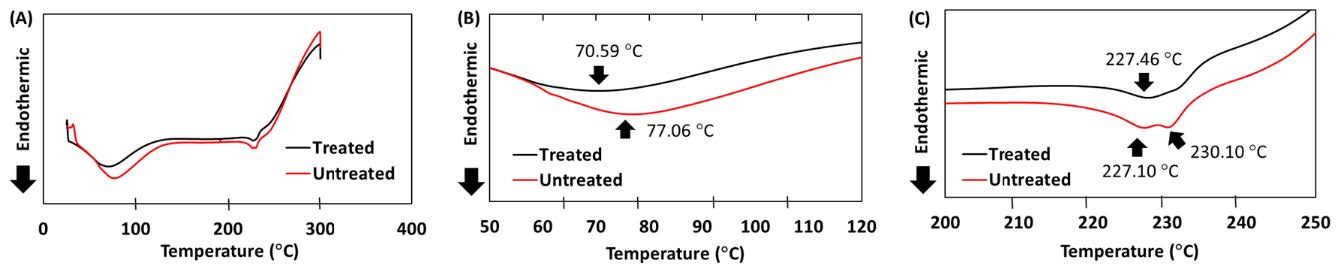
Figure 3. DSC thermogram of the shed snake skin after the skin permeation study.
The in vitro release profile indicated that the initial release rate of quercetin from the scar-reducing formulation (or Scar Q plus), the scar-reducing formulation incorporating shallot-micellar extract, and the shallot-micellar extract were not significantly different. The release rate of quercetin from the shallot-water extract was the lowest, while the release rate of the control was undetectable. The skin permeation via the snake skin indicated that the quercetin did not permeate the snake skin in this study. Thus, the mechanism of skin permeability was investigated. The FTIR spectra of untreated and treated skin with formulation exhibited greater broadening of peaks near 2,850 cm-1 and 2,920 cm-1 (Figure 2A). These results indicate that the C-H stretching at wavenumbers 2,920 and 2,850 cm-1 (Figure 2B) and the amide peaks at wavenumbers 1,200-1,700 cm-1 (Figure 2C) of skin treated with formulation were not different from that of intact untreated skin. The endothermic peak of the skin treated with formulation (70.59°C) was markedly different from that of the untreated skin (control) (77.06°C) (Figure 3B). Meanwhile, the peak at 230°C of the treated skin was absent (Figure 3C).
DISCUSSION
In formulating a pharmaceutical product for scar reduction, our focus revolved around three crucial criteria: the efficacy in reducing scars, the safety profile for the skin, and the stability of the formulation. Various scar-reducing formulations available in the market come in different forms, such as gels (Chanprapaph et al., 2012), silicone gels (Napavichayanun et al., 2022; Wananukul et al., 2013), creams (Esoje, Muazu, and Madu, 2016), patched (Conti et al., 2020; Prager and Gauglitz, 2018), spray (Ahmed et al., 2023) and serums. Recognizing the importance of customer satisfaction, our formulation prioritized a visually appealing appearance, characterized by a white serum with a mild aroma and features that make it non-sticky, easy to apply, and spread. Prior to evaluating the scar-reducing efficacy, a comprehensive assessment of skin safety and formulation stability was conducted. DermScan Asia Co., Ltd. performed a non-irritation test involving 20 healthy volunteers for our scar-reducing formulation, revealing no irritation.
The physicochemical characteristics of the scar-reducing formulation highlighted that the pH range of 5.0 to 5.2 aligns with the optimal levels for healthy physiological skin (pH 4 to 6) (Lukić et al., 2021). Furthermore, quercetin, a pivotal active ingredient, exhibited stability under conditions of pH < 6 (Kim et al., 2023). Consequently, the pH of the scar-reducing formulation, incorporating Allium cepa and quercetin, met the required criteria. Viscosity plays a critical role in the development of topical dosage forms, as it is essential for ensuring patient compliance and assessing the physical stability of the formulation during production and shelf-life (Chiarentin et al., 2023). With a viscosity of 91 Pa on day 1, the scar-reducing formulation demonstrated non-sticky properties and ease of application and spreadability. The viscosity tended to increase over subsequent days, although, by day 90, there was no significant difference compared to the initial viscosity. The total quercetin content in the scar-reducing formulation was under the label of 0.01% quercetin, and the remaining quercetin was also confirmed stable under the criteria of ±10% compared to day 1. While the antioxidant activity of the formulation was still high and stable. This result revealed that Allium cepa may have other antioxidant compounds, not only quercetin. Anthocyanins, chalcones, flavones, flavanones, flavanols, flavanonols, and isoflavones, which were subclasses of flavonoids and derivatives, were the most abundant flavonoids in Allium capa (Marefati et al., 2021). The Allium cepa extract exhibited antioxidant activity despite lacking detectable quercetin content, potentially falling below the lower limit of detection (< 1 µg/mL); thus, 0.01% quercetin was incorporated in the scar-reducing formulation to ensure activity. Furthermore, the addition of quercetin may serve against the degradation of Allium cepa extract. The quercetin content and antioxidant activity were crucial for ensuring the therapeutic efficacy of the scar-reducing formulation. These attributes confirmed that the antioxidant activity related to the scar-reducing process still occurs. Quercetin inhibits the overactive IGF-1 and TGF-β pathways, reducing fibroblast proliferation, collagen production, and ECM formation in keloids. Thus, quercetin shows potential in scar management by decreasing excessive ECM and scar formation (Poetschke and Gauglitz, 2020).
The 10 to 12% Allium cepa extract topical formulation was the well-known concentration of Allium cepa extract in various commercial products (Chanprapaph et al., 2012; Prager and Gauglitz, 2018). To improve the positive deviation from disappointing outcomes observed in clinical studies, the authors designed to formulate the 15% Allium cepa-micellar extract and 0.01% quercetin. In this study, a micellar delivery system was utilized to improve the efficacy and stability of Allium cepa extract (Wang et al., 2016). Besides acting as a key active ingredient, the inclusion of 0.01% quercetin compound may enhance the stability of the scar-reducing formulation as an antioxidant, thereby preserving both the formulation and the Allium cepa-micellar extract. This observation was supported by the consistent presence of quercetin, with percentages of 101.68 ± 0.93 and 103.59 ± 0.37 % (w/w), remaining at 25 and 40 °C on day 90, consistent with levels observed on day 1. The restoration at 4°C was not recommended for this product due to the potential risk of decreased solubility and quercetin precipitation (Srinivas et al., 2010; Bhatia et al., 2022). To evaluate the stability of scar-reducing formulation, various physicochemical characteristics were simultaneously assessed. The United States Pharmacopeia (USP 46, 2023) indicated the criterion for the topical formulation was ±10% label amount, which pH, viscosity, quercetin remaining and antioxidant activity were felt in the criterion of ±10% compared to day 1. The stability study suggested that clinical efficacy may occur when the physical and chemical stability of the formulation is stable throughout the shelf-life of the product.
The in vitro release studies provided valuable insights into the scar-reducing formulation's performance. The comparable release rate of quercetin from the scar-reducing formulation (15% Allium cepa-micellar extract plus 0.01% quercetin), scar-reducing formulation plus 15% shallot-micellar extract and shallot-micellar extract and their superiority over the shallot-water extract, the Allium cepa extract and the commercial product suggest the potential of the formulated product for effective skin permeation. In a preliminary study, quercetin could not be detected in the Allium cepa extract. Thus, the shallot-micellar extract and shallot-water extract were included in the in vitro release. The quercetin content varied among the formulations. The scar-reducing formulation containing 15% Allium cepa-micellar extract plus 0.01% quercetin had a concentration of 0.0118% (w/w). When 15% shallot-micellar extract was added to the scar-reducing formulation, the quercetin content increased to 0.0123% (w/w). Shallot-micellar extract alone contained 0.0033% (w/w) quercetin. Shallot-water extract showed a lower concentration of 0.0008% (w/w) quercetin. Both Allium cepa extract and the commercial product had undetectable levels of quercetin (0.0000% w/w). HPLC analysis revealed that shallot extract could serve as a promising alternative source of natural quercetin (Charoenchai et al., 2017; Adeyemo et al., 2023). The limited solubility of quercetin poses a challenge to its utilization in pharmaceutical formulations (Vigneshwari et al., 2023). Therefore, the micellar system of quercetin in Allium cepa and shallot extract was thoroughly investigated in this study. Although the quercetin content in the Allium cepa extract, shallot-micellar, and water extract was below detectable levels, their antioxidant activity was still observed (data not show). Therefore, all formulations were essential for conducting the in vitro release studies. The release rate of quercetin from the shallot-micellar extract was greater than that of the shallot-water extract. The application of micellar-assisted extraction may essential aspect for further investigation (Duangjit et al., 2023a; Duangjit et al., 2023b). This study confirms that the quercetin content can be released from the scar-reducing formulation (Scar Q Plus), the scar-reducing formulation incorporating shallot-loaded micelle, and the shallot-loaded micelle.
In vitro skin permeation was conducted to confirm the risk of nanotechnology (e.g., micelle formulation) (Schneider et al., 2009). The snakeskin was chosen for this study due to its structural similarity to human skin, including thin, flat cells and intercellular phospholipids, and its ethical ease of collection without harming the animal (Abd et al., 2016). Moreover, previous studies have shown similar permeability coefficients for certain drugs, validating their use for initial screening (Rigg and Barry, 1990; Megrab, Williams, and Barry, 1995). In this study, the quercetin-loaded scar-reducing formulation and shallot-micelle extraction could not permeate the snakeskin, ensuring no risk of systemic absorption and minimizing safety concerns. Interestingly, a previous study suggested that quercetin in micelles of sodium dodecyl sulfate and ammonium dodecyl sulfates (anionic surfactants) could penetrate the skin through pores due to its larger head group size, reducing steric hindrance and allowing better micellization with quercetin (Vigneshwari et al., 2023). However, the micelle utilized in our study was polysorbate 20 (a nonionic surfactant), and the model membrane lacked pores (snakeskin). These comparisons suggest that while anionic surfactant as ammonium dodecyl sulfate could enhance quercetin's skin permeability, the use of Tween 20 ensures safety by preventing unwanted skin penetration and irritation, making it a promising choice for scar reduction formulations. Therefore, the observed skin permeation of quercetin in our study contradicted previous research findings. It's plausible that the type and charge of the micelle, as well as the skin model utilized, may influence the permeation potential. The skin safety of the topical formulation was confirmed again by FTIR spectra and DSC thermogram. The FTIR spectra of contact untreated skin have no significant difference compared to the treated skin. A CH2 symmetry, CH2 asymmetry, amide I, amide II, and amide III peak show that the treated skin was similar to the contact untreated skin. However, the skin disruption was observed under the DSC thermogram.
The DSC thermogram confirmed that the peaks near 70 to 80 °C and 227 to 230 °C were minor changes. This phenomenon may indicate that the scar-reducing formulation affects the microstructure of the skin by disrupting the protein in the skin at the peak near 70 to 80 °C and disrupting the solid lipid of the skin at the peak near 227 to 230 °C. The stratum corneum lipid of the snake skin exists as a solid gel at the peak near 227 to 230 °C. In the DSC study, when the skin was treated with a scar-reducing formulation, which exists as a gel state formulation, the thermal properties shifted in the minor. The peak near 230°C of skin treated with formulation was absent compared to the untreated skin. The change in endothermic peak suggests an increase in gross fluidity of the stratum corneum lipids (Duangjit et al., 2011).
This result was consistent with the general view that the mechanism of action of the topical formulation was attributed to the alteration of the lipid organization and an increase in lipid lamellae disorder in the stratum corneum. The skin permeation mechanism studies revealed the skin permeation mechanism via stratum corneum lipid alteration. FTIR and DSC were a combined potential technique to investigate the microstructure of the skin that was treated with a gentle formulation that significantly affected the skin barrier of the stratum corneum (Duangjit, et al., 2023).
CONCLUSION
Our findings provide evidence that the scar-reducing formulation based on the commercial packaging of plastic tubes (15 g) and paper boxes was stable under the recommended storage condition at 25°C, without exceeding 40°C for 90 days. This recommendation is important for preserving the formulation's integrity. The positive stability behavior observed in this study supports its potential for effective scar management, although further clinical studies are necessary to confirm its efficacy throughout its shelf life. The potential of the formulation as a reliable intervention for scar prevention and improvement was suggested by its efficacy in consistently delivering active ingredients (15% Allium cepa-micellar extract, 0.01% quercetin) and maintaining stability. The unique attributes of the formulated product may be further elucidated through a comparison with other commercial formulations, especially those containing Allium cepa and quercetin. Future comparative studies have the potential to provide a comprehensive understanding of the strengths and advantages of different formulations. The necessity for long-term stability assessment and the relatively short duration of the study were acknowledged as essential limitations in investigating this new product in the field of scar-reducing formulation. An enhanced understanding of the formulation's performance over time will be attained through extended clinical studies and real-world applications.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Office of the Higher Education Commission and the Thailand Research Fund (Grant no. RGNS 64–237) for financial support. Grateful thanks also go to the Faculty of Pharmaceutical Sciences, Ubon Ratchathani, Thailand, for supporting facilities and equipment. I would like to express my sincere gratitude to the Industrial Technology Advisors Program, Ubon Ratchathani University Network (ITAP UBU) and the Next Chapter Co., Ltd. for their invaluable contributions to the initiation of the product prototype. Their insights, dedication, and collaborative efforts have been instrumental in laying the foundation for this innovative project. Their support and expertise have greatly enriched the development process. The authors are truly thankful for the opportunity to work alongside such committed and talented individuals. In addition, the authors would like to thank the Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand, for kindly providing the shed snake skin used in the pre-formulation study.
AUTHOR CONTRIBUTIONS
Sureewan Duangjit contributed to the conceptualization and original draft preparation, performed the statistical analysis and data visualization, wrote the manuscript and supported the funding acquisition. Suphaphon Jandum and Pakapol Palakun assisted in conducting the experiments. Worranan Rangsimawong, Phaijit Sritananuwat, Tipada Samseethong and Sureewan Bumrungthai assisted in the methodology and edited the manuscript. Kusuma Jitsaeng supported the extraction methodology and edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
Abd E., Yousuf S., Pastore M., Telaprolu K., Mohammed Y., Namjoshi S., Grice J., and Roberts M. 2016. Skin models for the testing of transdermal drug, Clinical Pharmacology: Advances and Applications. 2016(8): 163-176.
Adeyemo A.E., Omoba O.S., Olagunju A.I., and Josiah S.S. 2023. Assessment of nutritional values, phytochemical content, and antioxidant properties of shallot (Allium ascalonicum L.) leaf and bulb. Measurement: Food. 10: 100091.
Ahmed N., Deveswaran R., Parasuraman P., Bharath S., Priyanka C., and Santosh P.S. 2023. Topical delivery of natural extract for accelerated wound healing, Materials Today: Proceedings. 80: 481-491.
Ang L.F., Yam M.F., Fung Y.T.T., Kiang P.K., and Darwin Y. 2014. HPLC method for simultaneous quantitative detection of quercetin and curcuminoids in traditional chinese medicines. Journal of Pharmacopuncture. 17(4): 36-49.
Bhatia N.K., Raj Tomar V., Ishika, Kishor S., and Deep S. 2022. Effect of pH and temperature on physicochemical properties, aggregation behaviour and degradation kinetics of quercetin and baicalein in nearly aqueous media. Journal of Molecular Liquids. 366(15): 120236.
Chakraborty A.J., Uddin T.M., Matin Zidan B.M.R., Mitra S., Das R., Nainu F., Dhama K., Roy A., Hossain M.J., Khusro A., and Emran T.B. 2022. Allium cepa: A treasure of bioactive phytochemicals with prospective health benefits. Evidence-Based Complementary and Alternative Medicine. 2022: 4586318.
Chanprapaph K., Tanrattanakorn S., Wattanakrai P., Wongkitisophon P., and Vachiramon V. 2012. Effectiveness of onion extract gel on surgical scars in Asians. Dermatology Research and Practice. 2012: 212945.
Charoenchai L., Pathompak P., Phetmanee T., and Meksuriyen D. 2017. Relationships between chemical compositions of shallot extracts and antioxidant activity. RSU International Research Conference. 2017: 97-104.
Chiarentin L., Cardoso C., Miranda M., and Vitorino C. 2023. Rheology of complex topical formulations: An analytical quality by design approach to method optimization and validation, Pharmaceutics. 15(7): 1810.
Conti V., Corbi G., Iannaccone T., Corrado B., Giugliano L., Lembo S., Filippelli A., and Guida M. 2020. Effectiveness and tolerability of a patch containing onion extract and allantoin for cesarean section scars. Frontiers in Pharmacology. 11: 569514.
Duangjit S., Kenmat P., Pantamas W., Sriananuwat P., Rangsimawong W., Samseethong T., Jitsaeng K., and Bamrungthai S. 2023a. Comparative extraction of quercetin yield from shallot using organic solvent and non-organic solvent: Types and concentrations. p. 219-226. In: Rattanachaikunsopon P. (eds) Proceeding of UBU 2023, The 17th Annual Meeting of Ubon Ratchathani University Conference. Ubon Ratchathani, 20 Jul-21 Jul 2023. Ubon Ratchathani University, Ubon Ratchathani.
Duangjit S., Opanasopit P., Rojanarata T., and Ngawhirunpat T. 2011. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. Journal of Drug Delivery. 2011: 418316.
Duangjit S., Pantamas W., Kenmat P., Sriananuwat P., Rangsimawong W., Samseethong T., Jitsaeng K., and Bamrungthai S. 2023b. Development of extraction method of quercetin from shallot: A combination of ultrasound-assisted extraction and micellization using response surface methodology. p.227-237. In: Rattanachaikunsopon P. (eds) Proceeding of UBU 2023, The 17th Annual Meeting of Ubon Ratchathani University Conference. Ubon Ratchathani, 20 Jul-21 Jul 2023. Ubon Ratchathani University, Ubon Ratchathani.
Duangjit S., Takayama K., Bumrungthai S., Mahadlek J., Ngawhirunpat T., and Opanasopit P. 2023. Development of invaethosomes and invaflexosomes for dermal delivery of clotrimazole: optimization, characterization and antifungal activity. Pharmaceutical Development and Technology. 28(7): 611-624.
Esoje E., Muazu J., and Madu S. 2016. Formulation and in-vitro assessment of cream prepared from Allium cepa L., bulb. Asian Journal of Pharmaceutical Science & Technology. 6(1): 1-5.
Hosnuter M., Payasli C., Isikdemir A., and Tekerekoglu B. 2007. The effects of onion extract on hypertrophic and keloid scars. Journal of Wound Care. 16(6): 251-254.
Kim H.T., Yoo M., Yang E.-J., Song K.-S., Park E.J., and Na D.H. 2023. The importance of pH for the formation of stable and active quercetin–polyamidoamine dendrimer complex. Bulletin of the Korean Chemical Society., 44(4): 363-369.
Lukić M., Pantelić I., and Savić S.D. 2021. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics. 8(3): 69.
Marefati N., Ghorani V., Shakeri F., Boskabady M., Kianian F., Rezaee R., and Boskabady M.H. 2021. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharmaceutical Biology. 59(1): 287-302.
Megrab N.A., Williams A.C., and Barry B.W. 1995. Oestradiol permeation through human skin and silastic membrane: Effects of propylene glycol and supersaturation. Journal of Controlled Release. 36(3): 277-294.
Mounir R., Alshareef W.A., El Gebaly E.A., El-Haddad A.E., Ahmed A.M.S., Mohamed O.G., Enan E.T., Mosallam S., Tripathi A., Selim H.M.R.M., Bukhari S.I., Alfaraj R., Ragab G.M., El-Gazar A.A., and El-Emam S.Z. 2023. Unlocking the power of onion peel extracts: Antimicrobial and anti-inflammatory effects improve wound healing through repressing notch-1/nlrp3/caspase-1 signaling. Pharmaceuticals. 16(10): 1379.
Napavichayanun S., Vasuratna A., Santibenchakul S., Cherdchom S., and Aramwit P. 2022. Evaluating efficacy and safety of the topical silicone gel containing onion extract in the treatment of post-cesarean surgical scars. Journal of Cosmetic Dermatology. 21(7): 2908-2915.
Poetschke J., and Gauglitz G.G. 2020. Onion Extract. in L. Téot, et al. (eds.), Textbook on scar management: State of the art management and emerging technologies. Springer Cham, Cham.
Prager W., and Gauglitz G.G. 2018. Effectiveness and safety of an overnight patch containing Allium cepa extract and allantoin for post-dermatologic surgery scars. Aesthetic Plastic Surgery. 42(4): 1144-1150.
Rigg P.C., and Barry B.W. 1990. Shed snake skin and hairless mouse skin as model membranes for human skin during permeation studies. Journal of Investigative Dermatology. 94(2): 235-240.
Schneider M., Stracke F., Hansen S., and Schaefer U.F. 2009. Nanoparticles and their interactions with the dermal barrier. Dermato-Endocrinology. 1(4): 197-206.
Shin J., Cho J.T., Park S.I., and Jung S.N. 2019. Combination therapy using non-ablative fractional laser and intralesional triamcinolone injection for hypertrophic scars and keloids treatment. International Wound Journal. 16(6): 1450-1456.
Srimai K., and Akarapisan A. 2023. Occurrence, identification and preliminary biological control of bulb rot of onion (Allium cepa). Chiang Mai Journal of Science. 50(3):e2023031.
Srinivas K., King J.W., Howard L.R., and Monrad J.K. 2010. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. Journal of Food Engineering. 100(2): 208-218.
United States Pharmacopeia and National Formulary (USP 46-NF 41). United States Pharmacopeial Convention; 2023.
Vigneshwari R., Sivakumar K., Parinamachivayam G., Ragavendran V., Rajesh P., and Dash S. 2023. A proof of concept for the micellization of quercetin with anionic surfactants: Effect of counter ion. Journal of Molecular Liquids. 388: 122671.
Wallace H.A., Basehore B.M., and Zito. P.M. 2023. Wound healing phases. StatPearls Publishing: Treasure Island. Statpearls Publishing, LLC, Florida.
Wananukul S., Chatpreodprai S., Peongsujarit D., and Lertsapcharoen P. 2013. A prospective placebo-controlled study on the efficacy of onion extract in silicone derivative gel for the prevention of hypertrophic scar and keloid in median sternotomy wound in pediatric patients. Journal of The Medical Association of Thailand. 96(11): 1428-1433.
Wang W., Sun C., Mao L., Ma P., Liu F., Yang J., and Gao Y. 2016. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends in Food Science & Technology. 56: 21-38.
Yuan X., Shen J., Chen L., Wang L., Yan Q., and Zhang J. 2021. Onion extract gel is not better than other topical treatments in scar management: A meta-analysis from randomised controlled trials. International Wound Journal. 18(3): 396-409.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Sureewan Duangjit1, 2, *, Suphaphon Jandum1, Pakapol Palakun1, Worranan Rangsimawong1, 2, Phaijit Sritananuwat1, 2, Tipada Samseethong1, 2, Kusuma Jitsaeng1, 2, and Sureewan Bumrungthai1, 2
1 Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand.
2 Innovation in Drug and Extract of Agriculture (IDEA) Research Group, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand.
Corresponding author: Sureewan Duangjit, E-mail: sureewan.d@ubu.ac.th
ORCID: Sureewan Duangjit: https://orcid.org/0000-0002-6187-9368
Total Article Views
Editor: Wipawadee Yooin
Chiang Mai University, Thailand
Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: April 13, 2024;
Revised: September 9, 2024;
Accepted: September 12, 2024;
Online First: October 1, 2024

