
Implementing Sediment Delivery Model-Orodispersible Tablet (SeDeM-ODT) Expert System in Cetirizine Dihydrochloride Orally Disintegrating Mini-Tablets (ODMTs) Formulation
Phennapha Saokham and Karnkamol Trisopon*Published Date : October 1, 2024
DOI : https://doi.org/10.12982/NLSC.2024.066
Journal Issues : Number 4, October-December 2024
Abstract In recent years, pediatric medicine has shifted towards solid oral dosage forms, moving away from liquid formulations to create tailored platforms for children. Orally disintegrating mini-tablets (ODMTs), with a diameter below 3 mm, dissolve rapidly in the mouth and represent a promising option. This study aimed to develop ODMTs using various co-process excipients, assessing their suitability with the Sediment Delivery Model-Orally Disintegrating Tablet (SeDeM-ODT) expert system. Favorable Index of Good Compressibility and Bucodispersibility (IGCB) values (> 5) were found for F-MELT® and co-process rice starch (CR), utilizing for ODMT formulation. Cetirizine dihydrochloride (CET) was evaluated for direct compression suitability, revealing flowability deficiencies. Therefore, co-process excipient quantities were adjusted based on flowability compensation. CET-ODMTs containing F-MELT® exhibited higher drug loading capacity than CR (5 and 2.5 mg/table, respectively). Uniformity of mass, content uniformity, and friability met acceptance criteria with no significant differences between formulations. F-MELT®-based CET-ODMTs showed slower wettability due to magnesium stearate lubrication. Ultimately, the SeDeM-ODT expert system facilitated the excipient selection and formulation development, resulting in ODMTs meeting requirements with minimal experimentation.
Keywords: SeDeM-ODT, Cetirizine dihydrochloride, Co-process rice starch, Orally disintegrating mini-tablets, F-MELT®
Funding: This research project was supported by Faculty of Pharmacy, Chiang Mai University.
Citation: Saokham, P. and Trisopon, K. 2024. Implementing sediment delivery model-orodispersible tablet (SeDeM-ODT) expert system in cetirizine dihydrochloride orally disintegrating mini-tablets (ODMTs) formulation. Natural and Life Sciences Communications. 23(4): e2024066.
INTRODUCTION
Tablets represent the most favorable solid dosage form in the oral administration route due to their convenience. However, size and palatability have been notable concerns, particularly in pediatric and geriatric patients. Furthermore, young children, particularly those under the age of 6, may struggle to swallow conventional tablets or capsules (European Medicines Agency, 2006). Various solid dosage forms, including oral granules, pellets, and orodispersible solid forms, have been developed to address these challenges. Mini-tablets have emerged as a promising solution for pro-schoolers (European Medicines Agency, 2013). Defined as tablets with a diameter of 3 mm or less (Lennartz and Mielck, 1998), minitablets offer versatility in administration, either taken directly or suspended in semisolid or liquid media. Numerous studies verify the swallowability of mini-tablets in young children and mini-tables also offer advantages over other formulations (Thomson et al., 2009; Spomer et al., 2012; Klingmann et al., 2013; Mitsui et al., 2022; Miyazaki et al., 2022). Another innovation in pediatric medication delivery is orally disintegrating mini-tablets (ODMTs), defined by the United States Food and Drug Administration (US FDA) as solid dosage forms that disintegrate in saliva within 30 seconds without requiring chewing or liquids (United States Food and Drug Administration, 2008). ODMTs offer a promising solution for infants and toddlers due to their rapid disintegration and ease of administration (Krause and Breitkreutz, 2008).
Like orally disintegrating tablets (ODTs), ODMTs can undergo various manufacturing processes such as lyophilization, molding, and direct compression. Direct compression is the most favorable among these methods due to its convenience and cost-effectiveness. Manufacturing of mini-tablets is feasible using conventional tablet presses equipped with multiple-tip tooling. However, the efficacy of this technique is based on the characteristics of both active pharmaceutical ingredients (APIs) and excipients, including their flowability, compressibility, and compatibility (Shukla et al., 2009; Stoltenberg and Breitkreutz, 2011). Consequently, selecting excipients that exhibit superior direct compression properties is paramount in the formulation development process. The Sediment Delivery Model (SeDeM) expert system presents a pioneering approach to tablet formulation and formulation studies, established by the Quality by Design (QbD) paradigm described in the International Council for Harmonisation (ICH) guideline Q8 (R2) Pharmaceutical development. The system provides the physical characteristics of APIs, excipients, and the final powder mixture regarding their suitability for direct compression. By suppressing conventional micrometric analyses, SeDeM comprehensively evaluates direct compression parameters, thereby providing a comprehensive assessment of a powder’s suitability for direct compression (Suñé Negre et al., 2011). Once the profiles of the APIs and proposed excipients are determined, the final formulation and the proportions of the requisite excipients are evaluated through mathematical computations. A novel export system known as SeDeM-ODT was recently introduced in ODT formulation. The SeDeM-ODT expert system integrates the characteristic attributes of the SeDeM expert system with an additional parameter relating to disintegration properties. Therefore, it provides recommended ODT formulations with a high probability of successful compression and rapid disintegration (Aguilar-Díaz et al., 2012; Aguilar et al., 2013). Various studies have utilized both expert systems to explore individual excipients and APIs, streamlining tablet formulation processes (Campiñez et al., 2016; Wan et al., 2019; Khan et al., 2022; Castañeda Hernández et al., 2023). Consequently, the present study endeavors to validate the applicability of SeDeM-ODT in developing ODMTs that conform to ODT specifications.
Co-processed excipients, comprised of a combination of two or more compendial or non-compendial excipients, are engineered to modify physical properties without affecting chemical properties (Bhatia et al., 2022). Typically, these excipients demonstrate superior properties compared to the physical mixture of individual components. Ideally, they blend plastic and brittle deforming materials to prevent compression-related issues such as capping and lamination. Classified based on their constituents, co-processed excipients fall into four main groups: starch-based, cellulose-based, sugar-based, and miscellaneous (Rojas et al., 2012). Several commercially available sugar-based co-processed excipients, including F-MELT®, Ludiflash®, GalenIQTM 721, Pharmaburst® 500, and PROSOLV® ODT G2, have been promoted for ODT formulations (Woyna-Orlewicz et al., 2023). Furthermore, several studies have proposed novel co-process excipient composed of various composites. Amping these, a promising candidate is rice starch-based co-process excipient produced using a spray-dried technique (Trisopon et al., 2020). This excipient contains an optimal ratio of cross-linked carboxymethyl rice starch and silicon dioxide, which imparts potential as a multifunctional excipient for direct compression processes. This study investigated MCC-based, sugar-based, and rice starch-based co-processed excipients. Following the selection of co-processed excipients with optimal characteristics, CET-ODMTs were formulated. Subsequently, the physicomechanical properties of those ODMTs were evaluated to ensure compliance with ODT requirements.
Cetirizine dihydrochloride (CET) is a selective H1 receptor antihistamine drug with rapid onset and prolonged efficacy (Portnoy and Dinakar, 2004). It is authorized for administration to European children aged two years and older and to children in the United States aged six months and above for the treatment of allergic rhinitis and chronic spontaneous urticaria. (Parisi et al., 2020). Marketed under the tradename Zyrtec® by Pfizer, Inc., in various dosage forms. Eurand Pharmaceuticals, Inc. has pioneered the development of CET ODT utilizing a combination of microcaps® and advanTab® technologies. (Douroumis et al., 2011). Despite the pediatric suitability of CET, conventional ODT sizes may pose swallowing difficulties. ODMTs present a promising alternative. To the best of our knowledge, however, no ODMT formulations are commercially available. Hence, this study aimed to formulate CET-ODMTs with a minimal excipient composition utilizing the facilitate of SeDeM-ODT expert system.
MATERIALS AND METHODS
Materials
F-MELT® Type C was provided by Fuji Chemical Industry Co., Ltd, while PROSOLV® ODT G2 and PROSOLV® SMCC 90 were purchased from JRS Pharma, USA, as direct compressible commercial co-processed excipients. Spray-dried co-processed rice starch (CR) obtained from Trisopon (Trisopon et al., 2020) were utilized as customized co-process excipients. Cetirizine dihydrochloride (CET) was purchased from Ambeed, Inc., USA. Methanol HPLC grade was obtained from RCI Labscan, Thailand, deionized (DI) water was produced by Optima 10, ELGA Labwater, United Kingdom, and other chemicals such as methylene blue were analytical grade.
Characterization of co-processed excipients using the SeDeM-ODT expert system
Four co-processed excipients were selected as representatives of commercial and experimental categories. PROSOLV® and PROSOLV® ODT G2, which combine microcrystalline cellulose (MCC) axillary excipients, were chosen for their suitability in direct compression and ODT formulation, respectively. F-MELT® type C was included as a representative sugar-based co-processed excipient recognized for its claimed versatility as a universal excipient for ODT formulation. Lastly, co-processed rice starch, developed through a spray-dried process, was selected as a representative of rice starch-based co-processed excipients.
The SeDeM-ODT methodology comprises six factors derived from 15 parameters, five of them (i.e., Dimension, Compressibility, Flowability/Powder flow, Lubricity/Stability, and Lubricity/Dosage) align with those in the SeDeM expert system proposed by Suñé-Negre and colleagues (Suñé-Negre et al., 2008; Suñé Neģre et al., 2011; Suñé Neģre et al., 2013; Suñé-Negre et al., 2014). Based on prior research (Aguilar-Díaz et al., 2009; Aguilar-Díaz et al., 2012; Aguilar et al., 2013), the 15 parameters, categorized into six incidence factors through experimental and mathematically quantitative determination of powder characterization, at least in triplicate, were as follows:
Dimension: Bulk density (Da) and Tapped density (Dc) measured in accordance with the European Pharmacopeia (2.9.34). An accurate mass (M) of the power was poured into a 100-ml graduated cylinder. The bulk volume (Va) was then recorded, and bulk density was calculated using the equation provided in Table 1. The powder-filled graduated cylinder was subsequently tapped continuously using a Jolting volumeter (Stav 2003, Erweka, Germany). The tapped volume (Vc) was recorded once the difference between the volume after 500 taps and 1250 taps was less than 2 ml. Finally, the tapped density was determined from mass (M) and tapped volume (Vc) using the equation provided in Table 1.
Compressibility: Inter-particle porosity (Ie) and Carr Index (IC) were calculated based on the bulk and tapped densities using the equation provided in Table 1. The Cohesion Index (Icd) was used to indicate the maximum compressive stress that a tablet can withstand. For this test, 100 mg of powder was compressed to a 5-mm flat-face tablet using a hydraulic tablet press (C, Carver, USA) under a compression force of 0.5 ton. The tablet’s crushing strength (N) was then measured using a tablet hardness tester (PTB-311 PharmaTest, Germany).
Flowability/Powder flow: Hausner ratio (IH) was calculated based on the bulk and tapped densities to assess the flowability of powder, using the equation provided in Table 1. The angle of repose (α) and powder flow (t˝) were evaluated using a 10-cm fixed-height funnel, following the guidance of the European Pharmacopeia (2.9.36). An accurate mass of powder was poured into a closed-end funnel mounted on a stand, allowing the powder to flow freely through the funnel’s orifice by gravity. The time taken for the powder to flow through the funnel, expressed in seconds related to 100 g of powder, was recorded as t˝. Additionally, the angle of repose was calculated based on the height (h) and radius (r) of the powder pile using the equation provided in Tablet 1.
Lubricity/Stability: Loss on drying (%HR) of approximately 1 g of powder was determined using a moisture analyzer (MX-50, A&D, Japan). An accurate initial mass (M1) was placed into an aluminum pan and heated at 105 ± 2 °C until a constant mass (M2) was obtained. The loss on drying was then calculated using the equation provided in Table 1. Hygroscopicity (%H) was measured by placing a pre-weight 2.5-cm diameter polyethylene cup containing approximately 250 mg of powder (H1) into a sealed container maintained at 75 ± 5% relative humidity at ambient temperature. After 24 hours, the cup was re-weighed (H2), and the percentage of the weight gain was calculated using the equation provided in Table 1, to determine the hygroscopicity of the powder.
Lubricity/Dosage: Particle size < 45 μm (%Pf) or the percentage of particle smaller than 45 μm represented the proportion of powder that passed through a 45 μm sieve. This test was conducted using a sieve vibrator (AS 200 Control, Retsch, Germany) at 1.0 g for 10 min with approximately 20 g of powder. Homogeneity Index (Iθ) was evaluated using the same sieve vibrator with four different sieve sizee (45, 106, 212, and 355 μm). The Homogeneity Index was then calculated based on fraction of mass (Fmn) using the equation provided in Table 1.
Disgregability: The 5-mm flat-face tablets were prepared in the same manner as in the Cohesion Index study. Effervescence (DE), representing the disintegration time of tablet in deionized (DI) water, was determined by placing each tablet into a beaker containing an excess amount of DI water at ambient temperature. The time, in minutes, was recorded at the point when the tablet completely disintegrated. Disintegration time with disk (DCD) and Disintegration time without disk (DSD) were determined using the United State Pharmacopoeia (USP) disintegration apparatus, with DI water controlled at 37 ± 2 °C. The tablet disintegration times were observed and recorded once the tablet had fully disintegrated and passed through the sieve at the bottom of the disintegrating basket.
The powder characterization of four co-processed excipients was determined using the SeDeM-ODT expert system. Conversely, twelve powder parameters, derived from the five factors of the SeDeM expert system, were utilized to evaluate cetirizine dihydrochloride. The application of the SeDeM expert system to the API is anticipated to yield favorable disgregability properties, facilitated by the bucodispersibility characteristics of the co-processed excipients.
Determination of the index parameter (IP), parameter profile index (IPP), and index of good compressibility and bucodispersibility (IGCB)
Fifteen parameters were converted to diagram radius (r) using established equations validated by Aguilar and coworkers (Aguilar-Díaz et al., 2009; Aguilar-Díaz et al., 2012; Aguilar et al., 2013) (Table 1). The SeDeM-ODT diagram consisted of 15-sided polygons plotted with radius scales ranging from 0 to 10. Subsequently, the six incidence factors of the SeDeM-ODT expert system were calculated on the basis of these radii, as per prior research (Aguilar-Díaz et al., 2009; Aguilar-Díaz et al., 2012; Aguilar et al., 2013). These radius values also facilitated the determination of three indexes: Index Parameter, IP (Equation 1), Parameter Profile Index; IPP (Equation 2), and Index of Good Compressibility and Bucodispersibility; IGCB (Equation 3).

where No.P ≥ 5 donates the number of parameters with a value equal to or higher than 5, while No.Pt refers to the total number of parameters studied. The acceptability threshold for IP is set at an equal to or higher than 0.5.
IPP = Mean radius of all parameters (2)
The acceptability threshold for IPP would correspond to equal or higher than 5.
IGCB = IPP × ƒ (3)
where f represents the ratio of the polygon area to the circle area. For 15-sided polygons, f is 0.971. To calculate the Good Compressibility Index (IGC) for 12-sided polygons, f is 0.952. The acceptance threshold for both IGCB and IGC is set at five or higher. While IGCB indicates a good aptitude for compression and good disintegration properties, IGC represents the compressibility of the powder.
Table 1. Parameters and their conversion equations for the SeDeM-ODT expert system.
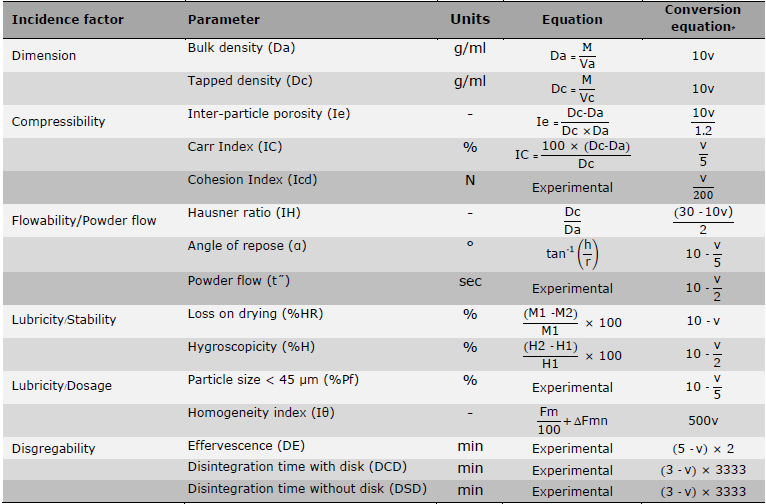
Note: * The value of v in the conversion equation indicates the experimentally or mathematically determined value of the corresponding SeDeM-ODT parameter.
Formulation of cetirizine dihydrochloride orally disintegrating mini-tablets (CET-ODMTs) using the SeDeM-ODT expert system
The SeDeM-ODT expert system was employed for the formulation development of direct compressible ODMTs containing CET. The deficiency factor of cetirizine dihydrochloride concerning the SeDeM expert system was evaluated. Additionally, co-processed excipients were chosen based on their IGCB values. A final formulation of each selected excipients, comprising a combination of CET and co-processed excipients, was determined through mathematical evaluation to compensate the deficiency factor of the CET according to Equation 4 proposed by Suñé-Negre (Suñé-Negre et al., 2008; Suñé Neģre et al., 2011; Suñé Neģre et al., 2013; Suñé-Negre et al., 2014).
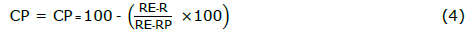
where CP represents the percentage of corrective co-processed excipients. R donates the incidence value of the deficiency to be obtained in the blend, while RE donates the incidence value of the deficiency of the corrective co-processed excipients. Finally, RP donates the incidence value of the deficiency factor in the CET to be corrected.
Furthermore, various CP values were calculated based on the variation in the target radius value to be obtained in the blend. Consequently, the R values of other incidence factors (except deficiency one) were recalculated based on the obtained CP calculated from the target radius value of deficiency incidence factor. Finally, the optimal R of CET ODMTs formulations was determined.
The compositions of CET ODMTs formulation were determined based on the optimal R, with a tablet weight of 14 mg and a batch size of 200 tablets. CET-ODMTs were fabricated using the direct compression method. Cetirizine dihydrochloride was uniformly blended with the calculated co-processed excipient employing the geometric dilution method for 10 min in a polyethylene bag. Subsequently, 1% magnesium stearate and 2% talc were incorporated and mixed for an additional 2 min. The resulting powder blend was then compressed using an eccentric tableting machine (CMT 12, Charatchai, Thailand) with 3-mm round, flat-face bevel edge tooling.
Evaluation of physicomechanical properties of CET-ODMTs
The uniformity of mass was determined following the procedure outlined in USP <905> Uniformity of dosage units. Thirty ODMTs were sampled, and each of the 20 tablets was individually weighed. The standard deviation values are calculated, and the acceptance criterion was not more than 10%. According to USP <905>, the degree of uniformity in the amount of the CET among the ODMTs required a content uniformity method. Ten randomly sampled tablets were individually weighed and dissolved in DI water to prepare a 50 μg/ml concentration. Each sample was then filtered through a 0.45 μm nylon syringe filter (Labfil, China), and the amount of CET was analyzed in triplicate using a Hewlett-Packard HP1100 High-Performance Liquid Chromatography (HPLC) system equipped with an Agilent Eclipse XDB-C18 (5 μm, 4.5 x 150 mm) column. The mobile phase consisted of 70%v/v methanolic solutions with a 1 ml/min flow rate. UV detection was performed at 230 nm. The linearity was established over the 5-200 μg/ml range with r2 = 0.9999. Subsequently, the acceptance value (AV) was calculated, with the maximum allowed acceptance value (L1) set at 15.0.
Tablet thickness was manually measured using a digital outside micrometer (3109-24A, INSIZE, Germany) and conducted on ten tablets. The radial crushing strength of 10 tablets was determined using a texture Analyzer (TA.XTplus, Stable Micro System, UK) equipped with a 2 mm diameter flat-face cylinder probe (P/2). The test speed was set to 0.1 mm/s with 5 g of trigger force. The maximum fracture force causing tablet breaks was recorded and converted to radial crushing strength by using the equation of flat-face tablets regarding Fell and Newton (Fell and Newton, 1970) as follow:
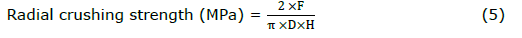
where F is the fracture force (N), D is the diameter (mm), and H is the thickness of the mini-tablet.
The friability was conducted following the procedures outlined in the USP <1216> Tablet friability with slight modifications as described by Hellberg and colleagues (Hellberg et al., 2021). Twenty-four tablets, corresponding to 310 mg, were dedusted and accurately weighed before and after undergoing 100 rotations at 25 rpm of friability apparatus (PTF 20E, Pharma Test, Germany). 6.3 g of 2-mm glass beads were added to decrease the number of tested tablets. The friability was then calculated and expressed as a percentage. The acceptable friability criterion was set at less than 1.0%.
Due to the small size of the ODMTs, determining disintegration time using standard apparatus was challenging. The disintegration time of the ODMTs was measured in a well of 24-well plate. After placing the tablet, each well was filled with 2 ml phosphate buffer saline pH 6.8 (37 ± 2 °C). The time until complete disintegration was recorded. It was noted that this test was carried out in static conditions. Additionally, wetting time was tested, assuming that a shorter wetting time indicates a shorter disintegration time of ODMTs. A filter paper (Whatman®, Germany) with a diameter of 5 mm was placed in each well of a 24-well plate. Then, 30 μl of 0.1%w/v methylene blue (Merck, USA) was added. A tablet was placed into the colored filter paper, and wetting time was recorded when the methylene blue reached the upper part of the table. The experiments of disintegration time and wetting time were conducted using six tablets. Although both tests relied on visual inspection, it was ensured that all ODMTs were determined on the same day by the same individual to mitigate any potential variation.
Statistical analysis
The results were expressed as mean ± standard deviation (SD), and 95% confidence intervals were applied. Statistically, the significant difference was determined using a two-tailed Student’s t-test, considering a P-value below 0.05. Statistical computations were conducted utilizing Microsoft Excel (Office 365, version 2403).
RESULTS
Characterization of the co-processed excipients using the SeDeM-ODT expert system
The co-processed excipients, including PROSOVL® SMCC 90, PROSOVL® ODT G2, F-MELT®, and co-processed rice starch (CR), were characterized with the SeDeM-ODT expert system. Subsequently, the radius values of fifteen parameters for these excipients are plotted, and their corresponding SeDeM-ODT diagrams are presented in Figure 1. Additionally, the individual SeDeM-ODT incidence factors were detailed in Table 2. Finally, the IP, IPP, and IGCB for the four co-processed excipients (Table 1) are calculated. Based on the acceptance criteria of IGCB (more than 5),
F-MELT® and CR emerged as suitable co-processed excipients for further ODMT formulation.
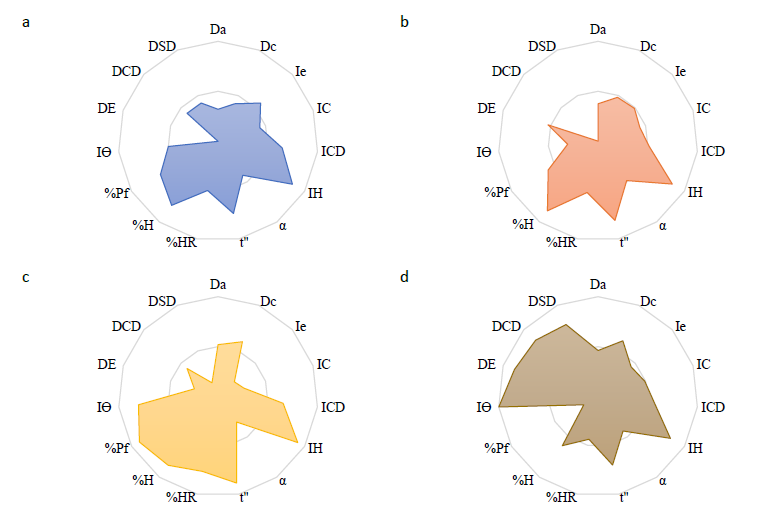
Figure 1. SeDeM-ODT diagrams of PROSOVL® SMCC 90 (a), PROSOVL® ODT G2 (b), F-MELT® (c), and co-processed rice starch (d).
Table 2. The SeDeM-ODT factors, including parameter index (IP), parameter profile index (IPP), and index of good compressibility and bucodispersibility (IGCB) for four co-processed excipients.
|
Incidence factors |
PROSOVL® SMCC 90 |
PROSOVL® ODT G2 |
F-MELT® type C |
CR |
|
Dimension |
3.65 |
4.27 |
5.60 |
5.35 |
|
Compressibility |
5.52 |
4.82 |
3.82 |
5.01 |
|
Flowability |
6.73 |
7.20 |
7.08 |
6.55 |
|
Lubricity/Stability |
6.48 |
6.95 |
8.08 |
5.23 |
|
Lubricity/Dosage |
5.83 |
4.41 |
8.57 |
5.83 |
|
Disgregability |
2.79 |
1.75 |
2.73 |
8.37 |
|
IP |
0.67 |
0.47 |
0.60 |
0.60 |
|
IPP |
5.72 |
4.84 |
5.69 |
6.17 |
|
IGCB |
4.99 |
4.70 |
5.53 |
5.99 |
Note: Bold indicated higher than the acceptable values.
Characterization of cetirizine dihydrochloride (CET) using the SeDeM expert system
Cetirizine dihydrochloride (CET) was evaluated for 12 parameters according to the SeDEM expert system (Figure 2). Subsequently, five incidence factors and three indexes were calculated (Table 3).
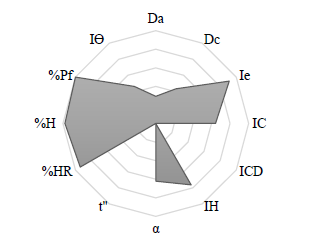
Figure 2. SeDeM diagrams of cetirizine dihydrochloride.
Table 3. The SeDeM expert system factors, including parameter index (IP), parameter profile index (IPP), and index of good compressibility (IGC) for cetirizine dihydrochloride.
|
Incidence factors |
Cetirizine dihydrochloride |
|
Dimension |
3.63 |
|
Compressibility |
5.19 |
|
Flowability |
4.62 |
|
Lubricity/Stability |
9.59 |
|
Lubricity/Dosage |
7.31 |
|
IP |
0.58 |
|
IPP |
5.87 |
|
IGC |
5.59 |
Note: Bold indicated higher than the acceptable values.
Formulation of cetirizine dihydrochloride orally disintegrating minitablets (CET-ODMTs)
According to the SeDeM results, CET exhibited adequate properties for direct compression technique. It represented a compressible, limited hygroscopic API with good homogeneity in particle size, although its flowability emerged as a critical factor in direct compression application. Therefore, the utilization of excipients with superior flowability was required. Analysis of SeDeM-ODT incidence factors and diagrams revealed that four co-processed excipients optimize flowability for CET formulations. However, one drawback of ODMT formulations was their disintegration. Among these excipients, only CR demonstrated acceptable values of disgregability factor and IGCB. Furthermore, although F-MELT® exhibited promising IGCB value, its disgregability factor value did not meet acceptable standards. Consequently, CR and F-MELT® emerged as the excipients of choice for CET ODMT formulations. The required percentage of F-MELT® and CR is determined using Equation 4 to enhance flowability while maintaining balance across other SeDeM-ODT factors. Initially, the percentage of corrective excipient (CP) was calculated based on the flowability/powder flow factor, i.e., deficiency factor. Various CP values were calculated based on the variation in the target radius value (R) to be obtained in the blend, ranging from 4.65 to 6.55. Values outside this range resulted in negative CP values, indicating infeasibility. The relationship between the target radius values in the blend and the calculated CP was illustrated in Figure 3a. Subsequently, other SeDeM-ODT factors were recalculated per the resulting percentage of corrective excipient, ensuring acceptability across all incidence factors, as presented in Figure 3b-3e.
For CR, it was observed that the increasing corrective flowability/powder flow factor (R), from 4.65 to 6.55, correlated with a rise in the percentage of corrective CR (Figure 3a Close circle), resulting in dimensional increments (Figure 3b Close circle) and lubricity/dosage incidence factor values (Figure 3e Close circle) while reductions in compressibility (Figure 3c Close circle) and lubricity/stability incidence factor value (Figure 3d Close circle). Conversely, for F-MELT®, varying R from 4.65 to 7.05 increased the CP value (Figure 3a Open circle) and dimension incidence factor values (Figure 3b Open circle) but decrease compression (Figure 3c Open circle), lubricity/stability (Figure 3d Open circle), and lubricity/dosage incidence factor values (Figure 3e Open circle). Ultimately, the optimal percentage of F-MELT® and CR required to enhance flowability and maintain balance across SeDeM-ODT incidence factors were calculated when R = 6.2, as outlined in Table 4.
Table 4. The quantities of co-processed rice starch (CR) and F-MELT® necessary for cetirizine dihydrochloride orally disintegrating mini-tablets (CET-ODMTs) formulation and the corresponding SeDeM-ODT factors calculated concerning the corrective excipient when R = 6.2.
|
|
CR |
F-MELT® |
|
CP (% corrective excipient) |
82 |
62 |
|
Dimension |
5.04 |
4.86 |
|
Compressibility |
5.04 |
4.34 |
|
Flowability |
6.20 |
6.15 |
|
Lubricity/Stability |
6.02 |
8.65 |
|
Lubricity/Dosage |
6.09 |
8.09 |

Figure 3. Relationship between target radius value of flowability (R) and various calculated incidence factor values of co-processed rice starch (•) and F-MELT® (○).
While all incident factor values of the CR-based formulation remained within the acceptable limit, the values corresponding to the dimension and compressibility incidence factors of the F-MELT®-based formulation were less than acceptable criteria, i.e., less than 5. Therefore, those two factors should be carefully monitored during tableting. Consequently, the formulation of CET-ODMTs (Figure 4) involved the incorporation of either corrective CR or F-MELT®, blended with CET and lubricants, as outlined in Table 5.
Table 5. The formulation components of co-processed rice starch (CR)-based and F-MELT®-based cetirizine dihydrochloride orally disintegrating mini-tablets (CET-ODMTs).
|
Component |
Milligram per tablet |
|
|
CR-based |
F-MELT®-based |
|
|
CET |
2.5 |
5.0 |
|
CR |
11.1 |
- |
|
F-MELT® type C |
- |
8.6 |
|
Magnesium stearate |
0.1 |
0.1 |
|
Talc |
0.3 |
0.3 |

Figure 4. Flat-face bevel edge 3-mm orally disintegrating mini-tablet containing cetirizine dihydrochloride.
The targeted weight for both formulations was established at 14 mg per tablet, with a permissible deviation of 10%, resulting in a 12.6 to 15.6 mg range. It was worth noting that F-MELT®-based CET-ODMTs exhibited twice the drug loading compared to CR-based CET-ODMTs. Remarkably, both formulations exhibited the capacity for compression without necessitating pre-compression force application. Notably, no instances of capping or lamination were observed, particularly in the F-MELT®-based formula.
Evaluation of cetirizine dihydrochloride orally disintegrating minitablets (CET-ODMTs)
Both CR- and F-MELT®-based CET-ODMTs complied with the uniformity of mass and content uniformity criteria according to the corresponding chapters in USP <2091> and <905> (Table 6). Compliance with the uniformity of mass of single-dose preparation was achieved when the standard deviation was below 10%, demonstrating that the flowability of blend powder was sufficient and uniform. Regarding uniformity of dosage unit, both formulas exhibited an AV value below 15, indicating that the content uniformity of CET-ODMTs fell within acceptable limits. While the drug content of F-MELT®-based formulations adhered to the acceptance threshold (90.0-110.0% labeled amount) specified in the cetirizine dihydrochloride orally disintegrating tablets monograph, it was observed to be significantly lower compared to alternative formula.
The radial tensile strength and friability indicated adequate mechanical robustness for tableting. Both formulas had tensile strength of more than 3 MPa with friability of < 1%, reflecting the mechanical strength and stability of ODMTs under external force. Notably, CR-based CET-ODMTs demonstrated significantly higher tensile strength than another formulation (P < 0.05).
The CR-based formula's wetting time and disintegration time were approximately 20 seconds, whereas a significantly longer time was observed in F-MELT®-based CET-ODMTs.
Table 6. Characterization of cetirizine dihydrochloride orally disintegrating mini-tablets (CET-ODMTs).
|
Evaluation parameter |
CR-based |
F-MELT®-based |
|
Uniformity of mass (mg; n = 20) |
14.00 ± 0.77 |
13.90 ± 1.10 |
|
Uniformity of dosage unit (AV; n = 10) |
9.02 |
3.64 |
|
Uniformity of content (%; n = 10) |
107.48 ± 3.76* |
97.46 ± 1.52 |
|
Radial tensile strength (MPa; n = 10) |
6.40 ± 0.95* |
3.87 ± 0.87 |
|
Friability (%; n = 24) |
0.85 |
0.42 |
|
Wetting time (sec; n = 6) |
20.80 ± 12.20 |
526.50 ± 52.80* |
|
Disintegration time (sec; n = 6) |
17.20 ± 8.70 |
389.40 ± 51.40* |
Note: * indicated a significantly higher value at P-value < 0.05.
DISCUSSION
According to the SeDeM Expert System of CET, it was suggested that improvements were needed for dimension and flowability incidence factors. As ODMTs are miniature-sized tablets, it was unnecessary to improve the dimension incidence factor. Additionally, with acceptable factor values (> 5), it was suggested that CET exhibited homogeneity and limited hygroscopic properties, with good compressibility, rendering it suitable for the direct compression method. The acceptable values of IP, IPP, and IGC further supported this.
The required percentage of F-MELT® or CR, calculated regarding flowability compensation, suggested that although the flowability of CET was improved by incorporating CR, it may potentially impact the compressibility, moisture stability, and blend homogeneity of the blended powder. Consequently, considering the dimension factor implications on tablet size and tableting capacity, the inclusion of F-MELT® may influence both the size and compressibility of the mini-tablets. This could lead to compressibility-related issues such as capping or lamination, necessitating careful monitoring during the tableting process. However, the absence of capping and lamination in the F-MELT®-based formulation suggested that it possessed sufficient compressibility despite the compressibility incidence factor values falling below the acceptance threshold. This underscored the robustness and viability of the formulation in achieving the desired mini-tablet characteristics and performance parameters. Furthermore, compared to the CR-based formula, the twofold increase in loading capacity of the F-MELT®-based formulation may be attributed to the dimension factor value of F-MELT® falling below the acceptance criteria.
According to the physicomechanical properties of these two formulations, the significant difference in radial tensile strength may be attributed to the marginally elevated compaction pressure employed during manufacturing. Notably, the F-MELT®-based formulation exhibited delayed disintegration, potentially attributable to magnesium stearate, a hydrophilic excipient. Kuck and Breitkreutz (Kuck and Breitkreutz, 2022) proposed that this disparity could be attributed to a hydrophobic layer of magnesium stearate, utilized as a lubricant, exerting a significant impact on the mannitol-based co-processed excipients within the ODMT formulation. Conversely, a previous study (Douroumis et al., 2011) reported that CET ODTs containing mannitol as a diluent, combined with various superdisintegrants, exhibited disintegration times of less than 10 seconds. This suggested that the hydrophobic effect of magnesium stearate may be dependent on tablet size.
Substitution with alternative lubricating agents, such as sodium stearyl fumarate, may mitigate this effect. Additionally, it was noteworthy that the disintegration test was conducted under static conditions due to the diminutive size of the ODMTs, which may introduce variations from the standard technique described in the pharmacopeia.
CONCLUSION
The SeDeM-ODT expert system served as an effective tool for systematically selecting excipients suitable for mini-tablet formulations, considering six incidence factors (dimension, compressibility, flowability, lubricity/stability, lubricity/dosage, and disgregability) along with three parameter indexes: parameter index, parameter profile index, and index of good compressibility and bucodisperisibility. Additionally, this study demonstrated the application of the SeDeM-ODT expert system in identifying appropriate co-processed excipients for the formulation of cetirizine dihydrochloride orally disintegrating mini-tablets (CET-ODMTs). The results indicated that co-processed rice starch and F-MELT® type C emerged as a promising excipient. The formulation of CET-ODMTs was devised based on mathematical computations utilizing the SeDeM-ODT expert system, resulting in uniformity of content and weight. Mechanical properties such as tensile strength and friability met robust manufacturing requirements.
ACKNOWLEDGEMENTS
The authors were grateful to Fuji Chemical Industries Co, Ltd (Japan) and Maxway Company Limited (Thailand) for providing F-MELT® type C.
AUTHOR CONTRIBUTIONS
Phennapha Saokham assisted in conceptualization, performed the data acquisition and analysis, and wrote the original draft. Karnkamol Trisopon designed conceptualization, reviewed, and edited the final draft. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aguilar-Díaz, J.E., García-Montoya, E., Pérez-Lozano, P., Suñe-Negre, J.M., Miñarro, M., and Ticó, J.R. 2009. The use of the SeDeM diagram expert system to determine the suitability of diluents–disintegrants for direct compression and their use in formulation of ODT. European Journal of Pharmaceutics and Biopharmaceutics. 73(3): 414-423.
Aguilar-Díaz, J.E., García-Montoya, E., Suñe-Negre, J.M., Pérez-Lozano, P., Miñarro, M., and Ticó, J.R. 2012. Predicting orally disintegrating tablets formulations of ibuprophen tablets: An application of the new SeDeM-ODT expert system. European Journal of Pharmaceutics and Biopharmaceutics. 80(3): 638-648.
Aģuilar, J.E., Montoya, E.G., Lozano, P.P., Negre, J.M.S., Carmona, M.M., and Grau, J.R.T. 2013. New SeDeM-ODT expert system: An expert system for formulation of orodispersible tablets obtained by direct compression. p. 137-154. In J.E. Aģuilar [ed] Formulation tools for pharmaceutical development. Woodhead Publishing.
Bhatia, V., Dhingra, A., Chopra, B., and Guarve, K. 2022. Co-processed excipients: Recent advances and future perspective. Journal of Drug Delivery Science and Technology. 71: 103316.
Campiñez, M., Casas, M., and Caraballo, I. 2016. Characterisation of the ability of carbamazepine for processing it through direct compression applying the new expert system SeDeM. International Journal of Clinical Pharmacology & Pharmacotherapy. 1: 105.
Castañeda Hernández, O., Domínguez-Robles, J., Caraballo, I., Bernad, M.J., and Melgoza Contreras, L.M. 2023. Comparison between polymeric excipients using SeDeM expert system in combination with mathematical modeling and quality control tools. Journal of Drug Delivery Science and Technology. 86: 104750.
Douroumis, D.D., Gryczke, A., Schminke, S. 2011. Development and evaluation of cetirizine HCl taste-masked oral disintegrating tablets. AAPS PharmSciTech. 12(1): 141-151.
European Medicines Agency. 2006. Committee for medicinal products for human use 2006 reflection paper: Formulation of choice for the paediatric population, emea/chmp/peg/194810/2005. London, United Kingdom: 28 July 2006.
European Medicines Agency. 2013. Guideline on pharmaceutical development of medicines for paediatric use. EMA/CHMP/QWP/805880/2012 Rev 2. London: Committee for Medicinal Products for Human Use (CHMP). Paediatric Committee (PDCO).
Fell, J.T. and Newton, J.M. 1970. Determination of tablet strength by the diametral-compression test. Journal of Pharmaceutical Sciences. 59(5): 688-691.
Hellberg, E., Westberg, A., Appelblad, P., and Mattsson, S. 2021. Evaluation of dissolution techniques for orally disintegrating mini-tablets. Journal of Drug Delivery Science and Technology. 61: 102191.
Khan, A., Majeedullah, Qayum, M., Ahmad, L., Ahmad Khan, S., and Abbas, M. 2022. Optimization of diluents on the basis of SeDeM-ODT expert system for formulation development of ODTs of glimepiride. Journal of Drug Delivery Science and Technology. 33(2): 103389.
Klingmann, V., Spomer, N., Lerch, C., Stoltenberg, I., Frömke, C., Bosse, H.M., Breitkreutz, J., and Meissner, T. 2013. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. The Journal of Pediatrics. 163(6): e1721.
Krause, J. and Breitkreutz, J. 2008. Improving drug delivery in paediatric medicine. Pharmaceutical Medicine. 22(1): 41-50.
Kuck, J. and Breitkreutz, J. 2022. Impact of lubrication on key properties of orodispersible minitablets in comparison to conventionally sized orodispersible tablets. European Journal of Pharmaceutics and Biopharmaceutics. 180:71-80.
Lennartz, P. and Mielck, J.B. 1998. Minitabletting: Improving the compactability of paracetamol powder mixtures. International Journal of Pharmaceutics 173(1): 75-85.
Mitsui, N., Hida, N., Kamiya, T., Yamazaki, T., Miyazaki, K., Saito, K., Saito, J., Yamatani, A., Ishikawa, Y., Nakamura, H. et al. 2022. Swallowability of minitablets among children aged 6–23 months: An exploratory, randomized crossover study. Pharmaceutics. 14(1): 198.
Miyazaki, K., Hida, N., Kamiya, T., Yamazaki, T., Murayama, N., Kuroiwa, M., Kurata, N., Ishikawa, Y., Yamashita, S., Nakamura, H. et al. 2022. Comparative acceptability of mini-tablets, fine granules, and liquid formulations in young children: An exploratory randomized crossover study. Journal of Drug Delivery Science and Technology. 70:103154.
Parisi, G.F., Leonardi, S., Ciprandi, G., Corsico, A., Licari, A., Miraglia Del Giudice, M., Peroni, D., Salpietro, C., and Marseglia, G.L. 2020. Cetirizine use in childhood: An update of a friendly 30-year drug. Clinical and Molecular Allergy. 18: 2.
Portnoy, J.M. and Dinakar, C. 2004. Review of cetirizine hydrochloride for the treatment of allergic disorders. Expert Opinion on Pharmacotherapy. 5(1): 125-135.
Rojas, J., Buckner, I., and Kumar, V. 2012. Co-proccessed excipients with enhanced direct compression functionality for improved tableting performance. Drug Development and Industrial Pharmacy. 38(10): 1159-1170.
Shukla, D., Chakraborty, S., Singh, S., and Mishra, B. 2009. Mouth dissolving tablets I: An overview of formulation technology. Scientia Pharmaceutica. 77(2): 309-326.
Spomer, N., Klingmann, V., Stoltenberg, I., Lerch, C., Meissner, T., and Breitkreutz, J. 2012. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Archives of Disease in Childhood. 97(3): 283.
Stoltenberg, I., Breitkreutz, J. 2011. Orally disintegrating mini-tablets (ODMTs) – a novel solid oral dosage form for paediatric use. European Journal of Pharmaceutics and Biopharmaceutics. 78(3): 462-469.
Suñé-Negre, J.M., Pérez-Lozano, P., Miñarro, M., Roig, M., Fuster, R., Hernández, C., Ruhí, R., García-Montoya, E., Ticó, J.R. 2008. Application of the SeDeM diagram and a new mathematical equation in the design of direct compression tablet formulation. European Journal of Pharmaceutics and Biopharmaceutics. 69(3): 1029-1039.
Suñé-Negre, J.M., Roig, M., Fuster, R., Hernández, C., Ruhí, R., García-Montoya, E., Pérez-Lozano, P., Miñarro, M., and Ticó, J.R. 2014. New classification of directly compressible (dc) excipients in function of the SeDeM diagarm expert system. International Journal of Pharmaceutics. 470(1-2): 15-27.
Suñé Neģre, J.M., Encarna García, M., Pilar Pérez, L., Johnny, E.A.D.a., Manel Roig, C., Roser Fuster, G.a., Montserrat Miñarro, C., and Josep, R.T.G. 2011. SeDeM diagram: A new expert system for the formulation of drugs in solid form. In p. Ch. 2. V. Petrică [ed] Expert systems for human, materials and automation. Rijeka: IntechOpen.
Suñé Neģre, J.M., Roiģ Carreras, M., García, R.F., Montoya, E.G., Lozano, P.P., Aģuilar, J.E., Carmona, M.M., and Ticó Grau, J.R. 2013. SeDeM diagram: an expert system for preformation, characterization and optimization of tablets obtained by direct compression. p. 109-135. In J.E. Aģuilar [ed] Formulation tools for pharmaceutical development. Woodhead Publishing. Thomson, S.A., Tuleu, C., Wong, I.C., Keady, S., Pitt, K.G., and Sutcliffe, A.G. 2009. Minitablets: New modality to deliver medicines to preschool-aged children. Pediatrics. 123(2): e235-e238.
Trisopon, K., Kittipongpatana, N., Kittipongpatana, O.S. 2020. A spray-dried, co-processed rice starch as a multifunctional excipient for direct compression. Pharmaceutics. 12(6): 518.
United States Food and Drug Administration. 2008. Guidance for industry: orally disintegrating tablets. USA: Department of Health and Human Services.
Wan, S., Yang, R., Zhang, H., Li, X., Gu, M., Guan, T., Ren, J., Sun, H., and Dai, C. 2019. Application of the SeDeM expert system in studies for direct compression suitability on mixture of rhodiola extract and an excipient. AAPS PharmSciTech. 20(3): 105.
Woyna-Orlewicz, K., Brniak, W., Tatara, W., Strzebońska, M., Haznar-Garbacz, D., Szafraniec-Szczęsny, J., Antosik-Rogóż, A., Wojteczko, K., Strózik, M., and Kurek, M. et al. 2023. Investigating the impact of co-processed excipients on the formulation of bromhexine hydrochloride orally disintegrating tablets (ODTs). Pharmaceutical Research. 40(12): 2947-2962.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Phennapha Saokham and Karnkamol Trisopon*
Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Karnkamol Trisopon, E-mail: karnkamol.t@cmu.ac.th
ORCID: Phennapha Saokham: https://orcid.org/0000-0003-4581-7498
Total Article Views
Editor: Nisit Kittipongpatana
Chiang Mai University, Thailand
Article history:
Received: June 26, 2024;
Revised: August 15, 2024;
Accepted: September 23, 2024;
Online First: October 1, 2024

