
Plant Beneficial Desert Actinomycete, Modestobacter caceresii KNN 45-2bT, Promote Growth of Tomato (Lycopersicum esculentum Mill.) under Drought Condition
Feiyang Xie, Barbara Andrews, Juan A. Asenjo, Michael Goodfellow, and Wasu Pathom-aree*Published Date : October 1, 2024
DOI : https://doi.org/10.12982/NLSC.2024.063
Journal Issues : Number 4, October-December 2024
Abstract Drought stress is currently the most serious challenge to global food security and agricultural productivity. Desert actinobacteria have gained attention as potential candidates for enhancing plant growth in water stress environments. In this regard, a desert actinomycete, namely Modestobacter caceresii strain KNN 45-2bT, was selected to investigate its plant growth promoting abilities and drought tolerance. Next, this desert strain was inoculated to tomato (Lycopersicum esculentum Mill.) under drought, and the results included increases in root length, shoot and root fresh weight, shoot and root dry weight, total fresh weight and dry weight, fruit weight, proline accumulation, total soluble sugar content and trolox content. Stress treatments on the bacterized tomato plants also resulted in the reduction of hydrogen peroxide accumulation. Putative proteins coding sequences conferring plant growth promoting (PGP) traits (IAA production, phosphate solubilization, siderophore production, nitrogen fixation) and drought response (biosynthesis of proline metabolism, oxidative and osmotic stress response) were also detected from their genomic analyses. In conclusion, these results provide credence to the idea that the inoculation of tomato plants with plant beneficial desert actinomycete is an effective method of combating the negative effects of drought stress.
Keywords: Desert actinobacteria, Drought stress, Field trial, Tomato, Plant growth promotion
Funding: This project was funded through the Graduate PhD’s Degree Program in Applied Microbiology, Department of Biology, Faculty of Science, Chiang Mai University, under the CMU Presidential Scholarship scheme and CMU Short Term Research Fellowships in Overseas from Research Administration Center, Chiang Mai University.
Citation: Xie, F., Andrews, B., Asenjo, J.A., Goodfellow, M. and Pathom-aree, W. 2024. Plant beneficial desert actinomycete, Modestobacter caceresii knn 45-2bt, promote growth of tomato (Lycopersicum esculentum Mill.) under Drought Condition. Natural and Life Sciences Communications. 23(4): e2024063.
INTRODUCTION
Drought is one of the most significant abiotic stresses that has a detrimental impact on plant growth and development and is recognized as a common feature of climate change that occurs on a global scale (Cotrina Cabello et al., 2023). Plants are sessile organisms whose biochemical, ecological, molecular, morphological, and physiological characteristics may rapidly decline in response to drought stress (Naikwade, 2023). All of these detrimental outcomes are influenced by water deficit conditions owing to reduced turgor, enzyme activities, and energy supplementation. Plant growth promoting (PGP) actinomycetes have been frequently proven to have special abilities to enhance plant development for sustainable agriculture in order to bestow such severe living conditions on plants (Faddetta et al., 2023; González et al., 2023). Typically, desert biomes are a rich source of various actinomycetes that are cultivable and have a high level of drought resistance and potential as plant growth promoters (Mohammadipanah and Wink, 2016; Selim et al., 2019; Xie and Pathom-Aree, 2021). The genera Arthrobacter, Cryobacterium, Frondihabitans, Kocuria, Microbacterium, Rhodococcus and Streptomyces has been established as plant beneficial actinomycetes (Goswami et al., 2014; Gaete et al., 2020). They potentially release PGP properties such as indole-3-acetic acid (IAA) production, phosphate solubilization, siderophore production, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity, along with nitrogen fixation. Desert actinomycetes also have complex adaptation mechanisms that allow them to survive in arid conditions, which contributes to their common drought tolerance. As Selim et al. (2019) recently reported, a Saudi Arabia Desert strain, Streptomyces sp. AC 5 has successfully promoted maize (Zea mays L.) growth under drought conditions, with high amounts of PGP traits. Moreover, the growth of other plants also can benefit from the inoculation of desert actinomycetes, including groundnut (Goswami et al., 2014), durum wheat (Allali et al., 2019), tomato (Abdelmoteleb and González-Mendoza, 2020) and sunflower (Zahra et al., 2020).
Tomato (L. esculentum Mill.) is a widely grown crop that is susceptible to drought and ranks second to the potato in terms of global vegetable production (Cammarano et al., 2022). Tomato plays an important role in human nutrition supplementation, reducing the risk of certain cancers and cardiovascular illnesses. It is rich in antioxidants and minerals, including carotenoids, vitamin C, E, and phenolic compounds (Ullah et al., 2016). However, the growth and productivity of tomato, were seriously threatened by unexpected effects of climate change, notably drought stress. Compared to the 1980 - 2009 baseline period, global tomato production will decline by 6% by 2050 (Cammarano et al., 2022). Therefore, the goal of the present study was to determine how tomato plants withstand water scarcity with the help of an actinomycete isolated from Atacama Desert: M. caceresii strain KNN 45-2bT (Busarakam et al., 2016). Protein encoding sequences conferring PGP traits and drought response were also identified.
MATERIAL AND METHODS
Plant growth promoting actinomycete
A desert actinomycete, M. caceresii strain KNN 45-2bT (Busarakam et al., 2016), was previously isolated from the Yungay core region of the Atacama Desert. This desert strain was selected and used as bioinoculant to promote growth of tomato (L. esculentum Mill.) under drought stress. The selection of this strain was based on its potential plant growth promoting properties and strong drought tolerance ability.
In vitro plant growth promoting properties of desert actinomycete
Indole‑3‑acetic acid (IAA) production
IAA production was estimated by the standard colourimetric method as described by Rangseekaew et al. (2022) with some modifications. Actinomycete was grown on ISP2 agar plates and incubated at 37°C for 7 days. Two agar plugs (5 mm diameter) of each isolate were added to ISP2 broth (5 ml), supplemented with 2 mg/mL L-tryptophan, and incubated at 30°C on a shaker (200 rpm) for 7 days in the dark, as described by Glickmann and Dessaux (1995). For IAA production under drought, ISP2 broth was supplemented with 405 g/L sorbitol to adjust the water activity (aw) to 0.919. One millilitre of supernatant was obtained by centrifugation at 12,000 rpm for 5 min, then vigorously mixed with 2 ml of Salkowski’s reagent (50 ml sterile water; 50 ml 70% HClO4; 2 ml 0.5M FeCl3) and incubated for 30 min in the dark. The absorbance was measured at 530 nm with a microplate spectrophotometer (Revelation Spectra MRTM version 4.29; DYNEX Technologies). The concentration of IAA produced by the isolate was estimated from a standard curve generated using a pure IAA standard (Mohite, 2013).
Phosphate solubilization
Quantitative analysis of phosphate solubilization was performed following the method described by Fiske and Subbarow (1925). Two agar plugs (5 mm diameter) were inoculated into 25 ml Pikovskaya (PVK) broth containing 0.5 % (w/v) tricalcium phosphate (10 g glucose; 5g Ca3(PO4)2; 0.2g NaCl; 0.2g KCl; 0.1g MgSO4; 0.0025g MnSO4; 0.0025g Fe2(SO4)3; 0.5g ((NH4)2SO4; 20g agar; 1L sterile water; Pikovskaya, 1948), and incubated with shaking (120 rpm) for 7 days at 28°C. After incubation, the supernatant (500 µl) was collected by centrifugation at 12,000 rpm for 10 min and then combined with 10% tri-chloroacetic acid (500 µl), as well as 4 ml of colour reagent (6NH2SO4: 2.5% (NH4)MO7O24·4H20 : 10% C6H8O6 : H2O = 1:1:1:2). The mixture was incubated at room temperature (about 28°C) for 15 min. Autoclaved PVK broth was used as a blank. The absorbance was measured at 820 nm using a microplate spectrophotometer (Revelation Spectra MRTM version 4.29; DYNEX Technologies). The pH value of each culture broth was measured by a pH meter (Mettler Toledo FiveEasyTM pH/mV bench meter). The concentration of released P in PVK broth was estimated using a standard curve (Lasudee et al., 2018). For phosphate solubilization under drought, PVK broth was supplemented with 405 g/L sorbitol to adjust the water activity (aw) to 0.919.
Siderophore production
Two agar plugs (5 mm diameter) were inoculated into 5 ml of King’s B broth (10g proteose peptone; 10ml glycerol; 1.5g K2HPO4; 1.5g MgSO4; 1L sterile water; pH 7.2; Schwyn and Neilands, 1987) for incubation in a shaker (120 rpm) for 7 days at 28°C. Quantitative estimation of siderophore production was determined by ferric perchlorate assay for hydroxamate-type siderophores (Atkin et al., 1970) and Arnow assay for catecholate-type siderophores (Arnow, 1937). For hydroxamate siderophores, 0.5 ml of supernatant was collected after centrifugation at 12,000 rpm for 10 min at 25°C. The supernatant was mixed with 2.5 ml ferric perchlorate (5 mM Fe(ClO4)3 : 0.1M HClO4 = 1:1) and incubation at room temperature for 5 min. Sterile King’s B broth (0.5 ml) was used as the blank. After incubation, the absorbance (OD480) was measured by a microplate spectrophotometer (Revelation Spectra MRTM version 4.29; DYNEX Technologies) followed by the estimation of the amount of hydroxamate siderophores from a standard curve. For catecholate siderophores, 1 ml of supernatant was mixed with 1 ml 0.5M HCl and 1 ml nitrite-molybdate
(2g sodium nitrite; 2g sodium molybdate; 20 ml sterile deionized water). The mixture was incubated at room temperature for 5 min after adding 1 ml 1M NaOH. The absorbance at 500 nm was measured after incubation. The amount of catecholate-type siderophores was estimated based on the standard curve. For siderophore production under drought, King’s B broth was supplemented with 405 g/L sorbitol to adjust the water activity (aw) to 0.919.
Nitrogen fixation
M. caceresii strain KNN 45-2bT (50 µl of cell suspension at 108 CFU/ml) was inoculated on Jensen’s agar, a nitrogen-free medium to determine its ability to fix atmospheric nitrogen (Jensen, 1942; Balagurunathan et al., 2020). For nitrogen fixing ability under drought, Jensen’s agar plate was supplemented with 405 g/L sorbitol to adjust the water activity (aw) to 0.919. All plates were incubated at 28°C for 10 days. Growth on the plate indicated nitrogen fixing ability.
Plate assay for drought tolerance
M. caceresii strain KNN 45-2bT was cultured on 10% tryptic soy agar supplemented with nine concentrations of sorbitol to adjust the values of the water activity (aw) as described by Lasudee et al. (2018). Growth appearance on the plates at aw ≤ 0.919 was considered as drought tolerance.
Growth promotion of tomato under drought
Field trial
M. caceresii strain KNN 45-2bT showed potent in vitro PGP properties and was selected for promoting tomato (L. esculentum Mill.) growth under drought condition. The experiment was carried out for 5 months (30/11/2020-23/04/2021) in a greenhouse. The tomato seeds were sterilized by sequentially immersed in 2% (v/v) sodium hypochlorite for 1 min, 95% (v/v) ethanol for 1 min and 70% (v/v) ethanol for 1 min, and then washed with sterile distilled water for 1 min (repeated three times). The selected strains were grown on ISP2 agar at 37°C for 10–14 days. Inoculum (108 CFU/ml) were prepared by mixing biomass of the isolates from the ISP2 plates with sterile distilled water. Two treatments were prepared: 1) control (non-bacterial inoculation) and 2) tomato plants inoculated with M. caceresii KNN 45-2bT. The seedlings were watered once a day with tap water for one month. One millilitre of the inoculum (108 CFU/ml) was further pipped around the center of each tomato plant near the root once a week for 5 months. All seedlings were transferred to bigger pots (containing 5 kg of planting material) after one month and grown until harvesting time (about 5 months). The planting material was composed of black soil: rich husk: coconut coir compost = 1:1:1. The physicochemical characteristics were as follows: 0.68% calcium, 0.14% magnesium, 0.24% phosphorus, 0.8% potassium and 9.75% total organic matter, pH 6.32 and 0.60 ds/m of electrical conductivity (EC). One millilitre of the inoculum (108 CFU/ml) and control (water) was monthly added to each pot. Pots were arranged in a completely randomized arrangement. The drought condition was set up by supplying 50% of water daily, while 100% water irrigation was considered normal condition.
Root colonization
Root colonization assay was used to evaluate the interactions between actinomycete and host plants, as described by Cao et al. (2004). Roots of tomato plants were washed with running water to remove soil particles. Washed roots were surface sterilized by sequential immersion in 70% (v/v) ethanol for 5 min, followed by sodium hypochlorite solution (0.9 %, w/v, available chlorine) for 20 min. Surface-sterilized roots were washed three times in sterile distilled water to remove surface sterilization agents. Surface-sterilized tomato roots (1 g) were cut and crushed in 9 ml of sterile sodium chloride solution (0.85% w/v) to prepare root suspension. Serial dilutions (10-1~10-8) were prepared from the root suspension, and spread on ISP2 agar plates supplemented with 25µg/ml nalidixic acid and 100 µg/ml ketoconazole, incubated at 37°C for 14 days. Colonies with the same appearance as the inoculated strain (M. caceresii strain KNN 45-2bT) were counted and confirmed by 16S rRNA gene sequencing. Additionally, three decontaminated roots were randomly selected and placed on ISP2 agar without antibiotic supplement for surface sterility checking.
Measurement of growth parameters
At the end of the field trial, selected growth parameters were determined as evidence of plant growth promoting potential of selected actinomycete. Parts of tomato leaves, including the second-youngest leaves, were collected for further biochemical tests. Tomato plants were collected and washed with running water to remove soil. After cleaning, roots and shoots were separated to measure their length, fresh and dry weight. The roots of plants were maintained in 5 ml of TE buffer and stored at - 20°C for root colonization assay. The weights of tomato fruits were also measured after harvest and maintained at - 20°C for determination of vitamin C content and antioxidant activity. All the experiments were set up as three replicates for each tomato plant. Figures were visualized using Image GP (https://www.bic.ac.cn/BIC/#/) (Chen et al., 2022).
Proline content in tomato was assayed by the colourimetric method as described by Bates et al. (1973). Tomato leaves (100 mg) were crushed by a sterile pestle and mortar with 3 ml of 95% ethanol, followed by an overnight incubation at room temperature. The supernatant was collected by centrifugation at 1,500 rpm for 10 min. The supernatant (200 µl) was mixed with 300 µl of sterile DI water, and 2 ml of ninhydrin reagent (1.25g ninhydrin; 30 ml acetic acid glacial; 20 ml 1M phosphoric acid; mixed at 70°C and kept stock in 4°C) in a glass tube. All samples were boiled at 100°C for 1 h before being transfer to the ice bath to stop the reaction. The sample was added to 6 ml toluene and vortexed for 10 seconds, then incubated at room temperature for 10 min. The top organic layer (1 ml) was collected and measured at 520 nm by a spectrophotometer. Toluene was used as the blank. The proline content of tomato plants was estimated from a standard curve.
Total chlorophyll and carotenoid contents were quantified using a modified method of Arnon and Whatley (1949). Leaf samples (500 mg) were cut into small pieces and mixed with 5 ml of methanol by vortexing, then overnight incubation in the dark. The supernatant (200 µl) was collected by centrifugation at 5000 rpm for 10 min, followed by the measurement of optical density at 480, 663 and 645 nm by a spectrophotometer. Solutions without leaves were used as blank. Chlorophyll and carotenoid contents were calculated using the equations shown below:
Chlorophyll a (mg/L) = 12.7× OD663 - 2.69× OD645 ×V / (1,000 × W)
Chlorophyll b (mg/L) = 22.9 ×OD645 - 4.68×OD663× V / (1,000 × W)
Total chlorophyll (mg/L) = 20.2 ×OD645 +18.2×OD663 × V / (1,000 ×W)
Carotenoid (mg/L) = 4.695× OD480 - 0.268 ×V / (1,000 × W)
Total soluble sugar (TSS) was determined by the technique reported by Shukla et al. (2012). Tomato leaves (100 g) were cut into small pieces and mixed with 3 ml of 80% ethanol, incubated overnight at room temperature. Supernatant was collected by centrifugation at 12,000 rpm for 15 min. The collected supernatant (500 µl) was mixed with 500 µl of 5% (w/w) phenol, 1.5 ml of 95% sulfuric acid. The mixture was incubated in the dark for 15–20 min at room temperature. The solution colour was changed from light yellow to dark yellow, then the optical density was measured at 520 nm using a spectrophotometer. Eighty percent ethanol was used as the blank. The amount of total soluble sugar content was estimated from a standard curve.
Hydrogen peroxide (H2O2) detection was carried out using the histochemical method as described by Hernández et al. (2001) and Gowtham et al. (2020) and quantitative estimation by the method of Velikova et al. (2000). To quantify H2O2, the second-youngest leaves of tomato (25 mg) were cut into small pieces and mixed with 750 µl of 0.1% (w/v) of trichloroacetic acid, and incubated at 4°C overnight. The supernatant was collected after centrifugation at 10,000 rpm for 15 min. The supernatant (500 µl) was mixed with 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M potassium iodine. Solutions without leaves were used as blank. The optical density was measured at 390 nm. The H2O2 content was estimated from a standard curve. Additionally, the whole second-youngest leaves were also used to detect H2O2 accumulation by soaking in 2 mg/mL aqueous 3,3-diaminobenzidine (DAB) (GoldBio, USA) for 4 h under light condition (Romero-Puertas et al., 2004). After incubation, leaves were boiled with 70% ethanol to remove chlorophyll, then the reddish-brown precipitates were produced by the H2O2–DAB reaction, which was observed under a stereomicroscope for staining (Hernández et al., 2001; Azad and Kaminskyj, 2016; Gowtham et al., 2020).
The vitamin C content of tomato fruits was determined using the 2,6-dichlorophenol indophenol titrimetric method, as mentioned by Nielsen (2017). Several tomato fruits were cut into small pieces and completely homogenized by a blender. The homogenized sample (10 g) was mixed with 0.4% oxalic acid and transferred to a 100 ml volumetric flask to bring up the volume to 100 ml. Fruit juices were filtered through Whatman qualitative filter paper No.1. Tomato juice sample (10 ml) was added to the Erlenmeyer flask and titrated with the dye solution (0.04% w/v of 2,6-dichlorophenol indophenol) until a light, but distinct rose-pink colour developed and persisted at least for 5 seconds. The flask was continuously swirling during the titration process. The final burette reading was recorded and used to calculate the volume of the dye used for each sample. The vitamin C content was determined from a standard curve.
The antioxidant activity of tomato fruits was determined using the diphenylpicrylhydrazyl (DPPH) assay (Erge and Karadeniz, 2011). Tomato fruits were completely dried in an oven at 60°C for about 3–5 days, and crushed by a pestle and mortar. The dried fruit sample (1 g) was dissolved with 10 ml of 60% ethanol and homogenized in a sonicator bath for 30 min. The mixture (600 µl) was added to 1.8 mL of DPPH solution: Tris buffer: 85% ethanol (1:1:1) and incubated in a dark for 30 min. Six hundred mL of 60% ethanol was used as a control. Absorbance was measured at 525 nm by a spectrophotometer. The percentage decrease in the absorbance of the DPPH radical solution was calculated using the equation shown below, which can be used to express the percentage quenching of DPPH radical to indicate the antioxidant activity of tomato fruits. The standard curve was generated using various concentrations of standard Trolox instead of the sample. The total antioxidants in tomato fruits were determined from the standard curve and expressed as µmol Trolox equivalent per gram fresh weight.
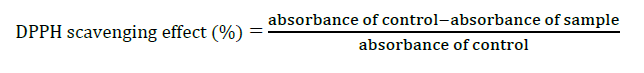
Genomic analysis of selected desert actinomycete
Whole genome sequence of M. caceresii strain KNN 45-2bT was mined for genes responsible for plant growth promotion and drought tolerance mechanisms using the RAST annotation server (Aziz et al., 2008) and analyzed through SEED viewer (https://rast.nmpdr.org/seedviewer.cgi; Overbeek et al., 2014) PRISM3 (http://magarveylab.ca/prism/; Skinnider et al., 2017) and antiSMASH version 6.0.0 (https://antismash.secondarymetabolites.org; Blin et al., 2021) with default options.
Statistical Analysis
All experimental data were expressed as the mean value of at leaset three replications ± standard deviation (SD). The significant differences between the means of all samples were statistically analyzed by IBM® SPSS® Statistics (version 28.0.0.0). The data obtained from plant growth promotion and measurement of growth parameters were analyzed using 2-factorial in completely randomized designs (CRD) and Ducan’s multiple range tests at P < 0.05.
RESULTS
In vitro plant growth promoting properties
M. caceresii strain KNN 45-2bT exhibited potential PGP traits in both normal and drought conditions, as stated in Table 1. Compared to stressful conditions, M. caceresii KNN 45-2bT normally produced 0.57 to 26 times statistically significantly higher levels of IAA, phosphorus, and siderophores in each corresponding culture broth. The findings indicated that M. caceresii KNN 45-2bT had better PGP activities in non-stressed environments than drought treatments. The most prominent PGP trait was phosphate solubilization, followed by siderophore production and IAA production. Additionally, M. caceresii KNN 45-2b contained higher levels of catecholates compared to hydroxamates. The strain also grew effectviely on Jensen’s agar, even at aw=0.919, indicating positive nitrogen fixation. It is important to note that drought stress significantly affected all PGP characteristics as low activity levels were observed at aw=0.919.
Plate assay for drought tolerance ability
M. caceresii strain KNN 45-2bT showed significant levels of drought tolerance in plate assay by growing in media with reduced water availability, especially at aw=0.919 (Table 2). As a result, M. caceresii strain KNN 45-2bT was selected as bioinoculants for promoting tomato growth under drought condition in terms of it was drought-tolerant and had high activities in PGP properties.
Table 1. Plant growth promoting properties of Modestobacter caceresii strain KNN 45-2bT under normal (aw=0.998) and drought (aw=0.919) conditions.
|
Strain |
Water activity (aw) |
IAA production (µg/mL) |
Phosphate solubilization |
Siderophore production |
Nitrogen fixation |
||
|
P released in PVK broth (mg/L) |
pH |
Hydroxamate-type (µmol/L) |
Catecholate-type (µmol/L) |
||||
|
Modestobacter
caceresii KNN 45-2bT |
0.998 |
0.30 ± 0.05b |
8.73 ± 5.47b |
5.19 ± 0.23a |
4.17 ± 2.50a |
4.39 ± 0.80b |
++++ |
|
0.919 |
0.17 ± 0.02a |
0.33 ± 0.04a |
5.94 ± 0.03b |
3.33 ± 1.44a |
0.70 ± 0.30a |
+++ |
|
Note: a,b indicated significant difference in statistical analyses tested by SPSS Independent Samples T-Test (P<0.05), n=3.
Table 2. Growth of Modestobacter caceresii strain KNN 45-2bT on 10% tryptic soy agar plates under reduced water activity.
|
Sorbitol (g/L) |
0 |
85 |
175 |
285 |
405 |
520 |
605 |
660 |
780 |
|
Water activity (aw) |
0.998 |
0.986 |
0.976 |
0.957 |
0.919 |
0.897 |
0.857 |
0.844 |
0.807 |
|
Modestobacter caceresii strain KNN 45-2bT |
++++ |
+++ |
+++ |
++ |
++ |
+ |
+ |
+ |
+ |
Note: +: poorly growth; ++: moderately growth; +++: growth well; ++++: strongly growth
Growth promotion of tomato under drought
Root colonization
The root colonization efficiency of M. caceresii KNN 45-2bT was assessed under both drought and normal conditions. Notably, the numbers of colonized in tomato roots was increased under drought conditions compared to normal conditions, as depicted in Figure 1. This indicated that M. caceresii KNN 45-2bT has an enhanced ability to colonize roots in response to drought stress, likely aiding the tomato’s adaptation to water-limited environments. Conversely, while root colonization was also successful under normal conditions, the density of actinomycetes was comparatively lower. This variation in colonization patterns highlights the robust adaptability of M. caceresii KNN 45-2bT and its potential to support plant growth and resilience in arid conditions.
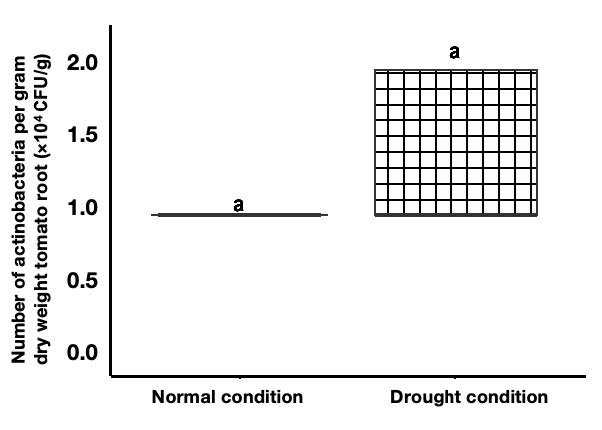
Figure 1. Numbers of re-isolated Modestobacter caceresii strain KNN 45-2bT (cfu’s per gram dry weight roots with standard deviations) isolated from root of tomato plants inoculated with Modestobacter caceresii strain KNN 45-2bT prior to incubation at 28 °C for 2–4 weeks.
Morphological growth parameters
Measurement of shoot and root. Figure 2 illustrated the impact of inoculation with M. caceresii KNN 45-2bT on plant growth compared to a control under normal and drought conditions. The results indicated that inoculation significantly enhanced various plant growth parameters, including root length, shoot and root fresh weight, shoot and root dry weight, under drought conditions (Figure 2). Specifically, inoculated plants exhibited significantly greater shoot biomass (both fresh and dry weights) than the control under drought condition. This suggested that strain KNN 45-2bT promoted shoot growth and helped maintain it even under water stress. While root length showed minimal differences between treatments, inoculated plants displayed a slight increase in root length, fresh and dry weights under drought conditions. This suggested that the strain contributed to better root development when water availability was limited, although the effect was less pronounced than on shoot growth. Inoculated plants consistently achieved higher total fresh and dry weights compared to the control. This indicated a general improvement in overall plant biomass due to the desert strain KNN 45-2bT inoculation, especially under drought condition where growth was typically constrained. Overall, inoculation with a desert strain KNN 45-2bT significantly enhanced plant growth, particularly shoot and root biomass, under drought conditions. The desert strain appeared to confer some level of drought resistance, as evidenced by less reduction in growth metrics under drought conditions compared to the control. These positive results suggested that strain KNN 45-2bT had strong potential as bio-inoculant for improving tomato growth and resilience, particularly in environments subject to water stress.
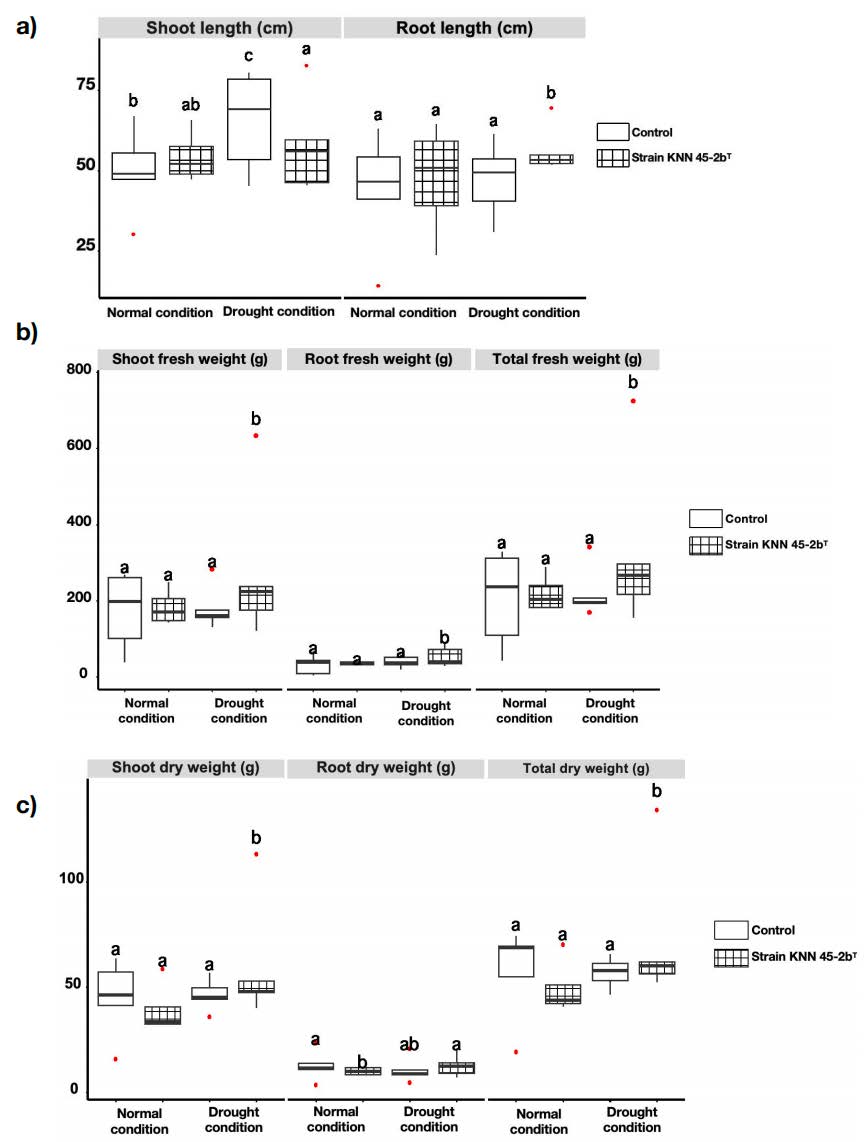
Figure 2. Morphological growth parameters of shoots and roots of tomato plants inoculated with control (non-inoculation) and Modestobacter caceresii strain KNN 45-2bT, grown in a greenhouse under normal and drought condition: a) shoot length and root length; b) shoot fresh weight, root fresh weight and total fresh weight of shoot and root; c) shoot dry weight, root dry weight and total dry weight of shoot and root.
Measurement of fruit weight. It is obvious that M. caceresii KNN 45-2bT had beneficial effects for increasing the amount of fruit weight of tomato plants under drought condition (Figure 3). Compared to the control, tomato plants inoculated with M. caceresii KNN 45-2bT showed significantly higher amount of fruit weight in water stressed environments. It is worth noting that, in contrast to normal condition, tomatoes treated with the desert strain under drought condition displayed dramatically enhanced fruit weight, whereas non-inoculated plants showed lower growth once supplied with reduced water. The results indicated that M. caceresii KNN 45-2bT could enhance the growth of fruit weight of tomato while tolerating drought stress.
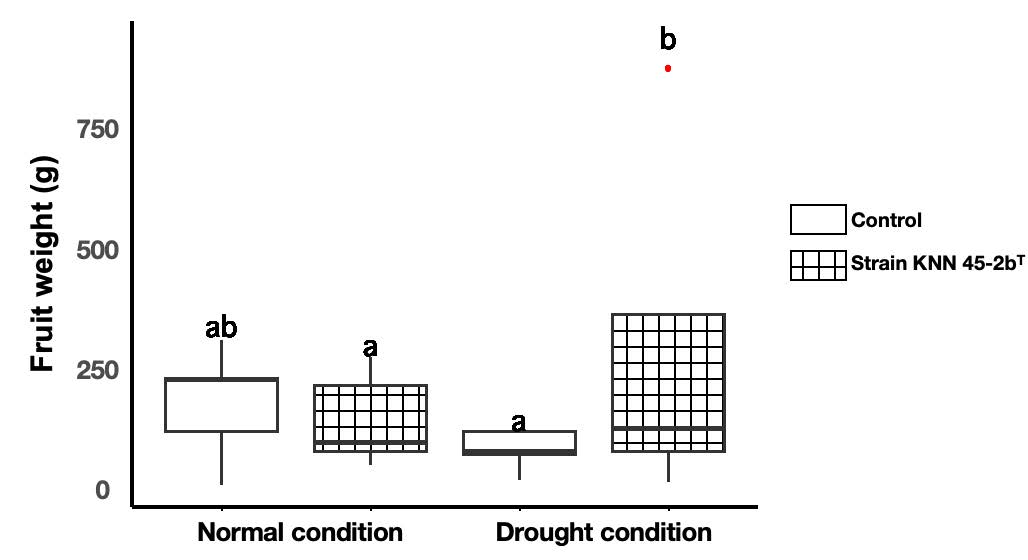
Figure 3. Fruit weight of tomato plants inoculated with control (non-inoculation) and Modestobacter caceresii strain KNN 45-2bT, grown in a greenhouse under normal and drought conditions.
Biochemical growth parameters
Measurement of biochemical parameters in tomato leaves. Figure 4 provided comparative data of the biochemical responses of tomato leaves inoculated with strain M. caceresii KNN 45-2bT against a control treatment under normal and drought conditions. The parameters analyzed include proline content, hydrogen peroxide levels, chlorophyll content, carotenoid content, and total soluble sugars. Proline content, a key osmoprotectant, was significantly higher in inoculated plants under both normal and drought conditions compared to the control, with the highest levels under drought stress. This suggested that strain KNN 45-2bT enhances proline accumulation, contributing to improved stress tolerance. Inoculated plants exhibited significantly lower hydrogen peroxide levels under both conditions, indicating reduced oxidative stress. This pointed to the strain’s potential role mitigating reactive oxygen species (ROS) damage. Meanwhile, the reddish-brown precipitates shown in Figure 5 suggested a decreased accumulation of hydrogen peroxide of inoculated plant leaves compared to the control under drought conditions. Inoculated plants maintained higher chlorophyll content under normal conditions, and slightly higher carotenoid content under drought stress compared to the control. This suggested better photosynthetic efficiency and protective pigment accumulation. Total soluble sugar (TSS) content was significantly higher in inoculated plants, particularly under drought conditions, which indicated enhanced osmotic adjustment and energy reserves. These results indicated that desert strain significantly strengthens plant resilience to drought by enhancing both biochemical stress responses, making it a promising bio-inoculant for improving plant performance in challenging environments.
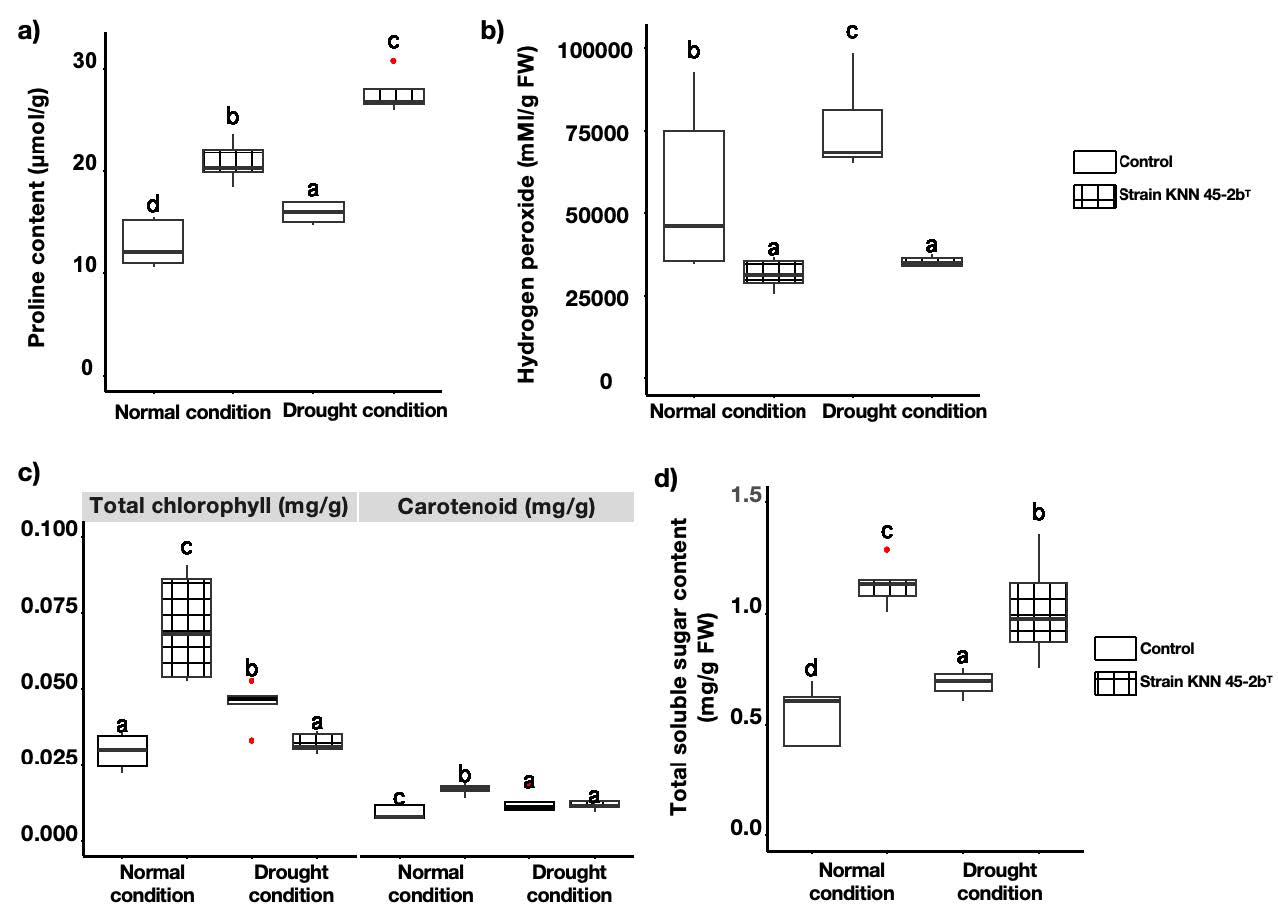
Figure 4. Biochemical parameters of tomato plants inoculated with control (non-inoculation) and Modestobacter caceresii strain KNN 45-2bT, grown in a greenhouse under normal and drought conditions: a) proline content; b) hydrogen peroxide content; c) total chlorophyll content and carotenoid content; d) total soluble sugar content.
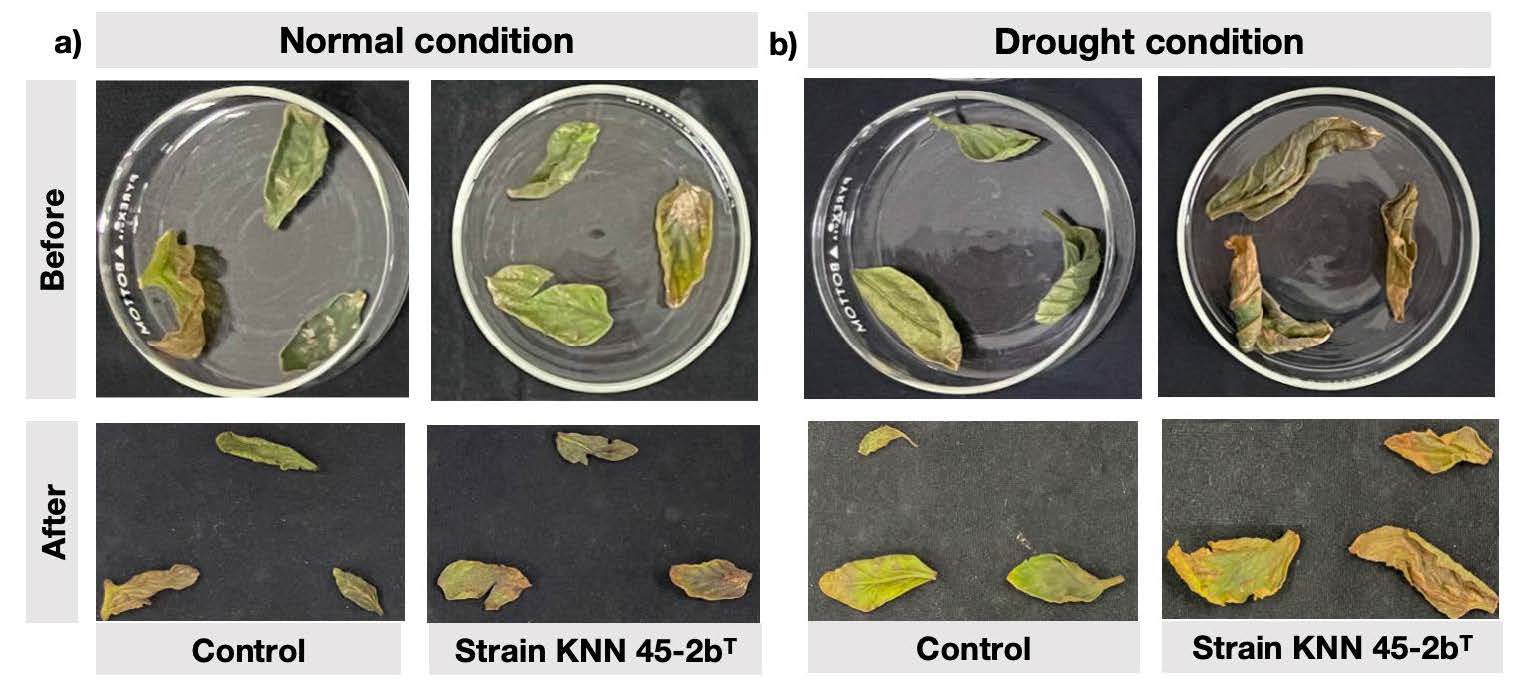
Figure 5. Localization of hydrogen peroxide accumulation of leaves from tomato plants inoculated with control (non-inoculation) and Modestobacter caceresii strain KNN 45-2bT, grown in a greenhouse under a) normal and b) drought conditions.
Measurement of biochemical parameters in tomato fruits. Trolox content, a measure of antioxidant capacity, was consistently higher in inoculated plants under both conditions, suggesting enhanced antioxidant defenses (Figure 6 a). Inoculated plants showed reduced ascorbic acid levels under drought conditions, possibly due to its utilization in combating oxidative stress (Figure 6 b). These findings showed that M. caceresii KNN 45-2bT had beneficial impacts on Trolox concentrations under drought, whereas it had detrimental effects on the ascorbic acid concentrations in tomato plants during both conditions.
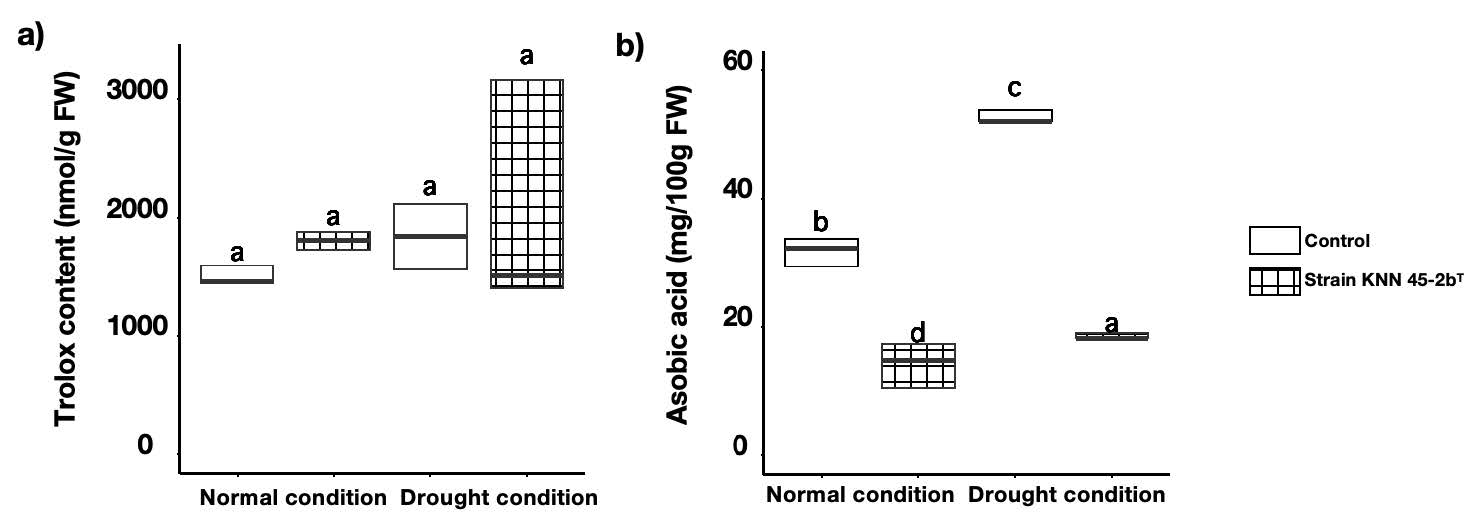
Figure 6. Trolox content a) and ascorbic acid b) of tomato plants inoculated with control (non-inoculation) and Modestobacter caceresii strain KNN 45-2bT, grown in a greenhouse under normal and drought conditions.
Genomic analyses for plant growth promoting potential and drought tolerance mechanisms
Number of genes responsible for plant growth promotion and drought stress response mechanisms were mined from the whole genome sequences of M. caceresii strain KNN 45-2bT, as concluded in Table 3. Gene annotation of M. caceresii KNN 45-2bT revealed 1,935 coding sequences which was further assigned to 434 subsystems.
Table 3. Putative proteins coding sequences conferring plant growth promoting (PGP) traits were detected in the draft genome of Modesetobacter caceresii KNN 45-2bT using RAST website.
|
Plant growth promoting traits |
Protein coding sequences conferring PGP traits |
|
Modesetobacter caceresii KNN 45-2bT |
|
|
Plant growth promoting properties |
|
|
Indole-3-acetic acid (IAA) production |
Tryptophan synthesis:
|
|
Phosphate solubilization |
High affinity phosphate transporter and control of PHO regulon:
Phosphate metabolism:
|
|
Siderophore production |
Siderophore assembly kit:
Heme, hemin uptake and utilization systems in Gram-positives:
ABC transporter [iron.B12.siderophore.hemin]:
Encapsulating protein for DyP-type peroxidase and ferritin-like protein oligomers:
|
|
Nitrogen fixation |
Cyanate hydrolysis:
Allantoin utilization:
Nitrate and nitrite ammonification:
Ammonia assimilation:
Denitrifying reductase gene clusters:
|
|
Potassium solubilization |
Potassium homeostasis:
|
|
Chitinase production |
Chitin and N-acetylglucosamine utilization:
|
|
|
|
|
Stress response |
|
|
Proline metabolism
|
Proline synthesis:
A hypothetical protein related to proline metabolism:
Proline, 4-hydroxyproline uptake and utilization:
|
|
Oxidative stress |
NADPH: quinone oxidoreductase 2:
Glutathione: non-redox reactions:
CoA disulfide thiol-disulfide redox system:
Redox-dependent regulation of nucleus processes:
Glutaredoxins:
Glutathione analogs: mycothiol:
Oxidative stress:
|
|
Oxidative stress |
Protection from reactive oxygen species:
Glutathionylspermidine and trypanothione:
|
|
|
Osmoregulation:
Choline and betaine uptake and betaine biosynthesis:
|
|
Trehalose metabolism |
Trehalose biosynthesis:
Trehalose uptake and utilization:
|
|
SigmaB stress response |
SigmaB stress response regulation:
|
|
Detoxification |
Nucleoside triphosphate pyrophosphohydrolase MazG:
Nudix proteins: (nucleoside triphosphate hydrolases):
Housecleaning nucleoside triphosphate pyrophosphatases:
Uptake of selenate and selenite:
|
|
Cold shock |
Cold shock, CspA family of proteins:
|
|
Heat shock |
Heat shock dnaK gene cluster extended:
|
|
Other stress response |
Flavohaemoglobin:
Bacterial hemoglobins:
Hfl operon:
Carbon starvation:
|
Among the 96 genes associated with plant growth promotion in M. caceresii KNN 45-2bT, they were participated in the biosynthesis of IAA, phosphate solubilization, siderophores, nitrogen fixation, potassium solubilization and chitinase. Genes involved in tryptophan synthesis were responsible for IAA production in two desert strains. Genes encoded for phosphate solubilization included high affinity phosphate transporter and control of phosphate (PHO) regulon and phosphate metabolism were also detected in two desert strains. In particular, the biosynthesis of siderophores was mainly coded by siderophore assembly kit, heme, hemin uptake and utilization systems in Gram-positives, ABC transporter [iron.B12.siderophore.hemin] and encapsulating protein for DyP-type peroxidase and ferritin-like protein oligomers were recorded in M. caceresii KNN 45-2bT. Protein coding sequences essential for nitrogen fixation in the desert strain, namely allantoin utilization, ammonia assimilation, nitric oxide synthase, cyanate hydrolysis, nitrate and nitrite ammonification, denitrifying reductase gene clusters.
It is important to note that M. caceresii KNN 45-2bT was well-equipped with multi-stress response-related genes that functioned in cooperation to facilitate the adaptive mechanisms of drought stress. These, for instance, code for proline metabolism, oxidative stress, osmotic stress, trehalose metabolism, UV defence activity, sigma B stress response, detoxification, cold shock, heat shock and other stress response. Proline synthesis, a hypothetical protein related to proline metabolism and proline, 4-hydroxyproline uptake and utilization in two desert strains were identified as groups of protein coding sequences relevant to the biosynthesis of proline metabolism. Numerous encoding sequences, including protection from reactive oxygen species, glutathione: non-redox reactions, redox-dependent regulation of nucleus processes, osmoregulation choline and betaine uptake and betaine biosynthesis, were responsible for oxidative and osmotic stress responses.
DISCUSSION
It is well known that actinomycetes have a unique capacity to promote plant growth for sustainable agriculture. Cultivable microbial diversity in desert biomes is a rich source of diverse actinomycetes with a high level of drought tolerance and potential as plant growth promoters. In this study, M. caceresii strain KNN 45-2bT from the Atacama Desert with abilities to promote plant growth (PGP) and withstand drought stress, was examined. This desert strain was further selected to promote tomato (L. esculentum Mill.) growth under drought condition. Its beneficial effects were evidently noted from the increased growth of tomato plants under drought condition, including the significantly enhanced root length, fresh weights of shoot and root, dry weights of shoot and root, total fresh and dry weights, proline contents, chlorophyll and carotenoid contents, total soluble sugar contents and Trolox contents, as well as, the significantly decreased hydrogen peroxide. Strong evidences were also provided by the abundance of encoding sequences identified in M. caceresii strain KNN 45-2bT, indicating its ability to encourage plant growth under drought condition.
IAA is an auxin phytohormone that is synthesized after L-tryptophan is degraded by PGP actinomycetes (Myo et al., 2019). It is crucial for the growth and development of plants, especially for the development of shoots and roots as well as fruit. Desert actinomycetes can promote plant growth by producing IAA has been reported by several studies, such as Kocuria turfanensis 2M4 (Goswami et al., 2014), Nocardiopsis dassonvillei MB22 (Allali et al., 2019), Streptomyces netropsis A-ICA (Abdelmoteleb and González-Mendoza, 2020). In the present study, M. caceresii strain KNN 45-2bT produced significant amounts of IAA, albeit the quality was affected by drought stress. PGP actinomycetes generally synthesize IAA via L-tryptophan-dependent mechanisms (Myo et al., 2019), as evidenced here that genes encoding for tryptophan synthesis was presented in M. caceresii KNN 45-2bT. The improved shoot and root developments as well as the enhancement of fruit weight that were seen in the inoculated tomato plants under drought condition may therefore be positively impacted by the IAA produced by M. caceresii KNN 45-2bT.
A significant micronutrient is phosphorus (P), which can be released by PGP actinomycetes via secreting phosphatase to release P linked to organic compounds as well as releasing organic acids (Kalayu, 2019). Phosphate solubilization might therefore be seen as a key mechanism for improving the uptake of available P and lowering pH during plant-microbe interactions (Kalayu, 2019). Several actinomycetes isolated from Atacama Desert including Arthrobacter sp. AF3, Cryobacterium sp. S5, Frondihabitans sp. R8, Microbacterium spp. M1-B and M2-A, and Rhodococcus sp. D4 have been reported to solubilize inorganic phosphates (Gaete et al., 2020). Similarly, M. caceresii KNN 45-2bT was also capable of phosphate solubilization under non-stress and stressed conditions. Genes encoding for high affinity phosphate transporter, regulation of the PHO regulon, and phosphate metabolism were found in M. caceresii KNN 45-2bT (26 genes), all of which are essential for the solubilization of phosphate. Nocardiopsis dassonvillei MB22 isolated from the Sahara Desert had the ability to solubilize inorganic phosphates, enhanced the growth of durum wheat (Allali et al., 2019). Another phosphate solubilizing desert strain, Streptomyces netropsis A-ICA, was also found to successfully promote the growth of tomato. Regarding these earlier studies, the ability of M. caceresii KNN 45-2bT to solubilize phosphate enabled it to effectively stimulate tomato growth as observed in this study.
Iron (Fe) is a crucial nutritional component that serves in the growth of plants by participating in the biological processes of nitrogen fixation, biosynthesis of chlorophyll, and photosynthesis (Kobayashi and Nishizawa, 2012). The acquisition of ferric irons was facilitated by siderophores, specifically hydroxamate- and catecholate-type siderophores (Krewulak and Vogel, 2008). Recently, siderophore producing actinomycetes from the desert have been continually reported, such as Cryobacterium sp. S5, Pseudarthrobacter spp. M1 (Gaete et al., 2020), Streptomyces sp. AC5 (Selim et al., 2019) and Streptomyces sp. MM40 (Solans et al., 2022). Among them, Streptomyces sp. AC5 significantly enhanced the growth of maize under drought condition (Selim et al., 2019). In the current investigation, it was also demonstrated that M. caceresii KNN 45-2bT positively produced siderophores at aw=0.998 and aw=0.919, respectively. Similar concentrations of hydroxamate from M. caceresii KNN 45-2bT were found in both non-stressed (4.17 µmol/l) and stressed conditions (3.33 µmol/l), albeit with the catecholates were also affected by the drought treatment. It's noteworthy to highlight that while 16 siderophore-producing genes were present in M. caceresii KNN 45-2bT. In addition, the genomic analyses of M. caceresii KNN 45-2bT revealed nine genes encoding for the siderophore assembly kit, three genes for heme, hemin uptake and utilization systems in Gram-positive bacteria, three genes for the ABC transporter [iron.B12.siderophore.hemin], and one gene for the encapsulating protein for DyP-type peroxidase and ferritin-like protein oligomers. The increase in photosynthesis (total soluble sugar content) of inoculated tomatoes in water-stressed condition possibly showed that M. caceresii KNN 45-2bT might promote plant growth by generating siderophores during drought condition.
Nitrogen (N) is an important nutrient for plant growth since it participates in the biological process of chlorophyll biosynthesis and photosynthesis (Kour et al., 2019). Biological nitrogen fixation (BNF) is a process that uses nitrogenase to prevent N2 from being converted to ammonia (NH3), which is required for plant nitrogen absorption to maximize productivity (Franche et al., 2009). It has been suggested that several desert actinomycetes are prospective nitrogen-fixing promoters with the potential to enhance plant development. For example, the genera Arthrobacter, Microbacterium, Paeniglutamicibacter and Streptomyces (Gaete et al., 2020; Nafis et al., 2019). In the current research, M. caceresii KNN 45-2bT was viable candidates for nitrogen fixation since both strains grew well on nitrogen-free media at aw=0.998 and aw=0.919. In addition, a number of protein-coding sequences related to nitrogen fixation, primarily associated with subgroups of allantoin utilization, ammonia assimilation, and ammonium transporter, were found in the genome of M. caceresii KNN 45-2bT. Moreover, the capacity of M. caceresii KNN 45-2bT to fix nitrogen might be seen as one of the key PGP mechanisms for tomato growth, especially in water deficit environments.
As ethylene has detrimental effects in large quantities, it is a gaseous phytohormone that can effectively promote plant development at low concentrations (Souza et al., 2015). The ACC deaminase activity of PGP actinomycetes can be a stress response mechanism that greatly reduces excessive levels of ethylene-induced by drought stress (Farajzadeh et al., 2012). A study reported by Zahra et al. (2020) revealed that members of the Streptomyces genus efficiently mitigated ethylene levels via ACC deaminase to promote the growth of sunflower. However, M. caceresii KNN 45-2bT showed no ACC deaminase activity based on the screening assay results. Additionally, their genomic analyses also did not identify any gene related to ACC deaminase activity. These results strongly suggested that M. caceresii KNN 45-2bT did not adjust to drought stress via ACC deaminase activity but rather via alternative stress response mechanisms.
Root colonization is an essential factor for the observation of the survival rate of actinomycetes in plants under drought condition. Etesami et al. (2014) and Qin et al. (2017) has reported that a substantial number of inoculants were successfully re-isolated, which suggested the probable survival of PGP actinomycetes within the roots. Similarly, the successful re-isolation of M. caceresii KNN 45-2bT suggested that this desert strain had the ability to colonize the roots of tomato plants. It is surprising that a higher amount of M. caceresii KNN 45-2bT were recorded from drought-treated tomato plants, which suggested that M. caceresii KNN 45-2bT could survive and flourish under drought.
It is well-known that drought stress will result in the supply of nutrients for plant growth being limited, which will further disrupt a variety of physiological processes in plants (Bogati and Walczak, 2022). Proline is an effective osmoprotectant and osmoregulator that protects plants from harmful stresses, and proline accumulation in plants can be used as a key predictor of water stress. Increasing proline levels have been established as an essential drought tolerance mechanism involved in several plants, such as rice (Pandey and Shukla, 2015), pepper (Anjum et al., 2012) and tomato (Ahammed et al., 2020). Under water-stressed condition, considerable increases in proline levels were detected in tomato plants inoculated with M. caceresii KNN 45-2bT. In addition, possible proline metabolism genes were identified in this desert strain, with roles including proline synthesis, proline, 4-hydroxyproline absorption and utilization, and a speculative protein associated with proline metabolism. Additionally, it is known that proline accumulation can be triggered by hydrogen peroxide (Yang et al., 2009). Hydrogen peroxide (H2O2) is sensitive to water deficit and is commonly generated by reactive oxygen species (ROS) via photorespiration. Exogenous H2O2 can lead to a repaid accumulation of cellular proline as a drought response. However, H2O2 is also a toxic cellular metabolite, excessive accumulation of H2O2 can result in cell death in plants exposed to oxidative stress. Compared to normal condition, H2O2 contents of tomato with the inoculation of M. caceresii KNN 45-2bT, was significantly decreased under water-stressed environments. The decrease of H2O2 enhanced plant growth facing drought stress. Three genes were involved in the ROS defence mechanisms of M. caceresii KNN 45-2bT that encoded for the H2O2 accumulation, namely peroxidase, superoxide dismutase [Cu-Zn] precursor and catalase. Additionally, soluble sugars, such as fructose, glucose and sucrose, also can be regarded as an osmotic adjuster to enhance the growth of plants that facing drought stress (Dien et al., 2019). Total soluble sugar (TSS) contents have been revealed to be a significant component in the modulation of drought response in German chamomile (Salehi et al., 2016), rice (Dien et al., 2019), and tomato (Živanović et al., 2020). Under drought condition, the increasing levels of TSS were observed from both tomato plants inoculated with M. caceresii KNN 45-2bT. These results indicated that M. caceresii KNN 45-2bT could mitigate the accumulation of proline and H2O2, together with TSS in drought-stressed tomato plants.
The limited absorption of light energy can result in chlorophyll that cannot be released through photosynthesis, which is a consequence of drought stress (Macar and Ekmekçi, 2008). Carotenoids, which serve as light-harvesting pigments, can prevent the degradation of chlorophyll and membrane deterioration (Macar and Ekmekçi, 2008). Chlorophyll contents and carotenoids can be considered adaptive features of the drought response earlier studies showed the negative consequences of stress on plants, including chickpea (Macar and Ekmekçi, 2008) and pepper (Khazaei et al., 2020). Similarly, tomato plants treated with M. caceresii KNN 45-2bT maintained higher chlorophyll content under normal conditions, and increased carotenoids on leaves under both normal and drought conditions compared to the control, This indicated greater photosynthetic efficiency and protective pigment accumulation. Surprising that there is none of the genes encoding for the biosynthesis of chlorophyll and carotenoids in M. caceresii KNN 45-2bT. Above results demonstrated that total chlorophyll and carotenoids were being positively influenced by M. caceresii KNN 45-2bT in tomato plants.
Antioxidant activity is a major mechanism of plants to mitigate the negative effects of drought stress through scavenging protecting antioxidant enzymes and ROS (Wegener et al., 2015). In this study, two antioxidative variations were investigated. Ascorbic acid content was examined for vitamin C and Trolox content was presented for lipid-soluble antioxidants. The tomato plants treated with M. caceresii KNN 45-2bT showed an increase in Trolox concentrations under normal condition, albeit with inoculated tomatoes, either in well-watered or water-stressed condition, both had lower amount of ascorbic acid compared to non-inoculated plants. For the oxidative stress response, 39 genes were encoded in M. caceresii KNN 45-2bT. The genomic investigations of this desert strain revealed the presence of several oxidative stress response enzymes, including those involved in reactive oxygen species defence, NADPH: quinone oxidoreductase 2, glutathione analogues, and mycothiol. M. caceresii KNN 45-2bT, however, lacked the enzymes necessary for the biosynthesis of ascorbic acid and Trolox. These results indicated that M. caceresii KNN 45-2bT was capable of potential antioxidant activity, but those activities were not involved in the synthesis of ascorbic acid and Trolox in tomato plants.
It's interesting to note that genes encoding other functioning enzymes, such as those that solubilize potassium and generate chitinase, can also be beneficial for PGP. Despite the fact that two desert strains have certain plant growth characteristics, the tolerance mechanism for drought could be described by the complex stress response, which is performed by osmotic adjustment, antioxidative defence, molecular events, and stress proteins. The production of trehalose and proline metabolism, as well as the control of osmotic stress, are all parts of the osmotic adjustment for M. caceresii KNN 45-2bT. The oxidative stress response is a component of antioxidant defences. Molecular events included the sigma B stress response, detoxification, cold shock, and heat shock. The remaining genes were regarded as additional stress proteins.
CONCLUSION
In conclusion, the results presented above offer strong evidence for the potential of desert actinomycete, M. caceresii KNN 45-2bT, to promote plant development under drought condition. Inoculation of this selected desert strain can enhance tomato (L. esculentum Mill.) development under stressed environments. The significantly increasing morphological (root length, shoot and root fresh weights, shoot and root dry weights, total fresh and dry weights, fruit weights) and biochemcial parameters (proline accumulation, total soluble sugar content, chlorophyll and carotenoid contents), together with the decreased hydrogen peroxide, were recorded from water-stressed treatments. The exceptional survival of isolates under stressful condition was revealed via root colonization. The genomic investigations of M. caceresii KNN 45-2bT also indicated that this desert strain contained a number of genes encoding for PGP traits and stress response, particularly for drought resistance.
ACKNOWLEDGEMENTS
This research was supported by the Graduate PhD’s Degree Program in Applied Microbiology, Department of Biology, Faculty of Science, Chiang Mai University, under the CMU Presidential Scholarship scheme. WP is grateful for financial supported from Chiang Mai University through the CMU Short Term Research Fellowships in Overseas programme.
AUTHOR CONTRIBUTIONS
W.P. conceived the project, designed the research methodology, data analysis, funding acquisition and supervised F.X. who carried out all experiments and prepared the initial draft of the paper. M.G., W.P., J.A.A. B.A. and F.X. revised and edited the manuscript. M.G. B.A. and J.AA. organised the collection of the environmental samples from the Atacama Desert. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdelmoteleb, A. and González-Mendoza, D. 2020. A novel streptomyces rhizobacteria from desert soil with diverse anti-fungal properties. Rhizosphere. 16: 100243.
Ahammed, G. J., Li, X., Wan, H., Zhou, G., and Cheng, Y. 2020. SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Scientia Horticulturae. 270: 109444.
Allali, K., Goudjal, Y., Zamoum, M., Bouznada, K., Sabaou, N., and Zitouni, A. 2019. Nocardiopsis dassonvillei strain MB22 from the Algerian Sahara promotes wheat seedlings growth and potentially controls the common root rot pathogen Bipolaris sorokiniana. Journal of Plant Pathology. 101(4): 1115–1125.
Anjum, S. A., Farooq, M., Xie, X., Liu, X., and Ijaz, M. F. 2012. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Scientia Horticulturae. 140: 66–73.
Arnon, D. I. and Whatley, F. 1949. Determination of total chlorophyll and carotenoids content. Crop Science. 110(2865): 554–556.
Arnow, L. E. 1937. Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine-tyrosine mixtures. Journal of Biological Chemistry. 118(2): 531–537.
Atkin, C. L., Neilands, J. B., and Phaff, H. J. 1970. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. Journal of Bacteriology. 103(3): 722–733.
Azad, K. and Kaminskyj, S. 2016. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis. 68(1): 73–78.
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., Formsma, K., Gerdes, S., Glass, E. M., and Kubal, M. 2008. The RAST server: Rapid annotations using subsystems technology. BMC Genomics. 9(1): 75.
Balagurunathan, R., Radhakrishnan, M., Shanmugasundaram, T., Gopikrishnan, V., and Jerrine, J. 2020. Evaluation of actinobacteria for agricultural applications. In: Balagurunathan, R., Radhakrishnan, M., Shanmugasundaram, T., Gopikrishnan, V., and Jerrine, J. (eds) Protocols in actinobacterial research. (pp.165-174). Springer, New York, NY.
Bates, L. S., Waldren, R. P., and Teare, I. D. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil. 39(1): 205–207.
Blin, K., Shaw, S., Kloosterman, A. M., Charlop-Powers, Z., van Wezel, G. P., Medema, M. H., and Weber, T. 2021. AntiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Research. 49(W1): W29-W35.
Bogati, K. and Walczak, M. 2022. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy. 12(1): 189.
Busarakam, K., Bull, A. T., Trujillo, M. E., Riesco, R., Sangal, V., van Wezel, G. P., and Goodfellow, M. 2016. Modestobacter caceresii sp. nov., novel actinobacteria with an insight into their adaptive mechanisms for survival in extreme hyper-arid Atacama Desert soils. Systematic and Applied Microbiology. 39(4): 243–251.
Cammarano, D., Jamshidi, S., Hoogenboom, G., Ruane, A. C., Niyogi, D., and Ronga, D. 2022. Processing tomato production is expected to decrease by 2050 due to the projected increase in temperature. Nature Food. 3(6): 437–444.
Cao, L., Qiu, Z., You, J., Tan, H., and Zhou, S. 2004. Isolation and characterization of endophytic Streptomyces strains from surface‐sterilized tomato (Lycopersicon esculentum) roots. Letters in Applied Microbiology. 39(5): 425–430.
Chen, T., Yong-Xin L., and Luqi H. 2022. ImageGP: An easy-to-use data visualization web server for scientific researchers. Imeta. 1: e5.
Cotrina Cabello, G. G., Ruiz Rodriguez, A., Husnain Gondal, A., Areche, F. O., Flores, D. D. C., Astete, J. A. Q., Camayo-Lapa, B. F., Yapias, R. J. M., Jabbar, A., and Yovera Saldarriaga, J. 2023. Plant adaptability to climate change and drought stress for crop growth and production. CABI Reviews. 2023.
Dien, D. C., Mochizuki, T., and Yamakawa, T. 2019. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Production Science. 22(4): 530–545.
Erge, H. S. and Karadeniz, F. 2011. Bioactive compounds and antioxidant activity of tomato cultivars. International Journal of Food Properties. 14(5): 968–977.
Etesami, H., Hosseini, H. M., Alikhani, H. A., and Mohammadi, L. 2014. Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. Journal of Plant Growth Regulation. 33(3): 654–670.
Faddetta, T., Polito, G., Abbate, L., Alibrandi, P., Zerbo, M., Caldiero, C., Reina, C., Puccio, G., Vaccaro, E., and Abenavoli, M. R. 2023. Bioactive metabolite survey of actinobacteria showing plant growth promoting traits to develop novel biofertilizers. Metabolites. 13(3): 374.
Farajzadeh, D., Yakhchali, B., Aliasgharzad, N., Sokhandan-Bashir, N., and Farajzadeh, M. 2012. Plant growth promoting characterization of indigenous Azotobacteria isolated from soils in Iran. Current Microbiology. 64(4): 397–403.
Fiske, C. H. and Subbarow, Y. 1925. The colorimetric determination of phosphorus. Journal of Biological Chemistry. 66(2): 375–400.
Franche, C., Lindström, K., and Elmerich, C. 2009. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant and Soil. 321(1): 35–59.
Gaete, A., Mandakovic, D., and González, M. 2020. Isolation and identification of soil bacteria from extreme environments of Chile and their plant beneficial characteristics. Microorganisms. 8(8): 1213.
Glickmann, E. and Dessaux, Y. 1995. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Applied and Environmental Microbiology. 61(2): 793–796.
González, F., Santander, C., Ruiz, A., Pérez, R., Moreira, J., Vidal, G., Aroca, R., Santos, C., and Cornejo, P. 2023. Inoculation with Actinobacteria spp. isolated from a hyper-arid environment enhances tolerance to salinity in lettuce plants (Lactuca sativa L.). Plants. 12(10): 2018.
Goswami, D., Pithwa, S., Dhandhukia, P., and Thakker, J. N. 2014. Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA-producing bacteria isolated from saline desert. Journal of Plant Interactions. 9(1): 566–576.
Gowtham, H. G., Singh, B., Murali, M., Shilpa, N., Prasad, M., Aiyaz, M., Amruthesh, K. N., and Niranjana, S. R. 2020. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiological Research. 234: 126422.
Hernández, J. A., Ferrer, M. A., Jiménez, A., Barceló, A. R., and Sevilla, F. 2001. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. its relation with salt-induced necrotic lesions in minor veins. Plant Physiology. 127(3): 817–831.
Jensen, H. L. 1942. Nitrogen fixation in leguminous plants. II. Is symbiotic nitrogen fixation influenced by Azotobacter?. Proceedings of the Linnean Society of New South Wales. 67: 205–212.
Kalayu, G. 2019. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. International Journal of Agronomy. 2019. 4917256.
Khazaei, Z., Esmaielpour, B. and Estaji, A. 2020. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L.) plants. Physiology and Molecular Biology of Plants. 26(8): 1649–1662.
Kobayashi, T. and Nishizawa, N. K. 2012. Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology. 63: 131–152.
Kour, D., Rana, K. L., Yadav, A. N., Yadav, N., Kumar, V., Kumar, A., Sayyed, R. Z., Hesham, A. E.-L., Dhaliwal, H. S. and Saxena, A. K. 2019. Drought-tolerant phosphorus-solubilizing microbes: Biodiversity and biotechnological applications for alleviation of drought stress in plants. In: Sayyed, R., Arora, N., Reddy, M. (eds) Plant growth promoting rhizobacteria for sustainable stress management. Microorganisms for Sustainability. vol 12. (pp. 255-308) Springer. Singapore.
Krewulak, K. D. and Vogel, H. J. 2008. Structural biology of bacterial iron uptake. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1778(9): 1781–1804.
Lasudee, K., Tokuyama, S., Lumyong, S. & Pathom-Aree, W. (2018). Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai jasmine rice (Oryza sativa). Frontiers in Microbiology. 9: 1247.
Macar, T. K. and Ekmekçi, Y. 2008. PSII photochemistry and antioxidant responses of a chickpea variety exposed to drought. Zeitschrift Für Naturforschung C. 63(7–8): 583–594.
Mohammadipanah, F. and Wink, J. 2016. Actinobacteria from arid and desert habitats: Diversity and biological activity. Frontiers in Microbiology. 6: 1541.
Mohite, B. 2013. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. Journal of Soil Science and Plant Nutrition. 13(3): 638–649.
Myo, E. M., Ge, B., Ma, J., Cui, H., Liu, B., Shi, L., Jiang, M. and Zhang, K. 2019. Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiology. 19(1): 155.
Nafis, A., Raklami, A., Bechtaoui, N., El Khalloufi, F., El Alaoui, A., Glick, B. R., Hafidi, M., Kouisni, L., Ouhdouch, Y., and Hassani, L. 2019. Actinobacteria from extreme niches in Morocco and their plant growth-promoting potentials. Diversity. 11(8): 139.
Naikwade, P. V. 2023. Plant responses to drought stress: Morphological, physiological, molecular approaches, and drought resistance. In: Nivas M. Desai, Manasi Patil, Umesh R. Pawar (eds) Plant metabolites under environmental stress. (pp. 149–183). Apple Academic Press. New York.
Nielsen, S. S. 2017. Vitamin C determination by indophenol method. In Food analysis laboratory manual (pp. 143–146). Springer.
Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., Edwards, R. A., Gerdes, S., Parrello, B., and Shukla, M. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Research. 42(D1): D206–D214.
Pandey, V. and Shukla, A. 2015. Acclimation and tolerance strategies of rice under drought stress. Rice Science. 22(4): 147–161.
Pikovskaya, R. I. 1948. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species, Mikrobiologiya. 17: 362–370.
Qin, S., Feng, W.-W., Wang, T.-T., Ding, P., Xing, K. and Jiang, J.-H. 2017. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant and Soil. 416(1): 117–132.
Rangseekaew, P., Barros-Rodríguez, A., Pathom-Aree, W. and Manzanera, M. 2022. Plant beneficial deep-sea actinobacterium, Dermacoccus abyssi MT1.1T promote growth of tomato (Solanum lycopersicum) under salinity stress. Biology. 11(2): 191.
Romero‐Puertas, M. C., Rodríguez‐Serrano, M., Corpas, F. J., Gomez, M. del, Del Rio, L. A., and Sandalio, L. M. 2004. Cadmium‐induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant, Cell & Environment. 27(9): 1122–1134.
Salehi, A., Tasdighi, H., and Gholamhoseini, M. 2016. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pacific Journal of Tropical Biomedicine. 6(10): 886–891.
Schwyn, B. and Neilands, J. B. 1987. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry. 160(1): 47–56.
Selim, S., Hassan, Y. M., Saleh, A. M., Habeeb, T. H., and AbdElgawad, H. 2019. Actinobacterium isolated from a semi-arid environment improves the drought tolerance in maize (Zea mays L.). Plant Physiology and Biochemistry. 142: 15–21.
Shukla, S., Mehta, A., Mehta, P., and Bajpai, V. K. 2012. Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Experimental and Toxicologic Pathology. 64(7–8): 807–811.
Skinnider, M. A., Merwin, N. J., Johnston, C. W. and Magarvey, N. A. 2017. PRISM 3: Expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Research. 45(W1): W49–W54.
Solans, M., Pelliza, Y. I. and Tadey, M. 2022. Inoculation with native actinobacteria may improve desert plant growth and survival with potential use for restoration practices. Microbial Ecology. 83(2): 380–392.
Souza, R. de, Ambrosini, A., and Passaglia, L. M. P. 2015. Plant growth-promoting bacteria as inoculants in agricultural soils. Genetics and Molecular Biology. 38: 401–419.
Ullah, U., Ashraf, M., Shahzad, S. M., Siddiqui, A. R., Piracha, M. A. and Suleman, M. 2016. Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil & Environment. 35(1): 65-75.
Velikova, V., Yordanov, I. and Edreva, A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Science. 151(1): 59–66.
Wegener, C. B., Jansen, G., and Jürgens, H.-U. 2015. Bioactive compounds in potatoes: Accumulation under drought stress conditions. Functional Foods in Health and Disease. 5(3): 108–116.
Xie, F. and Pathom-Aree, W. 2021. Actinobacteria from desert: Diversity and biotechnological applications. Frontiers in Microbiology. 12: 765531.
Yang, S.-L., Lan, S.-S., and Gong, M. 2009. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. Journal of Plant Physiology. 166(15): 1694–1699.
Zahra, T., Hamedi, J. and Mahdigholi, K. 2020. Endophytic actinobacteria of a halophytic desert plant Pteropyrum olivieri: Promising growth enhancers of sunflower. 3 Biotech. 10(12): 514.
Živanović, B., Milić Komić, S., Tosti, T., Vidović, M., Prokić, L., and Veljović Jovanović, S. 2020. Leaf soluble sugars and free amino acids as important components of abscisic acid—Mediated drought response in tomato. Plants. 9(9): 1147.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Feiyang Xie1, Barbara Andrews2, Juan A. Asenjo2, Michael Goodfellow3, and Wasu Pathom-aree4, *
1 Doctor of Philosophy Program in Applied Microbiology (International Program) in Faculty of Science, Chiang Mai University, under the CMU Presidential Scholarship, Chiang Mai, Thailand.
2 Department of Chemical Engineering, Biotechnology and Materials, Centre for Biotechnology and Bioengineering (CeBiB), University of Chile, Beaucheff 851, Santiago, Chile.
3 School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne NE1 7RU, UK.
4 Research Center of Microbial Diversity and Sustainable Utilization, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand.
Corresponding author: Wasu Pathom-aree, E-mail: wasu215793@gmail.com
ORCID: Wasu Pathom-aree: https://orcid.org/0000-0003-0299-3731
Total Article Views
Editor: Priraya Rithaporn
Chiang Mai University, Thailand
Article history:
Received: November 8, 2023;
Revised: November 20, 2023;
Accepted: November 23, 2023;
Online First: October 1, 2024

