
PM2.5-Bound Fatty Acids and Oxidative Potential: A Year-Round Study in Chiang Mai, Upper Northern Thailand
Teetawat Santijitpakdee, Tippawan Prapamontol*, Pitakchon Ponsawansong, Sawaeng Kawichai, Wenhuai Song, Fang Cao, and Yan-lin ZhangPublished Date : September 23, 2024
DOI : https://doi.org/10.12982/NLSC.2024.064
Journal Issues : Number 4, October-December 2024
Abstract Air pollution study mostly targeted in components of fine particulate matter (PM2.5, particulate matter with aerodynamic diameter ≤ 2.5 µm) but their health implication is still scarce. Fatty acids could exhibit surfactant properties of PM2.5. We investigated the oxidative potential (OP) of PM2.5-bound fatty acids in Chiang Mai, upper northern Thailand: a year-round study in 2021. A dithiothreitol (DTT), an acellular assay, was used to evaluate the OP of eighty-nine PM2.5 filters collected from January to December 2021. A series of PM2.5-bound fatty acids i.e., C14:0–C30:0 fatty acids were determined by gas chromatography/mass spectrometry (GC/MS). Total fatty acids (C14:0–C30:0 and C18:1, C18:2) accounted for 498.7 ± 196.1 and 206.9 ± 112.8 ng/m³ in high biomass burning (HBB) and low biomass burning (LBB) periods, respectively. Mean values of OPDTTv (OP normalized by air volume of sampling) in HBB and LBB periods were 0.13 ± 0.06 and 0.03 ± 0.03 nmol/min/m³, respectively. Long chain saturated fatty acids (C22:0-26:0) mostly showed moderately significant correlation with OPDTTv (r = 0.33-0.47, P < 0.01) in HBB period but showed no significant correlation in LBB period. Meanwhile, linoleic acid (C18:2) was the only individual compound among unsaturated fatty acids that significantly correlated with OPDTTv (r = 0.39, P < 0.01). These findings suggest that PM2.5-bound fatty acids, particularly during open-biomass burning period, contribute to variations in oxidative potential, potentially influencing the health risks associated with PM2.5 exposure.
Keywords: PM2.5, Air pollution, Fatty acids, Oxidative potential, Chiang Mai
Funding: This research was funded by Research Institute for Health Sciences (RIHES), Chiang Mai University (CMU) Grant Number 001/2566 to T.S. PM2.5 sampling and PM2.5 chemical analyses essential for this research were funded by The National Research Council of Thailand (project: Determination of secondary aerosol sources of ambient PM2.5 in Chiang Mai city: A year-round campaign in 2021, project ID: 31249 to T.P.)
Citation: Santijitpakdee, T., Prapamontol, T., Ponsawansong, P., Kawichai, S., Song, W., Cao, F., and Zhang, Y-L. 2024. PM2.5-bound fatty acids and oxidative potential: A year-round study in Chiang Mai, upper northern Thailand. Natural and Life Sciences Communications. 23(4): e2024064.
INTRODUCTION
PM2.5 (particulate matter 2.5) is an important constituent of air pollution that can impact human health (World Health Organization, WHO, 2021). Acute or chronic effects on human health may become apparent depending on the level and duration of exposure to PM2.5. Short-term exposure to high levels of PM2.5 can lead to respiratory issues such as coughing, wheezing, and shortness of breath (Sugiyama et al., 2020), while long-term exposure has been linked to more serious health conditions such as cardiovascular disease, lung cancer, and even premature death(Kloog et al., 2013; Beelen et al., 2014; Shi et al., 2016). Epidemiological studies have shown a correlation between elevated levels of PM2.5 and health issues such as hospital admissions and mortality(Atkinson et al., 2014; Shi et al., 2016; Lee et al., 2019; Ren et al., 2021). PM2.5 can affect humans by serving as an oxidant precursor. The reactive oxygen species (ROS) and free radicals from PM2.5 can cause sickness by altering the body's antioxidant balance when they enter the human body(Boogaard et al., 2012; Pisoschi and Pop, 2015).
The evaluation of the oxidative potential (OP) of PM2.5 is crucial as it provides information on the particles' capacity to produce ROS. Oxidative potential can be utilized to assess the ability of PM2.5 to oxidize certain compounds. Dominutti et al. (2023) reviewed several assays, each with unique strengths. The dithiothreitol (DTT) assay is sensitive to organic compounds and transition metals, making it effective for analyzing OP. The ascorbic acid (AA) assay primarily detects transition metals, offering complementary insights to DTT. The 2,7-Dichlorofluorescein (DCFH) assay is valuable for studying specific ROS mechanisms, while the Ferrous-orange xylenol (FOX) assay provides potential insights into less explored oxidative processes. The direct ROS-quantification assay directly measures ROS, offering clear data on OP. Xu et al. (2024) focused on two spectrophotometric methods, highlighting the DTT assay’s sensitivity to ROS and ability to quantitatively measure OP, though it can be influenced by water-soluble ions. The AA assay complements DTT by responding differently to oxidants, expanding the understanding of OP. Both assays rely on spectrophotometric techniques, with absorbance measured at 412 nm.
The DTT assay was selected as a technique to measure oxidative potential because of its common usage and practical benefits. This technique is commonly used in research due to its ease of operation on a small scale in a laboratory, delivering quick results in a controlled setting at little expense. the DTT sensitive response toward sources with the presence of trace metals and organic compounds. Additionally, the DTT assay has been shown to correlate well with other assays(Gao et al., 2020; Dominutti et al., 2023), establishing it as a dependable and practical option for evaluating the oxidative potential of PM2.5. Furthermore, several research used of DTT to analyze the components of PM2.5, climate change, and the spatial variations in PM2.5 resulting from natural and anthropogenic sources(Bates et al., 2018; Cesari et al., 2019; Farahani et al., 2022; Shen et al., 2022). Previous research indicated that in the eastern U.S., fire sources had the most significant impact on OP (29%), with spatial and seasonal variations primarily attributed to fires and vehicle emissions, particularly diesel exhaust(Bates et al., 2018). In Beijing, biomass burning is the dominant source of OP levels during the heating season, whereas secondary aerosol formation is the most important source during non-heating seasons (Ma et al., 2018). Reduced vehicle uses during the COVID-19 pandemic reduced PM2.5 emissions, but biomass burning, and secondary aerosols continued to have an impact on OP levels. (Paraskevopoulou et al., 2022; Pietrogrande et al., 2023). Moreover, the importance of the OP level was highlighted as a measure of PM2.5 risks, alongside the Air Quality Index (AQI) scale. They found that days with "good" AQI levels showed extremely high OP concentrations, similar to those on "unhealthy" days(Carolina et al., 2023). Park et al. (2024) proposed using the OP of PM2.5 to predict the health effect of PM2.5, considering the contribution of secondary organic aerosols and dust. Both studies emphasized the significance of OP levels in understanding the risks associated with PM2.5.
Organic carbon (OC) comprises a combination of constituents and is released directly into the air from biogenic or anthropogenic sources, including plants and combustion emissions or generated through the atmospheric oxidation of biogenic or anthropogenic volatile organic compounds (VOCs), resulting in the formation of less volatile substances that deposit into the condensed phase (Liu et al., 2017). Previous studies found that OC accounted for approximately 10% to 70% of the total fine aerosol mass (Jacobson et al., 2000).
Lipid components are constituents of aerosols. Previous studies found them to comprise of 20% to 80% (Zheng et al., 1997; Chrysikou and Samara, 2009). Fatty acids can originate from human activities such as biomass burning, fossil fuel combustion, and cooking operations, as well as from natural sources like plant waxes, vegetation waste, soil dust, and microorganisms (Boreddy et al., 2018; Cheng et al., 2019; Ren et al., 2020). Fatty acids can occur in emission from wildfires where there is the greatest fresh biomass (Ma et al., 2024). Fatty acids can act as surface-organic coatings and molecular packing of the monolayers at the air–aqueous interface (Cheng et al., 2019). Additionally, unsaturated fatty acids can increase secondary oxidation in the atmosphere (Yang et al., 2016). These reactions contribute to the formation of ROS and enhance oxidative potential in PM2.5 (Rossignol et al., 2016). These compounds can serve as indicators of the sources because they are stable and have a long lifespan as well as possessing unique characteristics from different origins (Bush and McInerney, 2013). Palmitic (C16:0) and oleic acids (C18:1) have minimal effect on surface tension but can increase surface tension. Monounsaturated fatty acids, like oleic acid (C18:1), have less impact compared to lysophospholipids, while arachidonic acid (C20:4), despite being highly unsaturated, is generally limited in its effect due to low pulmonary surfactant levels(Hite et al., 2005). Therefore, investigation of the components of particulate matter (PM) with fatty acids can provide specific insights into the origins and diversity of PM transformation pathways in the atmosphere.
Over the past decade, haze issues have occurred in Chiang Mai, northern Thailand. The daily increase of PM2.5 always occurs between January and April. This is caused by the extensive combustion of biomass from both local burning and long-range transport. Furthermore, the geographical features of the Chiang Mai in the Chiang Mai-Lamphun basin accumulate the concentration of air pollution when compared to a flat area. This topographical configuration inhibits the dispersion of pollutants, impeding their effortless dissemination. (Chunram. et al., 2015; Kawichai et al., 2020; Mueller et al., 2020; Kawichai et al., 2022; Kraisitnitikul et al., 2024; Song et al., 2022; Amnuaylojaroen et al., 2023;Ponsawansong et al., 2023). The annual average of PM2.5 for the 10 years (2012-2021) of Chiang Mai ranges from 21 to 33 μg/m3. It's noteworthy that these levels consistently exceeded Thailand's annual PM2.5 average standard, which is set at 15 µg/m³. (Pollution Control Department, 2023). Chiang Mai area experienced a notable increase in both illness occurrences and the mortality rate during the severe haze campaign (Pothirat et al., 2019a, 2019b). In a previous study on OP in the Chiang Mai area, it was revealed that there existed a positive correlation between the OP level and 4-5 ring polycyclic aromatic hydrocarbons (PAHs), as well as inorganic ions. A substantial contribution to OP was identified from biomass burning, constituting 57.9% of its source identified by PMF model(Ponsawansong et al., 2023).In addition, they found that exposure to ambient PM2.5 resulted in elevated urinary hydroxylated-PAH levels, which were associated with increased urinary malondialdehyde (MDA) levels, indicating that oxidative damage and inflammation may be the underlying health effects (Ponsawansong et al., 2024).
The present study aimed to investigate the fatty acids and oxidative potential of PM2.5 over a regionally representative site in Chiang Mai City. This investigation will encompass various components, including fatty acids (C14:0-C30:0) and carbonaceous components all of which are constituents bound to PM2.5. The study will focus on establishing correlations and understanding the relationships between these components with the oxidative potential (OP) activity of PM2.5.
MATERIAL AND METHODS
Description of the sampling area
Ambient PM2.5 samples were collected in Chiang Mai City. The altitude of the sampling site is 332 meters above the mean sea level. The air sampler was located on the rooftop of the Research Institute for Health Sciences at the campus site of Chiang Mai University (CMU) (18°47'42.80"◦N, 98°57'28.41"◦E), which is in an urban zone of Chiang Mai City. It is in the Chiang Mai-Lamphun Basin, a large basin that causes accumulated air pollution. Seasonal stratification was divided into two periods including high- and low-biomass burning periods. The high biomass burning period was identified by hotspot data from NASA’s Fire Information for Resource Management System (FIRMS) showed more than 100 active hotspots per month in the Chiang Mai area, based on Near Real-Time (NRT) data (NASA FIRMS, https://firms.modaps.eosdis.nasa.gov/).
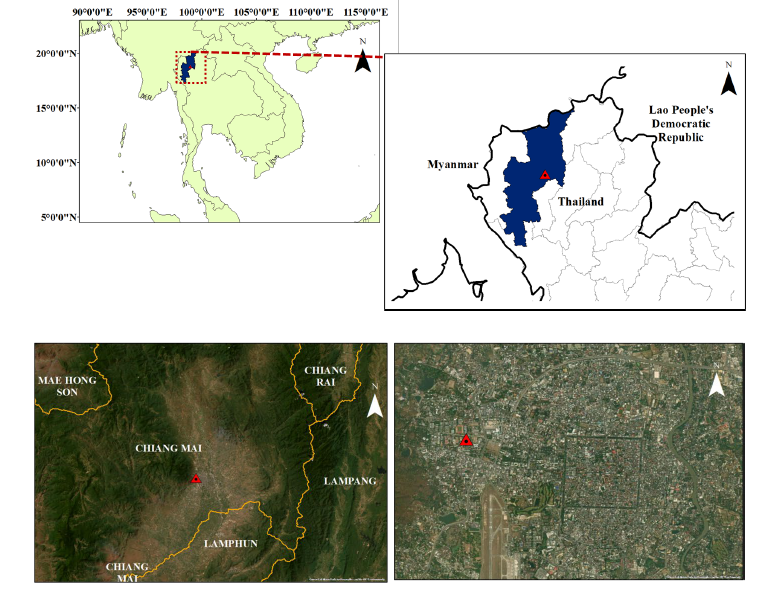
Figure 1. Location of sampling sites.
PM2.5 sample collection
The total number of ambient PM2.5 samples was 89, from January to December 2021. Ambient PM2.5 samples were collected by using quartz filters (2.2 μm, 90 mm diameter, QM-A grade, Whatman, Kent, UK) and a medium-volume sampler (model KC-120H, Qingdao Laoshan Co., Ltd., China) with a flow rate of 100 L/minute for 24 hours and three field blanks during the campaign. The quartz filters were heated to 450˚C for 6 hours in an electric furnace (model 8,2/1100 LHM01, SNOL, Utena, Lithuania). Before sampling, the filters were conditioned for 24 hours in a controlled environment. The environment had a relative humidity of 20 ± 2% and a temperature of 25 ± 2 °C. After conditioning, the filters were weighed using an analytical balance (5 places, AB 135 S/Fact, Mettler Toledo, Ohio, USA). After sampling, the filters were reweighted, wrapped with aluminum foil, and stored in sealed containers in the freezer at − 20˚C until analysis.
Measurement of oxidative potential (OP)
The OP measurement on PM2.5 was performed using the protocol previously described by Ponsawansong et al. (2023); Wang et al. (2019). Briefly, a sample filter (Ø 2 cm., 3.14 cm2) was extracted with ultrapure water using an ultrasonicator. The solution obtained from the filter was mixed with 0.1 M, pH 7.4 potassium phosphate buffer (K2HPO4, Loba, Mumbai, India; KH2PO4, Supelco, Darmstadt, Germany) and 1 mM dithiothreitol (DTT) solution (Calbiochem, Darmstadt, DE). The solution was incubated at 37°C. The solution was transferred into a centrifuge tube pre-filled with 10% trichloroacetic acid (Sigma-Aldrich, St. Louis, MO, USA) at 5-minute intervals and mixed with 10 mM DTNB (5,5'-dithiobis (2-nitrobenzoic acid)) solution (Sigma-Aldrich, St. Louis, MO, USA) and 0.4 M Tris buffer with 20mM EDTA (pH 8.9) buffer (Bio Basic, Markham, Ontario, Canada). The absorbance of 5-thio-2-nitrobenzoic acid (TNB) formed was measured at wavelength 412 nm using a UV-Vis spectrophotometer (model SPECTROstar® Nano, BMG LABTECH, Geylang, Singapore). A positive control, consisting of 0.24 µM phenanthrenequinone (PQN) (Sigma-Aldrich, St. Louis, MO, USA), was included in the assay to validate the assay's performance. Additionally, a blank quartz filter was used to account for any background levels of reactive oxygen species (ROS). The results obtained from the oxidative potential by DTT assay (OPDTT) were normalized using the total volume of sampled air (OPDTTv).
Analyses of PM2.5 components
Analysis of fatty acids
The fatty acids analysis on PM2.5 was performed using the protocol previously described by Haque et al. (2019). Briefly, the filter sample (Ø 1 cm., 0.79 cm2). The filter sample was extracted three times using ultrasonication for 10 minutes each time. Each extraction used a 5 mL mixture of dichloromethane and methanol in a 2:1 ratio (CNW, Shanghai, China). The extracted solution was filtered through quartz wool in a Pasteur glass pipette to remove all insoluble matrix. The solution was concentrated using a rotary evaporator, which removes the solvent under vacuum at a temperature of 25°C. The remaining extract was then dried using a light stream of nitrogen heat at 50°C. The dried extract was derivatized with 50 μl of N, O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylsilyl chloride (Sigma-Aldrich, St. Louis, MO, USA) and 10 μL of pyridine (Aladdin, CA., USA) in a glass vial (1.5 mL) with a Teflon screw cap at 75°C for 3 hours. 40 µL of 1.43 ppm tridecane (Sigma-Aldrich, St. Louis, MO, USA) was added to the derivatives as an internal standard.
The solution was injected into the gas chromatography system (model 7890B Agilent Technologies, California, USA) and mass spectrometry detector (model 5977A MSD Agilent Technologies, California, USA) using the spitless mode, with an injector temperature of 280ºC. The GC oven temperature was set at 50°C for 2 min, then the oven temperature was increased from 50 to 120°C at 30°C min-1 and then to 300°C at 6°C min-1, maintaining isotherm at 300°C for 16 min. Separate the compounds on a DB-5MS fused silica capillary column (30 m long × 0.25 mm inner diameter × 0.5 µm film thickness) with a helium gas carrier ( flow rate 1.0 mL min-1) (Haque et al., 2019). Acquire the signal using a mass spectrometer at 70 eV in electron impact (EI) mode with a scan range of 50–650 Daltons (Da). The field blank filters were analyzed by the procedure described above. The target compounds were not detected in the blank filters.
Analysis of organic carbon (OC) and elemental carbon (EC)
OC/EC analysis was performed using the protocol previously described by Chow et al. (2007). Briefly, the sampled filters were placed in a desiccant for 24 hours to remove moisture. The sampled filters (Ø 0.8 cm., 0.50 cm2) were analyzed the carbonaceous content of organic carbon (OC) and elemental carbon (EC) using the Multi-wavelength Thermal/Optical carbon analyzer (model DRI 2015, Magee Scientific, Berkeley, USA). Measurements of standards were compared using an 1800 ppm sucrose solution (Glentham Life Sciences, Corsham, United Kingdom) in 0.4 M hydrochloric acid (Carlo Erba Reagents SRL, Milan, Italy). The OC and EC content were analyzed using the IMPROVE_A standard method. The OC content was determined using 100% helium carrier gas, while the EC content was determined using a mixture of 98% helium and 2% oxygen. The carbonaceous content was determined by reversibly calculating it based on the total area of the sampled filter and the total air volume. The analytical detection limit (LOD) for total carbon was 0.2 ng C/cm2 of the filter blank.
Analysis of water-soluble organic carbon (WSOC)
The WSOC analysis was performed using the protocol previously described by Haque et al. (2019). The sampled filters (Ø 1.6 cm., 2.01 cm2) were extracted by sonication in ultrapure water (18.2 MΩ·cm, Milli-Q) in 15 mL for 30 minutes. The extracts were filtered using 0.22 μm PTFE syringe filters in glass bottles. The filtrates were analyzed for water-soluble organic carbon (WSOC) using a Total Organic Carbon Analyzer (model: TOC-L CPH CN200, Shimadzu Corporation, Kyoto, Japan). Total water-soluble organic carbon (TOC) is calculated by extracting inorganic water-soluble carbon (IC) from total water-soluble carbon (TC) and is labeled as WSOC. The detection of TC and IC by the nondispersive infrared (NDIR) gas analyzer is calibrated using the standard solutions of potassium hydrogen phthalate (C8H5KO4) and sodium carbonate-bicarbonate mixture (Na2CO3+NaHCO3; 1:1 mole/mole), respectively. The analytical detection limit (LOD) for WSOC was 0.22 mg C/L from blank SD. The WSOC concentration of sample was subtracted by the WSOC of blank filters.
Statistical analysis
All descriptive statistics were computed using Python programming language (Python Software Foundation, version 3.12.0, Delaware, USA). The Mann Whitney U test was conducted to check for statistically significant differences in OP responses and the concentrations of PM2.5 components. Moreover, univariate analysis was applied by computing the Spearman's rank correlation coefficients (r) to investigate the association of the OP responses with the PM2.5 chemical components. The significance level for all statistical tests was established at a P value of less than 0.05.
RESULTS
PM2.5 and fatty acids concentrations detected in PM2.5 from CM, 2021
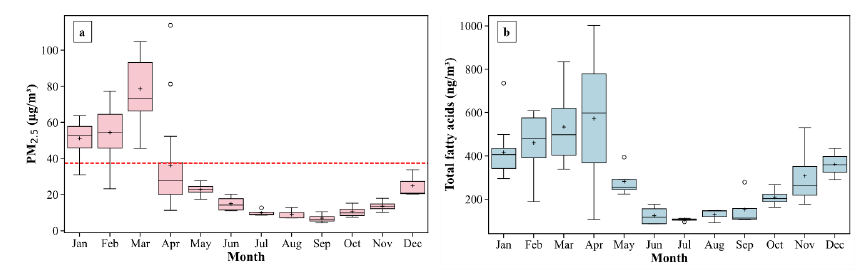
Figure 2. Time series of monthly average of PM2.5 concentrations in Chiang Mai city in 2021 (a). Time series of monthly average total fatty acids (b).
The mass concentration of PM2.5 was higher in HBB period than in LBB period with the highest monthly mean value (78.66 ± 18.31 μg/m³) observed in March (Figure 2a), which was possibly attributed to increased biomass burning activities during this period. Conversely, during LBB period from May to December, a general decrease in PM2.5 was noted, indicating a reduction in biomass burning activities and potentially improved air quality. April experienced a considerable decrease compared to March. From May forward, the PM2.5 concentrations consistently decreased, reaching their lowest concentrations in September of 6.91 ± 2.61 μg/m³. Overall, the annual average PM2.5 concentrations was at 41.54 ± 28.26 μg/m³. The data showed a notable daily PM2.5 concentrations exceeded the Thailand NAAQs. (37.5 μg/m³). There are 45 days in throughout the year (12.3%), indicating considerable harm to population health.
The time series data for total fatty acids across different months in year-round 2021 shows significant variability in Figure 2(b). March and April exhibit higher average concentrations, with means of approximately 534.5 ± 156.2 ng/m³ and 572.5 ± 301.6 ng/m, respectively, and wide ranges that include some of the highest revealed concentrations. April notably has the highest value of 1,002.14 ng/m³, indicating a highest in fatty acids during this month. In contrast, In LBB period including June, July, and August show lower concentrations, with June having the lowest mean of 125.1 ± 45.7 ng/m³ and the minimum concentration of 86.67 ng/m³, suggesting a seasonal trend. This seasonal pattern could be influenced by several factors such as changes in sources of PM2.5, atmospheric conditions, or human activities affecting the concentrations of fatty acids throughout the year. November and December have moderate concentrations, with November showing a broad range from 176.8 to 530.2 ng/m³ and December showing a range from 290.6 to 435.1 ng/m³. This indicates that the concentration of fatty acids in PM2.5 is subject to significant seasonal variations.
Chemical components of PM2.5
Table 1. Concentration of OPDTTv and chemical components of PM2.5 from Chiang Mai in 2021.
|
Components |
Mean ± SD |
Median |
Range |
|
PM2.5 (µg/m³) |
41.54 ± 28.25 |
41.46 |
4.34 - 113.77 |
|
OPDTTv (nmol/min/m³) |
0.10 ± 0.06 |
0.12 |
0.01 - 0.32 |
|
WSOC (µg/m³) |
7.08 ± 5.74 |
6.20 |
0.36 - 23.29 |
|
OC (µg/m³) |
11.29 ± 8.59 |
10.23 |
1.19 - 37.74 |
|
EC (µg/m³) |
1.67 ± 0.99 |
1.69 |
0.26 - 3.53 |
|
Total saturated fatty acid (ng/m³) |
339.00 ± 182.37 |
340.72 |
54.62 - 773.45 |
|
Total unsaturated fatty acid (ng/m³) |
61.33 ± 84.41 |
35.87 |
1.16 - 436.82 |
|
Total fatty acid (ng/m³) |
400.33 ± 220.85 |
393.82 |
86.67 - 1002.14 |
Figure 3 shows that the HBB period, from January to April, leads to peak PM2.5 concentrations and hotspot counts, with more than 100 hotspot counts per month in the Chiang Mai area. In contrast, the LBB period, from May to December, results in significantly lower PM2.5 concentrations and hotspot counts.
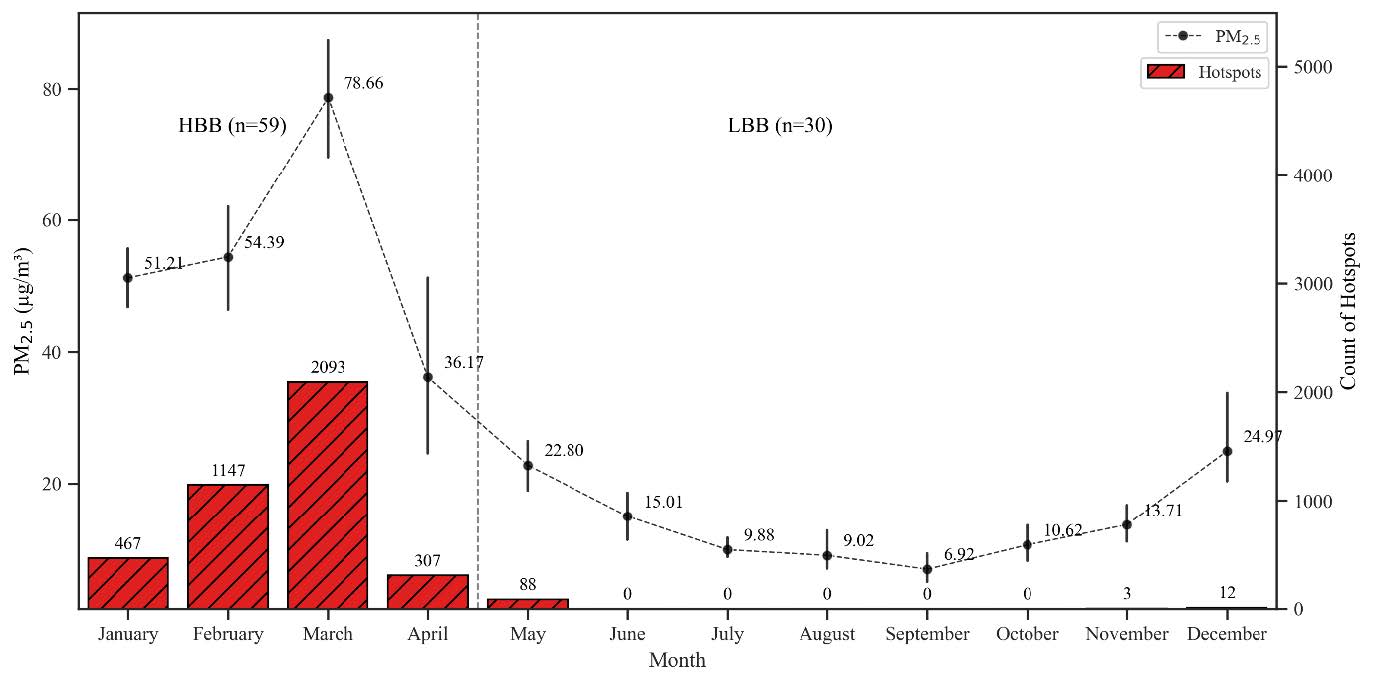
Figure 3. Time series of hotspot counts and monthly average PM2.5 concentrations in Chiang Mai during 2021.
The study revealed significantly higher concentrations of carbonaceous components during the HBB period compared to the LBB period. OC has the strongest positive correlation with PM2.5 concentrations, followed by EC, and WSOC. Comparing the concentrations of fatty acids between HBB and LBB periods revealed significant differences in the composition and concentration of various fatty acids, shown in Table 2. The mean concentrations of total fatty acids exhibited are significantly higher in HBB compared to LBB periods, indicating potentially greater contributions from lipid-rich sources or enhanced atmospheric oxidation in HBB period. Specifically, individual fatty acids such as myristic acid (C14), pentadecylic acid (C15), palmitic acid (C16) and stearic acid (C18) showed significantly elevated concentrations in HBB period, suggesting a higher abundance of shorter-chain fatty acids originating from lipid combustion or biogenic emissions. Conversely, certain fatty acids like margaric acid (C17), nonadecylic acid (C19), and arachidic acid (C20) showed higher concentrations in LBB period, indicating potential differences in emission sources or atmospheric processing pathways influencing their abundance in this LBB period.
Several fatty acids, such as pentadecylic acid (C15), nonadecylic acid (C19), heneicosylic acid (C21), behenic acid (C22), tricosylic acid (C23), lignoceric acid (C24), and pentacosylic acid (C25) exhibited remarkably high Spearman’s rank correlation coefficients ranging from 0.892 to 0.937 (P<0.01). These strong positive correlations suggested a robust correlation between the presence of these fatty acids and higher concentrations of PM2.5 in the atmosphere.
Table 2. Concentration of OPDTTv and chemical components of PM2.5 in HBB period and LBB period from CM, 2021.
|
Components |
HBB period (Jan to Apr 2021) |
LBB period (May to Dec 2021) |
Mann-Whitney U test |
||
|
Mean ± SD |
Median |
Mean ± SD |
Median |
P value |
|
|
OPDTTv (nmol/min/m³) |
0.13 ± 0.05 |
0.14 |
0.04 ± 0.04 |
0.02 |
<0.01 |
|
PM2.5 (µg/m³) |
55.5 ± 24.3 |
53.27 |
13.9 ± 7.00 |
12.19 |
<0.01 |
|
OC (µg/m³) |
15.3 ± 7.87 |
14.76 |
3.45 ± 2.00 |
2.95 |
<0.01 |
|
EC (µg/m³) |
2.13 ± 0.89 |
2.24 |
0.79 ± 0.45 |
0.64 |
<0.01 |
|
WSOC (µg/m³) |
9.70 ± 5.35 |
9.08s |
1.93 ± 1.26 |
1.48 |
<0.01 |
|
Total fatty acid (ng/m³) |
498.7 ± 196.1 |
466.0 |
206.9 ± 112.8 |
177.2 |
<0.01 |
|
Total saturated fatty acid |
425.1 ± 150.3 |
388.9 |
169.6 ± 104.8 |
146.2 |
<0.01 |
|
Total low molecular fatty acids (LFAs) |
344.2 ± 109.1 |
335.6 |
111.6 ± 57.8 |
91.83 |
<0.01 |
|
Myristic acid (C14:0) |
34.62 ± 29.18 |
30.83 |
3.10 ± 2.51 |
2.33 |
<0.01 |
|
Pentadecylic acid (C15:0) |
10.50 ± 8.65 |
8.45 |
1.24 ± 1.25 |
0.83 |
<0.01 |
|
Palmitic acid (C16:0) |
162.98 ± 44.92 |
162.6 |
27.47 ± 13.79 |
22.28 |
<0.01 |
|
Margaric acid (C17:0) |
40.96 ± 46.34 |
29.10 |
62.00 ± 45.77 |
49.58 |
<0.01 |
|
Stearic acid (C18:0) |
85.12 ± 43.25 |
82.18 |
14.26 ± 6.67 |
11.59 |
<0.01 |
|
Nonadecylic acid (C19:0) |
3.70 ± 2.78 |
3.42 |
0.62 ± 0.41 |
0.49 |
<0.01 |
|
Arachidic acid (C20:0) |
6.25 ± 3.54 |
5.64 |
2.90 ± 2.36 |
1.85 |
<0.01 |
|
Total high molecular fatty acids (HFAs) |
80.97 ± 61.26 |
67.53 |
57.96 ± 66.43 |
27.42 |
<0.05 |
|
Heneicosylic acid (C21:0) |
2.93 ± 2.28 |
2.93 |
0.03 ± 0.03 |
0.02 |
<0.01 |
|
Behenic acid (C22:0) |
6.74 ± 5.27 |
5.99 |
0.58 ± 0.39 |
0.43 |
<0.01 |
|
Tricosylic acid (C23:0) |
3.80 ± 3.23 |
2.85 |
0.10 ± 0.09 |
0.08 |
<0.01 |
|
Lignoceric acid (C24:0) |
13.30 ± 9.77 |
11.34 |
0.56 ± 0.88 |
0.38 |
<0.01 |
|
Pentacosylic acid (C25:0) |
2.84 ± 2.62 |
2.18 |
0.06 ± 0.06 |
0.03 |
<0.01 |
|
Cerotic acid (C26:0) |
13.90 ± 15.09 |
10.05 |
0.89 ± 3.03 |
0.34 |
<0.01 |
|
Carboceric acid (C27:0) |
5.39 ± 4.33 |
4.66 |
11.49 ± 16.23 |
2.77 |
0.738 |
|
Montanic acid (C28:0) |
14.81 ± 12.55 |
12.35 |
11.3 ± 13.47 |
4.99 |
0.088 |
|
Nonacosylic acid (C29:0) |
11.22 ± 15.79 |
5.86 |
32.22 ± 41.38 |
14.72 |
<0.01 |
|
Melissic acid (C30:0) |
6.11 ± 6.17 |
4.26 |
0.72 ± 0.60 |
0.51 |
<0.01 |
|
Total unsaturated fatty acid |
73.56 ± 101.12 |
35.87 |
37.28 ± 16.29 |
35.91 |
0.728 |
|
Oleic acid (C18:1) |
49.48 ± 109.4 |
5.00 |
36.46 ± 16.07 |
35.04 |
<0.01 |
|
Linolenic acid (C18:2) |
24.07 ± 16.35 |
23.48 |
0.82 ± 0.46 |
0.76 |
<0.01 |
Oxidative potential of PM2.5 components
The correlation analysis between various fatty acids and other PM2.5 components and OPDTTv is shown in Table 3 over a year-round sampling period, and during specific periods, namely, HBB and LBB periods. The concentrations of PM2.5 showed a strong positive correlation with OPDTTv, particularly over the year and during the HBB period indicating its significant contribution to elevated OPDTTv. The findings reveal that several carbonaceous components i.e., OC, EC, and WSOC and fatty acids, showed strong positive correlations with OPDTTv throughout the year. Specifically, pentacosylic acid (C25:0), pentadecylic acid (C15:0), tricosylic acid (C23:0), heneicosylic acid (C21:0), myristic acid (C14:0), cerotic acid (C26:0), behenic acid (C22:0), and linolenic acid (C18:2) exhibit significant positive correlations with OPDTTv year-round and HBB period indicating their contribution to the OP of PM2.5 across all seasons especially in HBB period. Notably, oleic acid (C18:1) exhibits a significant negative correlation with OPDTTv.
Table 3. Spearman’s rank correlation coefficient (r) of OPDTTv and PM2.5 components
|
Components |
Spearman’s rank correlation coefficient (r) |
|||||
|
|
Year |
HBB |
LBB |
|||
|
PM2.5 |
0.666** |
0.512** |
-0.072 |
|||
|
OC |
0.661** |
0.504** |
-0.051 |
|||
|
EC |
0.593** |
0.418** |
0.011 |
|||
|
WSOC |
0.655** |
0.510** |
-0.144 |
|||
|
Myristic acid (C14:0) |
0.619** |
0.336* |
-0.016 |
|||
|
Pentadecylic acid (C15:0) |
0.663** |
0.528** |
-0.023 |
|||
|
Palmitic acid (C16:0) |
0.483** |
-0.055 |
-0.189 |
|||
|
Stearic acid (C18:0) |
0.587** |
0.119 |
0.001 |
|||
|
Nonadecylic acid (C19:0) |
0.578** |
0.466** |
-0.249 |
|||
|
Arachidic acid (C20:0) |
0.362** |
0.159 |
-0.113 |
|||
|
Heneicosylic acid (C21:0) |
0.652** |
0.371** |
-0.064 |
|||
|
Behenic acid (C22:0) |
0.614** |
0.419** |
-0.083 |
|||
|
Tricosylic acid (C23:0) |
0.657** |
0.45** |
-0.18 |
|||
|
Lignoceric acid (C24:0) |
0.577** |
0.327* |
-0.013 |
|||
|
Pentacosylic acid (C25:0) |
0.666** |
0.458** |
0.014 |
|||
|
Cerotic acid (C26:0) |
0.609** |
0.469** |
-0.019 |
|||
|
Melissic acid (C30:0) |
0.499** |
0.387** |
-0.181 |
|||
|
Oleic acid (C18:1) |
-0.511** |
-0.099 |
0.055 |
|||
|
Linolenic acid (C18:2) |
0.645** |
0.386** |
-0.16 |
|||
|
** = P < 0.01 * = p < 0.05 |
||||||
DISCUSSION
The monthly average of PM2.5 concentrations in 2021 was higher in the HBB period than in the LBB period with the highest value in March. The annual average PM2.5 concentrations (41.54 ± 28.26 μg/m³) exceeded Thailand NAAQs (15 μg/m³) by approximately 3-fold, indicating potential public health risks. Seasonal variations of fatty acids concentrations were also observed. PM2.5 concentrations varied considerably, Thepnuan et al. (2018) reporting the highest concentration of 64.3 ± 17.6 µg/m³ from February to April 2016. This contrasts with the lower level of 38.7 ± 18.2 µg/m³ documented by Kawichai et al. (2024) from January to May 2017. Song et al. (2022) observed PM2.5 concentrations of 55.5 ± 20.1 µg/m³ from February to March 2018, similar this study's findings of 55.5 ± 24.3 µg/m³ for January to May 2021. OC concentrations also showed significant differences over various periods at 23.6 ± 8.1 µg/m³ in 2016 (Thepnuan et al., 2018). In comparison, Kawichai et al. (2024) and Song et al. (2022) reported much lower concentrations at 10.0 ± 4.89 µg/m³ in 2017 and 10.2 ± 4.1 µg/m³ in 2018, respectively. This study found intermediate OC concentrations were 15.3 ± 7.87 µg/m³, OC in 2021 increase from the 2017 and 2018 data but still lower than the 2016 highest. EC consistently showed lower concentrations than OC across all studies. In 2016 noted EC concentrations of 2.85 ± 0.98 µg/m³, while in 2017 reported 1.35 ± 0.68 µg/m³, and in 2018 found 1.2 ± 0.6 µg/m³. This study showed EC concentrations at 2.13 ± 0.89 µg/m³. Additionally, WSOC concentrations showed an increasing trend, with 7.61 ± 4.08 µg/m³ in 2017, 8.0 ± 3.4 µg/m³ in 2018, and 9.70 ± 5.35 µg/m³ in 2021, indicating a gradual increase in WSOC content over the years. Strong correlations between PM2.5 and specific chemical components like levoglucosan, K, and NH4 can indicate biomass burning as a major source of PM2.5 (Thepnuan et al., 2018). Additionally, the correlation between WSOC and OC suggests primary combustion emissions significantly influence WSOC levels (Kawichai et al., 2024)
Table 4. Carbonaceous components studies in Chiang Mai City (µg/m³).
|
Year |
PM2.5 |
OC |
EC |
WSOC |
References |
|
Feb to Apr, 2016 |
64.3 ± 17.6 |
23.6 ± 8.1 |
2.85 ± 0.98 |
N.A. |
Thepnuan et al. (2018) |
|
Jan to May, 2017 |
38.7 ± 18.2 |
10.0 ± 4.89 |
1.35 ± 0.68 |
7.61 ± 4.08 |
Kawichai et al. (2024) |
|
Feb to Mar, 2018 |
55.5±20.1 |
10.2 ± 4.1 |
1.20 ± 0.60 |
8.00 ± 3.40 |
Song et al. (2022) |
|
Jan to April, 2021 |
55.5 ± 24.3 |
15.3 ± 7.87 |
2.13 ± 0.89 |
9.70 ± 5.35 |
This study |
Among several studies of fatty acids were shown in Table 5, New Delhi, India, is notable for its exceptionally high concentrations of PM10 (373 µg/m³) during March (haze period)(Kang et al., 2016). In addition, in this study, and Nanjing, China was reported by Haque et al. (2019), exhibit PM2.5 concentrations of 41.54 and 66.00 µg/m³ respectively. However, rural regions like Melpitz, Germany, and Alaska, USA, also encounter significant PM2.5 pollution (Alves et al., 2012; Boreddy et al., 2018).
This study revealed that fatty acid concentrations stand out with a high concentration of 400.33 ng/m³, significantly surpassing heights concentrations in Nanjing, China (76.3 ng/m³), Melpitz, Germany (85.0 ng/m³), and Alaska, USA (48.72 ng/m³ in cold period and 82.2 ng/m³ in warm period). This variation may stem from diverse emission sources, atmospheric conditions, and local activities influencing the composition of fatty acids. Urban areas like New Delhi and Nanjing may see higher concentrations due to vehicular emissions and industrial activities, while rural regions like Melpitz and Alaska may have lower but still significant levels attributed to agricultural practices and biomass burning.
Table 5. Comparisons of PM2.5 -bound fatty acids in Chiang Mai City with other studies.
|
City |
Study Year |
Season (month) |
PM (µg/m³) |
Fatty acids (ng/m³) |
References |
|
Chiang Mai, TH (Chiang Mai City) |
2021 |
Year-round (Jan -Dec) |
PM2.5 |
400.3 ± 220.80 |
This study |
|
Nanjing, CN (urban area) |
2014-2015 |
Winter |
PM2.5 |
76.30 |
Haque et al. (2019) |
|
Melpitz, Germany (rural area) |
2008 |
Spring (May) |
PM2.5 |
85.0 ± 21.30 |
Alves et al. (2012) |
|
Alaska, USA (rural area) |
2008-2009 |
Year round (Jun to Jun) |
TSP |
Warm (82.20 ± 103.00) Cold (48.72 ± 32.80) |
Boreddy et al. (2018) |
|
New Delhi, India (urban area) |
2012 |
Mar (Haze) |
PM10 |
1,458.00 |
Kang et al. (2016) |
Our study shows the Spearman's rank correlation coefficients (r) for OPDTTv and fatty acids, indicating the relationship between these fatty acids and particulate matter pollution. HFAs such as pentacosylic acid (C25:0) and tricosylic acid (C23:0), are primarily from vegetation combustion (Fu et al., 2013; Hughen et al., 2004) and show strong positive correlations with OPDTTv throughout the year. In contrast, LFAs, such as pentadecylic acid (C15:0) and myristic acid (C14:0), emitted from microbial and algal in soil or marine to aerosol (Haque et al., 2019), also exhibit significant positive correlations. During HBB periods, the correlations remain positive but generally lower. LFAs such as pentadecylic acid (C15:0) and nonadecylic acid (C19:0) still show significant correlations with OPDTTv. The notable exceptions are oleic acid (C18:1) consistently shows a negative correlation, oleic acid (C18:1) was observed to be lower during HBB period than at LBB period, suggesting an enhanced emission to the atmosphere at LBB period and a higher oxidation rate during HBB period (Kawamura and Gagosian, 1987). The divergent behavior of oleic acid (C18:1) compared to linolenic acid (C18:2), which shows significant positive correlations highlights the different emission sources and atmospheric behaviors of these fatty acids. Saturated fatty acids (C22:0–26:0) might contribute to OP during the biomass burning period due to their stability and ability to interact with reactive species over time.
Table 6 summarizes the relevant studies on the OP of PM2.5 in other cities worldwide. Ponsawansong et al. (2023) reported that in Chiang Mai, Thailand, OPDTTv was strongly influenced by 4-5 ring PAHs like fluoranthene and pyrene during the dry season, particularly during haze episodes. Chiang Mai experiences these haze episodes predominantly during the dry season, characterized by elevated PM2.5 concentrations largely due to biomass burning activities. Secondary inorganic aerosols, including ions such as NH4+, SO42–, and NO3–, also showed strong positive correlations with OPDTTv activity. Pietrogrande et al. (2023) reported that in Milan, Italy, the OP was driven by pollution from biomass burning (traced by OC and levoglucosan) and vehicular traffic (indicated by redox-active metals like Cr, Mn, Fe, Cu, Zn, and Pb) during late winter. The Po Valley experiences stable weather conditions during winter, leading to weak atmospheric mixing and the accumulation of pollutants in the lower atmosphere, resulting in higher concentrations of particulate matter and oxidative potential. Paraskevopoulou et al. (2022) reported that in Athens, Greece, OP was primarily driven by local biomass burning and regional transport, particularly linked to SO42− concentrations. Athens experiences significant wood burning for domestic heating during the coldest period of winter, contributing to intense smog events and impacting air quality. Cui et al. (2023) reported that in Changzhou, China, OP was closely correlated with OC, WSOC, and SIA, with transition metals like Cu, Mn, and Zn strongly correlated with OP. In Changzhou, secondary inorganic aerosols, such as NO₃⁻, SO₄²⁻, and NH₄⁺, were significant in spring, driven by favorable weather conditions for chemical reactions. The study also noted higher pollution levels in spring compared to summer. In this study, OP was strongly correlated with OC, EC, and WSOC, especially during haze and biomass burning periods. In Chiang Mai City, Thailand, the dry season was associated with high PM2.5 concentrations due to biomass burning and reduced air dispersion, exacerbating the oxidative potential of PM2.5 components.
PM2.5-bound fatty acids contribute to OP through photolytic reactions, generating reactive radicals like hydroxyl radicals (OH•) when exposed to UV light. These radicals trigger further reactions with surrounding organic molecules, forming unsaturated aldehydes and acids that play a key role in secondary organic aerosol (SOA) production. Interactions between these unsaturated compounds and oxidants like ozone (O₃) lead to the formation of low-volatility products, further enhancing SOA and ROS generation(Rossignol et al., 2016; Alpert et al., 2017) are demonstrated in Figure 4. This radical-driven chemistry is particularly relevant during HBB period, explaining the strong correlation between fatty acids and OP. The ROS generated by these reactions can interact with biological tissues, causing oxidative stress and damaging essential cellular components like DNA, proteins, and lipids. Such oxidative stress is associated with various health issues, including inflammation, respiratory diseases, and cardiovascular conditions (Albano et al., 2022), emphasizing the significant role of fatty acids in the health impacts of air pollution.
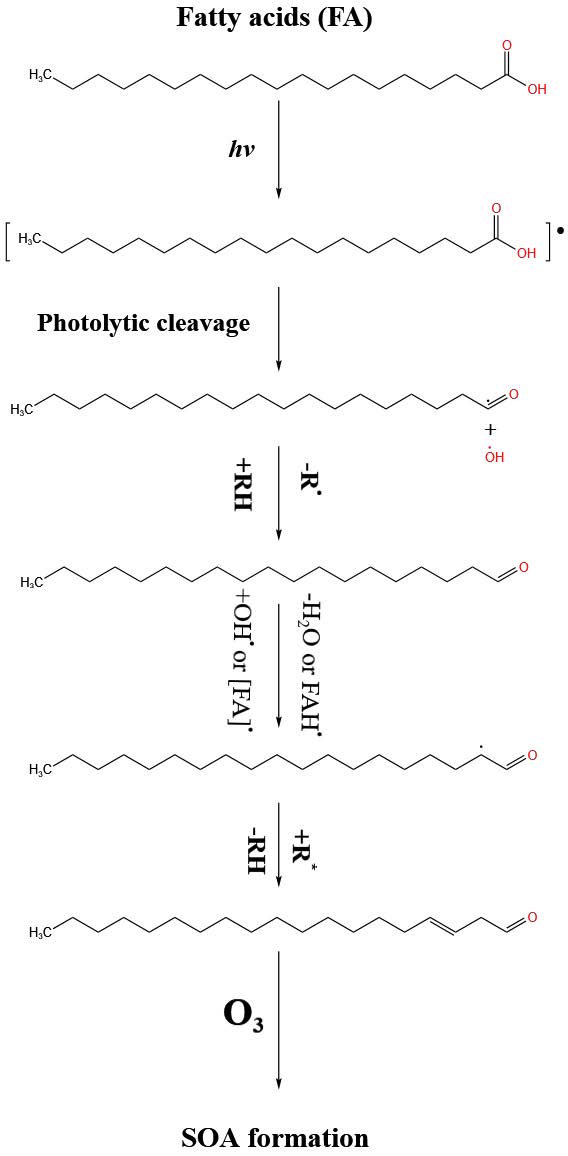
Figure 4. Proposed photochemical reaction mechanism including ozonolysis of products resulting in SOA formation.
In addition to generating ROS, fatty acids exhibit surfactant properties that influence the behavior of these reactive molecules (Cheng et al., 2019). lipids can adhere to hydrophobic particles seen in natural lung surfactants (Kumar and Bohidar, 2010; Konduru et al., 2018). As surfactants, fatty acids can alter the solubility and distribution of ROS in the epithelial lining fluid of the lungs (Hite et al., 2005). PM2.5 can be incorporated into lung surfactant either as individual particles or as aggregates, with or without a lipid coating. The surfactant's defense system can initiate clearance to remove the impinging particulate matter. PM2.5 particles can potentially infiltrate lung surfactant and enter capillaries. PM2.5 interaction with bilayer vesicles hinders the metabolism of lung surfactant. Additionally, lipids can also transfer to the surface of the pulmonary membrane and influence its activity within the alveoli. This transfer also captures a portion of lung surfactant(Wang et al., 2020). The combined effects of ROS generation and surfactant properties make fatty acids a significant contributor to the OP of PM2.5.
Table 6. Comparisons of the OP of Chiang Mai PM2.5 with others study.
|
City |
Year |
Month |
Season |
PM2.5 (µg/m³) |
OPDTTv (nmol/min/m³) |
References |
|
Chiang Mai, TH (Saraphi District) |
2018 |
Jan – Apr (N=79) |
Dry |
64.30 ± 47.30 |
1.75 ± 2.48 |
Ponsawansong |
|
Milan, IT |
2019 |
Feb – Mar (N= NA) |
Late winter |
25.20 ± 8.90 |
0.21 ± 0.13 |
Pietrogrande |
|
Athens, GR |
2020 |
Mar – May (N=NA) |
Late winter |
16.67 ± 6.78 |
0.22 ± 0.11 |
Paraskevopoulou et al. (2022) |
|
Changzhou, CN |
2021 |
Mar – Apr (N=48) |
Spring |
91.44 ± 27.18 |
1.16 ± 0.58 |
Cui et al. (2023) |
|
Chiang Mai, TH (Chiang Mai City) |
2021 |
Jan – Apr (N=59) |
Dry |
55.58 ± 24.33 |
0.13 ± 0.06 |
This study |
CONCLUSION
This study investigated the OP of PM2.5-bound fatty acids in Chiang Mai, located in upper northern Thailand, throughout the year 2021. Chiang Mai faces annual haze problems, with PM2.5 concentrations peaking between January and April due to extensive biomass burning from both local sources and long-range transport. The region's geographical features, particularly the Chiang Mai-Lamphun basin, exacerbate air pollution compared to flat areas. To our knowledge, this is the first study to report the presence of PM2.5-bound fatty acids and their OP in upper northern Thailand, highlighting the role of fatty acids in contributing to OP. Our focus was on establishing correlations and understanding the relationships between these components and the OP activity of PM2.5.
We found that PM2.5 concentrations increased significantly during the high biomass burning period, especially in March, where the highest average concentration was observed. This peak was likely due to increased biomass burning activities. Conversely, during the low biomass burning period from May to December, PM2.5 concentrations decreased, with an annual average concentration lower than the high biomass burning period. Notably, daily PM2.5 concentrations exceeded Thailand's national air quality standards on several occasions, indicating significant health risks. Total fatty acids accounted for higher concentrations during the high biomass burning period compared to the low biomass burning period. The OP was also higher during the high biomass burning period. Long-chain saturated fatty acids showed a moderately significant correlation with OP during the high biomass burning period, but not during the low biomass burning period. April had the highest concentration of total fatty acids and, correspondingly, the highest OP. Higher fatty acids strongly correlate with OP, particularly during high biomass burning periods, suggesting a significant risk of oxidative stress when inhaled into the lungs. These fatty acids can act as surfactants, dissolving other ROS in PM2.5 and interacting with the epithelial lining fluid, which is elevated during lung inflammation. Additionally, they serve as surface-organic coatings and can undergo secondary oxidation in the atmosphere, further contributing to ROS formation. Our findings highlight the importance of assessing PM2.5-induced oxidative stress in upper northern Thailand for its health implications.
ACKNOWLEDGEMENTS
I would like to acknowledge the financial support partially provided by the Research Institute for Health Sciences (RIHES), Chiang Mai University (CMU), Grant Number 001/2566. Furthermore, I am grateful for the research funding provided by the National Research Council of Thailand (project: Determination of Secondary Aerosol Sources of Ambient PM2.5 in Chiang Mai City: A Year-Round Campaign in 2021, project ID: 31249) to TP. This support enabled me to conduct the necessary PM2.5 sampling and PM2.5 chemical analyses essential for this research. I am thankful to the School of Ecology and Applied Meteorology, the Atmospheric Environment Center, and the Joint Laboratory for International Cooperation on Climate and Environmental Change, Ministry of Education (ILCEC), Nanjing University of Information Science & Technology (NUIST), Nanjing, China, for providing the facility to analyze samples.
AUTHOR CONTRIBUTIONS
Conceptualization, T.S. and T.P.; methodology, P.P.; validation, S.K.; formal analysis, T.S.; investigation, T.P.; resources, Y.Z. and F.C.; data curation, T.S.; writing-original draft preparation, T.S.; writing-review and editing, T.P.; supervision, S.K., W.S., Y.Z. and F.C.; project administration, T.P.; funding acquisition, T.S. and T.P. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Albano, G. D., Gagliardo, R. P., Montalbano, A. M., and Profita, M. 2022. Overview of the mechanisms of oxidative stress: Impact in inflammation of the airway diseases. Antioxidants. 11(11): 2237.
Alpert, P. A., Ciuraru, R., Rossignol, S., Passananti, M., Tinel, L., Perrier, S., Dupart, Y., Steimer, S. S., Ammann, M., Donaldson, D. J., and George, C. 2017. Fatty acid surfactant photochemistry results in new particle formation. Scientific Reports.7(1): 12693.
Alves, C., Vicente, A., Pio, C., Kiss, G., Hoffer, A., Decesari, S., Prevôt, A. S. H., Minguillón, M. C., Querol, X., Hillamo, R., Spindler, G., and Swietlicki, E. 2012. Organic compounds in aerosols from selected European sites – Biogenic versus anthropogenic sources. Atmospheric Environment. 59: 243-255.
Amnuaylojaroen, T., Kaewkanchanawong, P., and Panpeng, P. 2023. Distribution and meteorological control of PM2.5 and its effect on visibility in northern Thailand. Atmosphere. 14(3): 538.
Atkinson, R. W., Kang, S., Anderson, H. R., Mills, I. C., and Walton, H. A. 2014. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax. 69(7): 660.
Bartoli, M., Novelli, F., Costa, F., Malagrinò, L., Melosini, L., Bacci, E., Cianchetti, S., Dente, F., Di Franco, A., and Vagaggini, B. 2011. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators of Inflammation. 2011: 891752.
Bartoli, M. L., Novelli, F., Costa, F., Malagrinò, L., Melosini, L., Bacci, E., Cianchetti, S., Dente, F. L., Di Franco, A., Vagaggini, B., and Paggiaro, P. L. 2011. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators of Inflammation. 2011(1): 891752.
Bates, J. T., Fang, T., Verma, V., Zeng, L., Weber, R. J., Tolbert, P. E., Abrams, J. Y., Sarnat, S. E., Klein, M., Mulholland, J. A., and Russell, A. G. 2019. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environmental Science & Technology. 53(8): 4003-4019.
Bates, J. T., Weber, R. J., Verma, V., Fang, T., Ivey, C., Liu, C., Sarnat, S. E., Chang, H. H., Mulholland, J. A., and Russell, A. 2018. Source impact modeling of spatiotemporal trends in PM2.5 oxidative potential across the eastern United States. Atmospheric Environment. 193: 158-167.
Beelen, R., Stafoggia, M., Raaschou-Nielsen, O., Andersen, Z. J., Xun, W. W., Katsouyanni, K., Dimakopoulou, K., Brunekreef, B., Weinmayr, G., Hoffmann, B., et al., 2014. Long-term exposure to air pollution and cardiovascular mortality: An analysis of 22 European cohorts. Epidemiology. 25(3): 368-378.
Boogaard, H., Janssen, N. A., Fischer, P. H., Kos, G. P., Weijers, E. P., Cassee, F. R., van der Zee, S. C., de Hartog, J. J., Brunekreef, B., and Hoek, G. 2012. Contrasts in oxidative potential and other particulate matter characteristics collected near major streets and background locations. Environmental Health Perspectives. 120(2): 185-191.
Boreddy, S. K. R., Haque, M. M., Kawamura, K., Fu, P., and Kim, Y. 2018. Homologous series of n-alkanes (C19-C35), fatty acids (C12-C32) and n-alcohols (C8-C30) in atmospheric aerosols from central Alaska: Molecular distributions, seasonality and source indices. Atmospheric Environment. 184: 87-97.
Bush, R. T. and McInerney, F. A. 2013. Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochimica et Cosmochimica Acta. 117: 161-179.
Carolina, M., Carlos, A. M., A, and G. 2023. The oxidative potential of airborne particulate matter in two urban areas of Chile: More than meets the eye. Environment International. 107866.
Cesari, D., Merico, E., Grasso, F. M., Decesari, S., Belosi, F., Manarini, F., De Nuntiis, P., Rinaldi, M., Volpi, F., Gambaro, A., Morabito, E., and Contini, D. 2019. Source apportionment of PM2.5 and of its oxidative potential in an industrial suburban site in south Italy. Atmosphere. 10(12): 758.
Cheng, S., Li, S., Tsona, N. T., George, C., and Du, L. 2019. Insights into the headgroup and chain length dependence of surface characteristics of organic-coated sea spray aerosols. ACS Earth and Space Chemistry. 3(4): 571-580.
Chow, J. C., Watson, J. G., Chen, L. W. A., Chang, M. C. O., Robinson, N. F., Trimble, D., and Kohl, S. 2007. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. Journal of the Air & Waste Management Association. 57(9): 1014-1023.
Chrysikou, L. and Samara, C. 2009. Seasonal variation of the size distribution of urban particulate matter and associated organic pollutants in the ambient air. Atmospheric Environment. 43: 4557-4569.
Chunram., N., Vinitketkumnuen., U., Deming., R. L., and Kamens., R. M. 2007. Distributions of fine particulate matter (PM2.5) in the ambient air of Chiang Mai-Lamphun Basin. Journal of Yala Rajabhat University, 2(1): 1-13.
Cui, Y., Zhu, L., Wang, H., Zhao, Z., Ma, S., and Ye, Z. 2023. Characteristics and oxidative potential of ambient PM2.5 in the Yangtze river delta region: Pollution level and source apportionment. Atmosphere. 14: 425.
Dominutti, P. A., Borlaza, L. J. S., Sauvain, J.-J., Ngoc Thuy, V. D., Houdier, S., Suarez, G., Jaffrezo, J.-L., Tobin, S., Trébuchon, C., Socquet, S., Moussu, E., Mary, G., and Uzu, G. 2023. Source apportionment of oxidative potential depends on the choice of the assay: insights into 5 protocols comparison and implications for mitigation measures [10.1039/D3EA00007A]. Environmental Science: Atmospheres. 3(10): 1497-1512.
Farahani, V. J., Altuwayjiri, A., Pirhadi, M., Verma, V., Ruprecht, A. A., Diapouli, E., Eleftheriadis, K., and Sioutas, C. 2022. The oxidative potential of particulate matter (PM) in different regions around the world and its relation to air pollution sources. Environmental Science: Atmospheres Journal. 2(5): 1076-1086.
Fu, P. Q., Kawamura, K., Chen, J., Charrière, B., and Sempéré, R. 2013. Organic molecular composition of marine aerosols over the Arctic Ocean in summer: Contributions of primary emission and secondary aerosol formation. Biogeosciences. 10(2): 653-667.
Gao, D., Godri Pollitt, K., Mulholland, J., Russell, A., and Weber, R. 2020. Characterization and comparison of PM2.5 oxidative potential assessed by two acellular assays. Atmospheric Chemistry and Physics. 20: 5197-5210.
Haque, M. M., Kawamura, K., Deshmukh, D. K., Fang, C., Song, W., Mengying, B., and Zhang, Y. L. 2019. Characterization of organic aerosols from a Chinese megacity during winter: Predominance of fossil fuel combustion. Atmospheric Chemistry and Physics 19(7): 5147-5164.
Hite, R. D., Seeds, M. C., Jacinto, R. B., Grier, B. L., Waite, B. M., and Bass, D. A. 2005. Lysophospholipid and fatty acid inhibition of pulmonary surfactant: Non-enzymatic models of phospholipase A2 surfactant hydrolysis. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1720(1): 14-21.
Hughen, K. A., Eglinton, T. I., Xu, L., and Makou, M. 2004. Abrupt tropical vegetation response to rapid climate changes. Science. 304(5679): 1955-1959.
Jacobson, M. C., Hansson, H. C., Noone, K. J., and Charlson, R. J. 2000. Organic atmospheric aerosols: Review and state of the science. Reviews of Geophysics. 38(2): 267-294.
Kang, M., Fu, P., Aggarwal, S., Kumar, S., Zhao, y., Sun, Y., and Wang, Z. 2016. Size distributions of n-alkanes, fatty acids and fatty alcohols in springtime aerosols from New Delhi, India. Environmental Pollution. 219: 957-966.
Kawamura, K. and Gagosian, R. B. 1987. Implications of ω-oxocarboxylic acids in the remote marine atmosphere for photo-oxidation of unsaturated fatty acids. Nature. 325(6102: 330-332.
Kawichai, S., Prapamontol, T., Cao, F., Song, W., and Zhang, Y.-L. 2024. Characteristics of Carbonaceous species of PM2.5 in Chiang Mai city, Thailand. Aerosol and Air Quality Research. 24(4): 230269.
Kawichai, S., Prapamontol, T., Cao, F., Song, W., and Zhang, Y. 2022. Source identification of PM2.5 during a smoke haze period in Chiang Mai, Thailand, using stable carbon and nitrogen isotopes. Atmosphere. 13: 1149.
Kawichai, S., Prapamontol, T., Chantara, S., Kanyanee, T., Wiriya, W., and Zhang, Y. 2020. Seasonal variation and sources estimation of PM2.5-bound PAHs from the ambient air of Chiang Mai city: An all-year-round study in 2017. Chiang Mai Journal of Science.47(5): 958-972.
Kloog, I., Ridgway, B., Koutrakis, P., Coull, B. A., and Schwartz, J. D. 2013. Long- and short-term exposure to PM2.5 and mortality: Using novel exposure models. Epidemiology. 24(4): 555-561.
Konduru, N. V., Damiani, F., Stoilova-McPhie, S., Tresback, J. S., Pyrgiotakis, G., Donaghey, T. C., Demokritou, P., Brain, J. D., and Molina, R. M. 2018. Nanoparticle wettability influences nanoparticle–phospholipid interactions. Langmuir. 34(22): 6454-6461.
Kraisitnitikul, P., Thepnuan, D., Chansuebsri, S., Yabueng, N., Wiriya, W., Saksakulkrai, S., Shi, Z., and Chantara, S. 2024. Contrasting compositions of PM2.5 in northern Thailand during La Niña (2017) and El Niño (2019) years. Journal of Environmental Sciences. 135: 585-599.
Kumar, P. and Bohidar, H. B. 2010. Interaction of soot derived multi-carbon nanoparticles with lung surfactants and their possible internalization inside alveolar cavity. Indian Journal of Experimental Biology. 48(10): 1037-1042.
Lee, S., Lee, W., Kim, D., Kim, E., Myung, W., Kim, S.-Y., and Kim, H. 2019. Short-term PM2.5 exposure and emergency hospital admissions for mental disease. Environmental Research. 171: 313-320.
Liu, B., Li, T., Yang, J., Wu, J., Wang, J., Gao, J., Bi, X., Feng, Y., Zhang, Y., and Yang, H. 2017. Source apportionment and a novel approach of estimating regional contributions to ambient PM2.5 in Haikou, China. Environmental Pollution. 223: 334-345.
Ma, Y., Cheng, Y., Qiu, X., Cao, G., Fang, Y., Wang, J., Zhu, T., Yu, J., and Hu, D. 2018. Sources and oxidative potential of water-soluble humic-like substances (HULISWS) in fine particulate matter (PM2.5) in Beijing. Atmospheric Chemistry and Physics. 18(8): 5607-5617.
Ma, Y. J., Xu, Y., Yang, T., Xiao, H. W., and Xiao, H. Y. 2024. Measurement report: Characteristics of nitrogen-containing organics in PM2.5 in Ürümqi, northwestern China - differential impacts of combustion of fresh and aged biomass materials. Atmospheric Chemistry and Physics. 24(7): 4331-4346.
Mueller, W., Loh, M., Vardoulakis, S., Johnston, H. J., Steinle, S., Precha, N., Kliengchuay, W., Tantrakarnapa, K., and Cherrie, J. W. 2020. Ambient particulate matter and biomass burning: An ecological time series study of respiratory and cardiovascular hospital visits in northern Thailand. Environmental Health. 19(1): 77.
Papaharalambus, C. A. and Griendling, K. K. 2007. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends in cardiovascular medicine. 17(2): 48-54.
Paraskevopoulou, D., Bougiatioti, A., Zarmpas, P., Tsagkaraki, M., Nenes, A., and Mihalopoulos, N. 2022. Impact of COVID-19 lockdown on oxidative potential of particulate matter: Case of athens (Greece). Toxics. 10(6): 280.
Park, M., Lee, S., Lee, H., Denna, M., Jang, J., Oh, D., Bae, M.-S., Jang, K.-S., and Park, K. 2024. New health index derived from oxidative potential and cell toxicity of fine particulate matter to assess its potential health effect. Heliyon. 10: e25310.
Pietrogrande, M. C., Colombi, C., Cuccia, E., Dal Santo, U., and Romanato, L. 2023. Seasonal and spatial variations of the oxidative properties of ambient PM2.5 in the Po Valley, Italy, before and during COVID-19 lockdown restrictions. International Journal of Environmental Research and Public Health. 20(3): 1797.
Pisoschi, A. M. and Pop, A. 2015. The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry. 97: 55-74.
Pollution Control Department, T. 2023. Air4thai. http://air4thai.pcd.go.th/webV3/#/Home
Ponsawansong, P., Prapamontol, T., Rerkasem, K., Chantara, S., Tantrakarnapa, K., Kawichai, S., Li, G., Fang, C., Pan, X., and Zhang, Y. 2023. Sources of PM2.5 oxidative potential during haze and non-haze seasons in Chiang Mai, Thailand. Aerosol and Air Quality Research. 23(10): 230030.
Ponsawansong, P., Prapamontol, T., Singkaew, J., Li, G., and Pan, X. 2024. Significantly elevated urinary OH-PAHs and oxidative damage concentrations attributable to PM2.5 exposure: A panel study of preaging females in Chiang Mai, Thailand. Natural and Life Sciences Communications. 23(1): e2024015.
Pothirat, C., Chaiwong, W., Liwsrisakun, C., Bumroongkit, C., Deesomchok, A., Theerakittikul, T., Limsukon, A., Tajarernmuang, P., and Phetsuk, N. 2019a. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. Journal of Thoracic Disease. 11(7): 3070-3083.
Pothirat, C., Chaiwong, W., Liwsrisakun, C., Bumroongkit, C., Deesomchok, A., Theerakittikul, T., Limsukon, A., Tajarernmuang, P., and Phetsuk, N. 2019b. Influence of particulate matter during seasonal smog on quality of life and lung function in patients with chronic obstructive pulmonary disease. International Journal of Environmental Research and Public Health. 16(1): 106.
Ren, L., Wang, Y., Kawamura, K., Bikkina, S., Haghipour, N., Wacker, L., Pavuluri, C. M., Zhang, Z., Yue, S., Sun, Y., Wang, Z., Zhang, Y., Feng, X., Liu, C.-Q., Eglinton, T. I., and Fu, P. 2020. Source forensics of n-alkanes and n-fatty acids in urban aerosols using compound specific radiocarbon/stable carbon isotopic composition. Environmental Research Letters. 15(7): 074007.
Ren, Z., Liu, X., Liu, T., Chen, D., Jiao, K., Wang, X., Suo, J., Yang, H., Liao, J., and Ma, L. 2021. Effect of ambient fine particulates (PM2.5) on hospital admissions for respiratory and cardiovascular diseases in Wuhan, China. Respiratory Research. 22(1): 128.
Rossignol, S., Tinel, L., Bianco, A., Passananti, M., Brigante, M., Donaldson, D. J., and George, C. 2016. Atmospheric photochemistry at a fatty acid–coated air-water interface. Science. 353(6300): 699-702.
Shen, J., Taghvaee, S., La, C., Oroumiyeh, F., Liu, J., Jerrett, M., Weichenthal, S., del Rosario, I., Ritz, B., Zhu, Y., and Paulson, S. 2022. Aerosol oxidative potential in the greater Los Angeles area: Source apportionment and associations with socioeconomic position. Environmental Science & Technology, 56: 17795-17804.
Shi, L., Zanobetti, A., Kloog, I., Coull, B. A., Koutrakis, P., Melly, S. J., and Schwartz, J. D. 2016. Low-concentration PM2.5 and mortality: Estimating acute and chronic effects in a population-based study. Environmental Health Perspectives. 124(1): 46-52.
Song, W., Zhang, Y., Zhang, Y., Cao, F., Rauber, M., Salazar, G., Kawichai, S., Prapamontol, T., and Szidat, S. 2022. Is biomass burning always a dominant contributor of fine aerosols in upper northern Thailand? Environment International. 168: 107466.
Sugiyama, T., Ueda, K., Seposo, X. T., Nakashima, A., Kinoshita, M., Matsumoto, H., Ikemori, F., Honda, A., Takano, H., Michikawa, T., and Nitta, H. 2020,. Health effects of PM2.5 sources on children's allergic and respiratory symptoms in Fukuoka, Japan. Science of the Total Environment. 709: 136023.
Thepnuan, D., Chantara, S., Lee, C.-T., Lin, N.-H., and Tsai, Y. I. 2018. Molecular markers for biomass burning associated with the characterization of PM2.5 and component sources during dry season haze episodes in Upper South East Asia. Science of the Total Environment. 658: 708-722.
Wang, F., Liu, J., and Zeng, H. 2020. Interactions of particulate matter and pulmonary surfactant: Implications for human health. Advances in Colloid and Interface Science. 284: 102244.
Wang, J., Lin, X., Lu, L., Wu, Y., Zhang, H., Lv, Q., Liu, W., Zhang, Y., and Zhuang, S. 2019. Temporal variation of oxidative potential of water soluble components of ambient PM2.5 measured by dithiothreitol (DTT) assay. Science of the Total Environment. 649: 969-978.
World Health Organization(WHO). 2021. WHO global air quality guidelines. 2021.
Xu, Y., Liu, Q., and Schauer, J. J. 2024. Quantifying the levels and oxidative potential of submicron carbon black in plant leaves. Atmospheric Pollution Research. 15(1): 101954.
Yang, F., Kawamura, K., Chen, J., Ho, K., Lee, S., Gao, Y., Cui, L., Wang, T., and Fu, P. 2016. Anthropogenic and biogenic organic compounds in summertime fine aerosols (PM2.5) in Beijing, China. Atmospheric Environment. 124: 166-175.
Zheng, M., Wan, T. S. M., Fang, M., and Wang, F. 1997. Characterization of the non-volatile organic compounds in the aerosols of Hong Kong— identification, abundance and origin. Atmospheric Environment. 31(2): 227-237.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Teetawat Santijitpakdee1, 2, 3, Tippawan Prapamontol1, *, Pitakchon Ponsawansong1, Sawaeng Kawichai1, Wenhuai Song3, Fang Cao3, and Yan-lin Zhang3
1 Environment and Health Research Group, Research Institute for Health Sciences (RIHES), Chiang Mai University, Chiang Mai 50200, Thailand.
2 School of Health Sciences Research (SHSR), Research Institute for Health Sciences (RIHES), Chiang Mai University, Chiang Mai 50200, Thailand.
3 School of Ecology and Applied Meteorology, Nanjing University of Information Science & Technology, Nanjing 210044, China; Atmospheric Environment Center, Joint Laboratory for International Cooperation on Climate and Environmental Change, Ministry of Education (ILCEC), Nanjing University of Information Science & Technology, Nanjing 210044, China.
Corresponding author: Tippawan Prapamontol, E-mail:tippawan.prapamontol@cmu.ac.th
ORCID:
Teetawat Santijitpakdee: https://orcid.org/0000-0002-7856-4987
Tippawan Prapamontol: https://orcid.org/0000-0001-7132-3235
Total Article Views
Editor: Sirasit Srinuanpan
Chiang Mai University, Thailand
Article history:
Received: June 27, 2024;
Revised: September 10, 2024;
Accepted: September 12, 2024;
Online First: September 23, 2024

