
Development of Toothpaste Containing Morus alba Stem Extract and Its Antimicrobial Activity Against Oral Pathogens
Tasana Pitaksuteepong*, Angkanee Chatthong, Anyaphat Meepian, and Jantipa JobsriPublished Date : September 18, 2024
DOI : https://doi.org/10.12982/NLSC.2024.060
Journal Issues : Number 4, October-December 2024
Abstract Oxyresveratrol has been reported to combat the microorganisms that lead to gingival inflammation and tooth decay. The previous study found that Morus alba stems contained a high amount of oxyresveratrol compared to other parts of the tree. Therefore, the objectives of this research were to develop a toothpaste containing M. alba stem extract (MSE) and to evaluate its antimicrobial activity against oral pathogens. The ethanolic extract was first prepared, and its oxyresveratrol content was analyzed using high performance liquid chromatography (HPLC). Various toothpastes containing MSE were developed and characterized for the required characteristics. The toothpastes that satisfied the requirements were then chosen and subjected to a stability test. Lastly, the effectiveness of MSE and the most stable toothpaste in suppressing oral pathogens was assessed. Analysis of the MSE showed that oxyresveratrol was present at 8.74% by weight. The toothpaste containing 0.25% MSE was successfully developed. It showed good physical and chemical characteristics: the desired color, odor, and taste, with the right quantity of foam, a pH in the range of 5.5–10.5, and did not exhibit phase separation. Stability testing showed that it retained the required characteristics. MSE inhibited Porphyromonas gingivalis more effectively than Streptococcus mutans. The minimum inhibitory concentration (MIC) values of MSE against P. gingivalis and S. mutans were 62.50 µg/mL and 4 mg/mL, whereas the minimum bactericidal concentration (MBC) values were 125 µg/mL and 4 mg/mL. The toothpaste containing MSE effectively inhibited the growth of P. gingivalis and S. mutans, the causes of periodontitis and tooth decay.
Keywords: Morus alba stem extract, Toothpaste, Antimicrobial activity, Oral pathogens, Periodontitis
Funding: The authors are grateful for the research funding provided by Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok, Thailand.
Citation: Pitaksuteepong, T., Chatthong, A., Meepian, A., and Jobsri, J. 2024. Development of toothpaste containing Morus alba stem extract and its antimicrobial activity against oral pathogens. Natural and Life Sciences Communications. 23(4): e2024060.
INTRODUCTION
Toothpaste is a cosmetic that is essential to oral health. It reduces dental issues and fights oral pathogens. Oral pathogens play roles in the etiology of several oral diseases, including dental caries, gingivitis, periodontitis, and endodontic infection. Among these pathogens, Streptococcus mutans and Porphyromonas gingivalis are major microorganisms that cause dental caries and periodontitis (Capasso and Supuran, 2016). S. mutans, a gram-positive anaerobic bacterium, is the main pathogen responsible for dental caries and dental plaque (biofilm) formation. S. mutans digests sucrose into glucose and fructose. These monosaccharide sugars enter the glycolytic metabolic pathway and fermentation processes, where they are converted to pyruvate and then to lactic and other acids. These acids can dissolve enamel and dentin and facilitate the formation of cavities. In addition, it produces three glucosyltransferases (Gtfs) enzymes (GtfB, GtfC, and GtfD) (Zhang et al., 2021). GtfB produces mainly insoluble glucans containing α-1,3-linked glucose; GtfC produces a mixture of insoluble and soluble glucans (α-1,6-linked glucose); and GtfD synthesizes mostly soluble glucans (Paes Leme et al., 2006). The water-insoluble glucans contribute to the adhesion of bacteria to dental surfaces and facilitate bacterial colonization (Zhang et al., 2021). Conversely, the water-soluble glucans serve as backup energy when exogeneous fermentable carbohydrates are depleted, producing acids as by-products as mentioned above. These acids lower the pH of the dental plaque, leading to the demineralization of tooth enamel and subsequently the formation of cavities (Zhang et al., 2021). In addition, S. mutans can also cause serious oral health issues, such as mouth pain, tooth abscesses, poor nutritional status (Zhang et al., 2022), and inflamed pulp linked to severe dental caries (Nomura et al., 2016). P. gingivalis is a gram-negative anaerobic bacterium and plays a critical role in the progression of periodontal disease. Periodontal disease can range in severity from gingivitis (i.e., a moderate and reversible inflammation of the gingiva) to chronic deterioration of connective tissues, which forms periodontal pockets and eventually leads to tooth loss (How et al., 2016; Gerits et al., 2017; Supandi et al., 2023). Although more than 500 different bacterial species have been found to be present in human subgingival plaque, extensive studies have revealed that P. gingivalis is the main cause of chronic periodontitis (How et al., 2016).
Oxyresveratrol, or trans-2, 3', 4, 5'-tetrahydroxystilbene, is a natural compound found in various plants, such as grapes, Artocarpus lakoocha, and Morus alba, or white mulberry. Oxyresveratrol has been reported to inhibit the growth of S. mutans and the production of water-insoluble glucans, thereby reducing the formation of biofilm (Wu et al., 2020). It has also been found to suppress the production of acids by S. mutans during carbohydrate fermentation, which is crucial for preventing the demineralization of tooth enamel and the development of cavities. Wu et al. (2022) reported that oxyresveratrol, in conjunction with Lactobacillus casei, has been demonstrated to reduce the ability of S. mutans to adhere to tooth surfaces and inhibit the growth of biofilm formation of S. mutans. Oxyresveratrol has also shown promising effects against P. gingivalis by inhibiting its growth (Phoolcharoen et al., 2013) and attenuating the inflammatory response (Phoolcharoen et al., 2013; Sekiya et al., 2018), which is crucial for preventing tissue destruction and the progression of periodontal disease.
Apart from antimicrobial effects, oxyresveratrol also possesses anti-inflammatory effects. It has been shown to suppress LPS-induced inflammatory responses in the RAW264.7 murine macrophage cell line by downregulating the activity of various inflammatory signalling pathways, including NF-κB and MAPK pathways, which play crucial roles in orchestrating inflammatory responses (Lee et al., 2015). In the human periodontal ligament (hPDL) cell model, it was proven to suppress the mRNA and protein expression of the pro-inflammatory cytokines IL-6, IL-8, and IL-1ß (Phoolcharoen et al., 2013; Yiemwattana et al., 2018).
Our previous study determined quantities of oxyresveratrol in various parts of the mulberry tree (Thongsuk, 2007). The results found that the stems have the maximum amount of oxyresveratrol compared to that obtained from the twigs, with the least quantity in the leaves. We also confirmed the anti-inflammatory activities of M. alba stem extract (MSE) using LPS-stimulated RAW 264.7 cells (Soonthornsit et al., 2017). It attenuates the inflammation through the inhibition of nitric oxide production by suppressing mRNA and protein expression of the inducible nitric oxide synthase (iNOS). It has also been shown to inhibit cyclooxygenase (COX)-2 mRNA expression. Overall, the extract prepared from M. alba stems, which contains a high amount of oxyresveratrol, holds promise as a potential therapeutic agent for preventing and treating oral diseases, including dental caries and periodontal disease. Therefore, the objectives of this research were to further develop a toothpaste containing MSE and to evaluate its antimicrobial activity against oral pathogens.
MATERIAL AND METHODS
Materials
Brain Heart Infusion (BHI) Agar, and L-cysteine hydrochloride were obtained from HiMedia Laboratories Pvt. Ltd., Maharashtra, India. Tryptic Soy Broth (TSB) Agar and yeast extract were bought from Becton, Dickinson and Company, Maryland, USA. Vitamin K1, and hemin were purchased from Sigma Chemical Co., St. Louis, Missouri, USA. Horse blood was received from Oxoid Ltd., Cheshire, UK. Oxyresveratrol (purity 98%) was supplied by Chanjao Longevity Co., Ltd., Bangkok, Thailand. Sodium fluoride was received from Ajax Finechem, Australia. Carboxymethylcellulose (CMC FVH6, viscosity 2400–2600 mPa.s at 2% solution), was purchased from Changshu Wealthy Sciences & Technology Co., Ltd., China. Hydrated silicas (Zeodent® 113 and Zeodent®165) (Evonik Operations GmbH, Essen, Germany) were received as gifts from Jebsen & Jessen Ingredients (Thailand) Ltd. (Bangkok, Thailand). Cocamidopropyl betaine (CAPB) was bought from Galaxy Surfactants Ltd. (Maharashtra, India). PEG-40 hydrogenated castor oil (PEG-40) was obtained from BASF SE (Ludwigshafen, Germany). Tocopheryl acetate was obtained from Sigma-Aldrich, Massachusetts, USA. Butylated hydroxytoluene (BHT) was bought from Namsiang Co., Ltd., Bangkok, Thailand.
Preparation of Morus alba stem extract (MSE)
The stems of M. alba (var. Buriram 60) were supplied by the Queen Sirikit Sericulture Center, Phrae Province, Thailand. The sample stems were chopped and dried in a hot air oven at a controlled temperature of 50°C. The extraction protocol followed the method previously described by Soonthornsit et al. (2017). The chopped and dried stems (500 g) were macerated in 3,500 mL of 80% ethanol. The extraction process was repeated twice. The filtrates were collected, pooled, and dried using a rotary evaporator (Buchi, Flawil, Switzerland), followed by drying in a hot air oven (Memmert GmbH, Schwabach, Germany) at 50°C. The yield of the extraction was calculated based on the dry weight of the plant materials.
HPLC analysis of oxyresveratrol in MSE
Oxyresveratrol content in the MSE was quantified by HPLC analysis using the method described by Yhirayha et al. (2021) with some modifications. An HPLC system, the Nexera LC-40 (Shimadzu, Kyoto, Japan), was equipped with a photodiode array detector (PDA). The detection wavelength was 320 nm. An analysis was performed on a C18(2) LC column (Phenomenex Luna, 5 µm, 150 × 4.6 mm, Torrance, USA) at a controlled temperature of 30°C using the isocratic mode. Acetonitrile mixed with 0.05M phosphate buffer pH 3 (ratio 1:3) was used as the mobile phase at a flow rate of 1 mL/min. A standard oxyresveratrol was dissolved in methanol to obtain concentrations ranging from 5 to 50 µg/mL. The stock solution of MSE was prepared by dissolving the extract in methanol at a concentration of 0.25 mg/mL.
Formulation of toothpastes containing MSE
The preparation process for the toothpaste containing MSE started with preparing the liquid base by dissolving water-soluble ingredients, including sodium fluoride and sodium benzoate, in water. This solution was added to a rheology modifier that had been pre-wetted with humectants like glycerin or sorbitol. After allowing the mixture to swell for 30–60 min, MSE and an antioxidant (tocopheryl acetate or BHT), which were dissolved in PEG-40 hydrogenated castor oil, were combined. Subsequently, each powder ingredient was added, one at a time, and mixed well. Flavoring and coloring agents were added and mixed thoroughly. Surfactants were added last and mixed gently to avoid foaming.
Evaluation of toothpaste
The physical and chemical characteristics of each of the various toothpaste samples were determined as follows.
Organoleptic evaluation
The organoleptic properties of the formulated toothpaste, including appearance, color, odor, and phase separation, were evaluated based on the criteria outlined by Thai Industrial Standard (TIS) S41-2019 (TISI, 2019), which state that an herbal toothpaste shall be homogeneously semi-solid, show no separation, and be free of foreign matter. It should have a good natural color from the ingredients used and no other undesirable odors such as musty, rancid, or spoiled. Although the taste of a toothpaste did not specify this requirement, it was noted in this study.
Spreadability
A 1 g sample of each toothpaste was placed on a glass plate 10 x 10 cm and 0.5 cm thick. Another identical-sized glass plate was used to cover the toothpaste, and a 1,000-gram weight was placed on top for 30 sec. The diameter of the spread on the plate was measured. A commercial herbal toothpaste was used as the control sample to benchmark the spreadability.
pH measurement
One gram of each toothpaste was weighed into a test tube. Three grams of water were then added and thoroughly mixed. The pH of the suspension was measured at 25 ± 1 °C within 10 min using a pH meter. The pH must be in the range of 5.5–10.5 as outlined by (TIS) S41-2019 (TISI, 2019).
Foam-forming test
The sample preparation was the same as for the pH test. However, the shaking was performed by placing the tube on a vortex mixer (Genie-2, Scientific Industries, Inc., USA) using touch-mode operation for 1 min. The level of foam produced was immediately measured and again after the tube was allowed to stand for 1, 3, and 5 min. The foam height at t = 0 min indicates foamability, while the foam height at t = 1, 3, and 5 min indicates foam stability.
The formulated toothpastes that exhibited favorable physical and chemical characteristics were chosen and subjected to a stability test.
Determination of stability
According to Thai Industrial Standard recommendations, heating-cooling cycling tests were used to determine the stability of each toothpaste (TIS S41-2019). The toothpaste samples, filled in flexible plastic tubes, were kept at 4 ± 2 °C for 24 hours and then stored at 45 ± 2 °C for 24 hours. This process was performed for four cycles. Subsequently, the samples were allowed to cool to room temperature. Their organoleptic properties, pH, and foaming properties were then examined using the procedure mentioned previously, compared with their initial characteristics.
In addition, “How hard or easy is the toothpaste to squeeze from the tube?” was determined using the TA.XTplus texture analyzer (Stable Micro Systems, Surrey, UK) equipped with a 30 kg load cell. A cylinder aluminium probe, 20 mm in diameter (P/20 probe), was used. The parameters for the testing included a pre-test speed of 1 mm/sec, a test speed of 2 mm/sec, and a post-test speed of 10 mm/sec. The trigger force of 5 g was set, and the compression distance of 15 mm was targeted. The experiments were performed at 25°C. Peak forces of the force-time curves were recorded via Texture Exponent software (version 2.0.7.0 Stable Microsystems).
Following the stability tests which were carried out on the toothpastes containing MSE that had passed the characteristics tests, the toothpaste that remained unchanged was chosen and further tested for its antimicrobial activities.
Antimicrobial activity testing
The experiment protocols for the antimicrobial activities against Porphyromonas gingivalis (ATCC 33277) and Streptococcus mutans (ATCC 25175) were reviewed and approved by the Naresuan University Institutional Biosafety Committee (NUIBC MI 66-04-10).
The determination of the minimum inhibitory concentration (MIC) of P. gingivalis and S. mutans was performed by the broth macrodilution method in accordance with the previously described method (Yiemwattana et al., 2018) and CLSI standards (CLSI, 2020), with some modifications. A stock solution of MSE was prepared by dissolving the extract in a 2% DMSO solution. The MSE toothpaste was prepared by dispersing it in a culture medium. Two-fold dilutions were performed in 1 mL of supplemented TSB for P. gingivalis and 1 mL of BHI for S. mutans. The ingredients of the supplemented TSB were 30 mg/mL of tryptic soy broth, 5 mg/mL of yeast extract, 0.5 mg/mL of L-cysteine hydrochloride, 1 µg/mL of vitamin K1, 5 µg/mL of hemin, and 50 mg/mL of horse blood. After that, each bacterial inoculum with a 0.5 McFarland scale density was added to each tube to give a final concentration of 2.5 x 106 CFU/mL for P. gingivalis and 1 x 106 CFU/mL for S. mutans. The tubes were incubated under anaerobic conditions at 37°C for 18–24 hours for P. gingivalis and 48–72 hours for S. mutans. The growth of bacteria is indicated by the turbidity or the formation of a pellet of bacteria at the bottom of the tubes. The lowest concentration of the tested samples that inhibited the visible growth of the bacteria was recorded as the MIC.
Following the MIC determination, the MBC was determined by taking aliquots from the tubes that showed no visible growth of bacteria and inoculating them on supplemented TSB blood agar plates for P. gingivalis and on BHI agar plates for S. mutans using the drop plate method. Nutrient agar shared the same composition as nutrient broth, except 15% of agar powder was added. The plates were incubated and observed for bacteria forming colonies for 3-5 days. The minimum concentration of the test sample that kills 99.9% or more of the test microorganisms in the original inoculum was defined as the MBC (Rahmanian et al., 2015).
An uninoculated medium was used as a negative control, and a chlorhexidine solution (0.2%) was used as a positive control. Inoculated medium was employed as a bacterial growth control. Additionally, the 2% DMSO vehicle for MSE was utilized as a vehicle control.
Statistical analysis
The statistical analysis for before and after stability tests was determined by the paired t-test. A P-value < 0.05 was considered a statistically significant difference.
RESULTS
Extraction yield and oxyresveratrol content
The extract prepared from the stems of M. alba was dark brown in color, with a percentage yield of 5.5% of the dried plant material. The amount of oxyresveratrol in the extract was 8.74%.
Formulation of toothpaste
Toothpastes generally consist of abrasive, humectant, rheology modifier, surfactant, therapeutic agent, preservative, flavoring agent, sweetener, coloring agent, and water. In this study, they were prepared using Zeodent® 113 as an abrasive, glycerin or sorbitol as a humectant (to prevent toothpaste from drying out), CMC or Zeodent®165 as a rheology modifier, sodium lauroyl sarcosinate or CAPB as a surfactant, sodium fluoride as an anti-cavity agent, sodium benzoate as a preservative, spearmint as a flavoring agent, maltitol as a sweetener, brilliant blue as a coloring agent, and water as a solvent. MSE was the therapeutic agent used for its potential for preventing and treating dental caries and periodontal disease. The formulations, coded variously as Toothpastes A to H, are shown in Table 1.
Table 1. Ingredients of toothpastes containing M. alba stem extract formulated.
|
|
Quantity (%w/w) |
|||||||
|
A |
B |
C |
D |
E |
F |
G |
H |
|
|
Glycerin |
54.83 |
39.50 |
30.27 |
23.14 |
25.14 |
- |
- |
- |
|
Sorbitol |
- |
- |
- |
- |
- |
36.48 |
29.61 |
29.61 |
|
Maltitol |
2.52 |
12.57 |
17.64 |
17.64 |
17.64 |
- |
8.82 |
8.82 |
|
CMC |
0.45 |
0.45 |
0.45 |
0.21 |
0.21 |
0.58 |
0.58 |
0.58 |
|
MSE |
0.25 |
0.25 |
0.25 |
0.25 |
0.25 |
0.25 |
0.25 |
0.25 |
|
PEG-40 |
7.00 |
7.00 |
7.00 |
7.00 |
5.00 |
0.83 |
1.67 |
1.67 |
|
Tocopheryl acetate (Vitamin E) |
- |
- |
- |
- |
- |
0.50 |
0.50 |
- |
|
BHT |
- |
- |
- |
- |
- |
- |
- |
0.50 |
|
Sodium fluoride |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
|
Sodium benzoate |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
|
Zeodent 113® |
20.00 |
20.00 |
20.00 |
20.00 |
20.00 |
20.00 |
20.00 |
20.00 |
|
Zeodent 165® |
3.50 |
5.00 |
4.00 |
4.00 |
4.00 |
4.00 |
1.67 |
1.67 |
|
Titanium dioxide |
0.50 |
1.00 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
|
Sodium lauroyl sarcosinate |
1.65 |
1.65 |
2.40 |
4.50 |
4.50 |
4.50 |
3.00 |
3.00 |
|
CAPB |
- |
- |
- |
- |
- |
- |
1.50 |
1.50 |
|
Flavoring agent |
1.10 |
1.10 |
1.16 |
1.16 |
1.16 |
1.20 |
1.20 |
1.20 |
|
Brilliant blue |
- |
- |
- |
- |
- |
- |
0.37 |
0.37 |
|
Water qs to |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
Glycerin and sorbitol are the most frequently used humectants in toothpaste. The glycerin-based toothpastes (i.e., Toothpastes A–E) were formulated. They showed a homogeneous and creamy texture without phase separation and were yellowish-brown in color (Table 2). The desirable level of sweetness was achieved using maltitol, which is a sugar alcohol that is not metabolized by oral bacteria (Godswill, 2017). Toothpastes A–E had spreadability ranging from 3.9 to 5.9 cm. Their pH was about 7, which was in the acceptable range specified by TIS S41-2019, i.e., 5.5–10.5. The foaming ability of Toothpastes A–D was low and was enhanced when the content of PEG-40 hydrogenated castor oil was decreased, as obtained in Toothpaste E (Table 2). As seen in Table 2, the spreadability of Toothpaste E was nearly identical to that of the commercial toothpaste used as a benchmark. Also, it demonstrated the highest foamability (i.e., foam height at t = 0 min). Toothpaste E was therefore selected and subjected to the stability test.
Table 2. Organoleptic properties, pH, spreadability and foam formation of toothpaste developed compared to the commercial toothpaste (n = 2).
|
Parameter |
Commercial |
A |
B |
C |
D |
E |
F |
G |
H |
|
Appearance |
- |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
|
Color |
- |
yellowish brown |
yellowish brown |
yellowish brown |
yellowish brown |
yellowish brown |
yellowish brown |
mint green |
mint green |
|
Odor |
- |
light mint smell |
light mint smell |
pleasant smell and feel fresh |
pleasant smell and feel fresh |
pleasant smell and feel fresh |
pleasant smell and feel fresh |
pleasant smell and feel fresh |
pleasant smell and feel fresh |
|
Taste |
- |
unpalatable, light sweet |
sweetness increase |
palatable, fresh, and cool |
palatable, fresh, and cool |
palatable, fresh, and cool |
bitter, fresh, and cool |
palatable, fresh, and cool |
palatable, fresh, and cool |
|
Spreadability (cm) |
5.10 |
5.90 |
4.20 |
4.60 |
3.90 |
5.00 |
4.90 |
4.90 |
5.10 |
|
pH |
- |
7.24 |
7.07 |
7.12 |
7.05 |
7.11 |
7.20 |
7.00 |
7.32 |
|
Foam height (cm) |
|
|
|
|
|
|
|
||
|
t = 0 min |
2.60 |
0.20 |
0.10 |
0.50 |
0.50 |
0.90 |
1.10 |
2.70 |
2.40 |
|
t = 1 min |
2.50 |
0.20 |
0.10 |
0.50 |
0.50 |
0.80 |
1.1 |
2.60 |
1.90 |
|
t = 3 min |
2.40 |
0.20 |
0.10 |
0.40 |
0.50 |
0.60 |
1.00 |
2.40 |
1.80 |
|
t = 5 min |
2.30 |
0.20 |
0.10 |
0.40 |
0.50 |
0.50 |
1.00 |
2.30 |
1.50 |
It was noted that the appearance, color, odor, taste, and pH of Toothpaste E remained unchanged throughout the stability test (Figure 1, Table 3). At this point, a TA.XTplus texture analyzer fitted with a cylinder aluminum probe (20 mm in diameter) was used to measure the force required to extrude toothpaste from a tube. Following the stability test, the extrusion force required to extrude Toothpaste E increased significantly (10,748.10 ± 887.42 g) from the initial value (8,444.50 ± 574.76 g).
Next, Toothpastes (F–H) were further developed using sorbitol as the humectant. To enhance foam formation, the content of PEG-40 hydrogenated castor oil was further decreased, and the water component was increased by taking out maltitol from the formulation. Although the foamability of Toothpaste F only slightly increased, foam stability (i.e., foam height at t = 1, 3, and 5 min) greatly improved over that of Toothpaste E (Table 2). However, a bitter taste developed in Toothpaste F and became dominant. This is because glycerin and sorbitol have similar sweetness levels but are lower than those of maltitol. The sweetness value relative to the sucrose of glycerin is 0.6, sorbitol is 0.6, and maltitol is 0.9 (Godswill, 2017). Also, the reduction in PEG-40 hydrogenated castor oil content caused difficulty in the solubilization of MSE so the PEG-40 hydrogenated castor oil content was increased. Maltitol was re-added to the formulation to improve its palatableness. An antioxidant, tocopheryl acetate was added to Toothpaste F and G, while BHT was added to Toothpaste H to prevent unpleasant smell and taste due to chemical alteration in a product in the long term. Cocamidopropyl betain (CAPB), an amphoteric surfactant, was added to both Toothpaste G and H. CAPB does not have as strong foamability as an anionic surfactant such as sodium lauroyl sarcosinate but is commonly used as a foam booster to improve the foamability and stability of various products. CAPB was combined with sodium lauroyl sarcosinate to enhance the foaming capability of both Toothpaste G and H. It was observed that both Toothpaste G and H were also found to have acceptable organoleptic characteristics, and the spreadability of Toothpaste G was 4.9 and Toothpaste H was 5.1 cm (Table 2). The pH of both formulations was in the acceptable range. The foamability and stability of both formulations were greatly enhanced (Table 2).
Following the stability test, Toothpaste G did not show phase separation (Figure 1). Its color and taste remained unchanged, but it smelled rancid (Table 3). On the other hand, Toothpaste H remained stable throughout the stability test (Figure 1, Table 3), so it was chosen for further investigation on its antimicrobial properties.
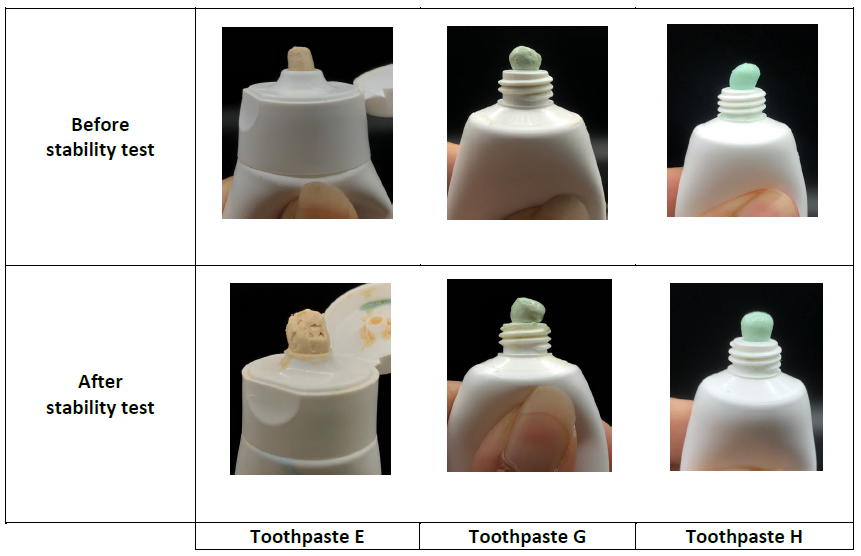
Figure 1. Appearance and color of Toothpastes E, G, and H before and after stability test.
Table 3. Characteristics of toothpaste containing M. alba stem extract (Toothpaste E, G, and H) before and after stability study (n = 3).
|
Parameters |
|
Before stability test |
|
|
After stability test |
|
|
Toothpaste E |
Toothpaste G |
Toothpaste H |
Toothpaste E |
Toothpaste G |
Toothpaste H |
|
|
Organoleptic property |
||||||
|
Appearance |
homogeneous, creamy |
homogeneous, creamy |
homogeneous, creamy |
not change |
not change |
not change |
|
Color |
yellowish brown |
mint green |
mint green |
not change |
not change |
not change |
|
Odor |
pleasant smell & fresh |
pleasant smell & fresh |
pleasant smell & fresh |
not change |
Rancid smell |
not change |
|
Taste |
palatable, fresh, and cool |
palatable, fresh, and cool |
palatable, fresh, and cool |
not change |
not change |
not change |
|
pH |
7.11 ± 0.05 |
6.99 ± 0.01 |
7.31 ± 0.01 |
7.19 ± 0.02 |
7.15 ± 0.00 |
7.36 ± 0.01 |
|
Foam height (cm) |
||||||
|
t = 0 min |
0.87 ± 0.21 |
2.70 ± 0.29 |
2.15 ± 0.23 |
1.43 ± 0.33 |
4.05 ± 0.35 |
3.03 ± 0.57 |
|
t = 1 min |
0.80 ± 0.16 |
2.60 ± 0.29 |
2.10 ± 0.12 |
1.33 ± 0.37 |
3.92 ± 0.35 |
2.89 ± 0.74 |
|
t = 3 min |
0.57 ± 0.05 |
2.43 ± 0.25 |
2.03 ± 0.21 |
1.20 ± 0.36 |
3.70 ± 0.29 |
2.60 ± 0.80 |
|
t = 5 min |
0.50 ± 0.00 |
2.30 ± 0.22 |
1.92 ± 0.16 |
1.17 ± 0.39 |
3.60 ± 0.29 |
2.28 ± 0.76 |
|
Extrudability (g) |
8,444.50 ± 574.76 |
2,454.53 ± 108.76 |
2,012.30 ± 44.98 |
10,748.10 ± 887.42* |
2,743.57 ± 161.85 |
1,587.23 ± 165.59 |
Note: * P < 0.05
Antimicrobial activity
As the negative control, the uninoculated medium did not show any colonies. The positive control, a 0.2% chlorhexidine solution, demonstrated bactericidal action against P. gingivalis and S. mutans. Conversely, bacterial growth was observed in the inoculated medium without MSE (i.e., the bacterial growth control). Furthermore, the MSE vehicle (i.e., 2% DMSO) exhibited no antimicrobial activity against any of the two oral pathogens.
MSE showed antimicrobial activity against the tested oral pathogens. However, it showed stronger inhibitory activity against P. gingivalis than S. mutans (Table 4). The MIC and MBC of MSE against P. gingivalis were 0.0625 and 0.1250 mg/mL, respectively. Its MIC and MBC values against S. mutans, however, were 4 mg/mL. MSE 0.0625 mg, 0.125 mg, and 4 mg are equivalent to MSE toothpaste 25 mg, 50 mg, and 1,600 mg, respectively. Nevertheless, Toothpaste H demonstrated total inhibition against P. gingivalis and S. mutans even after being diluted to 7.8125 mg/mL (or MSE 0.02 mg/mL) and 62.5 mg/mL (or MSE 0.16 mg/mL).
Table 4. Antimicrobial activity of M. alba stem extract (MSE) and Toothpaste H containing MSE at 0.25% w/w (n = 3).
|
Sample |
Porphyromonas gingivalis |
Streptococcus mutans |
||
|
MIC |
MBC |
MIC |
MBC |
|
|
(mg/mL) |
(mg/mL) |
|||
|
M. alba stem extract (MSE) |
0.0625 |
0.1250 |
4.0000 |
4.0000 |
|
Toothpaste H |
<7.8125 |
<7.8125 |
<62.5000 |
<62.5000 |
|
Note: Controls for the antimicrobial tests were uninoculated medium, inoculated medium, medium with 2% DMSO without MSE, and 0.2% chlorhexidine solution. |
||||
DISCUSSION
In this study, toothpastes containing MSE at 0.25% w/w with the desired characteristics were formulated. The content of MSE in the toothpaste was set at 0.25% w/w. This is because the recommended amount of toothpaste is pea-sized, or 0.25 g (EFSA, 2005), but 0.7 g is typically used (Ponikvar, 2008). Therefore, the dosage of the MSE toothpaste in this study was aimed at between 0.25 and 0.5 g, containing 0.625 and 1.25 mg of MSE extract per usage. This range permits dilution during brushing because it is five to ten times greater than the MBC value (i.e., 0.125 mg/mL).
The desired characteristics of a toothpaste are a homogeneous and creamy texture, a pleasant smell, being fresh and cool, as well as being palatable. Apart from these, a toothpaste’s viscosity is a crucial feature that has an impact on customer satisfaction. The toothpaste should extrude out of the tube into a ribbon that can maintain its ribbon shape on a toothbrush’s bristles without collapsing.
The spreadability and extrudability were used as indicators for toothpastes’ viscosity. It was observed that the viscosity of the toothpastes was dependent mainly on the ratio of liquid components in the mixture, especially humectant, maltitol, and water. Humectant is one of the essential ingredients in toothpaste, which can prevent water loss and provide creamy structure. A large amount of water loss can cause the toothpaste to dry out, leading to hardening paste in the tube. Glycerin was used as the humectant in Toothpaste A–E, while sorbitol was used as the humectant in Toothpaste F–H. Both glycerin and sorbitol also act as thickening and sweetening agents. Maltitol, a sugar alcohol, was used as the sweetener. It provides a cooling sensation because, upon dissolving in water, an endothermic (heat-absorbing) reaction occurs. Like glycerin, due to its viscous nature, maltitol also acts as a rheology modifier. The sugar alcohols, including glycerin, sorbitol, and maltitol, were used in this because they were not metabolized by oral bacteria. Therefore, they do not cause tooth decay (Bradshaw and Marsh, 1994; Godswill, 2017). Water was used as the solvent for water-soluble components and to adjust the final weight of the formulation. The viscosity properties of Toothpaste H were similar to those of the commercial toothpaste, as evidenced by its spreadability (5.1 cm) and extrudability (2,012.30 ± 44.98 g). The commercial toothpaste has a spreadability of 5.1 cm and an extrudability of 1919.2 ± 128.79 g. Following the stability test, the extrudability of Toothpaste H remained unchanged, suggesting that Toothpaste H possesses an appropriate viscosity.
The pH must be in the range of 5.5–10.5. This is because enamel is the outermost layer of the tooth. The main component of tooth enamel is calcium hydroxyapatite (Ca5(PO4)3(OH) or Ca10(PO4)6(OH)2), which naturally demineralizes, especially in environments with a pH value lower than 5.5 (called critical pH) (Amaechi and Loveren, 2013). All toothpastes developed in this study showed a pH of around 7, suggesting a low risk of enamel demineralization.
Many customers prefer toothpaste with high foaming ability since it gives a feeling of deep cleaning. Foam height determined at t = 0 min indicated foam ability, while that determined at the following time points indicated foam stability. Surfactant is known to facilitate the formation of foam by reducing the surface tension of a liquid. It can also enhance the stability of foam by inhibiting the coalescence of bubbles. In addition, lowering the surface tension property of the surfactant helps to remove debris from the surface of teeth. Thus, it functions as a cleansing agent. It also helps to disperse the toothpaste in the mouth. Sodium lauroyl sarcosinate, an anionic surfactant, was used as the surfactant in this study. Anionic surfactants are widely used as primary surfactants because they typically produce larger amounts of foam than other types of surfactants. However, some of them are known to be irritating, including sodium lauryl sulfate. Sodium lauroyl sarcosinate is mild and biodegradable. From the results, an increasing amount of sodium lauroyl sarcosinate could increase foam height to some extent. However, in combination with CAPB, foam forming ability was enhanced. The synergistic effect of sodium lauroyl sarcosinate (an anionic surfactant) and CAPB (a zwitterionic surfactant) has been reported by Wei et al. (2020).
The antimicrobial activities of MSE against P. gingivalis and S. mutans were clearly shown, indicating that it has therapeutic potential for preventing and treating periodontal disease and dental caries. Toothpaste H containing MSE at 0.25% w/w possessed strong bactericidal activities against P. gingivalis and S. mutans even after being diluted and stronger than the extract itself. This may be attributed to the toothpaste base. As mentioned above, the toothpaste base contains preservatives and surfactants, which may exert antimicrobial effects (Lindstedt et al., 1990; Yapar et al., 2017).
CONCLUSION
Toothpaste containing MSE at 0.25% w/w with the desired characteristics was successfully developed. It effectively inhibited the growth of P. gingivalis and S. mutans. Therefore, it has potential to be used for preventing and treating periodontal disease and dental caries. Nevertheless, further investigations into the cytotoxicity of MSE toothpaste in 3D oral tissue models or in volunteers are recommended.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Pharmaceutical Sciences, Naresuan University for providing financial support and facilities. Thanks to Mr. Pan Sathanthung, Mr. Somkiet Chaiphan, and Ms. Sakawrat Taptonglang, the scientists of the Faculty of Pharmaceutical Sciences, Naresuan University; Ms. Thanwarat Suebwongfag, the scientist of the Cosmetics and Natural Products Research Center, Naresuan University; and Ms. Kusuma Jamdee, the scientist of the Faculty of Dentistry, Naresuan University, for the technical support. The authors would also like to acknowledge the Queen Sirikit Sericulture Center, Phrae Province, and Jebsen & Jessen Ingredients (Thailand) Ltd. for supporting some raw materials. Thanks also to Mr Roy Morien of the Naresuan University Graduate School for his editing of the grammar, syntax and general English expression in this manuscript.
AUTHOR CONTRIBUTIONS
Angkanee Chatthong and Anyaphat Meepian designed and conducted the experiments and data analysis. Tasana Pitaksuteepong designed the experiments, supervised the project, and wrote the manuscript. Jantipa Jobsri was responsible for antimicrobial activity against Porphyromonas gingivalis. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Amaechi, B.T., and Loveren, C.V. 2013. Fluorides and non-fluoride remineralization systems. Monographs in Oral Science. 23: 15-26.
Bradshaw, D.J., and Marsh, P.D. 1994. Effect of sugar alcohols on the composition and metabolism of a mixed culture of oral bacteria grown in a chemostat. Caries Research. 28(4): 251-256.
Capasso, C., and Supuran, C.T. 2106. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Current Topics in Medicinal Chemistry. 16(21): 2359-2368.
CLSI, Performance Standards for Antimicrobial Susceptibility Testing, Approved Standard, 30th ed., CLSI document M100. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite2500, Wayne, Pennsylvania 19087, USA, 2020.
European Food Safety Authority (EFSA). 2005. Opinion of the scientific panel on dietetic products, nutrition and allergies (NDA) on a request from the commission related to the tolerable upper intake level of fluoride. EFSA Journal, 3(3): 192.
Gerits, E., Verstraeten, N., and Michiels, J. 2017. New approaches to combat Porphyromonas gingivalis biofilms. Journal of Oral Microbiology. 9(1): 1300366.
Godswill, A.C. 2017. Sugar alcohols: Chemistry, production, health concerns and nutritional importance of mannitol, sorbitol, xylitol, and erythritol. International Journal of Advanced Academic Research. 3(2): 31-66.
How, K.Y., Song, K.P., and Chan, K.G. 2016. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Frontiers in Microbiollogy. 7:53.
Lee, H.S., Kim, D.H, Hong, J.E., Lee, J.Y., and Kim, E.J. 2015. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Human & Experimental Toxicology. 34(8): 808-818.
Lindstedt, M., Allenmark, S., Thompson, R.A., and Edebo, L. 1990. Antimicrobial activity of betaine esters, quaternary ammonium amphiphiles which spontaneously hydrolyze into nontoxic components. Antimicrobial Agents and Chemotherapy. 34(10): 1949-1954.
Nomura, R., Ogaya, Y., and Nakano, K. 2016. Contribution of the collagen-binding proteins of Streptococcus mutans to bacterial colonization of inflamed dental pulp. PLoS One 11: e0159613.
Paes Leme, A.F., Koo, H., Bellato, C.M., Bedi, G., and Cury, J.A. 2006. The role of sucrose in cariogenic dental biofilm formation—New insight. Journal of Dental Research. 85(10): 878-887.
Phoolcharoen, W., Sooamponb, S., Sritularaka, B., Likhitwitayawuida, K., Kuvatanasuchatid, J., and Pavasante, P. 2013. Anti-periodontal pathogen and anti-inflammatory activities of oxyresveratrol. Natural Product Communications. 8(5): 613-616.
Ponikvar, M. 2008. Chapter 12 -Exposure of humans to fluorine and its assessment. P.487-549. In A. Tressaud (ed) Fluorine and Health. Elsevier, B.V.
Rahmanian, N., Jafari, S.M., and Wani, T.A. 2015. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends in Food Science and Technology. 42: 150-172.
Sekiya, M., Shimoyama, Y., Ishikawa, T., Sasaki, M., Futai, M., Nakanishi-Matsui, M. 2018. Porphyromonas gingivalis is highly sensitive to inhibitors of a proton-pumping ATPase. Biochemical and Biophysical Research Communications. 498: 837-841.
Soonthornsit, N., Pitaksutheepong, C., Hemstapat, W., Utaisincharoen, P., and Pitaksuteepong, T. 2017. In vitro anti-inflammatory activity of Morus alba L. stem extract in LPS-stimulated RAW 264.7 cells. Evidence-Based Complementary and Alternative Medicine. 2017: 3928956.
Supandi S.K., Elvandari, A., Bargowo, L., and Wijaksana, I.K.E. 2023. Nigella sativa extract on gingival epithelium exposed to LPS Porphyromonas gingivalis and its impact on the expression of TLR-4 and NF-κB in vivo study. Natural and Life Sciences Communications. 22(4): e2023061.
Thai Industrial Standard Institute (TISI), Ministry of Industry. Thai SMEs Standard TIS S41-2019. Herbal Toothpaste.
Thongsuk, P. In vitro and clinical study of mulberry extract for skin whitening product [M.S. thesis], Naresuan University, Phitsanulok, Thailand, 2007.
Wei, H., Zhang, R., Lei, Z., and Dang, L. 2020. Synergistic effect of cocamidopropyl betaine and sodium lauroyl sarcosinate. Transactions of Tianjin University. 27(1): 366-376.
Wu, J., Fan, Y., Wang, X., Jiang, X., Zou, J., and Huang, R. 2020. Effects of the natural compound, oxyresveratrol, on the growth of Streptococcus mutans, and on biofilm formation, acid production, and virulence gene expression. European Journal of Oral Sciences. 128(1): 18-26.
Wu, J., Jiang, X., Yang, Q., Zhang, Y., Wang, C., and Huang, R. 2022. Inhibition of Streptococcus mutans biofilm formation by the joint action of oxyresveratrol and Lactobacillus casei. Applied and Environmental Microbiology. 88(9): e02436-e024321.
Yapar, S., Ates, M., and Özdemir, G. 2017. Preparation and characterization of sodium lauroyl sarcosinate adsorbed on cetylpyridinium-montmorillonite as a possible antibacterial agent. Applied Clay Science. 150: 16-22.
Yhirayha, C., Wittaya-Areekul, S., and Pitaksuteepong, T. 2021. Lamellar lyotropic liquid crystal phases as a carrier for skin delivery of Morus alba stem extract. Key Engineering Materials. 901: 67-72.
Yiemwattana, I., Chaisomboon, N., and Jamdee.K. 2018. Antibacterial and anti-inflammatory potential of Morus alba stem extract. The Open Dentistry Journal. 12: 265-274.
Zhang, Q. Ma, Q., Wang, Y., Wu, H., and Zou, J. 2021. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. International Journal of Oral Science. 13:30.
Zhang, Y., Fang, J., Yang, J., Gao, X., Dong, L., Zheng, X., Sun, L., Xia, B., Zhao, N., Ma, Z., and Wang, Y. 2022. Streptococcus mutans-associated bacteria in dental plaque of severe early childhood caries. Journal of Oral Microbiology. 14(1): 2046309.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tasana Pitaksuteepong1, 2, *, Angkanee Chatthong1, Anyaphat Meepian1, and Jantipa Jobsri3
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
2 Center of Excellence for Innovation in Chemistry, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
3 Department of Oral Biology, Faculty of Dentistry, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Tasana Pitaksuteepong, E-mail: tasanap@nu.ac.th
ORCID: Tasana Pitaksuteepong: https://orcid.org/0000-0001-5641-5430
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: April 22, 2024;
Revised: August 23, 2024;
Accepted: September 6, 2024;
Online First: September 18, 2024

