
Anti-Inflammatory Activity and Wound Healing Ability of Coconut Oil Mouthwash on Gingival Fibroblast Cell In Vitro
Peerachat Marasri, Siriwoot Sookkhee, Phenphichar Wanachantararak*, and Darunee Owittayakul*Published Date : September 17, 2024
DOI : https://doi.org/10.12982/NLSC.2024.059
Journal Issues : Number 4, October-December 2024
Abstract Coconut oil-pulling therapy is used for maintaining oral health. The procedure has benefits for the prevention of oral disease, including dental caries, oral malodor, bleeding gums. However, virgin coconut oil (VCO) has unsatisfying oily taste. Therefore, coconut oil mouthwash (CoMW) was recently developed. This in vitro study aimed to evaluate the anti-inflammatory activity of coconut oil mouthwash consisting of 60% v/v virgin coconut oil (VCO), 30% v/v propylene glycol (PG), and 10% v/v distilled water on human gingival fibroblast (HGF) cells compared with the activity on murine macrophage (Raw 264.7) cells. The cytotoxicity of CoMW, 0.12% chlorhexidine gluconate (CHX), VCO, and PG was assessed. IC50 concentration of CoMW, CHX, and PG were 1:8 (v/v), 1:32 (v/v), and 1:16 (v/v), respectively. All tested concentrations of VCO had no impact on cell viability. Their anti-inflammatory effects of each IC50 concentration were further studied. Notably, the IC50 concentration of CHX also significantly inhibited nitric oxide production in lipopolysaccharide (LPS)- activated Raw 264.7 cells. Moreover, the IC50 concentrations of CoMW, VCO, PG, and CHX could suppress the interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and cyclooxygenase-2 (COX-2) gene expressions in LPS-activated HGF cells, while also enhancing the cell migration of HGF cells as likely to the effect observed with the IC50 concentration of CHX. Wound healing ability of CoMW was also demonstrated after testing with a scratch assay. These findings indicated promising potential for coconut oil mouthwash as an effective agent in reducing inflammation and facilitating wound healing.
Keywords: Anti-inflammation, Cytotoxicity, Coconut oil mouthwash, Gingival fibroblast cell, Wound healing
Funding: This research project was financially supported by the Research Fund for Postgraduates of the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Citation: Marasri, P., Sookkhee, S., Wanachantararak, P., and Owittayakul, D. 2024. Anti-inflammatory activity and wound healing ability of coconut oil mouthwash on gingival fibroblast cell in vitro. Natural and Life Sciences Communications. 23(4): e2024059.
INTRODUCTION
Inflammation is a crucial defense mechanism of the body, aimed at eliminating harmful stimuli and initiating the healing process. Typically, during acute inflammation, the host immune system reacts to rapid onset, and resolves the issue within a few days. However, if acute inflammation persists unresolved, it can escalate into chronic inflammation, contributing to various long-term health issues (Furman et al., 2019). In the oral cavity, a multitude of pathogenic microorganisms colonize, leading to plaque-related diseases, notably periodontitis. This condition involves the immune system-mediated process of osteoclastogenesis and soft tissue destruction caused by tissue lytic enzymes. Pathogens trigger the release of immune system mediators that can severely damage the gingiva, periodontal ligament, and alveolar bone. Inflammatory mediators play a vital role in cellular communication, exerting endocrine, paracrine, and autocrine activities (AlQranei and Chellaiah, 2020). They are released by specific cells and influence the behavior of many others, regulating functions like cell proliferation, differentiation, immune responses, and inflammation. Interleukin-1β (IL-1β), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), and nitric oxide, numerous other inflammatory mediators are involved in periodontitis (Tanabe et al., 2023).
Due to the significant role of nitric oxide synthesis in the host inflammatory response, this cellular response is induced by LPS, the outer membrane of Gram-negative bacteria, through Toll-Like Receptor 4 (TLR4) on the cell membrane of macrophages (Palmieri et al., 2020). Additionally, RAW 264.7 cells treated with LPS or LPS/IFN-γ can produce nitric oxide through iNOS (Suriyaprom et al., 2023), and are widely used as a model primary macrophage (Merly and Smith, 2017). Therefore, the LPS-activated RAW 264.7 was selected for using in our experiment.
Pro-inflammatory mediators, including IL-1β, IL-6, and COX-2, are known to be regulated through the NF-кB signaling pathway (Liu et al., 2017; Shih et al., 2018). These mediators are released from human gingival fibroblast (HGFs) stimulated by LPS, causing damage to the surrounding gingival and periodontal tissues (Naruishi, 2022). Consequently, we investigated the effects of three types of mouthwash at the sub-IC50 concentrations on the induction of pro-inflammatory mediators, including IL-1β, IL-6 and COX-2 in both non-LPS and LPS-stimulated HGF cells.
Wound healing is a process of repairing soft tissues and skin after infection or injury, consisting of inflammatory, proliferative and remodeling phases (Quazi et al., 2022). The cell migration is one of the key steps during the proliferative phase and is crucial for effective wound healing (Landén et al., 2016).
Furthermore, therapeutic agents that suppress the production and secretion of inflammatory mediators have been shown to reduce periodontal inflammation (Scheres et al., 2011; Kang et al., 2016; Ramadan et al., 2020). Mouthwash plays a role as an adjunct to maintaining oral hygiene. A commonly used therapeutic mouthwash is 0.12% chlorhexidine gluconate (CHX), known for its effectiveness in reducing plaque and gingivitis due to its broad antimicrobial spectrum, bactericidal and bacteriostatic effects, antifungal properties, and prolonged therapeutic effect. However, CHX has been reported to cause dental stains, metallic taste, and oral irritation. These drawbacks have spurred the development of natural alternatives (Nittayananta et al., 2008; Shrestha et al., 2011; Brookes et al., 2020).
Coconut oil pulling, done for 10-20 minutes in the morning, harnesses the beneficial properties of coconut oil such as its antiplaque (Peedikayil et al., 2015), anti-gingivitis (Peedikayil et al., 2015), antibacterial (Nitbani et al., 2022), and anticandidal (Chanpa et al., 2023) properties. Previously research said that wounds treated with virgin coconut oil (VCO) healed faster, increased collagen tissue, increased fibroblast proliferation, and neovascularization of wounds (Kappally et al., 2015). Coconut oil is rich in medium-chain fatty acids (MCFA) like caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid, and linoleic acid (Boateng et al., 2016). These MCFAs, especially butyric (C4) and decanoic (C10) acid possess anti-inflammatory properties (Joshi et al., 2020; Sam et al., 2021) by influencing the gene expression of inflammatory cytokines. Certain MCFAs reduce the gene expression of IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) in plasma (de Jong et al., 2014). Additionally, monolaurin, lauric acid, capric acid, oleic acid, and linoleic acid have been found to reduce the gene expression of IL-1β, IL-6, and COX-2 genes (Lin et al., 2017; Famurewa et al., 2020).
Recently, an innovative coconut oil mouthwash was developed, which consisted of 60% VCO, 30% propylene glycol, and 10% distilled water to combat C. albicans biofilms (Intarakaewsri et al., 2020). The study showed that this mouthwash was equally effective as nystatin, with inhibition percentages of 83.75 ± 5.75 and 82.36 ± 4.61, respectively. However, the anti-inflammatory activity of this mouthwash has not been explored. Therefore, this present study aimed to investigate its anti-inflammatory activity of coconut oil mouthwash on HGF cells with parameters used in the present study included % cell cytotoxicity, nitric oxide level, IL-1β, IL-6, and COX-2 gene expression levels, and evaluate the wound healing ability of CoMW on HGF cells.
MATERIALS AND METHODS
Ethics
The study was reviewed and approved the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University (Document No.5/2022).
Preparation of coconut oil mouthwash
Cold-pressed coconut oil (CoCo Delight, Lot NP630107, GPO; Pathumthani, Thailand) was selected as VCO. CoMW used in the present study was prepared from VCO, 0.45-microns filtered propylene glycol USP (Srichand United Dispensary Co., Ltd., Bangkok, Thailand), and distilled water in a specific volume ratio (Intarakaewsri et al., 2020).
Human gingival cell culture
HGF cells were obtained from a healthy 20-year-old female donor who underwent the third molar extraction at the Faculty of Dentistry, Chiang Mai University, Thailand.
Gingival tissues were washed with Dulbecco’s Modified Eagle Medium (DMEM) (1TFS-1CC-11995065, GibcoTM; Grand Island, NY, USA) containing 1% penicillin-streptomycin (10,000 Units/mL penicillin and 10,000 µg/mL streptomycin) (1TFS-1CC-15140122, GibcoTM; Grand Island, NY, USA), then cut into 1-3 mm3 pieces and cultured in 35-mm culture dishes with DMEM complete medium which was supplemented with 10% fetal bovine serum (FBS) (1TFS-1RS-10270106, GibcoTM; Grand Island, NY, USA) and 1% penicillin-streptomycin. Cells were incubated at 37°C with 5% CO2, with medium changes every three days until cells reached 80% confluence. Subsequently, cells were harvested and treated with 0.25% trypsin-EDTA solution (1TFS-1CC-15400054, GibcoTM; Grand Island, NY, USA). HGF cells at passages between three to six were used in all experiments (Costa et al., 2019; Karlis et al., 2024).
Determination of cytotoxicity test using MTT assay
The MTT test, using 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) (Cat.No.M5655, Sigma-Aldrich, Saint Louis, MO, USA), was conducted to assess cell viability. HGF cells (1*105 cells/well) were seeded into 96-well plates with complete medium and incubated at 37°C with 5% CO2 for 24 hours to allow for cell attachment.
After incubation, cells were treated with various concentrations of CoMW, VCO, PG, and 0.12% CHX (provided by Faculty of Dentistry, Chiang Mai University), using two-fold serial dilutions ranging from 1:2 to 1:2,048 for 24 hours. Cells treated with a complete medium served as the control group.
Five mg/mL of MTT was dissolved in phosphate-buffered saline (PBS) (1TFS-1CC-20012027, GibcoTM; Grand Island, NY, USA) at pH 7.4, and 50 µL of MTT solution was added to each well for three hours of incubation. After incubation period, all solutions were removed, and the 1:1 v/v solution of 100 µL of dimethylsulfoxide (DMSO) (AR Grade 1054, 67-68-5, RCL Labscan Ltd, Bangkok, Thailand) and ethanol (AR Grade 1069, 64-17-5, RCL Labscan Ltd, Bangkok, Thailand) were added to each well for 15 minutes. Subsequently, the absorbance at 540 nm was measured using an ELISA microplate reader (TECAN Sunrise, Mannedorf, Switzerland).
The percentages of cell viability were calculated using the following equation:
% Cell viability = [Absorbance of sample/Absorbance of control] x 100
The half maximum inhibitory concentration (IC50) was used to determine the cytotoxic concentrations (Costa et al., 2019).
Determination of anti-inflammatory activity using the nitric oxide (NO) inhibition assay
The present study utilized RAW 264.7 cells which purchased from the American Type Culture Collection (CRL-2278, ATCC®, Manassas, VA, USA). Both RAW 264.7 cells and HGF cells were cultured in complete medium at 37°C with 5% CO2.
Nitrate production was assessed using the Griess reaction. Cells (1×105 cells/well) were seeded in 24-well plates with DMEM medium (phenol red-free, 10% FBS), and then incubated at 37°C with 5% CO2 for 24 hours. Subsequently, cells were treated with CoMW, VCO, PG, and CHX at their IC50 concentration, with and without 10 µg/mL Escherichia coli serotype 0127: B8-extracted LPS (LPS) (L8654 Sigma-Aldrich, Saint Louis, MO, USA) for 24 hours.
Nitrite accumulation in the culture supernatants was determined using the Griess Reagent Kit (1TFS-1CP-G-7921, Invitrogen by Thermo Fisher Scientific, Molecular Probes Inc., Eugene, OR, USA) following the manufacturer's instructions. One hundred fifty µL of culture supernatants were mixed with 20 µL of Griess reagent and 130 µL of distilled water in a 96-well plate for 30 minutes at room temperature.
Cells treated with medium alone served as the negative control, while cells treated with medium and LPS (LPS-activated RAW 264.7 cells or HGF cells) served as the positive control. The absorbance at 540 nm was measured using an ELISA microplate reader, nitrite amounts were determined using a standard sodium nitrite curve (0–1,000 μM) (Costa et al., 2019).
Gene expression measurement of pro-inflammatory mediator genes by qPCR technique
To quantify gene expression, we investigated how CoMW, VCO, PG, and CHX at IC50 concentrations affect the mRNA expression of pro-inflammatory mediators (IL-1β, IL-6, and COX-2) in LPS-activated HGF cells using real-time quantitative polymerase chain reaction (qPCR).
HGF cells were seeded into six-well plates at a density of 1×105 cells/well with a complete medium and incubated at 37°C with 5% CO2 for 24 hours. Then, they were treated with DMEM without phenol red supplemented with 10% FBS, along with CoMW, VCO, PG, and 0.12% CHX at IC50 concentrations, in the presence or absence of 10 µg/mL LPS. Non-LPS-activated HGF cells treated with medium served as the negative control, while LPS-activated HGF cells treated with medium served as the positive control.
After treatment, total RNA was extracted using the IllustraTM RNAspin Mini RNA Isolation Kit (45-001-161, Thermo Fisher Scientific, Wilmington, DE, USA) following the manufacturer's instructions. RNA quality was assessed by measuring the absorbance ratio at 260/280 nm using NanoDrop2000 spectrophotometry (ND-2000, Thermo Fisher Scientific, Wilmington, DE, USA). Subsequently, total RNA was reverse-transcribed to cDNA using the ReverTra AceTM qPCR RT Master Mix with gDNA remover Kit (TYB-FSQ-301S, TOYOBO, Osaka, Japan).
Gene expressions of IL-1β, IL-6, and COX-2 were analyzed with GAPDH as the housekeeping gene using the Light Cycler® 480 Real Time-PCR system (05015278001, Roche Applied Science, Mannheim, Germany). The oligonucleotide primers were shown in Table 1 (Bio Basic Inc., Markham, Ontario, Canada).
For the real-time PCR reactions, eight µL of diluted cDNA and 10 µL of SensiFASTTM SYBR Master Mix – No ROX Kit (BIO-98005, Bioline Ltd., London, UK) were used. The oligonucleotide primers for IL-1β, IL-6, and COX-2 were added, with one µL of forward primer and one µL of reverse primer, totalling 20 µL.
The PCR reaction protocol involved pre-incubation at 95°C for five minutes, followed by 40 cycles of amplification at 95°C for five seconds, annealing at 60°C for ten seconds, and extension at 72°C for 20 seconds. A melting curve analysis confirmed product specificity from 60 to 95°C.
Gene expression levels were normalized to GAPDH using the 2–∆∆Ct method (Costa et al., 2019; Harshitha and Arunraj, 2021).
Table 1. Primers names, nucleotide sequences, product sizes, annealing temperature, cycles used, and references in this study.
|
Primer names |
Nucleotide sequences (5’-3’) |
Product sizes (bp) |
Annealing (°C) |
Cycles |
References |
|
Interleukin-1 beta (IL-1β) |
(F) GCACGATGCACCTGTACGAT (R) CACCAAGCTTTTTTGCTGTGAGT |
64 |
65.4 |
40 |
NM_000576.3 |
|
Interleukin-6 (IL-6) |
(F) GGTACATCCTCGACGGCATCT (R) GCCTCTTTGCTGCTTTCAC |
79 |
62.6 |
40 |
NM_000600.5 |
|
Cycloxygenase-2 (COX-2) |
(F) CCCTTGGGTGTCAAAGGTAA (R) GCCCTCGCTTATGATCTGTC |
169 |
62.9 |
40 |
NM_000963.1 |
|
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
(F) AAATCCCATCACCATCTTCCAGGAGC (R) CATGGTTCACACCCATGACGAACA |
200 |
68.2 |
40 |
NM_002046.7 |
Note: Catalogue number of each nucleotide primer was 1816537765 (F) and 1816537766 (R) for IL-1β primer; 1816537759 (F) and 1816537760 (R) for IL-6; 1816537773 (F) and 1816537774 (R) for COX-2 primer, respectively.
Determination of cell migration by scratch assay
HGF cells (2×104 cells/well) were plated onto 24-well plates with complete medium and incubated at 37°C with 5% CO2 for 24 hours until reaching 80% confluence. The cell monolayer was manually scratched with a sterile 200 µL pipette tip to create the wound area. After washing three times with DMEM to remove detached cells, cells were treated with CoMW and CHX at IC50 concentrations. Cells treated with a complete medium served as the control.
Cell migration were monitored by capturing images every 8 hours for 36 hours using an inverted microscope Leica DFC3000 G equipped with a CCD camera and software LAS X (Leica Microsystems, Wetzlar, Germany). Image analysis was performed using ImageJ software (ImageJ bundled with Java 1.8.0_172), and the percentage of the initial wound area at hour zero was calculated by comparing the extracted data to the control using the following equation (Venter and Niesler, 2019):
% wound healing = 1 - (total wound area at specific time / initial wound area) x 100
Statistical analysis
The data were presented as mean ± standard deviation (SD). Statistical analysis was conducted using IBM SPSS Statistics software version 26.0 for Windows. One-way analysis of variance (ANOVA) was initially performed, followed by Tukey’s Honestly Significant Difference (HSD) and Dunnett's test for multiple comparisons. Statistical significance was set at the levels of P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001.
RESULTS
Determination of cytotoxicity effect
The cell viability of HGF cells treated with different concentrations of CoMW, VCO, PG, and CHX using a two-fold serial dilution was determined by the MTT assay, and the IC50 values were used to assess cytotoxicity. The IC50 values of CoMW, PG, and CHX were 1:8, 1:16, and 1:32 (v/v), respectively. In contrast, various concentrations of VCO did not interfere on cell viability as shown in Figure 1. Therefore, VCO at a concentration of 1:1 (v/v) was chosen for further experiments.
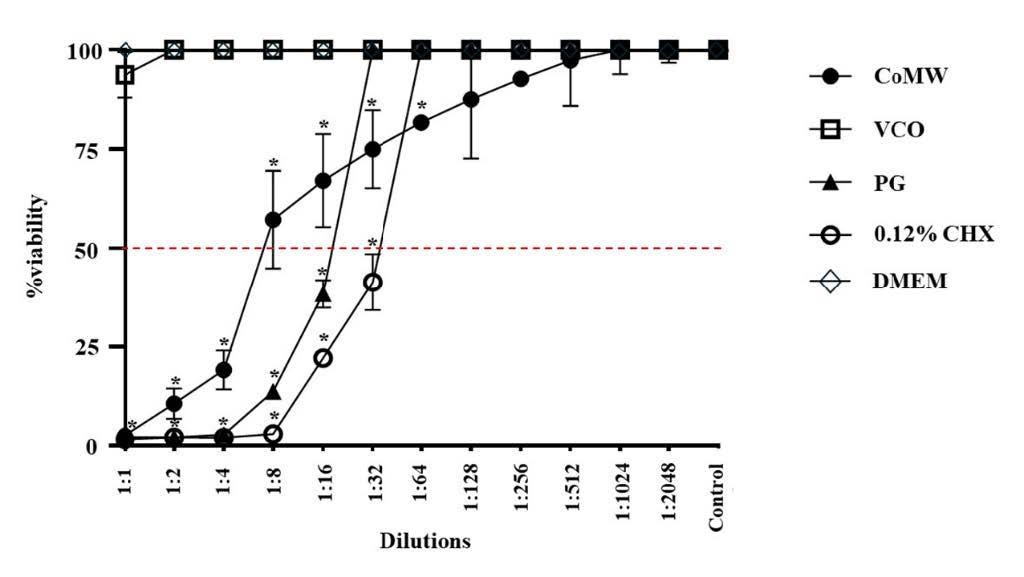
Figure 1. Cytotoxic Effects of CoMW, VCO, PG, and 0.12% CHX at Various Dilutions on HGF Cells. Each concentration was tested in triplicate using the MTT assay. All data are presented as the mean ± SD of three independent experiments. *, significant difference at P ≤ 0.001 compared to the DMEM or control group.
Anti-inflammatory activity using Griess assay
The nitric oxide (NO) production in LPS-activated HGF cells and RAW 264.7 cells treated with IC50 concentrations of CoMW, VCO, PG, and CHX was assessed by measuring nitrite concentrations using the Griess assay.
Results of LPS-activated HGF cells group indicated no toxicity compared to HGF cells treated with medium alone. Additionally, NO levels were significantly higher in LPS-activated HGF cells treated with IC50 of CoMW and VCO compared to untreated LPS-activated HGF cells, as shown in Figure 2 (P < 0.01 and < 0.001, respectively). LPS-activated RAW 264.7 cells exhibited a significant increase in NO levels compared to non-LPS-activated RAW 264.7 cells (P < 0.001). The inhibitory effect of substances at IC50 concentrations on NO production is illustrated in Figure 2.
The IC50 concentrations of CoMW and PG did not significantly differ from the positive control group. However, the IC50 of CHX significantly reduced NO production from LPS-activated Raw 264.7 cells to 287.75 ± 6.61 µmol compared to the positive control at 394.42 ± 22.51 µmol (P < 0.001). Interestingly, the IC50 of VCO led to a significant increase in NO levels compared to the positive control group (P < 0.001).
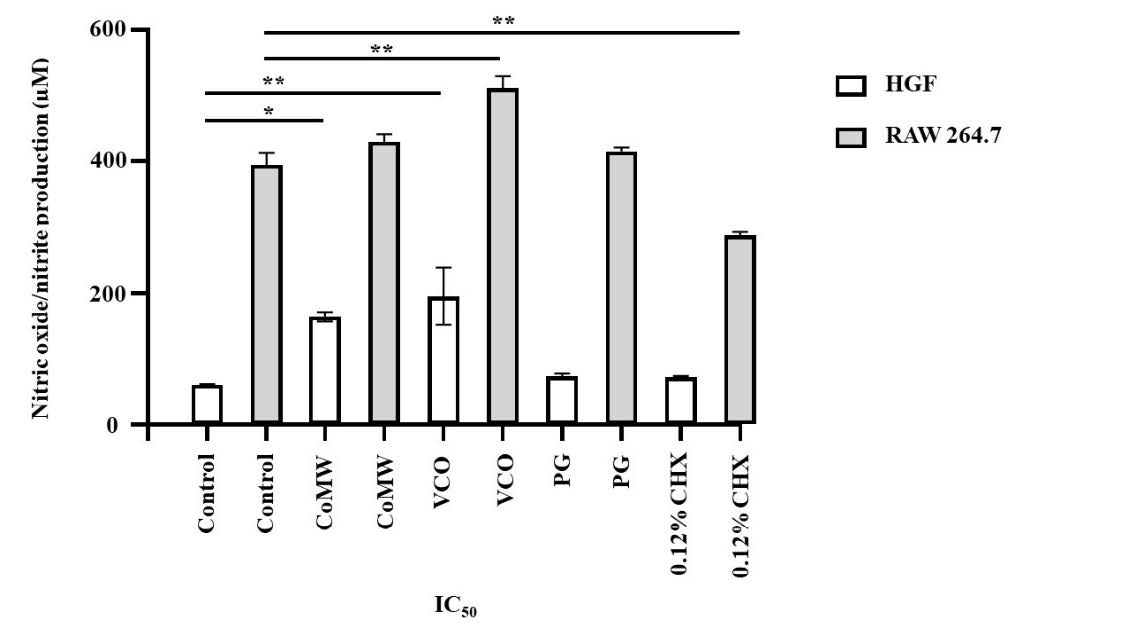
Figure 2. Effects of CoMW (1:16 v/v), VCO (1:1 v/v), PG (1:16), and 0.12% CHX (1:32 v/v) at IC50 Concentrations on LPS-Activated NO Production in (white box) HGF and (grey box) Raw 264.7 cells. All data are presented as the mean ± SD of three independent experiments. *, P < 0.01; **, P < 0.001 when compared to the positive control cells.
Gene expression measurement of pro-inflammatory mediator genes by qPCR technique
The HGF cells were treated with CoMW, VCO, PG, and CHX at IC50 concentrations, either in the presence or absence of 10 µg/mL LPS. The gene expressions of pro-inflammatory mediators, including IL-1β, IL-6, and COX-2 were determined by real-time qPCR.
As shown in Figure 3, the results indicated that LPS-activated HGF cells treated with medium (the positive control) showed significantly elevated expression (P < 0.001) compared to non-LPS-activated HGF cells in medium (the negative control). In non-LPS-activated groups, treatment with CoMW, VCO, PG, and CHX at IC50 concentrations did not significantly affect the gene expression of IL-1β, IL-6, and COX-2 compared to the negative control.
However, in LPS-activated groups, treatment with CoMW and VCO at IC50 concentrations significantly reduced the gene expression of IL-6 and COX-2(P < 0.001 and P ≤ 0.05, respectively) compared to the positive control. Treatment with IC50 concentrations of CHX significantly inhibited (P < 0.001) the gene expression of IL-1β, IL-6, and COX-2 while treatment with IC50 concentration of PG did not significantly reduce the gene expression of IL-1β, IL-6, and COX-2 compared to the positive control group.
Similarly, treatment with IC50 concentrations of CHX significantly inhibited (P < 0.001) the gene expression of IL-1β and IL-6 compared to treatment with CoMW and inhibited (P < 0.001) the gene expression of IL-6 compared to treatment with VCO. It was noteworthy that the gene expression of IL-6 was ten times higher than the gene expression of IL-1β and COX-2.
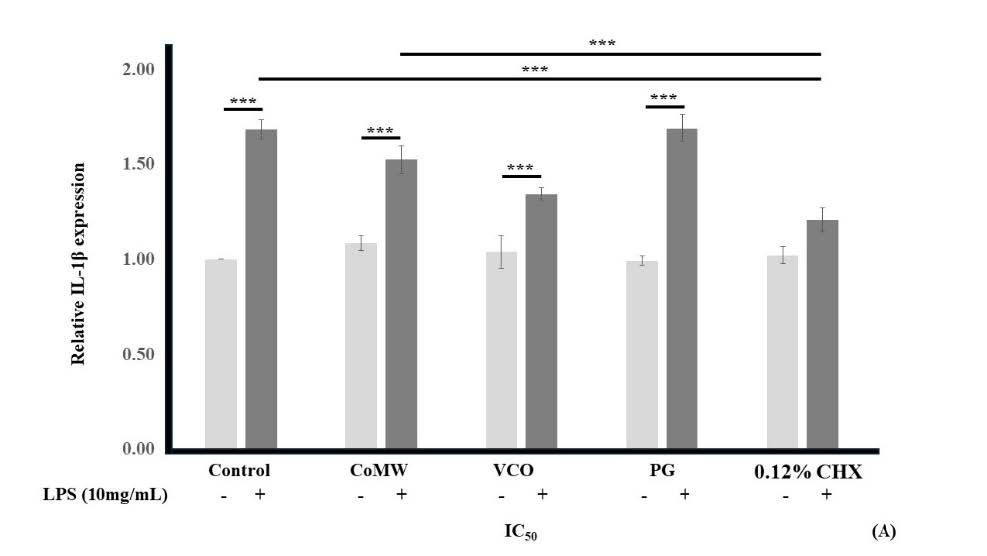
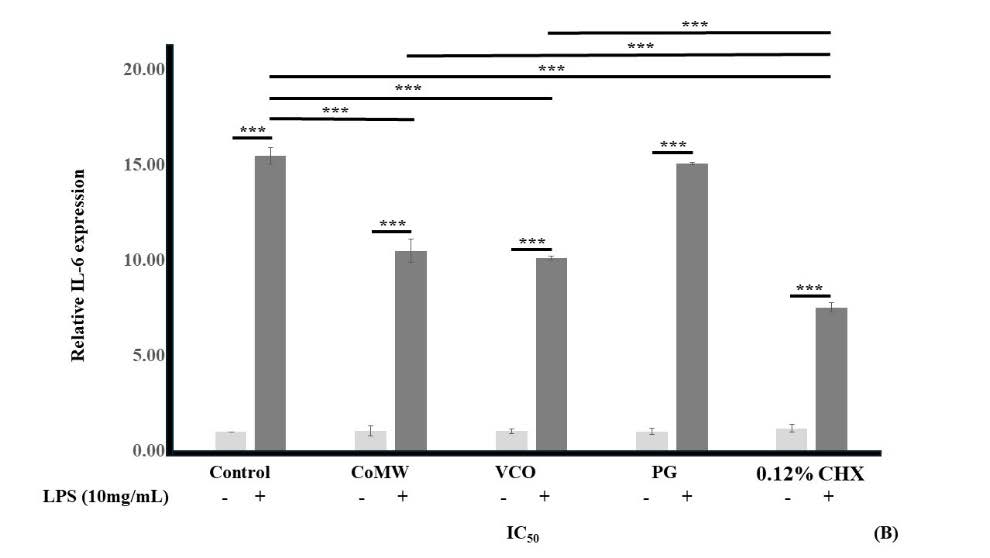
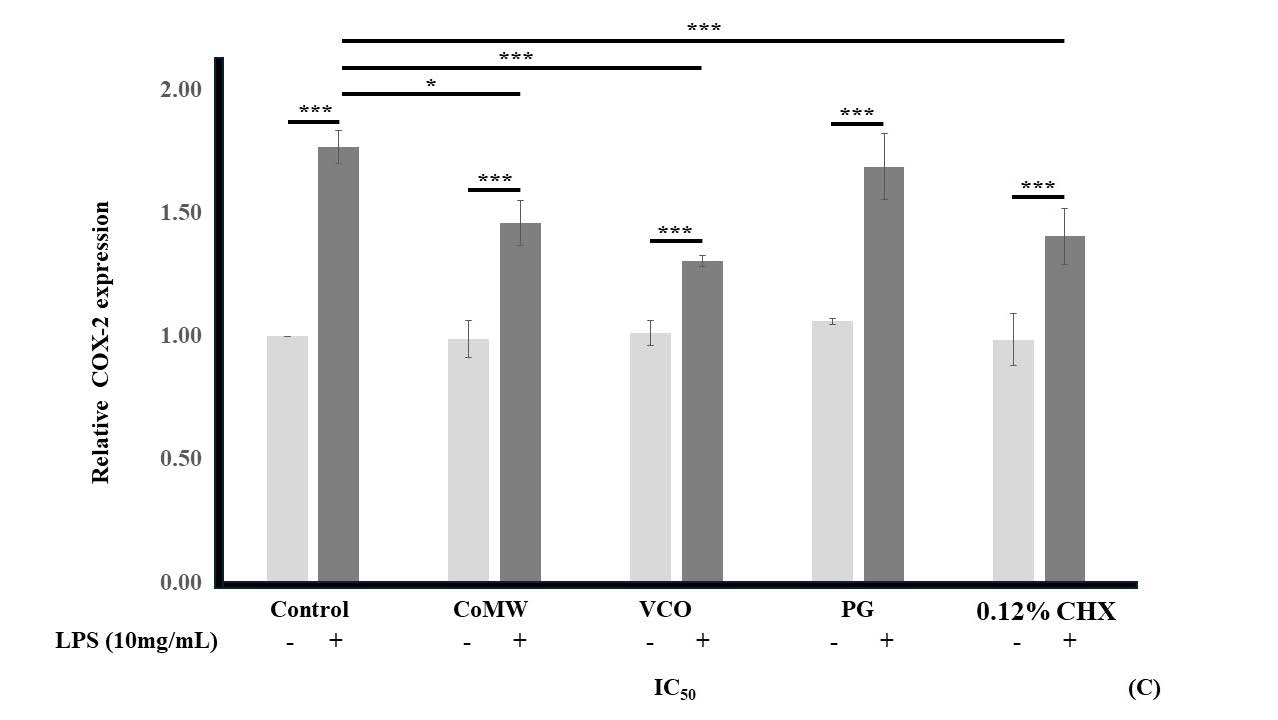
Figure 3. Effects of CoMW, VCO, PG, and CHX at IC50 concentration in the presence or absence of 10 µg/mL LPS on (A) IL-1β, (B) IL-6, and (C) COX-2 gene expression in HGF cells, and (D) Melting point analysis of each gene which compared with GAPDH. All data are expressed as the mean ± standard deviation (N=3). * and ***, significant difference at P < 0.05 and P < 0.001, respectively.
Measurement of HGF migration
The percentage of wound healing of HGF cells treated with the IC50 concentration of CoMW and CHX was measured by the scratched widths every eight hours, as shown in Figure 4. The results indicated that migrations were observed to rapidly increase in the first eight hours and then slowed from 36 to 48 hours (Figure 4a). Complete wound closures were recorded after 48 hours of treatment with the IC50 concentration of CoMW and CHX, with values of 90.50 ± 5.36 % and 90.71 ± 2.15 %, respectively, while the control group exhibited a value of 64.01 ± 2.02 (Figure 4b). The IC50 concentration of CoMW and CHX significantly increased the migration of HGF cells compared to the non-treated cells (the control group) in all periods (Figure 4c).
The results indicated that the wound healing rates of the IC50 concentration of CoMW and CHX were significantly different from the control group every eight hours (P < 0.001).
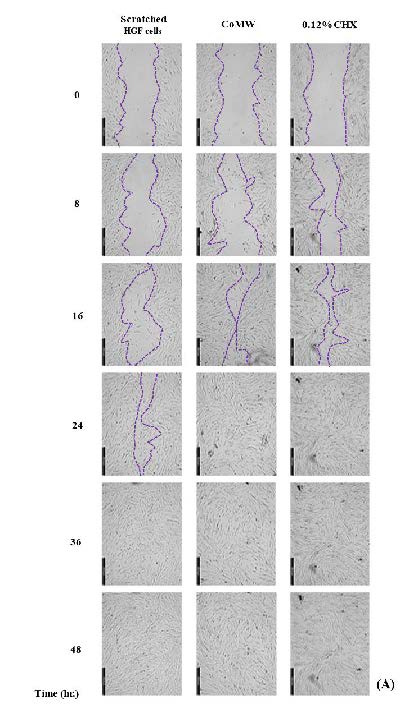
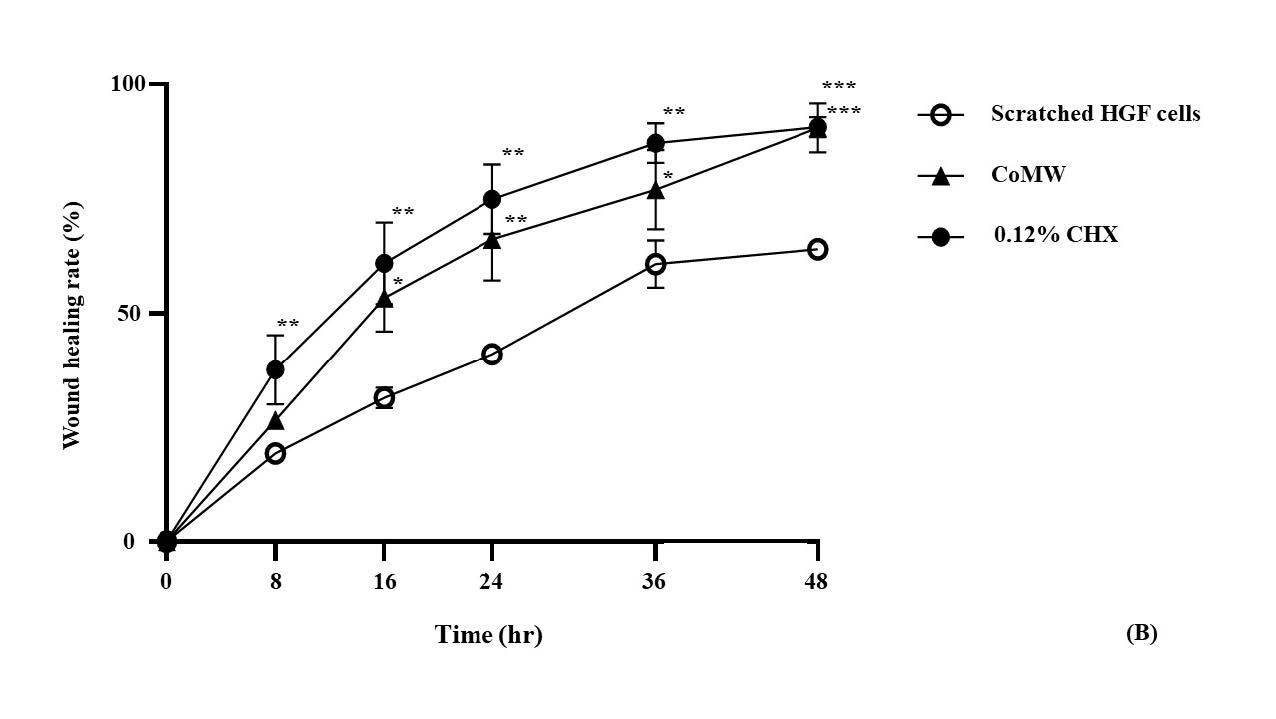
Figure 4. The wound closure rate of HGF cells treated with the IC50 concentration of CoMW and CHX was measured by calculating the scratch area every eight hours. (A) Representative images of wound closure indicating cell migration in each period, (B) The percentage of wound closure was calculated by the remaining cell-free area in each period, expressed as a percentage of the initial scratch area at time zero, with the extract compared to the control group for 48 hours. All data were expressed as the mean ± SD of solutions (n=3). *, **, and ***, significant differences at P < 0.05, P < 0.01, and P < 0.001 after comparison between samples at each time point.
DISCUSSION
CHX mouthwash is a commonly used as an antiseptic oral rinse that is effective against a wide range of bacteria and fungi. Its primary active ingredient disrupts the cell membranes of microorganisms, leading to cell death. It is highly effective in reducing dental plaque accumulation. It helps to prevent plaque formation and maintain oral hygiene (Herrera, 2013; Brookes et al., 2020). CHX can be used as a part of the treatment for gingivitis to reduce inflammation and promote gingival healing. Additionally, a regular use of CHX can prevent the development of gingivitis in individuals at risk, such as those with poor oral hygiene or a history of gingivitis (James et al., 2017). Along with professional dental treatments, CHX can help control the bacterial infection associated with periodontal disease, and reduce the bacterial amount in the oral cavity, thereby addressing the underlying cause of bad breath and providing a temporary solution for this common problem, and improving oral health outcomes. While CHX is primarily known for its antibacterial properties, it also exhibits some antifungal activity. It can reduce the population of Candida albicans in the oral cavity (Brookes et al., 2020), thereby lowering the risk of developing oral candidiasis or preventing its recurrence in susceptible individuals. However, prolonged or excessive use can lead to side effects such as staining of the teeth, altered taste perception, and oral irritation (McCoy et al., 2008). It has been associated with mucosal irritation, including irritation of the gingiva, tongue, and oral mucosa.
Additionally, CHX exhibits concentration-dependent cytotoxicity at high concentrations. It can cause a direct damage to cells by disrupting cell membranes. This disruption results in leakage of cellular contents, loss of cell function, and ultimately cell death. The mechanism of action involves CHX's ability to interact with phospholipids in the cell membrane, leading to membrane disruption and cellular damage (Liu et al., 2018).
CHX was able to reduce cellular proliferation and increase collagen deposition as well as the expression of proapoptotic molecule and fibrotic marker expression, and myofibroblast differentiation. It also reduced the expression of RAC1 and trigger expression of SERPINE1 and TIMP1, showing “scar wound healing response” pattern, which are essential steps in the wound healing process (Pilloni et al., 2021). This study assessed the in vitro effects of CHX on gingival tissue. It reveals that CHX may inhibit the proliferation and migration of certain types of cells involved in the wound-healing process, such as fibroblasts and keratinocytes. These inhibitory effects could potentially delay wound closure and tissue regeneration. However, the extent of this inhibition may vary depending on factors such as concentration of CHX, exposure time, and the specific cell types involved in the process (Wyganowska-Swiatkowska et al., 2016).
Coconut oil pulling is a traditional oral health practice which can reduce plaque formation and prevent gingivitis (Peedikayil et al., 2015). However, scientific studies investigating the effectiveness of coconut oil pulling for gingivitis prevention are limited, and further research is needed to confirm its benefits. Coconut oil contains medium-chain fatty acid components have antimicrobial, inhibit the growth of bacteria in mouth (Peedikayil et al., 2016), and antifungal activity (Akula et al., 2021). The medium-chain fatty acids, including lauric acid, capric acid, and caprylic acid, which have been shown to exhibit antifungal activity against Candida species. These fatty acids disrupt the cell membranes of candidal cells, leading to their destruction (Akula et al., 2021). Therefore, using coconut oil as a mouthwash may reduce the population of Candida in the oral cavity, potentially aiding in the treatment of oral candidiasis and prevention of the recurrence of oral candidiasis in individuals prone to recurrent infections. Regular use of coconut oil may contribute to maintain a healthy balance of microorganisms in the mouth and promoting oral hygiene, thus reducing the risk of future episodes of oral thrush.
The CoMW is composed of 60% VCO, 30% PG, and 10% distilled water. The cytotoxicity, anti-inflammatory, and wound-healing activity of VCO, PG, and 0.12% CHX were investigated. Initially, exposure of HGF cells to cytotoxic compounds can lead to uncontrolled cell death, apoptosis, or inhibition of growth and division, resulting in decreased cell proliferation (Brookes et al., 2020).
In this study, HGF cells were treated with two-fold serial dilutions of VCO. The results showed that cell viability remained above 90% at all dilutions. The IC50 concentrations of CoMW, PG, and 0.12% CHX were 1:16 (v/v), 1:16 (v/v), and 0.001871:32 (v/v), respectively. All dilutions of VCO were non-cytotoxic to HGF cells. The anti-inflammatory activities of the IC50 concentrations of CoMW, VCO, PG, and CHX were examined against LPS-induced HGF and RAW264.7 cells using the nitric oxide assay. The findings demonstrated that the IC50 concentrations of CoMW and VCO increased NO production in LPS-activated HGF and RAW 264.7 cells, whereas the IC50 concentrations of CHX had the opposite effect, reducing nitric oxide production in LPS-activated RAW 264.7 cells. The NO productions in the LPS-activated RAW 264.7 cells treated with IC50 concentrations of CoMW and PG were not significantly different. Therefore, the IC50 concentration of CHX exhibited the most potent anti-inflammatory effect in LPS-activated RAW264.7 cells. No significant difference was observed in nitric oxide production between the non-LPS-activated HGF cells treated with IC50 concentrations of PG, CHX and the non-LPS-activated HGF cells. NO is a signaling molecule that plays a key role in the pathogenesis of inflammation. There are biphasic effects on HGF cells. Low NO concentrations stimulate the proliferation of HGF cells and their differentiation into myofibroblast cells, which are involved in wound contraction and tissue repair. However, high concentrations of NO inhibit cell growth, differentiation, and extracellular matrix production, ultimately leading to cell apoptosis (Baek et al., 2015).
Lauric acid (C12:0), a component of coconut oil, has been associated with the activation of NF-κB and the expression of inducible NO (Chen et al., 2022). If the IC50 concentration of CoMW had influenced NO production during the inflammatory response, it could potentially triggered inflammation through alternative pathways.
The gene expression analysis of this study revealed that LPS-activated HGF cells treated with IC50 concentrations of CHX suppressed the gene expression of IL-1β, IL-6, and COX-2. Similarly, IC50 concentrations of VCO also inhibited the gene expression of IL-6 and COX-2 in LPS-activated HGF cells. In contrast, IC50 concentrations of PG did not significantly impact gene expression in LPS-activated HGF cells.
The anti-inflammatory effect of the IC50 concentrations of CoMW was mainly attributed to the concentration of VCO. The IC50 concentrations of CoMW contained 3.725% VCO (v/v), and although its anti-inflammatory effect reduced gene expression, it might not have been sufficient to regulate IL-1β production. Additionally, it decreased the gene expression of IL-6 and COX-2 in HGF cells.
Wound healing is a normal biological process in the human body achieved through four precisely and highly programmed phases: hemostasis, inflammation, proliferation, and remodeling. All four phases must occur in the proper sequence and time frame for successful wound healing (Guo and Dipietro, 2010). Meanwhile, the IC50 concentration of CHX was 0.001875% (v/v). Within a 24-hour incubation period, these substances boosted the migration of HGF cells and maintained wound healing at approximately 90% after 48 hours. The active ingredient in CoMW's wound healing properties was found to be VCO. Lauric acid and monolaurin, present in VCO, played a pivotal role in the wound-healing process by increasing the proliferation of HGF cells and stimulating cell migration. Furthermore, Lauric acid and monolaurin influenced COX-2 levels, which are involved in angiogenesis and cell migration (Wilkinson and Hardman, 2020). This effect is similar to the previous study with nitric oxide, which is increased through polyphenol stimulation (Silalahi et al., 2019; Serreli and Deiana, 2023).
The observation regarding the IC50 concentration of CHX highlights its dual role in wound healing. At low dose, CHX acts as a debriding agent at non-cytotoxic levels, aiding in wound healing and creating a favorable cellular environment for regulating proliferation and migration. However, at high doses, CHX, being cytotoxic, hindered cell proliferation and could potentially delay wound healing.
In this study, treating HGF cells with the IC50 concentrations of CoMW and CHX, did not affect cell viability. The observed effect on cell migration could be attributed to the downregulation of inflammatory gene expression which likely facilitated faster healing compared to untreated cells.
CONCLUSION
This study evaluated the in vitro anti-inflammatory activities and wound-healing effects of a concentration of 1:16 (v/v) CoMW. The results demonstrated that the CoMW exhibited anti-inflammatory activities by reducing the gene expression of IL-6 and COX-2. The level of reduction was not significantly less than that observed with CHX. Additionally, both the 1:16 (v/v) CoMW and 1:32 (v/v) of 0.12% CHX similarly promoted faster wound healing in HGF cells compared to non-treated cells. Therefore, the developed CoMW formula could be used as an adjunct treatment for oral inflammation.
ACKNOWLEDGEMENTS
The authors are grateful to the Faculty of Dentistry, Chiang Mai University, Thailand, for providing instruments. We would like to thank Thanapat Sastraruji at the Dental Research Center, Faculty of Dentistry, Chiang Mai University, for his invaluable statistical advice and research guidance.
AUTHOR CONTRIBUTIONS
Peerachat Marasri wrote the research proposal, conducted the experiments, analyzed the statistical data, and drafted the manuscript. Siriwoot Sookkhee consulted on the research experiment scope, provided the conceptualization, designed the methodology, contributed to data visualization, analyzed the statistical data, and wrote and proofread the manuscript. Phenphichar Wanachantararak provided the conceptualization, designed the methodology and validated the experiments. Darunee Owittayakul initiated the research experiment scope, submitted the research funding, and provided consultation throughout the research process. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Akula, S. T., Nagaraja, A., Ravikanth, M., Kumar, N. G. R., Kalyan, Y., and Divya, D. 2021. Antifungal efficacy of lauric acid and caprylic acid – derivatives of virgin coconut oil against Candida albicans. Biomedical and Biotechnology Research Journal. 5(2): 229-234.
AlQranei, M. S. and Chellaiah, M. A. 2020. Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms. Journal of Oral Biosciences. 62(2): 123-130.
Baek, M. W., Seong, K. J., Jeong, Y. J., Kim, G. M., Park, H. J., Kim, S. H., et al. 2015. Nitric oxide induces apoptosis in human gingival fibroblast through mitochondria-dependent pathway and JNK activation. International Endodontic Journal. 48(3): 287-297.
Boateng, L., Ansong, R., Owusu, W. B., and Steiner-Asiedu, M. 2016. Coconut oil and palm oil's role in nutrition, health and national development: A review. Ghana Medical Journal. 50(3): 189-196.
Brookes, Z. L. S., Bescos, R., Belfield, L. A., Ali, K., and Roberts, A. 2020. Current uses of chlorhexidine for management of oral disease: A narrative review. Journal of Dentistry. 103: 103497.
Chanpa, P., Owittayakul, D., Wanachantararak, P., Chaiyana, W., and Sookkhee, S. 2023. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Natural and Life Sciences Communications. 22(1): 1-16.
Chen, X., Kim, D. I., Moon, H. G., Chu, M., and Lee, K. 2022. Coconut oil alleviates the oxidative stress-mediated inflammatory response via regulating the mapk pathway in particulate matter-stimulated alveolar macrophages. Molecules. 27(9): 2898.
Costa, C. R. R., Amorim, B. R., Silva, S., Acevedo, A. C., Magalhães, P. O., and Guerra, E. N. S. 2019. In vitro evaluation of Eugenia dysenterica in primary culture of human gingival fibroblast cells. Brazilian Oral Reserach. 33: e035.
de Jong, A. J., Kloppenburg, M., Toes, R. E. M., and Ioan-Facsinay, A. 2014. Fatty acids, lipid mediators, and T-cell function. Frontiers in Immunology. 5: 483.
Famurewa, A. C., Maduagwuna, E. K., Folawiyo, A. M., Besong, E. E., Eteudo, A. N., Famurewa, O. A., et al. 2020. Antioxidant, anti-inflammatory, and antiapoptotic effects of virgin coconut oil against antibiotic drug gentamicin-induced nephrotoxicity via the suppression of oxidative stress and modulation of iNOS/NF-ĸB/caspase-3 signaling pathway in Wistar rats. Journal of Food Biochemistry. 44(1): e13100.
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. 2019. Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 25(12): 1822-1832.
Guo, S. and Dipietro, L. A. 2010. Factors affecting wound healing. Journal of Dental Research. 89(3): 219-229.
Harshitha, R. and Arunraj, D. R. 2021. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochemistry and Molecular Biology Education. 49(5): 800-812.
Herrera, D. 2013. Chlorhexidine mouthwash reduces plaque and gingivitis. Evidence-Based Dentistry. 14(1): 17-18.
Intarakaewsri, T., Owittayakul, D., and Wanachantararak, P. 2020. Development of virgin coconut oil mouthwash against Candida albicans biofilms. Chiang Mai Dental Journal. 41(3): 55-64.
James, P., Worthington, H. V., Parnell, C., Harding, M., Lamont, T., Cheung, A., et al. 2017. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database of Systematic Reviews. 3(3): Cd008676.
Joshi, S., Kaushik, V., Gode, V., and Mhaskar, S. 2020. Coconut oil and immunity: What do we really know about it so far? Journal of the Association of Physicians of India. 68(7): 67-72.
Kang, W., Hu, Z., and Ge, S. 2016. Healthy and inflamed gingival fibroblasts differ in their inflammatory response to Porphyromonas gingivalis lipopolysaccharide. Inflammation. 39(5): 1842-1852.
Kappally, S., Shirwaikar, A., and A, S. 2015. Coconut oil- a review of potential applications. Hygeia Journal for Drugs and Medicine. 7(2): 34-41.
Karlis, G. D., Schoenmaker, T., Tsoromokos, N., Veth, O. E., Loos, B. G., and de Vries, T. J. 2024. Passaging of gingival fibroblasts from periodontally healthy and diseased sites upregulates osteogenesis-related genes. Human Cell. 37(1): 193-203.
Landén, N. X., Li, D., and Ståhle, M. 2016. Transition from inflammation to proliferation: A critical step during wound healing. Cellular and Molecular Life Sciences. 73(20): 3861-3885.
Lin, T. K., Zhong, L., and Santiago, J. L. 2017. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. International Journal of Molecular Sciences. 19(1): 70.
Liu, J. X., Werner, J., Kirsch, T., Zuckerman, J. D., and Virk, M. S. 2018. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. Journal of Bone and Joint Infection. 3(4): 165-172.
Liu, T., Zhang, L., Joo, D., and Sun, S. C. 2017. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2: 17023.
McCoy, L. C., Wehler, C. J., Rich, S. E., Garcia, R. I., Miller, D. R., and Jones, J. A. 2008. Adverse events associated with chlorhexidine use: Results from the department of veterans affairs dental diabetes study. Journal of the American Dental Association. 139(2): 178-183.
Merly, L. and Smith, S. L. 2017. Murine RAW 264.7 cell line as an immune target: are we missing something? Immunopharmacology and Immunotoxicology. 39(2): 55-58.
Naruishi, K. 2022. Biological roles of fibroblasts in periodontal diseases. Cells. 11(21): 3345.
Nitbani, F. O., Tjitda, P. J. P., Nitti, F., Jumina, J., and Detha, A. I. R. 2022. Antimicrobial properties of lauric acid and monolaurin in virgin coconut oil: A review. ChemBioEng Reviews. 9(5): 442-461.
Nittayananta, W., DeRouen, T. A., Arirachakaran, P., Laothumthut, T., Pangsomboon, K., Petsantad, S., et al. 2008. A randomized clinical trial of chlorhexidine in the maintenance of oral candidiasis-free period in HIV infection. Oral Diseases. 14(7): 665-670.
Palmieri, E. M., McGinity, C., Wink, D. A., and McVicar, D. W. 2020. Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites. 10(11): 229.
Peedikayil, F. C., Remy, V., John, S., Chandru, T. P., Sreenivasan, P., and Bijapur, G. A. 2016. Comparison of antibacterial efficacy of coconut oil and chlorhexidine on Streptococcus mutans: An in vivo study. Journal of International Society of Preventive Community Dentistry. 6(5): 447-452.
Peedikayil, F. C., Sreenivasan, P., and Narayanan, A. 2015. Effect of coconut oil in plaque related gingivitis - a preliminary report. Nigerian Medical Journal. 56(2): 143-147.
Pilloni, A., Ceccarelli, S., Bosco, D., Gerini, G., Marchese, C., Marini, L., et al. 2021. Effect of chlorhexidine digluconate in early wound healing of human gingival tissues. a histological, immunohistochemical and biomolecular analysis. Antibiotics (Basel). 10(10): 1192.
Quazi, A., Patwekar, M., Patwekar, F., Mezni, A., Ahmad, I., and Islam, F. 2022. Evaluation of wound healing activity (excision wound model) of ointment prepared from infusion extract of polyherbal tea bag formulation in diabetes-induced rats. Evidence-Based Complementary and Alternative Medicine. 2022: 1372199.
Ramadan, D. E., Hariyani, N., Indrawati, R., Ridwan, R. D., and Diyatri, I. 2020. Cytokines and chemokines in periodontitis. European Journal of Dentistry. 14(3): 483-495.
Sam, Q. H., Ling, H., Yew, W. S., Tan, Z., Ravikumar, S., Chang, M. W., et al. 2021. The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids. International Journal of Molecular Sciences. 22(12): 6453.
Scheres, N., Laine, M. L., Sipos, P. M., Bosch-Tijhof, C. J., Crielaard, W., de Vries, T. J., et al. 2011. Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis. Journal of Periodontal Research. 46(4): 407-416.
Serreli, G. and Deiana, M. 2023. Role of dietary polyphenols in the activity and expression of nitric oxide synthases: A review. Antioxidants (Basel). 12(1): 147.
Shih, T. L., Liu, M. H., Li, C. W., and Kuo, C. F. 2018. Halo-substituted chalcones and azachalcones-inhibited, lipopolysaccharited-stimulated, pro-inflammatory responses through the TLR4-mediated pathway. Molecules. 23(3): 597.
Shrestha, A., Rimal, J., Rao, A., Sequeira, P. S., Doshi, D., and Bhat, G. K. 2011. In vitro antifungal effect of mouth rinses containing chlorhexidine and thymol. Journal of Dental Sciences. 6(1): 1-5.
Silalahi, J., Yuandani, Y., Meliala, D., Margata, L., and Satria, D. 2019. The activity of hydrolyzed virgin coconut oil to increase proliferation and cyclooxygenase-2 expression towards on NIH 3T3 cell line in wound healing process. Macedonian Journal of Medical Sciences. 7(19): 3164-3168.
Suci, P. A. and Tyler, B. J. 2002. Action of chlorhexidine digluconate against yeast and filamentous forms in an early-stage Candida albicans biofilm. Antimicrobial Agents and Chemotherapy. 46(11): 3522-3531.
Suriyaprom, S., Srisai, P., Intachaisri, V., Kaewkod, T., Pekkoh, J., Desvaux, M., et al. 2023. Antioxidant and anti-inflammatory activity on LPS-stimulated RAW 264.7 macrophage cells of white mulberry (Morus alba L.) leaf extracts. Molecules. 28(11): 4395.
Tanabe, N., Tomita, K., Manaka, S., Ichikawa, R., Takayama, T., Kawato, T., et al. 2023. Co-stimulation of AGEs and LPS induces inflammatory mediators through PLCγ1/JNK/NF-κB pathway in MC3T3-E1 cells. Cells. 12(10): 1383.
Venter, C. and Niesler, C. U. 2019. Rapid quantification of cellular proliferation and migration using ImageJ. BioTechniques. 66(2): 99-102.
Wilkinson, H. N. and Hardman, M. J. 2020. Wound healing: Cellular mechanisms and pathological outcomes. Open Biology. 10(9): 200223.
Wyganowska-Swiatkowska, M., Kotwicka, M., Urbaniak, P., Nowak, A., Skrzypczak-Jankun, E., and Jankun, J. 2016. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. International Journal of Molecular Medicine. 37(6): 1594-1600.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Peerachat Marasri1, Siriwoot Sookkhee2, Phenphichar Wanachantararak3, *, and Darunee Owittayakul1, *
1 Department of Family and Community Dentistry, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Phenphichar Wanachantararak, E-mail: phenphichar.w@cmu.ac.th
Darunee Owittayakul, E-mail: darunee.o@cmu.ac.th
ORCID:
Siriwoot Sookkhee: https://orcid.org/0000-0002-8724-6008
Phenphichar Wanachantararak: https://orcid.org/0000-0002-0594-0398
Darunee Owittayakul: https://orcid.org/0009-0004-7004-6204
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: March 26, 2024;
Revised: August 28, 2024;
Accepted: September 5, 2024;
Online First: September 17, 2024

