
Antioxidant, Antibacterial and Immunomodulatory Properties of Algerian Phoenix dactylifera
Afaf Sakhri*, Fatma Zahra Sakhri, and Chawki BensouiciPublished Date : September 16, 2024
DOI : https://doi.org/10.12982/NLSC.2024.056
Journal Issues : Number 4, October-December 2024
Abstract The aim of the current study is to evaluate the antioxidant, antibacterial, and phagocytic activities of the hydroethanolic extract of Phoenix dactylifera fruit variety Mech degla. The antioxidant activity was determined through DPPH assay, ferrous ions chelating assay, and CUPRAC assay. The antibacterial activity against four bacteria (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae), was evaluated using the disc diffusion method, while the in vivo phagocytic activity was measured using carbon clearance method. The results showed that the extract exhibited good antioxidant activity with the IC50 values of 0.89 ± 0.3 g/l and 0.56 ± 0.24 g/l for DPPH and ferrous ions chelating activities, respectively, and the A0.5 value of 1.45 ± 0.15 g/l in the CUPRAC assay. Moreover, the extract showed notable antibacterial activity against all tested strains with the highest zone of inhibition (23.3 ± 0.3 mm) against S. pneumonia. Besides the extract did not show any significant toxicity, it was able to stimulate the phagocytic activity with a significantly faster carbon clearance rate at the dose of up to 100 mg/kg compared to the control group. Our results suggested that the Phoenix dactylifera fruit represents a promising source for the development of safe and effective immunomodulators as remedies to prevent and treat various microbial and immune-related diseases.
Keywords: Antibacterial activity, Antioxidant activity, Immunomodulators, Phagocytic activity, Phoenix dactylifera.
Citation: Sakhri, A., Sakhri, F. Z., and Bensouici, C. 2024. Antioxidant, antibacterial and immunomodulatory properties of algerian Phoenix dactylifera. Natural and Life Sciences Communications. 23(4): e2024056.
INTRODUCTION
Fruits are universally known as a source of health benefits due to their richness of active constituents such as antioxidant compounds which can reduce the risks of free-radical mediated diseases such as cancer, coronary heart diseases, and neurodegenerative diseases including Parkinson’s and Alzheimer’s diseases (Kim et al., 2015; Al-Alawi et al., 2017). Bioactive compounds presented in fruits have received great attention through their ability to modify the activity of the immune system, which can provide new and potent immunomodulators for the treatment of several diseases including infectious diseases (Alhazmi et al., 2021).
Phoenix dactylifera (PD, Arecaceae family), commonly known as date palm is one of the most popular and traditionally cultivated fruit trees distributed mainly in arid regions of the Middle East and North Africa (Gantait et al., 2018; Al-Shwyeh, 2019; Echegaray et al., 2020). The date fruit is considered as an ideal source for human nutrition due to its composition of essential nutrients, including carbohydrates, minerals, proteins, and fibers. In addition, its richness in bioactive compounds such as anthocyanins, phenolics, carotenoids, procyanidins, vitamins and flavonoids may elucidate its common use in folk medicine for the treatment of many diseases (Al-Alawi et al., 2017; Alshwyeh, 2020; Ourradi et al., 2021).
Epidemiological evidence, coupled with clinical studies strongly support the assertion that dietary consumption of date fruit can favorably modulate serum glucose and lipid level, as well as markers associated with inflammation, diabetes, and cardiovascular diseases (Rock et al.,2009; Ali Haimoud et al.,2016; Alalwan et al., 2020). Additionally, it has been shown to reduce the need for labor intervention (Nasiri et al., 2019). In the same context, in vitro and in vivo studies have revealed a wide range of biological activities of date fruit including gastroprotective (Al-Qarawi et al., 2005), nephroprotective (El Arem et al., 2014), , antiparasitic (Albakhit et al., 2016), antimicrobial (Qadoos et al., 2017), cardioprotective (Alhaider et al., 2017), anti-inflammatory (El Hilaly et al., 2018), antiviral (Allahyari et al., 2021), hepatoprotective (Fatani et al., 2022) and anticancer effects (Abdelbaky et al., 2023).
As for its health benefits, date fruit has an important socio-economic role in many countries. In terms of world production, Algeria is the fourth-largest producer of dates after Egypt, Saudi Arabia and Iran with 1.152 million tons in 2020 (FAO, 2020). In addition, the exploitation of dates in diverse areas of food and health processed products such as jams, jellies, and syrups is rapidly increasing (Echegaray et al., 2020; Farag et al., 2021).
In Algeria, most Algerian palm oases are located in the regions south of the Saharan Atlas Mountains. Several studies have focused on identifying the main properties of different Algerian date fruit varieties from the provinces of Ghardaia (Mansouri et al., 2005; Tassoult et al., 2021), Ouargla (Zineb et al., 2012), Biskra (Benmeddour et al., 2013), and Adrar (Abdul-Hamid et al., 2018). Whereas, none of the existing data reported about the date varieties of M’doukal region (gate of the desert). Moreover, while most studies were focused on evaluating the antioxidant activities of different date fruit varieties (Al-Jasass et al., 2015; Assirey, 2021; Bano et al., 2022), the exploration of their immunomodulatory properties has not been extensively conducted. The present study was performed to evaluate the antioxidant, antimicrobial and immunomodulatory activities of the date palm fruit Mech Degla variety from the M'Doukal region.
MATERIAL AND METHODS
Materials
Plant material
Fresh samples of date fruit, Mech Degla variety, were collected at their mature stage (Tamr stage) from different fully grown date palms in a private farm located in M’doukal, Batna Province, North-Eastern Algeria with coordinates of 35°7′18″N 5°10′56″E. Date fruits were packaged, transported to the laboratory, and stored at 4°C. Identification of the samples was determined based on the information provided by experienced farmers and confirmed at the department of Biology, University of Batna 2.
Microorganisms test
Four clinically isolated bacteria, including two Gram negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and two Gram positive bacteria (Staphylococcus aureus and Streptococcus pneumoniae), were obtained from the laboratory of Clinical Microbiology, University Hospital Centre Benflis Touhami Batna- Algeria.
Experimental animals
Adult albino male mice, 2–2.5 months old, with a weight range between 25 and 33 g were provided from the animal stock facility of Pasteur Institute in Algiers-Algeria. The animal studies were approved by the ethics committee of institutional animals and conducted according to Executive Decree n°10–90 completing the Executive Decree n°04–82 of the Algerian Government, and the ethical principles and guidelines for the purpose of control and supervision of experiments on animals (OECD, 2002).
Methods
Sample preparation and extraction
PD fruits were rinsed in tap water, pitted, air-dried under shadow then ground to a fine powder using a grinder. A quantity of 50 g was extracted with 70%v/v ethanol for 24 h at room temperature (25°C) using a mechanical shaker. The macerate was filtered using Whatman No.1 to discard the precipitate. After completing three repeated extraction processes, the filtrate was concentrated on a rotary evaporator. The obtained PD extract was stored at -20°C for further analysis.
Antioxidant activity
DPPH assay
The antioxidant activity of PD extract was evaluated using 2,2-diphenyl-1-picryl hydrazyl (DPPH) assay as reported by Majhenič et al. (2007) with slight modifications. Different concentrations of PD extract (dissolved in methanol) were prepared. A volume of 2,800 µl of methanolic solution of DPPH (6x10-5 M) was mixed with 200 µl of each concentration and incubated in the darkness at 37°C. Twenty minutes later, the absorbance was measured at 517 nm using a spectrophotometer against blank. Ascorbic acid was used as a standard. The percentage of the DPPH scavenging activity was calculated using the following equation:
% inhibition = [(A0 –A1)/A0] x 100
Where A0 represents the absorbance of the blank sample, and A1 represents the absorbance of the extract/standard
IC50 (the concentration required to scavenge 50% of free radicals) was calculated from the regression equation, prepared from the concentration of samples and percentage inhibition of free radical formation.
Ferrous ions chelating capacity
The chelating activity of the extract on Fe2+ was measured in accordance with Tel et al. (2013). In brief, 40 μl of FeCl2 reagent (0.2 mM) was added to 80 μl of the extract solution (dissolved in ethanol at different concentrations). The reaction was initiated by the addition of 80 μl ferene (0.5 mM). The mixture was shaken vigorously and left at room temperature. After 10 min, the absorbance was measured at 593 nm. Ethylenediamine tetra-acetic acid (EDTA) was used as an antioxidant standard.
The ability of the PD extract to chelate ferrous ion was calculated using the formula:
Fe2+chelating activity (%) = [(A0−A1) ∕A0] x100
In which, A0 is the absorbance of the blank sample and A1 is the absorbance of the PD extract/standard.
Cupric reducing antioxidant capacity (CUPRAC)
The CUPRAC was measured according to Apak et al. (2004) with slight modifications. In each well of 96 well plates, a mixture of 50 μl of copper (II) chloride solution (10 mM), 50 µl of neocaproine solution (7.5 mM), and 60 μl of ammonium acetate buffer (1 M, pH 7.0) was added. Then, 40 μl of the sample extracts dissolved in methanol at different concentrations were added. The well plates were shaken vigorously and left at room temperature. After one hour, the absorbance of the mixed solution was recorded at 450 nm. Butylated hydroxyanisole (BHA) was used as antioxidant standard. The presence of antioxidant compounds reduced the cupric ions to cuprous ions, resulting in the formation of a stable complex between neocuproine and cuprous ions. The results were given as A0.50 (g/l) corresponding to the concentration indicating 0.50 absorbance intensity. A higher absorbance indicates a higher reducing capacity of antioxidants.
Antibacterial assay
The disc diffusion method was used to evaluate the antibacterial activity of PD extract as described by Islam et al. (2013) with slight modifications. Sterilized paper discs (6 mm) were impregnated with 10 μl of PD extract dissolved in DMSO (200 mg/ml) and dried aseptically. The impregnated discs were placed on the surface of plates that were previously inoculated with bacterial suspensions (0.5 McFarland standards) and incubated at 35 ± 2°C for 24 h. Chloramphenicol (10 µg/disc) was used as a positive control. While, discs impregnated with 10 μl of DMSO and clean discs (without solvent) were used as negative controls. After incubation, the antibacterial activity was evaluated by measuring the diameter of the inhibition zone surrounding the discs.
Acute toxicity study
The oral acute toxicity study of PD extract was carried out as per the OECD guideline 423 (OECD, 2002). In brief, a single animal was administered a high dose of the extract (1,000 mg/kg) and observed for up to 48 h. Following the absence of toxicity signs (body weight variation, salivation, tremors, convulsions, diarrhea, death.) an additional four animals were administered the same dose and observed for comparison with the normal group. A control group of mice (n = 5) received distilled water in the same volume as that of the treated group. All animas were closely observed at a regular interval for 14 days.
In vivo phagocytic activity
The phagocytic activity of PD extract was evaluated in vivo according to Sahu et al. (2010) with slight modifications. Mice were divided into seven groups of five animals each. Animal in the control group (CON) received the vehicle (0.9%w/v NaCl), whereas animals in other groups received i.p of PD extract suspended in 0.9%w/v normal saline at doses of 30, 50, 70, 100, 150, and 200 mg/kg body weight, respectively. After 5 days of treatment, mice have injected with carbon ink suspension (10 µl/g body weight) through the tail vein. A blood sample from each mouse (25 μl) was drawn from the orbital vein at 0 and 15 min and immediately lysis in 2 ml of sodium carbonate solution (0.1%). The absorbance of the mixture was measured at 675 nm.
The phagocytic activity is expressed by the phagocytic index K which is calculated by the following equation: K = (ln (OD1) – ln (OD2)) /(t2-t1)
Where, OD1 and OD2 are the optical densities at t1=0 min and t2=15min, respectively.
Statistical analysis
All data are presented as the mean ± SD with each in vitro assay conducted in triplicate samples. One-way analysis of variance (ANOVA) followed by a Tukey's multiple comparison test was used to assess statistical significance using SPSS software (version 25.0). The values of P< 0.05 were considered statistically significant.
RESULTS
Plant extraction yield
The date fruits were extracted with 70%v/v ethanol and the obtained yield from 50 g was 44.10%.
Antioxidant activities of PD extract
The antioxidant activity of the PD extract evaluated through the three previously mentioned methods, is presented in Table 1. In all assays, antioxidant activity increased in a dose dependent manner. The PD extract displayed robust free-radical-scavenging activity, ranging from 60.51% to 70.17% of DPPH radicals scavenged. Moreover, the extract exhibited efficient chelation of ferrous ions and demonstrated notable reducing activity of copper ions. However, the PD extract showed less antioxidant activities than those observed with the standard solutions.
Table 1. Antioxidant activity of Phoenix dactylifera fruit extract.
|
|
IC50 (g/L) |
A0.5 (g/L) |
|
|
DPPH |
Fe2+ ion chelating |
CUPRAC |
|
|
PD extracta |
0.890 ± 0.300 |
0.560 ± 0.240 |
1.450 ± 0.150 |
|
Ascorbic acid |
0.110 ± 0.020 |
- |
- |
|
BHAb |
- |
- |
0.003 ± 0.002 |
|
EDTAc |
- |
0.010 ± 0.004 |
- |
Note: Values are means ± standard deviation of three parallel measurements. a: Phoenix dactylifera extract, b: butylated hydroxyanisole, c: ethylenediamine tetra-acetic acid.
Antibacterial activity
The antibacterial activity of PD extract against four strains of human pathogenic bacteria is presented in Table 2. The extract exhibited an inhibitory growth activity against all tested strains at a concentration of 200 mg/ml. The results showed maximum inhibition against Gram positive bacteria, with the highest zone of inhibition recorded against S. pneumonia (23.3 ± 0.3 mm). The lowest zone of inhibition was observed against E.coli with 14.1 ± 0.3 mm.
Table 2. Antibacterial activity of Phoenix dactylifera fruit extract.
|
|
Zone of inhibition (mm) |
|
|
PD extracta |
Positive controlb |
|
|
Escherichia coli |
14.1 ± 0.3 |
18.1 ± 0.2 |
|
Pseudomonas aeruginosa |
17.9 ± 0.3 |
21.2 ± 0.3 |
|
Staphylococcus aureus |
19.4 ± 0.4 |
22.8 ± 0.4 |
|
Streptococcus pneumoniae |
23.3 ± 0.3 |
24.7 ± 0.2 |
Note: Values are the means of three replicates ± SD, a: Phoenix dactylifera extract, b: Chloramphenicol (10 µg/disc).
Acute toxicity study
The extract was found to be safe up to 1,000 mg/kg body weight, and all mice were free of any toxicity signs during the observation period of 14 days.
In vivo phagocytic activity
As shown in Figure 1, the phagocytic index exhibited a notable increase in a dose-dependent manner. Mice pretreated with PD extract at a dose of 150 mg/ml demonstrated a significantly higher phagocytic index (P<0.001) compared to CON and the groups treated with 30, 50, and 70 mg/kg doses. Notably, there was no significant difference observed between the 150 mg/kg and 200 mg/kg doses. Accordingly, the half-time carbon in the bloodstream significantly decreased in a dose-dependent manner (Figure 2), with the shortest durations recorded at the dose of 150 mg/mg, with the corresponding value of 8.2 min. This value was significantly different from that recorded for the control group (25.4 min) with P<0.001.
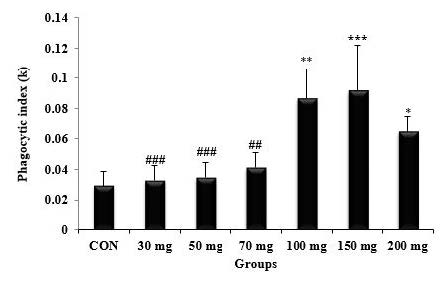
Figure 1. Effect of Phoenix dactylifera fruit extract on phagocytic activity.
n = 5, values are the mean ± SD, *P˂ 0.05, **P˂ 0.01, ***P˂ 0.001 vs control group. ##P˂ 0.01, ###P˂ 0.001 vs 150 mg/kg group.
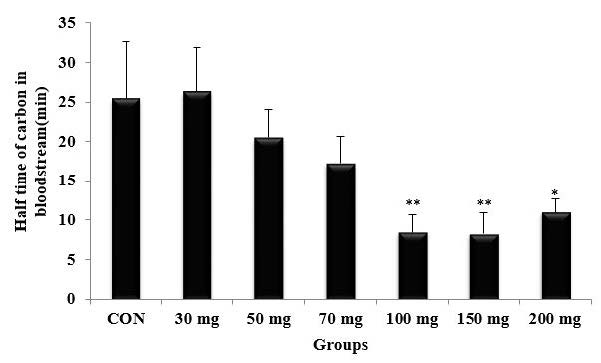
Figure 2. Effect of Phoenix dactylifera fruit extract on the half time of carbon in the bloodstream. n = 5, values are the mean ± SD, *P˂0.05, **P˂0.01 vs control group.
DISCUSSION
The association between healthy consumption of fruit and low risk of chronic diseases has drawn increased interest. Numerous studies have underscored the natural compounds' ability to suppress free-radical generation and reducing oxidative stress (Hussain et al., 2020). They are also seen as promising sources for novel medicines intended to enhance the immune function and resist microbial growth. Therefore, our study was undertaken to provide insight into a less-studied variety of date fruits with potential antioxidant, antimicrobial, and immunomodulatory activities.
The date fruit was extracted with 70%v/v ethanol to obtain a higher extraction which yielded a higher extraction rate and a diverse array of compounds known for their versatile biological activities (Kadum et al., 2019).
Due to the extensive biodiversity among plant varieties, antioxidant molecules may act through three principal mechanisms: scavenging or reducing free radicals and chelation of transition metals. Thus, it is recommended to evaluate the in vitro antioxidant capacity of tested samples using various analytical methods (Mamache et al., 2020). In our study, the antioxidant activity of the PD extract was evaluated using three different methods (DPPH, Ferrous chelating and CUPRAC assays). The DPPH assay is easy, rapid, inexpensive and commonly applied to determine the antioxidant capacity of the natural products with the advantage of being unaffected by side reactions (Zihad et al., 2021; Marliani et al., 2022). It is based on the measurement of the scavenging capacity of antioxidants found in the extract towards DPPH radical free radicals (Jadid et al., 2017). Ferrous ion chelating activity is very important since it reduces the metal concentration and the subsequent oxidative damage. Chelating agents could reduce redox potential thereby stabilizing the oxidized metal ion (Gulcin and Elwasel, 2022). In the CUPRAC method, the CUPRAC reagent (chromogenic), bis(neocuproine)copper(II) chloride (Cu(II)-Nc) is reduced by antioxidants present in the plant extract generating a colored product (cuprak chromophore) bis(neocuproine) copper(I) cation (Cu(I)-Nc) (Özyürek et al., 2011). Furthermore, the CUPRAC assay provides a better evaluation of in vitro antioxidant ability (Hachani et al., 2018). Even though these methods were based on different mechanisms of the antioxidant assay, the PD extract showed an important antioxidant activity in all the tests. This result was in agreement with Mansouri et al. (2005), Benmeddour et al. (2013), and Zhang et al. (2017) who reported that PD extract has a proton donating ability and could serve as free radical inhibitors or scavengers activity. Numerous studies have linked the presence of phenolics, carotenoids, and anthocyanins in date fruit with its antioxidant capacity (Benmeddour et al., 2013; Al-Mamary et al., 2014; Zihad et al., 2021). Mansouri et al. (2005) demonstrated the presence of p-coumaric, ferulic and sinapic acids and some cinnamic acid derivatives in different varieties of Algerian date fruits. Another study of Kadum et al. (2019) correlated the strongest antioxidant activity of Piyarom and Rabbi varieties to the higher concentration of ascorbic acid, epicatechin, citric acid, and gallic acid in these varieties compared to Deglet Nour, Ajwa, and Anbara varieties. Hence, we propose that the antioxidant activity of date fruit is due to the synergistic and additive effects of multiple compounds present in the extract.
Bacterial infections have been of major concern to public health due to the spread of antimicrobial resistance. Regarding their ability to produce a variety of bioactive compounds, many medicinal plants have been tested and identified as good sources of natural antimicrobial substances and as a potential treatment of infections by resistant pathogens (Manandhar et al., 2019). The PD extract showed an inhibitory growth activity against all tested strains (E. coli, P. aeruginosa, S. aureus, and S. pneumoniae). This was in line with prior studies (Al-Dhaihan and Bhat, 2012; El Sohaimy et al., 2015; Sani et al., 2017) reported the sensitivity of both Gram positive and Gram negative bacteria to date fruit extract. In addition, several studies (Bonilla et Sobral, 2017; Likittrakulwong et al., 2023) reported that Gram negative bacteria are more resistant to plant extract which is in agreement with our findings. The variation on susceptibility to PD extract might be attributed to the presence of an outer complex membrane in Gram negative bacteria which blocked the passage of active components present in the extract such as phenolics, flavonoids and terpenoids (Mai-Prochnow et al., 2016; Tangjitjaroenkun et al., 2017). Moreover, phenolic compounds may interact with proteins of the microbial cell membrane and/or enzymes causing cell lysis (Mostafa et al., 2018).
Despite decades that 35% of modern drugs are based on natural products such as Aspirin (Ephedrine), and Paclitaxel (Artemisinin) (Calixto, 2019), natural immunomodulators are less studied. Immunomodulators stimulate the body’s natural defense against pathogens and maintain immune-system homeostasis. The reticuloendothelial system (RES, also known as the mononuclear phagocytic system, or MPS) is a diffuse system of phagocytic cells that provides the first body’s line of defense. The main role of RES is to capture and destroy unwanted microorganisms and enhance the innate (nonspecific) immune response. In our study, the carbon clearance test was realized to evaluate the effect of PD extract on RES. When ink containing colloidal carbon is administered intravenously, macrophages engulf the carbon particles of the ink, and the rate at which the carbon particles are cleared from the bloodstream is known as the phagocytic index (George et al., 2014). The increase in the phagocytic index after injection of PD extract reflects the enhancement of the phagocytosis activity of the RES. Our results are in line with prior studies that showed an increment in the phagocytic index when animals were pretreated with medicinal plants (Patel and Asdaq, 2010; Chan-Zapata et al., 2018; Alves et al., 2020; Sakhri et al., 2021). In addition, a study conducted by Karasawa et al. (2011) demonstrated that the hot water extract of date fruit stimulates IL-12 produced by macrophages, which leads to the activation of NK cells and differentiation of naive T cells into Th1 cells thereby enhancing the cellular immune system. The immunomodulatory activity of PD extract could be attributed to various phytoconstituents. Elhazmi et al, (2021) reported that polysaccharides, terpenoids, flavonoids, alkaloids, glycosides, and lactones are the most important phytochemicals, which provide the immunomodulation activity of medicinal plants. Therefore, date fruit could be used as an effective immunomodulator when the immune system is compromised to improve both innate and cell-mediated immune response or for preventing some diseases.
CONCLUSION
The scientific results obtained from this study enhance our knowledge of the health-promoting effects of date fruit in the protection against the oxidation of biological macromolecules and indicate potential antibacterial effects against microbial infections. In addition, these results lead us to believe that the PD extract represents a promising tool for the development of safe and effective immunomodulators as remedies to prevent and treat inflammatory and immune-related diseases. Further purification and isolation of the bioactive compounds in PD extract would provide possible identification of the mechanism of action and possible lead compounds for the development of new drugs.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Hadjira Sakhri for the contribution in collecting fruit samples.
AUTHOR CONTRIBUTIONS
Afaf Sakhri designed the study, Afaf Sakhri, Fatma Zahra Sakhri and Chawki Bensouici performed the experiments, Afaf Sakhri analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
REFERENCES
Abdelbaky, A.S., Tammam, M.A., Ali, M.Y., Sharaky, M., Selim, K., Semida, W.M., Abd El-Mageed, T.A., Ramadan, M.F., Oraby, H.F., Diab, Y.M. 2023. Antioxidant and anticancer assessment and phytochemical investigation of three varieties of date fruits. Metabolites. 13: 816.
Abdul‐Hamid, N.A., Maulidiani, M., Mediani, A., Yahya, U.I.I., Ismail, I.S., Tham, C.L., Shadid, K. and Abas, F. 2018. Physicochemical characteristics, nutritional composition, and phytochemical profiles of nine Algerian date palm fruit (Phoenix dactylifera L.) varieties. Journal of Food Biochemistry. 42: e12663.
Al-Alawi, R. A., Al-Mashiqri, J. H., Al-Nadabi, J. S., Al-Shihi, B. I., and Baqi, Y. 2017. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Frontiers in Plant Science. 8: 845.
Al-Daihan, S., and Bhat, R. S. 2012. Antibacterial activities of extracts of leaf, fruit, seed and bark of Phoenix dactylifera. African Journal of Biotechnology. 11: 10021-10025.
Al-Jasass, F. M., Siddiq, M., and Sogi, D. S. 2015. Antioxidants activity and color evaluation of date fruit of selected cultivars commercially available in the United States. Advances in Chemistry. 2015: 1-5.
Al-Mamary, M., Al-Habori, M., and Al-Zubairi, A. S. 2014. The in vitro antioxidant activity of different types of palm dates (Phoenix dactylifera) syrups. Arabian Journal of Chemistry. 7: 964-971.
Al-Qarawi, A. A., Abdel-Rahman, H., Ali, B. H., Mousa, H. M., and El-Mougy, S. A. 2005. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. Journal of Ethnopharmacology: 98: 313-317.
Al-Shwyeh, H.A. 2019. Date palm (Phoenix dactylifera L.) fruit as potential antioxidant and antimicrobial agents. Journal of Pharmacy and Bioallied Sciences. 11:1-11.
Alalwan, T.A., Perna, S., Mandeel, Q.A., Abdulhadi, A., Alsayyad, A.S., D’Antona, G., Negro, M., Riva, A., Petrangolini, G., Allegrini, P. and Rondanelli, M. 2020. Effects of daily low-dose date consumption on glycemic control, lipid profile, and quality of life in adults with pre-and type 2 diabetes: A randomized controlled trial. Nutrients.12: 217.
Albakhit, S., Khademvatan, S., Doudi, M., and Foroutan-Rad, M. 2016. Antileishmanial activity of date (Phoenix dactylifera L.) fruit and pit extracts in vitro. Journal of Evidence-Based Complementary & Alternative Medicine. 21: NP98-NP102.
Alhaider, I. A., Mohamed, M. E., Ahmed, K. K. M., and Kumar, A. H. 2017. Date palm (Phoenix dactylifera) fruits as a potential cardioprotective agent: The role of circulating progenitor cells. Frontiers in Pharmacology. 8: 592.
Alhazmi, H.A., Najmi, A., Javed, S.A., Sultana, S., Al Bratty, M., Makeen, H.A., Meraya, A.M., Ahsan, W., Mohan, S., Taha, M.M. and Khalid, A. 2021. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Frontiers in Immunology. 12: 637553.
Ali Haimoud, S., Allem, R., and Merouane, A. 2016. Antioxidant and anti‐inflammatory properties of widely consumed date palm (Phoenix dactylifera L.) Fruit varieties in Algerian oases. Journal of Food Biochemistry. 40: 463-471.
Allahyari, S., Pakbin, B., Amani, Z., Mahmoudi, R., Hamidiyan, G., Peymani, A., Qajarbeygi, P. and Mousavi, S., 2021. Antiviral activity of Phoenix dactylifera extracts against herpes simplex virus type 1: An animal study. Comparative Clinical Pathology. 30: 945-951.
Alshwyeh, H. A. 2020. Phenolic profiling and antibacterial potential of Saudi Arabian native date palm (Phoenix dactylifera) cultivars. International Journal of Food Properties. 23: 627-638.
Alves, S.F., Gomes, C.M., de Oliveira, M.G., de Andrade, W.M., Moreira, L.C., Borges, L.L., da Silva, C.C., de Souza, G.O., da da Silva, V.B., Valadares, M.C. and Bara, M.T.F.F. 2020. Cytotoxicity, phagocytic activity, and leishmanicidal potential of extract standardized in geranylgeraniol obtained from the fruit of Pterodon emarginatus Vogel. Pharmacognosy Magazine. 16: 140.
Apak, R., Güçlü, K., Özyürek, M., and Karademir, S. E. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry. 52: 7970-7981.
Assirey, E.A. 2021. The chemical composition, total phenolic and antioxidant content of four date palm Saudi cultivars. Journal of Taibah University for Science. 15:282-287.
Bano, Y., Rakha, A., Khan, M.I., Asgher, M. 2022. Chemical composition and antioxidant activity of date (Phoenix dactylifera L.) varieties at various maturity stages. Food Science and Technology. 42:e29022.
Benmeddour, Z., Mehinagic, E., Le Meurlay, D., and Louaileche, H. 2013. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: A comparative study. Journal of Functional Foods. 5: 346-354.
Bonilla, J., and Sobral, P. J. D. A. 2017. Antioxidant and antimicrobial properties of ethanolic extracts of Guarana, Boldo, Rosemary and Cinnamon. Brazilian Journal of Food Technology. 20: e2016024.
Calixto, J. B. 2019. The role of natural products in modern drug discovery. Anais da Academia Brasileira de Ciências. 91: e20190105.
Chan-Zapata, I., Canul-Canche, J., Fernández-Martín, K., Martín-Quintal, Z., Torres-Romero, J.C., Lara-Riegos, J.C., Ramírez-Camacho, M.A. and Arana-Argáez, V.E. 2018. Immunomodulatory effects of the methanolic extract from Pouteria campechiana leaves in macrophage functions. Food and Agricultural Immunology. 29: 386-399.
Echegaray, N., Pateiro, M., Gullon, B., Amarowicz, R., Misihairabgwi, J. M., and Lorenzo, J. M. 2020. Phoenix dactylifera products in human health–A review. Trends in Food Science & Technology. 105: 238-250.
El Arem, A., Thouri, A., Zekri, M., Saafi, E. B., Ghrairi, F., Zakhama, A., and Achour, L. 2014. Nephroprotective effect of date fruit extract against dichloroacetic acid exposure in adult rats. Food and Chemical Toxicology. 65: 177-184.
El Hilaly, J., Ennassir, J., Benlyas, M., Alem, C., Amarouch, M. Y., and Filali-Zegzouti, Y. 2018. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. Journal of King Saud University-Science. 30: 519-526.
El Sohaimy, S. A., Abdelwahab, A. E., Brennan, C. S., and Aboul-Enein, A. M. 2015. Phenolic content, antioxidant and antimicrobial activities of Egyptian date palm (Phoenix dactylifera L.) fruits. Australian Journal of Basic and Applied Sciences. 9: 141-147.
FAO. (2020). FAOSTAT statistical database. Livestock primary. From https://www.fao.org/faostat/en/#compare (Accessed 05 February 2023).
Farag, M. A., Otify, A., and Baky, M. H. 2021. Phoenix Dactylifera L. date fruit By-products outgoing and potential novel trends of phytochemical, nutritive and medicinal merits. Food Reviews International. 39: 488-510
Fatani, A.M., Baothman, O.A., Shash, L.S., Abuaraki, H.A., Zeyadi, M.A., Hosawi, S.B., Altayb, H.N. and Abo-Golayel, M.K. 2022. Hepatoprotective effect of date palm fruit extract against doxorubicin intoxication in Wistar rats: In vivo and in silico studies. Asian Pacific Journal of Tropical Biomedicine. 12: 357.
Gantait, S., El-Dawayati, M. M., Panigrahi, J., Labrooy, C., and Verma, S. K. 2018. The retrospect and prospect of the applications of biotechnology in Phoenix dactylifera L. Applied Microbiology and Biotechnology. 102: 8229-8259.
George, A., Chinnappan, S., Choudhary, Y., Bommu, P., and Sridhar, M. 2014. Immunomodulatory activity of an aqueous extract of Polygonum minus Huds on Swiss albino mice using carbon clearance assay. Asian Pacific Journal of Tropical Disease. 4: 398-400.
Gulcin, İ., and Alwasel, S. H. 2022. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes. 10: 132.
Hachani, S., Hamia, C., Boukhalkhal, S., Silva, A. M., Djeridane, A., and Yousfi, M. 2018. Morphological, physico-chemical characteristics and effects of extraction solvents on UHPLC-DAD-ESI-MSn profiling of phenolic contents and antioxidant activities of five date cultivars (Phoenix dactylifera L.) growing in Algeria. NFS Journal. 13: 10-22.
Hussain, M. I., Farooq, M., and Syed, Q. A. 2020. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)–A review. Food Bioscience. 34: 100509.
Islam, S., Akhtar, M., Parvez, S., Alam, J., and Alam, F. M. 2013. Antitumor and antibacterial activity of a crude methanol leaf extract of Vitex negundo L. Archives of Biological Sciences. 65: 229-238.
Jadid, N., Hidayati, D., Hartanti, S. R., Arraniry, B. A., Rachman, R. Y., and Wikanta, W. 2017. Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. PP. 020019. In: Setiawan, E., Muzaki, F. K., Saputro, T. B., Sa’adah, N. N., Desmawati, I., Ashuri, N. M., and Alami, N. H. (eds) Proceeding of International Biology Conference 2016: Biodiversity and Biotechnology for Human Welfare. Surabaya, 15 Oct-2016. AIP Conference Proceedings. 1854.
Kadum, H., Hamid, A. A., Abas, F., Ramli, N. S., Mohammed, A. K. S., Muhialdin, B. J., and Jaafar, A. H. 2019. Bioactive compounds responsible for antioxidant activity of different varieties of date (Phoenix dactylifera L.) elucidated by 1H-NMR based metabolomics. International Journal of Food Properties. 22: 462-476.
Karasawa, K., Uzuhashi, Y., Hirota, M., and Otani, H. 2011. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. Journal of Agricultural and Food Chemistry. 59: 11287-11293.
Kim, G. H., Kim, J. E., Rhie, S. J., and Yoon, S. 2015. The role of oxidative stress in neurodegenerative diseases. Experimental Neurobiology. 24: 325.
Likittrakulwong, W., Chanburee, S., Kitpot, T., Ninjiaranai, P., and Pongpamorn, P. 2023. Phytochemical properties, in vitro antimicrobial, and bioactive compounds of banana peel extractions using GC-MS. Natural and Life Sciences Communications. 22: e2023021.
Mai-Prochnow, A., Clauson, M., Hong, J., and Murphy, A. B. 2016. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Scientific Reports. 6: 38610.
Majhenič, L., Škerget, M., and Knez, Ž. 2007. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chemistry. 104: 1258-1268.
Mamache, W., Amira, S., Ben Souici, C., Laouer, H., and Benchikh, F. 2020. In vitro antioxidant, anticholinesterases, anti‐α‐amylase, and anti‐α‐glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. Journal of Food Biochemistry. 44: e13472.
Manandhar, S., Luitel, S., and Dahal, R. K. 2019. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. Journal of Tropical Medicine. 2019:1895340.
Mansouri, A., Embarek, G., Kokkalou, E., and Kefalas, P. 2005. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chemistry. 89: 411–420.
Marliani, N., Artika, I. M., and Nurcholis, W. 2022. Optimization extraction for total phenolic, flavonoid contents, and antioxidant activity with different solvents and UPLC-MS/MS metabolite profiling of Justicia gendarussa Burm. Chiang Mai University Journal of Natural Sciences. 21: e2022046.
Mostafa, A. A., Al-Askar, A. A., Almaary, K. S., Dawoud, T. M., Sholkamy, E. N., and Bakri, M. M. 2018. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi Journal of Biological Sciences. 25: 361-366.
Nasiri, M., Gheibi, Z., Miri, A., Rahmani, J., Asadi, M., Sadeghi, O., Maleki, V., and Khodadost, M. 2019. Effects of consuming date fruits (Phoenix dactylifera Linn) on gestation, labor, and delivery: An updated systematic review and meta-analysis of clinical trials. Complementary Therapies in Medicine. 45: 71-84.
OECD (2002), test N°2.423: Acute oral toxicity-acute toxic class method, OECD guidelines for testing of chemicals, section 4, OECD publishing, Paris.
Ourradi, H., Ennahli, S., Martos, M.V., Hernadez, F., Dilorenzo, C., Hssaini, L., Elantari, A., Hanine, H. 2021. Proximate composition of polyphenolic, phytochemical, antioxidant activity content and lipid profiles of date palm seeds oils (Phoenix dactylifera L.). Journal of Agriculture and Food Research. 6:100217.
Özyürek, M., Güçlü, K., Tütem, E., Başkan, K.S., Erçağ, E., Çelik, S.E., Baki, S., Yıldız, L., Karaman, Ş. and Apak, R. 2011. A comprehensive review of CUPRAC methodology. Analytical Methods. 3: 2439-2453.
Patel, P., and Asdaq, S. M. B. 2010. Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharmaceutical Journal. 18: 161-165.
Qadoos, H. A., Dhafari, H. S., Al Marzooqi, D. A., Kumarappan, A., and Nazir, A. 2017. Phenolic content and antimicrobial activities of date palm (Phoenix dactylifera L.) fruits and leaves. Food Biology. 6: 11-15.
Rock, W., Rosenblat, M., Borochov-Neori, H., Volkova, N., Judeinstein, S., Elias, M., and Aviram, M. 2009. Effects of date (Phoenix dactylifera L., Medjool or Hallawi Variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status: A pilot study. Journal of Agricultural and Food Chemistry. 57: 8010-8017.
Sahu, M. S., Mali, P. Y., Waikar, S. B., and Rangari, V. D. 2010. Evaluation of immunomodulatory potential of ethanolic extract of Roscoea procera rhizomes in mice. Journal of Pharmacy and Bioallied Sciences. 2: 346.
Sakhri, F. Z., Zerizer, S., and Bensouici, C. 2021. Evaluation of the antioxidant, antidiabetic and immunomodulatory activity of Cydonia oblonga fruit extract. Chiang Mai University Journal of Natural Sciences. 20: e2021052.
Sani, N. M., Abdulkadir, F., and Mujahid, N. S. 2017. Antimicrobial activity of Phoenix dactylifera (date palm) on some selected members of Enterobacteriaceae. Bayero Journal of Pure and Applied Sciences. 10: 36-39.
Tangjitjaroenkun, J., Tangchitcharoenkhul, R., Yahayo, W. and Supabphol, R. 2017. In vitro antimicrobial and cytotoxic activities of mangrove actinomycetes from eastern Thailand. Chiang Mai Journal of Science. 44: 322-337.
Tassoult, M., Kati, D. E., Fernández-Prior, M. Á., Bermúdez-Oria, A., Fernandez-Bolanos, J., and Rodríguez-Gutiérrez, G. 2021. Antioxidant capacity and phenolic and sugar profiles of date fruits extracts from six different Algerian cultivars as influenced by ripening stages and extraction systems. Foods. 10: 503.
Tel, G., Deveci, E., Küçükaydın, S., Özler, M. A., Duru, M. E., and Harmandar, M. 2014. Evaluation of antioxidant activity of Armillaria tabescens, Leucopaxillus gentianeus and Suillus granulatus: The mushroom species from Anatolia. Eurasian Journal of Analytical Chemistry. 8: 136-147.
Zhang, C. R., Aldosari, S. A., Vidyasagar, P. S., Shukla, P., and Nair, M. G. 2017. Health-benefits of date fruits produced in Saudi Arabia based on in vitro antioxidant, anti-inflammatory and human tumor cell proliferation inhibitory assays. Journal of the Saudi Society of Agricultural Sciences. 16: 287-293.
Zihad, S.N.K., Uddin, S.J., Sifat, N., Lovely, F., Rouf, R., Shilpi, J.A., Sheikh, B.Y., and Göransson, U. 2021. Antioxidant properties and phenolic profiling by UPLC-QTOF-MS of Ajwah, Safawy and Sukkari cultivars of date palm. Biochemistry and Biophysics Reports. 25: 100909.
Zineb, G., Boukouada, M., Djeridane, A., Saidi, M., and Yousfi, M. 2012. Screening of antioxidant activity and phenolic compounds of various date palm (Phoenix dactylifera) fruits from Algeria. Mediterranean Journal of Nutrition and Metabolism. 5: 119-126.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Afaf Sakhri1, 2*, Fatma Zahra Sakhri3, and Chawki Bensouici4
1 Laboratoire de Mycologie, de Biotechnologie et de l’activité Microbienne (LaMyBAM), Université des Frères Mentouri Constantine-1, Constantine, Algeria.
2 Department of Medicine, Faculty of Medicine, University of Batna-2, Batna, Algeria.
3 Higher National School of Biotechnology, Constantine, Algeria.
4 Centre de Recherche en Biotechnologie, Constantine, Algeria.
Corresponding author: Afaf Sakhri, E-mail: afsakhri@hotmail.fr; a.sakhri@univ-batna2.dz
ORCID: Afaf Sakhri: https://orcid.org/0000-0002-7266-6464
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: April 26, 2023;
Revised: March 24, 2024;
Accepted: August 28, 2024;
Online First: September 16, 2024

