
Enhanced Transdermal Delivery of Rhein In Vitro Using a Dissolving Microneedle Patch
Kamchai Saepang, Nithi Imon, Sorasak Plangsorn, Tiyada Khuanrupsuan, Benjaporn Buranrat, Tasana Pitaksuteepong, and Supavadee Boontha*Published Date : August 23, 2024
DOI : https://doi.org/10.12982/NLSC.2024.051
Journal Issues : Number 4, October-December 2024
Abstract Transdermal delivery presents an attractive option for rhein (RN) to circumvent the undesirable laxative effect associated with oral administration. However, the stratum corneum (SC) acts primarily as a barrier against RN permeation across the skin. Thus, dissolving microneedle patches (DMNPs) serve as an effective tool for enhancing skin permeation by bypassing the SC. This study focused on preparing a RN-loaded DMNP and evaluating its efficacy in enhancing transdermal RN delivery. The DMNP was fabricated from a 10% (w/w) aqueous polyvinyl alcohol solution using the micro-molding technique. The efficiency of the prepared DMNP in delivering RN across neonatal porcine skin was compared in vitro with a film as a control. The DMNP effectively pierced a skin-simulating Parafilm® M stack and neonatal porcine skin. The DMNP completely dissolved within 30 min after skin insertion. A 1 cm × 1 cm piece of the DMNP contained 96.5 ± 2.0 μg of RN. In a light-protected drug release study, the DMNP released 79.44 ± 2.0% of RN within 8 h and then achieved approximately 100% of RN release at 24 h. The in vitro permeation study showed that the DMNP exhibited a significantly greater RN permeation than the film. The amount of RN permeated at 24 h from the DMNP was 31.6 ± 2.4 μg/cm2, corresponding to a flux of 1.24 ± 0.10 μg/cm2/h, whereas RN permeation from the film was undetectable. Therefore, the DMNP exhibits promising potential for enhancing transdermal RN delivery in vitro and could be used in further in vivo studies.
Keywords: Rhein, Transdermal delivery, Dissolving microneedles, Polyvinyl alcohol
Funding: This research was supported by University of Phayao and Thailand Science Research and Innovation Fund (Fundamental Fund 2024).
Citation: Saepang, K., Imon, N., Plangsorn, S., Khuanrupsuan, T., Buranrat, B., Pitaksuteepong, T., and Boontha, S. 2024. Enhanced transdermal delivery of rhein in vitro using a dissolving microneedle patch. Natural and Life Sciences Communications. 23(4): e2024051.
INTRODUCTION
Rhein (RN), an active metabolite of diacerein for anti-inflammation in the treatment of osteoarthritis, is 4, 5-dihydroxyanthraquinone-2-carboxylic acid found in the roots of rhubarb (Rheum rhabarbarum L.; family Polygonaceae) (Bhat, 2020; Tamura et al., 2001). It has been traditionally used as a laxative and a stomach drug. Presently, various pharmacological activities of RN have been reported, including hepatoprotective, nephroprotective, chondroprotective, anti-inflammatory, antioxidant, anticancer, antidiabetic, antimicrobial, purgative and lipid-lowering activities (Zhou et al., 2015). Despite its wide-ranging pharmacological benefits, its clinical utility is limited due to poor stability and water solubility. RH contains the hydroxyl and carboxyl groups that render it highly light-sensitive in aqueous solutions and susceptible to oxidation-induced decomposition (Jiang et al., 2023). Its water solubility has been reported to be 0.002 mg/mL (Cheng et al., 2015), leading to poor absolute bioavailability of 35%-56% (Mandawgade et al., 2016). Importantly, undesirable laxative side effects associated with unabsorbed and localized RN in the colon can cause discomfort for the patient and low patient compliance (Mandawgade et al., 2016; Sun et al., 2018). Therefore, non-oral RN formulations have been proposed to overcome these problems. Transdermal delivery seems highly preferable due to its patient-friendly nature, its ability for self-administration, and its ability to circumvent the laxative side effects of oral RN that is unabsorbed and localized in the colon (Prausnitz and Langer, 2008). However, poor skin permeation of RN across the stratum corneum (SC) raises concerns regarding the feasibility of transdermal RN delivery due to the SC’s primary barrier function (Feng et al., 2016). Delivery systems have been investigated for improving intradermal (Ebada et al., 2021) and transdermal delivery of RN (Ebada et al., 2021; Al-Hamyari et al., 2024).
Microneedles (MNs) are minimally invasive devices that have become well-known as patient-friendly delivery solutions for difficult-to-deliver drugs. MNs represent an array of micron-size needles with a certain mechanical strength and a length less than 1 mm arranged on a baseplate. The MNs rupture the SC and reversibly form microchannels in the skin after being applied, thus allowing more drug molecules to diffuse to the dermal microcirculation with no pain (Henry et al., 1998). MNs have been categorized into 5 types: solid, hollow, coated, dissolving, and hydrogel-forming MNs. Dissolving MNs (DMNs) have gain considerable attention due to their ease of preparation and improved safety as compared with other kinds of microneedles (coated, metal, or hollow MNs). The DMNs are fabricated from water-soluble polymers, such as poly (vinyl alcohol) (PVA) (Ando et al., 2024), sodium carboxymethyl cellulose (CMC-Na) (Lee et al., 2011), hyaluronic acid (HA) and poly (vinylpyrrolidone) (PVP) (González-Vázquez et al., 2017). The DMNs can be designed as a patch, i.e., a dissolving microneedle patch (DMNP), in which drug molecules are incorporated into a soluble polymeric matrix (Zare et al., 2021). Following application to the skin, the needles of the DMNP dissolve rapidly and release the encapsulated drugs without producing biohazardous waste (Zare et al., 2021; Zhao et al., 2023). Currently, there is a great deal of interest in using DMNPs for transdermal therapeutic delivery (Quinn et al., 2016). Given the SC-restricted RN permeation, the DMNP emerges as a valuable tool for enhancing transdermal RN delivery. The DMNP temporarily ruptures the SC and creates microchannels in the skin, which facilitates RN delivery into the blood circulation and allows for systemic therapeutic effects on deeper or more distant tissues. Therefore, the DMNP appears to be beneficial for RN in the treatment of diseases such as hepatic disease, osteoarthritis, diabetes, atherosclerosis, and various cancers (Zhou et al., 2015). However, no studies have been conducted to investigate the enhanced transdermal RN using DMNP.
The present study aimed to prepare a DMNP loaded with RN. PVA was selected as a polymer matrix to form DMNP due to its well-established safety and bio-compatibility. Additionally, it was expected that transdermal delivery of RN would be enhanced by the prepared DMNP through microchannels of MN-ruptured skin (i.e., SC). For this purpose, the prepared RN-loaded DMNP was evaluated in vitro for the transdermal delivery of RN including skin insertion test, drug release and skin permeation across neonatal porcine skin, which has biochemical similarities to human skin. This is the first report on transdermal delivery of RN using a DMNP.
MATERIALS AND METHODS
Materials
RN (98% purity) was purchased from Xi’an Mainherb Biotech Co., Ltd. (Shannxi, China). Polyvinylalcohol (PVA, MW 13,000-23,000, 87-89% hydrolyzed) was obtained from Sigma-Aldrich Inc. (St. Louis, MO). Sodium carboxymethyl cellulose (CMC-Na, 1990 cps at 1% w/v, 25°C) was provided by Jining Fortune Biotech Co., Ltd (Shandong, China). Methanol, high-performance liquid chromatography (HPLC) grade was supplied by RCl labscan (Bangkok, Thailand). All other chemicals, including triethanolamine, phosphoric acid, sodium chloride, sodium azide, sodium dihydrogen phosphate, disodium hydrogen phosphate, hydrochloric acid, sodium hydroxide and deionized (DI) water were of analytical grade.
Neonatal porcine skin was used as a surrogate membrane for human skin in in vitro skin insertion and permeation studies. The neonatal piglets were provided by a farm located in Phayao province within 24 h after death. The abdominal skin area was carefully shaved and excised with a scalpel. The subcutaneous fat was removed using scissors. The obtained skin was then washed and immersed in 0.01 M phosphate buffered saline (PBS) pH 7.4 for 1 h, then wrapped in aluminum foil and stored at -20 °C until use. The skin was thawed and equilibrated with 0.01 M PBS pH 7.4 for 30 min before beginning each study. The Animal Experimentation Ethics Committee of the University of Phayao (No. UP-AE: 2-001-66) approved the protocol for the use of neonatal porcine skin.
Preparation of RN-loaded DMNP
In an alkaline solution, RN becomes more soluble (Yingngam et al., 2019). Consequently, 500 μL of 1% (w/w) of an aqueous triethanolamine solution was used to dissolve 3 mg of RN. The RN solution (500 μL) was then added to 3 g of an aqueous polymer solution containing 10% (w/w) PVA and 1% (w/w) CMC-Na. Subsequently, 0.3 g of the polymer solution containing RN was placed in a MN mold and filled into the mold cavities by centrifugation at 4500 rpm for 15 min. The polymer solution filled in the MN mold was left to dry under ambient conditions for 24 h. The dried DMNP was demolded, cut into a 1 cm × 1 cm square and stored in a desiccator until further use. The intended amount of RN to incorporate into a 1 cm × 1 cm square DMNP was 100 μg.
Characterization of the DMNP
Needle appearance of DMNP
The needle morphologies of the DMNP were observed under a light microscope (Olympus CX31, Olympus Co., Tokyo, Japan).
In vitro skin insertion test
In vitro skin insertion was conducted using Parafilm® M (PF) and neonatal porcine skin. PF was employed as a skin-simulating material for the DMNP insertion study, as reported in a previous study (Larrañeta et al., 2014). In the case of using PF, the DMNP was manually inserted into an 8-layer PF stack using thumb force for 30 sec. The number of holes created in each PF layer was determined. Additionally, the shape and height of needles were compared before and after PF insertion to evaluate the mechanical strength of the DMNP against thumb force application. For the study involving neonatal porcine skin insertion, the same procedure was replicated with the skin to examine the micropores created in the skin after insertion. To visualize the micropores, the skin treated with the DMNP was stained with 1% (w/v) methylene blue solution.
Dissolution kinetics of needles
RN-loaded DMNPs were applied to neonatal porcine skin. At predetermined time intervals, the DMNPs were withdrawn from the skin. A disappearance of the needle shaft due to the dissolution of needles was observed for each time interval under a light microscope (Olympus CX31, Olympus Co., Tokyo, Japan).
Determination of drug content
The determination of RN in the DMNP was performed by dissolving a 1 cm × 1 cm square of the DMNP in a vial containing 10 mL of deionized water. The resulting solution was filtered through a 0.22 μm syringe membrane filter. The RN content in the filtrate was then analyzed by the validated HPLC method.
In vitro drug release study
In vitro release of RN was carried out using Franz diffusion cells. Spectra-Por®, 3,500 MWCO dialysis membrane (Spectrum Medical Industries, Los Angeles, CA, USA) was used as a membrane separating the DMNP and releasing medium. To simulate physiological fluid and ensure sink conditions, 0.01 M PBS pH 7.4 was used as the releasing medium. The solubility of RN in a phosphate buffer at pH 7.4 was reported to be 431.65 μg/mL (Qin et al., 2018). The receiver compartment of the Franz diffusion cells was filled with 4.5 mL of 0.01 M PBS pH 7.4, which was thermostatically maintained at 37 ± 1 °C. Aliquots of 0.3 mL were taken at 0, 0.5, 1, 2, 4, 6, 8, 20, and 24 h and replaced by 0.3 mL of fresh releasing medium. The samples were diluted to an appropriate concentration and then analyzed using the validated HPLC method. In vitro release studies were conducted under both light-unprotected and protected conditions.
Photostability study of RN
Photostability of RN was investigated in 0.01 M PBS pH 7.4. An RN solution was prepared by dissolving a 1 cm × 1 cm square of the DMNP in a vial containing 10 mL 0.01 M PBS pH 7.4. The solution was stored under both light-unprotected and light-protected conditions with continuous stirring throughout the experiment. Temperature was thermostatically maintained at 37 ± 1 °C. Aliquots of 0.3 mL were collected at predetermined time intervals of 0, 2, 4, 6, 8, 20, and 24 h and analyzed for drug concentration using the HPLC method. The percentage remaining of RN in the solution was calculated based on the initial concentration. Stability profiles were plotted between the percentage remaining of RN versus time.
In vitro permeation study
The permeation of RN across neonatal porcine skin was investigated in vitro using Franz diffusion cells. The skin was shaved and equilibrated in 0.01 M PBS pH 7.4 for 30 min. Before mounting onto the Franz diffusion cells, the equilibrated skin was placed on a 6-mm silicone block with the dermis side facing downwards. The skin surface was then blotted dry using a lint-free tissue paper. The DMNP was applied to the skin with thumb force for 30 sec and secured in place using adhesive tape to prevent the DMNP from being ejected due to the relaxation of constricted skin. Subsequently, the skin assembled with the DMNP was mounted onto the Franz diffusion cells consisting of donor and receiver compartments. The receiver compartment was filled with 4.5 mL 0.01 M PBS pH 7.4. The temperature of the receiver compartment was maintained at 37 °C by a water circulating system to mimic physiological blood circulation and help achieve a normal skin surface temperature (32 ± 1 °C). After 24 h, the amount of RN permeated across the skin into the receiver compartment was quantified by HPLC analysis. As a control, the permeation of RN from a simple film comprising the same composition as the DMNP was also investigated for comparison. Flux of RN was calculated by dividing the amount of RN permeated in the receiver compartment per unit area by time.
HPLC analysis
A reverse phase HPLC method for RN quantification was modified from a previous study (Madni et al., 2020). Hypersil® BDS C18 column (150 mm × 4.6 mm, 0.5 μm particle size) was used in this experiment. A mixture of methanol and 0.01% (v/v) phosphoric acid was used in a 75:25 ratio to make up the mobile phase of the experiment. Flow rate was set at 1 mL/min. The injection volume was 20 μL. Detection wavelength was 254 nm. Prior to use in the content analysis, these HPLC conditions were validated as per the International Committee of Harmonization (ICH) guidelines (ICH Harmonised Tripartite Guideline, 2005). The calibration range was 0.025 to 25 μg/mL with R2 of 0.9998. Limit of detection (LOD) and quantification (LOQ) were 0.0200 ± 0.0004 and 0.0600 ± 0.0011 μg/mL, respectively.
Data analysis
Data were reported as mean ± standard deviation (SD). Statistical analysis was conducted using an independent t-test with a significance level of P<0.05 to compare the differences between the two groups of data.
RESULTS
PVA is a frequently used water-soluble polymer for preparing DMNPs. Previous studies have demonstrated that DMNPs made from PVA solutions with concentrations ranging from 10% to 30% (w/w) exhibit sufficient mechanical strength to create micropores in the skin (Nguyen et al., 2018; Cheng et al., 2022; Ando et al., 2024). In addition, the PVA-based DMNP matrix dissolves in the skin without causing appreciable toxicity upon application (Zhang et al., 2021). In the present study, a 10% (w/w) PVA solution was homogenously mixed with an alkaline RN solution and subjected to centrifugation to prepare the RN-loaded DMNP using the micro-molding technique.
Characterization of RN-loaded DMNP
Figure 1 shows a micrograph of needles located on the baseplate of the prepared RN-loaded DMNP. The needles exhibited a conical shape with pointed tips and their length was 576 ± 9 μm (n = 6).
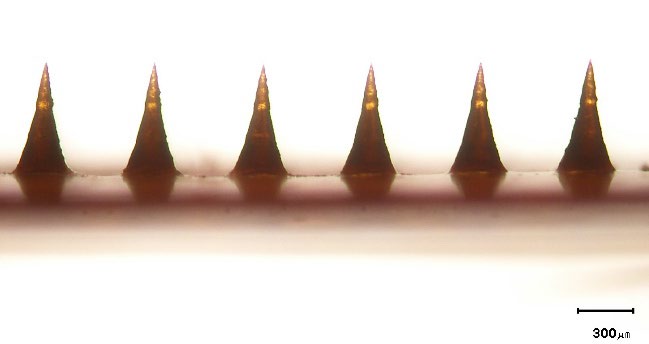
Figure 1. Representative micrograph of needles on the baseplate of the rhein-loaded dissolving microneedle patch. The scale bar represents 300 μm.
The insertion capability of the RN-loaded DMNP into the 8-layer PF stack is shown in Figure 2. The RN-loaded DMNP penetrated the 1st to 4th PF layers. Figure 3 compares the RN-loaded DMNP before and after thumb-forced compression into the 8-layer PF stack. No change in the length of needles was observed between before insertion into the 8-layer PF stack (Figure 3a) and after removal from the 8-layer PF stack (Figure 3b). Upon insertion into the neonatal porcine skin for 30 sec, micropores formed on the skin surface as depicted in Figure 4a. These micropores became clearly visible after staining with methylene blue solution as shown in Figure 4b.
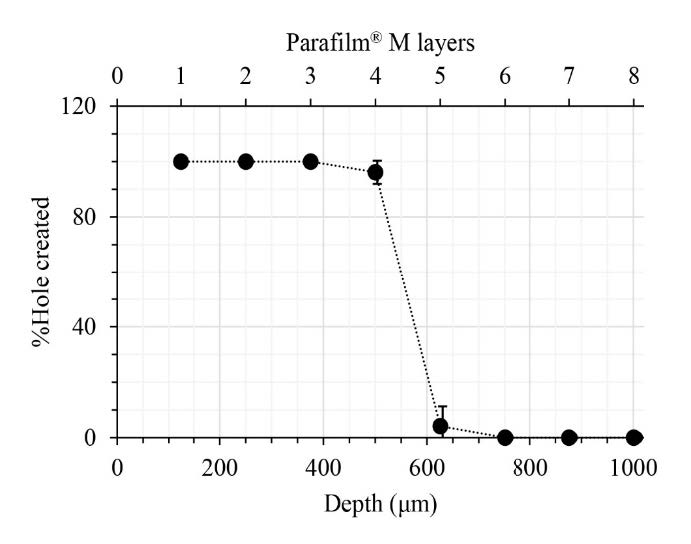
Figure 2. Penetration profile of a rhein-loaded dissolving microneedle patch insertion into the 8-layer Parafilm® M stack. Data represents mean ± SD (n = 3).
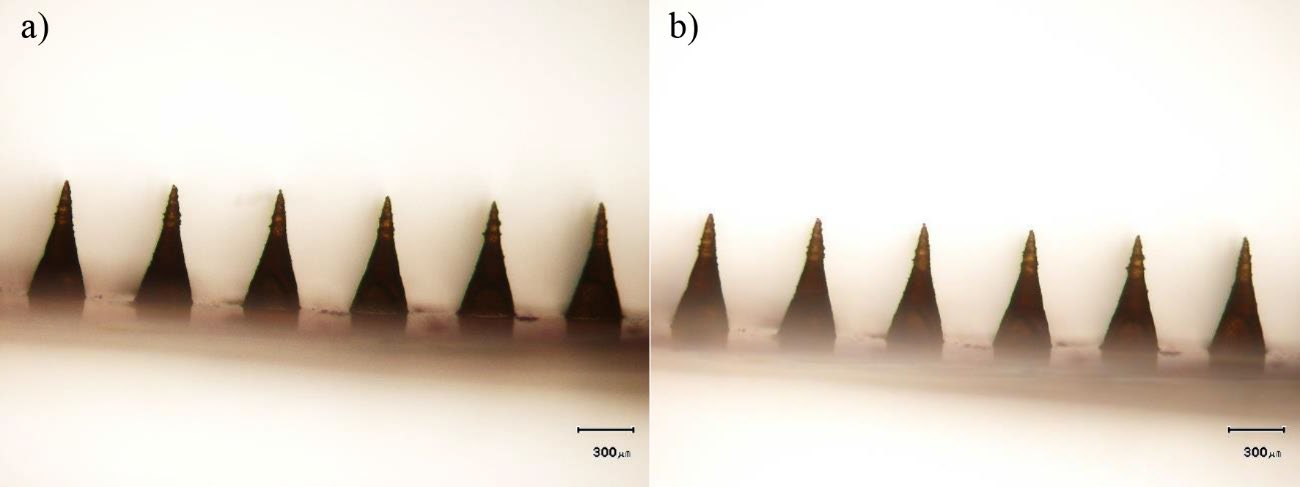
Figure 3. Representative micrographs of needles on the baseplate of rhein-loaded dissolving microneedle patch: a) before and b) after insertion into Parafilm® M. The scale bars represent 300 μm.
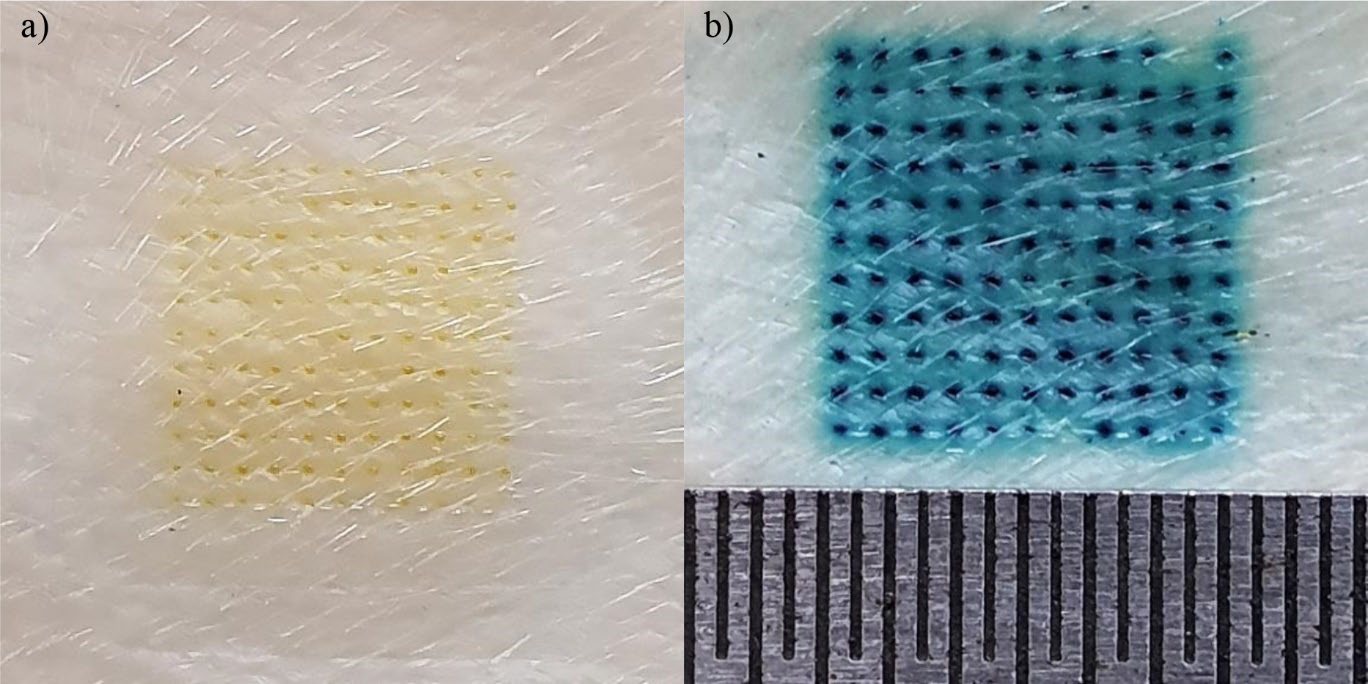
Figure 4. Photographs of skin after rhein-loaded dissolving microneedle patch removal: a) without and b) with methylene blue staining. Figure b shows the scale ruler with intervals of 0.5 mm.
Figure 5 presents the dissolution kinetics of the needles inserted into the skin. The length of the needles gradually decreased over time until they completely disappeared within 30 min.
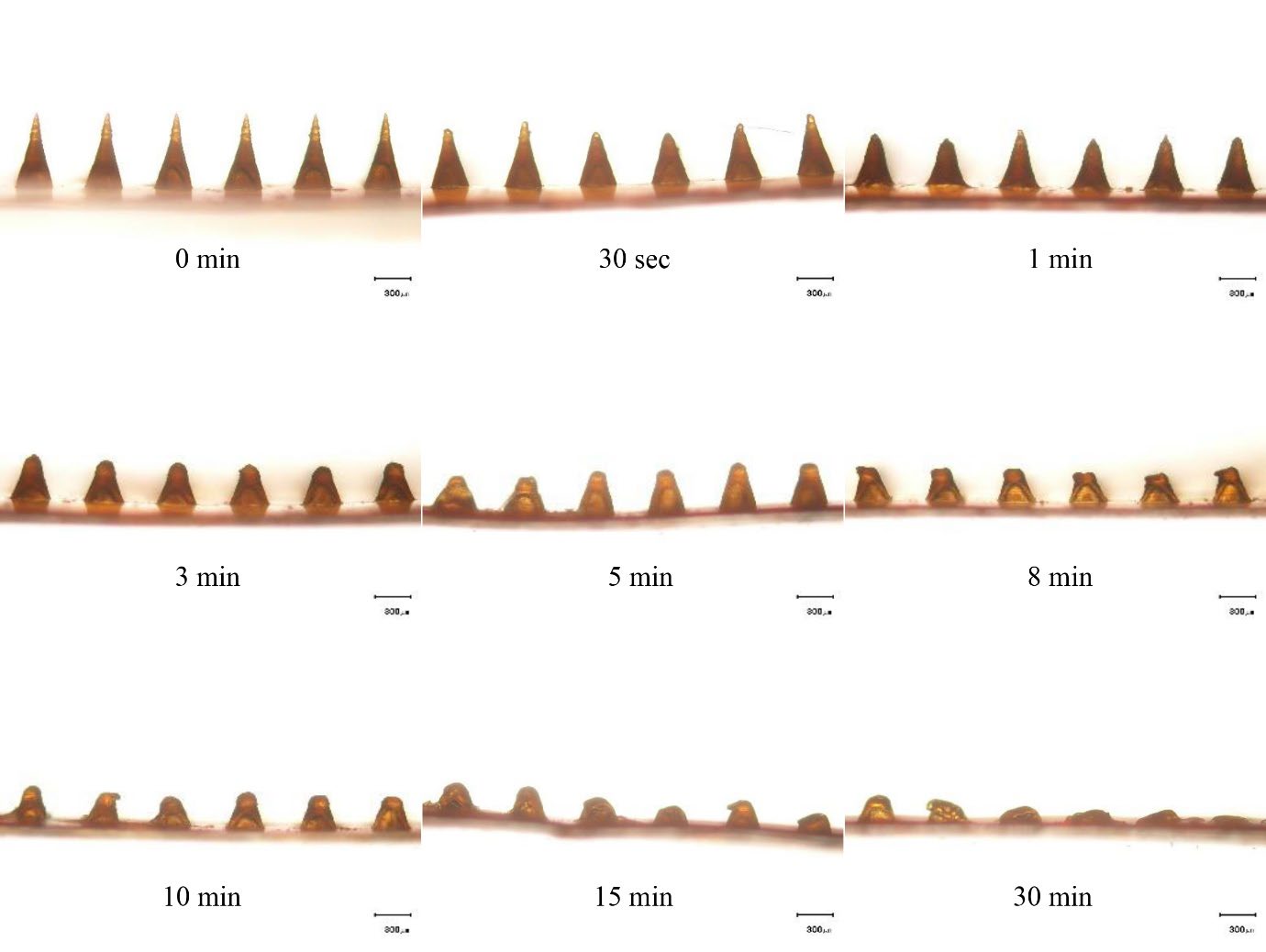
Figure 5. Light micrographs of the rhein-loaded dissolving microneedle patch after removal from neonatal porcine skin at different time intervals. The scale bars represent 300 μm.
Determination of drug content
The content of RN measured in the DMNP was found to be 96.5 ± 2.0 μg/cm2, which was 96.5 ± 2.0 % of the intended loading amount (100 μg/cm2).
In vitro drug release study
Figure 6 presents the release profiles of RN from the DMNP over a 24-h period under conditions both light-unprotected and light-protected. The percentage of the cumulative released amount of RN under conditions protected from light was significantly higher than that under conditions unprotected from light (P<0.05). Under the conditions unprotected from light, the RN released from the DMNP at 8 h and 24 h was 42.7 ± 10.4 % and 44.5 ± 2.9 %, respectively. On the other hand, RN released from the DMNP at 8 h and 24 h, under conditions protected from light was 79.4 ± 8.6 % and 102.9 ± 8.6 %, respectively.
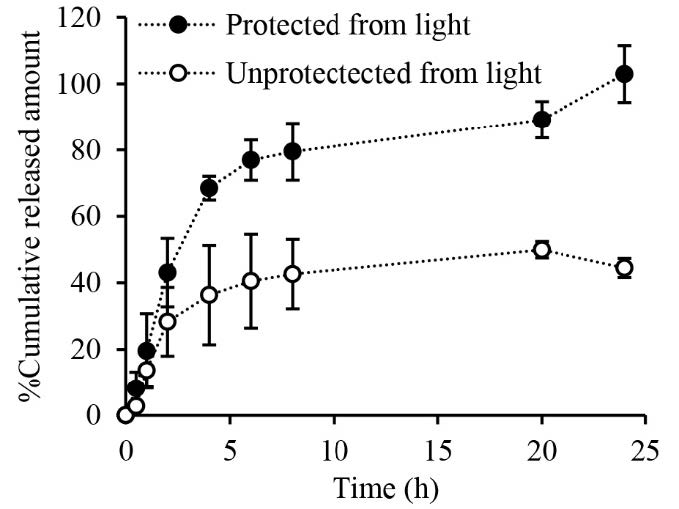
Figure 6. Profiles of rhein released from the dissolving microneedle patch. Data represents mean ± SD (n ≥ 3).
Photostability study of RN
A lower cumulative release of RN from the DMNP under conditions unprotected from light is hypothesized to be due to the degradation of RN. Therefore, a separate photostability study was conducted to elucidate this hypothesis. Under conditions protected from light, the percentage remaining of RN in the solution was essentially the same throughout the experiment (Figure 7). At 24 h, the percentage remaining of RN was 95.4 ± 3.5 %. In contrast, the percentage remaining of RN decreased with time when the solution was exposed to light. At 24 h, only about 36.4 ± 6.5 % remaining of RN was observed.
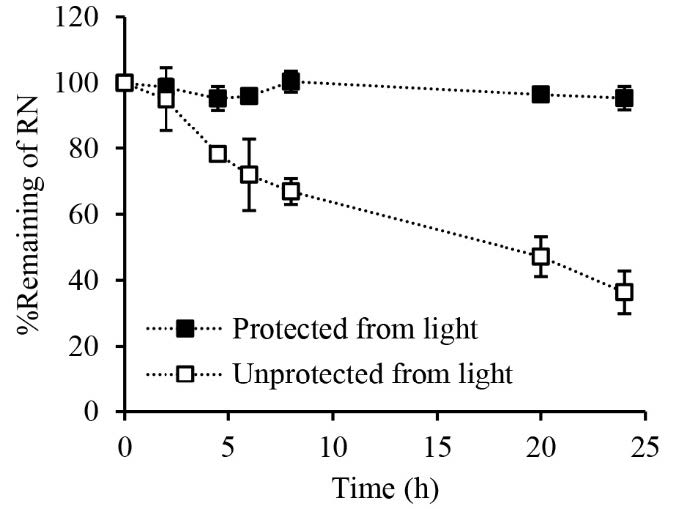
Figure 7. Stability of rhein in 0.01 M phosphate-buffered saline under conditions unprotected and protected from light. Data represents mean ± SD (n = 3).
In vitro permeation study
Permeation parameters of RN permeated across neonatal porcine skin after application of the DMNP and the film for 24 h are shown in Figure 8. The permeation of RN was significantly enhanced with the use of the DMNP compared to the film for both permeated amount and flux (P<0.05). The amount of RN permeated from the film was undetectable and the flux could not be determined. In contrast, the amount of 31.6 ± 2.4 μg/cm2 RN permeated from the DMNP was detected. The flux of RN obtained from the DMNP over the 24-h period was 1.24 ± 0.10 μg/cm2/h.
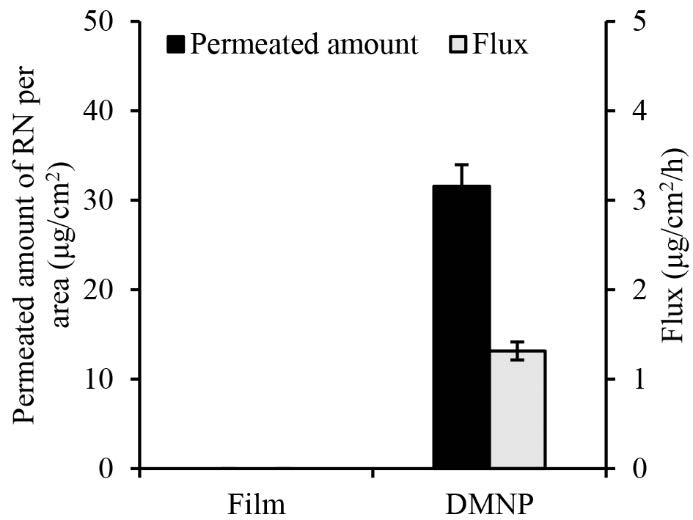
Figure 8. Rhein permeation from the dissolving microneedle patch. Data represents mean ± SD (n = 3).
DISCUSSION
Transdermal delivery of RN offers great potential to circumvent the laxative side effects of orally administered RN that is unabsorbed and localized in the colon. However, the SC is the most significant barrier that must be overcome for successful transdermal RN delivery (Feng et al., 2016). DMNPs serve as a valuable tool for puncturing the SC and facilitating RN permeation. In this study, the DMNP containing RN was prepared from a 10% (w/w) solution of PVA. The selection of PVA as the polymer matrix of the DMNP is based on its water solubility, biocompatibility and mechanical strength (Nguyen et al., 2018; Zhang et al., 2021). RN is poorly soluble in water (0.002 mg/mL) but its solubility improves in an alkaline solution (Cheng
et al., 2015; Yingngam et al., 2019). To achieve a homogeneous mixture of RN and PVA solution for DMNP preparation, RN was first dissolved in the triethanolamine solution before being mixed with the PVA solution.
For the characterization studies of the prepared DMNP, various parameters were examined, including needle appearance, insertion capability, dissolution kinetics of needles in the skin and in vitro release. In this study, the DMNP was prepared using the micro-molding technique that the shape and length of the needles are controlled by the microstructures of the mold (Wang et al., 2016). The MN mold used in the present study contains conical needle-shaped cavities with a length of 600 μm. Microscopic examination of the prepared DMNP revealed that the shape of the needles, i.e., cone-shaped needles (Figure 1), is consistent with the microstructure of the mold cavity used. The length of the needles (576 ± 9 μm) observed in the present study was nearly identical to that of the original mold (600 μm). This minor deviation in the needle length from the mold cavity length can be attributed to the volumetric shrinkage of the PVA solution due to water loss during drying. This occurrence can commonly be found in the fabrication process of polymer MNs (Yang et al., 2012).
PF has been identified as a suitable skin simulant for a facile, rapid and reliable insertion test of MNs (Larrañeta et al., 2014). Accordingly, the insertion capability of the DMNP was first assessed by manually inserting the prepared DMNP into the 8-layer PF stack. The mechanical strength of the DMNP was also determined by comparing any changes in needle shape and height before and after compression of thumb force into the 8-layer PF stack. The DMNP showed good insertion capability reaching the 4th PF layer (Figure 2). One layer of the PF stack has been verified to be 127 μm thick (Larrañeta et al., 2014), indicating the possible penetration of the DMNP into the skin with a depth of 504 μm. Human skin consists of SC (10-15 μm thick), viable epidermis (50-100 μm thick) and dermis (1-2 mm thick) (Andrews et al., 2013). The PF insertion study suggests that the DMNP could reach the viable epidermal and dermal layers. This capability enables RN delivery into the deeper skin layers and facilitates systemic absorption through the dermal microcirculation (Alkilani et al., 2015). No deformation of needles after the PF insertion study (Figure 3) indicates adequate mechanical strength against thumb compression force for MN application to the skin. To ensure the effectiveness of the insertion capability of the DMNP, the DMNP was further inserted into the neonatal porcine skin. From the insertion capability study using neonatal porcine skin, the successful micropore formation and the presence of methylene blue-stained micropores on the skin (Figure 4) prove that the DMNP effectively pierced the SC and created the aqueous microchannels in the skin. The creation of micropores has been investigated as the permeation-promoting mechanism of MNs (Li et al., 2023). Therefore, the prepared DMNP in the present study is a potentially viable means for a further in vitro permeation study.
The RN-loaded DMNP was designed to deliver RN transdermally through dissolution of the polymer matrix and release of the encapsulated RN after insertion into the skin. Prior to application in the in vitro permeation study, the dissolution kinetics of the DMNP in the skin were evaluated by applying the DMNP to neonatal porcine skin and monitoring a reduction in needle length over different time intervals. The observation of the in vitro dissolution kinetics of the needles inserted into the skin indicates that the needle tips start to dissolve immediately (i.e., 30 sec) and fully dissolve within 30 min (Figure 5), suggesting the potential for rapid drug release after skin application. RN content analysis indicated that a total of 96.5 ± 2.0 μg RN was available for delivery from a 1 cm × 1 cm square-shaped DMNP. The in vitro release study revealed a biphasic pattern of RN released from the DMNP. This release pattern exhibited a fast release phase within the first 4 h, followed by a slower release phase extending up to 24 h (Figure 6). Importantly, RN was almost released (102.9 ± 8.6%) from the DMNP under conditions protected from light whereas RN released from the DMNP decreased approximately 2.3 folds (44.5 ± 8.6%) under conditions unprotected from light. Under identical conditions except light exposure, the significant difference in RN release profiles suggests that light induced the degradation of RN when released from the DMNP into the releasing medium (0.01 M PBS pH 7.4). This phenomenon can be attributed to the photosensitivity of RN in nature. The presence of hydroxyl and carboxyl groups in its structure makes RN prone to oxidation and decomposition (Petralito et al., 2009; Jiang et al., 2023). To further elucidate the light-induced RN loss during the release study, a separate photostability study of RN in 0.01 M PBS pH7.4 was then performed. The degradation kinetics of RN in 0.01 M PBS pH7.4 under conditions unprotected and protected from light (Figure 7) clarify that the significant lower RN release under conditions unprotected from light primarily results from photodegradation of RN in the releasing medium. Based on the dissolution kinetics and light-protected release studies, the results suggest that the DMNP in the present study readily dissolves in the interstitial fluid and releases RN from the DMNP matrix after insertion into the skin.
To demonstrate the effectiveness of the prepared DMNP in enhancing RN permeation across the skin, the RN-loaded DMNP was evaluated for in vitro permeation across neonatal porcine skin under conditions protected from light to protect RN degradation. The significant increase in RN permeation from the DMNP compared to that from the film (Figure 8) indicates the capability of the DMNP to enhance RN permeation. These results suggest that MN-ruptured skin is responsible for the enhanced transdermal delivery of RN. Specifically, the skin becomes more permeable to RN when the SC is microporated by the DMNP, which potentially enables RN to exert its effects on deeper or more distant tissues. The mechanism of RN delivery improved by the DMNP can be attributed to the dissolution of the DMNP, which allows RN to be released from the DMNP into the skin. Subsequently, the released RN permeates across the skin through the aqueous microchannels created by the DMNP. This finding is consistent with the enhancement mechanism of MNs elucidated in several studies (Gomaa et al., 2012; Li et al., 2023). As a result, the DMNP has the potential to enhance transdermal RN delivery. However, further studies, such as in vivo pharmacokinetic studies, and skin irritation tests are required before clinical application.
CONCLUSION
This study prepared and evaluated the RN-loaded DMNP for transdermal delivery. PVA was used as the main polymer matrix for DMNP preparation. The prepared DMNP effectively penetrated the skin, created microchannels, dissolved and released the loaded RN. Compared to the film, the DMNP significantly increased permeation of RN across neonatal porcine skin in vitro. Accordingly, the DMNP offers a significant advantage for enhancing transdermal RN delivery. Nevertheless, further studies, such as in vivo studies and skin irritation tests, are required for successful application.
ACKNOWLEDGEMENTS
This research was supported by University of Phayao and Thailand Science Research and Innovation Fund (Fundamental Fund 2024). The authors thank the School of Pharmaceutical Sciences, University of Phayao for providing instruments.
AUTHOR CONTRIBUTIONS
Nithi Imon, Sorasak Plangsorn and Tiyada Khuanrupsuan assisted in conducting the experiments. Kamchai Saepang conceptualized and conducted the experiments, analyzed and visualized the data and wrote the first draft of the manuscript. Benjaporn Buranrat and Tasana Pitaksuteepong reviewed the manuscript and offered insightful criticism and helped with the manuscript revisions. Supavadee Boontha supervised the study, reviewed and edited the original draft manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Al-Hamyari, B., Wang, L., Wang, H., Alafifi, J.H., Kang, S., Wang, Y., Zhang, H., Lv, H., Liao, D., Sun, X., et al. 2024. Development, optimization, and characterization of rhein loaded nanoemulgel for treatment of osteoarthritis. Journal of Drug Delivery Science and Technology. 92: 105330.
Alkilani, A.Z., McCrudden, M.T., and Donnelly, R.F. 2015. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 7(4): 438-470.
Ando, D., Miyatsuji, M., Sakoda, H., Yamamoto, E., Miyazaki, T., Koide, T., Sato, Y., and Izutsu, K.I. 2024. Mechanical characterization of dissolving microneedles: factors affecting physical strength of needles. Pharmaceutics. 16(2): 200.
Ando, D., Ozawa, A., Sakaue, M., Yamamoto, E., Miyazaki, T., Sato, Y., Koide, T., and Izutsu, K.I. 2024. Fabrication and characterization of dissolving microneedles for transdermal drug delivery of apomorphine hydrochloride in Parkinson's disease. Pharmaceutical Research. 41(1): 153-163.
Andrews, S.N., Jeong, E., and Prausnitz, M.R. 2013. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharmaceutical Research. 30(4): 1099-1109.
Bhat, R. 2020. Bioactive compounds of rhubarb (Rheum species). p.239-254. In H. N. Murthy and K. Y. Paek (eds). Bioactive compounds in underutilized vegetables and legumes. Springer Cham, Switzerland.
Cheng, A., Sun, W., Xing, M., Zhang, S., and Gao, Y. 2022. The hygroscopicity of polymer microneedles on the performance of dissolving behavior for transdermal delivery. International Journal of Polymeric Materials and Polymeric Biomaterials. 71(1): 72-78.
Cheng, Y., Wang, D., Zhang, Z., and Wang, Z. 2015. Solubility and solution thermodynamics of rhein in eight pure solvents from (288.15 to 313.15) K. RSC Advances. 5(98): 80548-80552.
Ebada, H.M.K., Nasra, M.M.A., Elnaggar, Y.S.R., and Abdallah, O.Y. 2021. Novel rhein–phospholipid complex targeting skin diseases: Development, in vitro, ex vivo, and in vivo studies. Drug Delivery and Translational Research. 11(3): 1107-1118.
Ebada, H.M.K., Nasra, M.M.A., Elnaggar, Y.S.R., Nassra, R.A., Solaiman, A.A., and Abdallah, O.Y. 2021. Novel rhein integrate transphytosomes as non-invasive local therapy for osteoarthritis to ameliorate cartilage deterioration in mia-arthritic rats. Colloids and Surfaces B: Biointerfaces. 202: 111713.
Feng, L., Ding, M.H., Shen, Q., Sun, Q.H., and Shi, S.L. 2016. Percutaneous permeability of rhein at shenque acupoint in vitro. China Journal of Chinese Materia Medica. 41(8): 1546-1552.
Gomaa, Y.A., El-Khordagui, L.K., Garland, M.J., Donnelly, R.F., McInnes, F., and Meidan, V.M. 2012. Effect of microneedle treatment on the skin permeation of a nanoencapsulated dye. Journal of Pharmacy and Pharmacology. 64(11): 1592-1602.
González-Vázquez, P., Larrañeta, E., McCrudden, M.T.C., Jarrahian, C., Rein-Weston, A., Quintanar-Solares, M., Zehrung, D., McCarthy, H., Courtenay, A.J., and Donnelly, R.F. 2017. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. Journal of Controlled Release. 265: 30-40.
Henry, S., McAllister, D.V., Allen, M.G., and Prausnitz, M.R. 1998. Microfabricated microneedles: A novel approach to transdermal drug delivery. Journal of Pharmaceutical Sciences. 87(8): 922-925.
ICH Harmonised Tripartite Guideline. 2005. Validation of analytical procedures: Text and methodology Q2(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva.
Jiang, Y., Zang, K., Sun, J., Chandrapala, J., Brennan, C., Majzoobi, M., and Zeng, X.A. 2023. Characterization and stability investigation of rhein encapsulated microcapsules using different enteric biopolymers with pullulan and Jiuzao glutelin conjugates via maillard reaction. Food Research International. 172: 113135.
Larrañeta, E., Moore, J., Vicente-Pérez, E.M., González-Vázquez, P., Lutton, R., Woolfson, A.D., and Donnelly, R.F. 2014. A proposed model membrane and test method for microneedle insertion studies. International Journal of Pharmaceutics. 472(1): 65-73.
Lee, J.W., Choi, S.O., Felner, E.I., and Prausnitz, M.R. 2011. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 7(4): 531-539.
Li, H., Peng, Z., Song, Y., Dou, M., Lu, X., Li, M., Zhai, X., Gu, Y., Mamujiang, R., Du, S., et al. 2023. Study of the permeation-promoting effect and mechanism of solid microneedles on different properties of drugs. Drug Delivery. 30(1): 2165737.
Madni, M.A., Khan, M., Rehman, M., Tahir, N., Rai, N., Parveen, F., Saeed, M., Rahim, M., and Shah, H. 2020. Reverse phase high performance liquid chromatographic-diode array detection method for quantification of rhein in microsample of rabbit plasma and application to the pharmacokinetic study. Journal of the Chemical Society of Pakistan. 42: 866-874.
Mandawgade, S.D., Kulkarni, S., Pal, A., Srivastava, S., Padhi, B.K., and Raghuvanshi, R.S. 2016. Development and pharmacokinetic evaluation of new oral formulations of diacerein. Current Drug Delivery. 13(1): 83-89.
Nguyen, H.X., Bozorg, B.D., Kim, Y., Wieber, A., Birk, G., Lubda, D., and Banga, A.K. 2018. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. European Journal of Pharmaceutics and Biopharmaceutics. 129: 88-103.
Petralito, S., Zanardi, I., Memoli, A., Annesini, M.C., and Travagli, V. 2009. Solubility, spectroscopic properties and photostability of rhein/cyclodextrin inclusion complex. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 74(5): 1254-1259.
Prausnitz, M.R., and Langer, R. 2008. Transdermal drug delivery. Nature Biotechnology. 26(11): 1261-1268.
Qin, F., Cai, Y., and Yu, J. 2018. Determination of equilibrium solubility and apparent oil/water partition coefficients of rhein. Herald of Medicine. 37(1): 88-91.
Quinn, H.L., Larrañeta, E., and Donnelly, R.F. 2016. Dissolving microneedles: safety considerations and future perspectives. Therapeutic Delivery. 7(5): 283-285.
Sun, L.L., Jiang, H.B., Liu, B.Y., Li, W.D., Du, A.L., Luo, X.Q., and Li, X.Q. 2018. Effects of rhein on intestinal transmission, colonic electromyography and expression of aquaporin-3 by colonic epithelium cells in constipated mice. International Journal of Clinical and Experimental Pathology. 11(2): 614-623.
Tamura, T., Kosaka, N., Ishiwa, J., Sato, T., Nagase, H., and Ito, A. 2001. Rhein, an active metabolite of diacerein, down-regulates the production of pro-matrix metalloproteinases-1, -3, -9 and -13 and up-regulates the production of tissue inhibitor of metalloproteinase-1 in cultured rabbit articular chondrocytes. Osteoarthritis and Cartilage. 9(3): 257-263.
Wang, Q.L., Zhu, D.D., Chen, Y., and Guo, X.D. 2016. A fabrication method of microneedle molds with controlled microstructures. Materials Science and Engineering C: Materials for Biological Applications. 65: 135-142.
Yang, S., Feng, Y., Zhang, L., Chen, N., Yuan, W., and Jin, T. 2012. A scalable fabrication process of polymer microneedles. International Journal of Nanomedicine. 7: 1415-1422.
Yingngam, B., Zhao, H., Baolin, B., Pongprom, N., and Brantner, A. 2019. Optimization of ultrasonic-assisted extraction and purification of rhein from Cassia fistula pod pulp. Molecules. 24(10): 2013.
Zare, M.R., Khorram, M., Barzegar, S., Sarkari, B., Asgari, Q., Ahadian, S., and Zomorodian, K. 2021. Dissolvable carboxymethyl cellulose/polyvinylpyrrolidone microneedle arrays for transdermal delivery of amphotericin B to treat cutaneous leishmaniasis. International Journal of Biological Macromolecules. 182: 1310-1321.
Zhang, X.P., Wang, B.B., Li, W.X., Fei, W.M., Cui, Y., and Guo, X.D. 2021. In vivo safety assessment, biodistribution and toxicology of polyvinyl alcohol microneedles with 160-day uninterruptedly applications in mice. European Journal of Pharmaceutics and Biopharmaceutics. 160: 1-8.
Zhao, W., Zheng, L., Yang, J., Ma, Z., Tao, X., and Wang, Q. 2023. Dissolving microneedle patch-assisted transdermal delivery of methotrexate improve the therapeutic efficacy of rheumatoid arthritis. Drug Delivery. 30(1): 121-132.
Zhou, Y.X., Xia, W., Yue, W., Peng, C., Rahman, K., and Zhang, H. 2015. Rhein: A review of pharmacological activities. Evidence-Based Complementary and Alternative Medicine. 2015: 578107.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kamchai Saepang1, 2, Nithi Imon1, Sorasak Plangsorn1, Tiyada Khuanrupsuan1, Benjaporn Buranrat2, 3, Tasana Pitaksuteepong4, and Supavadee Boontha1, 2, *
1 Division of Pharmacy and Technology, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
2 Research Group in Herbal and Development of Formulation and Delivery Systems for Elderly Adults and Cancer Treatment, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
3 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand.
4 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Supavadee Boontha, E-mail: supavadee.bo@up.ac.th
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: April 6, 2024;
Revised: July 24, 2024;
Accepted: August 1, 2024;
Online First: August 23, 2024

