
Gingival Crevicular Fluid Volume and Protein Concentration: A Biomarker Tool for Predicting Periodontal Diseases Progression and Severity
Mohammed Faisal Ali and Ghada Ibrahim Taha*Published Date : August 23, 2024
DOI : https://doi.org/10.12982/NLSC.2024.055
Journal Issues : Number 4, October-December 2024
Abstract Periodontitis represents a significant public health challenge due to its complex pathogenesis and prevalence. This inflammatory disorder, driven by an interplay between microbial agents and the host immune response, emphasizes the critical need for early and accurate diagnostics. This study aims to measure the volume of GCF using Periotron® and potential biomarkers (NETs and IL-23) to predict periodontal disease severity and progression.
In this cross-sectional study, 90 subjects (30 controls, 30 with Stage 1 periodontitis, and 30 with Stage 3 periodontitis) were involved at the Periodontics Department of the Dental College Teaching Hospital at Baghdad University. Samples were collected from November 2023 to May 2024. Participants were assessed for periodontal parameters, including Clinical Attachment Loss (CAL), Bleeding on Probing (BOP), and Probing Depth (PD). Gingival Crevicular Fluid (GCF) samples were collected and analyzed to measure the levels of NETs and IL-23 by enzyme-linked immunosorbent assay (ELISA). The GCF volumes were quantified using the Periotron® 8010 device.
A significant increase significantly in levels of NETs and IL-23 were found in stage 3 periodontitis (73.90 ± 6.58), (5.018 ± 1.081) compared to those in stage 1 and healthy controls (43.17 ± 4.47), (3.083 ± 0.569), (19.43 ± 4.63), (1.431 ± 0.586). The increased biomarker levels correlated significantly with higher GCF volumes
(P <0.05).
The findings suggest that NETs and IL-23 are significantly elevated in severe cases of periodontitis and may serve as reliable biomarkers for assessing disease progression. Their quantification could support the development of tailored treatment strategies, potentially improving patient outcomes.
Keywords: Periodontitis, Neutrophil Extracellular Traps, IL-23, Gingival Crevicular Fluid, Periotron®
Citation: Ali, A. F. and Taha, G. I. 2024. Gingival crevicular fluid volume and protein concentration: A biomarker tool for predicting periodontal diseases progression and severity. Natural and Life Sciences Communications. 23(4): e2024055.
INTRODUCTION
Periodontitis is a multifactorial and complex inflammatory disorder that affects the supporting structures of the teeth, leading to progressive attachment loss and bone resorption. This inflammatory disease, if left untreated, can culminate in tooth loss. It is well-recognized that the pathogenesis of periodontitis is modulated by the interaction between microbial agents and the host immune response (Talib and Taha, 2024).
While bacteria are fundamental initiators of the disease, the damage to the periodontal tissues is primarily mediated by the host's inflammatory and immune responses (Hajishengallis, 2014).
To craft an efficacious treatment strategy, understanding the epidemiology of periodontitis is crucial, as it underscores both the probability of the disease's presence and the diagnostic test's utility. Current data suggests a tiered prevalence: approximately 10% of adults grapple with "severe" periodontitis (stage III or IV), another 10% maintain periodontal health, and the remaining 80% exhibit symptoms of either gingivitis or mild to moderate periodontitis (Stage I or II). Notably, while the diagnosis of stages III and IV periodontitis can be straightforward, differentiating between gingivitis and milder forms of periodontitis is more nuanced, necessitating an evaluation of interdental Clinical Attachment Loss (CAL). Such differentiation is paramount, as it aids in distinguishing gingivitis from periodontitis. Thus, the cornerstone of any periodontal health assessment should be the early detection of attachment or bone loss (Tonetti and Sanz, 2019).
Periodontitis diagnosis relies on a comprehensive clinical examination, which includes assessing medical and dental history, tooth mobility, and radiographic evaluation. Clinical examinations primarily concentrate on the assessment of dental cleanliness, gingival condition, probing depth, bleeding upon probing, clinical attachment loss, and alveolar bone condition. Further diagnostic methods may include periodontal microbiological testing, systemic health evaluations through blood analysis, and histological examinations (Trindade et al., 2014). However, the main limitation of these methods is their inherent subjectivity, as they depend on the examiner. The biological mechanisms driving progression from health to disease remain ambiguous, highlighting the urgent need to identify and validate reliable biomarkers. These biomarkers should ideally detect the early stages of periodontitis, potentially even before clinical symptoms manifest, to facilitate timely and effective treatment (Preianò et al., 2020).
Diagnostic tests are generally not essential for distinguishing between inflamed and healthy sites since clinical indices of inflammation adequately correlate with histological inflammation in the periodontium. Nevertheless, from a diagnostic standpoint, it is crucial to differentiate between actively inflamed-progressive sites and less severe, inflamed but stable sites (Ngo et al., 2010). It is now recognized that outcomes of the immune/inflammatory responses in saliva and gingival crevicular fluid (GCF), along with behavioral and environmental factors, significantly influence the clinical presentation of periodontal diseases (Salahuddin and Taha, 2023). Extensive research has focused on identifying molecular markers of periodontitis, with numerous genes, transcripts, proteins, and metabolites linked to the disease. Consequently, GCF has emerged as a key focus in the molecular profiling of periodontal disease (Fernández-Reyes et al., 2023).
The Periotron is a sophisticated electronic device designed to measure gingival crevicular fluid (GCF), periodontal pocket fluid, salivary flow, and saliva thickness. It operates using micro-moisture metering, where an electronic transducer detects the moisture level of a paper strip, called Periopaper, and converts it into a digital readout (Talib and Taha, 2024). The electrical conductivity changes with the moisture level of the strip, allowing the Periotron to convert these readings into clinical conditions and scores, which are then recorded using the gingival index (Fernández-Reyes et al., 2023). The volume of GCF is correlated with the gingival index and probing depth at various tooth sites (Bhardwaj and Prabhuji, 2013). Research has demonstrated that peri-implant crevicular fluid (PICF) volumes are significantly higher at sites with peri-implant diseases than at clinically healthy sites, showing positive correlations with all clinical indices (probing depth, plaque index, gingival index, and bleeding on probing) (Bielemann et al., 2018; Fernández-Reyes et al., 2023).
A new model, the Periotron® 8010, is now available on the market. This model measures the electrical capacitance of the wet paper strip, allowing for the quantification of the amount of gingival crevicular fluid (GCF) and saliva collected on Periopaper® in periotron units (PU). The interaction between the charges of opposite polarity on the electrodes generates an electric field, which in turn influences the polarity of the molecules and thus modifies the capacitance (Ito et al., 2021). Periopaper® also helps detect microbiological or biochemical changes in the GCF (Gunpinar et al., 2017; Pappe et al., 2021). Measuring GCF volume is vital for diagnosing the severity of periodontal disease. Although calibration studies have confirmed the reliability and reproducibility of previous Periotron® models (Fernández-Reyes et al., 2023).
Neutrophils are central to the immune system's defence against microbial pathogens but can also contribute to tissue injury and sterile inflammation. Neutrophils, a type of white blood cell known as leukocytes, play a critical role in responding to infections. They can release Neutrophil Extracellular Traps (NETs)—web-like structures aimed at trapping and neutralizing pathogens (Arteaga-Henríquez et al., 2022). NETs consist of DNA structures adorned with cytosolic, granule, and nuclear protein (Brinkmann et al., 2004). These NETs, secreted by activated neutrophils, play a crucial role in the immune response against infections, functioning to capture and neutralize a variety of pathogens (Arteaga-Henríquez et al., 2022; Panase et al., 2023).
Cytokines are small, soluble proteins that play vital roles in modulating the immune response and inflammation. Periodontal tissue components, including fibroblasts, epithelial cells, and endothelial cells, actively contribute to the production of cytokines during inflammatory reactions (Esteves Lima et al., 2021). The health of the gums relies on the local equilibrium between reactive and suppressor immune cells, as well as their cytokines and mediators. The cytokines IL-6, IL-8, and IL-12 have proinflammatory properties and promote the breakdown of bone tissue, whereas IL-10 has anti-inflammatory actions (Husham and Taha, 2024).
IL-23, primarily synthesized by dendritic cells (DCs) and epithelial cells, has distinctive functions in the development and proliferation of memory T lymphocytes (Yang et al., 2019; Ali and Taha, 2023). IL-23 is known to have a crucial impact on the development and proliferation of Th17 cells (Yu et al., 2021). IL-23 is linked to several inflammatory conditions and has a role in oral disorders (Bianchi and Rogge, 2019).
The researchers demonstrated a substantial increase in the expression of IL-23 messenger RNA (mRNA) in periodontal lesions compared to control locations (Bunte and Beikler, 2019). Both IL-22 and IL-23 are essential mediating cytokines for T-cell-mediated inflammatory responses. The objective of this study is to assess the levels of neutrophil extracellular traps (NETs) and IL-23 in the GCF of patients with Stage 1 and Stage 3 periodontitis and to compare these levels with those in healthy controls. Additionally, to evaluate GCF volume using the Periotron® across all groups to predict periodontal disease progression.
MATERIALS AND METHODS
Study design
This case-control study was conducted at the Periodontics Department of the Dental College Teaching Hospital at Baghdad University, where samples were collected from November 2023 to May 2024. A total of 90 participants was included, divided into two main groups: the study group and the healthy control group. The study group, consisting of 60 patients, was further divided into two subgroups, with 30 patients diagnosed with stage 1 Periodontitis and another 30 with stage 3 Periodontitis. Additionally, 30 individuals with healthy gingiva comprised the healthy control group.
Inclusion criteria:
1. Participants aged 18-60 years.
2. Clinical diagnosis patients of mild or severe periodontitis by a dental surgeon.
3. Absence of autoimmune diseases or Systemic diseases
For control group, absence of clinical signs of periodontal disease and confirmed by a dental surgeon.
Exclusion criteria:
1. Individuals with systemic diseases that can affect periodontal status (e.g., diabetes, rheumatoid arthritis, cardiovascular diseases).
2. Participants with a history of taking antibiotics or anti-inflammatory drugs within the past 3 months.
3. Pregnant or lactating women.
4. Individuals with a history of periodontal treatment or surgery within the past six months.
5. Smokers or individuals using tobacco products.
6. Individuals with oral conditions other than periodontitis (e.g., oral ulcers, tumors).
7. Individuals undergoing orthodontic treatment.
Sample size
It was calculated using the online tool EPITOOLS (Sergeant, 2018), (https://epitools.ausvet.com.au/casecontrols) at alpha level 0.05 for a 95% confidence interval. The calculated sample size for periodontitis (stage 1 and stage 3) groups is 60 with a 2:1 ratio, while it will be 30 for healthy control. So, the total sample size would be 90 (30 control, 30 stage 1 and 30 stage 3 periodontitis groups.
Ethical approval
The study protocol was approved by the scientific committee at the Basic Science Department/College of Dentistry/University of Baghdad, (Reference number: 862, Project number: 862823, Date: 23/11/2023), and all patients were given a piece of detailed information about the study's objectives and informed consent was signed to represent the patient's acceptance in order to indicate their agreement for involved in the study.
Diagnostic categories and oral examination
Periodontitis is categorized based on Ababneh et al., (2019) (Ababneh et al., 2019), into two stages: Stage 1 Periodontitis, which typically presents with 1-2 mm of Clinical Attachment Loss (CAL) and probing depths between 4-5 mm, and Stage 3 Periodontitis, which involves more than 5 mm of CAL with probing depths greater than 6 mm. During oral examinations, several clinical parameters are utilized to assess the presence, severity, and progression of periodontitis. Bleeding on Probing (BOP) occurs when a gentle insertion of a periodontal probe into the sulcus or pocket around a tooth lead to bleeding in inflamed tissues, indicating inflammation and active disease (Lang et al., 1986). Probing Depth (PD) is measured using a calibrated periodontal probe to determine the depth of the sulcus or pocket from the gingival margin to the base of the pocket, with depths greater than 3 mm considered pathological and indicative of advancing periodontitis (Armitage, 2004). Clinical Attachment Loss (CAL) is measured from a fixed point on the tooth, typically the cementoenamel junction (CEJ), to the base of the pocket, incorporating both gingival recession and probing depth to provide a comprehensive assessment of tissue loss (Cairo et al., 2011).
Sample collection
Patients were prepared ninety minutes prior to the sample collection, which took place between 9:00 and 11:00 a.m. They were instructed to refrain from eating and tooth brushing before the procedure. The targeted sites underwent a cleansing process involving rinsing with water, isolation with cotton rolls, and a gentle air spray. "Perio Paper" strips were used to collect fluid samples from the test groups. Supragingival plaque was removed using dry gauze before placing standard paper strips in the sulcus, where they remained for 30 seconds. Any strips that showed signs of blood staining were excluded from the sample set. To maintain the integrity of the samples, the paper strips were immediately placed into sterile Eppendorf tubes containing 0.5 ml of preservative phosphate Buffer Saline (PBS). The tubes were then centrifuged at 3,000 rpm for 10 minutes and stored at -80 °C until they were ready for laboratory analysis (Bhardwaj and Prabhuji, 2013). The detection of Human Neutrophil Extracellular Traps (NET) and Human IL-23 biomarkers was conducted using the Human Enzyme-linked immunosorbent Assay (ELISA) quantitative immunoassay kits (Lot Nos:E23XMB286 and E23UMS852, Feiyuo company, respectively).
Gingival crevicular fluid (GCF) and calculate quantification of mediator concentration by Periotron
An electronic method has been devised for measuring the fluid collected on a “blotter” (Periopaper, PerioPaper®, Oraflow Inc., New York, USA) with the use of an electronic transducer (Periotron, Oraflow Inc., New York, USA). The moisture content of the paper strip influences the conductivity of an electric current and enables a digital output. The data collected by the Periotron device may be translated into specific clinical circumstances and then documented using the gingival index (Table 1) (Attar et al., 2018).
Weighing the strip
Pre-weighed strip is inserted into the gingival crevice & then determined the amount of fluid collected by weighing the sample strip.
Table 1. Translating the Periotron result into the corresponding clinical circumstances and gingival index (Attar et al., 2018).
|
Periotron Reading |
Gingival Inflammation Level |
Gingival Index |
|
(0 – 20) |
Healthy |
0 |
|
(21- 40) |
Mild |
1 |
|
(41- 80) |
Moderate |
2 |
|
(81 – 200) |
Severe |
3 |
Statistical analysis
The data processing utilized Statistical Package for Social Sciences (SPSS) version 26 and Microsoft Excel 2019. Statistical analyses included Chi-square and Mann-Whitney U test, Analysis of Variation-ANOVA as well as Kruskal-Wallis H tests. The significance level was set at p>0.05 for non-significant results and p<0.05 for significant results.
RESULTS
Thirty patients with stage 1 Periodontitis had a mean age of 36.37 ± 9.197, thirty patients with stage 3 Periodontitis had a mean age of 36.60 ± 11.047, and thirty healthy controls had a mean age of 33.60 ± 9.171. Furthermore, there was a non-significant male predominance (56.7%) among the study groups, no statistically significant differences (P=0.426), (P=1.000) in age or sex existed between study groups. As demonstrated in Figure 1.
Figure 1. Frequency of demographic data for sex between groups.
The mean ±SD values of the percentage of BOP in the stage 3 periodontitis group and stage 1 periodontitis group were (2.35 ± 0.50) and (2.15 ± 0.56) which is non-significant (P >0.05). As shown in Table 2.
While the result of both CAL & PD parameters showed statistically significant differences (P=0.0001), (P=0.003) between the stage 1 and stage 3 periodontitis. The mean ± SD values of CAL were (1.73 ± 0.45), (5.50 ± 0.51), and the mean ± SD values of PD were (4.63 ± 0.49), (5.13 ± 0.73), respectively.
Table 2. Descriptives statistic of Clinical Periodontal Parameters in study groups.
|
|
N |
Mean |
Std. Deviation |
Std. Error |
F |
P -value |
||
|
BOP |
Stage 1 |
30 |
2.150 |
0.560 |
0.100 |
2.243 |
0.140 |
|
|
Stage 3 |
30 |
2.350 |
0.500 |
0.090 |
||||
|
CAL |
Stage 1 |
30 |
1.730 |
0.450 |
0.080 |
923.444 |
0.000 |
|
|
Stage 3 |
30 |
5.500 |
0.510 |
0.090 |
|
|||
|
PD |
Stage 1 |
30 |
4.630 |
0.490 |
0.090 |
9.695 |
0.003 |
|
|
Stage 3 |
30 |
5.130 |
0.730 |
0.130 |
||||
The descriptive statistics of NETs, as shown in Table 3, revealed statistically significant differences in the mean rank of NETs across the study groups (Stage 1 and Stage 3) compared to the control group. The highest Mean Rank concentration was found in Stage 3 (73.90 ± 6.58), followed by Stage 1 (43.17 ± 4.47), and then the control group (19.43 ± 4.63), with a p-value less than 0.05, indicating statistical significance.
The comparisons of NETs between each group are illustrated in Table 4. There were statistically significant differences in NETs levels among all comparisons. The control group versus Stage 1 periodontitis group showed a Z-value of -4.908 with a P -value of 0.000. Similarly, the control group versus Stage 3 periodontitis group had a Z-value of -6.653 with the same P -value. The comparison between Stage 1 and Stage 3 periodontitis groups also indicated a significant difference, with a Z-value of -5.943 and a P -value of 0.000.
Table 3. Summary statistics for the studied groups concerning of NETs.
|
NETs pg/ml |
Control group |
Stage 1 |
Stage 3 |
Kruskal-Wallis H |
P- value |
|
Mean Rank |
19.43 |
43.17 |
73.90 |
65.560 |
0.0001 |
|
Median |
133.78 |
164.31 |
224.30 |
||
|
SD |
25.37 |
24.46 |
36.06 |
|
|
|
SE |
4.63 |
4.47 |
6.58 |
|
|
|
Note: Significant, P <0.05, non-significant, (P >0.05).,SD: Standard Deviation, SE: Standard Erarr, df=2 |
|||||
Table 4. Multiple comparison of NETs (pg/ml) level between study groups and control group.
|
Combination (*) |
Groups |
NETs |
|
Mann-Whitney U test |
||
|
Control group X Stage 1 |
Z-value |
-4.908 |
|
P-vlaue |
0.000 |
|
|
Significant |
HS |
|
|
Control group X Stage 3 |
Z-value |
-6.653 |
|
P-vlaue |
0.000 |
|
|
Significant |
HS |
|
|
Stage 1X Stage 3 |
Z-value |
-5.943 |
|
P-vlaue |
0.000 |
|
|
Significant |
HS |
The mean ±SD values of IL-23 highest in stage 3 periodontitis were (5.018 ± 1.081) followed by stage 1(3.083 ± 0.569), while the low mean showed in control group (1.431 ± 0.586), with a statistical analysis a highly significant different at P -value <0.05 (P =0.0001). As shown in Table 5.
Table 5. Disruptive statistic of IL-23 (pg/ml) in study groups and control group.
|
IL-23 pg/ml |
Groups |
|
|||
|
Control group |
Stage 1 |
Stage 3 |
F |
P -value |
|
|
Mean |
1.431 |
3.083 |
5.018 |
158.078 |
0.0001 |
|
Std. Deviation |
±0.586 |
±0.569 |
±1.081 |
|
|
|
Std. Error of Mean |
±0.107 |
±0.104 |
±0.197 |
|
|
|
Minimum |
0.509 |
1.938 |
3.068 |
|
|
|
Maximum |
2.710 |
4.871 |
7.367 |
|
|
Gingival crevicular fluid (GCF) μl value by Periotron
The results of this study revealed a significant elevation in the median levels of Gingival crevicular fluid (GCF) value among Stage 3 Periodontitis (0.70 pg/ml) followed by Stage 1 Periodontitis (0.10 pg/ml) compared to the control group (0.04 pg/ml). The statistical analysis revealed a significant difference (P=0.000), as observed in Table 6.
Table 6. Summary statistics for the studied groups concerning of GCF μl value.
|
GCF μl |
Control group |
Stage 1 |
Stage 3 |
Kruskal-Wallis H |
P-value |
|
Mean Rank |
15.63 |
45.37 |
75.50 |
79.293 |
0.0001 |
|
Median |
0.04 |
0.10 |
0.70 |
||
|
Minimum |
0.03 |
0.06 |
0.40 |
||
|
Maximum |
0.07 |
0.15 |
0.90 |
||
|
SD |
0.01 |
0.02 |
0.16 |
|
|
|
SE |
0.00 |
0.00 |
0.03 |
|
|
|
Note: Significant, p<0.05, non-significant, (p>0.05)., SD: Standard Deviation, SE: Standard Erarr, df=2 |
|||||
For comparing between stage 1, stage 3 and control groups using Mann-Whitney U test in Table 7. The results showed significant differences (P=0.0001) with respect to the GCF value in comparison between stage 1 group and control group. Similarly, when comparing stage 3 and control groups, results indicated significant differences (P=0.0001).
Finally, when comparing between stage 1 and stage 3 groups, results demonstrated significant differences were noted at P=0.0001.
Table 7. Comparison of gingival crevicular fluid (GCF) levels (μl) between control group, stage 1 and stage 3 periodontitis.
|
Combination (*) |
Groups |
GCF μl |
|
Mann-Whitney U test |
||
|
Control group X Stage 1 |
Z-value |
-6.650 |
|
P-vlaue |
0.000 |
|
|
Control group X Stage 3 |
Z-value |
-6.718 |
|
P-vlaue |
0.000 |
|
|
Stage 1 X Stage 3 |
Z-value |
-6.681 |
|
P-vlaue |
0.000 |
Table 8 evaluates the validity of various biomarkers for predicting the progression of stage 3 periodontitis using Receiver Operator Characteristics (ROC) Analysis, as shown in Table 9. NETs show an optimal cut-off point of 196.471 with an Area Under the Curve (AUC) of 0.947, indicating excellent accuracy. They have a sensitivity of 90% and a specificity of 83.3%, with an overall accuracy rate of 86.66% and a statistically significant p-value of 0.000. IL-23 presents a cut-off point of 3.66500 with an AUC of 0.964, suggesting exceptional predictive power and high sensitivity and specificity of 96.7% and 90% respectively, leading to an overall accuracy of 93.33% and a p-value of 0.000. GCF is identified as an ideal biomarker with a perfect AUC of 1.000, demonstrating 100% sensitivity, specificity, and accuracy, highlighting its strong diagnostic potential, further supported by a significant p-value of 0.000.
Table 8. The validity of biomarkers for predicting progress stage 3 periodontitis patients.
|
Test Result Variable(s) |
Optimal cut-off point |
AUC |
Sensitivity % |
Specificity % |
Accuracy % |
P. value |
Asymptotic 95% Confidence Interval |
|
|
Lower Bound |
Upper Bound |
|||||||
|
NETs |
196.471 |
0.947 |
90.00 |
83.30 |
86.66 |
0.000 |
0.895 |
0.998 |
|
IL-23 |
3.665 |
0.964 |
96.70 |
90.00 |
93.33 |
0.000 |
0.917 |
1.000 |
|
GCF |
0.830 |
1.000 |
100.00 |
100.00 |
100 |
0.000 |
1.000 |
1.000 |
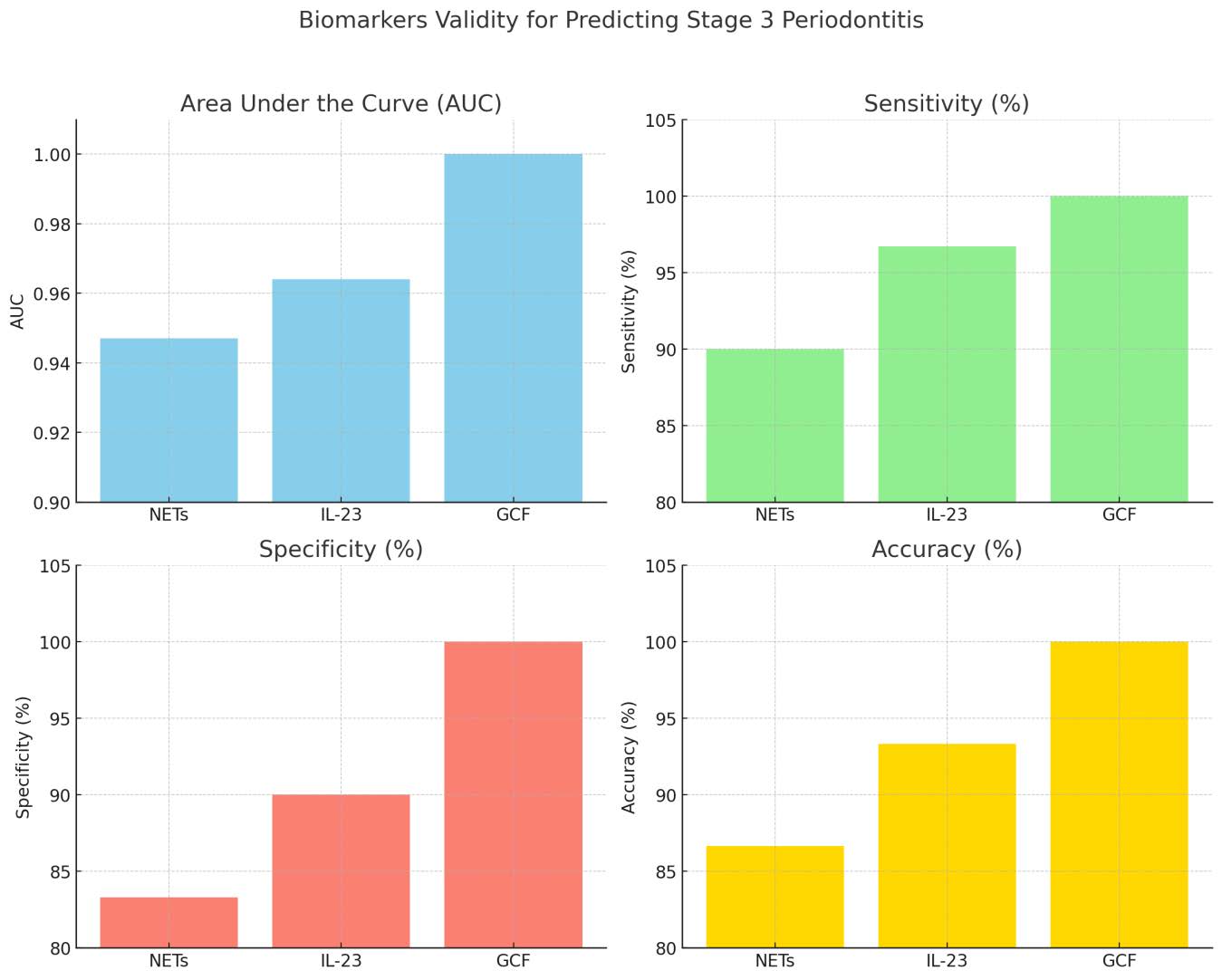
Figure 2. Receiver operator characteristics (ROC) chart of biomarkers.
DISCUSSION
Periodontitis is a chronic, infectious inflammatory disease characterized by the destructive interplay between periodontopathic bacteria and host immune responses. The presence of harmful bacteria, combined with an imbalance in cytokine levels—high in proinflammatory and low in anti-inflammatory cytokines—drives the progression of periodontal disease (Gemmell and Seymour, 2004). Furthering this, cytokines are released in response to lymphocytes infiltrating the periodontal lesions, which contributes to periodontal tissue damage (Takahashi et al., 2005).
Regarding age and sex, the findings of this study indicate no significant differences in the distribution of age or sex among the control group, Stage 1, and Stage 3 periodontitis groups. Although, males in this study are the majority in this study periodontitis compared to females. These suggest that the severity of periodontitis is not significantly associated with these demographic variables. Han and Park, (2018) in their study on age and sex in the prevalence of periodontitis among adults noted that age and sex were significant risk factors for periodontitis, particularly noting higher odds in older adults and males compared to their counterparts (Han and Park, 2018). The study found a significant effect of age on periodontal health. While Dye and Selwitz, (2005) concluded a significant effect of age on periodontal health and there was a notable difference in periodontal health between males and females, and reported males generally had poorer periodontal health compared to females (Dye and Selwitz, 2005). However, in the present study, sex did not show a significant difference in periodontitis prevalence between the groups. This might suggest that the role of sex could vary depending on other factors such as genetic predispositions or environmental exposures not accounted for in the study.
In the current study, the concentrations of Neutrophil Extracellular Traps (NETs) were analyzed across different stages of periodontitis. Findings revealed the highest median NETs concentration in Stage 3 periodontitis (224.30), followed by Stage 1 periodontitis (164.31), and the lowest in the control group (133.78), with statistically significant differences (P < 0.05) among the groups. These differences underscore the progressive increase in NETs levels correlating with the severity of periodontal disease. The present study consistent with Wang et al. (2021) found that NETs could be influenced by periodontal treatment, indicating their potential as a biomarker for periodontitis severity and therapeutic effects (Wang et al., 2021). Actually, NETs play a dual role in periodontal health. They restrict oral inflammation by eliminating pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), as suggested by Zhu et al. (2022) (Zhu et al., 2022). However, excessive NETs can disrupt homeostasis and contribute to periodontitis by protecting harmful bacteria within periodontal pockets, thus exacerbating tissue injury and disease progression (Yost et al., 2016). The relationship between NETs and periodontal pathogens is complex. Periodontal pathogens have evolved mechanisms to evade the immune response, benefiting from NETs. For instance, major Gram-negative periodontal pathogens such as P. gingivalis, P. intermedia, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans exhibit varying degrees of DNA degradation activity, with P. intermedia showing the highest (Doke et al., 2017). Moreover, P. gingivalis expresses Porphyromonas peptidylarginine deiminase (PPAD), which impairs NETs by altering peptidylarginine into citrulline, aiding its evasion from NET-mediated capture (Potempa et al., 2017; Stobernack et al., 2018). Interestingly, while high levels of NETs are linked to increased tissue injury and even mortality, as noted by Kaneko et al. (2018) (Kaneko et al., 2018), they are also implicated in exacerbating conditions like oral cancer (Jablonska et al., 2020).
Furthermore, the study by Pérez-Sánchez et al. (2017) emphasized the elevated serum NETs levels in patients with periodontitis and rheumatoid arthritis (RA), though the significance of circulating NETs in periodontitis remains debated. This calls for further research to clarify NETs' specific mechanisms and their regulation in periodontitis, considering both their protective roles and potential to promote disease under certain conditions (Pérez-Sánchez et al., 2017).
The result of Interleukin-23 (IL-23) levels across different study groups (control, Stage 1 periodontitis, and Stage 3 periodontitis) revealed a significant highest in the Stage 3 periodontitis group, followed by the Stage 1 group, and the lowest mean was observed in the control group. Furthermore, significant differences were found when comparing IL-23 levels between the Stage 1 group and the control group, as well as between the Stage 3 group and the control group. Moreover, significant differences were noted when comparing IL-23 levels between the Stage 1 and Stage 3 groups. IL-23 is a key cytokine involved in the inflammatory response and immune regulation, and its elevated levels in periodontitis patients suggest its role in the pathogenesis and progression of the disease. The study's findings are consistent with previous research that has demonstrated the importance of IL-23 in inflammatory diseases and its association with disease severity and activity (Cornelissen et al., 2009; Duvallet et al., 2011). The significant differences in IL-23 levels between the control, Stage 1, and Stage 3 groups underscore the potential of IL-23 as a biomarker for assessing the progression and severity of periodontitis. Other findings consistent with the present finding by Althebeti et al. (2018) demonstrated that IL-23 levels were elevated in locations affected by periodontal disease, suggesting that gingivitis and periodontitis are influenced by IL-23. The concentration of IL-23 in the blood and cervical gingival fluid was considerably greater in individuals with chronic periodontitis (Althebeti et al., 2018). IL-23 is released by immune cells called dendritic cells and macrophages in response to bacterial exposure. IL-23 stimulates the production of Th17 cells, which in turn create a cytokine that promotes inflammation (Himani et al., 2014). The IL-23 and Th17 pathways have a role in the development of rheumatoid arthritis (Ohyama et al., 2009). Rheumatoid arthritis is regarded as a suitable model for chronic periodontitis due to its analogous patterns of soft and hard tissue degradation (Mercado et al., 2000). Elevated levels of IL23 have been seen in areas where clinical attachment loss occurs, suggesting that IL-23 may play a role in the advancement of periodontitis. Ohyama et al. (2009) proposed that the IL-23-induced Th17 pathway has a significant impact on periodontal disease. They investigated the presence of cytokines and associated components in both periodontal lesions and control locations. (Ohyama et al., 2009). The findings indicated that the expression of IL23 was markedly elevated in periodontal lesions compared to control areas. These findings indicate that the IL-23-induced Th17 pathway is activated in inflammatory periodontal diseases. Allam et al. (2011) obtained tissue samples from the oral mucosa, as well as the upper and lower areas of chronic periodontitis, and subjected them to immunohistochemical analysis (Allam et al., 2011). Himani et al. (2014) assessed the concentrations of IL-23 in the gingival crevicular fluid (GCF) of individuals who were systemically healthy and had either a healthy periodontium, gingivitis, or chronic periodontitis. Their findings indicated that individuals with chronic periodontitis had the highest average IL-23 concentration in GCF, whereas those with a healthy periodontium had the lowest concentration (Himani et al., 2014).
The findings indicated that the rise in IL-23 levels in GCF was directly correlated with the extent of periodontal tissue injury. Given that the content of IL-23 in GCF (gingival crevicular fluid) increases in proportion to the degree of periodontal damage, it may be inferred that IL-23 may have a role in the development of periodontal disease (Himani et al., 2014). Lester et al. (2007) assessed the levels of IL-23 and IL-17 in the gingiva of both healthy sites and sites affected by chronic periodontal disease. Their findings demonstrated that the levels of IL-23 and IL-17 in the gums were considerably higher in locations with moderate and severe CAL compared to normal areas (Lester et al., 2007).
The result of the current study shows a progression-dependent increase in GCF levels from control to Stage 1 and Stage 3 periodontitis, with a marked elevation in Stage 3. This may be because GCF could serve as a biomarker for periodontitis severity. The elevation in GCF levels is likely due to an enhanced inflammatory response and immune activity within the periodontal tissue as the disease progresses. GCF is known to contain inflammatory cytokines and biomarkers that reflect the host's response to periodontal pathogens (Nair et al., 2022).
Several studies corroborate these findings as research has demonstrated that cytokines such as IL-6, IL-10, IL-17, IL-18, IL-21 and IL-23 are significantly elevated in the GCF of patients with more severe periodontal disease (Figueredo et al., 2008; Barros et al., 2016; Nair et al., 2022) this research supporting the idea that these cytokines found in GCF could serve as biomarkers for periodontal tissue destruction.
According to Preianò et al. (2020) and Nair et al. (2022) highlighted the potential of GCF as a source for discovering biomarkers for periodontal diseases, noting that various antimicrobial peptides and proteins in GCF play roles in the immune-inflammatory response (Preianò et al., 2020; Nair et al., 2022).
In addition to a study by Andronovici et al. (2022) that developed a gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis (Andronovici et al., 2022).
In addition to Alamri et al. (2023) found that scaling and root planning on interleukin-1β, interleukin-8, and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients (Alamri et al., 2023). These studies further emphasize the importance of GCF as a potential biomarker for periodontal diseases. However, conflicting studies have reported opposite results, such as Romano et al. (2022) reporting lower GCF levels in periodontitis compared to gingivitis (Romano et al., 2022). This discrepancy may be due to differences in study design, sample size, and patient populations.
According to Khurshid et al. (2017), it is suggested that GCF might potentially serve as a reservoir for identifying novel biomarkers associated with periodontitis/gingivitis. The oral cavity serves as a reservoir for the microbiome. When unfavourable changes occur in the oral cavity, it leads to pathological alterations, such as gingivitis, periodontitis, and dental caries (Khurshid et al., 2017).
Majeed et al., (2016) found that gingival crevicular fluid (GCF) may have a significant impact on both the diagnosis and prognosis of oral disorders, namely periodontal diseases. It is considered one of the least invasive methods to gather information on the health of periodontal tissue, including the state of the connective tissue and the extent of hard tissue damage (Nazar Majeed et al., 2016).
The study's findings support the notion that GCF levels are elevated in more severe stages of periodontitis, which could be a useful biomarker for diagnosing and monitoring the progression of periodontal diseases. Further research is needed to clarify the inconsistencies in the literature and to establish the most accurate and reliable biomarkers for periodontal diseases.
Limitations of study
The study has several limitations that should be considered. The small sample size may limit the generalizability of the findings, and the cross-sectional design restricts the ability to infer causal relationships between biomarkers and periodontitis progression. Additionally, participant variability, including differences in oral health habits, systemic conditions, and genetic predispositions, may impact the results. Potential confounders such as diet and medication use, along with limitations in the sensitivity and specificity of biomarker measurement techniques, could also affect the accuracy of the findings.
CONCLUSION
This study underscores the significant association of Neutrophil Extracellular Traps (NETs) and IL-23 with the severity of periodontitis. Elevated levels of these biomarkers were notably higher in patients with advanced periodontitis (stage 3), indicating their crucial role in the progression of this inflammatory disease. By assessing GCF volumes and specific inflammatory mediators, we can better predict disease progression, tailor treatment strategies, and ultimately improve patient outcomes.
ACKNOWLEDGEMENTS
The authors thank the College of Dentistry, University of Baghdad for providing instruments.
AUTHOR CONTRIBUTIONS
MFA: contributed to conceptualizing, validation, writing original draft, supervision, and project administration, also contributed to the methodology, formal analysis, resources, original draft, review, editing, and visualization. GIT: contributed to the methodology, formal analysis, resources, original draft, review, editing, and visualization.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ababneh, K. T., Maslamani, M. J., Abbadi, M. S., Taha, A. H., Karasneh, J. A., Sa’di, A. G., and Khader, Y. S. 2019. Risk indicators of aggressive periodontitis in a Jordanian population. BMC Oral Health. 19(1): 155.
Alamri, M. M., Antonoglou, G. N., Proctor, G., Balsa-Castro, C., Tomás, I., and Nibali, L. 2023. Biomarkers for diagnosis of stage III, grade C with molar incisor pattern periodontitis in children and young adults: A systematic review and meta-analysis. Clinical Oral Investigations. 27(9): 4929–4955.
Ali, D. and Taha, G. 2023. The effectivity of Pfizer vaccine on oral immunological biomarkers sIgA and interleukin-21. Microbes and Infectious Diseases. 5: 0–0.
Allam, J.-P., Duan, Y., Heinemann, F., Winter, J., Götz, W., Deschner, J., Wenghoefer, M., Bieber, T., Jepsen, S., and Novak, N. 2011. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. Journal of Clinical Periodontology. 38(10): 879–886.
Althebeti, G. R., Elfasakhany, F. M., and Talla, E. A. 2018. Evaluation of interleukin-23 in periodontal health and disease. International Journal of Health Sciences and Research. 8: 226–232.
Andronovici, A. M., Caruntu, I.-D., Onofriescu, M., Hurjui, L. L., Giusca, S.-E., Covic, A. S., Braescu, R., and Foia, L.-G. 2022. TNF-α, IL-1β, MMP-8 crevicular profile in patients with chronic kidney disease and periodontitis. Applied Sciences, 12(2): 736.
Armitage, G. C. 2004. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000. 34: 9–21.
Arteaga-Henríquez, G., Lugo-Marín, J., Gisbert, L., Setién-Ramos, I., Martínez-Gallo, M., Pujol-Borrell, R., and Ramos-Quiroga, J. A. 2022. Activation of the monocyte/macrophage system and abnormal blood levels of lymphocyte subpopulations in individuals with autism spectrum disorder: A systematic review and meta-analysis. International Journal of Molecular Sciences. 23(22): 14329.
Attar, N. B., Banodkar, A. B., Gaikwad, R. P., Sethna, G. D., Patil, C. L., and Simon, S. 2018. Evaluation of gingival crevicular fluid volume in relation to clinical periodontal status with periotron 8000. International Journal of Applied Dental Sciences. 4(1): 70–71.
Barros, S. P., Williams, R., Offenbacher, S., and Morelli, T. 2016. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000. 70(1): 53–64.
Bhardwaj, S. and Prabhuji, M. L. V. 2013. Comparative volumetric and clinical evaluation of peri-implant sulcular fluid and gingival crevicular fluid. Journal of Periodontal & Implant Science. 43(5): 233–242.
Bianchi, E. and Rogge, L. 2019. The IL-23/IL-17 pathway in human chronic inflammatory diseases-new insight from genetics and targeted therapies. Genes and Immunity. 20(5): 415–425.
Bielemann, A. M., Marcello-Machado, R. M., Del Bel Cury, A. A., and Faot, F. 2018. Systematic review of wound healing biomarkers in peri-implant crevicular fluid during osseointegration. Archives of Oral Biology. 89: 107–128.
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., Weinrauch, Y., and Zychlinsky, A. 2004. Neutrophil extracellular traps kill bacteria. Science. 303(5663): 1532–1535.
Bunte, K. and Beikler, T. 2019. Th17 Cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. International Journal of Molecular Sciences, 20(14): 3394.
Cairo, F., Nieri, M., Cincinelli, S., Mervelt, J., and Pagliaro, U. 2011. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: an explorative and reliability study. Journal of Clinical Periodontology. 38(7): 661–666.
Cornelissen, F., Mus, A. M., Asmawidjaja, P. S., van Hamburg, J. P., Tocker, J., and Lubberts, E. 2009. Interleukin-23 is critical for full-blown expression of a non-autoimmune destructive arthritis and regulates interleukin-17A and RORgammat in gammadelta T cells. Arthritis Research & Therapy. 11(6): R194.
Doke, M., Fukamachi, H., Morisaki, H., Arimoto, T., Kataoka, H., and Kuwata, H. 2017. Nucleases from prevotella intermedia can degrade neutrophil extracellular traps. Molecular Oral Microbiology. 32(4): 288–300.
Duvallet, E., Semerano, L., Assier, E., Falgarone, G., and Boissier, M.-C. 2011. Interleukin-23: A key cytokine in inflammatory diseases. Annals of Medicine. 43(7): 503–511.
Dye, B. A. and Selwitz, R. H. 2005. The relationship between selected measures of periodontal status and demographic and behavioural risk factors. Journal of Clinical Periodontology. 32(7): 798–808.
Esteves Lima, R. P., Atanazio, A. R. S., Costa, F. O., Cunha, F. A., and Abreu, L. G. 2021. Impact of non-surgical periodontal treatment on serum tnf-α levels in individuals with type 2 diabetes: A Systematic review and meta-analysis. The Journal of Evidence-Based Dental Practice. 21(2): 101546.
Fernández-Reyes, M., Márquez-Arrico, C.-F., Silvestre, F.-J., Perea-Galera, L., Silvestre-Rangil, J., and Rocha, M. 2023. Comparison of three fluids for calibration of the new Periotron® 8010. Medicina Oral, Patologia Oral y Cirugia Bucal, 28(6): e519–e524.
Figueredo, C. M., Rescala, B., Teles, R. P., Teles, F. P., Fischer, R. G., Haffajee, A. D., Socransky, S. S., and Gustafsson, A. 2008. Increased interleukin‐18 in gingival crevicular fluid from periodontitis patients. Oral Microbiology and Immunology. 23(2): 173–176.
Gemmell, E. and Seymour, G. J. 2004. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontology 2000. 35(1): 21–41.
Gunpinar, S., Alptekin, N. O., and Dundar, N. 2017. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 in patients with aggressive periodontitis. Oral Diseases. 23(6): 763–769.
Hajishengallis, G. 2014. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends in Immunology. 35(1): 3–11.
Halmilton, M.B., Pincus, E.L., Fion, A.D., and Fleischer, R.C. 1999. Universal linker and ligation procedures for construction of genomic DNA libraries enriched for microsatellites. Microbial Biotechnology. 27: 500507.
Han, K. and Park, J.-B. 2018. Clinical implications of age and sex in the prevalence of periodontitis in Korean adults with diabetes. Experimental and Therapeutic Medicine. 15(4): 3865–3873.
Himani, G. S., Prabhuji, M. L. V., and Karthikeyan, B. V. 2014. Gingival crevicular fluid and interleukin‐23 concentration in systemically healthy subjects: Their relationship in periodontal health and disease. Journal of Periodontal Research. 49(2): 237–245.
Husham, S. and Taha, G. 2024. Changes in levels of interleukin-6 (IL-6) and interleukin-8 (IL-8) in the serum of preterm delivery pregnant women affected by gingivitis. Journal of the Faculty of Medicine Baghdad. 65(4):362-367.
Ito, H., Numabe, Y., Hashimoto, S., Uehara, S., Wu, Y.-H., and Ogawa, T. 2021. Usefulness of hemoglobin examination in gingival crevicular fluid during supportive periodontal therapy to diagnose the pre-symptomatic state in periodontal disease. Clinical Oral Investigations. 25(2): 487–495.
Jablonska, E., Garley, M., Surazynski, A., Grubczak, K., Iwaniuk, A., Borys, J., Moniuszko, M., and Ratajczak-Wrona, W. 2020. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus - possible implications for the development of oral cancer. Immunobiology. 225(2): 151901.
Kaneko, C., Kobayashi, T., Ito, S., Sugita, N., Murasawa, A., Nakazono, K., and Yoshie, H. 2018. Circulating levels of carbamylated protein and neutrophil extracellular traps are associated with periodontitis severity in patients with rheumatoid arthritis: A pilot case-control study. PloS One, 13(2): e0192365.
Khurshid, Z., Mali, M., Naseem, M., Najeeb, S., and Zafar, M. 2017. Human gingival crevicular fluids (gcf) proteomics: An overview. Dentistry Journal. 5(1): 12.
Lang, N. P., Joss, A., Orsanic, T., Gusberti, F. A., and Siegrist, B. E. 1986. Bleeding on probing. A predictor for the progression of periodontal disease? Journal of Clinical Periodontology. 13(6): 590–596.
Lester, S. R., Bain, J. L., Johnson, R. B., and Serio, F. G. 2007. Gingival concentrations of interleukin‐23 and ‐17 at healthy sites and at sites of clinical attachment loss. Journal of Periodontology. 78(8): 1545–1550.
Mercado, F., Marshall, R. I., Klestov, A. C., and Bartold, P. M. 2000. Is there a relationship between rheumatoid arthritis and periodontal disease? Journal of Clinical Periodontology. 27(4): 267–272.
Nair, V., Grover, V., Arora, S., Das, G., Ahmad, I., Ohri, A., Sainudeen, S., Saluja, P., and Saha, A. 2022. Comparative evaluation of gingival crevicular fluid interleukin-17, 18 and 21 in different stages of periodontal health and disease. Medicina (Kaunas, Lithuania), 58(8): 1042.
Nazar Majeed, Z., Philip, K., Alabsi, A. M., Pushparajan, S., and Swaminathan, D. 2016. Identification of gingival crevicular fluid sampling, analytical methods, and oral biomarkers for the diagnosis and monitoring of periodontal diseases: A systematic review. Disease Markers. 2016: 1804727.
Ngo, L. H., Veith, P. D., Chen, Y.-Y., Chen, D., Darby, I. B., and Reynolds, E. C. 2010. Mass spectrometric analyses of peptides and proteins in human gingival crevicular fluid. Journal of Proteome Research. 9(4): 1683–1693.
Ohyama, H., Kato-Kogoe, N., Kuhara, A., Nishimura, F., Nakasho, K., Yamanegi, K., Yamada, N., Hata, M., Yamane, J., and Terada, N. 2009. The involvement of IL-23 and the Th17 pathway in periodontitis. Journal of Dental Research. 88(7): 633–638.
Panase, A., Thirabunyanon, M., Promya, J., Palić, D., and Chitmanat, C. 2023. Synergistic effects of Fructooligosaccharide and Bacillus subtilis dietary supplementation on growth, innate immune responses, and protection against Streptococcus agalactiae in juvenile nile tilapia (Oreochromis niloticus). Chiang Mai Journal of Science. 50(5): e2023050.
Pappe, C. L., Steckhan, N., Hoedke, D., Jepsen, S., Rauch, G., Keller, T., Michalsen, A., and Dommisch, H. 2021. Prolonged multimodal fasting modulates periodontal inflammation in female patients with metabolic syndrome: A prospective cohort study. Journal of Clinical Periodontology. 48(4): 492–502.
Pérez-Sánchez, C., Ruiz-Limón, P., Aguirre, M. A., Jiménez-Gómez, Y., Arias-de la Rosa, I., Ábalos-Aguilera, M. C., Rodriguez-Ariza, A., Castro-Villegas, M. C., Ortega-Castro, R., Segui, P., Martinez, C., Gonzalez-Conejero, R., Rodríguez-López, S., Gonzalez-Reyes, J. A., Villalba, J. M., Collantes-Estévez, E., Escudero, A., Barbarroja, N., and López-Pedrera, C. 2017. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. Journal of Autoimmunity. 82: 31–40.
Potempa, J., Mydel, P., and Koziel, J. 2017. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nature Reviews Rheumatology. 13(10): 606–620.
Preianò, M., Savino, R., Villella, C., Pelaia, C., and Terracciano, R. 2020. Gingival crevicular fluid peptidome profiling in healthy and in periodontal diseases. International Journal of Molecular Sciences. 21(15): 5270.
Romano, F., Iaderosa, G., Corana, M., Perotto, S., Baima, G., Di Scipio, F., Abbadessa, G., Mariani, G. M., Aimetti, M., and Berta, G. N. 2022. Comparing ionic profile of gingival crevicular fluid and saliva as distinctive signature of severe periodontitis. Biomedicines. 10(3): 687.
Salahuddin Jasim, S. and Ghada Ibrahim Taha. 2023. Comparison between HSV-1 Ag detection techniques by ELISA and real-time PCR in breast cancer patients suffering from periodontitis. Journal of the Faculty of Medicine Baghdad. 65(3): 227–233.
Sergeant, E.S.G., 2018. Epitools epidemiological calculators. Ausvet Pty Ltd.
Stobernack, T., du Teil Espina, M., Mulder, L. M., Palma Medina, L. M., Piebenga, D. R., Gabarrini, G., Zhao, X., Janssen, K. M. J., Hulzebos, J., Brouwer, E., Sura, T., Becher, D., van Winkelhoff, A. J., Götz, F., Otto, A., Westra, J., and van Dijl, J. M. 2018. A secreted bacterial peptidylarginine deiminase can neutralize human innate immune defenses. MBio. 9(5): e01704-e1718.
Takahashi, K., Azuma, T., Motohira, H., Kinane, D. F., and Kitetsu, S. 2005. The potential role of interleukin‐17 in the immunopathology of periodontal disease. Journal of Clinical Periodontology. 32(4): 369–374.
Talib, E. Q. and Taha, G. I. 2024. Involvement of interlukin-17A (IL-17A) gene polymorphism and interlukin-23 (IL-23) level in the development of peri-implantitis. BDJ Open. 10(1): 12.
Tonetti, M. S. and Sanz, M. 2019. Implementation of the new classification of periodontal diseases: Decision‐making algorithms for clinical practice and education. Journal of Clinical Periodontology. 46(4): 398–405.
Trindade, F., Oppenheim, F. G., Helmerhorst, E. J., Amado, F., Gomes, P. S., and Vitorino, R. 2014. Uncovering the molecular networks in periodontitis. Proteomics Clinical Applications. 8(9–10): 748–761.
Wang, J., Zhou, Y., Ren, B., Zou, L., He, B., and Li, M. 2021. The role of neutrophil extracellular traps in periodontitis. Frontiers in Cellular and Infection Microbiology. 11: 639144.
Yang, P., Qian, F.-Y., Zhang, M.-F., Xu, A.-L., Wang, X., Jiang, B.-P., and Zhou, L.-L. 2019. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. Journal of Leukocyte Biology. 106(6): 1233–1240.
Yost, C. C., Schwertz, H., Cody, M. J., Wallace, J. A., Campbell, R. A., Vieira-de-Abreu, A., Araujo, C. V, Schubert, S., Harris, E. S., Rowley, J. W., Rondina, M. T., Fulcher, J. M., Koening, C. L., Weyrich, A. S., and Zimmerman, G. A. 2016. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. The Journal of Clinical Investigation. 126(10): 3783–3798.
Yu, S., Tripod, M., Atasoy, U., and Chen, J. 2021. HuR plays a positive role to strengthen the signaling pathways of CD4(+) T cell activation and Th17 cell differentiation. Journal of Immunology Research. 2021: 9937243.
Zhu, X., Huang, H., and Zhao, L. 2022. PAMPs and DAMPs as the bridge between periodontitis and atherosclerosis: The potential therapeutic targets. Frontiers in Cell and Developmental Biology. 10: 856118.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Mohammed Faisal Ali and Ghada Ibrahim Taha*
Department of Basic Sciences, College of Dentistry, University of Baghdad, Baghdad, Iraq.
Corresponding author: Ghada Ibrahim Taha, E-mail: ghada_ibraheem@codental.uobaghdad.edu.iq
ORCID: Ghada Ibrahim Taha: https://orcid.org/0000-0002-1958-1939
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: July 24, 2024;
Revised: August 10, 2024;
Accepted: August 14, 2024;
Online First: August 23, 2024

