
Effects of Occlusion Cuff Pressures for Determining Brachial Artery Flow-Mediated Dilation in Healthy Thai Adults
Kanpiraya Nithitsuttibuta, Jaruta Kunritt, Suchart Kiatwattanacharoen, and Sainatee Pratanaphon*Published Date : August 9, 2024
DOI : https://doi.org/10.12982/NLSC.2024.050
Journal Issues : Number 4, October-December 2024
Abstract Variability in cuff occlusion pressure used during flow-mediated dilation (FMD) measurement may cause differences in FMD results and discomfort sensation. The objective of this study was to compare FMD data and sensations of pain and numbness using three levels of occlusion cuff pressures commonly used for FMD assessment in healthy adults. A within-subject design was conducted in 12 healthy adults aged 20-40 years. FMD was measured using brachial artery Doppler ultrasound at three different occlusion pressures: 25 and 50 mmHg above systolic blood pressure (SBP) and 200 mmHg. Pain and numbness were recorded using the Numeric Pain Rating Scale and the Visual Analog Scale, respectively, at baseline and every minute during the 5-minute occlusion and 3 minutes after cuff deflation. The results showed that %FMD and mean blood flow velocity after using cuff pressure at 25 mmHg above SBP (10.11 ± 2.58% and 11.52 ± 3.96 cm/s) were comparable to those of 50 mmHg above SBP (10.08 ± 2.70% and 12.54 ± 4.31 cm/s) and at 200 mmHg (10.15 ± 2.74% and 12.28 ± 4.30 cm/s) (all P >0.05). The pain intensity was significantly greater at 200 mmHg compared to 25 mmHg at the first, second, and fourth minutes during occlusion (all P <0.05). The numbness scale was not different among the three pressure conditions. In conclusion, the minimum occlusion pressure above SBP by 25 mmHg generated an insignificant FMD response compared to the other two cuff pressures, caused minor pain, and may be the favorable pressure for determining endothelium-dependent FMD in healthy Thai adults.
Keywords: Flow-mediated dilation, Occlusion cuff pressure, Pain sensation, Adults
Funding: The authors are grateful for the research funding provided by the Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand.
Citation: Nithitsuttibuta, K., Kunritt, J., Kiatwattanacharoen, S., and Pratanaphon, S. 2024. Effects of occlusion cuff pressures for determining brachial artery flow-mediated dilation in healthy Thai adults. Natural and Life Sciences Communications. 23(4): e2024050.
INTRODUCTION
Flow-mediated dilation (FMD), a surrogate marker of endothelium-dependent vasodilator function, is considered an important early event in preceding structural atherosclerotic changes (Moens et al., 2005; Thijssen et al., 2011). Studies have demonstrated a relationship between coronary endothelial function and the function of the brachial artery (Broxterman et al., 2019). Individuals with impaired brachial FMD had a significantly higher risk of cardiovascular events (CVs) than those with normal FMD (Heiss et al., 2022; Mućka et al., 2022). Moreover, the brachial FMD can independently predict long-term adverse CVs in healthy subjects (Shechter et al., 2014) and patients with cardiovascular disease (Inaba et al., 2010), which most of them may not be aware of their condition until an acute event (Sarriff et al., 2014). Therefore, monitoring endothelial function as a preventive measure for cardiovascular disease is important.
Endothelium-dependent FMD, which refers to the change in artery diameter in response to reactive hyperemia, can be measured using a standardized blood pressure occlusion (Bots et al., 2005). Elevations in blood flow-associated shear stress cause the vascular endothelium to release nitric oxide (NO), resulting in local arterial vasodilation. Thus, reduced NO bioavailability may play a role in the pathogenesis of vascular disease (Bots et al., 2005).
Factors known to affect FMD results include ultrasound settings, subject preparation, cuff size, occlusion site, and occlusion duration (Corretti et al., 2002; Harris et al., 2010; Thijssen et al., 2019). Additionally, the pressure of the occlusion cuff is crucial for FMD measurements, as it impacts the shear stress stimulus and the NO-dependent dilation response (Thijssen et al., 2019). Previous literature has reported variability in the occlusion cuff pressures used, with common ranges being 25–50 mmHg above systolic blood pressure (SBP) and between 200–300 mmHg (Thijssen et al., 2019). A meta-analysis revealed no significant difference in the mean value of FMD responses between occlusion pressures above or below 275 mmHg (Bots et al., 2005). Expert consensus guidelines recommend using cuff pressure exceeding 50 mmHg above SBP to prevent arterial inflow, noting that this pressure is well-tolerated and not associated with discomfort (Thijssen et al., 2019).
However, FMD results and the occurrence of discomfort sensations, including pain and numbness, in response to different cuff occlusion pressures during FMD assessments remains unclear. Additionally, it has been reported that pain perceptions can differ due to ethnic variations (Krupić et al., 2019; Rowell et al., 2011). Studies have shown that Asians tend to have lower sensory and pain thresholds but experience greater pain compared to both Caucasians and African Americans (Ramer et al., 1999; Rowell et al., 2011). Moreover, studies revealed that higher cuff occlusion pressure can increase discomfort, pain, and numbness during procedures as they compress nerves and blood vessels (Spitz et al., 2022). Appropriate use of cuff occlusion pressure may minimize these discomforts and the risks of injury associated with FMD measurement.
Therefore, this study aimed to compare FMD across three cuff occlusion pressures: 25 and 50 mmHg above SBP, and the typical occlusion at 200 mmHg. These pressures were selected based on previous research recommendations for inducing sufficient responses in FMD assessment. Specifically, 25 mmHg above SBP is the minimal recommended value, while 50 mmHg (moderate) and 200 mmHg (high) are commonly used pressures. Young healthy subjects were selected for this initial study to minimize the impact of confounding variables like age-related vascular changes (Seals et al., 2011), and comorbidities on FMD (Ray et al., 2023). Additionally, the scale of discomfort sensations, including pain and numbness, were compared among the three cuff pressures. We hypothesized that there would be no significant differences in FMD results between the three cuff occlusion pressures, while discomfort sensations would significantly differ between the pressures.
MATERIALS AND METHODS
Study design
Twelve healthy adults aged 20–40 years were voluntarily recruited via flyers placed at a university campus and around the community. All participants were recreationally active (≥ 600 metabolic equivalents (MET)-minutes per week) using the Global Physical Activity Questionnaire (GPAQ) (Cleland et al., 2014; Cortés et al., 2018). They were non-smokers and normotensive (<128/80 mmHg) (Whelton Paul et al., 2018). Participants did not have any known cardiac or medical conditions, diabetes, cardiovascular disease, peripheral artery disease, dyslipidemia, rheumatoid arthritis, and chronic kidney disease. None of the participants used any medications that interfered with the cardiovascular system (Thijssen et al., 2009). Participants who were unable to fully participate in the study were excluded. Before the commencement of the study, all participants signed informed consent documents approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (RAS; 0056), and the Thai Clinical Trials Registry (TCTR 20230822004).
Experimental design
A repeated-measures within-subject design was used. Eligible participants were randomly assigned to the order of occlusion cuff pressure for FMD assessment by a simple sampling method. Three levels of occlusion cuff pressure, at 25 and 50 mmHg above SBP and 200 mmHg, were examined with a 30-minute rest interval between each measurement. The outcome measures were FMD and levels of pain and numbness. FMD was determined at baseline and after the release period, while the levels of pain and numbness were recorded at baseline, during occlusion, and during release periods.
Brachial artery FMD assessments
The procedure of FMD assessment was conducted according to a previous study (Corretti et al., 2002; Sturm et al., 2009). Participants were asked to fast and refrain from a high-fat diet, caffeine, vitamin C, and exercise for at least 8–12 hours the night before the FMD assessment. Additionally, women were not to be in their menstrual period. FMD was assessed using a Doppler color ultrasound, pulsed wave Doppler mode (Xario 100, Toshiba, Japan) in a temperature-controlled room at 25°C with dim light. After 15 minutes of resting in a supine position, participants were instructed to abduct the arm at a 90o angle. An 11-MHz linear probe with the insonation angle at 60 degrees with sample volume to the vessel was scanned on the right brachial artery (Corretti et al., 2002; Sturm et al., 2009). This was conducted in a longitudinal section 4.5 cm above the antecubital fossa, ensuring the maximum lumen diameter (Wisløff et al., 2007). The scans were performed at the same time of day by a trained technician. The intra-rater reliability of the FMD measurement was within an acceptable range (ICCs = 0.90, P < 0.001). The standard error of measurement and the minimal detectable change were 0.92 and 2.54, respectively. The baseline of the brachial artery diameter (FMDbase) and mean blood flow velocity (MBFVbase) were measured using pulse wave Doppler at the well-rested stage. A sphygmomanometer cuff (Model HM-1101, Japan) was placed around the forearm and inflated for 5 minutes (Agewall et al., 2001; Corretti et al., 2002). After cuff release, the images were continuously recorded every 30 seconds for 2 minutes to measure the peak diameter of the brachial artery (FMDpeak) (Corretti et al., 2002; Rodriguez-Miguelez et al., 2016). The absolute maximal diameter of the brachial artery at baseline (FMDbase) and after the release of cuff occlusion (FMDpeak) was calculated for %FMD by the formula (FMDpeak - FMDbase)/FMDbase) × 100. In addition, mean blood flow velocity at baseline (MBFVbase) and after the release of cuff occlusion (MBFVpost-occlusion) were determined (Corretti et al., 2002).
Discomfort symptoms
Pain was assessed by a Numeric Pain Rating Scale (NPRS) from 0 (no pain) to 10 (worst imaginable) (Edwards, 2005; Katz and Melzack, 1999), while numbness was determined by a Visual Analog Scale (VAS) from 0 (none) to 10 (unbearable) (Hongwen et al., 2018). Both variables were recorded at baseline and every minute for 5 minutes during the 5-minute occlusion and were continuously recorded at 30-second intervals until the symptoms dissipated after cuff deflation. The 30-minute rest period between the three cuff pressures trials was designed to prevent habituation, ensuring that each pain assessment was not influenced by the previous stimulus (Rankin et al., 2009). Additionally, a 30-second interval was chosen for monitoring pain to capture significant changes, as studies have found that peak pain sensation typically occurs within 2 minutes (Estebe et al., 2000; Mitrichev et al., 2024).
Anthropometric data
Body weight (BW) was measured by weight scale (Omron HN289, Japan). Height was measured using a wall-mounted stadiometer (Health-O-Meter 402 KL, IL) to the nearest 0.1 cm, and body mass index (BMI) was subsequently calculated by using a formula: BMI = BW (kg)/height2(m) (Senaratna et al., 2017).
Sample size calculation
There is no evidence studying the effects of occlusion pressure on FMD. Thus, the sample size of this study was estimated based on statistical principles (Maher et al., 2013) using G*Power 3.1.9.4 software. The sample size for FMD using two-way repeated analysis of ANOVA was computed with the effect size of 0.4, and the number of measurements was 3 with a power of 80% and a significance level of 0.05. Therefore, the total number of subjects was 12. The sample size for the pain scale using a two-way repeated measure ANOVA was calculated with an effect size of 0.4, with 12 repeated measurements, a power of 80%, and a significance level of 0.05. This calculation yielded a sample size of 6 individuals. Similarly, for the numbness measure, which was repeated 16 times, a sample size of 5 individuals was required. Consequently, the total number of participants in the study was 12 individuals.
Statistical analysis
All data was presented as mean ± SD. The normality and homogeneity of each variable were confirmed before the analyses using the Shapiro–Wilk test. One-way ANOVA was used to compare FMD among three occlusion pressures, while two-way repeated measured ANOVA within subjects was used for the pain and numbness scales. Bonferroni post hoc analysis was used to perform all pairwise comparisons between condition means. All p-values at 0.05 were considered to indicate statistical significance. All statistical procedures were performed using SPSS 17.0 (NY, USA). Partial Eta squared (η2) were classified as small, medium, and large (0.01, 0.06, and 0.14, respectively) (Richardson, 2011).
RESULTS
Subject characteristics
Age, gender, weight, height, body mass index (BMI), systolic and diastolic blood pressure, resting heart rate, and physical activities are presented in Table 1.
Table 1. Subject characteristics.
|
Subject characteristics |
Mean ± SD |
|
Age (year) |
28.08 ± 4.76 |
|
Gender (male/female) |
50/50 |
|
Weight (kg) |
64.28 ± 11.21 |
|
Height (cm) |
168.42 ± 7.89 |
|
BMI (kg/m2) |
22.59 ± 3.09 |
|
Systolic blood pressure (mmHg) |
118 ± 6.02 |
|
Diastolic blood pressure (mmHg) |
79.33 ± 6.77 |
|
Resting heart rate (beats/min) |
80.83 ± 13.76 |
|
Physical activity (METs/week) |
1677 ± 1346 |
Note: Values are presented as means ± SD, with gender shown as a percentage.
Vascular parameters
There were no statistically significant differences in the baselines of vascular diameter (F=0.319, P=0.73), MBFVbase (F=1.546, P=0.24), MBFVpost (F=2.180, P=0.14), and the percentage change of FMD (F=0.050, P=0.95) between these three levels of occlusion pressure (Table 2).
Table 2. Flow-mediated dilation response before and after three levels of occlusion pressures.
|
Value |
Occlusion Pressure |
F test |
P |
ɳ2 |
||
|
25 mmHg of supra SBP |
50 mmHg of supra SBP |
At 200 mmHg |
||||
|
Baseline diameter (mm) |
3.48 ± 0.62 |
3.46 ± 0.62 |
3.46 ± 0.67 |
0.31 |
0.73 |
0.28 |
|
Peak arterial diameter (mm) |
3.84 ± 0.66 |
3.81 ± 0.70 |
3.79 ± 0.72 |
0.65 |
0.48 |
0.06 |
|
MBFVbase (cm/s) |
2.63 ± 0.73 |
2.76 ± 0.85 |
2.84 ± 0.96 |
1.54 |
0.24 |
0.12 |
|
MBFVpost (cm/s) |
11.52 ± 3.96 |
12.54 ± 4.31 |
12.28 ± 4.30 |
2.18 |
0.14 |
0.17 |
|
FMD (%) |
10.11 ± 2.58 |
10.08 ± 2.7 |
10.15 ± 2.74 |
0.05 |
0.95 |
0.00 |
Note: Represented as means ± SD, s; second, ɳ2; partial Eta squared, cm; centimeter, mm; millimeter, mmHg; millimeter of mercury, FMD (%); percentage change of flow-mediated dilation, SBP; systolic blood pressure, MBFVbase; mean blood flow velocity at baseline, and MBFVpost; means blood flow velocity post occlusion.
Discomfort symptoms
Pain
During the 5-minute occlusion, the mean value of the pain scale of occlusion pressure at 25 mmHg, 50 mmHg, and 200 mmHg were 1.83 ± 0.98, 2.34 ± 1.27, and 3.63 ± 1.87, respectively, while the maximum value of the pain scale of those mentioned pressures were 2.58 ± 0.59, 3.42 ± 0.63, and 5.08 ± 0.8, respectively. After deflation, the pain scale of all different pressures remarkedly decreased to lower than 3, gradually decreased, and disappeared within 3 minutes. Pain scale significantly interacted with the occlusion pressure and time (F=1.79, p=0.016). The main effects on the occlusion pressure (F= 3.56, P =0.039) and time (F=43.66, P <0.0001) were significant for the pain scale. A significant difference was observed only in the pain scale between occlusion pressures of 25 mmHg and 200 mmHg at the time points of 1, 2, and 4 minutes (all P < 0.05) (Figure 1).
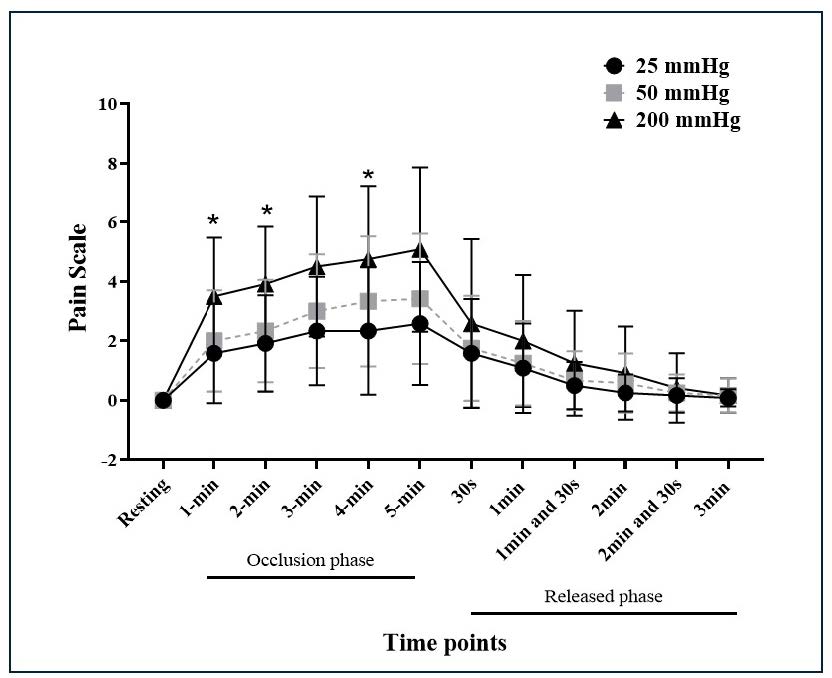
Figure 1. Pain scale for three levels of occlusion cuff pressures during the occlusion phase and released phase. * P < 0.05.
Numbness
During the 5-minute occlusion, the mean of the numbness scale of occlusion pressure at 25 mmHg, 50 mmHg, and 200 mmHg were 3.67 ± 2.02, 4.24 ± 2.38, and 4.72 ± 2.7, respectively, while the maximum value of the numbness scale of those mentioned pressures were 5.3 ± 0.71, 6.1 ± 0.59, and 7.36 ± 0.59, respectively. After deflation, the numbness scale gradually decreased from 6 to 0 within 5 minutes.
A significant main effect of time was observed for the numbness scale (F=17.4, P <0.0001). There was no main effect of occlusion pressure on the numbness scale (F=0.82, P=0.45). The numbness scale insignificantly interacted with the occlusion pressure and time (F=0.9, P=0.62) (Figure 2).
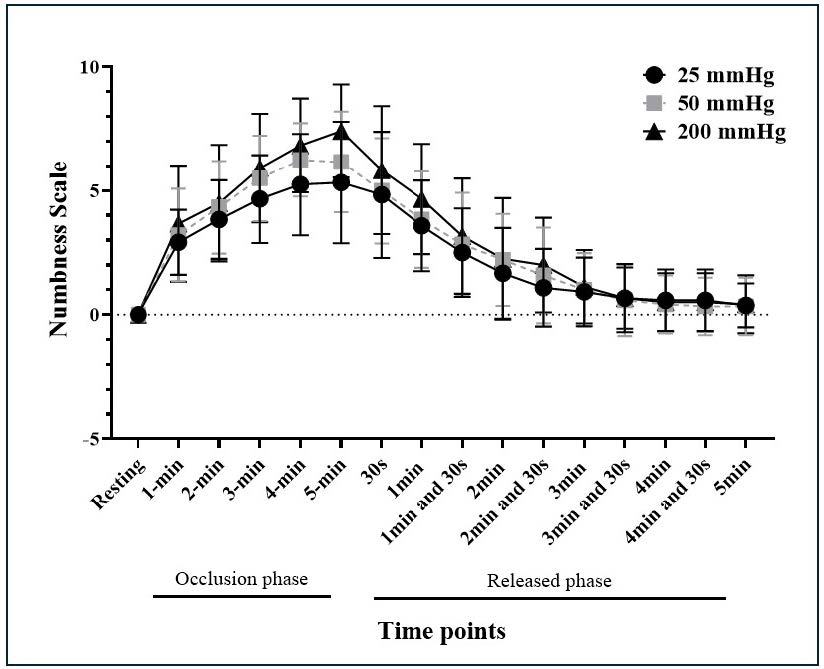
Figure 2. Numbness scale for three levels of occlusion cuff pressures during the occlusion phase and released phase. * P < 0.05.
DISCUSSION
The findings of this study demonstrated that an occlusion cuff pressure at 25 mmHg above SBP can induce mean values of %FMD and MBFVpost comparable to those of 50 mmHg above SBP and 200 mmHg. Notably, the pain sensation at a cuff pressure of 25 mmHg above SBP was significantly less than 200 mmHg during the first, second, and fourth minutes of cuff occlusion. The sensation of numbness was not affected by these occlusion cuff pressures.
Our findings align with those of previous study by McLay et al. (McLay, 2012), which found no significant differences in popliteal FMD measurements across five different occlusion pressures. In this study, the comparable results across pressure levels may be due to all the pressures used being above SBP. Such pressures are adequate to produce similar blood flow and shear stress, thereby inducing a NO response that causes vasodilation to a similar extent, as noted in previous research (Pyke and Tschakovsky, 2007; Thijssen et al., 2011). Additionally, our results support the majority of studies that used a cuff occlusion pressure of 200 mmHg or exceeding 50 mmHg above SBP, as per the expert consensus on FMD measurement (Thijssen et al., 2019). Importantly, we found that a cuff pressure of 25 mmHg above SBP is sufficient for measuring FMD in Thai adults.
Due to the lack of established reference ranges for FMD results, we compared the mean %FMD value of our participants to the reference value from Königstein et al. (2021), who used a cuff pressure of 50 mmHg above SBP (Königstein et al., 2021). Our findings showed a considerably higher %FMD value within the same age range. The high value can be considered normal, as an FMD value of less than 7.1% is used as a cut-off for diagnosing endothelial dysfunction (Maruhashi et al., 2020). Differences in ethnicity and geographical location between our participants and those in the Swiss study might account for these discrepancies. Another potential factor is the difference in physical activity (PA) levels. Our participants engaged in more PA (>600 METs/week) compared to those in the Königstein et al.’s study, where 95% of participants had at least 150 minutes of moderate-to-vigorous physical activity per week, roughly equivalent to 500 METs/week. Given that physical activity is known to improve endothelial function and slow the progression of vascular changes (Pahkala et al., 2011; Kozakova et al., 2013), this higher level of PA may explain the elevated %FMD values observed in our study.
Research on pain perception, which may vary by ethnicity, has reported that Asian individuals are more sensitive to pain than Caucasians and African American individuals (Ramer et al., 1999; Rowell et al., 2011). This contrasts with earlier studies predominantly involving White populations, which reported minimal discomfort or complications, with only a few participants experiencing pain or numbness (Bots et al., 2005; Thijssen et al., 2011). In our study, we observed a significant difference in pain sensation during cuff occlusion. A pressure of 200 mmHg produced an average pain score of 4.4 on the NPRS, approximately twice as much as the pain experienced at 25 mmHg above SBP (with an average NPRS score of 2.2) over the 5-minute occlusion period. A change of 1.65-2.0 points on the NPRS is considered a minimal clinically important difference, reflecting a meaningful change in pain status (Bahreini et al., 2020; Suzuki et al., 2020).
Additionally, numbness, a loss of sensation due to blood flow restriction, was observed in our participants but did not differ significantly across the three pressure levels. Di Maio et al. reported that increased cuff pressure enhances nociceptor activation (Di Maio et al., 2023), resulting in more intense pain as the pressure rises. In contrast, Daly et al. explained that numbness occurs when sensory nerves involved in touch and proprioception are compressed (Daly et al., 2012). Once these nerves are sufficiently compressed, additional increases in pressure do not significantly alter the sensation of numbness, which explains why numbness did not change with different pressure levels in the present study. Overall, these results suggest that a higher cuff pressure of 200 mmHg induces more pain compared to a lower pressure of 25 mmHg above SBP for Thai participants, although both pressures produce similar %FMD results. Therefore, using a minimum occlusion pressure of 25 mmHg above SBP, which effectively stimulates the FMD response while minimizing pain, may be a more practical approach for FMD assessment in healthy Thai adults.
The strength of this study lies in its novel approach to compare different cuff occlusion pressures commonly used for measuring FMD in healthy adults, considering both endothelial response and discomfort levels, such as pain and numbness. However, it is important to note that the FMD procedure used in this study elicits only a reactive hyperemic stimulus, which is predominantly reflects endothelium-dependent and NO-mediated vasodilation, rather than endothelium-independent vasodilation. Additionally, habituation typically occurs with repeated stimuli without adequate rest (Rankin et al., 2009). In our study, we mitigated this by inducing discomfort through a single sustained stimulus during each occlusion. Discomfort subsided within 3-5 minutes post-deflation, followed by a 25-minute rest before the next trial. This 30-minute rest period minimized habituation effects, ensuring reliable results regarding unpleasant sensations and their impact on outcome measures (Rankin et al., 2009). This adequate rest interval is another strength of our study.
There are some limitations to this study. Firstly, the absence of lipid profile and glycemic index measurements, which can influence vascular function, is a notable limitation. However, the crossover design of the study mitigates these concerns to some extent. Secondly, the within-subject design could potentially lead to carryover effects. To address this, we randomly assigned the order of the pressure conditions and included 30-minute rest intervals between each condition to ensure that arterial data returned to baseline levels. Finally, the small effect size observed in %FMD suggests that a larger sample size is necessary for more robust and reliable results. Further studies are warranted to confirm these findings.
CONCLUSION
Occlusion cuff pressures at 25 mmHg above SBP induced brachial FMD responses similar to those at 50 mmHg above SBP and 200 mmHg, but with less pain. These findings suggest that 25 mmHg above SBP is a practical occlusion pressure for FMD assessment in healthy Thai adults.
ACKNOWLEDGEMENTS
We acknowledge the support from the Program in Biomedical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University, through the CMU Presidential Scholarship.
AUTHOR CONTRIBUTIONS
Study design and concept– Kanpiraya Nithitsuttibuta and Sainatee Pratanaphon; Study conduct and data collection– Kanpiraya Nithitsuttibuta and Jaruta Kunritt; Technical support for vascular assessment– Suchart Kiatwattanacharoen; analysis, interpretation, and drafted manuscript– Kanpiraya Nithitsuttibuta. and Sainatee Pratanaphon. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Agewall, S., Doughty, R.N., Bagg, W., Whalley, G.A., Braatvedt, G., and Sharpe, N. 2001. Comparison of ultrasound assessment of flow-mediated dilatation in the radial and brachial artery with upper and forearm cuff positions. Clinical Physiology. 21(1): 9-14.
Bahreini, M., Safaie, A., Mirfazaelian, H., and Jalili, M. 2020. How much change in pain score does really matter to patients? The American Journal of Emergency Medicine. 38(8): 1641-1646.
Bots, M.L., Westerink, J., Rabelink, T.J., and de Koning, E.J. 2005. Assessment of flow-mediated vasodilatation (fmd) of the brachial artery: Effects of technical aspects of the fmd measurement on the fmd response. European Heart Journal. 26(4): 363-368.
Broxterman, R.M., Witman, M.A., Trinity, J.D., Groot, H.J., Rossman, M.J., Park, S.-Y., Malenfant, S., Gifford, J.R., Kwon, O.S., Park, S.H. et al. 2019. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension. 74(1): 208-215.
Cleland, C.L., Hunter, R.F., Kee, F., Cupples, M.E., Sallis, J.F., and Tully, M.A. 2014. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 14(1): 1255.
Corretti, M.C., Anderson, T.J., Benjamin, E.J., Celermajer, D., Charbonneau, F., Creager, M.A., Deanfield, J., Drexler, H., Gerhard-Herman, M., Herrington, D. et al. 2002. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. Journal of the American College of Cardiology. 39(2): 257-265.
Cortés, O.L., Moreno, K., Alvarado, P., Povea, C., Lloyd, M., and Dennis, R. 2018. Inactivity and its associated factors in adults scheduled for noncardiac surgery: The pamp phase i study. Rehabilitation Nursing. 43(2): 81-87.
Daly, B.P., Eichen, D.M., Bailer, B., Brown, R.T., and Buchanan, C.L. 2012. Central nervous system. p.454-459. In: V.S. Ramachandran [ed] Encyclopedia of human behavior. 2nd ed. Academic Press, San Diego.
Di Maio, G., Villano, I., Ilardi, C.R., Messina, A., Monda, V., Iodice, A.C., Porro, C., Panaro, M.A., Chieffi, S., Messina, G. et al. 2023. Mechanisms of transmission and processing of pain: A narrative review. International Journal of Environmental Research and Public Health. 20(4): 3064.
Edwards, R.R. 2005. Pain assessment. p.29-34. In: H.T. Benzon, S.N. Raja, R.E. Molloy, S.S. Liu, S.M. Fishman [eds] Essentials of pain medicine and regional anesthesia. 2nd ed. Churchill Livingstone, Philadelphia.
Estebe, J.P., Le Naoures, A., Chemaly, L., and Ecoffey, C. 2000. Tourniquet pain in a volunteer study: Effect of changes in cuff width and pressure. Anaesthesia. 55(1): 21-26.
Harris, Nishiyama, Wray, D.W., and Richardson, R.S. 2010. Ultrasound assessment of flow-mediated dilation. Hypertension. 55(5): 1075-1085.
Heiss, C., Rodriguez-Mateos, A., Bapir, M., Skene, S.S., Sies, H., and Kelm, M. 2022. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovascular Research. 119(1): 283-293.
Hongwen, Y., Pei, W., Nijuan, H., Peng, Z., Chunhua, L., Yuqi, L., Liangxiao, M., and Jiang, Z. 2018. A review of the methods used for subjective evaluation of De Qi. Journal of Traditional Chinese Medicine. 38(2): 309-314.
Inaba, Y., Chen, J.A., and Bergmann, S.R. 2010. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. International Journal of Cardiovascular Imaging. 26(6): 631-640.
Katz, J. and Melzack, R. 1999. Measurment of pain. Surgical Clinics of North America. 79(2): 231-252.
Königstein, K., Wagner, J., Frei, M., Knaier, R., Klenk, C., Carrard, J., Schwarz, A., Hinrichs, T., and Schmidt-Trucksäss, A. 2021. Endothelial function of healthy adults from 20 to 91 years of age: Prediction of cardiovascular risk by vasoactive range. Journal of Hypertension. 39(7): 1361-1369.
Kozakova, M., Balkau, B., Morizzo, C., Bini, G., Flyvbjerg, A., and Palombo, C. 2013. Physical activity, adiponectin, and cardiovascular structure and function. Heart and vessels. 28: 91-100.
Krupić, F., Čustović, S., Jašarević, M., Šadić, S., Fazlić, M., Grbic, K., and Samuelsson, K. 2019. Ethnic differences in the perception of pain: A systematic review of qualitative and quantitative research. Medicinski Glasnik. 16(1): 108-114.
Maher, J.M., Markey, J.C., and Ebert-May, D. 2013. The other half of the story: Effect size analysis in quantitative research. Cell Biology Education-A Journal of Life Science Education. 12(3):345-351.
Maruhashi, T., Kajikawa, M., Kishimoto, S., Hashimoto, H., Takaeko, Y., Yamaji, T., Harada, T., Han, Y., Aibara, Y., Mohamad Yusoff, F. et al. 2020. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. Journal of the American Heart Association. 9(2):e013915.
McLay, K.M. 2012. Reliability of flow-mediated dilation measures in the popliteal artery and implications for use in clinical and research practices. Master's Thesis. The University of Western Ontario, Ontario, Canada.
Mitrichev, A., Maunder, J., Jabur, A., Singh, P., Lees, D., Morse, L., and Parkinson, B. 2024. Randomised controlled trial of tourniquet associated pain generated in lower limb after exsanguination by esmarch bandage versus limb elevation. Journal of Orthopaedic Surgery and Research. 19(1): 276.
Moens, A.L., Goovaerts, I., Claeys, M.J., and Vrints, C.J. 2005. Flow-mediated vasodilation: A diagnostic instrument, or an experimental tool? Chest. 127(6): 2254-2263.
Mućka, S., Miodońska, M., Jakubiak, G.K., Starzak, M., Cieślar, G., and Stanek, A. 2022. Endothelial function assessment by flow-mediated dilation method: A valuable tool in the evaluation of the cardiovascular system. International Journal of Environmental Research and Public Health. 19(18): 11242.
Pahkala, K., Heinonen, O.J., Simell, O., Viikari, J.S., Rönnemaa, T., Niinikoski, H., and Raitakari, O.T. 2011. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. 124(18): 1956-1963.
Pyke, K.E. and Tschakovsky, M.E. 2007. Peak vs. total reactive hyperemia: Which determines the magnitude of flow-mediated dilation? Journal of Applied Physiology. 102(4):1510-1519.
Ramer, L., Richardson, J.L., Cohen, M.Z., Bedney, C., Danley, K.L., and Judge, E.A. 1999. Multimeasure pain assessment in an ethnically diverse group of patients with cancer. Journal of Transcultural Nursing. 10(2): 94-101.
Rankin, C.H., Abrams, T., Barry, R.J., Bhatnagar, S., Clayton, D.F., Colombo, J., Coppola, G., Geyer, M.A., Glanzman, D.L., Marsland, S. et al. 2009. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 92(2):135-138.
Ray A, Ch. Maharana K, Meenakshi S, Singh S. 2023. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sciences Review. 7:100084.
Richardson, J. 2011. Eta squared and partial eta squared as measures of effect size in educational research. Educational Research Review. 6(2):135-147.
Rodriguez-Miguelez, P., Seigler, N., and Harris, R.A. 2016. Ultrasound assessment of endothelial function: A technical guideline of the flow-mediated dilation test. Journal of Visualized Experiments. (110):54011.
Rowell, L., Mechlin, B., Ji, E., Addamo, M., and Girdler, S. 2011. Asians differ from non-hispanic whites in experimental pain sensitivity. European Journal of Pain. 15(7): 764-771.
Sarriff, A. M., Amin, A., and Mostafa, H. 2014. Public knowledge and awareness of cardiovascular diseases and the expected role of community pharmacists in the prevention and management of cardiovascular diseases in Penang, Malaysia. Chiang Mai University Journal of Natural Sciences. 13(3): 355-370.
Seals, D.R., Jablonski, K.L., and Donato, A.J. 2011. Aging and vascular endothelial function in humans. Clinical Science. 120(9): 357-375.
Senaratna, C.V., Perret, J.L., Lodge, C.J., Lowe, A.J., Campbell, B.E., Matheson, M.C., Hamilton, G.S., and Dharmage, S.C. 2017. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Medicine Reviews. 34: 70-81.
Shechter, M., Shechter, A., Koren-Morag, N., Feinberg, M.S., and Hiersch, L. 2014. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. American Journal of Cardiology. 113(1): 162-167.
Spitz, R.W. Wong, V., Bell, Z.W., Viana, R.B., Chatakondi, R.N., Abe, T., and Loenneke, JP. 2022. Blood flow restricted exercise and discomfort: A review. The Journal of Strength & Conditioning Research. 36(3): 871-879.
Sturm, W., Sandhofer, A., Engl, J., Laimer, M., Molnar, C., Kaser, S., Weiss, H., Tilg, H., Ebenbichler, C.F., and Patsch, J.R. 2009. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity (Silver Spring). 17(9): 1783-1788.
Suzuki, H., Aono, S., Inoue, S., Imajo, Y., Nishida, N., Funaba, M., Harada, H., Mori, A., Matsumoto, M., Higuchi, F. et al. 2020. Clinically significant changes in pain along the pain intensity numerical rating scale in patients with chronic low back pain. PLoS One. 15(3): e0229228.
Thijssen, D.H., Black, M.A., Pyke, K.E., Padilla, J., Atkinson, G, Harris, R.A., Parker, B., Widlansky, M.E., Tschakovsky, M.E., and Green, DJ. 2011. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American Journal of Physiology Heart and Circulatory Physiology. 300(1): H2-H12.
Thijssen, D.H., Bullens, L.M., van Bemmel, M.M., Dawson, E.A., Hopkins, N., Tinken, T.M., Black, M.A., Hopman, M.T., Cable, N.T., and Green, D.J. 2009. Does arterial shear explain the magnitude of flow-mediated dilation?: A comparison between young and older humans. American Journal of Physiology Heart and Circulatory Physiology. 296(1): H57-64.
Thijssen, D.H.J., Bruno, R.M., van Mil, A., Holder, S.M., Faita, F., Greyling, A., Zock, P.L., Taddei, S., Deanfield, J.E., Luscher, T. et al. 2019. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. European Heart Journal. 40(30): 2534-2547.
Whelton Paul, K., Carey Robert, M., Aronow Wilbert, S., Casey Donald, E., Collins Karen, J., Dennison Himmelfarb, C., DePalma, Sondra, M., Gidding, S., Jamerson Kenneth, A., Jones Daniel, W. et al. 2018. Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Journal of the American College of Cardiology. 71(19): e127-e248.
Wisløff, U., Støylen, A., Loennechen, J.P., Bruvold, M., Rognmo, Ø., Haram, P.M., Tjønna, A.E., Helgerud, J., Slørdahl, S.A., Lee, S.J. et al. 2007. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 115(24): 3086-3094.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kanpiraya Nithitsuttibuta1, Jaruta Kunritt1, Suchart Kiatwattanacharoen2, and Sainatee Pratanaphon1, *
1 Department of Physical Therapy, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Sainatee Pratanaphon, E-mail: sainatee.pra@cmu.ac.th
ORCID: Kanpiraya Nithitsuttibuta: https://orcid.org/0009-0002-6251-6910
Total Article Views
Editor: Waraporn Boonchieng,
Chiang Mai University, Thailand
Article history:
Received: December 18, 2023;
Revised: July 23, 2024;
Accepted: July 31, 2024;
Online First: August 9, 2024

