
Comprehensive Analysis of Conserved B-Cell Epitopes in DENV NS1 Protein for Enhanced Rapid Diagnostic Tests Development in Indonesia
Yunita Messe, Syahputra Wibowo, Hery Wijayanto, Wayan Tunas Artama, Fajar Sofyantoro, and Nastiti Wijayanti*Published Date : July 23, 2024
DOI : https://doi.org/10.12982/NLSC.2024.049
Journal Issues : Number 4, October-December 2024
Abstract Dengue virus (DENV) constitutes a formidable public health threat within tropical and subtropical regions. The escalation of cases in Southeast Asia, notably Indonesia, is a matter of considerable concern. Timely identification of DENV infection remains imperative for efficient disease management. The non-structural protein-1 (NS1), distinguished by its heightened immunogenicity and early detectability during infection, stands as a pivotal target for diagnostic modalities. The current investigation delves into the exploration of B-cell epitopes within Indonesian DENV NS1 protein isolates, with the overarching objective of enhancing the sensitivity and specificity of early detection systems, such as the Rapid Diagnostic Test (RDT). This study has successfully delineated five B-cell epitopes that exhibit conservation across DENV serotypes within Indonesian isolates (accessions number: QBB90021.1, QBE90252.1, QBB90023.1, and UDW38833.1). These epitopes were discerned through comprehensive screening leveraging the IEDB platforms. Noteworthy variations in antigenicity, allergenicity, and toxicity profiles were observed among these identified epitopes. Molecular docking analysis substantiated a robust binding affinity between the predicted epitope and the B-cell receptor. The ensuing in silico protein-peptide docking analyses offer valuable insights into potential B-cell epitopes on the Indonesian DENV NS1 protein. The identified epitopes, particularly the SQHNYRPGY epitope characterized by its antigenic potency and non-allergenic attributes, hold promise for advancing the development of sensitive and specific RDTs tailored for DENV detection in the Indonesian context. However, it is imperative to underscore the requisite for subsequent experimental validation to affirm the efficacy of these epitopes in diagnostic applications.
Keywords: Dengue, Immunogenicity, Antigenicity, Allergenicity, Immuno-informatics
Funding: This work was supported by Riset Inovatif Produktif (RISPRO) Invitasi No. PRJ-18/LPDP /LPDP.4/2022.
Citation: Messe, Y., Wibowo, S., Wijayanto, H., Artama, W.T., Sofyantoro, F., and Wijayanti, N. 2024. Comprehensive analysis of conserved b-cell epitopes in DENV NS1 protein for enhanced rapid diagnostic tests development in Indonesia. Natural and Life Sciences Communications. 23(4): e2024049.
INTRODUCTION
Dengue virus (DENV), a member of the Flavivirus genus within the Flaviviridae family, represents a significant threat to public health in tropical and subtropical regions across Asia, America, Africa, the Pacific, and the Caribbean (Roy and Bhattacharjee, 2021; Jantakee et al., 2023). Human infection with DENV is primarily vectored by female Aedes mosquitoes, including Aedes aegypti and Aedes albopictus (Higa, 2011; Surendran et al., 2022). Classified into four serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), these serotypes exhibit close phylogenetic relatedness, sharing approximately 65% amino acid identity (Tuiskunen Bäck and Lundkvist, 2013; Harapan et al., 2020). The distinct serotypes of DENV contribute to a spectrum of health complications in humans, elevating the propensity for severe outcomes. Within Southeast Asia, dengue cases witnessed a 46% escalation from 2015 to 2019, reaching 658,301, while the mortality rate experienced a modest 2% decline (from 1,584 to 1,555) during the same period (Utama et al., 2019). Indonesia, situated in Southeast Asia, grapples with endemic dengue infections, marked by sporadic outbreaks in various regions (Utama et al., 2019). Over the past five decades, Indonesia has observed a substantial increase in the incidence rate of dengue fever, exhibiting a cyclic pattern with peaks occurring approximately every 6 to 8 years, juxtaposed with a decreasing trend in the annual case fatality rate (Harapan et al., 2019; Gan et al., 2021).
The genomic composition of DENV comprises positive-sense single-stranded RNA (+ssRNA), encompassing three structural proteins (Envelope, pre-Membrane, Capsid) and seven non-structural proteins, including Non-Structural-1 (NS-1), NS-2A/2B, NS-3, NS-4A/4B, and NS-5 (Harapan et al., 2020; Hidayatullah et al., 2020). The NS1 protein, a highly conserved glycoprotein (43–48 kDa), is prominently present in-patient serum during the early clinical phase of the disease, spanning from day 1 to day 9 following the onset of fever. Synthesized in the rough endoplasmic reticulum of infected cells, NS-1 initially exists as a hydrophilic monomeric protein, undergoing subsequent transformation into a more hydrophobic homodimer. NS-1 manifests itself either intracellularly, at the plasma membrane, or through extracellular secretion. Notably, the presence of NS-1 at the plasma membrane contributes to its involvement in the immunopathological processes of infection. With the highest level of immunogenicity for inducing antibody formation, NS-1 can be detected as early as the second day of virus onset, thereby facilitating early diagnosis of dengue infection without relying on antibody formation (Rocha et al., 2017).
Various laboratory diagnostic methods for DENV infection include real-time reverse transcription-polymerase chain reaction (RT-PCR) for viral nucleic acid detection, enzyme-linked immunosorbent assay (ELISA) for viral antigen detection, and hemagglutination inhibition (HI), complement fixation test (CF), neutralization test, and ELISA for anti-dengue virus antibody detection. Rapid Diagnostic Tests (RDTs) have recently been developed, relying on the detection of NS1 antigen and anti-dengue antibodies in patient blood. However, the production of these RDTs in Indonesia remains heavily reliant on imported NS-1 antibody products. Tailoring specific NS-1 antibodies based on prevalent DENV strains in Indonesia holds promise for enhancing the sensitivity and specificity of locally produced RDTs, thus mitigating misdiagnosis (Jones et al., 2017). To initiate the production of NS-1 DENV antibodies, the first step involves a bioinformatics investigation of NS-1 gene sequences derived from Indonesian patient isolates, serving as the foundation for subsequent NS-1 antigen synthesis.
This study employed an in-silico approach, utilizing B-cell epitope prediction tools from the Immune Epitope Database (IEDB) to identify B-cell epitopes within the DENV NS-1 protein. Subsequent molecular docking studies ascertain the binding affinity between the predicted epitope peptides and the B-cell receptor of Mus musculus. HPEPDOCK, as highlighted by Weng et al., (2020), emerged as the optimal program for protein−peptide docking in this comprehensive study. The primary objective of this study was to scrutinize all Indonesian DENV serotype isolates, focusing on conserved regions in the NS-1 protein for preliminary investigations toward monoclonal antibody synthesis, with the aim of producing more sensitive and specific RDTs tailored for deployment in Indonesia.
MATERIALS AND METHODS
Study design
This study employed an in-silico methodology, leveraging diverse online databases, platforms, websites, and software tools. The data collection occurred in September 2023, utilizing resources from the UniProt and NCBI databases. Subsequently, a thorough interpretation and comparative analysis were conducted, integrating comprehensive literature reviews and information from online repositories.
Within the scope of this study, our analysis focused on the structural examination of the NS1 protein. We specifically engineered NS1 protein epitopes derived from dengue virus serotypes (DENV 1-4) originating from the Indonesian population. This analytical endeavor also encompassed the execution of peptide-protein docking procedures. This investigative approach adhered to the framework elucidated by Abesamis et al., (2022) and Biswas et al., (2022), with necessary modifications introduced for methodological refinement.
Analysis of Indonesian DENV NS1 protein sequences
The amino acid sequences of NS1 proteins pertaining to each Dengue virus (DENV) serotype were retrieved from UniProt (https://www.uniprot.org/) and NCBI (https://www.ncbi.nlm.nih.gov/) based on their respective accession numbers. Only NS1 protein sequences originating from Indonesian isolates were selected and obtained in FASTA format from the extensive database search. Subsequent analysis of these sequences employed the MEGA X software, utilizing the ClustalW tool for multiple sequence alignment and phylogenetic tree generation. The processed data obtained from the databases underwent further examination through VaxiJen 2.0 (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html), with a predefined threshold value set at 0.5. This step facilitated the prediction of antigenicity scores for the Indonesian NS1 protein corresponding to each DENV serotype. Notably, the sequences with the most recent year of data collection were selected as templates for the design of epitopes within the DENV NS1 protein. Each selected NS1 protein sequence, representing all DENV serotypes, was subsequently modelled into a three-dimensional structure in pdb format utilizing SWISSMODEL (https://swissmodel.expasy.org/). The resulting structures were subjected to detailed analysis using PDBsum (https://www.ebi.ac.uk/thorntonsrv/databases/pdbsum/Generate.html)
Prediction of B-cell epitopes
For each NS1 protein sequence pertaining to the Dengue Virus (DENV) serotypes, B-cell epitopes were predicted using the Epitope Prediction tools available on the Immune Epitope Database and Analysis Resource (IEDB) platform (http://tools.iedb.org/main/bcell/). This prediction involved the utilization of Bepipred Linear Epitope Prediction, Emini Surface Accessibility Prediction, and Kolaskar & Tongaonkar Antigenicity Prediction. Subsequently, the identified B-cell epitope candidates were transferred to Microsoft Excel for the purpose of ascertaining consensus and unique epitopes across all DENV serotypes (DENV 1-4), as outlined in studies by Abesamis et al. (2022) and Adnan et al. (2020). Furthermore, the three-dimensional structural of each candidate epitope were predicted employing the PEPFOLD algorithm (https://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py#forms::PEP-FOLD), followed by meticulous analysis through PDBsum (https://www.ebi.ac.uk/thornton-srv/databases/pdbsum/Generate.html), and visualization using pyMOL software (pymol.org), referencing the parameters outlined in Biswas et al. (2022) and Wibowo et al. (2022). Additionally, the allergenicity, antigenicity, and toxicity profiles of each epitope candidate were discerned through computational tools, namely AllerCatPro 2.0 (https://allercatpro.bii.a-star.edu.sg/), VaxiJen v2.0 (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html), and ToxinPred2 (https://webs.iiitd.edu.in/raghava/toxinpred2/batch.html), respectively.
Protein receptor characterization and preparation
In this study, we employed the B-cell antigen receptors sourced from Mus musculus (PDB ID: 8EMA) to predict epitopes intended for immunization of mice. Our subsequent research will focus on immunizing mice to elicit spleen-derived B cells that secrete antibodies against the NS1 DENV. These B cells will be fused with myeloma cells to generate hybridomas capable of producing monoclonal antibodies specific to the NS1 DENV antigen on a large scale. These antibodies are crucial for the development of Rapid Diagnostic Tests (RDTs).
The protein information was extracted from the Protein Data Bank (http://www.rcsb.org/pdb). To ensure the integrity of our analysis, the protein structure underwent meticulous pre-processing through the removal of ligands, heteroatoms, and solvent molecules, executed using the Discovery Studio 2016 Client application, culminating in the preservation of the structure in (.pdb) format. To further elucidate the intricate details of the protein-ligand binding sites, an exhaustive examination was performed using the PrankWeb tool (https://prankweb.cz/).
Molecular docking
The B-cell antigen receptor (8EMA) and its anticipated epitopes served as the receptor and ligand entities, respectively, in the conducted molecular docking investigation. The molecular docking procedure employed the HPEPDOCK algorithm, a tool demonstrated by Zhou et al., (2018). HPEPDOCK, a server distinguished for its hierarchical methodology, specializes in the blind docking of peptides to proteins. Within the docking algorithm, each identified peptide (epitope) was designated as a ligand, while the molecular structure of the B-cell was assigned as the receptor. The resultant binding affinities from each docking event were meticulously recorded. Subsequently, the PDB file format derived from the docking outcomes underwent pre-processing using both pyMOL software and Discovery Studio 2016 Client, facilitating the subsequent analytical stages.
Protein-protein docking assessment and validation
The interaction between the B cell antigen receptor and the predicted peptide (epitope) was determined using the Ligplot program integrated in PDBsum (https://www.ebi.ac.uk/ thorntonsrv/databases/pdbsum/Generate.html). Ligplot was used to examine the amino acid residues involved in the binding of protein-ligand complexes by 2-D schematic representation. Since the B-cell antigen receptor (8EMA) has no native ligand, ligand binding site prediction from protein structure was compared with the results from PrankWeb. These assessment and validation steps are necessary to provide better understanding about the functional behavior and potential interactions between epitope and B cell antigen receptor (Carbery et al., 2024).
RESULTS
Sequence analysis of Indonesian DENV NS1 protein
In this study, a dataset encompassing 72 sequences of the Dengue virus (DENV) NS1 protein from Indonesian isolates were obtained. The dataset includes sequences with the oldest sampling recorded in 1978 and the most recent in 2019. The sequences were systematically retrieved from authoritative repositories, including UniProt and NCBI, ensuring a balanced representation across all four DENV serotypes (DENV 1-4), with sequence counts of 23, 9, 22, and 18, respectively.
The analytical framework employed MEGA X, a robust bioinformatics tool tailored for the scrutiny of biological sequences. The computational examination involved a meticulous assessment of the sequences, culminating in the computation of the overall mean distance. Subsequently, a phylogenetic tree was constructed through the execution of 1,000 replications using the bootstrap method and the unweighted group method with arithmetic mean (UPGMA). Figure 1 portrays the resulting phylogram, generated using MEGA X. The findings of this investigation reveal the presence of closely related protein sequences, as indicated by a discernible total mean distance of 0.02. This observation underscores the intricate evolutionary relationships inherent within the Indonesian DENV NS1 protein sequences, providing valuable insights into their phylogenetic interplay.
The most recent collection data from each DENV serotype isolated in Indonesia, available in the online database, was chosen for further examination. Specifically, DENV-1, isolated in 2015 from Jambi (QBB90021.1), DENV-2, isolated in 2016 from Jambi (QBE90252.1), DENV-3, isolated in 2016 from Samarinda (QBB90023.1), and DENV-4, isolated in 2019 from Jember (UDW38833.1) were included in the analysis (Table 1, Figure 2). Subsequently, the VaxiJen v2.0 web-based server was employed to assess the antigenicity of each sequence.
The SWISSMODEL web service was employed to predict the 3-dimensional configuration of DENV 1-4 proteins. The outcome of the prediction indicated that there are no notable distinctions in the 3-dimensional structure of DENV 1-4. In terms of secondary structure composition, the proportions of Sheet, Beta hairpins, Beta bulges, Disulphides, Helix-helix interactors, and overall trends in the 3-dimensional DENV 1-4 proteins exhibit identical compositions. The only variation observed lies in the quantities of compositions within Beta turns, Gamma turns, and Helix (Table 2). However, these differences in the three types of secondary structure were deemed inconsequential.
The NS1 protein structure in all DENV subtypes consists of two protein chains, denoted as chains A and B, interconnected by distinct bonds. In the NS1 protein of DENV-1 and DENV-2, protein-protein interfaces exhibit a salt bridge, unlike DENV-3 and DENV-4 where no such bridge is present (Figure 3). Analysis of interactions in DENV-1 and DENV-2 reveals three types: salt bridge bonds, hydrogen bonds, and non-bonded contacts. Specifically, DENV-1 displays one salt bridge interaction, 17 hydrogen bond interactions, and 205 non-bonded contact interactions. In contrast, DENV-2 exhibits four salt bridge interactions, 29 hydrogen bond interactions, and 247 non-bonded contact interactions.
Conversely, analysis of DENV-3 and DENV-4 structures indicates only two types of interactions: hydrogen bonding and non-bonded contacts. DENV-3 and DENV-4 each show 25 hydrogen bond interactions, with 263 non-bonded contact interactions in DENV-3 and 237 in DENV-4. These interactions arise from the collaborative activity of two chains, forming a 3-dimensional structure of the NS1 serotype protein across DENV 1-4. The varying abundance of bonds reflects the distinct interaction profiles observed in each NS1 serotype.
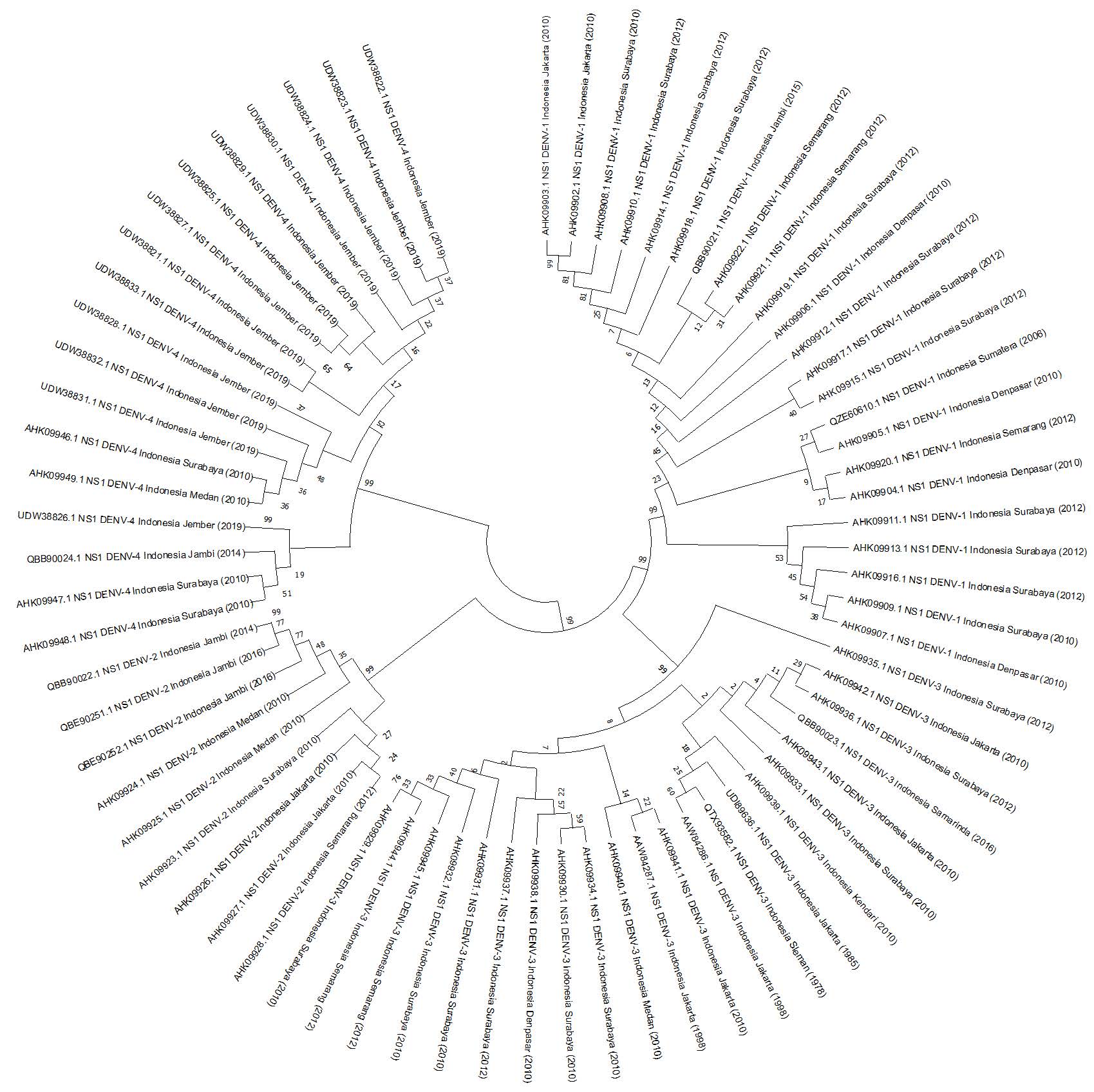
Figure 1. Analysis of evolutionary divergence of 72 sequences of the Non-Structural-1 (NS1) protein from available Dengue virus (DENV) data. The outcomes were visualized in a phylogenetic tree, with branches representing partitions not shown if their bootstrap values (1000 replicates) were less than 0.50.
Table 1. Selected sequences of Indonesian DENV NS1 protein.
|
Serotype |
Accessions UniProt |
Collection Date |
Country |
Antigenicity Score |
|
DENV-1 |
QBB90021.1 |
2015 |
Jambi, Indonesia |
0.5271 |
|
DENV-2 |
QBE90252.1 |
2016 |
Jambi, Indonesia |
0.6096 |
|
DENV-3 |
QBB90023.1 |
2016 |
Samarinda, Indonesia |
0.4869 |
|
DENV-4 |
UDW38833.1 |
2019 |
Jember, Indonesia |
0.4897 |
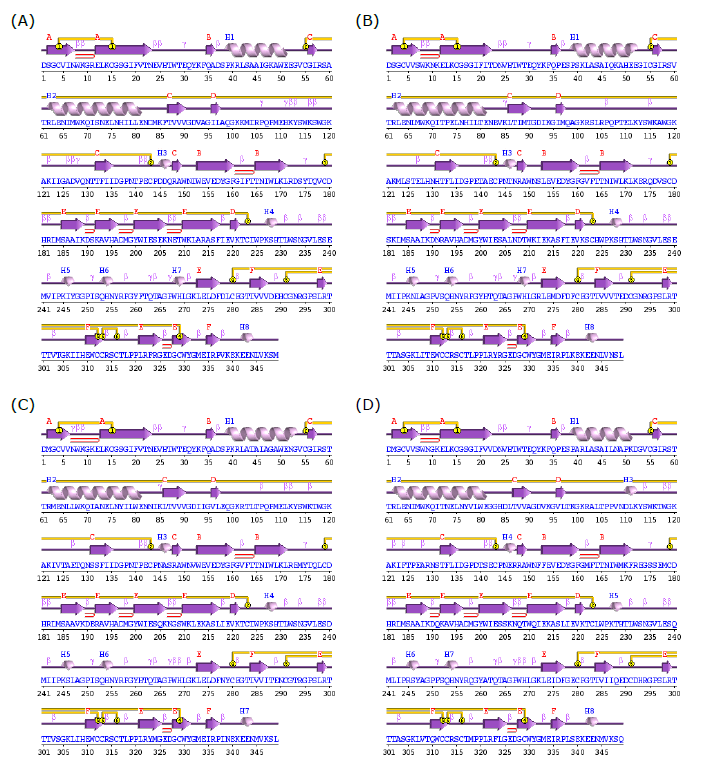
Figure 2. Predicted secondary structure of DENV NS1 protein Built by PDBsum. (A) DENV-1, (B) DENV-2, (C) DENV-3, and (D) DENV-4.
Table 2. The motifs in secondary structure of DENV NS1 protein.
|
Secondary Structure |
DENV-1 |
DENV-2 |
DENV-3 |
DENV-4 |
|
Sheets |
6 |
6 |
6 |
6 |
|
Beta hairpins |
6 |
6 |
6 |
6 |
|
Beta bulges |
3 |
3 |
3 |
3 |
|
Strands |
22 |
22 |
22 |
22 |
|
Helices |
8 |
8 |
7 |
8 |
|
Helix-helix interac |
1 |
1 |
1 |
1 |
|
Beta turns |
38 |
34 |
38 |
39 |
|
Gamma turns |
10 |
7 |
8 |
6 |
|
Disulphides |
6 |
6 |
6 |
6 |
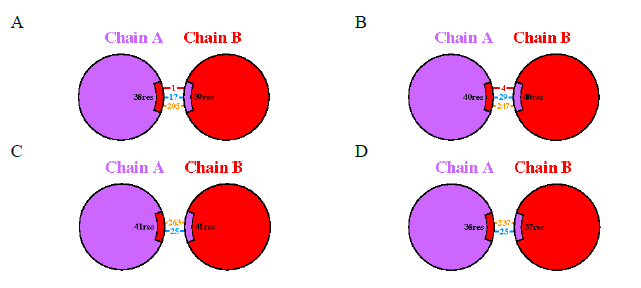
Figure 3. Predicted protein-protein interface of DENV NS1 protein Models Built by PDBsum. (A) DENV-1, (B) DENV-2, (C) DENV-3, and (D) DENV-4. Red line indicates salt bridges, blue line indicates hydrogen bonds, and orange line indicates non-bonded contacts.
B-cell epitope identification and conservancy analysis
The IEDB server was employed for predicting epitopes in each DENV subtype using three prediction models: Bepipred Linear Epitope Prediction, Emini Surface Accessibility Prediction, and Kolaskar & Tongaonkar Antigenicity Prediction. The alignment outcomes for all identified epitope candidates in IEDB indicate the conservation of five epitopes across all DENV subtypes—specifically, WTEQYKFQADSP, HTWTEQYKFQ, VHTWTEQYKFQ, SQHNYRPGY, and RGPSLRTT (Figure 4). The 3-dimensional protein structure analysis of these epitope candidates reveals a composition of α-helix and random coil shapes (Figure 5). Furthermore, an additional set of 32 data points collected from Salatiga between 2019 and 2022, encompassing DENV1-4 serotypes (unpublished data), also confirms the presence of these same epitopes.
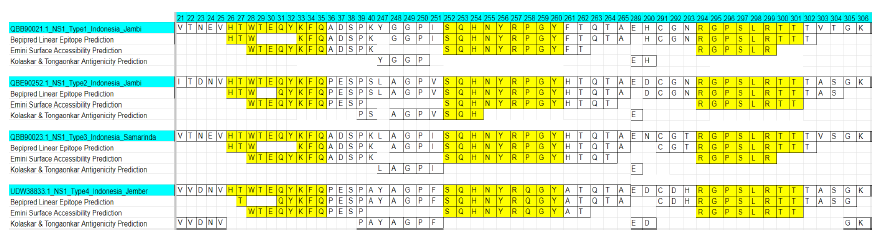
Figure 4. The consensus and unique region across all dengue virus serotypes (DENV 1-4).

Figure 5. The 3-dimensional structure of 5 candidate epitopes. (A) HTWTEQYKFQ, (B) RGPSLRTT, (C) SQHNYRPGY, (D) VHTWTEQYKFQ, and (E) WTEQYKFQADSP.
The assessment of allergenicity, antigenicity, and toxicity for each epitope candidate indicates that the SQHNYRPGY epitope exhibits optimal characteristics, specifically being Non-Allergen, Antigen, and Non-Toxin (Table 3). Evaluation of the five epitope predictions reveals that all five are non-allergenic, with antigenicity scores of -0.1449, -0.1544, -0.4471, 1.4265, and 0.3661. While the antigenic properties of four epitopes are non-antigenic, the SQHNYRPGY epitope stands out as antigenic. Additionally, toxicity analysis identifies the four epitopes as non-toxic, except for the RGPSLRTT epitope, which is classified as a toxin. Considering toxicity parameters is crucial to anticipate the characteristics of individual epitopes when introduced into the body, thereby preventing potential failures or instances of poisoning.
Table 3. Potential B-cell epitopes of Indonesian DENV NS1 protein.
|
Position |
Epitope Sequence |
Allergenicity |
Antigenicity |
Antigen/Non-Antigen |
Toxicity |
|
26-35 |
HTWTEQYKFQ |
Non-Allergen |
-0.1449 |
Non-Antigen |
Non-Toxin |
|
28-39 |
WTEQYKFQADSP |
Non-Allergen |
-0.1544 |
Non-Antigen |
Non-Toxin |
|
25-35 |
VHTWTEQYKFQ |
Non-Allergen |
-0.4471 |
Non-Antigen |
Non-Toxin |
|
252-260 |
SQHNYRPGY |
Non-Allergen |
1.4265 |
Antigen |
Non-Toxin |
|
294-301 |
RGPSLRTT |
Non-Allergen |
0.3661 |
Non-Antigen |
Toxin |
Characterization B-cell antigen receptor
This investigation utilized the B-cell antigen receptor sourced from Mus musculus (PDB ID: 8EMA) as the Receptor Binding Domain RBD, chosen through Ramachandran Plot analysis. The 8EMA protein was chosen due to 79.2% of its residues falling within the most favored regions of the Ramachandran plot, with an overall average G-factor of 0.16. Additionally, its resolution, as indicated by PDB ID 8EMA, is satisfactory at 8.20 Å. According to PROCHECK analysis, the G-factor, ideally ranging from 0 to 0.5, signifies high-quality models (Elengoe et al., 2014). Evaluation of protein-ligand binding sites revealed 20 such sites on the 8EMA protein (Figure 6). Proteins engaging in binding interactions with other proteins or ligands typically possess specific regions for interaction. Prankweb is a website designed to aid in identifying or predicting these regions, determining which pockets are conducive to ligand binding on a protein. A pocket, resembling a sac, serves as a space for a ligand molecule to interact, consisting of various residue sequences, each playing a role in the interaction with the ligand molecule. Analysis of the 8EMA protein on Prankweb identified 20 pocket regions predicted as sites for ligand bonding.
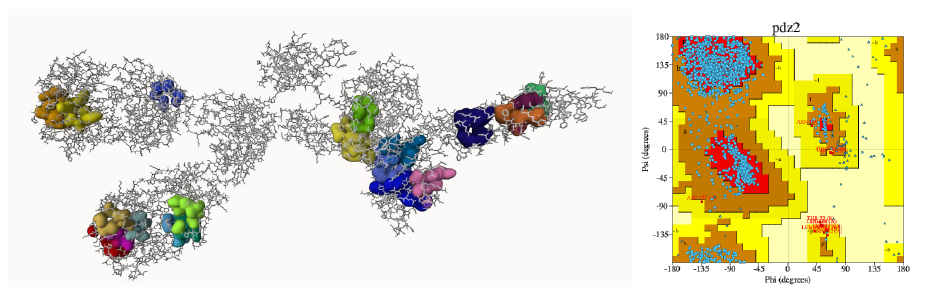
Figure 6. 8EMA protein-ligand binding sites pocket with Ramachandran plot.
Molecular docking
The HPEPDOCK server produced ten conformations, and the one exhibiting the highest binding energy score was selected (Figure 7). A lower binding energy indicates a stronger binding interaction between the B-cell receptor and the epitope. Table 4 displays the docking scores for peptide-receptor complexes.
To explore the interactions between predicted epitopes and the B-cell receptor protein compound (8EMA), molecular docking was conducted. Analysis using the HPEPDOCK web service revealed that five epitopes are predicted to be securely anchored in the 8EMA protein structure, with docking energy values of -228,832 kcal/mol, -203,593 kcal/mol, -216,141 kcal/mol, -215,566 kcal/mol, and -189,316 kcal/mol for epitope models 1-5 (Table 4). The HTWTEQYKFQ epitope, for instance, forms hydrogen bond interactions with five amino acid residues of the 8EMA protein (A_Ser74, A_Asp71, A_Tyr120, R_Tyr53, and R_Asn74) and establishes non-bonded contact interactions with eight amino acids. The chains present on the B-cell antigen receptor are denoted by the letters A, B, C, D, and R.
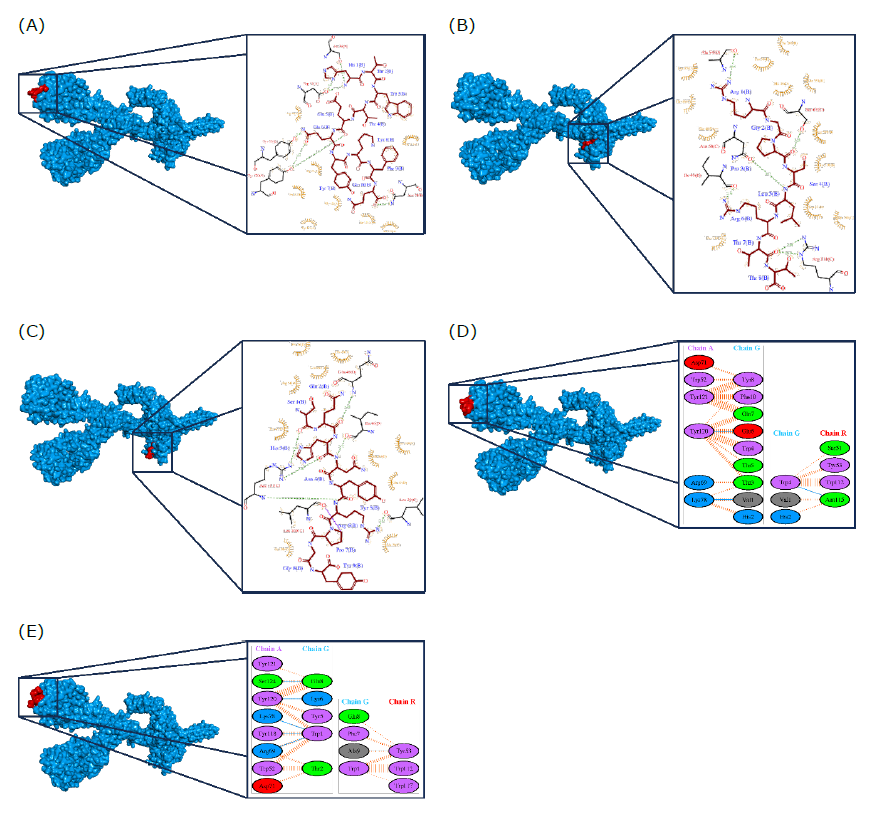
Figure 7. Docking simulation between protein-ligand interactions of B-cell antigen receptor from Mus musculus (PDB ID: 8EMA) and predicted epitope. (A) HTWTEQYKFQ, (B) RGPSLRTT, (C) SQHNYRPGY, (D) VHTWTEQYKFQ, and (E) WTEQYKFQADSP.
Table 4. Binding energy score between the B-cell Receptor (8EMA) and epitope
|
No. |
Epitope |
Binding Affinity (kcal/mol) |
|
1 |
HTWTEQYKFQ |
-228.832 |
|
2 |
WTEQYKFQADSP |
-203.593 |
|
3 |
VHTWTEQYKFQ |
-216.141 |
|
4 |
SQHNYRPGY |
-215.566 |
|
5 |
RGPSLRTT |
-189.316 |
Similarly, the RGPSLRTT epitope engages in hydrogen bond interactions with five residues (C_Ser67, C_Asn68, C_Arg111, D_Ala54, and D_Ile46) and non-bonded contact interactions with twelve residues. The SQHNYRPGY epitope forms hydrogen bond interactions with five residues (C_Arg111, C_Leu29, C_Leu109, D_Gln48, and D_Ile46), along with non-bonded interactions involving ten amino acid residues. The VHTWTEQYKFQ epitope establishes hydrogen bond interactions with three residues (A_Tyr120, A_Lys78, and R_Asn115) and non-bonded interactions with seven amino acid residues. Finally, the WTEQYKFQADSP epitope forms hydrogen bond interactions with six residues (A_Ser124, A_Tyr120, A_Lys78, A_Arg69, and R_Tyr53), and non-bonded contact interactions with five amino acid residues (Figure 8).
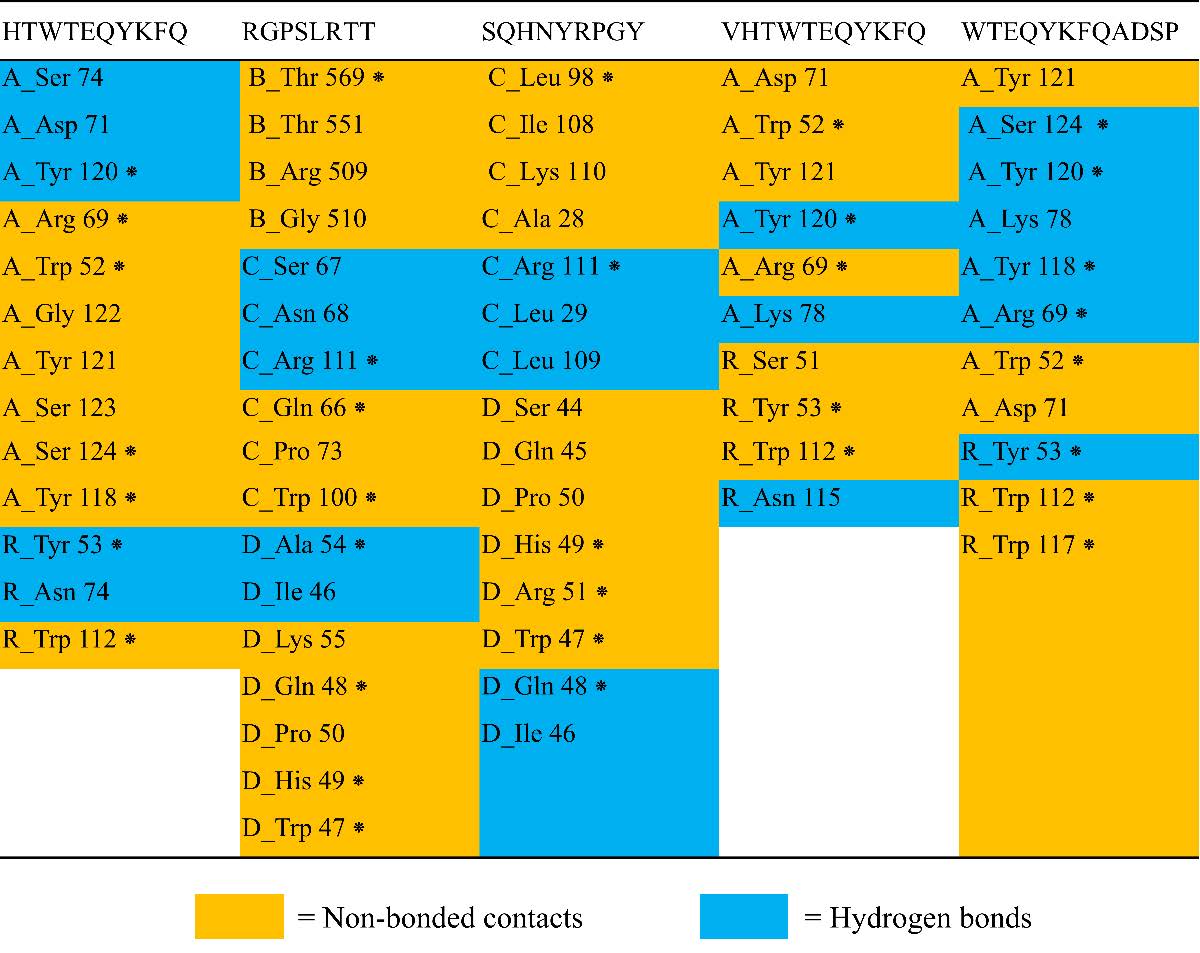
Figure 8. Residue bond formed of B-cell antigen receptor from Mus musculus (PDB ID: 8EMA) and predicted epitope. An asterisk (*) denotes the amino acid residues involved in the binding of protein-ligand complexes. The letters A, B, C, D, and R represent the respective chains of the B-cell antigen receptor. The accompanying number specifies the position of the amino acid residue on the B-cell antigen receptor. The abbreviations for amino acids are as follows: Ser = Serine, Asp = Aspartic Acid, Tyr = Tyrosine, Arg = Arginine, Trp = Tryptophan, Gly = Glycine, Asn = Asparagine, Thr = Threonine, Gln = Glutamine, Pro = Proline, Ala = Alanine, Ile = Isoleucine, Lys = Lysine, His = Histidine, and Leu = Leucine.
DISCUSSION
Constructing a phylogenetic tree serves as a model to understand the relationships and evolution of different sequences. Various algorithm models, including the UPGMA development model, are available for creating phylogenetic trees, with the UPGMA model being particularly known for its simplicity and quick study completion (Chen and Wang, 2022, p. 202). Analyzing 72 DENV NS-1 sequences revealed slight variations between the serotypes, showcased by a phylogenetic tree with an overall mean distance value of 0.22 and a standard error of 0.02, based on 1000 bootstrap replications. These findings contribute valuable insights into the genome structure of the DENV virus, enabling researchers to focus on specific serotypes for rapid diagnostic test predictions.
For further studies predicting rapid diagnostic test development, the latest data collection on DENV serotypes, specifically QBB90021.1 (DENV-1), QBE90252.1 (DENV-2), QBB90023.1 (DENV-3), and UDW38833.1 (DENV-2), was selected. Antigenicity evaluation using the VaxiJen v2.0 web service indicated differing antigenicity values among the serotypes, with DENV-1 and DENV-2 exceeding 0.5, while DENV-3 and DENV-4 fell below 0.5. Subsequently, a 3-dimensional structure was constructed for DENV 1-4 proteins using the Swiss-model web service, revealing structural similarities with variations in secondary structure elements. Analyzing interactions in the protein structures through PDBsum identified various bond interactions, such as salt bridges, hydrogen bonds, and non-bonded contacts. Stability assessments indicated predominant non-bonded contacts.
Epitope prediction for NS1 proteins of DENV 1-4, conducted using the IEDB web service, identified five conserved epitopes: WTEQYKFQADSP, HTWTEQYKFQ, VHTWTEQYKFQ, SQHNYRPGY, and RGPSLRTT. Subsequent analyses of allergenicity, antigenicity, and toxicity determined that the SQHNYRPGY epitope is antigenic, while RGPSLRTT is toxic. Molecular docking simulations indicated that all five epitopes exhibit favorable binding abilities, as evidenced by low binding energy values and the presence of hydrogen bonding interactions, suggesting strong protein-ligand complexes.
The binding energy value quantifies the energy required for the ligand (epitope) and the receptor (B-cell antigen receptor) to form a bonding interaction; lower or more negative values indicate a more favorable and easier bonding interaction (Boyles et al., 2020; Jones et al., 2021). Additionally, the analysis of bond interactions must consider hydrogen bonds in protein-ligand complexes, as these significantly impact the stability of the ligand binding structure. Consequently, the presence of residues in the active site is crucial for determining the optimal interaction (Bitencourt-Ferreira et al., 2019; Madushanka et al., 2023). The investigation shows that each predicted epitope has approximately five to six amino acid residues that form hydrogen bonds. Integrating the results from antigenicity analysis and docking simulations, epitope 4 (SQHNYRPGY) emerged as the most favorable and recommended candidate due to its antigenic properties, non-toxicity, non-allergenicity, and lower binding affinity.
Epitope mapping and bioinformatics analysis have revealed that the predicted epitopes encompass highly conserved regions of the NS1 proteins across all DENV serotypes. Epitopes that demonstrate antigenic properties without exhibiting toxicity can enhance the immunization process by eliciting a rapid B-cell response to produce specific antibodies (Kim et al., 2018; Salcedo et al., 2022). In our subsequent study, we will utilize these epitopes in the immunization of mice and the generation of hybridomas to discover specific monoclonal antibodies, facilitating the development of Rapid Diagnostic Tests (RDTs).
In conclusion, these comprehensive analyses support the recommendation to include at least four conserved epitopes from all DENV serotypes, especially from Indonesia, in the development of Rapid Diagnostic Tests for enhancing the sensitivity and specificity of locally produced RDTs.
CONCLUSION
This research involved the docking of five epitope candidates, which are conserved across all DENV serotypes, to the B-cell protein RBD, demonstrating a strong binding affinity. Additionally, an analysis of antigenicity, allergenicity, and toxicity profiles of these epitopes indicates that the SQHNYRPGY epitope is anticipated to enhance the creation of sensitive and specific Rapid Diagnostic Tests (RDTs) for detecting DENV in Indonesia. In conclusion, our findings have the potential to expedite the development of effective RDTs for all DENV serotypes, utilizing the NS1 protein, through further in vitro investigations in Indonesia.
AUTHOR CONTRIBUTIONS
Conceptualization: YM, SW, FS, NW; Data curation: YM, SW, FS, NW; Formal analysis: YM, SW, FS, NW; Funding acquisition: HW, WTA, FS, NW; Investigation: YM, SW, FS, NW; Methodology: YM, SW, NW; Supervision: HW, WTA, NW; Roles/Writing - original draft: YM, SW; Writing - review & editing: HW, WTA, FS, NW. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abesamis, L., Aliping, E., Armada, F., Danao, M., Del Valle, P., Regencia, Z., Baja, E., and Ligsay, A. 2022. In silico comparative analysis of predicted B cell epitopes against Dengue virus (serotypes 1–4) isolated from the Philippines. Vaccines. 10: 1259.
Adnan, M., Nuhamunada, M., Hidayati, L., and Wijayanti, N. 2020. In silico vaccine design against Dengue virus type 2 envelope glycoprotein. Hayati Journal of Biosciences. 27: 228.
Biswas, S., Mahmud, S., Mita, M.A., Afrose, S., Hasan, Md. R., Sultana Shimu, Mst. S., Saleh, Md. A., Mostafa-Hedeab, G., Alqarni, M., Obaidullah, A.J., and Batiha, G.E.-S. 2022. Molecular docking and dynamics studies to explore effective inhibitory peptides against the spike receptor binding domain of SARS-CoV-2. Frontiers in Molecular Biosciences. 8: 791642.
Bitencourt-Ferreira, G., Veit-Acosta, M., and De Azevedo, W. F. 2019. Hydrogen bonds in protein-ligand complexes. p. 93-107. In W. F. De Azevedo [ed] Docking screens for drug discovery.
Boyles, F., Deane, C. M., and Morris, G. M. 2020. Learning from the ligand: Using ligand-based features to improve binding affinity prediction. Bioinformatics. 36: 758–764.
Carbery, A., Buttenschoen, M., Skyner, R., Von Delft, F., and Deane, C. M. 2024. Learnt representations of proteins can be used for accurate prediction of small molecule binding sites on experimentally determined and predicted protein structures. Journal of Cheminformatics. 16: 32.
Chen, W., and Wang, H. 2022. Phylogenetic tree selection by testing substitution number in clade. Diversity. 14: 543.
Elengoe, A., Naser, M., and Hamdan, S. 2014. Modeling and docking studies on novel mutants (K71L and T204V) of the ATPase domain of human heat shock 70 kDa protein 1. International Journal of Molecular Sciences. 15: 6797–6814.
Gan, S. J., Leong, Y. Q., Bin Barhanuddin, M. F. H., Wong, S. T., Wong, S. F., Mak, J. W., and Ahmad, R. B. 2021. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: A review. Parasites & Vectors. 14: 315.
Harapan, H., Michie, A., Mudatsir, M., Sasmono, R. T., and Imrie, A. 2019. Epidemiology of dengue hemorrhagic fever in Indonesia: Analysis of five decades data from the National Disease Surveillance. BMC Research Notes. 12: 350.
Harapan, H., Michie, A., Sasmono, R. T., and Imrie, A. 2020. Dengue: A minireview. Viruses. 12: 829.
Hidayatullah, A., Putra, W. E., Salma, W. O., Muchtaromah, B., Permatasari, G. W., Susanto, H., Widiastuti, D., and Kismurtono, M. 2020. Discovery of drug candidate from various natural products as potential novel dengue virus nonstructural protein 5 (NS5) inhibitor. Chiang Mai University Journal of Natural Sciences. 20: e2021018.
Higa, Y. 2011. Dengue vectors and their spatial distribution. Tropical Medicine and Health. 39: S17–S27.
Jantakee, K., Panwong, S., Pandith, H., Tragoolpua, Y., and Panya, A. 2023. Stability of Terminalia bellirica extract and its combination effect with alpha-mangostin in inhibiting dengue virus infection. Chiang Mai Journal of Science. 50: 1–14.
Jones, D., Kim, H., Zhang, X., Zemla, A., Stevenson, G., Bennett, W. F. D., Kirshner, D., Wong, S. E., Lightstone, F. C., and Allen, J. E. 2021. Improved protein–ligand binding affinity prediction with structure-based deep fusion inference. Journal of Chemical Information and Modeling. 61: 1583–1592.
Jones, M., Legge, F., Lebani, K., Mahler, S., Young, P., Watterson, D., Treutlein, H., and Zeng, J. 2017. Computational identification of antibody epitopes on the Dengue virus NS1 protein. Molecules. 22: 607.
Kim, Y. H., Lee, J., Kim, Y.-E., Chong, C.-K., Pinchemel, Y., Reisdörfer, F., Coelho, J. B., Dias, R. F., Bae, P. K., Gusmão, Z. P. M., Ahn, H.-J., and Nam, H.-W. 2018. Development of a rapid diagnostic test kit to detect IgG/IgM antibody against Zika virus using monoclonal antibodies to the envelope and non-structural protein 1 of the virus. The Korean Journal of Parasitology. 56: 61–70.
Madushanka, A., Moura, R. T., Verma, N., and Kraka, E. 2023. Quantum mechanical assessment of protein–ligand hydrogen bond strength patterns: Insights from semiempirical tight-binding and local vibrational mode theory. International Journal of Molecular Sciences. 24: 6311.
Rocha, L., Alves, R., Caetano, B., Pereira, L., Mitsunari, T., Amorim, J., Polatto, J., Botosso, V., Gallina, N., Palacios, R., Precioso, A., Granato, C., Oliveira, D., Silveira, V., Luz, D., Ferreira, L., and Piazza, R. 2017. Epitope sequences in Dengue virus NS1 protein identified by monoclonal antibodies. Antibodies. 6: 14.
Roy, S. K., and Bhattacharjee, S. 2021. Dengue virus: Epidemiology, biology, and disease aetiology. Canadian Journal of Microbiology. 67: 687–702.
Salcedo, N., Reddy, A., Gomez, A. R., Bosch, I., and Herrera, B. B. 2022. Monoclonal antibody pairs against SARS-CoV-2 for rapid antigen test development. PLOS Neglected Tropical Diseases. 16: e0010311.
Surendran, S. N., Nagulan, R., Tharsan, A., Sivabalakrishnan, K., and Ramasamy, R. 2022. Dengue incidence and Aedes vector collections in relation to COVID-19 population mobility restrictions. Tropical Medicine and Infectious Disease. 7: 287.
Tuiskunen Bäck, A., and Lundkvist, Å. 2013. Dengue viruses – an overview. Infection Ecology & Epidemiology. 3: 19839.
Utama, I. M. S., Lukman, N., Sukmawati, D. D., Alisjahbana, B., Alam, A., Murniati, D., Utama, I. M. G. D. L., Puspitasari, D., Kosasih, H., Laksono, I., Karyana, M., Karyanti, M. R., Hapsari, M. M. D. E. A. H., Meutia, N., Liang, C. J., Wulan, W. N., Lau, C.-Y., and Parwati, K. T. M. 2019. Dengue viral infection in Indonesia: Epidemiology, diagnostic challenges, and mutations from an observational cohort study. PLOS Neglected Tropical Diseases. 13: e0007785.
Weng, G., Gao, J., Wang, Z., Wang, E., Hu, X., Yao, X., Cao, D., and Hou, T. 2020. Comprehensive evaluation of fourteen docking programs on protein–peptide complexes. Journal of Chemical Theory and Computation. 16: 3959–3969.
Wibowo, S., Costa, J., Baratto, M. C., Pogni, R., Widyarti, S., Sabarudin, A., Matsuo, K., and Sumitro, S. B. 2022. Quantification and improvement of the dynamics of human serum albumin and glycated human serum albumin with astaxanthin/astaxanthin-metal ion complexes: Physico-chemical and computational approaches. International Journal of Molecular Sciences. 23: 4771.
Zhou, P., Jin, B., Li, H., and Huang, S.-Y. 2018. HPEPDOCK: A web server for blind peptide–protein docking based on a hierarchical algorithm. Nucleic Acids Research. 46: W443–W450.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Yunita Messe1, Syahputra Wibowo1, 2, Hery Wijayanto3, Wayan Tunas Artama4, Fajar Sofyantoro1, and Nastiti Wijayanti1, *
1 Department of Tropical Biology, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
2 Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor 16911, Indonesia.
3 Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
4 Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
Corresponding author: Nastiti Wijayanti, E-mail: nastiti_wijayanti@ugm.ac.id
ORCID:
Yunita Messe: https://orcid.org/0000-0002-6465-6645
Syahputra Wibowo: https://orcid.org/0000-0003-3979-4277
Hery Wijayanto: https://orcid.org/0000-0003-2808-8270
Wayan Tunas Artama: https://orcid.org/0000-0002-8504-7558
Fajar Sofyantoro: https://orcid.org/0000-0003-0952-1956
Nastiti Wijayanti: https://orcid.org/0000-0001-6483-2816
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: March 28, 2024;
Revised: July 13, 2024;
Accepted: July 16, 2024;
Online First July 23, 2024

