
Stability, Irritation Property and Anti-aging Clinical Evaluation of Hydroethanolic Dimocarpus longan Leaf Extract-Loaded Niosomes
Pimjai Doungsaard, Sunee Chansakaow, Worrapan Poomanee, Busaban Sirithunyalug, and Pimporn Leelapornpisid*Published Date : June 27, 2014
DOI : https://doi.org/10.12982/NLSC.2024.046
Journal Issues : Number 3, July-September 2024
Abstract This study aimed to develop hydroethanolic Dimocarpus longan leaf extract (HDLE)-loaded niosomes for enhancing polyphenols’ stability and efficiency for cosmeceutical applications. Moreover, its irritation property was clinically evaluated. HDLE was loaded into niosomes of cholesterol, sorbitan laurate, and polysorbate 20 at a 1:1:1.5 molar ratio. The suitable extract-loaded capacity was 0.5% (w/w), which provided the percentage of entrapment efficiencies of gallic acid, ethyl gallate and ellagic acid at 37.77 ± 0.93, 49.76 ± 0.20, and 62.85 ± 1.55, respectively. The HDLE-loaded niosomes showed a unilamellar structure under a transmission electron microscope. The particle size range was 255.45-356.15 nm with a low polydispersity index at initial and after storage for three months. The remaining percentage of gallic acid, ethyl gallate and ellagic acid in HDLE-loaded niosomes at all storage temperatures after three months was significantly higher than in the solution. HDLE-loaded niosomes presented no irritation effect on the chorioallantoic membranes (CAMs). The serum containing HDLE-loaded niosomes showed no irritation by the 4-hr human patch test. After applying twice daily for 60 days of water-based serum containing the HDLE-loaded niosomes compared with a placebo and untreated skin on different areas of forearms, the result demonstrated that HDLE-loaded niosomes serum was significantly effective in skin moisturization, elasticity induction and wrinkle reduction. In conclusion, due to their efficacy and safety, HDLE-loaded niosomes can be used for cosmetic applications.
Keywords: Dimocarpus longan, Niosomes, Stability, Anti-aging, Clinical trial
Funding: The authors are grateful for the research funding provided by the CMU Presidential Scholarship, for providing financial support to conduct this study.
Citation: Doungsaard,P., Chansakaow, S., Poomanee, W., Sirithunyalug, B., and Leelapornpisid, P. 2024. Stability, irritation property and anti-aging clinical evaluation of hydroethanolic Dimocarpus longan leaf extract-loaded niosomes. Natural and Life Sciences Communications. 23(3): e2024046.
INTRODUCTION
Longan (Dimocarpus longan Lour.) is a tropical evergreen tree in the Sapindaceae family. This plant is mainly cultivated in northern Thailand (Paul et al., 2021). The longan leaf extract has been reported for its antioxidant potential where the 40% ethanol extract possessed a 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging value of 218.67 µmol Trolox/g, a 2,2'-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging value of 582.37 µmol Trolox/g and a ferric reducing antioxidant power (FRAP) value of 182.58 µmol Trolox/g (Chen et al., 2017). Previous research has shown that hydroethanolic D. longan leaf extract (HDLE) has potential as an anti-aging cosmeceutical application, demonstrated anti-radical activity in the experimentation of DPPH and H2O2 with 50% inhibitory concentration (IC50) values of 30.03 ± 7.64 and 71.40 ± 15.30 µg/ml, respectively. Moreover, HDLE inhibited the oxidation of lipids with IC50 of 537.01 ± 42.32 µg/ml. HDLE also attenuated hyaluronidase and collagenase enzymes, involving collagen and elastin breakdown in the skin, with IC50 of 234.80 ± 21.52 and 314.44 ± 62.14 mg/ml, respectively. In addition, it could inhibit matrix metalloproteinase (MMP)-2 and MMP-9 more effectively than gallic acid at a concentration of 1.0 mg/ml. The preliminary safety of HDLE for skin use was proven by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay on mouse embryonic fibroblast (BCRC 60071; ATCC CCL92) at 0.1-1.0 mg/ml (Doungsaard et al., 2020). The extract also possessed a high potential for nitric oxide scavenging with IC50 of 0.478 ± 0.033 mg/ml. HDLE could inhibit the tyrosinase enzyme with IC50 4.90 ± 0.50 and 6.60 ± 0.18 mg/ml for L-tyrosine and L-DOPA as substrates. The extract also exhibited a potent antiglycation activity with IC50 0.023 ± 0.003 mg/ml (Doungsaard et al., 2023). In addition, the anti-aging activities of HDLE related to ethyl gallate, a major polyphenol, were selected as a bioactive chemical marker. Gallic acid and ellagic acid were found in HDLE and considered markers for the chemical fingerprint of HDLE (Doungsaard et al., 2023). However, the polyphenol compounds were naturally unstable under light and temperature after storage, leading to decreased biological activities (Manosroi et al., 2012; Nitthikan et al., 2018). These problems affected the applications of HDLE in cosmetic products, and thus the potential delivery system as niosomes was selected to reduce this problem.
Niosomes are vesicular systems with a bilayer structure composed of non-ionic surfactants and cholesterol. These vesicles can entrap hydrophilic and hydrophobic compounds in a bilayer, composing an aqueous layer and vesicular membrane (Sankhyan and Pawar, 2012). Due to a remarkable vesicular delivery platform, niosomes have attracted the attention of researchers worldwide as a potential delivery system for cosmetics (Manosroi et al., 2013). In addition, niosomes have been demonstrated to enhance the physiochemical properties of the loaded compounds and improve the deliverability of active substances to the target skin layers (Chaikul et al., 2019). Their advantages include enhancing substances accumulated at the viable layer of skin (Rezaeiroshan et al., 2020). The bilayer structure of niosomes is similar to liposomes. Liposomes contain phospholipids with or without cholesterol, while niosomes are composed of cholesterol and non-ionic surfactants. Compared to liposomes, niosomes demonstrate greater stability toward oxidative degradation with low toxicity. No special methods are required to store and handle niosomes (Ge et al., 2019). In addition, niosomes are easy to prepare and scale up with the ultrasonic processing technique as facile, green, and cost-effective with low production cost (Khan et al., 2017). Therefore, niosomes are a beneficial formulation for loading the active compounds in this research.
To improve polyphenols' stability, HDLE-loaded niosomes were formulated using ultrasonic processing techniques. The physiochemical characteristics were determined, including particle size, polydispersity index, zeta potential, pH, entrapment efficiency, and remaining percentage. In addition, in vivo, the irritation and efficiency of HDLE-loaded niosomes were clinically investigated.
MATERIALS AND METHODS
Chemical and reagent
Ethanol (95%) was purchased from Liquor Distillery Organization (Chachoengsao, Thailand). Ellagic acid and gallic acid were purchased from Sigma-Aldrich (Steinhiem, Germany). Ethyl gallate was purchased from Tokyo Chemical Industry (Tokyo, Japan). Acetonitrile HPLC grade and 2-propanol were from RCI Labscan (Bangkok, Thailand). Ortho-phosphoric acid 85% was purchased from Merck (Darmstadt, Germany). Butylene glycol, disodium EDTA, glycerine, Spectrastat BHL®, and xanthan gum were purchased from Namsiang Co. Ltd. (Bangkok, Thailand). Sorbitan Laurate and Polysorbate 20 were purchased from Chanjao Longevity Ltd. (Bangkok, Thailand). Cholesterol was purchased from LOBA Chemie Pvt. Ltd. (Mumbai, India).
Plant materials
Mature leaves of Dimocarpus longan cv. E-Daw were harvested from a longan farm in Chiang Mai Province, Thailand, between April and May 2019. The leaves were washed, dried at 50°C for 24 hours, and ground into a powder using a grinder machine (Spring Green Evolution, Thailand). The powder was then passed through an 80-mesh sieve and packed into metalized bags. The packed powder was stored at room temperature until it was used.
Plant extraction
Briefly, 500 g of longan leaf powder was macerated with 50% ethanol in a ratio of 1:3 for 24 hours. The fluid was collected, and the remaining powder was extracted for another extraction. All filtrate was pooled and removed solvent using a rotary evaporator (R-300 Buchi®, Flawil, Switzerland) at the pressure of 50 mbar at 50°C (Doungsaard et al., 2020). The hydroethanolic longan leaf extract (HDLE) was kept at -20°C until use.
High-Performance Liquid Chromatography (HPLC) analysis
The HPLC analysis, employing the Hewlett Packard Agilent 1100 Series, was performed for the quantitation of gallic acid, ethyl gallate and ellagic acid in niosomes and serum preparations. The stationary phase was a C-18 column (250 x 4.6 mm, i.d. 5 µm, ODS-3, InertsilTM). The mobile phases were (A) acetonitrile and (B) 0.1% (v/v) ortho-phosphoric acid (pH 3). The gradient program was started from (A) 10% to 30% in 60 minutes, with a flow rate of 0.8 ml/min. Samples, including HDLE-loaded niosomes, unentrapped part of HDLE-loaded niosomes, HDLE solution, HDLE serum and HDLE-loaded niosomes serum were 2-fold diluted with 2-propanol before analysis. All samples passed through 0.22 µm nylon syringe filters. Each (20 µl) was injected, and compounds were detected at 280 nm (Doungsaard et al., 2020.). The experiment was done in triplicate. The reference standards used were gallic acid, ethyl gallate and ellagic acid.
Niosomes preparation
Niosomes, composed of cholesterol, sorbitan laurate, and polysorbate 20 at a 1:1:1.5 molar ratio, were produced by direct ultrasonication (Fraile et al., 2015). For HDLE-loaded niosomes, 0.25 g of cholesterol and 0.26 g of sorbitan laurate were weighed out in an Erlenmeyer flask. Then, 50 g of deionized water was added, and the mixture was stirred with a magnetic stirrer at 150 rpm for an hour. A separate flask containing 1.44 g of polysorbate 20, diluted with 7.00 g of deionized water and 0.5 g of HDLE, was stirred with a magnetic stirrer at 150 rpm for an hour. For blank niosomes, HDLE was replaced with deionized water. After mixing both flasks in a round-based glass container, deionized water was added to the final weight of 100.00 g. The sample was shaken for 10 minutes before the ultrasonication process. A high-intensity ultrasonic processor (Vibra-Cell VCX 130, Sonics & Materials Inc., USA) was used. The probe was immersed in the sample for a 20-minute effective time. The amplitude of the pulses was 50%, with pulses every 5 seconds (5 seconds on and 5 seconds off). The temperature of the niosomes at the end of the process was lower than 15°C.
Characteristics of unloaded niosomes and HDLE-loaded niosomes
pH measurement
The pH of the samples including HDLE solution (0.5% w/w in 1.44% w/w polysorbate 20), HDLE-loaded niosomes and unloaded niosomes were measured using a pH meter (PH60, APERA Instruments Co.Ltd., Shanghai, China) at 25ºC.
Particle size and the polydispersity index (PDI) measurements
The particle size distribution and PDI of samples were measured using a Zetasizer Nano Zs apparatus (Malvern Instruments Ltd., UK). The detection was set for backscattering detection at a scattering angle of 173º. Samples (1.5 cm3) were measured in DTS0012 square disposable polystyrene cells. Three replicates were done for each sample at 25 ± 0.5°C. (Rezaeiroshan
et al., 2020).
Zeta potential measurement
The zeta potential was measured using the Zetasizer Nano Zs apparatus (Malvern Instruments Ltd., UK). Samples were measured in DTS1070 disposable folded capillary cells. Three replicates were performed for each sample at 25 ± 0.5°C (Rezaeiroshan et al., 2020).
Particle morphology analysis
The particle morphological analysis of niosomes was performed using transmission electron microscopy (TEM) (JEOL JEM-1400). A drop of niosomes was placed on a copper grid. Then, a 2% (w/v) phosphotungstic acid solution was applied to the grid for two minutes. The sample was left overnight before analyzing by TEM (Fraile et al., 2015).
Entrapment efficiency determination
Entrapment efficiency was measured by the ultrafiltration method (Fraile et al., 2015). In brief, the unentrapped compounds were separated by centrifugal ultrafiltration (15 minutes, 7,500 g) using Amicon Ultra-4 units (Millipore) with ten kDa cutoff membranes. The initial samples were completely vesicle disrupted using 2-propanol with a ratio of 1:1 by volume. The concentration of gallic acid, ethyl gallate and ellagic acid in the initial samples and permeates were determined by HPLC. The following equation calculated the entrapment efficiency percentage.
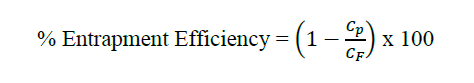
Where Cp is the concentration of the permeation after centrifugal ultrafiltration., CF is the concentration of the initial formulation (Before ultrasonication).
Stability study of HDLE-loaded niosomes
HDLE-loaded niosomes and solution were stored in transparent glass bottles at 4 ± 2°C, room temperature (RT or 25 ± 2°C) with/without light, and 45 ± 2°C for three months. The physical and HPLC analyses were analyzed after storage (Manosroi et al., 2012).
Irritation test by hen’s egg test-chorioallantoic membrane (HET-CAM) method
The HDLE-loaded niosomes were investigated for irritation potential using the HET-CAM assay (Rivero et al., 2021). The fertilized hen's eggs were placed in an incubator with a rotating tray. The eggs were incubated at 37.8 ± 0.3 °C and 58 ± 2% relative humidity and hand-rotated eggs five times daily until day 8. On incubation day 8, the eggs were turned the large end of the eggs upwards and there was no more hand rotation. On day 9, the air cell sections were cut with a rotating dentist saw blade, exposing the chorioallantoic membranes (CAMs). Then, 0.3 ml of sample was applied directly onto each CAM surface and observed the reactions for 300 seconds via a stereo microscope (Olympus, Tokyo, Japan). Negative, positive and vehicle controls were included in a 0.9% (w/v) NaCl solution, 1% (w/v) sodium lauryl sulfate and deionized water, respectively. The reaction time method was employed to obtain the irritation score. The endpoints that should be observed are hemorrhage, vascular lysis, and coagulation, recorded in seconds. Although the assay lasted 300 seconds in the protocols, the additional reaction information was observed for more than 60 minutes. The test results were evaluated as Irritation score (IS), calculated by the following equation.
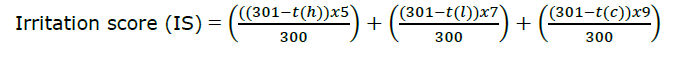
where t(h) is the time (sec) when the first sign of hemorrhage reaction (bleeding from the vessels) on CAM, t(l) is the time (sec) when first the sign of vascular lysis (blood vessel disintegration) on CAM, and t(c) is the time (sec) when the sign of coagulation formation (intra- and extra-vascular protein denaturation) on CAM.
Physiochemical stability of a topical serum containing HDLE solution and HDLE-loaded niosomes
Preparation of topical serum
The HDLE-loaded niosomes, unloaded niosomes or HDLE were incorporated into the water-based topical serum composed of 1% (w/w) of xanthan gum, 2% (w/w) of butylene glycol, 2% (w/w) of glycerin, 4% (w/w) of Spectrastat BHL®, and 0.4% (w/w) of disodium EDTA. Briefly, HDLE-loaded niosomes, unloaded-niosomes or HDLE were mixed with the base topical serum with gentle stirring at 1:1 ratio. In comparison, the placebo was added with deionized water instead of a niosomal formulation.
Physical stability of a topical serum
The serum containing HDLE, HDLE-loaded niosomes, unloaded niosomes and placebo was put in a tight container and stored under various conditions, including 4 ± 2°C, room temperature (RT or 25 ± 2°C) with/without light and 45 ± 2°C for three months. The color, phase separation and pH were observed (Manosroi et al., 2012).
Viscosity measurement
Viscosity measurements were obtained at 25°C using a Brookfield® Rheometer (Model: R/S-CPS) with a Bob & Cup (Model CC48).
Chemical stability of serum containing HDLE-loaded niosomes and HDLE solution
The percentages remaining of ethyl gallate after three months of storage were determined by HPLC. The serum samples were 2-fold diluted with 2-propanol. Then, the diluted samples were centrifuged at 7,500 g for 30 minutes. The upper solution was collected and further injected into the HPLC column.
Ex vivo skin permeation and deposition studies
Skin permeation study
Skin permeation study of HDLE-loaded niosomes was compared with HDLE-solution, containing an equivalent amount of HDLE. Franz diffusion cells (Logan DHC-6T, Logan Instrument Corp., USA) were used for ex vivo skin permeation study. The stillborn piglets’ skin was used as a membrane. Stillborn piglets were washed and dried with running tap water. Then, the skin was carefully removed subcutaneous fat and connective tissue. Before keeping in a freezer at -20°C, the pieces of skin were washed with 0.9% normal saline, dried, wrapped in tin foil and used within one month. Before the experiment, the skin was defrosted by soaking in 0.9% normal saline overnight at 4°C. After that, the skin was placed on the receiver chamber, with the dermis side facing the medium in the receiver chamber. One gram of each sample was applied, which was sandwiched with the donor compartment and receptor chamber of the Franz diffusion cell. The receptor medium was phosphate buffer (pH 5.5). The temperature stabilized at 32 ± 0.5° with continuous stirring at 150 rpm. The 500 µl of receptor medium (RM) was withdrawn at 30 minutes, 1, 2, 4, 6 and 8 hours. The volume of RM was maintained throughout the study (Nitthikan et al., 2018). The bioactive compounds of HDLE in the receptor medium were analyzed.
Deposition of samples in the skin
After the test, the interested polyphenols deposition in the test skin were investigated using HPLC. After removing the Franz diffusion cells, the skin was thoroughly cleaned with 0.9% (w/v) normal saline. To extract the samples in the stratum corneum (SC) layer, 20 pieces of 3M TM Micropore TM adhesive tape were used. Each tape was stuck to the skin for 10 seconds and then stripped out of the skin. The tape strips were extracted with 50% ethanol to quantify the number of bioactive compounds. The rest of the skin, called viable epidermis-dermis (VED), was extracted by cutting it into small pieces and extracting the deposit using 50% ethanol (Nitthikan et al., 2018). The percentage accumulative of interested compounds after 8 hours was calculated by the following equation.
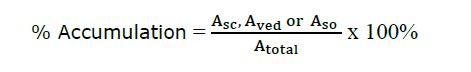
Where Asc, Aved, and Aso are amounts of interested compounds in stratum corneum (SC), viable epidermis-dermis (VED) and solution, respectively. A total is the total amount of interested compounds in 1 g of formulation.
Clinical trials for skin irritation and performance testing
These studies were conducted at the Faculty of Pharmacy, Chiang Mai, Thailand, from January to March 2023. The Ethics Committee of the Faculty of Pharmacy, Chiang Mai University in Thailand, approved the protocols, which follow the GCPs, international ethical guidelines, and applicable laws and regulations. The approval number is 025/2565/E. Thirty female volunteers aged 40-60 years were included in the study. The volunteers had been informed and signed consent. The exclusion criteria were volunteers who were receiving active skin treatment on the treatment site, visible skin abnormalities on the treated site, history of chronic skin disorders, history of allergy to skin care product compositions, pregnant, breastfeeding, receiving hormone supplements, routine smoker, participants of another clinical trial within 30 days before the study and presence of irritation in the skin irritation test.
The skin irritation test on human volunteers
The skin irritation test was a 4-hr human patch (Finn Chambers ®) test. The patch was separately mounted with 0.2 g of 2% (w/v) sodium lauryl sulfate (SLS), deionized water, the HDLE-loaded niosomes, the unloaded niosomes, HDLE-solution, and the blank serum (placebo). The patches were applied to marked regions on the upper outer arm, removed after a 4-hour application, and washed with physiological saline. The severity of erythema and edema at 1, 24, and 48 hours after patch removal was assessed using a 4-point scale. Erythema was graded from 0 (no erythema) to 4 (extreme redness), and edema was graded from 0 (no edema) to 4 (raised more than 1 mm and extending beyond the area of exposure). The PII value was calculated using the following equation (Jírová et al., 2010):

When PII values of 0-0.4 indicate no irritation, 0.5-1.9 indicates slight irritation, 2.0-4.9 indicates moderate irritation, and 5.0-8.0 indicates severe irritation.
Performance test of products on volunteers
The study design was a single-blinded, placebo-controlled trial. The left forearm was assigned to apply the HDLE-loaded niosomes serum (A) and separate it from the HDLE serum (B). The right forearm was assigned to apply the serum containing unloaded niosomes (C) and separate it from the serum base or placebo (D). The formulations were applied twice daily to 3x3 cm2 assigned areas for 60 days. The volunteers were not allowed to use other skincare on their arms for one week before and during the experiments. On the measuring days, the volunteers had to wait in an air-conditioned room at 25 ± 1ºC and 45 ± 5% relative humidity for 15 min before the skin analysis.
The performance of the products was evaluated by measuring skin hydration, whitening, elasticity, and wrinkles on day 0 and then again after days 30 and 60 of application. The skin moisture was evaluated using Arbitrary Corneometer® Units (AU). Skin whitening was investigated using a SkinColorCatch (Delfin Technologies, Finland). L* value was chosen for skin brightening assessment. Skin elasticity was determined using an Elastimeter® (Delfin Technologies, Finland). The instrument was pressed onto the skin, and a constant deformation value was measured in terms of the Instant Skin Elastimeter (ISE value). The skin surface microstructure of the treated skins was assessed using the Skin-Visiometer® SV700 with a Charge Couple Device (CCD) camera (Courage+Khazaka Electric GmbH, Germany). The parameters of the skin surface microstructure for wrinkle evaluation, including R1-5, surface and volume, were analyzed by the image analyzing program version 1.6.6.1 software (Courage+Khazaka Electric GmbH, Germany). The overall efficacy of each parameter was calculated as percentage of efficacy using the following equation (Kamma et al., 2019):
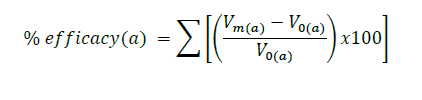
Vm(a) was the value of each anti-aging parameter after applying the formulation for 30 and 60 days. V0(a) was the value of each anti-aging parameter before applying the formulation.
Statistical analysis
All laboratory experiments were repeated in triplicate. The results of in vitro tests were presented as mean ± standard deviation (SD). The statistical analysis was performed using ANOVA at a significant level of P ≤ 0.05, with the Tukey test used for post hoc comparisons). The mean and standard error (SE) were presented in a clinical study. ANOVA (Tukey test) was applied to compare the difference in each intervention outcome, and the paired t-test was conducted to compare the difference between before and after treatment with each intervention. Data analysis was performed using SPSS (version 25.0, SPSS, Inc., USA).
RESULTS
Characteristics of HDLE-loaded niosomes and unloaded niosomes
HDLE-loaded and unloaded niosomes were prepared successfully with the ultrasonic processing technique. Unloaded niosomes presented the appearance of the white homogenous emulsion, while the HDLE-loaded niosomes presented the brownish homogenous emulsion. The pH, zeta potential, particle size and PDI of blank and HDLE-loaded niosomes are shown in Table 1.
Figure 1 shows the TEM images of HDLE-loaded and unloaded niosomes. The TEM images presented a unilamellar vesicle structure with a spherical shape and are in the nanosized range.
Table 1. The characteristics of unloaded niosomes and HDLE-loaded niosomes.
|
Formulation |
pH |
Zeta potential (mV) |
Particle size (nm) |
PDI |
|
Unloaded niosomes |
4.09 ± 0.02a |
-27.43 ± 0.92a |
193.57 ± 5.05a |
0.138 ± 0.026a |
|
HDLE-loaded niosomes |
4.90 ± 0.01b |
-21.35 ± 1.00b |
255.45 ± 3.48b |
0.250 ± 0.020b |
|
HDLE solution |
4.99 ± 0.01c |
- |
- |
- |
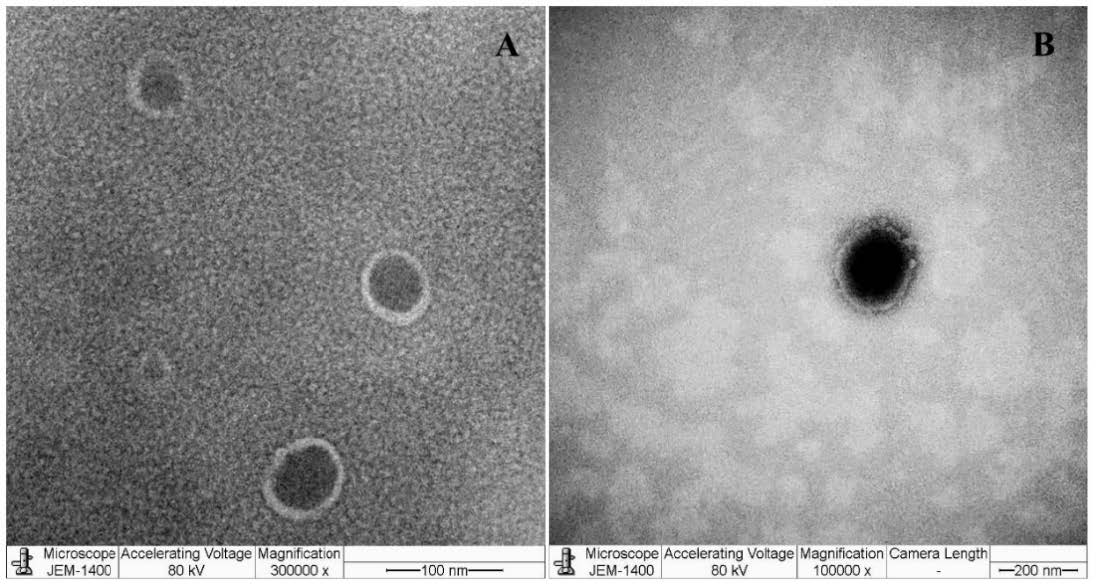
Figure 1. TEM images of niosomes of blank unloaded niosomes (A) and HDLE-loaded niosomes (B).
Entrapment efficiency determination
The entrapment efficiency of gallic acid, ethyl gallate and ellagic acid in HDLE-loaded niosomes were determined, and the values are presented in Table 2.
Table 2. Drug entrapment efficiency of niosomal formulation (n=3).
|
The interested compound in |
% Entrapment efficiency |
|
Gallic acid |
37.77 ± 0.93 |
|
Ethyl gallate |
49.76 ± 0.20 |
|
Ellagic acid |
62.85 ± 1.55 |
Note: Data are expressed as mean ± standard deviation (SD).
Stability study of niosomal formulation
Figure 2 presents the appearance of HDLE-loaded niosomes and solution after 90 days of storage. Both HDLE-loaded niosomes and solution had partial sedimentation; niosomes could be easily dispersed again. Table 3 shows the physical characteristics of HDLE-loaded niosomes after 90 days of storage. The pH values at all storage temperatures remained unchanged, while the color became darker than day 0, especially those kept at 45 ± 2°C. The particle size range was 255.45-356.15 nm with a low polydispersity index at initial and after storage for three months.
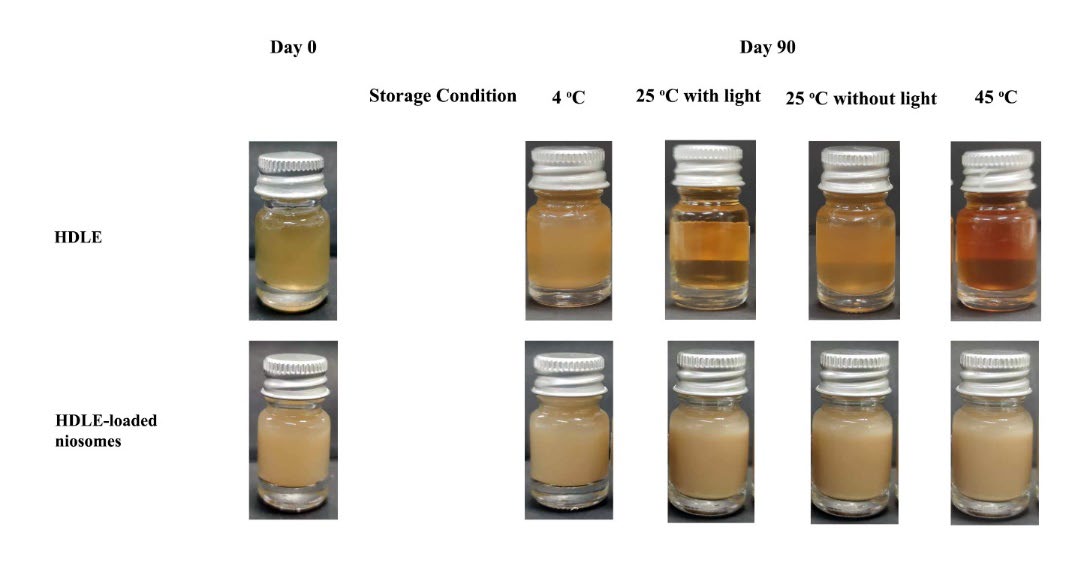
Figure 2. The appearance of HDLE solution and HDLE-loaded niosomes when kept at 4 ± 2°C, room temperature (RT or 30 ± 2°C) with/without light and 45 ± 2°C without light for three months.
Table 3. Characteristics of HDLE-loaded niosomes when kept at 4 ± 2°C, room temperature (RT or 30 ± 2°C) with/without light and 45 ± 2°C without light for three months.
|
Storage Condition |
pH |
Particle size (nm) |
PDI |
Zeta potential (mV) |
||||
|
Initial |
3-months |
Initial |
3-months |
Initial |
3-months |
Initial |
3-months |
|
|
4 ± 2°C |
4.90 |
4.88 ± 0.02 |
255.45 ± 3.48 |
280.15 ± 5.64 |
0.25 ± 0.02 |
0.285 ± 0.01 |
-21.35 ± 1.00 |
-19.40 ± 0.44 |
|
RT with light |
4.90 |
4.85 ± 0.03 |
255.45 ± 3.48 |
271.35 ± 5.80 |
0.25 ± 0.02 |
0.30 ± 0.04 |
-21.35 ± 1.00 |
-29.12 ± 1.33 |
|
RT without light |
4.90 |
4.88 ± 0.00 |
255.45 ± 3.48 |
256.15 ± 3.28 |
0.25 ± 0.02 |
0.26 ± 0.01 |
-21.35 ± 1.00 |
-29.08 ± 1.08 |
|
45 ± 2°C |
4.90 ± 0.01 |
4.83 ± 0.04 |
255.45 ± 3.48 |
356.15 ± 1.28 |
0.25 ± 0.02 |
0.34 ± 0.08 |
-21.35 ± 1.00 |
-32.40 ± 0.90 |
Figure 3 presents the remaining percentage of gallic acid, ethyl gallate and ellagic acid when kept at 4 ± 2°C, room temperature (RT or 30 ± 2°C) with/without light and 45 ± 2°C without light for three months.
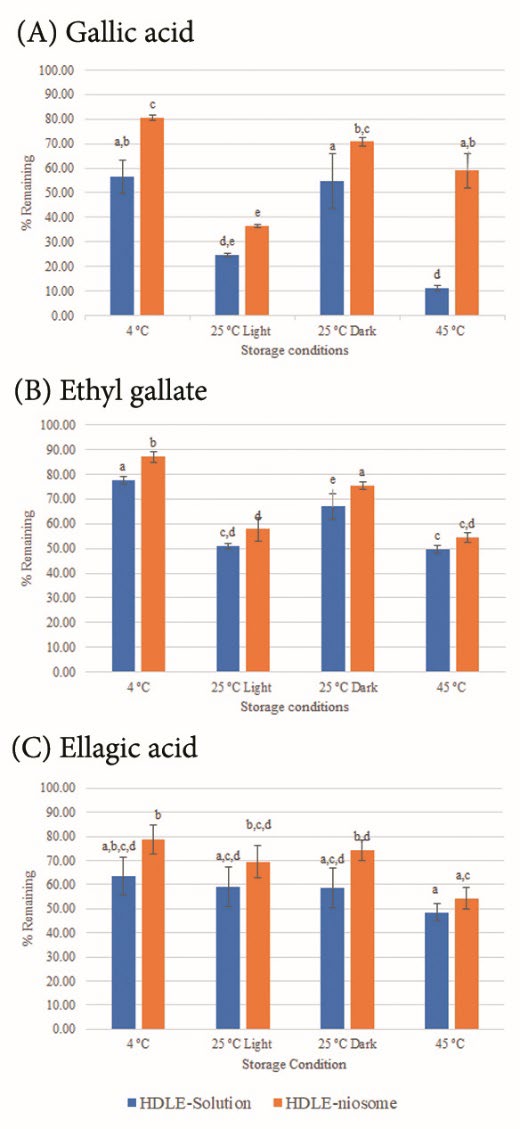
Figure 3. The remaining percentage of (A) gallic acid, (B) ethyl gallate, and (C) ellagic acid when kept at 4 ± 2°C without light, room temperature (RT or 30 ± 2°C) with/without light and 45 ± 2°C without light for three months. Data are expressed as mean ± standard deviation (SD). Different superscripts represent significant differences (P ≤ 0.05).
Irritation test by hen’s egg test-chorioallantoic membrane (HET-CAM) method
The results of the irritation test using the HET-CAM assay are shown in Figure 4. Sodium lauryl sulfate was used as a positive control as it could be in cosmetic products and causes acute skin irritation. When using the IS analysis method, the severe irritancy classification for a test substance is assigned when the value is greater than nine. SLS showed severe irritation, including vascular hemorrhage, lysis, and coagulation within 5 minutes, with an IS score of 10 ± 0.5. 0.9% (w/v) NaCl, deionized water, unloaded niosomes and HDLE-loaded niosomes presented no effect on vascular after 60 min of observation and interpreted as no irritation with an IS score of 0.0 ± 0.0.
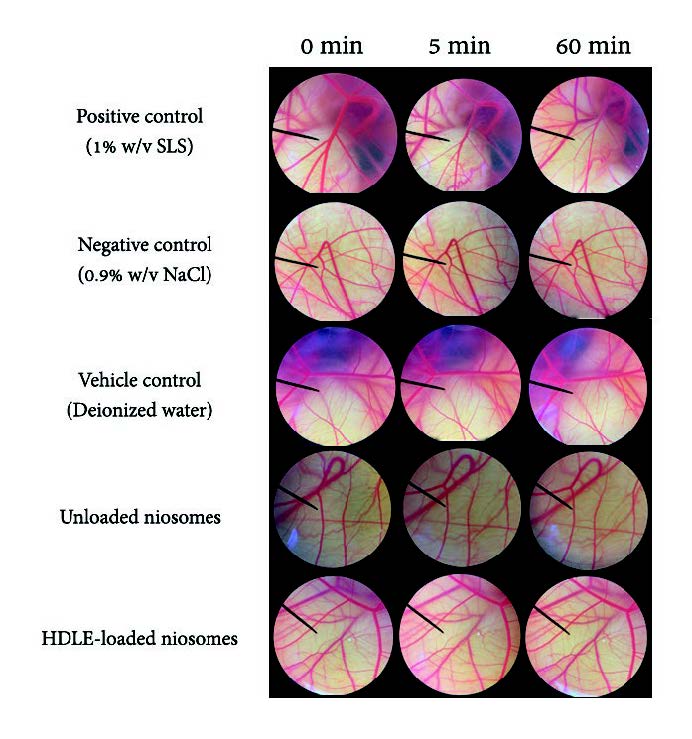
Figure 4. Effect of 1% (w/v) SLS, 0.9% (w/v) NaCl, Deionized water, unloaded niosomes and HDLE-loaded niosomes on chorioallantoic membranes (0 minutes, after 5 minutes, and 60 minutes exposure)
Physiochemical stability of topical serum containing HDLE-loaded niosomes, unloaded niosomes and placebo
The appearance of all serums is shown in Figure 5. The freshly prepared topical serum containing 0.25% (w/w) HDLE-loaded niosomes presented a cloudy brown appearance with pH 5.01 ± 0.01 and a viscosity value of 1.079 ± 0.098 Pa.s. The topical serum containing 0.25% (w/w) HDLE also presented a cloudy brown appearance with pH 5.00 ± 0.10 and a viscosity value of 0.979 ± 0.108 Pa.s. The topical serum containing 0.25% (w/w) unloaded niosomes had a cloudy white appearance with pH 4.20 ± 0.01 and a viscosity value of 1.157 ± 0.101 Pa.s. The placebo had a slightly cloudy white appearance with pH 5.25 ± 0.01 and a viscosity value of 1.385 ± 0.079 Pa.s. All formulations presented pseudoplasticity according to xanthan gum. The physical properties of all the serum, including pH and viscosity, were not significantly changed after three months of storage in all conditions. The HDLE-loaded niosomes and HDLE serum showed no separation, while the color became slightly darker.
For the chemical stability of serum, ethyl gallate was determined to assess the chemical stability of HDLE and HDLE-loaded niosomes serum because it is a potentially bioactive chemical marker for HDLE. Ethyl gallate is the most abundant compound in HDLE and has a strong positive linear relationship with various antioxidants and aging enzymatic inhibition (Doungsaard et al., 2023). After three months, the remaining ethyl gallate in HDLE serum were 85.35 ± 3.57, 55.23 ± 5.73, 80.35 ± 4.84 and 56.36 ± 5.47% and HDLE-loaded niosomes serum were 95.78 ± 2.35, 65.00 ± 8.42, 88.35 ± 4.67 and 69.57 ± 6.73% for storage conditions including 4 ± 2°C, room temperature (RT or 25 ± 2°C) with/without light and 45 ± 2°C, respectively. Ellagic acid, presented with the highest entrapment efficiency, was also designed for content evaluation. The remaining percentage of ellagic acid in HDLE serum were 75.57 ± 6.34, 62.58 ± 7.37, 62.46 ± 1.25 and 51.63 ± 9.36% and HDLE-loaded niosomes serum were 84.27 ± 4.74, 75.27 ± 3.94, 76.39 ± 7.25 and 63.85 ± 6.26% for storage conditions including 4 ± 2°C, room temperature (RT or 25 ± 2°C) with/without light and 45 ± 2°C, respectively. The HDLE-loaded niosomes serum showed higher stability of ethyl gallate and ellagic acid than the HDLE serum. Moreover, the ethyl gallate in HDLE and HDLE-loaded niosomes serum showed more stability than the solution and HDLE-loaded niosomes alone.
The result from 3-month storage indicated that the serum formulation could increase the durability of the bioactive compound. In addition, the result suggested that the serum should be kept in a light-resistant container and stored below 25 ± 2°C. For economic purposes, the formulations should be further determined for long-term stability to indicate expiratory date.
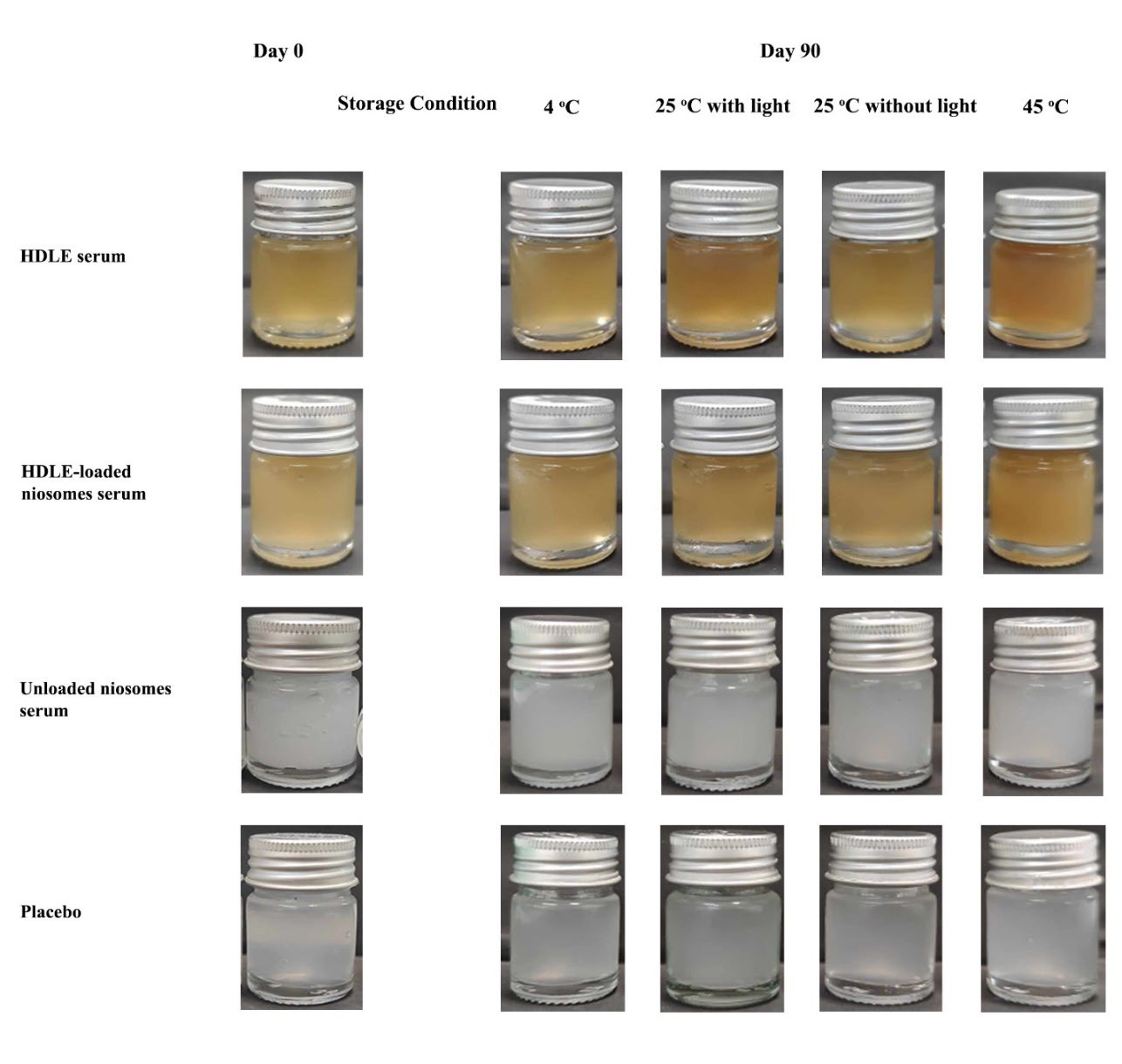
Figure 5. The appearance of all serums when kept at 4 ± 2°C, room temperature (RT or 25 ± 2°C) with/without light and 45 ± 2°C without light for three months.
Ex vivo skin permeation and deposition studies
The accumulative percentage of gallic acid, ethyl gallate and ellagic acid in skin permeation and deposition studies are shown in Figure 6.
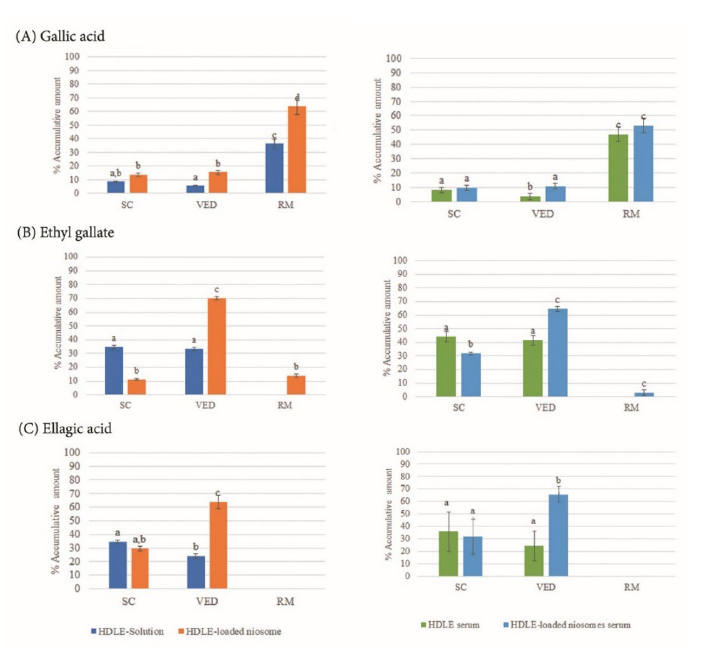
Figure 6. Accumulative amount percentage of gallic acid (A), ethyl gallate (B) and ellagic acid (C) in stratum corneum (SC), viable epidermis-dermis (VED) and receptor medium (RM). Data are expressed as mean ± standard deviation (SD). Different superscripts represent significant differences (P ≤ 0.05).
The skin irritation test on human volunteers
The PII value of 2% (w/v) SLS was 0.79, indicating slight irritation of positive control. All formulations, the HDLE-loaded niosomes, the unloaded niosomes, the HDLE-solution, and the blank serum (placebo), gave no irritation with PII values of 0.00, the same as deionized water. This result indicated that the HDLE-loaded niosomes, unloaded niosomes, HDLE solution and a blank serum (placebo) were safe for skin application.
Performance test of products on volunteers
The performance of the products was evaluated by measuring skin hydration, whitening, elasticity, and wrinkles on day 0 and after 30 and 60 days of application. The volar forearm was chosen for the performance test as it is less affected by the physical forces of stretching deformation (Manosroi et al., 2012). The final 30 subjects completed this study. All anti-aging parameters of test areas were measured and calculated for efficacy.
The efficacies of all interventions, including HDLE-loaded niosomes serum, unloaded niosomes serum, HDLE serum, placebo, and untreated area on skin hydration, were presented in Figure 7. The result exhibited that the unloaded niosomes serum significantly increases skin hydration after 30 and 60 days of application by about 10.41 ± 1.38 and 12.72 ± 1.71%, respectively. HDLE-loaded niosomes serum significantly increased the skin moisture content after 30 and 60 days of application by about 37.76 ± 0.95 and 35.32 ± 1.95%. HDLE serum significantly increased the skin moisture content after 30 and 60 days of application by about 25.59 ± 2.36 and 16.65 ± 1.93%.
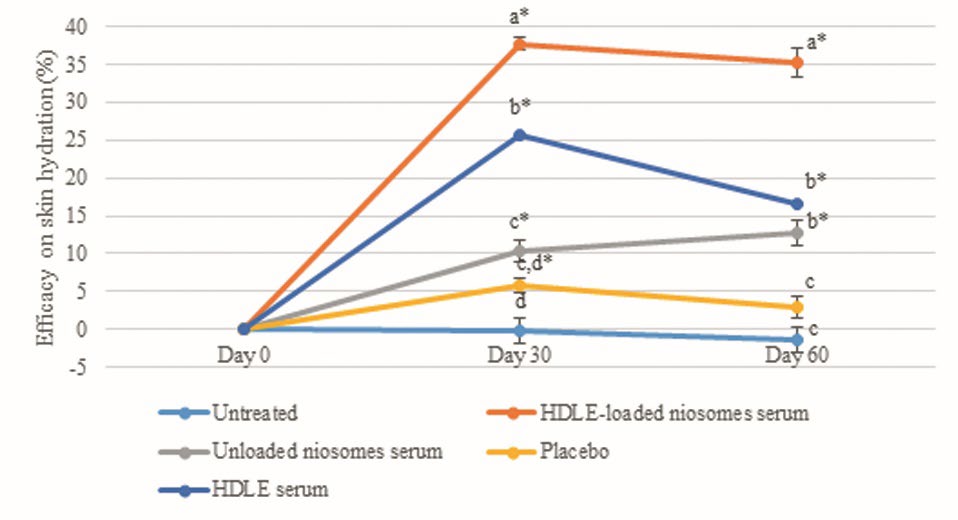
Figure 7. The percentage of efficacy on skin hydration was at days 0, 30, and 60 days after sample application. Data are expressed as mean ± standard error (SE). The values with different alphabets in the same day of measurement are significantly difference (P ≤ 0.05). * represents a significant difference from day 0 (P ≤ 0.05).
Regarding skin whitening, the HDLE-loaded serum could significantly brighten the skin by about 3.89 ± 0.23 and 3.98 ± 0.19% after 30 and 60 days of application. The HDLE serum could brighten the skin by about 1.2 ± 0.22 and 2.00 ± 0.16% after 30 and 60 days of application. However, the area treated with HDLE serum did not significantly brighten skin compared with unloaded niosomes serum and placebo after 60 days of application. Figure 8 shows that the HDLE and HDLE-loaded niosomes serum affected the lighter skin than the unloaded niosomes serum, placebo, and untreated area.
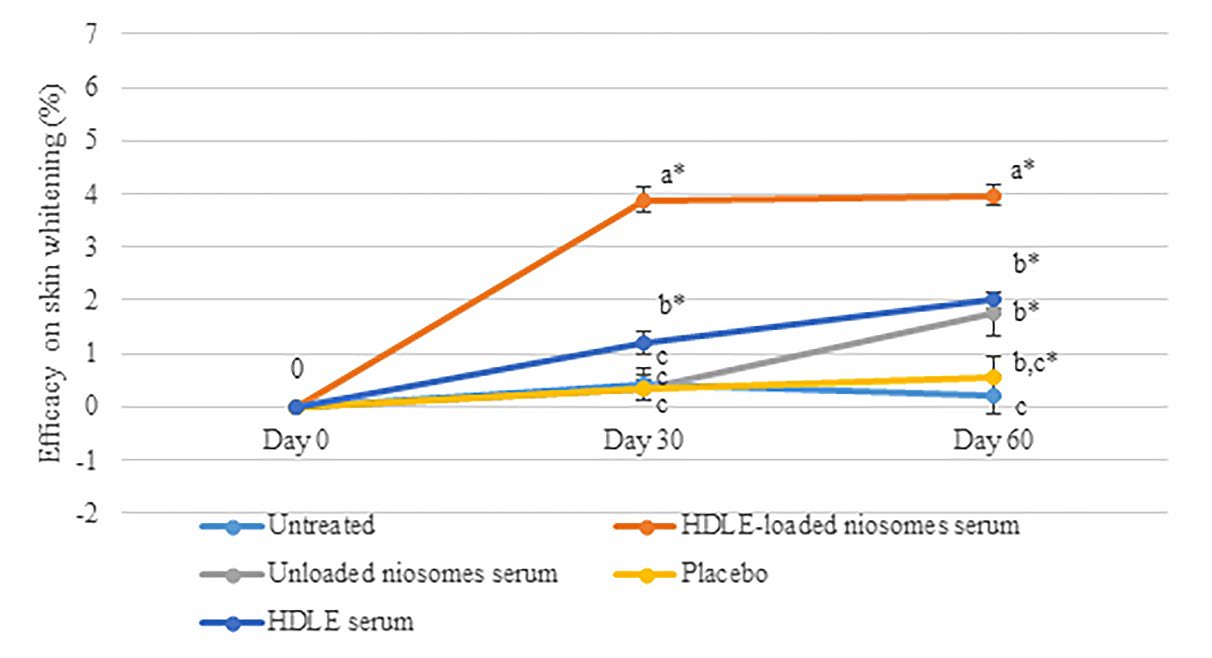
Figure 8. The percentage of the efficacy of skin whitening at day 0, 30, and 60 days after sample application. Data are expressed as mean ± standard error (SE). The values with different alphabets in the same day of measurement are significantly difference (P ≤ 0.05). * represents a significant difference from day 0 (P ≤0.05).
For skin elasticity, Figure 9 illustrates the efficacy of each formulation and the elasticity change of untreated area. The skin elasticity of the untreated area was significantly lower than the baseline, which was involved in the cell rejuvenation effects, usually occurring after 28-35 days (Manosroi et al., 2012). However, the HDLE loaded-niosomes serum could increase the skin elasticity by about 4.39 ± 2.13 and 13.43 ± 2.96% after 30 and 60 days of application. The HDLE serum could significantly increase the skin elasticity by about 7.10 ± 2.98% after 60 days of application. In addition, the efficacy of increasing skin elasticity of HDLE and HDLE-loaded niosome serum were significantly greater than that of unloaded niosome serum, placebo, and untreated area. The result showed that applying HDLE and HDLE-loaded niosomes could improve skin elasticity, an important parameter of skin aging.
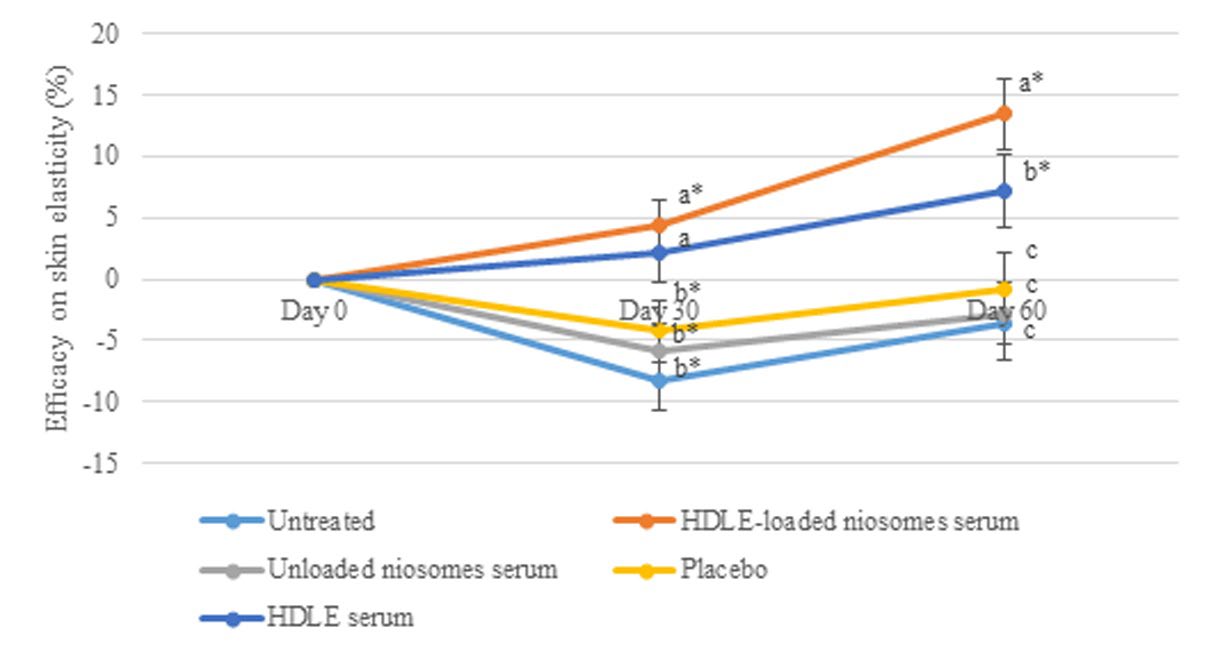
Figure 9. The efficacy on skin elasticity at days 0, 30, and 60 days after sample application. Data are expressed as mean ± standard error (SE). The values with different alphabets in the same day of measurement are significantly difference (P ≤ 0.05). * represents a significant difference from day 0 (P ≤ 0.05).
The efficacy of wrinkle reduction, including R1 (skin roughness), R2 (maximum roughness), R3 (average roughness), R4 (smoothness roughness), R5 (arithmetic average roughness), surface and volume, were statistically analyzed as shown in Figure 10(A)-(G). The HDLE-loaded niosomes serum presented the highest parameter reduction after 30 and 60 days of application. Additionally, the efficacies on every parameter value at Days 30 and 60 were significantly greater than the placebo and untreated skin. Figure 11 presents the depth of skin roughness and wrinkles on the skin of volunteers. The wrinkle was reduced, the primary lines were shallowed, and the skin looked more hydrated when applying the HDLE-loaded niosome serum compared with those before treatment. Compared with the related study, administration of longan leaf extract cream at the dose of 3.25%, 6.5% and 13% reported increased collagen density or decreased MMP-1 expression in a dose-dependent manner after UVB exposure. However, the result showed statistically no significant differences as there were some influence factors, including dose and bioavailability of active compounds (Mulyaningsih et al., 2019). In this study, the topical application of HDLE-loaded niosomes for at least 30 days significantly improved skin wrinkles, which could be from the various anti-aging activities of HDLE, especially antiglycation. The antiglycation activity could prevent structure protein damage, including collagen and elastin. Moreover, the reduction of glycosaminoglycan in the extracellular matrix was restrained, resulting in increased skin volume and decreased wrinkles. In addition, the unloaded niosomes present no interruption in permeation and deposit of bioactive polyphenol in the targeted skin.
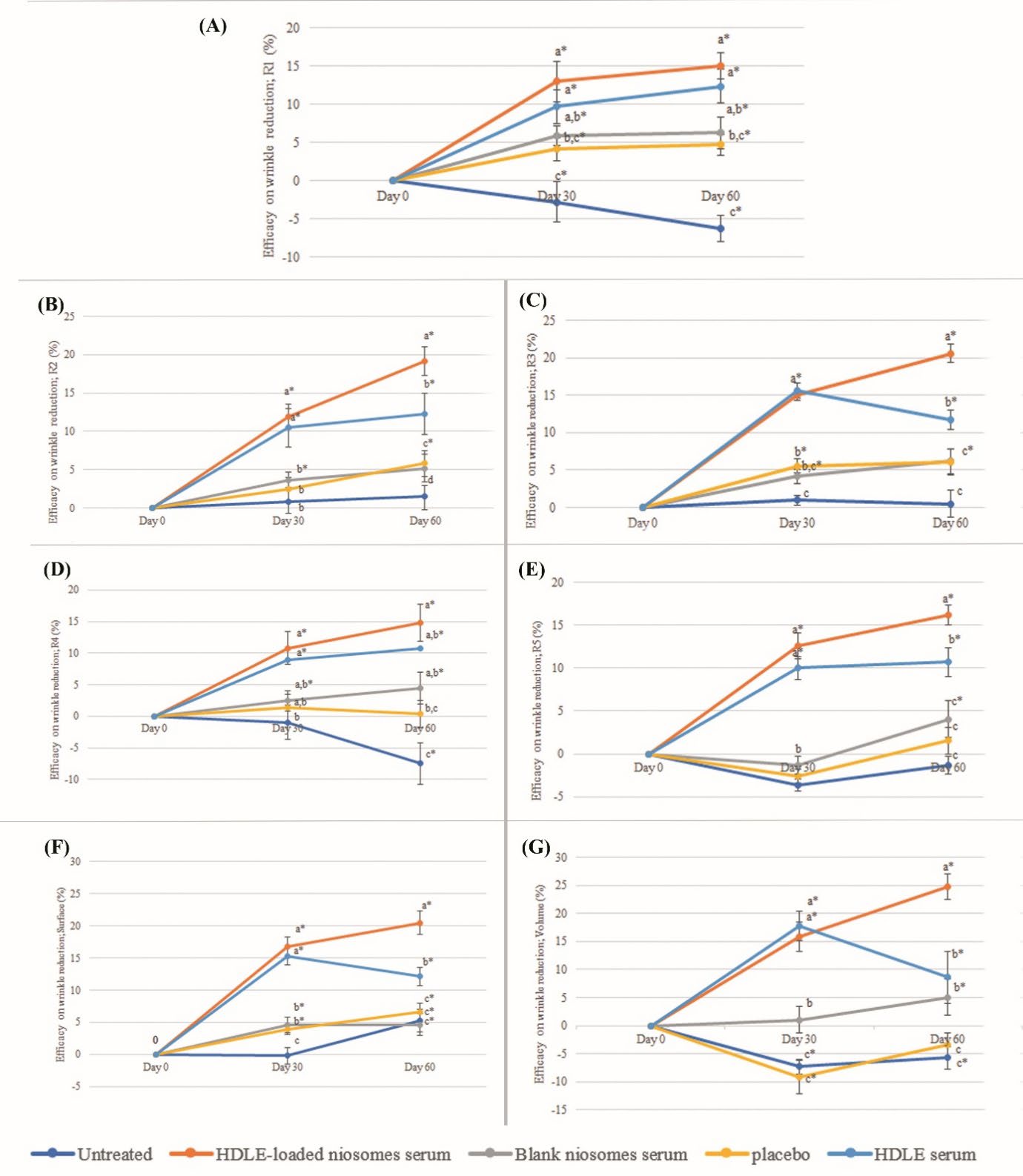
Figure 10. The efficacy on various wrinkle parameters, including R1 (A), R2 (B), R3 (C), R4 (D), R5 (E), Surface (F) and Volume at days 0 (G), 30 and 60 days after sample application. Data are expressed as mean ± standard error (SE). The values with different alphabets in the same day of measurement are significantly difference (P ≤ 0.05). * represents a significant difference from day 0 (P ≤ 0.05).
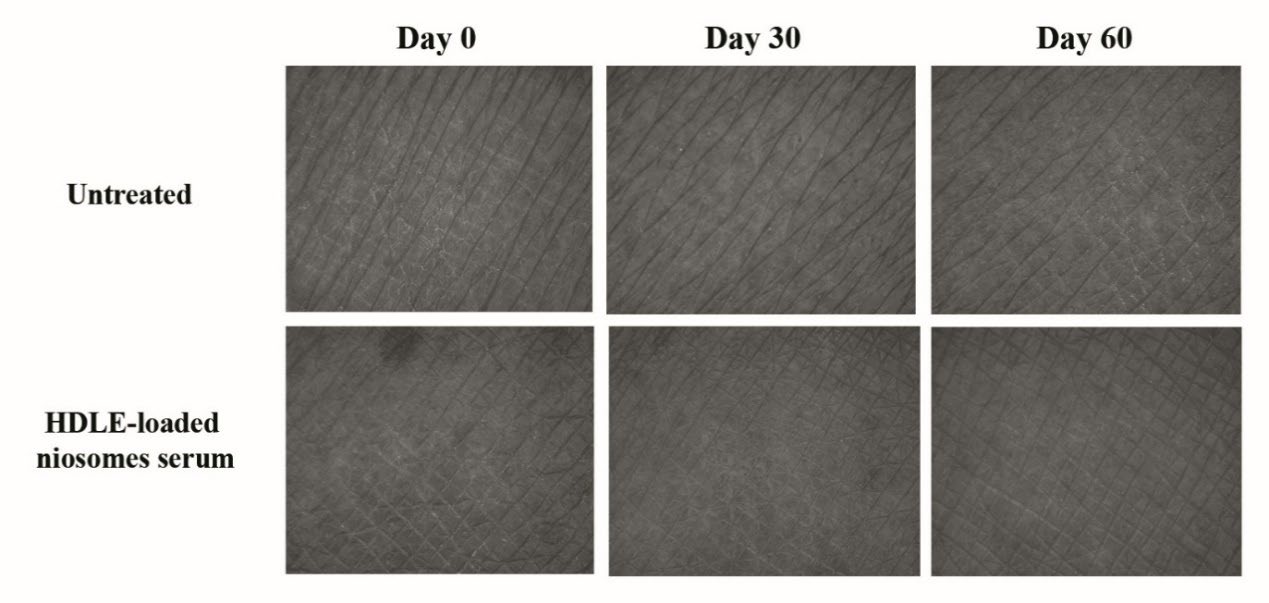
Figure 11. The depth of skin roughness and wrinkles on the skin of volunteers with untreated skin and skin after treatment with HDLE-loaded niosomes serum for 0, 30 and 60 days by Visiometer®.
DISCUSSION
Characteristics of HDLE-loaded niosomes and unloaded niosomes
HDLE-loaded and unloaded niosomes could be prepared with the ultrasonic processing technique. The pH of unloaded niosomes was 4.09 ± 0.02, while the HDLE-loaded niosomes was 4.90 ± 0.01. The pH of HDLE-loaded niosomes was higher than the unloaded niosomes according to the extract solution, which was higher in pH at about 4.99 ± 0.01. Although most active components were acidic compounds, including gallic acid and ellagic acid, the highest amount was ethyl gallate (predicted pKa about 8.04 ± 0.23), which could affect the overall the pH of extract solution. Furthermore, HDLE was a crude extract. From the phytochemical screening test, the more basic extract solution could be from saponins, which had a pH of about 4.83-5.1 (Supplementary data, Rai et al., 2023). The zeta potential of HDLE-loaded niosomes was nearly zero than the unloaded niosomes as the cation ionization of chemical compounds in extract involves the decrease in the negative charge of zeta potential by ion neutralization of surface charge. The chemical compounds in the extract might be absorbed onto the niosomes’ surface, decreasing the negative charge. The Zeta potential of blank and HDLE-loaded niosomes ranging from -28 to -21 mV is considered neutral particles (Barhoum and Makhlouf, 2018). The particle sizes in TEM images for the formulation were in good agreement with the DLS size measurement with a size of about 100 – 300 nm. The particle sizes and PDI of HDLE-loaded niosomes were larger than those of the unloaded niosomes. The increase is probably due to the interaction of chemical compounds in the extract with surfactant head groups, increasing the repulsion between the surfactant bilayers and thus increasing the vesicle size (Kazi et al, 2010). However, the size of the HDLE-loaded niosomes was still in the nanosized range, which is suitable for topical applications (Salvioni et al., 2021).
Entrapment efficiency determination
Ellagic acid was entrapped in niosomes in higher amounts than ethyl gallate and gallic acid., while the partition coefficient (Log P) of gallic acid, ethyl gallate and ellagic acid were 0.7, 1.3 and 1.05 (National Center for Biotechnology Information, 2021). The entrapment efficiency might involve the interaction of chemical compounds in an extract with the surfactant head group, as well as the polarity binding affinity. Furthermore, the size of the niosomes could also be involved in entrapment efficiency since smaller niosomes have a larger surface binding site of the surfactant head group (Barhoum and Makhlouf, 2018).
Stability study of niosomal formulation
For both HDLE-loaded niosomes and solution, light and heat could affect the color to become darker. The remaining percentages of all bioactive compounds decreased when the storage temperatures increased. Ethyl gallate was more chemically stable than gallic acid and ellagic acid. The result indicated that HDLE-loaded niosomes improved the stability of bioactive compounds compared to the solution as the remaining percentage of interested compounds at all storage temperatures in HDLE-loaded niosomes were higher than the solution. Storage at room temperature with light could reduce the number of bioactive compounds compared to without light. Thus, keeping the HDLE-loaded niosomes away from light and heat would be recommended.
Irritation test by hen’s egg test-chorioallantoic membrane (HET-CAM) method and a 4-hr human patch test
The HET-CAM is one of the most frequently used alternative tests for the prediction of the rinse-off and leave-on cosmetic products. The observation for IS calculation was usually for 300 seconds, mimicking the tear film turnover in humans, HDLE-loaded niosomes and unloaded niosomes presented no irritation sign in 300 seconds. Moreover, there were no irritation signs after 60 minutes of observation (Jirova et al., 2021). This method was observed to have a strong positive correlation with the clinical data. All the test products, including micellar water cleansers, eyelid sera, and shampoos, which were classified as non-irritants by HET-CAM protocols, were also safe in the product used in the clinical study. The product that was found to be non-safe in the product used in its usual way was also unsuitable by most of the HET-CAM protocols (Rivero et al., 2021). The 4-hr human patch test provides an opportunity to identify substances with significant skin irritation potential without using animals. The protocol is designed to avoid the production of more than mild irritant reactions and meets the highest ethical standards (Basketter et al, 1997). This methodology provides useful data regarding chemical irritation potential and appropriate chemical and product label needs. If the test materials present a statistical irritation score equally greater than 20% SLS, they would be classified as irritation to the skin (Robinson et al, 2001). HDLE was present with no irritation in the HET-CAM method in the previous study and confirmed its safety in a 4-hr human patch test (Doungsaard et al., 2023). Also, unloaded niosomes and HDLE-loaded niosomes present no irritation in both irritation tests. HDLE and HDLE-loaded niosomes were classified as non-irritation and proved their safety when applied in the performance test.
Ex vivo skin permeation and deposition studies
For the skin permeation study, HDLE-loaded niosomes presented a higher accumulation of gallic acid and ethyl gallate in the receiver medium (RM), indicating the improved permeation ability of HDLE-solution. For skin deposition, HDLE-loaded niosomes presented higher amounts of ethyl gallate and ellagic acid in the viable-epidermis dermis (VED) while retaining less amount in subcutaneous layers than HDLE-solution. Comparing HDLE-loaded niosomes serum and HDLE serum, the blank serum could reduce the permeation ability of each compound. HDLE-loaded niosome serum presented a higher amount of ethyl gallate and ellagic acid retaining in VED. Encapsulation of HDLE into niosomes can improve the deposition in the viable layers and enhance the skin cosmeceutical applications compounds in comparison with a simple extract solution, which can be more effective for the cosmeceutical application. As the skin is a complex organ with multiple layers to protect the organ from foreign matters outside the body, identifying the predominant permeation pathways is still under debate. It is generally believed that the molecular weight, lipophilicity, and charge of compounds are involved in skin permeation. Therefore, niosomes, a mixture of hydrophilic, hydrophilic, and charged compounds with nanosize, could enhance permeation and deposition (Salvioni et al., 2021). However, the study showed the penetration of ethyl gallate and gallic acid in the receptor medium, indicating the passage of substances into the systemic circulation. Additionally, the application of extract and loaded niosomes in cosmetic applications should be with some precautions. Gallic acid is a naturally occurring plant phenolic compound found in various edible fruits and vegetables. In an acute oral toxicity study in mice at orally 5000 mg/kg body weight, signs of lethal toxicity were not observed (Rajalakshmi et al., 2001). In a subacute toxicity study in F344 rats, orally 1,000 mg/kg was found to be non-toxic, indicating the safety of gallic acid (Niho et al, 2001). Ethyl gallate is an ester derived from gallic acid and ethanol, which is commonly used as a preservative in food, cosmetics, and pharmaceutical products. It has no toxicity at a dose of 20 mg/kg in albino Wistar rats. It is suggested that prolonged exposure to high doses of ethyl gallate may cause kidney and liver toxicity (Mohan et al, 2014). However, the observed toxic effects often occur at doses much higher than those used in cosmetic products.
Performance test of products on volunteers
The performance test of the products was evaluated through the skin parameters, including skin hydration, whitening, elasticity, and wrinkles on day 0 and after sample application on days 30 and 60. The efficacies on every parameter value at Days 30 and 60 were significantly greater than those of the placebo and untreated skin. The result demonstrated that the topical application of HDLE-loaded niosomes for at least 30 days significantly improved the skin parameters.
The increased skin moisture was associated with cholesterol in niosomes, known as occlusive moisturizing ingredients (Draelos, 2018). In addition, the efficacy of increasing skin hydration of HDLE and HDLE-loaded niosomes serum was significantly greater than that of unloaded niosome serum, placebo, and untreated area. Improving skin hydration may be associated with anti-hyaluronidase activity, which could reduce the hyaluronic acid degradation and the enhancing occlusive moisturizing effect of cholesterol (Draelos, 2018; Doungsaard et al., 2020).
For the skin-brightening effect, HDLE could inhibit tyrosinase activity, resulting in reduced melanin content (Doungsaard et al., 2023). Moreover, the extract has a significant absorption in UVB and some in the UVA solar spectrum; the sun filtering property could affect a whitening effect. According to skin permeation and skin deposition studies, niosomes deposited the HDLE in the superficial layer of skin where they acted as sun filtering agent.
The positive effect on elasticity and wrinkle reduction could be from the abilities of HDLE that could inhibit the activities of hyaluronidase, collagenase, MMP-2, and MMP-9, antiglycation as well as antioxidants through various mechanisms (Mulyaningsih et al., 2019; Doungsaard et al., 2020; Doungsaard et al., 2023). As a result, the breakdown and disorganization of skin structure were retard. Also, niosomes could enhance the permeation of bioactive compounds. Thus, HDLE-loaded niosomes could be appropriate choices for anti-aging cosmetic products that aim to provide a whitening effect.
CONCLUSION
This study has assessed the polyphenol stability, clinical irritation properties and in vivo anti-aging activity of HDLE-loaded niosomes. The niosomal formulation could retard the degradation of polyphenols compared to the HDLE solution after various storage conditions for three months. In addition, HDLE-loaded niosomes were confirmed for non-irritation by HET-CAM assay and a 4-hr human patch test. The efficacy of serum containing HDLE-loaded niosomes declared the improvement of skin hydration, elasticity, and wrinkles. Thus, HDLE-loaded niosomes can be used for cosmetic applications due to their good efficacy and safety.
ACKNOWLEDGEMENTS
The authors would like to express their sincere gratitude to the Ph.D. Degree Program in Pharmacy, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand 50200, under the CMU Presidential Scholarship, for providing financial support to conduct this study. Also, we would like to gratefully acknowledge the Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand 50200, for providing the facilities used in this study.
AUTHOR CONTRIBUTIONS
Pimporn Leelapornpisid designed, conducted all the experiments, performed the data visualization and wrote the manuscript. Pimjai Doungsaard assisted in conducting the experiments and performed the statistical analysis. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Barhoum, A. and Makhlouf A.S.H. 2018. Emerging applications of nanoparticles and architecture nanostructures. Amsterdam, The Netherlands: Elsevier. 255–278.
Basketter, D.A., Chamberlain, M., Griffiths, H.A., Rowson, M., Whittle, E., and York, M. 1997. The classification of skin irritants by human patch test. Food and Chemical Toxicology. 35(8): 845-852.
Chaikul, P., Khat-Udomkiri, N., Iangthanarat, K., Manosroi, J. and Manosroi, A. 2019. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. European Journal of Pharmaceutical Science. 131: 39-49.
Chen, G.L., Zhang, X., Chen, S.G., Han, M.D., and Gao, Y.Q. 2017. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the South of China. Journal of Functional Foods. 30:290-302.
Doungsaard, P., Chansakaow, S., Sirithunyalug, J., Shang-Chian, L., Wei-Chao, L. Chia-Hua, L. Kuan- Ha, L., and Leelapornpisid, L. 2020. In vitro biological activities of the anti-aging potential of Dimocarpus longan leaf extracts. Chiang Mai University Journal of Natural Science. 19(2): 235-251.
Doungsaard, P., Chansakaow, S., Poomanee, W., Sirithunyalug, S., Intasai, N. and Leelapornpisid, P. 2023. Antioxidant, anti-tyrosinase, antiglycation and safety of longan leaf extract for cosmeceutical application. Natural and Life Sciences Communications. 22(3): e2023052.
Doungsaard, P., Chansakaow, S., Poomanee, W., Sirithunyalug, B., Chaicjhit, S. and Leelapornpisid, P. 2023. Selection of bioactive chemical markers for anti-aging skin assessment of Dimocarpus longan Lour. leaf extract. Arabian Journal of Chemistry. 16(8): 104954.
Draelos, Z.D. 2018. The science behind skincare: Moisturizers. Journal of Cosmetic Dermatology. 17(2): 138-144.
Fraile, R., Geanta, R.M., Escudero, I., Benito, J.M. and Ruiz, M.O. 2015. Formulation of span 80 niosomes modified with SDS for lactic acid entrapment. Desalination Water Treatment. 56(13): 3463–3475.
Ge, X., Wei, M., He, S. and Yuan, W.E. 2019. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics. 11(2): 55.
Jírová, D., Basketter, D., Liebsch, M., Bendová, H., Kejlová, K., Marriott, M., and Kandárová, H. 2010. Comparison of human skin irritation patch test data with in vitro skin irritation assays and animal data. Contact Dermatitis. 62(2): 109–116.
Kamma, M., Wei-Chao, L., Lau, S-C., Chansakaow, S., and Leelapornpisid, P. 2019. Anti-aging cosmeceutical product containing of Nymphaea rubra Roxb.ex Andrews extract. Chiang Mai Journal of Science. 46(6): 1143-1160.
Kazi, K.M., Mandal, A.S., Biswas, N., Guha, A., Chatterjee, S., Behera, M., and Kuotsu, K. 2010. Niosome: A future of targeted drug delivery systems. Journal of Advance Pharmaceutical Technology and Research. 1(4): 374–380.
Khan, M.I., Madni, A., Hirvonen J., and L. Peltonen. 2017. Ultrasonic processing technique as a green preparation approach for diacerein-loaded niosomes. AAPS Journal. 18: 1554–1563.
Manosroi, A., Chutoprapat, R., Abe, M., Manosroi, W., and Manosroi, J. 2012. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharmaceutical Biology. 50(2): 208–224.
Manosroi, A., Chaikul, P., Abe, M., Manosroi, B. and Manosroi J. 2013. Melanogenesis of methyl myristate loaded niosomes in B16F10 melanoma cells. Journal of Biomedical Nanotechnology. 9(4): 626-638.
Mohan, S., Thiagarajan, K., Chandrasekaran, R., and Arul, J. (2014). In vitro protection of biological macromolecules against oxidative stress and in vivo toxicity evaluation of Acacia nilotica (L.) and ethyl gallate in rats. BMC Complementary and Alternative Medicine. 14: 257.
Mulyaningsih, R.E.M., Hussaana, A. and Chodidjah. 2019. The effect of administration of longan (Dimocarpus longan L. Steud) leaf extract cream on collagen density and MMP-1 expression in the dermis of BALB/C mice exposed to UV-B rays. Sains Medika. 10(2): 68-74.
National Center for Biotechnology Information, PubChem compound summary. 2021. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/.
Niho, N., Shibutani, M., Tamura, T., Toyoda, K., Uneyama, C., Takahashi, N., and Hirose, M. 2001. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food and Chemicol Toxicology. 39(11):1063-70.
Nitthikan, N., Leelapornpisid, P., Natakankitkul, S., Chaiyana, W., Mueller, M., Viernstein, H., and Kiattisin, K. 2018. Improvement of stability and transdermal delivery of bioactive compounds in green robusta coffee beans extract loaded nanostructured lipid carriers. Journal of Nanotechnology. 2018: 7865024.
Paul, P., Biswas, P., Dey, D., Saikat, A.S.M., Islam, M.A., Sohel, A., Hossain, R., Mamun, A.A., Rahman, M.A., Hasan, M.N. and Kim, B. 2021. Exhaustive plant profile of “Dimocarpus longan Lour” with significant phytomedicinal properties: A literature based-review. Processes. 9(10): 1803.
Rai, S., Kafle, A., Devkota, H.P. and Bhattarai, A. 2023. Characterization of saponins from the leaves and stem bark of Jatropha curcas L. for surface-active properties. Heliyon. 9(5): e15807.
Rajalakshmi, K., Devaraj, H., Niranjali, Devaraj, S.N. 2001. Assessment of the no-observed-adverse-effect level (NOAEL) of gallic acid in mice. Food and Chemical Toxicology. 39 (9): 919-922.
Rezaeiroshan, A., Saeedi, M., Morteza-Semnani, K., Akbari, J., Gahsemi, M. and Nokhodchi, A. 2020. Development of trans-ferulic acid niosome: An optimization and an in-vivo study. Journal of Drug Delivery Science and Technology. 59: 101854.
Robinson, M. K., McFadden, J. P., and Basketter, D. A. 2001. Validity and ethics of the human 4‐h patch test as an alternative method to assess acute skin irritation potential. Contact Dermatitis: Review Article. 45(1): 1-12.
Rivero, M.N., Lenze, M., Izaguirre, M., Pérez Damonte, S.H., Aguilar, A., Wikinski, S., and Gutiérrez, M.L. 2021. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products including cosmetics according to their surfactant composition. Food and Chemical Toxicology. 153: 112229.
Salvioni, L., Morelli, L., Ochoa, E., Labra, M., Fiandra, L., Palugan, L., Prosperi, D., and Colombo, M. 2021. The emerging role of nanotechnology in skincare. Advance in Colloid and Interface Science. 293: 102437.
Sankhyan, A. and Pawar, P. 2012. Recent trends in niosomes as vesicular drug delivery system, Journal of Applied Pharmaceutical Science. 2(6): 20–32.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Subplementary
QUALITY CONTROL OF PLANT MATERIAL AND HYDROETHANOLIC LONGAN LEAF EXTRACT
This research was a continuation of our related study, aimed at developing HDLE-loaded niosomes to enhance polyphenol stability and efficiency for cosmeceutical applications. In addition, the irritation and efficiency of HDLE and HDLE-loaded niosomes were clinically investigated. The evaluation of loss on drying, total ash, and acid-insoluble ash were performed to designate the quality specifications for plant materials. The UV-Vis spectra, phytochemical examine and HPLC analysis were performed for HDLE quality specifications, providing the repeatability of the experiment.
MATERIALS AND METHODS
Chemical and reagent
Ethanol (95%) was purchased from Liquor Distillery Organization (Chachoengsao, Thailand). Ellagic acid and gallic acid were purchased from Sigma-Aldrich (Steinhiem, Germany). Ethyl gallate was purchased from Tokyo Chemical Industry (Tokyo, Japan). Acetonitrile HPLC grade and 2-propanol were from RCI Labscan (Bangkok, Thailand). Ortho-phosphoric acid 85% was purchased from Merck (Darmstadt, Germany).
Plant materials
Mature leaves of Dimocarpus longan cv. E-Daw were harvested from a longan farm in Chiang Mai Province, Thailand, between April and May 2019. The leaves were washed, dried at 50ºC for 24 hours, and ground into a powder using a grinder machine (Spring Green Evolution, Thailand). The powder was then passed through an 80-mesh sieve and packed into metalized bags. The packed powder was stored at room temperature until it was used.
Evaluation of loss on drying
The two grams of longan leaf powder (W2) in a completely dried and weighed crucible (W1) were heated under controlled conditions at 105ºC until the weight was reduced to constant (W3). The percentage of loss on drying was calculated with the following equation (Thai Herbal Pharmacopoeia, 2019).
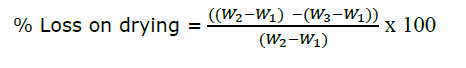
Evaluation of total ash
The sample from loss on drying evaluation (W3) was ignited to constant weight in a muffle furnace at a mild temperature at the beginning and then at 550 ± 25ºC. The constant weight (W4) was measured after prolonged ignition for more than 4 hours to obtain carbon-free ash. The percentage of total ash was calculated with the following equation, where the initial weight was equivalent to the final weight in loss on drying evaluation (Thai Herbal Pharmacopoeia, 2019).
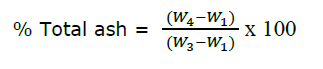
Evaluation of acid-insoluble ash
The sample from the total ash evaluation was boiled with 25 ml of 10% hydrochloric acid for 5 min. The insoluble matter was collected on ashless filter paper. Then, the insoluble matter and an ashless filter paper were ignited at a mild temperature at the beginning and then at 550 ± 25ºC. The constant weight (W5) was measured after prolonged ignition for more than 4 hours to obtain carbon-free ash. The percentage of acid-insoluble ash was calculated with the following equation; where the initial weight was equivalent to the final weight in loss on drying evaluation (Thai Herbal Pharmacopoeia, 2019).
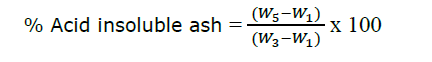
Plant extraction
Briefly, 500 g of longan leaf powder was macerated with 50% ethanol in a ratio of 1:3 for 24 hours. The fluid was collected, and the remaining powder was extracted for another extraction. All filtrate was pooled and removed solvent using a rotary evaporator (R-300 Buchi ®, Flawil, Switzerland) at the pressure of 50 mbar at 50ºC (Doungsaard et al., 2020). The hydroethanolic longan leaf extract (HDLE) was kept at -20ºC until use.
UV-visible spectrum of the extract
HDLE was dissolved with 50% ethanol at 200 µg/ml concentration. Then, the solution was placed in a 96-well plate with a UV transparent flat bottom (Corning Inc., New York, USA). Samples were scanned between 220 and 1000 nm using a SPECTROstar nano (BMG LABTECH GmbH, Germany).
Phytochemical examination
Phytochemical examination was carried out to all the extract using the standard methods as following described (Pandey and Tripathi, 2014).
Detection of alkaloids
Dragendroff’s, Mayer’s and Wagner’s reagents were used to detect alkaloids. The extract (0.1 g), dissolved or dispersed in 10 ml of 5% HCl, was boiled over a water bath and filtered. Six drops of reagent were added to the aliquot. Brownish-red or orange precipitate indicated the presence of alkaloids.
Detection of phenols
Ferric chloride was used as a reagent. The extract (0.1 g), dissolved or dispersed in 5 ml of ethanol, was added with 2-3 drops of 2% ferric chloride solution. The formation of bluish black color indicated the presence of phenols.
Detection of flavonoids
Shinoda’s test was performed to detect flavonoids. The extract (0.1 g), dissolved or dispersed in 5 ml of ethanol, was added magnesium ribbon and boiled over a water bath. After the water boiled, the 2 drops of concentrated HCL were added. The pink color scarlet solution indicated the presence of flavonoids.
Detection of tannins
Detection of tannin was performed using a gelatin test. The extract (0.1 g), dispersed in 5 ml of water, was filtered. Then, the 1% gelatin solution containing sodium chloride was added to the clear filtrate. The formation of white precipitate indicated the presence of tannins.
Detection of reducing sugar
Benedict’s reagent was used to detect reducing sugar. The extract (0.1 g) dispersed in 5 ml of water and filtered. Then, the filtrate was treated with Benedict’s reagent and heated over a water bath. The brick-red precipitate indicated the presence of reducing sugar contained in glycosides.
Detection of saponin
A frothing test was performed to detect saponin. The extract (0.1 g) was shaken with water in a graduated cylinder for 15 minutes. Formation of 1 cm layer of 10 minutes persistence foam indicated the presence of saponin.
Detection of triterpenes
Triterpenes was determined using Salkowski’s test. The extract (0.1 g) was treated with chloroform and filtered. The filtration was treated with a few drops of concentrated sulfuric acid. The appearance of golden yellow color indicated the presence of triterpenes.
High-Performance Liquid Chromatography (HPLC) analysis
The HPLC analysis, employing the Hewlett Packard Agilent 1100 Series, was performed for quality control of extract and the quantitation of gallic acid, ethyl gallate and ellagic acid in niosomes and serum preparations. The stationary phase was a C-18 column (250 x 4.6 mm, i.d. 5 µm, ODS-3, InertsilTM). The mobile phases were (A) acetonitrile and (B) 0.1% (v/v) ortho-phosphoric acid (pH 3). The gradient program was started from (A) 10% to 30% in 60 minutes, with a flow rate of 0.8 ml/min. HDLE was dissolved with 50% ethanol in a fingerprinting analysis of the extract.
RESULTS
Quality control of plant materials
The percentage of loss on drying represents the moisture content in longan leaf powder. The moisture influences deterioration in quality due to toxigenic fungi and damage from insects if the herbs are poorly dried and stored. The loss of drying of longan leaf powder was 4.82 ± 0.24%, which met the standard guidelines of loss on drying less than 10%. Total ash of longan leaf powder was 6.92 ± 0.16%. Total ash reveals how many minerals are physiologically contained in the plant powder and how many foreign materials have been mixed during processing. Acid-insoluble ash confirms contamination with soil and sand attached to the plant material during cultivation and processing. The acid-insoluble ash of longan leaf powder was 1.49 ± 0.09%, which also met the standard guidelines of less than 2.5% (Thai Herbal Pharmacopoeia, 2019).
Quality control of HDLE using UV-visible spectra profile
In this study, longan leaves, which were macerated using 50% (v/v) ethanol, yielded 14.98 ± 2.29% of a sticky crude extract with a brown appearance. The qualitative UV-VIS profile of HDLE in 50%(v/v) ethanol at 200 µg/ml concentration, the profile showed peaks at about 280 and 360 nm with the absorption of 1.630 and 0.437, respectively, as shown in Figure 1. The appearance of peaks in the region from 200 to 400 nm clearly indicates the presence of unsaturated groups and heteroatoms such as sulfur, nitrogen, and oxygen, which represent the containing of phenolic and flavonoid compounds (Jain et al., 2016)
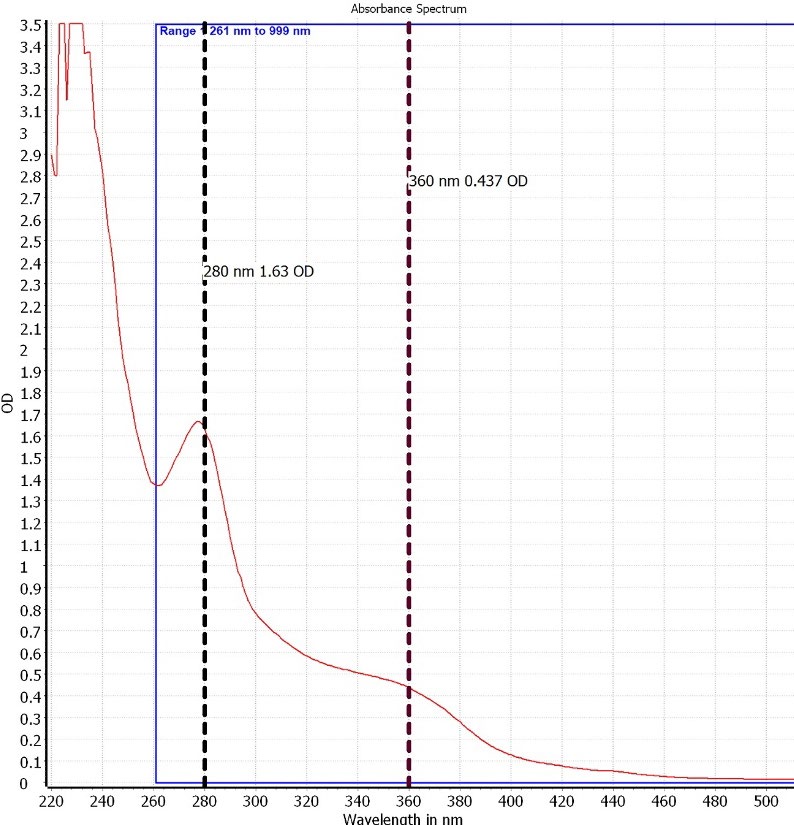
Figure 1. UV spectrum of HDLE in 50%(v/v) ethanol at 200 µg/ml concentration
Phytochemical examination of HDLE
HDLE were screened for their chemical constituents including alkaloids, phenolics, flavonoids, tannins, reduced sugar, saponins and triterpenes. Table 1 shows the phytochemical constituents of HDLE.
Table 1 Qualitative determination of constituents by phytochemical tests in HDLE.
|
Phytochemical examination |
HDLE |
|
Alkaloids |
+ |
|
Phenols |
+ |
|
Flavonoids |
+ |
|
Tannin |
+ |
|
Reducing Sugar |
+ |
|
Saponin |
+ |
|
Triterpenes |
+ |
Note: “+” indicates the present of each phytochemical compounds
HPLC Analysis
HPLC was performed as a comprehensive and quantifiable identification method for HDLE. Figures 2(A) and (B) show the HPLC chromatograms of the reference standards and HDLE. HDLE presented retention times of 6.587, 28.435, and 34.579 min, which were similar to standards, gallic acid (Rt of 6.737 min), ethyl gallate (Rt of 28.638 min) and ellagic acid (Rt of 34.579 min). According to the related study, these compounds were considered bioactive for the anti-aging assessment (Doungsaard et al., 2023). The assay method of each compound was validated before analysis, which is detailed in Table 2. The quantitation of gallic acid, ethyl gallate and ellagic acid in a gram of extract were 1.54 ± 0.71, 7.88 ± 0.94 and 4.62 ± 1.70 mg, respectively.
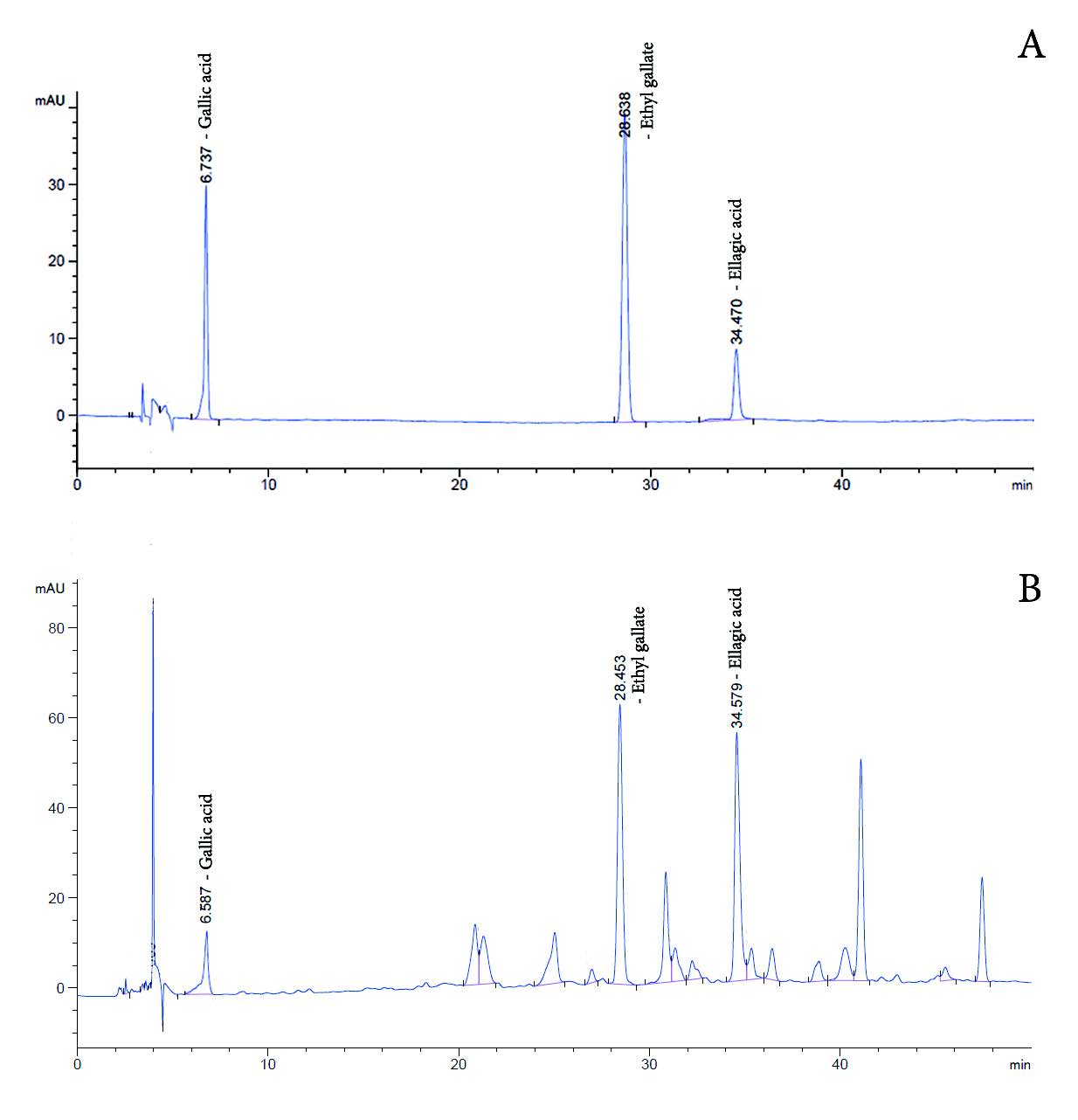
Figure 2. HPLC chromatogram, (A) the reference standards and (B) HDLE
Table 2 Methods of validation requirement: linearity, limit of detection (LOD), limit of quantitation (LOQ), and % RSD.
|
|
Linearity |
LOD (µg/ml) |
LOQ (µg/ml) |
% RSD |
|
Gallic acid |
R2 = 0.9996 |
0.391 |
12.500 |
0.015 |
|
Ethyl gallate |
R2 = 0.9998 |
0.098 |
1.562 |
1.326 |
|
Ellagic acid |
R2 = 0.9991 |
7.500 |
15.000 |
1.901 |
DISCUSSION
Quality control of plant material
The evaluation of loss on drying, total ash, and acid-insoluble ash was performed to designate the quality specifications for plant materials, providing the repeatability of the experiment. These tests provide valuable information about the overall purity of the plant material. High levels of ash may suggest the presence of inorganic contaminants or adulterants, such as sand, soil, or other extraneous materials. Moreover, it can help identify and authenticate plant materials. Different plant species tend to have characteristic ash values, and deviations from the expected range may indicate adulteration or substitution. Hence, the specification of Dimocarpus longan leaf cv. E-daw was 4.82 ± 0.24% of the loss on drying, 6.92 ± 0.16% of total ash and 1.49 ± 0.09% of acid-insoluble ash.
Quality control of HDLE
According to the quality control study, the specification of Dimocarpus longan leaf cv. E-daw was 4.82 ± 0.24% of the loss on drying, 6.92 ± 0.16% of total ash and 1.49 ± 0.09% of acid-insoluble ash. The HDLE extraction for skin aging and skin moisturizer should be prepared from powdered Dimocarpus longan Lour. (E-daw) leaves by maceration with 50 % ethanol. The extract characteristic should be a greenish-brown syrupy mass with characteristic odor. The UV-Vis spectra in 50% ethanol at 200 µg/ml concentration show peaks at about 280 and 360 nm with absorption of 1.630 and 0.437, respectively. The extract contained alkaloids, phenols, flavonoids, tannins, reducing sugar, saponin and triterpenes. The labeled amount of gallic acid, ethyl gallate and ellagic acid by HPLC is not less than 1.54 ± 0.71, 7.88 ± 0.94 and 4.62 ± 1.70 mg, respectively.
REFERENCE
Department of medical science, Ministry of Public health. Thai Herbal Pharmacopoeia, Bangkok; Office of National Buddism Press.2019.
Doungsaard, P., Chansakaow, S., Sirithunyalug, J., Shang-Chian, L., Wei-Chao, L. Chia-Hua, L. Kuan- Ha, L., and Leelapornpisid, L. 2020. In vitro biological activities of the anti-aging potential of Dimocarpus longan leaf extracts. Chiang Mai University Journal of Natural Science. 19(2): 235-251.
Doungsaard, P., Chansakaow, S., Poomanee, W., Sirithunyalug, B., Chaichit, S. and Leelapornpisid, P. 2023. Selection of bioactive chemical markers for anti-aging skin assessment of Dimocarpus longan Lour. leaf extract. Arabian Journal of Chemistry. 16(8): 104954.
Jain, P.K., Soni, A., Jain, P., and Bhawsar, J. 2016. Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. Journal of Chemical and Pharmaceutical Research. 8(2): 1-6.
Pandey, A. and Tripathi S. 2014. Concept of standardization, extraction and pre-phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry. 2(5): 115-119.
Pimjai Doungsaard1, 2, Sunee Chansakaow1, Worrapan Poomanee1, 2, Busaban Sirithunyalug1, and Pimporn Leelapornpisid1, 2, *
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200 Thailand.
2 Innovation Center for Holistic Health, Nutraceuticals and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200 Thailand.
Corresponding author: Pimporn Leelapornpisid, E-mail: pimporn.lee@cmu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: March 1, 2024;
Revised: June 14, 2024;
Accepted: June 18, 2024;
Online First: June 27, 2024

