
Fisetin Protects the Wistar Rats from the Oxidative and Inflammatory Damage Caused by Ethelene Glycol Induced Urolithiasis
Patnam Nageswari and Swathi Konda*Published Date : June 21, 2024
DOI : https://doi.org/10.12982/NLSC.2024.039
Journal Issues : Number 3, July-September 2024
Abstract Urolithiasis, commonly referred to as urinary stones, is the third most prevalent condition affecting the urinary system and affects 12% of people worldwide. In this investigation, we assessed the prospective flavonoid fisetin's anti-urolithiasis activity. Fisetin reduced the nucleation, aggregation, and crystallization of kidney stones in in vitro tests at dosages of 62.5, 125, 250, 500, and 1,000 ug/mL in a dose-dependent manner. Twenty-four male Wistar rats (n = 6) were induced with urolithiasis using ethylene glycol (0.75% v/v) with ammonium chloride (1% w/v) in drinking water for seven days. Except for the animals in the vehicle control and disease control groups, the remaining animals were supplemented with either Fisetin 15 mg/kg or 30 mg/kg body weight from days 7 to 21. On day 22, a urine evaluation showed that fisetin significantly increased the glycosaminoglycan (GAG) content, corrected urine pH, increased creatinine clearance, and increased urine volume. Additionally, fisetin decreased the levels of calcium, creatinine, uric acid, and N-acetyl glucouronidase (NAG) activity. Serum biochemical analysis revealed that fisetin reduced the excretion of creatinine, uric acid, and blood urea nitrogen (BUN). Further, fisetin reduced the content of total protein, calcium, oxalate, protein carbonyl content (PCC), malondialdehyde (MDA), tumor necrosis factor (TNF-α), interlukin-6 (IL-6), and increased the content of reduced glutathione (GSH), the activities of superoxide dismutase (SOD), and catalase (CAT). Histological findings also support our findings. Based on the above observations, fisetin can be a potential candidate for the treatment of urolithiasis.
Keywords: Urolithiasis, Creatinine, Fisetin, Urinary stones, Creatinine, Renal function
Funding: The authors are grateful for the research funding provided by University Grants Commission (UGC) (NFPwD) Scheme (Ref. No.: NFPwD-2018-2020-AND-8336) by the department of empowerment of persons with disabilities (Divyangjan), India.
Citation: Nageswari, P. and Konda, S. 2024. Fisetin protects the wistar rats from the oxidative and inflammatory damage caused by ethelene glycol induced urolithiasis. Natural and Life Sciences Communications. 23(3): e2024039.
INTRODUCTION
Urolithiasis is a complicated, multidimensional condition influenced by both intrinsic (such as age, sex, and inheritance) and extrinsic (such as geographic location, climate, diet, mineral content, and water intake) factors. Blood in the urine and discomfort in the back, groin, or abdomen are frequently brought on by kidney stones. Stone formation is associated with a decrease in urine volume or an increase in the excretion of substances such as calcium, urate, cystine, oxalate, phosphate, and xanthine. The kidney's urine-collecting area is where calculi grow, and they can be as small as a pinhead or as big as the renal pelvis. One in twenty people will have kidney stones at some point in their lives (0.5% annually in North America and Europe) (Worcester et al., 2006; Sofia et al., 2016). Being the third most common condition of the urinary system, affecting 2-3% of the general population. If left untreated, urinary calculi can have catastrophic health effects such as severe blockage, hydronephrosis, infection, and urinary tract bleeding. Tamsulosin and other alpha-blockers, phosphodiesterase-5 inhibitors (tadalafil), and calcium channel blockers (nifedipine) are often prescribed to relax the ureter's muscles, enabling patients to pass stones more quickly and with less discomfort (Saljoughian, 2020). To remove the calculi, typical methods include surgery, lithotripsy, and local calculus disruption with a high-power laser. Some interventional treatments, such as extracorporeal shock wave lithotripsy (ESWL), ureteroscopy (URS), or percutaneous nephrolithotomy (PNL), should be used to treat stones larger than 5 mm or stones that do not pass through (Butterweck and Saeed, 2009). A viable alternative therapy must be developed because the recurrence rate without preventative treatment (Pawar and Niraj, 2015). Churna, syrups, tablets, and capsules are often part of the traditional formulations used to treat urolithiasis. Many patients refuse to take these formulations as directed on a daily basis. Hence, there is a need to develop an alternative approach. Medicinal plants and their byproducts have been used for centuries to treat a variety of illnesses. However, synthetic drug use has become more common since the middle of the 20th century. The mounting evidence of synthetic drugs' adverse effects on public health has resulted in a growing trend of using medicinal plants as substitutes for synthetic drugs (Erdemli et al., 2020). Isolated actives from herbs are gaining special attention as ingredients in various formulations and dosage forms of herbal origin. Because of the unrivaled abundance of chemical variety, natural products like plant extracts, whether as pure chemicals or as standardized extracts, offer limitless prospects for the discovery of novel drugs. There are numerous compounds found in plants used in traditional medicine that can be used to treat both chronic and infectious disorders (Sasidharan et al., 2011). One of the major families of polyphenols are flavonoids, which have a variety of pharmacological functions, have antioxidant effects, and are known to support cardiovascular health. However, little is known about the function and illness of the kidneys as a result of flavonoids (Cao et al., 2022). Fisetin is a flavanol that has unique antioxidant capabilities in common with a wide variety of other plant polyphenols. Additionally, it demonstrates a particular biological activity of great relevance in relation to the cytoprotection of normal cells and the defense of functioning macromolecules against stress. Additionally, it has the potential to be an anti-inflammatory agent (Grynkiewicz et al., 2019). Numerous investigations have revealed the wide spectrum of pharmacological qualities that fisetin possesses, including antioxidant, anti-inflammatory, antibacterial, anti-osteoporotic, antidiabetic, and anti-carcinogenic actions (Antika and Rita 2021).
Fisetin can be found in many different fruits and vegetables, including apple, strawberry, persimmon, grape, onion, and cucumber, among others. Fisetin levels vary among fruits and vegetables (Kumar et al., 2019). Sahu et al. (2014) revealed the nephroprotective effect of fisetin in cisplatin-challenged rats.
The purpose of this study is to evaluate the anti-urolithiasis effect of fisetin against ethylene glycol (EG) induced urolithiasis in rat model. We have also investigated the influence of fisetin on oxidative and inflammatory damage in EG induced urolithiasis. Further, we have evaluated the efficacy of fisetin on cytokine content (IL-6 & TNF-α) in kidneys of rats induced with urolithiasis.
MATERIALS AND METHODS
In vitro anti-urolithiasis activity
Crystal nucleation assay
The nucleation assay was carried out as described by Hennequin et al. (1993) with some minor modifications (Hennequin et al., 1993). Briefly, calcium chloride (Cacl2; 5 mmol/L) and sodium oxalate (Na2C2O4; 7.5 mmol/L) were prepared in buffer containing Tris-HCl (0.05 mol/L) and sodium chloride (Nacl; 0.15 mol/L) at pH 6.5. Various quantities of fisetin (62.5, 250, 500, and 1,000 g/ml) were combined separately with eight milliliters of Cacl2 solution. Crystallization was initiated in the solution after the addition of 1 ml of sodium oxalate solution. The change in absorbance was measured at 620 nm using an UV spectrophotometer (UV-1,800, Shimadzu India Pvt. Ltd, New Delhi, India) for every 1 minute. The temperature was maintained at 37°C. a similar procedure was repeated for the control with distilled water in place of fisetin. All samples were assayed in triplicate. Cystone (1,000 ug/ml) was used as a reference standard. Percentage inhibition (%) of nucleation was then calculated by comparing the turbidity slope of different concentrations of fisetin/cystone with the control.
Crystal aggregation assay
The effect of fistein on calcium oxalate (CaOx) was studies as mentioned in Atmani and Khan, 2000 (Atmani and Khan, 2000). Briefly, fisetin (62.5, 250, 500, and 1000 µg/ml) and cystone (1000 ug/ml) were added to a Tris buffer (0.15 mol/L; pH-6.5) containing calcium oxalate monohydrate crystals (0.8 mg/ml) and NaCl 0.15 mol/L with constant stirring at 37°C. Optical density of the solution was measured at 620 nm using a UV spectrophotometer (UV-1,800, Shimadzu India Pvt. Ltd, New Delhi, India) at 10 min interval for 60 mins.
Crystallization assay
Twenty-five millilitres (ml) of artificial urine were prepared and maintained at room temperature (37°C) for 10 mins. Two millilitres (ml) urine sample was added to 50 µL of fisetin (1,000 ug/ml) or cystone (1,000 ug/ml). Reaction was initiated by the addition of 50 aliquot 2ml of urine sample in an Eppendorf tube followed by add 50 µL of sodium oxalate solution (0.1M). The reaction mixture was incubated at 37°C for 30 mins. Following incubation, contents were lyophilized to obtain dried pellets for scanning electron microscopy (SEM) (Carl Zeiss, Germany).
Crystal growth assay
The effect of fisetin (250, 500, and 1,000 µg/mL) on crystal growth was investigated according to the procedure described in literature (Nakagawa et al., 1985). Calcium oxalate monohydrate (COM) stone slurry (0.2 mg/ml) was prepared in 50 mM sodium acetate buffer (pH 5.7). Calcium chloride (Cacl2;1 mM) and sodium oxalate (1 mM) were prepared with 10 mM Tris-HCl containing 90 mM NaCl and the pH was adjusted to 7.2. One millilitre of fisetin (250, 500, and 1,000 µg/mL) or cystone (1,000 ug/ml) was added to a solution containing COM crystal seed (0.2 μl) and Cacl2 and sodium oxalate to evaluate the crystal growth inhibition. The optical density of the solution at 203 nm was measured using a UV-visible spectrophotometer (UV-1,800, Shimazdu India Pvt. Ltd, New Delhi, India), and the percent (%) inhibition of free oxalate content was calculated.
In vivo anti-urolithic activity
Chemicals
Fisetin was purchased from Doctor's Best, Inc., CA, USA. All other reagents used were of analytical grade and were purchased from licensed vendors.
Animals
Twenty-four adult male Wistar rats (200 ± 25 g) were purchased (Sri Venkateswara Enterprises, Bengaluru, India) and maintained and were cared in accordance with the guidelines of The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under standardized conditions (12-hours light/dark cycle, 24°C) and provided free access to regular chow (Chakkan food, Nav Maharashtra Oil Mills Pvt. Ltd., Pune, India) and purified drinking water ad libitum. The Institutional Animal Ethical Committee (IAEC), Sri Padmavathi Mahila Vishwavidyalayam (SPMVV), Tirupati, Andhra Pradesh, granted the necessary ethical permissions (No: CPCSEA/1677/SPMVV/IAEC/1-04).
Induction of urolithiasis
After 7 days of acclimatization, twenty-four rats were divided into four groups (n=6) and treated from days 7 to 21as follows. Group 1 (Vehicle Control): Received a normal diet, Group 2 (Disease Control): 0.5% Carboxy methyl cellulose (CMC), Group 3 (F-15): fisetin 15 mg/kg suspended in 0.5% CMC, Group 4 (F-30): fisetin 30 mg/kg suspended in 0.5% CMC. Except for the rats in the control group (Group 1), all animals received 0.75% ethylene glycol (W/V) I + 1% ammonium chloride (NH4Cl; V/V) in drinking water for 7 days.
Urine analysis
On days 7 and 21, 24-hour urine samples were collected from rats housed in individual metabolic cages after hydrating with 5 ml of drinking water. Urine volume was measured using a graduated measuring cylinder, and pH was evaluated using narrow range pH test strips. Further, urine samples were centrifuged at 2,500 rpm for 5 min analysed for the quantitative measurement of calcium (Cat# XSYS0007, Erba Mannheim, Germany), oxalate (Hodgkinson and Williams 1973), inorganic phosphate (Brenza and DeLuca, 2000), creatinine (Toora and Rajagopal, 2002), glycosaminoglycans (GAGs) (Chandrasekhar et al., 1987) and N-acetyl-glucosaminidase (NAG) (Wellwood et al., 1976). Creatinine clearance was calculated using the formula mentioned in the literature (Cockcroft and Gault, 1976).
Blood collection and necropsy
On day 22, blood samples were collected via retro-orbital plexus, and serum was separated by centrifugation at 1500 rpm for 15 min. All rats were euthanized under carbon dioxide (CO2) asphyxiation, and the kidneys were isolated. One kidney from each rat was snap frozen and stored at 80°C for biochemical analysis and the other was fixed in 10% neutral buffered formalin (NBF) for histopathological analysis.
Serum biochemistry
Serum samples were analysed for creatinine, blood urea nitrogen (BUN), uric acid and calcium using commercially available reagents in an automated biochemistry analyser (EM-200, Transasia Bio-Medicals Ltd., Vizag, India).
Analysis of kidney homogenates
Snap frozen kidneys were crushed, and the post-mitochondrial supernatants were prepared as mentioned in Shah et al., (2012) and were analysed for total protein content, superoxide dismutase (SOD) (Misra and Fridovich, 1972), catalase (CAT) (Aebi, 1972), and malondialdehyde (MDA) (Wills, 1966), and reduced glutathione (GSH) (Eyer and Podhradský, 1986). Total protein (Lowry et al., 1951), calcium (Michaylova and Kouleva, 1973), oxalate (Hodgkinson and Williams, 1973), and protein carbonyl content (PCC) (Levine et al., 1990). Further, kidney homogenates were analysed for tumour necrosis factor (TNF-α, Cat# KB3145, Krishen Biosystems, USA) and Interleukin-6 (IL-6, Cat# KB3068, Krishen Biosystems, USA) using commercially available ELISA kits.
Histopathology
Histopathological evaluations are carried out as mentioned in the literature (Sujatha et al., 2010). Formalin fixed tissues were washed in tap water overnight and dehydrated in a series of alcohols. Dehydrated tissues were cleared in xylene, embedded in paraffin blocks, trimmed, and sectioned (4-5 µm) using a microtome. Sections were deparaffinized, rehydrated in series of alcohols stained with haematoxylin and eosin (H&E) and mounted using DPX. The H&E-stained sections were carefully examined under the pathology microscope (CX41, Olympus, Evident Scientific Private Limited, India) and scored for crystal deposition and inflammation.
Statistical analysis
Six animals per group (n=6) provided us with the ‘E’ value of ‘20’. An ‘E’ value between 10 to 20 satisfies the sample size for animal studies (Charan and Khataria, 2023). Data were expressed as the mean ± SD of three and six observations for in vitro and in vivo studies, respectively. The data was analysed using one way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test in Graph pad prism version 5.0 (GraphPad Software, Boston). The statistical significance was set at P <0.05.
RESULTS
The present study shows the anti-urolithiasis activity of fisetin, in vitro and in vivo.
Fisetin reduces nucleation, aggregation and crystallization in vitro
Fisetin was tested at concentrations of 62.5, 125, 250, 500, and 1,000 ug/mL for its inhibitory potential on nucleation, aggregation, and crystallization, and the percent (%) inhibitions were dose-dependent. In the nucleation assay, the nucleation inhibition percent (%) was 3.2, 12.15, 26.47, 53.94, and 78.39 at concentrations of 62.5, 125, 250, 500, and 1,000 ug/mL, respectively. The mean % inhibitions in aggregation were 52.17, 140.87, 233.48, 499.57, and 839.57, respectively, at doses of 62.5, 125, 250, 500, and 1,000 ug/mL (Table 1). In the crystallization assay, analysis of scanning electron microscopy (SEM) images revealed that fisetin (1,000 ug/ml) reduced the size of crystals compared to the control group. The size of the crystals is smaller in the fisetin group, even if crystal formation is not low in this group (Figure 1).
Table 1. Percent (%) inhibition of crystallization in vitro.
|
Groups |
% Inhibition (mean ± SD of three experiments) |
||
|
Nucleation |
Aggregation |
Crystallization |
|
|
Fisetin-62.5 ug/mL |
3.21 ± 0.26 |
3.58 ± 0.40 |
52.17 ± 25.82 |
|
Fisetin 125 ug/mL |
12.15 ± 2.50 |
13.35 ± 0.40 |
140.87 ± 18.45 |
|
Fisetin 250 ug/mL |
26.47 ± 5.88 |
23.86 ± 1.10 |
233.48 ± 31.05 |
|
Fisetin 500 ug/mL |
53.94 ± 6.72 |
57.05 ± 1.16 |
499.57 ± 56.26 |
|
Fisetin-1000 ug/mL |
78.39 ± 3.84 |
81.36 ± 0.20 |
839.57 ± 20.64 |
|
Cystone – 1000 ug/ml |
54.15 ± 2.12 |
55.15 ± 1.77 |
718.76 ± 0.35 |
Note: Values represent the % inhibition as mean ± SD of three experiments vs. control.
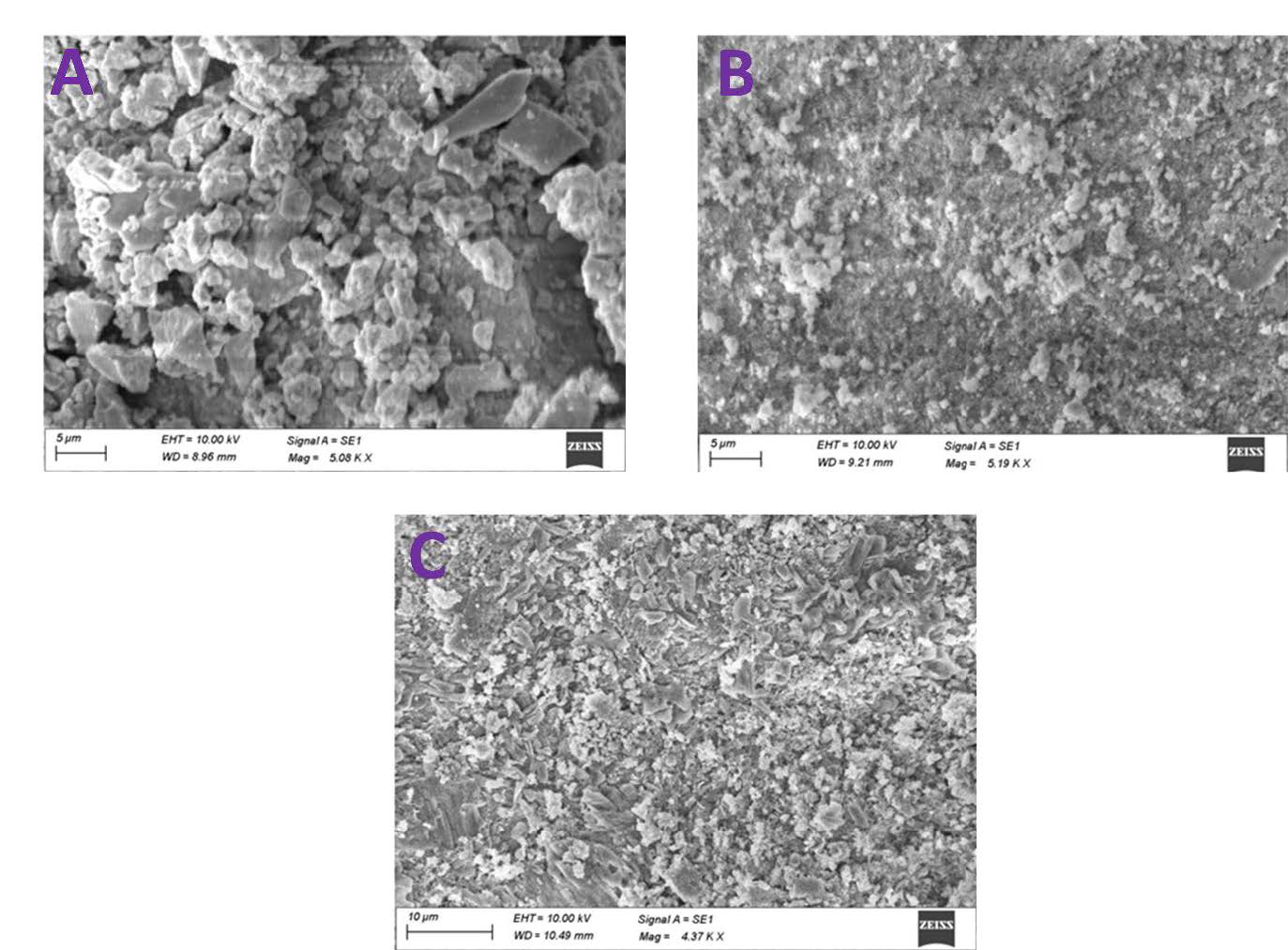
Figure 1. Scanning electron micrographs of CaOx crystals induced in artificial urine. A) Control, B) Fisetin (1,000 ug/ml) and C) Cystone (1,000 ug/ml).
Efficacy of fisetin on polyethylene glycol (EG)-induced urolithiasis in rats
The anti-urolithic activity of fisetin against EG-induced urolithiasis was investigated in a rat model. Following fisetin treatment, no significant changes were observed in the body weights of the rats across the groups. In kidney weight measurement, fisetin considerably reduced the weights of the kidneys of rats as compared to the EG control group in a dose-dependent manner. This highlights the anti-urolithiasis potential of fisetin in vivo (Table 2). In the control group, there was a significant increase in the pH of the urine in the EG control group in comparison with the vehicle group. Fisetin treatment (P <0.05) reduced the pH of the urine at both doses, i.e., 15 mg/kg and 30 mg/kg, when compared to EG control. Fisetin treatment significantly restored the reduced urine volume and corrected the elevated pH induced by EG.
Table 2. Effect of fisetin on weights of body and kidney’s in rats.
|
Groups |
Bodyweight (g) |
Kidney (g) |
|
|
Initial |
Final |
||
|
Vehicle |
195 ± 15.23 |
217 ± 24.72 |
0.54 ± 0.02 |
|
EG control |
210 ± 24.64 |
198 ± 13.14 |
0.89 ± 0.04 |
|
Fisetin- 15 mg/kg |
215 ± 24.93 |
205 ± 13.56 |
0.68 ± 0.03 |
|
Fisetin- 30 mg/kg |
226 ± 22.86 |
215 ± 24.14 |
0.47 ± 0.01 |
Note: Values represent mean ± SD. Analysed using one way ANOVA followed by bonferroni’s post hoc test;
* P <0.05 vs EG control, # indicated P <0.05 vs Vehicle
A key factor in kidney stone development is urinary pH. In addition, fisetin also normalized the weights of the kidneys and significantly enhanced the creatinine clearance when compared to EG control. In the EG control group, there was a significant (P <0.05) reduction in creatine clearance as compared to vehicle control. After fisetin supplementation, creatine clearance was significantly (P <0.05) increased in both the 15 mg/kg and 30 mg/kg groups when compared to the EG control group. Urea, a consistent compound of kidney stones and essential for maintaining urinary pH, was significantly elevated in the EG control group compared to the vehicle group. The elevated levels of urinary urea were significantly reduced in animals supplemented with fisetin in the 15 mg/kg and 30 mg/kg groups when compared with the EG control. Significant reductions in urinary urea and pH by fisetin (15 mg/kg or 30 mg/kg) contributed to considerably low levels of uric acid in fisetin-treated rats in a dose-dependent manner. Uric acid, one of the substances that can contribute to the formation of kidney stones, was also significantly increased in the EG control group when compared to the vehicle group. In the EG control group, there was a significant (P <0.05) increase in urinary calcium in rats compared to the vehicle group. Fisetin treatment significantly reduced the urinary calcium in rats at both doses, i.e., 15 mg/kg and 30 mg/kg, when compared to the EG group. Creatinine is a functional marker of the kidney. Urinary creatinine analysis revealed that creatinine levels were significantly (P <0.05) elevated in the EG control group in comparison with the vehicle group. In fisetin-treated groups, significantly (P <0.05) lower creatinine levels were observed in both the 15 mg/kg and 30 mg/kg groups. Fisetin at both doses, significantly inhibited the activity of urinary N-acetyl-beta-D-glucosaminidase (NAG) and enhanced the levels of glycosaminoglycans (GAGs) in both the 15 mg/kg and 30 mg/kg groups (Table 3). Serum biochemistry revealed that fisetin reduced the elevated levels of serum creatinine, calcium, and uric acid in both the 15 mg/kg and 30 mg/kg groups (Table 4). Fisetin treatment significantly reduced the average levels of total protein in serum and kidney homogenates. In addition, fisetin significantly reduced the levels of calcium, oxalates, and total protein in the kidney.
Fisetin at both the doses, significantly increased the activities of superoxide dismutase (SOD) and catalase (CAT) and reduced the levels of malondialdehyde (MDA) and protein carbonyl content (PCC) in both fisetin groups. Further, levels of reduced glutathione (GSH) were significantly higher in both fisetin (15 mg/kg and 30 mg/kg groups) (Table 5).
Fisetin also reduced the levels of tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) in the kidney homogenates (Table 6). Histological sections of the kidney showed the reduction in crystal deposition and almost no congestion or hemorrhage (Figure 2).
Table 3. Effect of fisetin on urine biochemical evaluations in rats.
|
Groups |
Urine (mL/24h) |
pH |
Creatinine Clearance (mL/min/hr) |
Urea (mg/dL) |
Uric acid (mg/dL) |
Calcium (mg/dL) |
Creatinine (mg/dL) |
NAG (µmol/L) |
GAG (mg/24h) |
|
Vehicle |
21.00 ± 1.10 |
6.10 ± 0.12 |
0.0820 ± 0.0074 |
48.57 ± 4.56 |
2.96 ± 0.69 |
4.27 ± 1.34 |
0.43 ± 0.19 |
23.57 ± 3.56 |
5.99 ± 1.24 |
|
EG control |
13.00 ± 1.65# |
9.33 ± 0.18# |
0.0180 ± 0.0066# |
87.24 ± 5.36# |
6.99 ± 1.34# |
7.95 ± 1.56# |
3.14 ± 0.26# |
57.23 ± 6.36# |
2.96 ± 0.59# |
|
Fisetin- 15 mg/kg |
36.00 ± 2.50* |
7.04 ± 0.13* |
0.0320 ± 0.0064* |
55.14 ± 3.87* |
3.45 ± 0.86* |
4.55 ± 1.23* |
1.56 ± 0.63* |
35.14 ± 4.17* |
3.65 ± 0.67* |
|
Fisetin- 30 mg/kg |
41.00 ± 3.96* |
6.69 ± 0.13* |
0.0430 ± 0.0071* |
47.24 ± 3.96* |
2.89 ± 0.56* |
3.56 ± 0.78* |
1.21 ± 0.43* |
27.64 ± 3.69* |
4.89 ± 0.65* |
Note: Values represent mean±SD. Analysed using one way ANOVA followed by bonferroni’s post hoc test; * P<0.05 vs EG control, # indicated P<0.05 vs Vehicle
Table 4. Effect of fisetin on serum creatinine, uric acid and blood urea nitrogen in rats.
|
Groups |
Creatinine(mg/dL) |
Uric acid (mg/dL) |
BUN (mg/dL) |
|
Vehicle |
1.81 ± 0.66 |
2.56 ± 0.89 |
17.21 ± 3.34 |
|
EG control |
4.04 ± 0.55# |
6.84 ± 1.33# |
38.95 ± 4.66# |
|
Fisetin- 15 mg/kg |
1.94 ± 0.67* |
3.76 ± 0.97* |
29.65 ± 3.33* |
|
Fisetin- 30 mg/kg |
1.24 ± 0.44* |
2.49 ± 0.77* |
19.46 ± 3.76* |
Note: Values represent mean ± SD. Analysed using one way ANOVA followed by bonferroni’s post hoc test; * P <0.05 vs EG control, # indicated P <0.05 vs Vehicle.
Table 5. Effect of Fisetin on tissue biochemical evaluations in rats.
|
Groups |
Total protein (units/g) |
Calcium (mg/g) |
Oxalate (μg/g) |
PCC nM/mg) |
SOD (Units/g) |
CAT (μM/min/g) |
GSH (μg/ g) |
LPO (nm MDA/ g) |
|
Vehicle |
1112.00 ± 44.90 |
0.26 ± 0.06 |
2.23 ± 0.74 |
4.99 ± 1.97 |
1112.00 ± 44.96 |
292.51 ± 31.63 |
52.14 ± 2.97 |
62.99 ± 1.97 |
|
EG control |
372.00 ± 73.33# |
0.76 ± 0.08 # |
11.34 ± 1.52# |
11.24 ± 2.14# |
372.00 ± 73.33# |
188.21 ± 25.40# |
23.93 ± 6.34# |
118.24 ± 5.12# |
|
Fisetin- 15 mg/kg |
687.10 ± 82.55* |
0.33 ± 0.07 * |
4.12 ± 0.72* |
5.13 ± 1.33* |
687.10 ± 82.55* |
213.26 ± 22.45* |
31.77 ± 4.77* |
77.13 ± 2.43* |
|
Fisetin- 30 mg/kg |
949.80 ± 62.84* |
0.21 ± 0.05* |
2.89 ± 0.90* |
4.21 ± 1.01* |
949.80 ± 32.84* |
229.56 ± 33.65* |
41.34 ± 7.64* |
64.21 ± 3.01* |
Note: Values represent mean ± SD. Analysed using one way ANOVA followed by bonferroni’s post hoc test; * P <0.05 vs EG control, # indicated P <0.05 vs Vehicle
Table 6. Effect of fisetin on inflammatory markers in kidney homogenates.
|
Groups |
TNF-α (pg/mg) |
IL-6(pg/mg) |
|
Vehicle |
989.45 ± 67.70 |
1192.61 ± 85.63 |
|
EG control |
1798.20 ± 103.33# |
1988.61 ± 122.84 # |
|
Fisetin- 15 mg/kg |
1387.18 ± 92.55* |
1313.26 ± 132.55 * |
|
Fisetin- 30 mg/kg |
1149.82 ± 112.54* |
1234.56 ± 123.85 * |
Note: Values represent mean ± SD. Analysed using one way ANOVA followed by bonferroni’s post hoc test;
* P <0.05 vs EG control, # indicated P <0.05 vs Vehicle
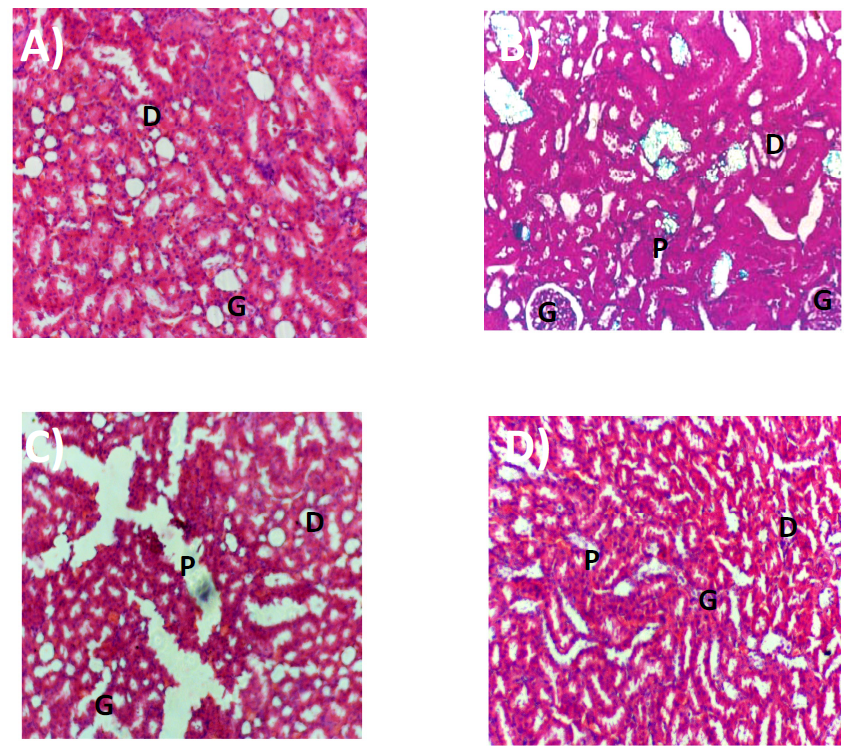
Figure 2. Photomicrographs of haematoxylin and eosin (H&E) sections rat kidneys in A-Vehicle, B-EG control, C-Fisetin-15 mg/kg, D-Fisetin-30 mg/kg. G-Glomerular capillary, P-Proximal tubule, D-Distal tubule.
DISCUSSION
We have presented the anti-urolithiasis activity of a potential flavonoid, fisetin in vitro and in vivo. Calculi, also known as urinary stones, are the third most common disease of the urinary system and afflict 12% of people globally, necessitating serious medical care for the duration of the patient's life (Leye et al., 2007). According to reports, calcium oxalate and calcium phosphate make up around 80% of urinary calculi, which can lead to urinary blockage, hydronephrosis, infection, and bleeding in the urinary tract system (Prein and Prien, 1968). Fisetin, tested at concentrations of 62.5, 125, 250, 500, and 1,000 ug/mL, showed its inhibitory potential on nucleation, aggregation, and crystallization in a dose dependent manner. Also, fisetin reduced the size of crystals compared to the control group.
In our in vivo study, fisetin treatment appears to be safe, as none of the study groups showed significant differences in body weights. Considerable reductions observed in the weights of the kidneys can be considered a reduction in the kidney stone size. Fisetin treatment significantly restored the reduced urine volume and corrected the elevated pH induced by EG. A key factor in kidney stone development is urinary pH. As the urine pH climbs, the supersaturation of calcium phosphate increases quickly. The combination of hypercalciuria and hypocitraturia in alkaline urine is a urinary factor that facilitates the production of these stones (Carvalho, 2018). In addition, fisetin significantly enhanced creatinine clearance, an important clinical marker to assess kidney function and stone formation (Worcester et al., 2006). The positive effect of fisetin on urinary pH is evident with a reduced level of urinary urea, which might be due to the reduced activity of urease to split ammonia into urea and carbon dioxide (CO2) (Alelign and Petros, 2018). Uric acid, one of the substances that can contribute to the formation of kidney stones, was also significantly reduced with fisetin treatment. Calcium dysregulation is one of the hallmark features of urolithiasis. Fisetin treatment significantly reduced the elevated levels of urinary calcium in rats at both doses, i.e., 15 mg/kg and 30 mg/kg. Further, fisetin significantly reduced the urinary excretion of calcium, which proved to be directly proportional to the quantity of calculus formation (Light et al., 1973). Creatinine is a functional marker of the kidney; the levels of urinary creatinine were significantly lower in both the 15 mg/kg and 30 mg/kg groups. Which indicates the reduction in the progression of kidney stones by fisetin (Shen at al., 2022). Fisetin also corrected renal tubular dysfunction, as evident by the inhibition of urinary NAG activity in the lysosomes of renal tubular cells (Bosomworth et al., 1999). Further, reduced stone formation is evident with enhanced levels of GAGs in the fisetin group. Reduced GAGs are a potential indicator of kidney stone formation (Dissayabutra et al., 2019). Serum biochemical analysis showed a fisetin-mediated reduction in the elevated levels of creatinine, calcium, and uric acid in both groups. Fisetin treatment significantly reduced EG-induced oxidative damage in kidneys by increasing the average levels of total protein in serum and kidney homogenates. Reduced deposition of calcium oxalate crystals is evident, along with a significant reduction in calcium, oxalates, and total protein in the kidney.
Fisetin reduced the EG-induced oxidative damage by increasing the activities of SOD and CAT and reducing the MDA levels in both fisetin (15 mg/kg and 30 mg/kg) groups. The body produces endogenous SOD, which can scavenge free radicals and excessive ROS. SOD levels can indicate how well the body can withstand oxidative stress. The breakdown of hydrogen peroxide (H2O2) into less harmful gaseous oxygen and water molecules is commonly accelerated by catalase in cells (Lobo et al., 2010). Lipid peroxidation, a degenerative biochemical process involving the generation of MDA, is significantly reduced with fisetin treatment (Lovel et al., 1995). The coexistence of mechanisms for the synthesis, breakdown, efflux, and absorption of reduced glutathione (GSH) is what makes the kidney, and especially the proximal tubules, special in terms of GSH homeostasis (Lash, 2005). Fisetin significantly improved kidney function by enhancing the tissue level of GSH. Fisetin-mediated reduction in ROS-induced oxidative damage is evident with the reduction in protein carbonyl content (PCC) in kidneys (Nocera et al., 2020). By halting the advancement of pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), in the kidney, fisetin also lessened inflammatory damage to the renal tubular cells (Huang et al., 2020). Kidney histological sections showed nearly no congestion or hemorrhage and a fisetin-mediated decrease in crystal deposition.
CONCLUSION
Fisetin, a biological flavonoid with an established safety profile, was tested for its anti-urolithiasis effect against EG-induced urolithiasis. Based on the above observations, we can conclude that fisetin might be a good candidate to treat kidney damage by reducing the progression of oxidative stress and inflammation. Further studies are warranted to establish the molecular mechanisms behind the anti-urolithic efficacy of fisetin.
ACKNOWLEDGEMENTS
Authors also thank Professor. Y. Indira Muzib, Pharmaceutics, Institute Pharmaceutical Technology (IPT), Sri Padmavathi Mahila Viswavidylayam, Tirupati, India.
AUTHOR CONTRIBUTIONS
These authors equally contributed to this work
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Aebi, H., 1984. Catalase in vitro. Methods in Enzymology. 105: 121-126.
Alelign, T., and Petros, B., 2018. Kidney stone disease: An update on current concepts. Advances in Urology. 2018: 3068365.
Antika, L.D., and Rita, M.D., 2021. Pharmacological aspects of fisetin. Asian Pacific Journal of Tropical Biomedicine. 11(1):1-9.
Atmani, F., and Khan, SR., 2000. Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. BJU International. 85(6):621-625.
Bosomworth, M.P., Aparicio, S.R., and Hay, A.W., 1999. Urine N-acetyl-beta-D-glucosaminidase--a marker of tubular damage? Nephrology Dialysis Transplantation. 14(3): 620-626.
Brenza, H.L., and DeLuca, H.F., 2000. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1, 25-dihydroxyvitamin D3. Archives of Biochemistry and Biophysics. 381(1): 143-152.
Butterweck, V., and Saeed, RK., 2009. Herbal medicines in the management of urolithiasis: alternative or complementary?. Planta Medica. 74(10): 1095-1103.
Cao, Y. L., and Lin J.H., Hammes, H.P., and Zhang, C., 2022. Flavonoids in treatment of chronic kidney disease. Molecules. 27(7): 2365.
Carvalho, M., 2018. Urinary pH in calcium oxalate stone formers: Does it matter? Brazilian Journal of Nephrology. 40(1): 06-07.
Chandrasekhar, S., Esterman, M.A., and Hoffman, H.A., 1987. Micro determination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Analytical Biochemistry. 161(1): 103-108.
Charan, J., and Kantharia, N. D. 2013. How to calculate sample size in animal studies?. Journal of Pharmacology & Pharmacotherapeutics. 4(4): 303–306.
Cockcroft, D.W., and Gault, M.H., 1976. Prediction of creatinine clearance from serum creatinine. Nephron. 16(1): 31-41.
Dissayabutra, T., Kalpongnukul, N., Chindaphan, K., Srisa-Art. M., Ungjaroenwathana, W., Kaewwongse, M., Iampenkhae, K., and Tosukhowong, P., 2019. Urinary sulfated glycosaminoglycan insufficiency and chondroitin sulfate supplement in urolithiasis. PLoS One. 14(3): e0213180.
Erdemli, M. E., Zayman, E., Erdemli, Z., Gul, M., Gul, S., and Gozukara Bag, H., 2020. Protective effects of melatonin and vitamin E in acetamiprid-induced nephrotoxicity. Environmental Science and Pollution Research International. 27(9): 9202-9213.
Eyer, P., and Podhradský, D., 1986. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman's reagent. Analytical Biochemistry. 153(1): 57-66.
Grynkiewicz, G., and Demchuk, O.M., 2019. New perspectives for fisetin. Frontiers in Chemistry. 7: 697.
Hennequin, C., Lalanne, V., Daudon, M., Lacour, B., and Drueke, T., 1993. A new approach to studying inhibitors of calcium oxalate crystal growth. Urology Research. 21(2): 101-108.
Hodgkinson, A., and Williams, A., 1973. An improved colorimetric procedure for urine oxalate. Clinca Chima Acta. 36(1): 127-132.
Huang, H., Wang, J., Li. X., Wu. W., Shi. K., and Zhang. C., 2020. Renoprotective effect of sulphate polysaccharide from brown algae on ethylene glycol-induced renal damage in rats. Medicine Research. 4(1-2): 190010.
Kumar, R., Kumar. R., Sharma. N., Vyas. M., Mahajan. S., Satija, S., Singh, S.K., Khursheed, R., Mehta, M., Khurana, S., and Khurana, N., 2019. Fisetin: A phytochemical with various pharmacological activities. Plant Archives. 19(2): 1012-1026.
Lash, L.H., 2005. Role of glutathione transport processes in kidney function. Toxicology and Applied Pharmacology. 204(3): 329-342.
Levine, R.L., Garland, D., Oliver, C.N., Amici, A., Climent, I., Lenz, A.G., Ahn, B.W., Shaltiel, S., and Stadtman, E,R., 1990. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 186: 464-78.
Leye, A., Philippe. J., William. R., and Robert, U., 2007. Renal stone disease. Medicine. 35(8): 415-419.
Light, I., Gursel, E., and Zinnser, H.H., 1973. Urinary ionized calcium in urolithiasis. Effect of cranberry juice. Urology. 1973; 1(1): 67-70.
Lobo, V., Patil. A., Phatak. A., Chandra. N., 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4(8): 118-126.
Lovell, M.A., Ehmann, W.D., Butler, S.M., and Markesbery, W.R, 1995. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology. 45(8): 1594-1601.
Lowry, O.H., Rosebrough N.J., Farr, A.L., and Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 193(1): 265-75.
Michaylova, V., and Kouleva, N., 1973. Arsenazo III as metallochromic indicator for complexometric determination of calcium in slightly alkaline medium. Talanta. 20(5): 453-458.
Misra, H.P., and Fridovich, I., 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 247(10): 3170-3175.
Nakagawa, Y., Abram, V., Parks, J.H., Lau, H.S., Kawooya, J.K., and Coe, F.L., 1985. Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. Journal of Clinical Investigation. 76(4): 1455-1462.
Nocera, I., Bonelli. F., Meucci. V., Rinnovati. R., Spadari. A., Intorre. L., Pretti. C., and Sgorbini. M., 2020. Evaluation of protein carbonyl content in healthy and sick hospitalized horses. Frontiers in Veterinary Sciences. 7: 582886.
Pawar, A.T., and Niraj, S.V., 2015. Anti-urolithiatic activity of standardized extract of Biophytum sensitivum against zinc disc implantation induced urolithiasis in rats. Journal of Advanced Pharmaceutical Technology & Research. 6(4): 176-182.
Prien, E.L., and Prien, E.L.Jr., 1968. Composition and structure of urinary stone. Amercian Journal of Medicine. 45(5): 654-672.
Sahu, B.D., Kalvala, A.K., Koneru, M., Mahesh, K.J., Kuncha, M., Rachamalla, S.S., and Sistla, R., 2014. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS One. 9(9): e105070.
Saljoughian, M., 2020. The management of urolithiasis, US Pharmacist. 45(9): 34-36.
Sasidharan, S., Chen, Y., Saravanan, D., Sundram, KM., and Yoga Latha, L., 2011. Extraction, isolation and characterization of bioactive compounds from plants' extracts. African Journal of Traditional, Complementary and Alternative Medicine. 8(1): 1-10.
Shen. X., Chen. Y., Zhang. Y., Xia, K., Chen. Y., and Hao. Z., 2022. The association of urine creatinine with kidney stone prevalence in US adults: Data from NHANES 2009-2018. Frontiers in Medicine (Lausanne). 29: 819738.
Sofia, N.H., Walter, T.M., and Sanatorium, T. 2016. Prevalence and risk factors of kidney stone. Global Journal for Research Analysis. 5(3): 183-187.
Sujatha, D., Ranganayakulu, D., Bharathi, K., and Prasad, K.V., 2010. Effect of ethanolic extract of Phyla nodiflora (Linn.) Greene against calculi producing diet induced urolithiasis. Indian Journal of Natural Products and Resources. 1(3): 314-321.
Toora, B.D., and Rajagopal, G. 2002. Measurement of creatinine by Jaffe's reaction--determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian Journal of Experimental Biology. 40(3): 352-354.
Wellwood, J.M., Price, R.G., Ellis, B.G., and Thompson, A.E., 1976. A note on the practical aspects of the assay of N-acetyl-beta-glucosaminidase in human urine. Clinica Chimica Acta. 69(1): 85-91.
Wiederkehr, M.R., and Moe, O.W., 2011. Uric acid nephrolithiasis: A systemic metabolic disorder. Clinical Reviews in Bone and Mineral Metabolism. 9(3-4): 207-217.
Wills, E.D., 1966. Mechanisms of lipid peroxide formation in animal tissues. Biochemical Journal. 99(3): 667-676.
Worcester, E. M., Parks, J. H., Evan, A. P., and Coe, F. L. 2006. Renal function in patients with nephrolithiasis. Journal of Urology. 176(2): 600–603.
Worcester, E.M., Parks, J.H., Evan, A.P., and Coe, F.L., 2006. Renal function in patients with nephrolithiasis. Journal of Urology. 176(2): 600-603.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Patnam Nageswari and Swathi Konda*
Institute of Pharmaceutical Technology, Sri Padmavati Mahila Viswavidyalayam, Tirupati, Sri Padmavathi Mahila Viswavidyalayam.
Corresponding author: Swathi Konda, E-mail: kswathi84@yahoo.co.in
Total Article Views
Editor:Nisit Kittipongpattana,
Chiang Mai University, Thailand
Article history:
Received: January 12, 2024;
Revised: May 10, 2024;
Accepted: May 20, 2024;
Online First: June 21, 2024

