
Applying Box-Behnken Design in Herbal Product Development: Semha-Pinas Plain Tablets
Jirapornchai Suksaeree and Chaowalit Monton*Published Date : June 17, 2024
DOI : https://doi.org/10.12982/NLSC.2024.041
Journal Issues : Number 3, July-September 2024
Abstract Phlegm production is a common symptom of upper respiratory tract infections and some allergies. A traditional Thai herbal formula called Semha-Pinas offers an alternative treatment option for managing this symptom. This study aimed to optimize the formulation of Semha-Pinas plain tablets using the Box-Behnken design. Three factors were identified as potentially influencing tablet properties: compressional force, quantity of spray-dried lactose, and quantity of croscarmellose sodium. Initial screening with a one-factor-at-a-time approach helped narrow down the relevant ranges for each factor. Subsequently, the Box-Behnken design was employed with compressional forces ranging from 1,500 to 2,500 psi, spray-dried lactose quantities from 0 to 10%, and croscarmellose sodium quantities from 2 to 4%. Results showed that the optimal formulation, achieving a hardness of 5 to 8 kP, disintegration time of at least 0.5 minutes, and friability of not more than 0.4%, was found at 1,500 psi compressional force, 6% spray-dried lactose, and 3% croscarmellose sodium. This optimized formulation exhibited the following characteristics: tablet weight of 381.23 ± 0.80 mg, diameter of 9.66 ± 0.01 mm, thickness of 4.20 ± 0.04 mm, hardness of 7.50 ± 0.41 kP, disintegration time of 0.67 ± 0.07 minute, and friability of 0.22% ± 0.04%. Verification data confirmed the accuracy of the predictions, with all percent errors falling below 10%. In conclusion, this study successfully applied the Box-Behnken design to develop Semha-Pinas plain tablets that meet the relevant pharmacopoeial criteria.
Keywords: Design of experiments, Design space, Direct compression, Optimization
Citation: Suksaeree, J. and Monton, C. 2024. Applying Box-Behnken design in herbal product development: Semha-Pinas plain tablets. Natural and Life Sciences Communications. 23(3): e2024041.
INTRODUCTION
A prominent symptom of both respiratory tract infections and irritation caused by allergens or pollutants, mucus-filled coughs can be treated through expectorants or mucolytics like Semha-Pinas, a traditional Thai medicine remedy. This unique herbal formulation combines six ingredients in equal proportions: Brassica spp. seeds, Blumea balsamifera (L.) DC. leaves, Terminalia chebula Retz. fruit pulps, Piper nigrum L. fruits, Citrus hystrix DC. fruit peels, and Coriandrum sativum L. fruits (Khun Sopit Banna Lak, 1961). Previously, Semha-Pinas was developed into effervescent tablets (Suksaeree et al., 2023) and orodispersible tablets (Suksaeree et al., 2024) that contained specific additives for each dosage form. A plain tablet is the simplest tablet dosage form. It does not require special additives and is easy to operate (Mura et al., 2019; Vijayakumar et al., 2023).
Conventional approaches to experimentation, like trial-and-error and one-factor-at-a-time methods, can be cumbersome and inefficient, especially when dealing with complex systems involving multiple factors. The one-factor-at-a-time method, while offering controlled manipulation of individual factors, often fails to reveal the optimal interactions between them, leading to suboptimal outcomes. However, the one-factor-at-a-time method has been routinely used nowadays (Nor et al., 2017; Abou-Taleb and Galal, 2018; Abdel-Rahman et al., 2020; Bhaturiwala et al., 2022; Xiao et al., 2023; Hegazy et al., 2024). This is where the Design of Experiments (DOE) emerges as a powerful solution (JMP Statistical Discovery LLC, 2022). Utilizing statistical design and analysis, DOE provides a structured and data-driven method for identifying the best combination of factors that affect the desired outcome. Compared to traditional methods, DOE significantly reduces the time, cost, and resources needed to achieve better results. Its effectiveness lies in its ability to not only show how each factor affects the outcome individually but also to uncover their interactions, leading to a comprehensive understanding of the response surface (Gibson, 2016; Steele, 2018). Moreover, DOE empowers the construction of robust statistical models, enabling confident prediction of the simultaneous impact of multiple factors and facilitating targeted optimization (JMP Statistical Discovery LLC, 2022). This approach has been popular in the field of pharmaceutical sciences, as evidenced by several studies (Jitrangsri et al., 2020; Duangjit et al., 2022; Jitrangsri et al., 2022; Suriyaamporn et al., 2023; Jaikham et al., 2024; Saepang et al., 2024).
The Box-Behnken design offers exceptional efficiency in exploring quadratic response surfaces with fewer experimental runs compared to other designs like central composite design and full factorial design. This effectiveness is particularly beneficial for dealing with complex formulations where minimizing the number of experimental trials is crucial due to resource constraints, such as time and material availability (National Institute of Standards and Technology, 2012). Therefore, this study employed the DOE approach using a Box-Behnken design to optimize the formulation of Semha-Pinas plain tablets. The authors expected that these findings would facilitate the development of effective Semha-Pinas plain tablets as a potential alternative treatment option for managing phlegm.
MATERIALS AND METHODS
Materials
Semha-Pinas extract was obtained from the previous works (Suksaeree et al., 2023; Suksaeree et al., 2024). Fumed silica was purchased from P.C. Drug Center, Bangkok, Thailand. Magnesium stearate was obtained from Changzhou Kaide Imp. & Exp. Co., Ltd., Changzhou, China. Microcrystalline cellulose (MCC) (Comprecel® M102) and spray-dried lactose (SDL) were purchased from Maxway Co., Ltd., Bangkok, Thailand. Croscarmellose sodium (CCS) was obtained from Onimax Co., Ltd., Bangkok, Thailand. Talcum was purchased from Nitika Pharmaceutical Specialities Pvt. Ltd., Nagpur, India.
Preparation of Semha-Pinas plain tablet
Each tablet contained 380 mg, with 10.53% Semha-Pinas extract (equivalent to 40 mg extract) as an active ingredient, 5.26% fumed silica as glidant and adsorbent, CCS (varied) as a disintegrant, SDL (varied) as a diluent, 1.32% magnesium stearate as a lubricant, 6.58% talcum as glidant and lubricant, and MCC, as diluent and binder, was used to adjust tablet weight to 100%. All ingredients were passed through a 40-mesh sieve, except talcum and magnesium stearate were passed through a 60-mesh sieve.
Semha-Pinas extract and fumed silica were first blended using a mortar and pestle. Separately, CCS, SDL, MCC, and magnesium stearate were premixed using the geometric dilution method. Both mixtures were then combined and blended for 5 min. Individual 380 mg portions of the powder mixture were weighed and compressed using a hydraulic press equipped with a pressure gauge. The resulting tablets were evaluated for weight (n=20), diameter (n=20), thickness (n=20), hardness (n=10), DT (n=6), and friability (n=20 total across two tests). Test methods were followed as described in a previous study (Suksaeree et al., 2024).
Screening factor levels by one-factor-at-a-time and experimental design by Box-Behnken design
Initial factor-level screening employed a one-factor-at-a-time approach. Compressional forces were tested at 1,000, 1,500, 2,000, and 2,500 psi; SDL quantities at 0, 5, 10, and 25%; and CCS quantities at 0, 1, 3, and 5%. Three levels of each factor were subsequently included in a Box-Behnken design (Table 1). Data analysis was conducted using Design-Expert® v.11 (Stat-Ease, Inc., MN, USA), generating response surfaces and analysis of variance (ANOVA) reports. Design spaces fulfilling the targeted tablet properties—hardness between 5-8 kP, DT not less than 0.5 min, and friability not exceeding 0.4%—were constructed. The optimal condition within the design space was selected, and model verification was performed by replicating the Semha-Pinas plain tablet preparation under the optimized condition. Percent error calculations were employed to evaluate the accuracy of the model.
Table 1. Factors and levels utilized in the Box-Behnken design for optimizing Semha-Pinas plain tablet properties.
|
Factors |
Levels |
||
|
-1 |
0 |
+1 |
|
|
Compressional force (psi) |
1,000 |
1,500 |
2,000 |
|
Quantity of SDL (%) |
0 |
5 |
10 |
|
Quantity of CCS (%) |
2 |
3 |
4 |
RESULTS
To screen for levels of factors influencing tablet properties, a one-factor-at-a-time method was employed. The effects of compressional force, SDL quantity, and CCS quantity on tablet properties are depicted in Figure 1. According to compressional force, a significant reduction in tablet thickness and friability was observed with increasing compressional force, alongside a significant increase in tablet hardness and prolonged DT. Increasing SDL quantity appeared to not affect tablet thickness or friability, but it slightly decreased tablet hardness and shortened DT, both significantly. Increasing CCS quantity led to a significant shortening of DT and a slight decrease in tablet thickness, with no apparent effect on hardness or friability.
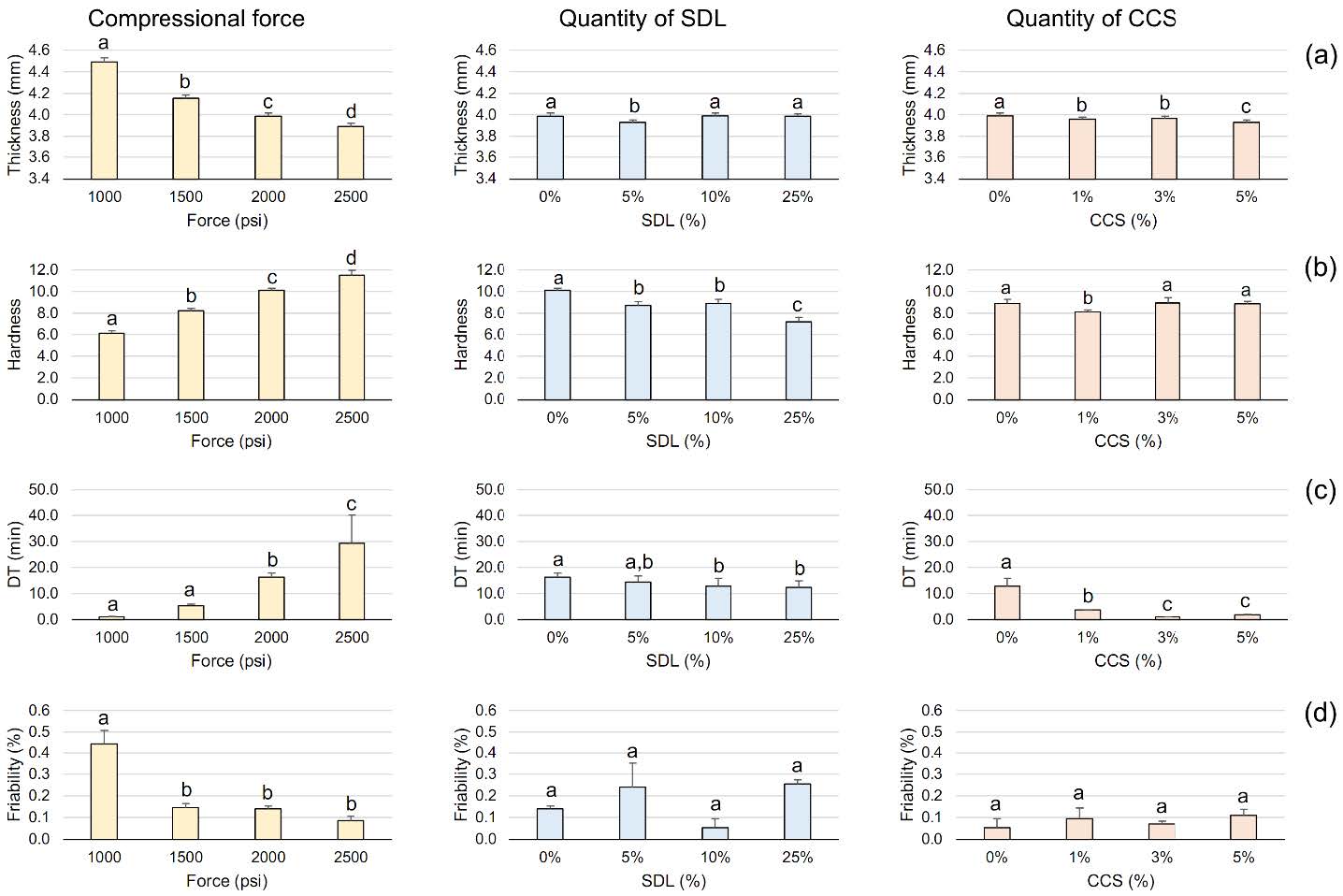
Figure 1. One-factor-at-a-time analysis of compressional force, quantity of SDL, and quantity of CCS on tablet (a) thickness, (b) hardness, (c) DT, and (d) friability. Different letters signify significant differences (P < 0.05).
The Box-Behnken design incorporated narrow ranges for each factor: compressional forces from 1,000 to 2,000 psi, SDL quantities from 0 to 10%, and CCS quantities from 2 to 4%. Figure 2 displays the response surfaces of tablet properties generated through this design, while Tables 2-5 present their corresponding ANOVA results. Increasing compressional force significantly decreased tablet thickness and friability while significantly increasing hardness and prolonging DT. Higher SDL quantities significantly decreased hardness but also significantly increased friability. The effect of CCS varied with compressional force. At low pressure, increasing CCS significantly prolonged DT. However, at medium and high compressional forces, increased CCS significantly shortened DT. In addition to the main effects, the interaction between compressional force and CCS quantity significantly affected DT. Notably, the quadratic term of compressional force also had a significant impact on friability.
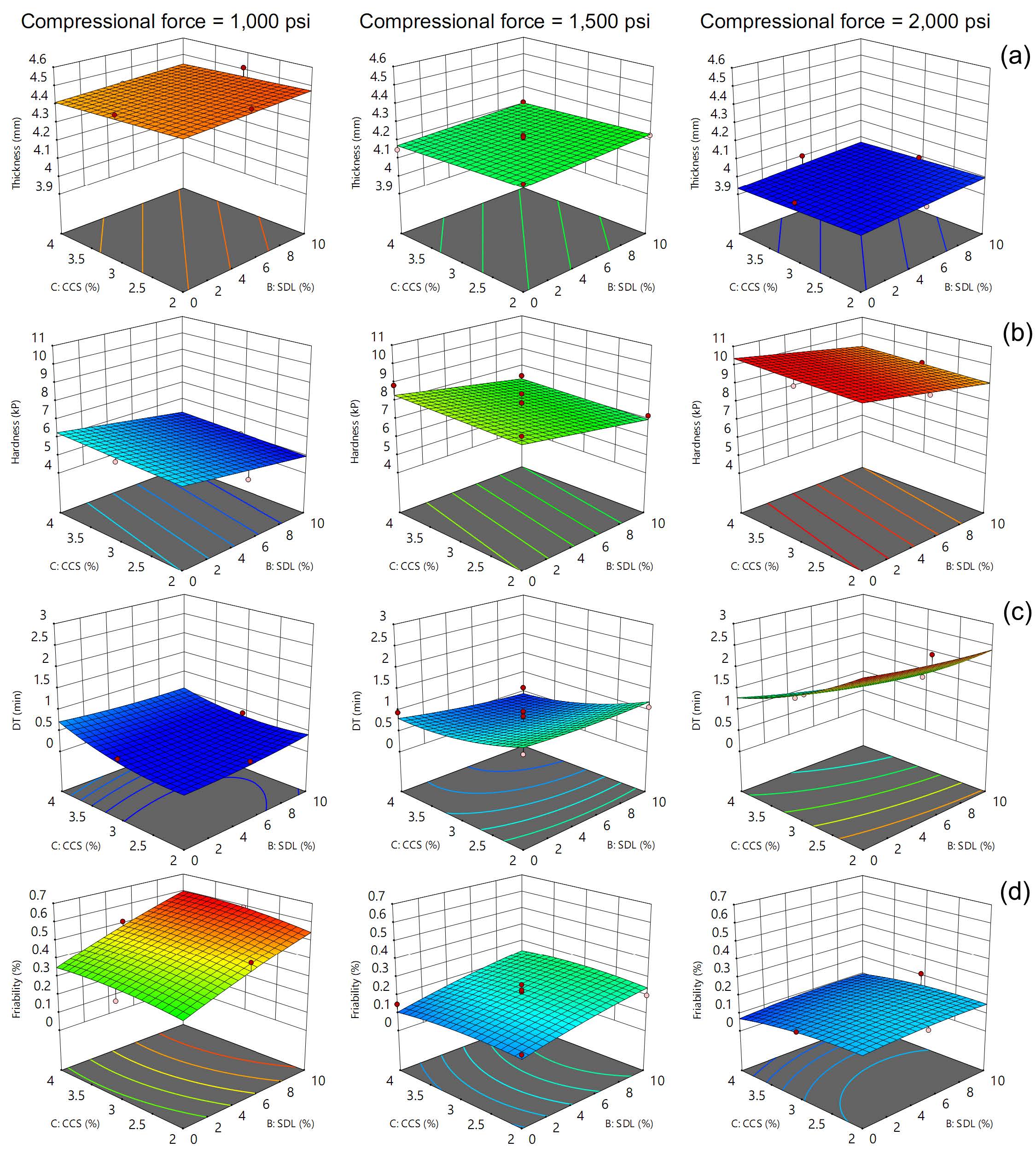
Figure 2. Response surfaces of tablet thickness (a), hardness (b), DT (c), and friability (d), based on Box-Behnken design, were generated for different applied compressional forces.
Table 2. ANOVA for the linear model of thickness.
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
P-value |
|
Model |
0.4599 |
3 |
0.1533 |
87.0800 |
< 0.0001* |
|
A-Force |
0.4560 |
1 |
0.4560 |
259.0200 |
< 0.0001* |
|
B-SDL |
0.0021 |
1 |
0.0021 |
1.2000 |
0.2932 |
|
C-CCS |
0.0018 |
1 |
0.0018 |
1.0200 |
0.3304 |
|
Residual |
0.0229 |
13 |
0.0018 |
||
|
Lack of Fit |
0.0128 |
9 |
0.0014 |
0.5607 |
0.7838 |
|
Pure Error |
0.0101 |
4 |
0.0025 |
||
|
Cor Total |
0.4828 |
16 |
Note: An asterisk (*) denoted significant values (P < 0.05)
Table 3. ANOVA for the linear model of hardness.
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
P-value |
|
Model |
35.7300 |
3 |
11.9100 |
72.1100 |
< 0.0001* |
|
A-Force |
33.2100 |
1 |
33.2100 |
201.0700 |
< 0.0001* |
|
B-SDL |
2.4200 |
1 |
2.4200 |
14.6500 |
0.0021* |
|
C-CCS |
0.1013 |
1 |
0.1013 |
0.6130 |
0.4477 |
|
Residual |
2.1500 |
13 |
0.1652 |
||
|
Lack of Fit |
1.4400 |
9 |
0.1605 |
0.9130 |
0.5849 |
|
Pure Error |
0.7030 |
4 |
0.1758 |
||
|
Cor Total |
37.8800 |
16 |
Note: An asterisk (*) denoted significant values (P < 0.05)
Table 4. ANOVA for the quadratic model of DT.
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
P-value |
|
Model |
5.4000 |
9 |
0.5996 |
12.8100 |
0.0014* |
|
A-Force |
3.3000 |
1 |
3.3000 |
70.5500 |
< 0.0001* |
|
B-SDL |
0.0903 |
1 |
0.0903 |
1.9300 |
0.2074 |
|
C-CCS |
0.7503 |
1 |
0.7503 |
16.0300 |
0.0052* |
|
AB |
0.0529 |
1 |
0.0529 |
1.1300 |
0.3230 |
|
AC |
0.8836 |
1 |
0.8836 |
18.8800 |
0.0034* |
|
BC |
0.0272 |
1 |
0.0272 |
0.5816 |
0.4706 |
|
A2 |
0.1638 |
1 |
0.1638 |
3.5000 |
0.1036 |
|
B2 |
0.0084 |
1 |
0.0084 |
0.1801 |
0.6840 |
|
C2 |
0.0944 |
1 |
0.0944 |
2.0200 |
0.1985 |
|
Residual |
0.3277 |
7 |
0.0468 |
||
|
Lack of Fit |
0.2080 |
3 |
0.0693 |
2.3200 |
0.2172 |
|
Pure Error |
0.1197 |
4 |
0.0299 |
||
|
Cor Total |
5.7200 |
16 |
Note: An asterisk (*) denoted significant values (P < 0.05)
Table 5. ANOVA for the quadratic model of friability.
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
P-value |
|
Model |
0.3230 |
9 |
0.0359 |
9.4500 |
0.0036* |
|
A-Force |
0.2278 |
1 |
0.2278 |
60.0100 |
0.0001* |
|
B-SDL |
0.0312 |
1 |
0.0312 |
8.2300 |
0.0240* |
|
C-CCS |
0.0003 |
1 |
0.0003 |
0.0823 |
0.7825 |
|
AB |
0.0121 |
1 |
0.0121 |
3.1900 |
0.1174 |
|
AC |
0.0030 |
1 |
0.0030 |
0.7968 |
0.4017 |
|
BC |
0.0001 |
1 |
0.0001 |
0.0263 |
0.8757 |
|
A2 |
0.0475 |
1 |
0.0475 |
12.5200 |
0.0095* |
|
B2 |
0.0005 |
1 |
0.0005 |
0.1404 |
0.7190 |
|
C2 |
0.0015 |
1 |
0.0015 |
0.3899 |
0.5521 |
|
Residual |
0.0266 |
7 |
0.0038 |
||
|
Lack of Fit |
0.0186 |
3 |
0.0062 |
3.1000 |
0.1518 |
|
Pure Error |
0.0080 |
4 |
0.0020 |
||
|
Cor Total |
0.3496 |
16 |
Note: An asterisk (*) denoted significant values (P < 0.05)
Figure 3 displays the design spaces achieving the desired tablet properties (hardness 5-8 kP, DT ≥ 0.5 min, friability ≤ 0.4%). Notably, the design space was narrow at 1,000 psi, absent at 2,000 psi, and broadest at 1,500 psi. Therefore, 1,500 psi was chosen as the optimal compressional force, resulting in the ideal formulation of 6% SDL and 3% CCS. Verification of the model's predictive capabilities was performed by replicating the preparation of Semha-Pinas plain tablets under the optimized condition. The results, as presented in Table 6, demonstrated remarkably low percent errors for all parameters, nearing 0%. This exceptional agreement between predicted and experimental values underscores the accuracy and reliability of the computer software's predictions (Duangjit et al., 2012; Duangjit et al., 2014).
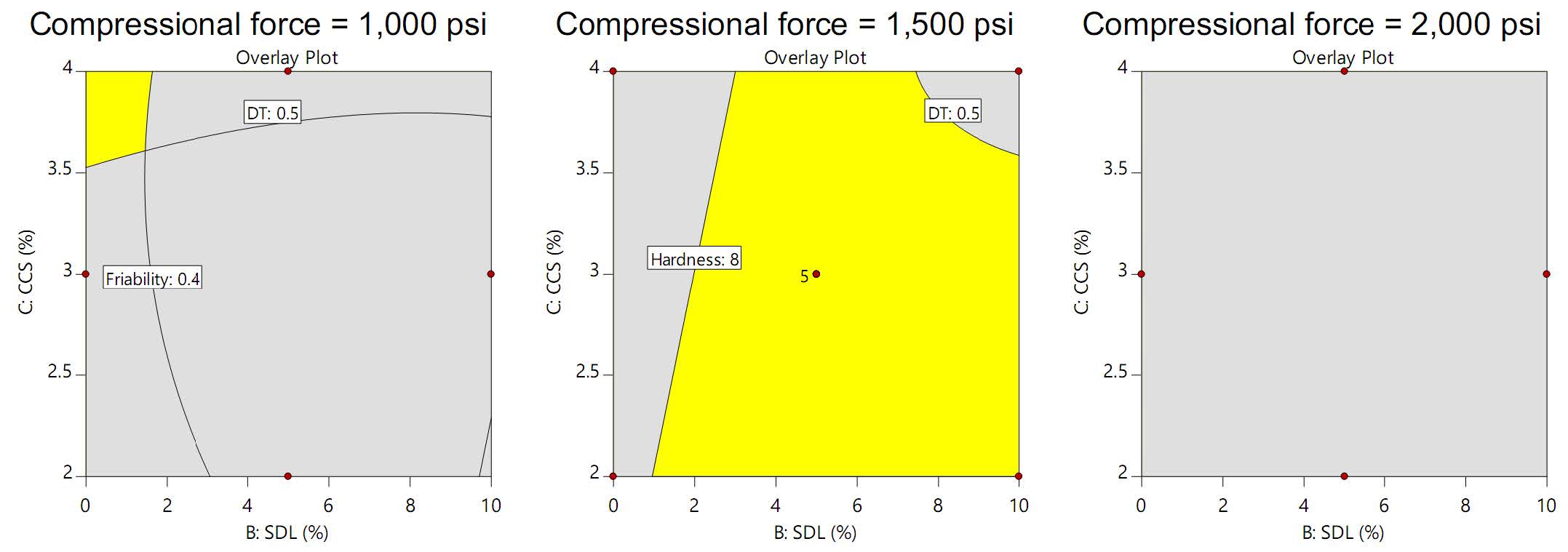
Figure 3. Design spaces for desired tablet properties (hardness 5-8 kP, DT ≥ 0.5 min, friability ≤ 0.4%) at different compressional forces.
Table 6. Verification of model predictions through experimental values, with percent error calculations for key tablet characteristics.
|
Parameters |
Predicted values |
Experimental values |
Error (%)* |
|
Weight (mg) |
- |
381.23 ± 0.80 |
- |
|
Diameter (mm) |
- |
9.66 ± 0.01 |
- |
|
Thickness (mm) |
4.21 |
4.20 ± 0.04 |
-0.24 |
|
Hardness (kP) |
7.56 |
7.50 ± 0.41 |
-0.80 |
|
DT (min) |
0.72 |
0.67 ± 0.07 |
-7.46 |
|
Friability (%) |
0.22 |
0.22 ± 0.04 |
0.00 |
* Error (%) = (Experimental value - Predicted value) × 100/Experimental value
DISCUSSION
In the past, the development of high-quality pharmaceutical products relied on a traditional method characterized by trial-and-error or one-factor-at-a-time approaches, resulting in various issues such as non-reproducibility, high costs, and time-consuming. To address these challenges, a novel concept known as DoE emerged. DoE entails conducting experimental designs based on relevant variables, coupled with statistical assessment of the acquired responses and exploration of the design space through mathematical or graphical analyses. Statistical assessment plays a crucial role in enhancing the quality of final products and meeting the escalating demand for products of superior quality and standards (Dhoot et al., 2019).
The Box-Behnken design provides outstanding efficiency in examining quadratic response surfaces, requiring fewer experimental runs than other designs such as the central composite design and the full factorial design. Without replication, the central composite design consists of 15 runs, the 33 full factorial design consists of 27 runs, while the Box-Behnken design consists of only 13 runs for three factors (National Institute of Standards and Technology, 2012). This demonstrates that the Box-Behnken design is the most economical method due to its minimal number of experimental runs.
While a one-factor-at-a-time screening identified a high compressional force of 2,500 psi as capable of achieving low friability, it also resulted in undesirable increases in hardness and prolonged DT. Therefore, the Box-Behnken design was limited to a narrower range of compressional forces, from 1,000 to 2,000 psi, to prioritize a balance between these properties. Similarly, a high SDL quantity of 25% significantly decreased hardness, prompting the Box-Behnken design to focus on a range of 0 to 10% for this factor. Finally, the one-factor-at-a-time screening revealed that omitting CCS entirely led to prolonged DT, while a high quantity of 5% did not significantly shorten DT. Consequently, CCS quantities were restricted to 2 to 4% in the Box-Behnken design to optimize DT within acceptable ranges.
One-factor-at-a-time screening and Box-Behnken design identified compressional force as the dominant factor governing multiple tablet properties, highlighting its pivotal role in formulation optimization (Manley et al., 2019; Marais et al., 2003; Monton et al., 2023a; Suksaeree et al., 2023). According to the Box-Behnken design data, our study found that tablet thickness and friability decreased, and hardness increased and DT prolonged, as compressional force increased, confirming the anticipated trends and aligning with previous research (Pimhataivoot et al., 2011; Monton et al., 2023b; Monton et al., 2024). In case SDL increased, another diluent (MCC) was decreased, therefore, the hardness decreased. MCC is used as a diluent and a binder for this formulation. A decrease in MCC content leads to a decrease in hardness, which was the effect of MCC aligns with previous research (Monton et al., 2023b).
CCS, a well-known tablet disintegrant, is insoluble in water but undergoes significant swelling (4-8 times its original volume) upon contact with water (Rowe et al., 2009). This remarkable fluid absorption and swelling capacity, which is intrinsic to CCS, significantly contributes to its effectiveness as a superdisintegrant (Hiremath et al., 2019). Compared to sodium starch glycolate, croscarmellose (in the form of calcium salt) exhibited less hardness and faster disintegration, which comparable to crospovidone, as determined using both static and dynamic modes (Singh et al., 2020). However, exceeding 5% CCS can lead to prolonged DT due to the formation of a viscous gel layer that hinders tablet breakdown (Hiremath et al., 2019). Studies have even shown that beyond 7.5%, CCS can dramatically prolong the DT of rapidly disintegrating tablets like aspirin, ibuprofen, and ascorbic acid (Desai et al., 2014). In our study, increasing CCS content surprisingly had opposite effects on DT depending on the compressional force. While it significantly shortened DT at medium and high pressures (1,500 and 2,000 psi), it curiously prolonged DT at the lowest pressure (1,000 psi). This seemingly paradoxical behavior could be explained by the interaction between compressional force and CCS quantity as shown in Table 4.
The design space criteria were established based on desired tablet properties and industry standards. A minimum DT of 0.5 min was chosen to ensure adequate breakdown but avoid excessively fast disintegrating, exceeding the expected characteristic of orodispersible tablets. While the typical friability limit for plain tablets is set at not more than 1.0%, the observed maximum friability in this study reached 0.6%. To balance achieving a suitable design space without excessive flexibility, a final friability limit of 0.4% was established.
CONCLUSION
This study successfully demonstrated the application of the Box-Behnken design to optimize the formulation of Semha-Pinas plain tablets for managing phlegm. The optimized formulation, comprising 1,500 psi compressional force, 6% SDL, and 3% CCS, achieved an ideal combination of desired tablet properties: hardness within the target range (5-8 kP), DT exceeding the minimum requirement (0.5 min), and friability well below the acceptable limit (0.4%). Verification data further cemented the robustness of the optimized formulation, with all predicted values closely matching the experimental values (percent errors < 10%). These findings indicated the potential development of effective Semha-Pinas plain tablets as an alternative treatment option for managing phlegm. Notably, the optimized formulation meets relevant pharmacopoeial criteria, ensuring quality and consistency for future production.
ACKNOWLEDGEMENTS
We would like to thank Ms. Chookwan Khunjaroen, Mr. Natrong Panjasanontachai, and Mr. Nattapong Yuwansri for their research assistance.
AUTHOR CONTRIBUTIONS
Jirapornchai Suksaeree: Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing. Chaowalit Monton: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdel-Rahman, M. A., Hassan, S. E. D., El-Din, M. N., Azab, M. S., El-Belely, E. F., Alrefaey, H. M. A., and Elsakhawy, T. 2020. One-factor-at-a-time and response surface statistical designs for improved lactic acid production from beet molasses by Enterococcus hirae ds10. SN Applied Sciences. 2: 573.
Abou-Taleb, K. A. and Galal, G. F. 2018. A comparative study between one-factor-at-a-time and minimum runs resolution-IV methods for enhancing the production of polysaccharide by Stenotrophomonas daejeonensis and Pseudomonas geniculata. Annals of Agricultural Sciences. 63: 173-180.
Bhaturiwala, R., Bagban, M., Mansuri, A., and Modi, H. 2022. Successive approach of medium optimization using one-factor-at-a-time and response surface methodology for improved β-mannanase production from Streptomyces sp. Bioresource Technology Reports. 18: 101087.
Desai, P. M., Er, P. X., Liew, C. V., and Heng, P. W. 2014. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech. 15: 1093–1104.
Dhoot, A. S., Fernandes, G. J., Naha, A., Rathnanand, M., and Kumar, L. 2019. Design of experiments in pharmaceutical development. Pharmaceutical Chemistry Journal. 53: 730-735.
Duangjit, S., Mehr, L. M., Kumpugdee-Vollrath, M., and Ngawhirunpat, T. 2014. Role of simplex lattice statistical design in the formulation and optimization of microemulsions for transdermal delivery. Biological and Pharmaceutical Bulletin. 37: 1948-1957.
Duangjit, S., Obata, Y., Sano, H., Kikuchi, S., Onuki, Y., Opanasopit, P., Ngawhirunpat, T., Maitani, Y., and Takayama, K. 2012. Menthosomes, novel ultradeformable vesicles for transdermal drug delivery: Optimization and characterization. Biological and Pharmaceutical Bulletin. 35: 1720-1728.
Duangjit, S., Rattanachithawat, N., Opanasopit, P., and Ngawhirunpat, T. 2022. Development and optimization of finasteride-cinnamon oil-loaded ethanol-free microemulsions for transdermal delivery. Journal of Drug Delivery Science and Technology. 69: 103107.
Gibson, M. (Ed.). 2016. Pharmaceutical preformulation and formulation: A practical guide from candidate drug selection to commercial dosage form (2 ed., Vol. 199). Informa Healthcare.
Hegazy, M. M., Badawi, A. A., El-Nabarawi, M. A., Eldegwy, M. A., and Louis, D. 2024. One factor at a time and factorial experimental design for formulation of L-carnitine microcapsules to improve its manufacturability. Heliyon. 10: e23637.
Hiremath, P., Nuguru, K., and Agrahari, V. 2019. Chapter 8 - material attributes and their impact on wet granulation process performance. P. 263-315. In A. S. Narang and S. I. F. Badawy (eds) Handbook of pharmaceutical wet granulation. Academic Press.
Jaikham, P., Leelapornpisid, P., and Poomanee, W. 2024. Development of transethosomes delivery system loaded with Bouea macrophylla Griffith seed kernel extract for cosmeceutical application. Natural and Life Sciences Communications. 23: E2024002.
Jitrangsri, K., Chaidedgumjorn, A., and Satiraphan, M. 2020. Supercritical fluid extraction (SFE) optimization of trans-resveratrol from peanut kernels (Arachis hypogaea) by experimental design. Journal of Food Science and Technology 57: 1486-1494.
Jitrangsri, K., Chaidedgumjorn, A., and Satiraphan, M. 2022. Experimental design for solid-liquid extraction from peanut kernel: Optimization through variability in antioxidant potential. Science, Engineering and Health Studies 16: 22050015.
JMP Statistical Discovery LLC. 2022. Design of experiments. Retrieved August 27, 2023 from https://www.jmp.com/en_ph/statistics-knowledge-portal/what-is-design-of-experiments.html
Khun Sopit Banna Lak. 1961. Kampee Phat Thai Phan Boran, Volume 3. Retrieved August 27, 2023 from https://be7herb.wordpress.com/
Manley, L., Hilden, J., Valero, P., and Kramer, T. 2019. Tablet compression force as a process analytical technology (PAT): 100% inspection and control of tablet weight uniformity. Journal of Pharmaceutical Sciences. 108: 485–493.
Marais, A. F., Song, M., and Villiers, M. M. d. 2003. Effect of compression force, humidity and disintegrant concentration on the disintegration and dissolution of directly compressed furosemide tablets using croscarmellose sodium as disintegrant. Tropical Journal of Pharmaceutical Research. 2: 125–135.
Monton, C., Chankana, N., Duangjit, S., Suksaeree, J., Naksuriya, O., Charoenchai, L., and Songsak, T. 2024. Fabrication and optimization of directly compressible self-emulsifying tablets containing cannabis extract obtained from supercritical carbon dioxide extraction. Applied Science and Engineering Progress. 17: 6973.
Monton, C., Keawchay, P., Pokkrong, C., Kamnoedthapaya, P., Navabhatra, A., Suksaeree, J., Wunnakup, T., Chankana, N., and Songsak, T. 2023a. Fabrication of direct compressible tablets containing Chatuphalathika extract obtained through microwave-assisted extraction: An optimization approach. Scientia Pharmaceutica. 91: 17.
Monton, C., Wunnakup, T., Suksaeree, J., Charoenchai, L., and Chankana, N. 2023b. Impact of compressional force, croscarmellose sodium, and microcrystalline cellulose on black pepper extract tablet properties based on design of experiments approach. Scientia Pharmaceutica. 91: 30.
Mura, P., Valleri, M., Baldanzi, S., and Mennini, N. 2019. Characterization and evaluation of the performance of different calcium and magnesium salts as excipients for direct compression. International Journal of Pharmaceutics. 567: 118454.
National Institute of Standards and Technology. 2012. NIST/SEMATECH e-Handbook of Statistical Methods. Retrieved May 25, 2024 from https://www.itl.nist.gov/div898/handbook/pri/section3/pri3363.htm
Nor, N. M., Mohamed, M. S., Loh, T. C., Foo, H. L., Rahim, R. A., Tan, J. S., and Mohamad, R. 2017. Comparative analyses on medium optimization using one-factor-at-a-time, response surface methodology, and artificial neural network for lysine–methionine biosynthesis by Pediococcus pentosaceus RF-1. Biotechnology & Biotechnological Equipment. 31: 935-947.
Pimhataivoot, P., Tipduangta, P., Sirithunyalug, B., and Sirithunyalug, J. 2011. Versatile compression force measuring system for rotary tablet presses. Chiang Mai University Journal of Natural Sciences. 10: 241-260.
Rowe, R. C., Sheskey, P. J., and Quinn, M. E. (Eds.). 2009. Handbook of Pharmaceutical Excipients (6th ed.). Pharmaceutical Press.
Saepang, K., Pitaksuteepong, T., Buranrat, B., and Boontha, S. 2024. Optimization of HPMC-based oral fast dissolving film of cetirizine dihydrochloride. Natural and Life Sciences Communications. 23: e2024007.
Singh, S. Y., Salwa, Shirodkar, R. K., Verma, R., and Kumar, L. 2020. Enhancement in dissolution rate of atorvastatin trihydrate calcium by formulating its porous tablet using sublimation technique. Journal of Pharmaceutical Innovation. 15: 498-520.
Steele, G. 2018. Quality by design (QbD) and the development and manufacture of drug substance. P. 61–95. In W. S. Schlindwein and M. Gibson (eds) pharmaceutical quality by design: a practical approach. John Wiley & Sons Ltd.
Suksaeree, J., Monton, C., Charoenchai, L., Chankana, N., and Wunnakup, T. 2023. Optimization of process and formulation variables for Semha–Pinas extract effervescent tablets using the Box–Behnken design. AAPS PharmSciTech. 24: 52.
Suksaeree, J., Monton, C., Navabhatra, A., Charoenchai, L., Chankana, N., and Naksuriya, O. 2024. Optimization of Semha-Pinas extract orodispersible tablets using response surface methodology. Applied Science and Engineering Progress. 17: 6944.
Suriyaamporn, P., Pamornpathomkul, B., Rojanarata, T., Patrojanasophon, P., Opanasopit, P., and Ngawhirunpat, T. 2023. Design and optimization of ganciclovir nanosuspension loaded in situ gelling mucoadhesive eye drops for herpetic keratitis. Natural and Life Sciences Communications. 22: e2023068.
Vijayakumar, J., Goudarzi, N. M., Eeckhaut, G., Schrijnemakers, K., Cnudde, V., and Boone, M. N. 2023. Characterization of pharmaceutical tablets by X-ray tomography. Pharmaceuticals. 16: 733.
Xiao, Q., Joseph, V. R., and Ray, D. M. 2023. Maximum one-factor-at-a-time designs for screening in computer experiments. Technometrics. 65: 220-230.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Jirapornchai Suksaeree1 and Chaowalit Monton2, 3, *
1 Department of Pharmaceutical Chemistry, College of Pharmacy, Rangsit University, Pathum Thani 12000, Thailand.
2 Drug and Herbal Product Research and Development Center, College of Pharmacy, Rangsit University, Pathum Thani 12000, Thailand.
3 Department of Pharmacognosy, College of Pharmacy, Rangsit University, Pathum Thani 12000, Thailand.
Corresponding author: Chaowalit Monton, E-mail: chaowalit@rsu.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: April 5, 2024;
Revised: May 25, 2024;
Accepted: June 11, 2024;
Online First: June 17, 2024

