
Morphological Properties of Poly (vinyl alcohol)/Gelatin contained Indonesian Carbonated Hydroxyapatite Abalone Nanofibrous Scaffold by Electrospinning
Mona Sari, Nilam Cahyati, and Yusril Yusuf*Published Date : June 11, 2024
DOI : https://doi.org/10.12982/NLSC.2024.037
Journal Issues : Number 3, July-September 2024
Abstract One strategy for dealing with bone defects is replicating and reconstructing artificial bone for bone tissue engineering (BTE) application, known as the scaffold. Bone consists of an extracellular matrix (ECM) which, at the nanoscale, has a fibrous structure that can be replicated in synthetic scaffolds using an electrospinning method. This work describes the analysis of the morphological properties of Poly (vinyl alcohol) (PVA)/Gelatin contained Indonesian carbonated hydroxyapatite (CHA) abalone nanoparticle scaffold by electrospun nanofiber. CHA nanoparticles were produced using a co-precipitation method, and nanofibrous PVA/Gelatin/CHA 5 wt% scaffold was fabricated by electrospinning. The synthesized CHA produced the same condition as B-type CHA, ensured by Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffractometer (XRD), and differential scanning calorimetry (DSC), and energy-dispersive X-ray spectroscopy (EDS) tests. The morphological properties of PVA/Gelatin/CHA are analyzed using Scanning Electron Microscopy (SEM). The SEM image indicated that PVA/Gelatin nanofiber tended to have a fine morphology without beads. The agglomeration of the PVA/Gelatin/CHA 5 wt% nanofiber scaffold can be neglected because it is still on the sub-micron scale (1 μm–100 nm). Moreover, adding CHA 5 wt% in the PVA/Gelatin matrix decreased the average fiber diameter. The diameter fiber results are within the fiber diameter range (100–450 nm) in native bone ECM. Therefore, PVA/Gelatin/CHA 5 wt% has the potential to serve as an alternative scaffold material for BTE application.
Keywords: Carbonated hydroxyapatite, Poly (vinyl alcohol), Gelatin, Nanofiber Scaffold, Electrospinning, Morphological properties, Bone tissue engineering
Funding: The authors are immensely grateful to the Directorate of Research Universitas Gadjah Mada (UGM) and UGM Reputation Improvement Team towards World Class University - UGM Quality Assurance Office through Post-Doctoral Program 2023 (No: 3662/UN1.P.II/Dit-Lit/PT.01.03/2023) and Directorate of Research Universitas Gadjah Mada through RTA Program 2023 (No: 5075/UN1.P.II/Dit-Lit/PT.01.01/2023) for financially supporting this research.
Citation: Sari, M., Cahyati, N., and Yusuf, Y. 2024.Morphological properties of poly (vinyl alcohol)/gelatin contained Indonesian carbonated hydroxyapatite abalone nanofibrous scaffold by electrospinning. Natural and Life Sciences Communications. 23(3): e2024037.
INTRODUCTION
Major fractures, deformities, losses, infections, and tumor resections are some serious bone diseases requiring treatment with orthopedic surgery (Asran et al., 2010). One strategy for dealing with severe bone defects is replicating and reconstructing artificial bone for various applications, known as the scaffold (Sari et al., 2021 (a)). The emergence of bone tissue damage cases encourages advances in bone tissue engineering (BTE) (Li et al., 2019). Therefore, bone tissue engineering (BTE) is increasingly essential in repairing bone defects and regenerating bone function. The three essential components in BTE are cells, scaffolds, and growth factors. Among these, the scaffold is essential in facilitating cell migration, formation, and movement by mimicking the shape and structure of the extracellular matrix (ECM) (Kim and Kim, 2014; Teixeira et al., 2020). Many procedures have been developed to fabricate bone scaffolds, consisting of porogen leaching (Sari et al., 2021 (a); Sari et al., 2021 (b); Sari et al., 2021 (c)), gas foaming (Manavitehrani et al., 2019), electrospinning (Perez-Puyana et al., 2018; Merkle et al., 2015; Merkle et al., 2014; Merkle et al., 2013), sponge templating (Imani et al., 2020; Jo et al., 2009), and freeze-drying (Govindan et al., 2020; Soltani et al., 2019). These methods produce a 3D scaffold structure with high porosity and interconnected macroporosity (pore size > 50 μm) (Januariyasa et al., 2020 (b)). However, the size of the microporosity is still around <10 μm, an essential characteristic in the osteogenesis process that modulates cell attachment, proliferation, and differentiation and provides a large surface area.
Bone consists of an ECM, which, at the nanoscale, has a fibrous structure arising from interactions between organic (mainly type-1 collagen) and inorganic (carbonate mineral apatite) components (Januariyasa et al., 2020). These nanoscale structures can be replicated in synthetic scaffolds using an electrospinning approach, one of the most emerging techniques for forming scaffolds with application in BTE, consisting of fabricating membranes formed by nanometric fibers (Januariyasa et al., 2020; Perez-Puyana et al., 2018; Wang et al., 2013) . The fibrous structure has high porosity, high surface area, and a structure similar to the original ECM in bone (Januariyasa et al., 2020; Wang et al., 2013). Therefore, electrospinning is the most effective technology for producing ECM-mimicking structures (Naghavi et al., 2015).
The electrospinning process presents several advantages over other traditional methods, such as high specific surface and nanofiber porosity, producing nanofibers with desired properties by changing their chemical or structural composition. In addition, some characteristics, such as nanofibers' diameter and morphological properties, can be adjusted by modifying the process parameters: the relationship between the polymer used, the voltage, the flow rate or the distance between the needle and the collector (Perez-Puyana et al., 2018). Moreover, the electrospinning approach can produce a variety of nanomaterials, including polymers, ceramics, metals, and nanofiber composites (Teo and Ramakrishna, 2009; Januariyasa et al., 2020). Many studies have been conducted to mimic bone structure at the nanoscale level using various polymers or bioceramic composites to form nanofiber scaffolds (Januariyasa et al. 2020; Rashedi et al., 2021; Ghazalian et al., 2022; Yuwono et al., 2022).
Composite and ceramic materials combined with synthetic and natural polymers effectively improve the osteogenetic properties of nanofiber scaffolds (Chen et al., 2019; Nemati et al., 2019; Ma et al., 2021). Based on previous literature, hydroxyapatite (HA), as human bone's main inorganic mineral component (Zhou and Lee, 2011; Ma et al., 2021; Sawada et al., 2021), has excellent osteoconductivity and bioactivity (Sari et al., 2021 (a)). When HA is deposited on the surface of biopolymeric nanofibers, it can mimic the bone structure to induce preosteoblasts to become osteogenic phenotypes through suitable cell-material interactions to enhance the biological activity (Chen et al., 2019). However, human bone comprises 65% carbonated hydroxyapatite (CHA) with a carbonate content ranging from 2–8 wt% (Januariyasa et al., 2020; Sari et al., 2021 (a)). CHA exhibits better biological properties due to its low crystallinity and increased surface area (Youness et al., 2017; Sari et al., 2022). Therefore, it exhibits superior bioactivity, which can be used in BTE applications. In addition, CHA is a promising material for inorganic components when it mimics the native structure of ECM, and several studies have investigated the effectiveness of CHA as a polymer matrix filler for BTE application (Januariyasa et al., 2020). This research used CHA based on biogenic materials from Indonesian abalone mussel shells (Haliotis asinina) as matrix composition with the polymer, as used in the previous study because it contains high concentrations of calcium carbonate (CaCO3) as a natural precursor for the fabrication of bioceramics, which is 90–95% CaCO3 (Sari et al., 2021 (a); Sari et al., 2021 (c)).
Most research results show that biopolymers, like proteins, prepared by the electrospinning approach have been selected as scaffolds for the repair and regeneration of bone tissue (Chen et al., 2019). The use of biopolymers, like proteins, presents the advantages of biodegradability and the ease of crosslinking (giving suitable mechanical resistance to membranes). The most used proteins are collagen, gelatin, elastin, and tropoelastin (Mithieux et al., 2013; Perez-Puyana et al., 2018), but gelatin is selected because of its biocompatibility and biodegradability, which are essential for BTE (Perez-Puyama et al., 2018). Gelatin, as a water-soluble protein (Wang et al., 2017), is generated by partial hydrolysis of collagen (the main component of native ECM) (Hoch et al., 2013), extracted from boiled bones, skin, and connective tissue of animals such as domesticated cows and pigs (Eysturskard et al., 2009). However, sometimes there are limitations related to the relatively low capacity to form protein fibers. Therefore, it is necessary to incorporate other polymers (synthetic polymers) that allow the electrospinning of polymer/protein solutions. Among the most widely used synthetic polymers are polystyrene (PS), poly (vinyl alcohol) (PVA), and polycaprolactone (PCL) (Linh et al., 2012; Perez-Puyana et al., 2018). PVA is a water-soluble synthetic, biodegradable, biocompatible, inexpensive, non-toxic, and highly flexible polymer with high tensile strength (Perez-Puyana et al., 2018; Yang et al., 2018; Januariyasa et al., 2020).
In this work, CHA was fabricated via co-precipitation using a precursor of CaO based on Indonesian abalone mussel shells. The physicochemical properties of this CHA are observed, consisting of its effect on the crystallographic properties, morphology, molar ratio of Ca/P, thermal properties, and chemical compositions of CHA products. Then, CHA with a concentration of 5 wt% is incorporated into a nanofibrous PVA/gelatin matrix to mimic the native ECM for BTE. The morphological properties of nanofiber scaffold CHA/PVA/gelatin also are analyzed through SEM.
MATERIALS AND METHODS
The experimental procedure consisted of two main stages: fabrication and physicochemical characterization of CHA from Indonesian abalone mussel shells and incorporation of CHA with the concentration of 5 wt% into a nanofibrous PVA/gelatin matrix to form PVA/gelatin/CHA 5 wt% via electrospinning method.
Materials
The materials for this study consist of the Indonesian abalone mussel shells as a natural precursor of CaCO3, the precursors of diammonium hydrogen phosphate ((NH4)2 HPO4), ammonium bicarbonate (NH4HCO3), and ammonium hydroxide (NH4OH) 25% solution used the same materials as previous research (Sari et al., 2021 (b); Sari et al., 2021 (c)). PVA with molecular weight 145.000 (fully hydrolyzed) and ethanol absolute for analysis were purchased from Merck (Merck KgaA, Darmstadt, Germany). Gelatin from bovine skin B-type was purchased from Sigma-Aldrich (Sigma-Aldrich Inc., MO, USA), and HyClone phosphate buffered saline solution was purchased from Cytiva (Cytiva, US). Water for injection was obtained from Ikapharmindo Putramas Company, Jakarta, Indonesia.
Fabrication of CHA based on Indonesian abalone mussel shells.
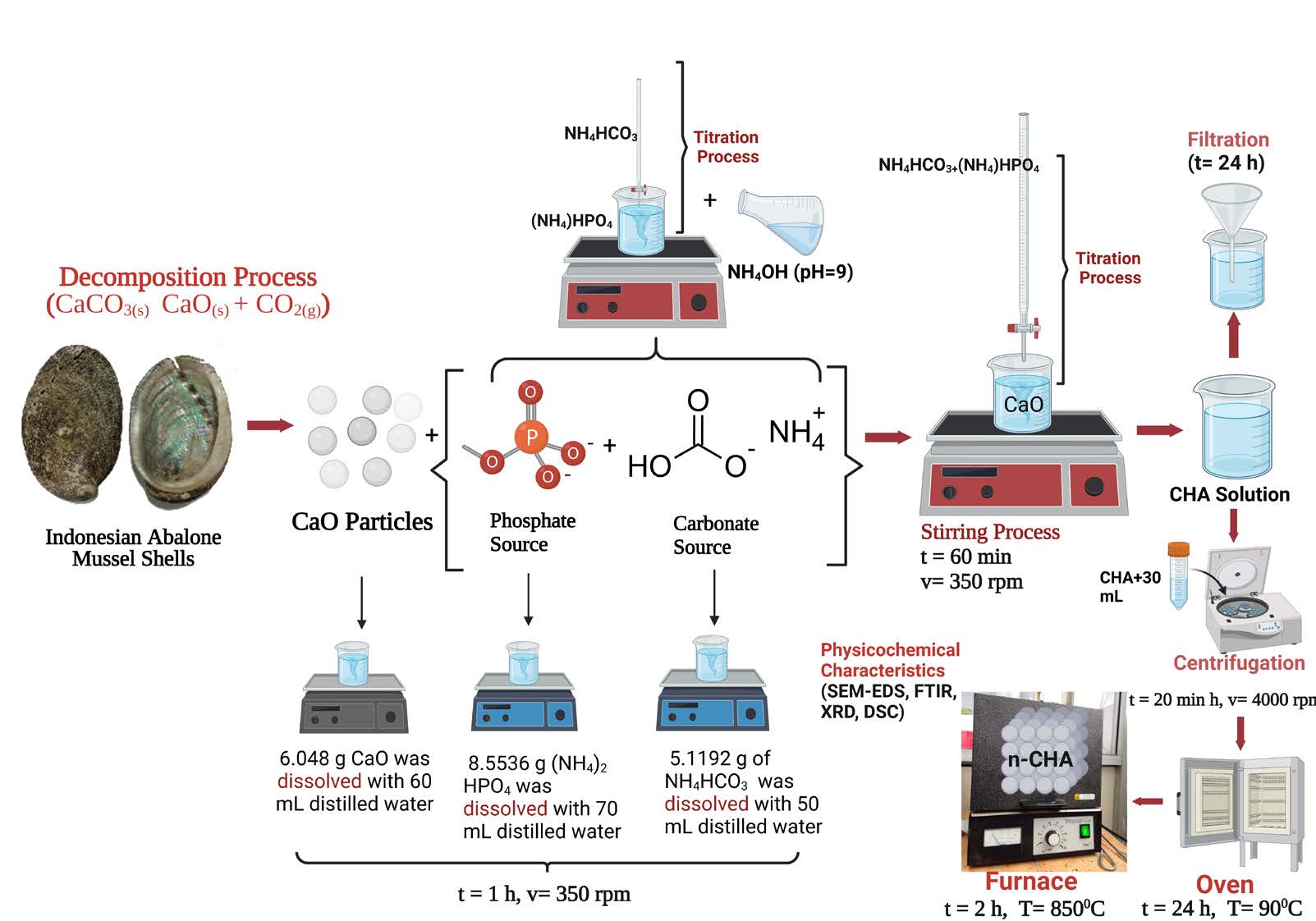
Figure 1. Schematic methods to fabricate and characterize CHA based on Indonesian abalone mussel shells using precipitation (Created with BioRender.com).
CaO and CHA have been fabricated in the previous study, so this work utilized those samples (Sari et al., 2021 (b); Sari et al., 2021 (c)). In this study, the CHA solution was stirred using a magnetic stirrer (Thermo Fisher Scientific, Waltham, MA, USA) at a velocity of 350 rpm for 60 min at a temperature of 60°C (Sari et al., 2021 (c)). CHA was calcinated at a temperature of 850°C for 2 h using a furnace (Vulcan, Yucaipa, CA, USA), so the CHA sample code is CHA-60 min-850°C. The schematic procedures to fabricate CHA from Indonesian abalone mussel shells can be seen in Figure 1.
Preparation of PVA/gelatin solution
An amount of 0.79 g of PVA was dissolved in 1% (v/v) ethanol/distilled water solution to prepare PVA 10 % (w/v) and stirred at a temperature of 60°C for 24 h. An amount of 0.87 g of gelatin was dissolved in 1% (v/v) ethanol/PBS to get gelatin 11 % (w/v) and stirred at a temperature of 40°C for 6 h. Then the PVA and gelatin solutions were mixed at a ratio of 50:15 (v/v) and stirred at 25°C for 24 h. All stirring velocities used 600–800 rpm, and the solution was sonicated at a temperature of 25°C for 1 h.
Preparation of PVA/gelatin/CHA 5 wt% composite solution.
An amount of 0, 12 grams of CHA powder was suspended in 10 mL of distilled water to prepare CHA 5 wt % of the total polymer mass in solution with stirring for 1 h at room temperature. All stirring velocity using 600-800 rpm. 1 gr of PVA crystals was added to the CHA suspension while stirring for 30 min at room temperature, then heated at 40–60 °C for 1.5 h and continued at room temperature for 3 h. PVA/CHA solution was mixed with 11 wt% gelatin solution with a ratio of 70:30, then stirred for 24 h at room temperature. The solution was sonicated for 1 h at 25°C to reduce CHA agglomeration in the solution, remove air bubbles and increase the homogeneity of the solution.
Fabrication of nanofibrous PVA/gelatin/CHA
At this stage, the experiment is modified from the previous study (Januariyasa et al., 2020 ). 6 mL of PVA/Gelatin and PVA/Gelatin/CHA solutions were put into a 12 ml plastic syringe fitted with a needle (the hole diameter is around 0.5–1 mm). The voltage used is approximately 10–20 kV, and a grounded collector, covered with aluminum, was put at a distance of 12–15 cm from the tip. The pump to push the solution is run manually and carefully with an average flow rate of 0.5-1 mL/h. The experiments were carried out at room temperature. The schematic of engineered morphological properties of nanofibrous CHA/PVA/Gelatin can be seen in Figure 2.
Physicochemical properties characterizations of CHA
Following the previous research (Sari et al., 2021 (a)), the physicochemical properties of the CHA-60 min-850°C were analyzed using SEM, EDS, XRD, FTIR, and DSC. The surface morphology description and particle size distribution calculation were analyzed using SEM (Joel JSM-6510LA-1400, Tokyo, Japan). First, all samples were embedded in a sample holder created from pure copper below vacuum to a pressure of 2.5 MPa and then coated with platinum by applying an ion coater. Next, the sample was incorporated into the SEM. CHA's particle size distribution based on 100 randomly selected measurements was computed using ImageJ software version 2006 (National Institutes of Health (NIH), Bethesda, MD, USA) (Sari et al., 2021 (c)).
EDS, incorporated in the SEM, was used to analyze the percentage of mineral elements in the CHA. These mineral results were applied to enumerate the molar ratio of Ca/P in the CHA-60 min-850°C. Calculation of the Ca/P ratio used the following equations (Sari et al., 2023)
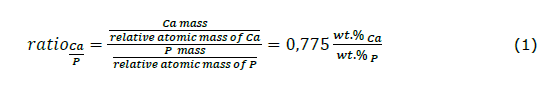
Crystallographic properties and functional groups of the CHA-60 min-850°C were specified by XRD (PAN analytical Type X’Pert Pro, Tokyo, Japan) and FTIR (Thermo Nicolet iS10, Tokyo, Japan), respectively. The XRD data were recorded in the range 2θ:10°-80° using Cu-Kα radiation at λ = 0.154 nm. The FTIR equipment was performed in the range of 400–4000 cm^(-1). The thermal properties of samples were measured by DSC (DSC-60 Plus Shimadzu, Tokyo, Japan) and taken using a flow rate of 20 mL/min, beginning from room temperature up to 600°C. The XRD pattern, FTIR spectra, and thermal properties spectra data were analyzed using OriginPro software version 2018 (OriginLab Corporation, Northampton, Massachusetts). FTIR spectra were also analyzed based on reference data using proper spectroscopy nomenclature (Sari et al., 2023; Sari et al., 2021 (c)).
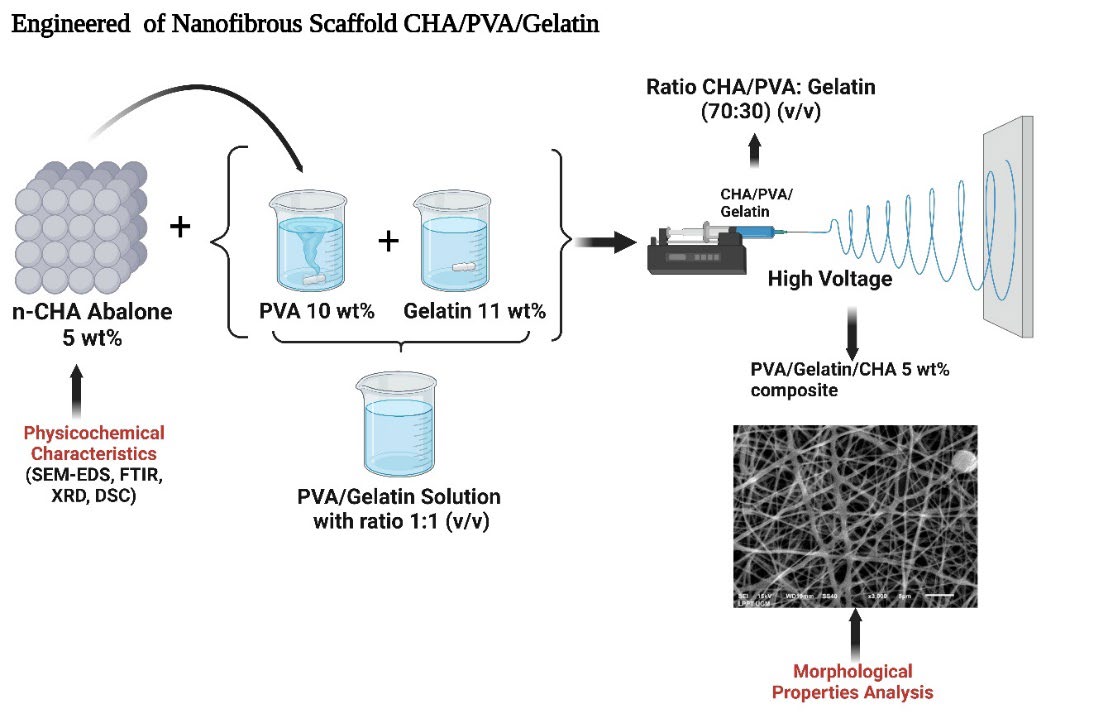
Figure 2. Schematic of engineered morphological properties of nanofibrous CHA/PVA/Gelatin (Created with BioRender.com).
Morphological properties of nanofibrous PVA/gelatin/CHA
The analysis of morphological properties includes a description of the morphology and a calculation of the average diameter of the fiber, also using SEM (JEOL JSM-6510LA-1400, Tokyo, Japan). The average diameter of the fibers was enumerated based on the measurement of 120 randomly selected fibers, adapted from previous research (Januariyasa et al., 2020 (b)), and analysis of diameter fiber data using ImageJ software version 2006 (National Institutes of Health (NIH), Bethesda, MD, USA).
RESULTS
Physicochemical properties characterizations of CHA
The physicochemical properties of CHA based on Indonesian abalone mussel shells are shown in Figure 3. Based on the morphology shown in Figure 3a, CHA had a small agglomerate line and a solid structure. Moreover, the CHA particles were rounded, and more regular shapes appeared at sizes below 1 μm. Based on Figure 3b, the CHA had an average particle size of (0.2 ± 0.003) nm with the same percentage distribution of the sample size at 98.5%. The EDS analysis for CHA produced a Ca/P molar ratio of 1.73, as shown in Figure 3c, which approaches the natural bone, i.e., 1.71 (Sari et al., 2021 (a)). This result was expected for CHA because the carbonate ions switched with phosphate ions in its crystal structure (Desai, 2007; Sari et al., 2021 (c)).
FTIR analysis was used to identify the functional groups in CHA, namely PO43, CO32, and OH-. The FTIR test spectra exhibited that the fabricated CHA was the B-type CHA phase, as ensured by the appearance of CO32- stretching at 1,463.5 and 875 cm-1 (Figure 3(d)). PO43- bending and stretching were observed at 602–571 and 1,092–963 cm-1, respectively. The proceeds of the FTIR spectra show OH- stretching at 3,642 and 3,572 cm-1. Moreover, the presence of two OH- absorptions is due to the possibility that there is still a residual Ca(OH)2 precursor. The absorption of H2O and OH- bending was inspected at 1,629 cm-1 and 631 cm-1, respectively. The analysis of the DSC pattern can be seen in Figure 3e: CHA generated a fusion temperature of 438.96°C and an enthalpy of 33.82 j/g (exothermic). DSC analysis justified the OH- stretching in CHA, as shown in the FTIR data. The analysis of the DSC pattern can be seen in Figure 3e: CHA generated a fusion temperature of 438.96°C and an enthalpy of 33.82 j/g (exothermic). DSC analysis justified the OH− stretching in CHA shown in the FTIR data.
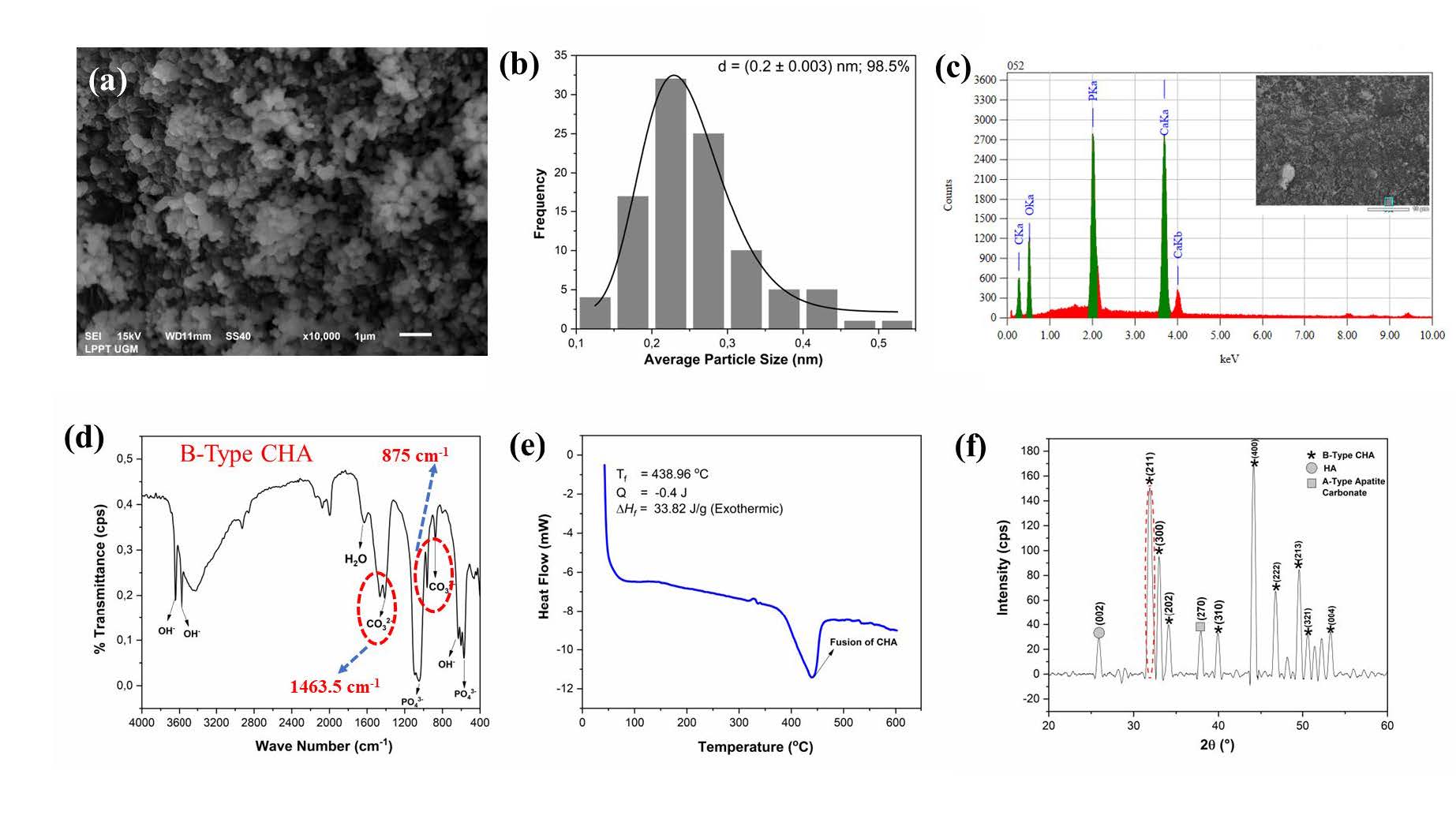
Figure 3. Physicochemical properties analysis of nano-CHA based on abalone mussel shells (a) morphology, (b) particle size distribution, (c) EDS, (d) FTIR spectra, and (e) temperature fusion, and (f) XRD pattern.
According to the XRD analysis (Figure 3f), the pattern formed had the crystal phase of B-type CHA, HA, and A-type apatite carbonate. The crystal phase of HA and A-type apatite carbonate emerged in the diffraction planes of (002) and (270), respectively. The properties of HA and CHA peaks were recognized according to previous research (Sari et al., 2021 (a)). The lattice parameters were computed as a = 9.67 Å and c = 6.84 Å. According to the XRD pattern, the exchange of carbonate ions into the HA lattice structure decreased the lattice parameter c. CHA resulted in crystallite size (s), microstrain (ε), and X-ray density (dx) of 21 ± 3 nm, 0.004, and 7.36 gr/cm3, respectively. In addition, the X-ray density value was appropriated to the lattice parameters of the CHA that displayed the atomic density per unit cell (Sari and Yusuf, 2018; Sari et al., 2021 (c)).
Morphological properties of nanofibrous PVA/gelatin/CHA
The morphology of PVA/Gelatin and PVA/Gelatin nanofibers with CHA 5 wt% can be seen in Figure 4. PVA/Gelatin nanofibers (Figure 4(a)) tend to have a fine morphology without beads. Obviously, the addition of CHA 5 wt% into the PVA/Gelatin matrix (Figure 4(b)) can increase the formation of agglomeration on nanofibers. However, the agglomeration of the PVA/Gelatin/CHA 5 wt% nanofiber scaffold can be neglected because it is still on the sub-micron scale (1 μm–100 nm) (Caldwell et al., 2022) . Moreover, the average fiber diameter decreased slightly from ~361 nm for PVA/Gelatin (Figure 4(c)) to ~265 nm for PVA/Gelatin/CHA 5 wt% (Figure 4(d)). Most gelatin nanofibers are in the range of 150-300 nm (Rashedi et al., 2021). These results follow the previous researcher who fabricated a polycaprolactone/chitosan nanofibers scaffold. The addition of CS (one of the biopolymers) decreased the diameter of nanofibers (Mosallanezhad et al., 2022).
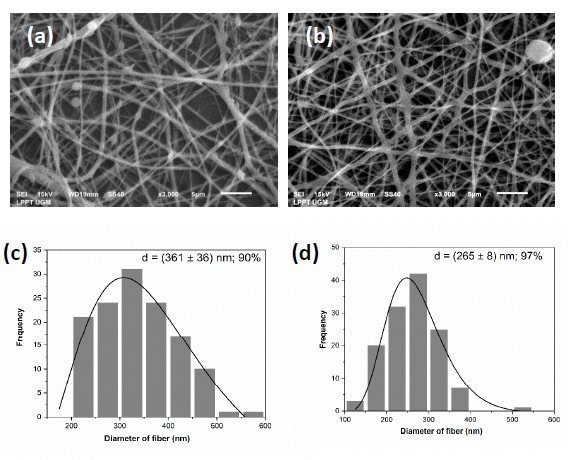
Figure 4. Representative SEM images of (a) PVA/gelatin and (b) PVA/Gelatin/CHA 5 wt% and fiber diameter distribution of (c) PVA/gelatin and (d) PVA/Gelatin/CHA 5 wt%.
DISCUSSION
The PVA/Gelatin nanofiber scaffolds are nano-sized, so electrospun fibers have the estimation of a high specific surface area, which is beneficial for BTE application (Perez-Puyana et al., 2018). The CHA nanoparticles were then incorporated into the PVA/Gelatin matrix to mimic bone structure at the nanoscale level (Januariyasa et al., 2020). In addition, the results of fiber diameter are within the range of fiber diameter (100–450 nm) in native bone ECM (Chaudhuri et al., 2016; Heydari et al., 2017; Januariyasa et al., 2020).
Introducing CHA nanoparticles may also form micro-capacitors, leading to a higher dielectric constant (Chaudhuri et al., 2016). The increase of these physical properties leads to a more substantial effect in the presence of electrical force produced by the electrical field between the needle intensity of the peaks of CHA relative to the broad peak of PVA/CS, as expected. The decrease in fiber diameter is probably caused by adding CHA nanoparticles with a higher solution conductivity (Chaudhuri et al., 2016; Heydari et al., 2017; Januariyasa et al., 2020) because CHA may be more conductive than PVA/Gelatin (Chaudhuri et al., 2016; Heydari et al., 2017; Januariyasa et al., 2020 (b)). CHA 5 wt% creates more CHA crystals in the nanofiber structure. In contrast, the PVA/gelatin peak decreased with increasing CHA content (Januariyasa et al., 2020.
The presence of CHA particles on nanofibers can disrupt the polymer macromolecular chains, causing a decrease in the number of polymer crystals (Kim et al., 2008). The interaction between the CHA particles and the polymer at the interface can also form an amorphous polymer layer (Sari et al., 2021 (c)). This interaction is caused by the hydrogen bonding of exposed hydroxyl groups on the CHA nanoparticle surfaces and the polymers (Januariyasa et al., 2020). Future research needs Fourier Transform Infrared (FTIR) spectroscopy to be characterized for deep analysis to know the chemical composition of PVA/Gelatin/CHA 5 wt%.It should be noted that the polymer chain near the CHA particles will crystallize when the composites are formed and cooled from the melt state, creating an interphase at the interface called trans crystalline (Zhang et al., 2009). The CHA crystals act as a nucleation agent and lead to more supercooling at the polymer/CHA interface due to the higher thermal conductivity of CHA compared to the polymers (PVA/Gelatin).
From a crystallization kinetics point of view, there is an energy competition between the entropic decrease from the low-order amorphous state to the high-order crystalline state and the surface energy minimization resulting from the reduced surface area. The crystal continues to grow until the system's free energy overcomes this energy barrier; the corresponding crystal size is called the critical size. If the nucleus size is less than the critical size, then the tension of the non-crystallized segments pulls the nucleus from the CHA surface; otherwise, the bonding of the CHA surface adheres to the nucleus, and the crystal continues to grow large (Zhang et al., 2009). This interaction also could reduce the crystallinity of the polymer (Januariyasa et al., 2020 (b)).
CONCLUSION
This study successfully fabricates CHA based on Indonesian abalone mussel shells with a molar ratio of Ca/P 1.73, which approaches the Ca/P molar ratio of natural bone. The synthesized CHA produced the same condition as B-type CHA, ensured by its crystallographic properties, morphology, the molar ratio of Ca/P, thermal properties, and chemical compositions. The CHA nanoparticles were incorporated and well dispersed in the PVA/CHA matrix, with negligible agglomeration. Adding CHA 5 wt% in the PVA/Gelatin matrix decreased the average fiber diameter because CHA may be more conductive than PVA/Gelatin. The diameter fiber results are within the fiber diameter range (100–450 nm) in native bone ECM.
ACKNOWLEDGEMENTS
The authors are immensely grateful to the Directorate of Research Universitas Gadjah Mada (UGM) and UGM Reputation Improvement Team towards World Class University - UGM Quality Assurance Office through Post-Doctoral Program 2023 (No: 3662/UN1.P.II/Dit-Lit/PT.01.03/2023) and Directorate of Research Universitas Gadjah Mada through RTA Program 2023 (No: 5075/UN1.P.II/Dit-Lit/PT.01.01/2023) for financially supporting this research. In addition, the authors would like to thank the Material Physics and Electronics Laboratory and the staff of Integrated Laboratory for Research and Testing Universitas Gadjah Mada, Indonesia, for supplying the facilities and technical assistance.
AUTHOR CONTRIBUTIONS
Mona Sari contributed to the study design, fabricated, and characterized PVA/Gelatin nanofiber scaffolds, drew all figures, interpreted the results, projected administration, drafted the manuscript, and revised the manuscript. Nilam Cahyati contributed to the fabrication and characterization of PVA/Gelatin nanofiber scaffolds and drafted the manuscript. Yusril Yusuf contributed to the study design, fabricated, and characterized PVA/Gelatin nanofiber scaffolds, drew all figures, interpreted the results, projected administration, drafted the manuscript, revised the manuscript, reviewed the manuscript, and supported the funding and resources. The authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Asran, A. S., Henning, S., and Michler, G. H. 2010. Polyvinyl alcohol-collagen-hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer. 51(4): 868–876.
Caldwell, J., Taladriz-Blanco, P., Lehner, R., Lubskyy, A., Ortuso, R.D., Rothen-Rutishauser, B., Petri-Fink, A. 2022. The micro- submicron- and nanoplastic hunt: A review of detection methods for plastic particles. Chemosphere. 293: 133514.
Chaudhuri, B., Mondal, B., Ray, S. K., and Sarkar, S. C. 2016. A novel biocompatible conducting polyvinyl alcohol (PVA)-polyvinylpyrrolidone (PVP)-hydroxyapatite (HAP) composite scaffolds for probable biological application. Colloids and Surfaces B: Biointerfaces. 143: 71–80.
Chen, P., Liu, L., Pan, J., Mei, J., Li, C., and Zheng, Y. 2019. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Materials Science and Engineering C. 97: 325–335.
Desai, A. Y. 2007. Fabrication and characterization of titanium-doped hydroxyapatite thin films. Dissertation (MSc). University of Cambridge.
Eysturskard, J., Haug, I. J., Ulset, A. S., and Draget, K. I. 2009. Mechanical properties of mammalian and fish gelatins based on their weight average molecular weight and molecular weight distribution. Food Hydrocolloids. 23(8): 2315–2321.
Ghazalian, M., Afshar, S., Rostami, A., Rashedi,S., and Bahrami, S.H. 2022. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 636: 128163.
Govindan, R., Gu, F. L., Karthi, S., and Girija, E. K. 2020. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Materials Today Communications. 22: 109865.
Heydari, Z., Mohebbi-Kalhori, D., and Afarani, M. S. 2017. Engineered electrospun polycaprolactone (PCL)/octacalcium phosphate (OCP) scaffold for bone tissue engineering. Materials Science and Engineering C. 81: 127–132.
Hoch, E., Hirth, T., Tovar, G. E. M., and Borchers, K. 2013. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. Journal of Materials Chemistry B. 1(41): 5675–5685.
Imani, S. M., Rabiee, S. M., Goudarzi, A. M., and Dardel, M. 2020. A novel modification for polymer sponge method to fabricate the highly porous composite bone scaffolds with large aspect ratio suitable for repairing critical-sized bone defects. Vacuum. 176: 109316.
Januariyasa, I. K., Ana, I. D., and Yusuf, Y. 2020. Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Materials Science and Engineering C. 107: 1–13.
Jo, I. H., Shin, K. H., Soon, Y. M., Koh, Y. H., Lee, J. H., and Kim, H. E. 2009. Highly porous hydroxyapatite scaffolds with elongated pores using stretched polymeric sponges as novel template. Materials Letters. 63(20): 1702–1704.
Kim, M. S., and Kim, G. 2014. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydrate Polymers. 114: 213–221.
Kim, G.M., Asran, A.S., Michler, G.H., Simon, P., and Kim, J.S. 2008. Electrospun PVA/HAp nanocomposite nanofibers: biomimetics of mineralized hard tissues at a lower level of complexity. Bioinspiration & Biomimetics. 3 (4): 046003.
Li, Y., Liao, C., and Tjong, S. C. 2019. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials. 9(4): 590.
Linh, N. T. B., and Lee, B. T. 2012. Electrospinning of polyvinyl alcohol/gelatin nanofiber composites and cross-linking for bone tissue engineering application. Journal of Biomaterials Applications. 27(3): 255–266.
Ma, P., Wu, W., Wei, Y., Ren, L., Lin, S., and Wu, J. 2021. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Materials & Design. 207: 109865.
Manavitehrani, I., Le, T. Y. L., Daly, S., Wang, Y., Maitz, P. K., Schindeler, A., and Dehghani, F. 2019. Formation of porous biodegradable scaffolds based on poly (propylene carbonate) using gas foaming technology. Materials Science and Engineering C. 96: 824–830.
Merkle, V. M., Tran, P. L., Hutchinson, M., Ammann, K. R., Decook, K., Wu, X., and Slepian, M. J. 2015. Core-shell PVA/gelatin electrospun nanofibers promote human umbilical vein endothelial cell and smooth muscle cell proliferation and migration. Acta Biomaterialia. 27: 77–87.
Merkle, V. M., Zeng, L., Slepian, M. J., and Wu, X. 2014. Core-shell nanofibers: Integrating the bioactivity of gelatin and the mechanical property of polyvinyl alcohol. Biopolymers. 101(4): 336–346.
Merkle, V., Zeng, L., Teng, W., Slepian, M., and Wu, X. 2013. Gelatin shells strengthen polyvinyl alcohol core-shell nanofibers. Polymer. 54(21): 6003–6007.
Mithieux, S. M., Wise, S. G., and Weiss, A. S. 2013. Tropoelastin - A multifaceted naturally smart material. Advanced Drug Delivery Review. 65 (4): 421–428.
Mosallanezhad, P., Nazockdast, H., Ahmadi, Z., and Rostami, A. 2022. Fabrication and characterization of polycaprolactone/chitosan nanofibers containing antibacterial agents of curcumin and ZnO nanoparticles for use as wound dressing. Frontiers in Bioengineering and Biotechnology. 10: 1027351.
Naghavi A., S., Moztarzadeh, F., Kargozar, S., Dodel, M., and Tahriri, M. 2015. Development of polyvinyl alcohol fibrous biodegradable scaffolds for nerve tissue engineering applications: In vitro study. International Journal of Polymeric Materials and Polymeric Biomaterials. 64(9): 474–480.
Nemati, S., Kim, S., Shin, Y.M., and Shin, H. 2019. Current progress in application of polymeric nanofibers to tissue engineering. Nano Convergence. 6: 36.
Perez-Puyana, V., Jiménez-Rosado, M., Romero, A., and Guerrero, A. 2018. Development of PVA/gelatin nanofibrous scaffolds for Tissue Engineering via electrospinning. Materials Research Express. 5(3): 035401.
Rashedi, S., Afshar, S., Rostami, A., Ghazalian, M., and Nazockdast, H. 2021. Co-electrospun poly(lactic acid)/gelatin nanofibrous scaffold prepared by a new solvent system: Morphological, mechanical and in vitro degradability properties. International Journal of Polymeric Materials and Polymeric Biomaterials. 70 (8): 545–553.
Sari, M., Ashilawati, A., Khoir, L. M., Wahyuningsih, R., and Yusuf, Y. 2023. Carbonated hydroxyapatite extracted from Indonesian’s eggshell biogenic wastes as bioceramic materials. Journal of Biomimetics, Biomaterials, and Biomedical Engineering. 62: 1–7.
Sari, M., Hening, P., Chotimah, Ana, I. D., and Yusuf, Y. 2021a. Bioceramic hydroxyapatite-based scaffold with a porous structure using honeycomb as a natural polymeric Porogen for bone tissue engineering. Biomaterials Research. 25(1): 2.
Sari, M., Hening, P., Chotimah, Ana, I. D., and Yusuf, Y. 2021b. Porous structure of bioceramics carbonated hydroxyapatite-based honeycomb scaffold for bone tissue engineering. Materials Today Communications. 26: 102135.
Sari, M., Kristianto, N. A., Chotimah, Ana, I. D., and Yusuf, Y. 2021c. Carbonated hydroxyapatite-based honeycomb scaffold coatings on a titanium alloy for bone implant application—physicochemical and mechanical properties analysis. Coatings. 11(8): 941.
Sawada, M., Sridhar, K., Kanda, Y., and Yamanaka, S. 2021. Pure hydroxyapatite synthesis originating from amorphous calcium carbonate. Scientific Reports. 11(1): 11546.
Soltani, M., Yousefpour, M., and taherian, Z. 2019. Porous fluorhydroxyapatite-magnesium-gelatin novel composite scaffold based on freeze-drying mechanism for bone tissue engineering application. Materials Letters. 244: 195–198.
Teixeira, M. A., Amorim, M. T. P., and Felgueiras, H. P. 2020. Poly(vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers. 12 (1): 1–33.
Teo, W. E., and Ramakrishna, S. 2009. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Composites Science and Technology. 69 (11–12): 1804–1817.
Wang, X., Ao, Q., Tian, X., Fan, J., Tong, H., Hou, W., and Bai, S. 2017. Gelatin-based hydrogels for organ 3D bioprinting. Polymers. 9 (9): 401.
Wang, X., Ding, B., and Li, B. 2013. Biomimetic electrospun nanofibrous structures for tissue engineering. Materials Today. 16 (6): 229–241.
Yang, S., Lei, P., Shan, Y., and Zhang, D. 2018. Preparation and characterization of antibacterial electrospun chitosan/poly (vinyl alcohol)/graphene oxide composite nanofibrous membrane. Applied Surface Science. 435: 832–840.
Youness, R. A., Taha, M. A., and Ibrahim, M. A. 2017. Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. Journal of Molecular Structure. 1150: 188–195.
Zhang, H., Lu, X., Leng, Y., Fang, L., Qu, S., Feng, B., Weng, J., and Wang, J. 2009. Molecular dynamics simulations on the interaction between polymers and hydroxyapatite with and without coupling agents. Acta Biomaterialia. 5: 1169–1181.
Zhou, H., and Lee, J. 2011. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomaterialia. 7 (7): 2769–2781.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Mona Sari1, Nilam Cahyati2, and Yusril Yusuf2, 3, *
1 Department of Physics Education, Faculty of Mathematics and Natural Science, Universitas Negeri Yogyakarta, Yogyakarta 55281, Indonesia.
2 Department of Physics, Faculty of Mathematics and Natural Science, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
3 Collaborative Research Center for Biomedical Scaffold, National Research and Innovation Agency of the Republic of Indonesia, Yogyakarta 55281, Indonesia.
Corresponding author: Yusril Yusuf, E-mail: yusril@ugm.ac.id
Total Article Views
Editor: Supon Ananta,
Chiang Mai University, Thailand
Article history:
Received: February 7, 2024;
Revised: May 13, 2024;
Accepted: May 20, 2024;
Online First: June 11, 2024

