
Nitric Oxide Inhibitory and Cytotoxic Activities of Spice Essential Oils. Nakuntwalai Wisidsri*, Suradwadee Thungmungmee, and Warachate Khobjai
Published Date : 2019-08-23
DOI : https://doi.org/10.12982/CMUJNS.2019.0026
Journal Issues :
Number 3 , July-September 2019
ABSTRACT
This study aims to investigate the effects of anise oil, lemongrass oil and cassia oil on nitric oxide production from nitric oxide donor and stimu- lated macrophage cells. Furthermore, this study also evaluates the cytotoxic effect of essential oils on cell viability of macrophage and human colorectal cancer cells. The results showed that anise oil and lemongrass oil presented higher nitric oxide scavenging capacity by reduction of nitrite formation from NO donor with IC50 of 406.90 and 413.50 µg/ml. In vitro study, cassia oil pre- sented lower NO scavenging capacity. For cellular study, lemongrass oil and cassia oil at concentration of 6.25-25 µg/ml and all concentration of anise oil (6.25-100 µg/ml) presented NO inhibitory activity with no cytotoxic effect of the macrophages. For the cancer cell study, lemongrass oil and cassia oil reduced cell viability of human colorectal cancer cells after 48 h of treatment with IC50 of 77.91 and 32.72 µg/ml, and IC50 was better in 72 h of treatment with 67.96 and 21.94 µg/ml. Nevertheless, anise oil displayed insignificant ef- fect on HT-29 cell viability. Anethole, citral and cinnamaldehyde were iden- tified as main composition of anise oil, lemongrass oil and cassia oil using gas chromatography-mass spectrometry (GC-MS). The results from this study suggested the different effects of essential oils on nitric oxide inhibition of in vitro and cellular study as well as the cytotoxic effect to macrophage and colorectal cancer cell. These results are beneficial for further study of anise oil, cassia oil and lemongrass oil in pharmaceuticals and natural therapies.
Keywords: Anise oil, Lemongrass oil, Cassia oil, Nitric oxide, Macrophage, Human colorectal cancer
INTRODUCTION
Nitric oxide (NO) is one of oxidative molecule which involved in in- flammatory processes and cancer development. Nitric oxide acts as a signaling molecule in the normal physiological control. However, under hypoxic condi- tions, NO is produced by the mitochondrial respiratory chain which can gen- erate other reactive nitrogen species (RNS). The excess production of NO causes of oxidative stress and chronic inflammation which further associated to increase the risk of several human cancer development (Reuter et al., 2010).
Nitric oxide (NO) is accumulated in chronic inflammatory processes by phagocytic cells, including macrophages. Excessive NO interacts with O2- to contribute cytotoxic oxidant peroxynitrite (ONOO-), which is a pow- erful oxidant to initiate lipid peroxidation and cleavage DNA, resulting in the risk of cancer (Choudhari et al., 2013). NO presents different roles in the development stage of cancers, including functions as a signaling mol- ecule in the regulation of cancer formation, progression and metastasis as well as application in cancer therapy. However, high level of NO modulates matrix metalloproteinase (MMP) expression in malignant cells to support tumor cell invasion in the cancer invasive stage. This event is supported by the previous study that found the angiogenesis in tumor cell was suppressed afterwards the inhibition of nitric oxide production (Cheng et al., 2014).
Three of spice essential oils (anise oil, lemongrass oil and cassia oil) have been demonstrated the pharmacological activities. Anise oil is separated from star anise (Illicium verum Hook. fil.) and commonly used in cooking and utilization for antifungal agents in medical and food (Matan et al., 2012). Anise oil and anise seed extract also presented anti-proliferative effect on gastric cancer cells (Rahamooz-Haghighi & Asadi, 2016).
Lemongrass oil is the volatile oil from Cymbopogon citratus D.C. (C. citratus) which has been used as a remedy for the treatment of various health conditions. In Asia and Africa, lemongrass oil has been used for pain and inflammation treatment, e.g. backache, sprains and anti-rheumatic as well as antiseptic and antitussive (Boukhatem et al., 2014). The pharmacological prop- erties of C. citratus have been exhibited antimicrobial and antifungal (Adukwu et al., 2016; Liakos et al., 2016) and anti-proliferative effect on human colon carcinoma (HCT-116), breast carcinoma (MCF-7 and MDA-MB 231), ovarian carcinoma (SKOV-3 and COAV), and a normal liver cell line (WRL 68) (Halabi & Sheikh, 2014) and HeLa and ME-180 cervical cell line (Ghosh, 2013).
Cassia oil is the volatile oil from the leaves, branches or barks of Cinnamomum cassia Blume (Lauraceae) and has been traditionally used as a spice and aromatic (Geng et al., 2011). The pharmacological activity of cassia oil were possessed antimicrobial (Ooi et al., 2006; Kavanaugh & Ribbeck, 2012; Swamy et al., 2016), antioxidant (Lin et al., 2003; Yang et al., 2012), cytotoxic and mutagenic effect in human HeLa epithelioid cervical cancer, A549 lung cancer, SK-OV-3 ovarian cancer, SK-MEL-2 melanoma, XF-498 central nerve system solid tumor, and HCT-15 colon tumor cell lines (Lee et al., 2004), and anti-inflammatory (Lee et al., 2005; Pannee et al., 2014) properties.
This research proposes to investigate the effects of anise oil, lemongrass oil and cassia oil in inhibition of nitric oxide the key molecule which involving in oxidative stress, inflammation and human colorectal cancer progression. Fur- thermore, this research also examines cytotoxicity of anise oil, lemongrass oil and cassia oil in RAW264.7 macrophage cells and HT-29 human colorectal cancer cells.
MATERIALS AND METHODS
Materials
Anise oil, lemongrass oil and cassia oil (leaves of Cinnamomum cassia) were obtained from Thai-China Flavours and Fragrance Industry Co., Ltd., (Bangkok, Thailand).
Nitric oxide (NO) radical scavenging ability of anise oil, lemongrass oil and cassia oil
In this research, sodium nitroprusside (SNP) (Sigma-Aldrich, USA) was used as the nitric oxide donor. Specifically, 10 mM of SNP in a phosphate buf- fer saline pH 7.4 (PBS pH 7.4) solution was incubated with 1 ml of variable concentrations of anise oil, lemongrass oil and cassia oil (62.5, 125, 250, 500, 1,000 µg/ml) at 25 ºC for 180 min. Approximately 100 µl of the testing solution was withdrawn to react with a Griess Reagent kit (Promega, USA) whereby the solution was reacted with 20 µl sulfanilamide for 10 min and then 20 µl N-1-napthylethylenediamine dihydrochloride for another 10 min. The reaction mixture absorbance was measured at 560 nm and the nitric oxide (NO) con- centrations were determined as the nitrite (NO2-) concentrations from the stan- dard curve of a standard nitrite solution. The 0.2% ethanol and L-ascorbic acid were used as the negative and positive controls, respectively. The NO scavenging capacity of anise oil, lemongrass oil and cassia oil were expressed as a percentage of nitrite formation inhibition using the following formula:
% NO2- formation inhibition = [(NO2- without essential oil – NO2- with
essential oil) / NO2- without essential oil] x 100
Cell culture
RAW264.7 murine macrophage cell line and HT-29 human colorectal cancer cell line were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA) and 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco, USA) and incubated at 37ºC in 5% CO2 / 95% air humidified incubator. The RAW264.7 cell was subcultured using cell scraper twice a week and HT-29 cell was subcultured using 0.25% trypsin-EDTA (Gibco, USA) trypsinization. The cell viability was determined using 0.4% trypan blue (Sigma-Aldrich, USA) with cell viability > 85% was used in all the experiments.
Determination of NO production in lipopolysaccharide (LPS)-stimulated macrophage
The RAW264.7 murine macrophage cells at 2x105 cell/ml were plated in 96-well plate for 24 h. The plated-cell were pretreated with various concen- trations of anise oil, lemongrass oil and cassia oil (6.25, 12.5, 25, 50 and 100 µg/ml) and incubated for another 24 h. The treated cells were stimulated with 1 µg/ml LPS and incubated for another 24 h. The NO concentrations were de- termined from nitrite (NO2-) in the stimulated-cell supernatant using a Griess reagent kit whereby 100 µl of the supernatant was reacted with 20 µl sulfanil- amide for 10 min and then with 20 µl N-1-napthylethylenediamine dihydro- choride for another 10 min. The reaction mixture absorbance was measured at 560 nm and the nitric oxide (NO) concentrations were determined as the nitrite (NO2-) concentrations from the standard curve of a standard nitrite solu- tion. The treated cell with 0.02% ethanol and 100 µM of dexamethasone each with 1 µg/ml LPS were respectively used as the negative and positive controls.
Determination of cell viability of RAW264.7 murine macrophage cells
The viability of the residual macrophage cells after the NO assay, given anise oil, lemongrass oil and cassia oil concentrations of 6.25, 12.5, 25, 50 and 100 µg/ml, were determined by resazurin (Sigma-Aldrich, USA) reduction assay, whereby the residual cells were incubated for 2 h at 37°C in 100 µl fresh DMEM containing 50 µg/ml resazurin. The reaction mixture absorbance was determined at 560 against 600 nm.
Determination of cell viability of HT-29 human colorectal cancer cells
The HT-29 cells at 5x104 cells/ml were plated in 96-well plates for 24 h. The cells were treated with various concentrations of anise oil, lemongrass oil and cassia oil at a concentration of 6.25, 12.5, 25, 50 and 100 µg/ml for another 48 and 72 h. The cell viability was evaluated by adding 50 µg/ml resazurin solution into each well. The reaction mixture absorbance was determined at 560 against 600 nm.
The cell viability of the RAW264.7 murine macrophage and HT-29 human colorectal cancer cells were presented as percentage cell viability by using the following formula:
% Cell viability = [(OD560 – OD600)sample / (OD560 – OD600)control] x 100
Analysis of essential oils
GC-MS analysis for essential oil compositions was performed on an Agilent 7890 GC system instrument equipped with HP-5MS (5% diphenyl 95% dimethylpolysiloxane) column (30 m x 0.25 mm x 0.25 µm) and interfaced to a 5975C inert XL MSD with Triple-Axis Detector. An injection volume of 5 µl was employed (split ratio of 20:1) injector temperature 110ºC. The column tempera- ture was increased from 110ºC (hold 1 min.) to 250ºC (hold 1 min.) in a rate 5ºC/ min. The outlet temperature was 250ºC. Mass spectra were taken at 70 eV and fragments from 40 to 700 m/z. MS transfer line temperature 250ºC. Identifica- tion of compounds was conducted using the database of the National Institute of Standards and Technology (NIST) library. The name, molecular weight, molecular formula, and area under the peak of the test material components were ascertained.
Data analysis
In this research, the experiments contain the NO scavenging ability, given anise oil, lemongrass oil and cassia oil concentration of 62.5, 125, 250, 500, 1,000 µg/ml; and the NO production and cell viability, given anise oil, lemongrass oil and cassia oil concentration of 6.25, 12.5, 25, 50 and 100 µg/ml; and the cytotox- icity of HT-29 cells, given anise oil, lemongrass oil and cassia oil concentration of 6.25, 12.5, 25, 50 and 100 µg/ml. Each experiment was carried out in tripli- cate. The statistical data were expressed as mean ± standard error of mean (SEM). The negative control group (ethanol) was compared against the experimental group using one-way ANOVA with Tukey’s Honestly Significant Difference (HSD) post hoc test, with the 5% (P<0.05) and 1% (P<0.01) significance levels.
RESULTS
Nitric oxide scavenging ability
This research found that NO scavenging capacity of anise oil, lemongrass oil and cassia oil were presented in the concentration of 62.5-1,000 µg/ ml (62.5, 125, 250, 500, 1,000 µg/ml). In the absence of essential oils, the negative control (0.2% ethanol) exhibited a high NO2- concentration while L-ascorbic acid (the positive control) inhibited the NO2- formation.
The results demonstrated that anise oil and lemongrass oil significantly reduced NO2-, achieving the scavenging performance in the range of 14.70±3.25 - 68.45±3.74% and 14.17±2.50 - 81.83±1.45%, respectively (Figure 1); and an IC50 of 406.90 and 413.50 µg/ml, respectively (Table 1), where IC50 is the inhibitory concentration at which the NO radicals are scavenged by 50%. Cassia oil revealed the percentage NO2- inhibition in the range of 3.44±2.19- 14.17±2.50%. The results revealed that anise oil and lemongrass oil showed higher percentage of NO2- inhibition while cassia oil presented lower per- centage of NO2- inhibition. By comparison, L-ascorbic acid (the positive con- trol) at 500 µg/ml achieved the NO2- inhibition performance of 98.22±0.85%.
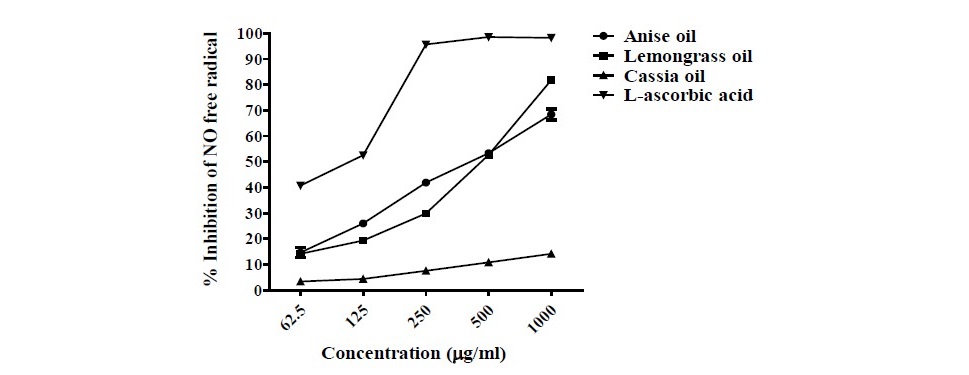
Figure 1. The NO (nitrite) inhibition efficiency (%) under variable essential oils (anise oil, lemongrass oil and cassia oil) concentrations, com- pared with essential oil-free ethanol (the negative control). The values are means ± SEM. L-ascorbic acid is used as the positive control.
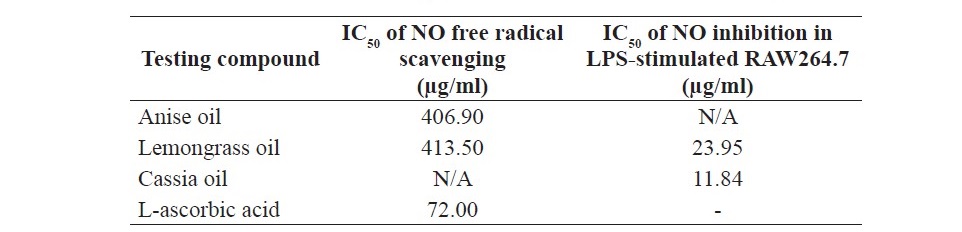
Table 1. The concentrations of anise oil, lemongrass oil, cassia oil and L-ascorbic acid at NO radical was scavenged 50% (IC50) and the concentrations of anise oil, lemongrass oil and cassia oil at NO production in LPS-stimu- lated RAW264.7 macrophage cells were inhibited 50% (IC50).
Nitric oxide inhibition and cell viability of LPS-stimulated RAW264.7 macrophage cells
Nitric oxide is commonly parameter which used as a marker for macro- phage activation. In this research, the RAW264.7 macrophage cells were treated with anise oil, lemongrass oil and cassia oil of variable concentrations (6.25, 12.5, 25, 50 and 100 µg/ml) for 24 h prior to stimulate with 1 µg/ml LPS. Furthermore, 0.02% ethanol and 100 µM of dexamethasone each with 1 µg/ml LPS were respec- tively used as the negative and positive controls. In Figure 2a, the experimental results demonstrated that the nitrite (NO2-) concentration in the LPS-stimulated macrophage were reduced in a concentration dependent manner. The NO2- in- hibition was presented with a higher performance in cassia oil-treated cells.
In Figure 2b, the cell viability of lemongrass oil- and cassia oil-treated cells are significantly reduced where the concentration of lemongrass oil and cassia oil reached 50 µg/ml while the cell viability of anise oil-treated cells was similar to that of control (0.02% ethanol). However, given the non- cytotoxicity of essential oils, the essential oil-treated cells could achieve high NO inhibition performance (lower nitrite concentrations) with the cell viability was similar to the control (0.02% ethanol). Therefore, NO inhibition performance with non-cytotoxicity was presented at a concentration of 6.25, 12.5 and 25 µg/ml of lemongrass oil- and cassia oil-treated cells.
Anise oil significantly reduced NO2-, achieving the NO inhibition performance with non-cytotoxicity in the range of 18.69±0.5 – 41.12±0.67% with concentration of 6.25, 12.5, 25, 50 and 100 µg/ml. Lemongrass oil and cassia oil significantly reduced NO2-, achieving the NO inhibition performance with non-cytotoxicity in the range of 6.76±1.74 – 47.59±2.15% and 12.79±0.34 – 80.49±0.34%, respectively (Figure 2a). An IC50 of lemongrass oil and cassia oil were 23.95 and 11.84 µg/ml, respectively (Table 1), where IC50 is the inhibitory concentration at which the NO is inhibited by 50%. By comparison, dexamethasone (the positive control) at 100 µM achieved the NO inhibitory performance of 64.50±0.85%.


Figure 2. The effects of variable anise oil, lemongrass oil and cassia oil concen- trations in 1µg/ml LPS-stimulated RAW264.7 macrophage: (a) nitrite concentrations (NO production), (b) cell viability. The values are means ± SEM and ** denote P<0.01. 0.02% ethanol and 100 µM of dexa- methasone are respectively used as the negative and positive controls.
Cell viability of HT-29 human colorectal cancer cells
In this research, the HT-29 human colorectal cancer cells were treated with anise oil, lemongrass oil and cassia oil of variable concentrations (6.26, 12.5, 25, 50 and 100 µg/ml) for 48 h and 72 h. In addition, 0.02% ethanol was used as negative control. The reduction of cell viability presented cytotoxici- ty performance of the spice essential oils to the human colorectal cancer cells. In Figure 3, the results revealed that the cell viability of lemongrass oil- and cassia oil-treated cell were reduced in a concentration dependent manner.
HT-29 human colorectal cancer cells were treated with the spice essential oils for 48 and 72 h. The cell viability was reduced significantly in lemongrass oil- and cassia oil-treated cells. Anise oil treated-cell affected insignificantly to HT-29 cell viability.
Lemongrass oil-treated cell for 48 h presented significant cytotoxicity at concentrations of 50 and 100 µg/ml, achieving the reduction of the cell viability in the range of 35.13±8.08 – 69.08±0.49%, compared with the negative control (0.02% ethanol). The cytotoxicity of lemongrass oil treated-cell was presented with a higher performance in 72 h. The cell viability of lemongrass oil-treated cell for 72 h were lower than that of 48 h treated-cell with presented significantly cytotoxicity at 25, 50 and 100 µg/ml, achieving the reduction of the cell viability in the range of 29.58±5.21 - 73.44±3.11%. However, the cell viability is not significantly different between 48 and 72 h of treatment (Figure 3). An IC50 of lemongrass oil-treated cell are 77.91 and 67.96 µg/ml for 48 and 72 h of treatment, respectively, where IC50 is the concentration at which the cell viability is reduced by 50% (Table 2).
The results demonstrated that the cytotoxicity was presented with a higher performance in cassia oil-treated cell. Cassia oil-treated cell for 48 h and 72 h presented cytotoxicity at a concentration of 12.5, 25, 50 and 100 µg/ml. After treating cell for 48 h, the cell viability of HT-29 cells was significantly reduced where compared with the negative control in the range of 25.29±8.94 - 54.88±11.52%. Cassia oil-treated cell for 72 h at a similar concentration of 48 h (25, 50 and 100 µg/ml) presented a reduction of cell viability in the range of 6.59±3.31 - 47.46±13.306% (Figure 3). The cytotoxicity was presented with a higher performance in 72 h of cassia oil treated-cell, however, the results were not significantly different between 48 and 72 h of treatment. An IC50 of cassia oil-treated cell for 48 and 72 h were 32.72 and 21.94 µg/ml, respectively (Table 2).

Figure 3. The effects of variable anise oil, lemongrass oil and cassia oil concentrations treated cell for 48 and 72 h on the cell viability of HT- 29 human colorectal cancer cells. The values are means ± SEM. * and ** respectively denote P<0.05 and P<0.01. 0.02% ethanol are used as the negative control.
Table 2. The concentrations of anise oil, lemongrass oil, cassia oil at HT-29 cell viability was reduced 50% (IC50).

Compositions of anise oil, lemongrass oil and cassia oil
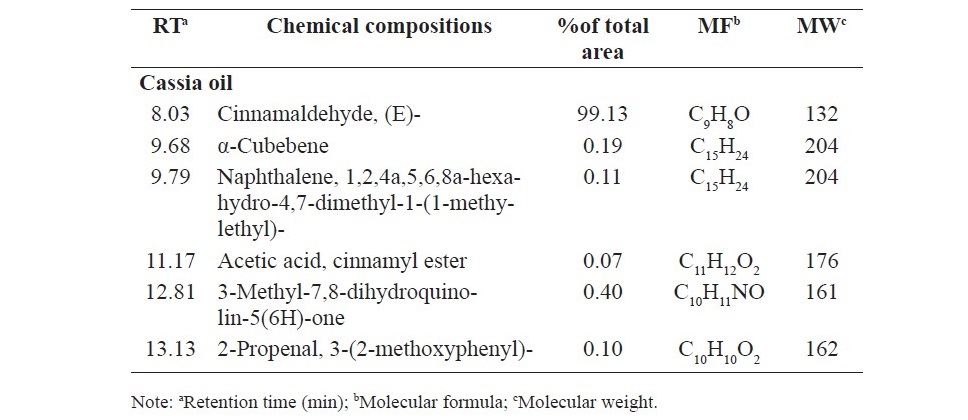
Chemical analysis of the components of anise oil, lemongrass oil and cassia oil by GC/MS led to identification by 13, 22 and 6 components, respectively. The main component of anise oil was anethole (95.72%), the main component of lemongrass oil was citral (47.91%) and β-citral (44.90%), and the main component of cassia oil was cinnamaldehyde (99.31%) (Table 3).
Table 3. Chemical compositions of anise oil, lemongrass oil and cassia oil.
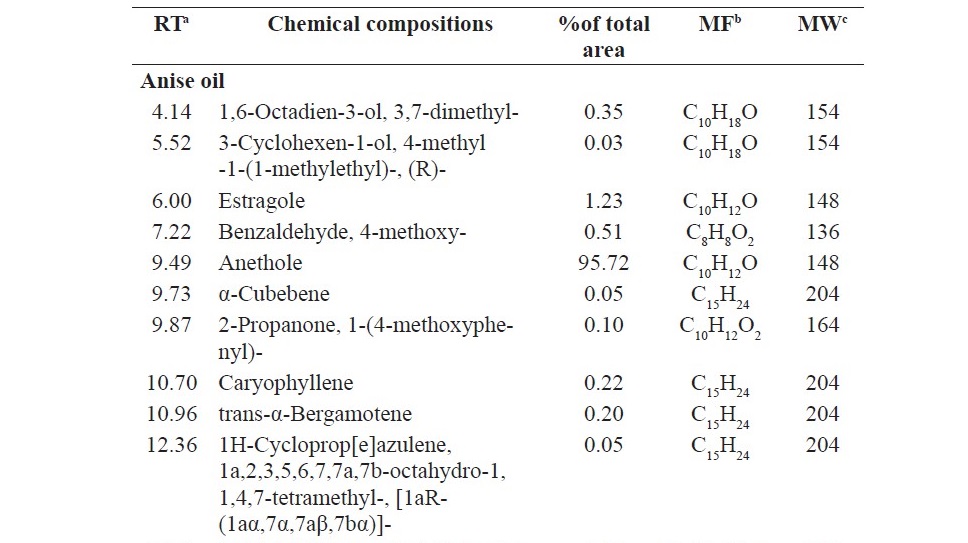
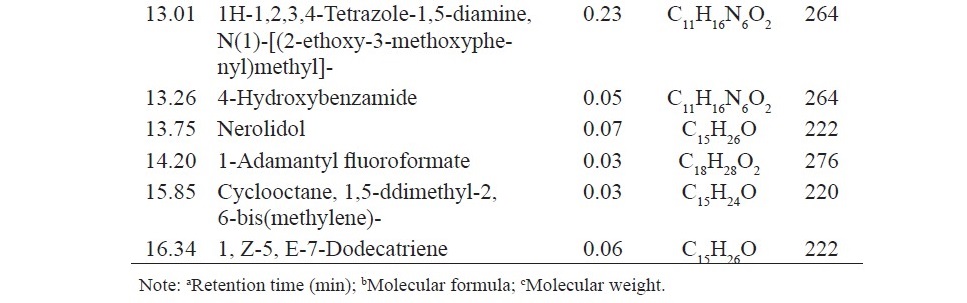
Table 3. Continued.
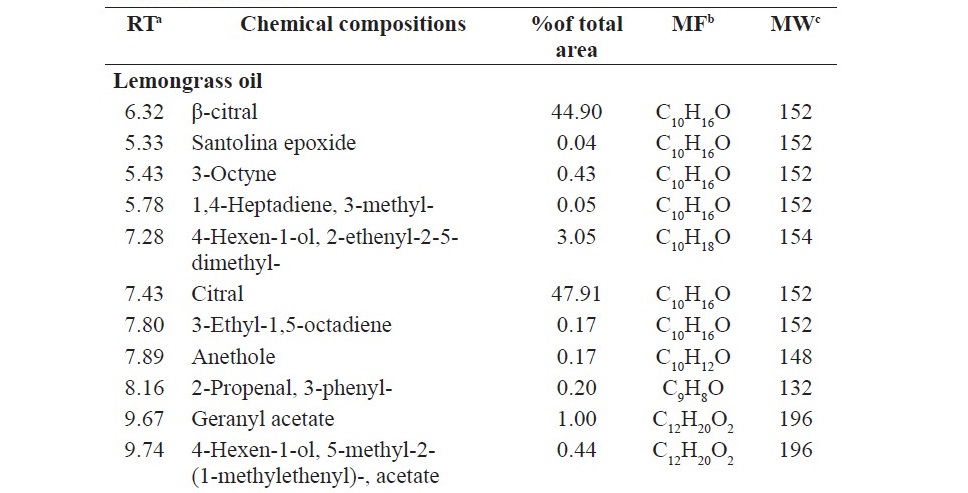
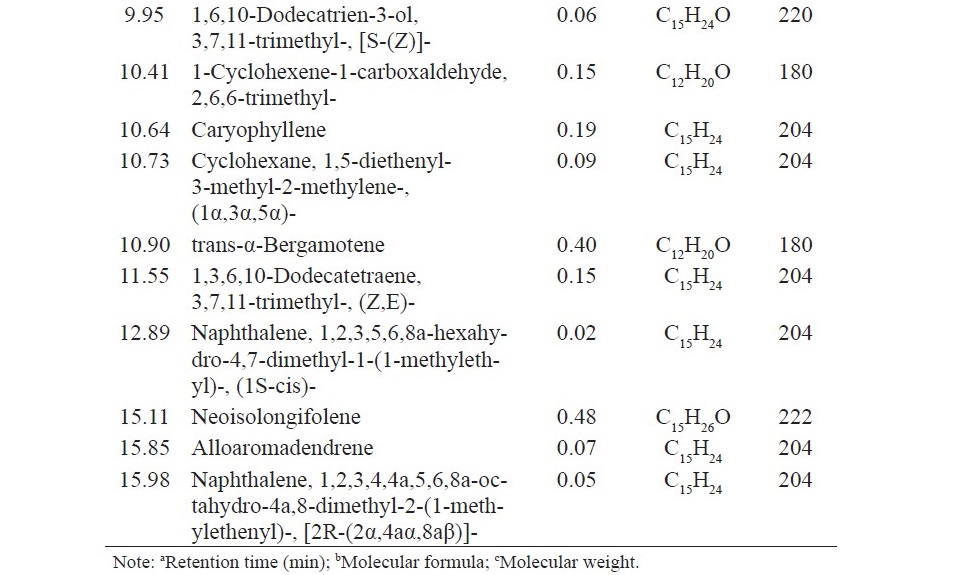
Table 3. Continued.
DISCUSSION
Nitric oxide (NO) is a free radical which produced during inflammatory processes by inducible nitric oxide synthase (iNOS) in macrophage cells. In general, NO functions in the regulation of defense responses against various pathogens (Joo et al., 2014). The excessive production of NO is found in chronic inflammation. The high concentration of NO reacts with oxygen and superoxide to produce peroxynitrite (ONOO-). Peroxynitrite is a powerful oxidant which reacts with proteins, lipids and DNA. The reaction of peroxynitrite induces oxidative and nitrosative changes in lipids resulting in peroxidation and DNA damage (Islam et al., 2015).
In general, nitric oxide scavengers compete with oxygen and decrease the NO production. The NO concentration is determined as nitrite (NO2-) concentra- tion which is a stable and a nonvolatile breakdown product of NO by using the Griess reagent kit. The experimental results demonstrated the ability of anise oil, lemongrass oil and cassia oil in scavenging NO free radical. These spice essential oils decreased nitrite formation from sodium nitroprusside (SNP), the nitric oxide donor. Therefore, anise oil, lemongrass oil and cassia oil may stand as NO scaven- ger. Especially, anise oil and lemongrass oil significantly reduced NO2- to achieve the NO scavenging performance which is remarkable as antioxidant agents.
In addition, macrophages produce inflammatory mediators, including NO in response to LPS. NO plays important roles in the inflammatory response.
However, the overproduction of NO can lead to cytotoxicity and chronic inflam- mation (Dzoyem et al., 2015). The unnecessary NO and inflammatory mediators are produced by continuously activated macrophages in chronic inflammatory processes. Chronic inflammation is associated with disease of cardiovascular, neurodegenerative disease and cancer (Okin & Medzhitov., 2012). Consequently, macrophage has been recognized as a target of anti-inflammatory treatment.
Anise oil, lemongrass oil and cassia oil also presented NO inhibitory effect on LPS-stimulated macrophage cells. In this current research, anise oil, lemongrass oil and cassia oil effectively inhibited the NO production in macro- phage. However, either lemongrass oil- or cassia oil-treated cell presented cytotoxicity in the macrophage cells when the concentration reached gather than 25 µg/ml while anise oil presented non-cytotoxicity although the concentration reached to 100 µg/ml. Therefore, it’s interesting that anise oil, lemongrass oil and cassia oil may be considered as an effective anti-inflammatory agent when applies in appropriate concentration to inhibit nitric oxide production from activated macrophage cells.
The cellular anti-inflammatory in macrophage cells of lemongrass oil in this current research according to previous study where lemongrass oil is presented anti-inflammatory activity in human fibroblast cells (Xuesheng and Parker, 2017). Lemongrass oil also presented anti-inflammatory in the animal model research where reduced skin inflammation in mice (Boukhatem et al., 2014). These results confirm that anti-inflammatory activity of lemongrass oil.
Cassia oil exhibits higher nitric oxide inhibition performance in acti-vated macrophage to present anti-inflammatory property. Cassia oil has been reported the anti-inflammatory property as well as its main active compound, cinamaldehyde. Both of cassia oil and cinnamaldehyde suppressed NO and proinflammatory cytokine production in activated macrophage. The previous study demonstrated that cassia oil and cinnamaldehyde inhibited IL-1β, IL-6, TNF-α, MCP-1, MIP1-α and COX-2 and PGES-1 expression in activated J774A.1 macrophage cell at a concentration of 1-20 μg/ml and revealed non- cytotoxicity to the cells by these concentrations (Pannee et al., 2014). This current study confirms that the inhibition of NO production in LPS-stimulated RAW264.7 macrophage cells to indicate anti-inflammatory property of cassia oil.
Chronic inflammation is also presented to link with different stages of cancer development, including stage of initiation, promotion, malignant conversion, invasion and metastasis (Grivennikov et al., 2010). As well as NO has been reported dual effects in both tumor growth promotion and tumoricidal effects. NO is the secondary messenger in signaling processes of cell apoptosis. In the other way, NO is involved in the invasive stage of cancer development. NO obtained from chronically inflamed tissue may lead to car- cinogenesis. Previous studies found that inflammation posed a risk factor of colorectal cancer progression. The overexpression of iNOS, an enzyme gen- erates NO production, was found to contribute progression of colon cancer (Wenzel et al., 2003). In addition, chronic inflammation-derived NO causes human colonic adenoma cell alteration into adenocarcinoma cells (Tazawa et al., 2013). Therefore, scavenging of nitric oxide formation and inhibition of nitric oxide production in macrophage are proposed to suppress chronic inflammation which may also useful in suppression of cancer development.
This study also displayed the cytotoxic effect of lemongrass oil and cassia oil on HT-29 human colorectal cancer cells. Lemongrass oil and cassia oil significantly reduced cell viability of HT-29 human colorectal cancer cells at concentrations of 25, 50 and 100 µg/ml and 12.5, 25, 50 and 100 µg/ml, respectively. These results suggested that lemongrass oil and cassia oil may involve in suppression of human colorectal cancer by inhibiting the factor of cancer progression and cytotoxic directly to the cells.
In addition, this current research provided an appropriate concentration of lemongrass oil and cassia oil were to be used for further study. Lemongrass oil- and cassia oil-treated cells can achieve high cytotoxicity performance in hu- man colorectal cancer cells, however, it’s also against macrophage cell viability. Consequently, it may concern of use for avoidance of normal cell cytotoxicity.
Findings from several studies in anticancer of lemongrass oil has been reported cytotoxicity in several cancer cells, such as anti-proliferative effect on human colon carcinoma (HCT-116), breast carcinoma (MCF-7 and MDA- MB 231), ovarian carcinoma (SKOV-3 and COAV) (Halabi & Sheikh, 2014) and HeLa and ME-180 cervical cell line (Ghosh, 2013). As well as cassia oil and cinnamaldehyde were also reported cytotoxicity in some cancer cells, such as human oral squamous cell carcinoma (HSC-3) (Chang et al., 2017), head and neck squamous cell carcinoma (FaDu, Detroit-562 and Scc-25 cells) (Yang et al., 2015). Furthermore, the derivative of cinnamaldehyde, 2’-hydrocycin- namaldehyde has been reported the growth inhibition effect on SW620 colon cancer cell via inactivation of AP-1 signaling (Lee et al., 2007). The results from this research extend to present anticancer property of lemongrass oil and cassia oil in another cancer cell, HT-29 human colorectal cancer.
The present research showed that anise oil contains mainly anethole 95.72% according to previous study which reported in Aly et al. (2016). Further- more, this current study also showed that lemongrass oil contains mainly citral and β-citral 47.91% and 44.90%, respectively as well as that was found in Katsukawa et al. (2010). In addition, cassia oil in this research contains mainly cinnamaldehyde 99.13% as well as those was found in previous study (Pannee et al., 2014). The variation of the main compound in each study may be due to difference in parts of the plant, geographical region, ages of the plant, harvest seasons, and method of oil extraction.
CONCLUSION
This experimental research has evaluated nitric oxide inhibition property of variable concentrations of anise oil, lemongrass oil and cassia oil through scavenging ability of NO free radical and inhibition of NO production in the LPS-stimulated RAW264.7 macrophage cells and cell viability; and the cytotox- icity through the reduction of the cell viability of HT-29 human colorectal cancer cells; and identified the compositions. The experimental results revealed that the scavenging performance was presented higher capacity in anise oil and lemon- grass oil, respectively. While the inhibition of NO production (presented as the nitrate concentrations) in the LPS-stimulated macrophage cells was presented in anise oil-, lemongrass oil- and cassia oil-treated RAW264.7 macrophage cells. The non-cytotoxicity in macrophage cell is presented in all concentration of anise oil (6.25-100 µg/ml) and a concentration of 6.25, 12.5 and 25 µg/ml of lemongrass oil and cassia oil. The cytotoxicity in HT-29 human colorectal cancer cells is pre- sented at a concentration of 12.5, 25, 50 and 100 µg/ml where the cell viability of the treated cell was reduced different from the control. The HT-29 cytotoxicity was presented a higher performance in cassia oil- and lemongrass oil-treated cell. These results suggested that the concentration of lemongrass oil and cassia oil above 25 µg/ml may impact to the normal cells (presented cytotoxicity in macrophage cells). Furthermore, the experimental results revealed that the main compound of anise oil, lemongrass oil and cassia oil were anethole, citral and cinnamaldehyde, respectively. The findings demonstrate the usefulness of spices containing essential oils as radical scavenger, anti-inflammatory agents and herb- based anticancer pharmaceuticals targeting the inhibition of NO production.
ACKNOWLEDGEMENTS
The authors would like to deep gratitude to Thai Traditional Medicine College, Rajamangala University of Technology Thanyaburi (RMUTT) and the Department of Pharmacology, Faculty of Medicine, Chulalongkorn University for funding and laboratory support. Appreciation also goes to Assistant Professor Dr. Wacharee Limpanasitthikul, for her kindly advices.
REFERENCES
Adukwu, E.C., Bowles, M., Edwards-Jones, V., and Bone, H. 2016. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Applied Microbiology and Biotechnology. 100(22): 9619-9627. https://doi.org/10.1007/s00253-016- 7807-y
Aly, S., Sabry, B., Shaheen, M., and Hathout, A. 2016. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. Journal of the Saudi Society of Agricultural Sciences. 15: 20-27. https://doi.org/10.1016/j.jssas.2014.05.003
Boukhatem, M.N., Ferhat, M.A., Kameli, A., Saidi, F., and Kebir, H.T. 2014. Lemon grass (Cymbopogon citratus) essential oil as a potent anti- inflammatory and antifungal drugs. Libyan Journal of Medicine.9. https://doi.org/10.3402/ljm.v9.25431
Chang, W.L., Cheng, F.C., Wang, S.P., Chou, S.T., and Shih, Y. 2017. Cinnamomum cassia essential oil and its major constituent cin- namaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Environmental Toxicology. 32(2): 456-468. https://doi.org/10.1002/tox.22250
Cheng, H.W., Wang, L., Mollica, M., Re, A.T., Wu, S.Y., and Zuo, L. 2014. Nitric oxide in cancer metastasis. Cancer Letters. 353(1): 1-7. https:// doi.org/10.1016/j.canlet.2014.07.014
Choudhari, S.K., Chaudhary, M., Bagde, S., Gadbail, A.R., and Joshi, V. 2013. Nitric oxide and cancer: A review. World Journal of Surgical Oncology. 11: 118. https://doi.org/10.1186/1477-7819-11-118
Dzoyem, J.P., Tsamo, A.T., Melong, R., Mkounga, P., Nkengfack, A.E., McGaw, L.J., and Eloff, J.N. 2015. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biological Research. 48: 57. https://doi.org/10.1186/s40659-015-0049-0
Geng, S.L., Cui, Z.X., Huang, X.C., Chen, Y.F., Xu, D., and Xiong, P. 2011. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Industrial Crops and Products. 33(1): 248-252. https://doi.org/10.1016/j.indcrop.2010.10.018
Ghosh, K. 2013. Anticancer effect of lemongrass oil and citral on cervical cancer cell lines. Pharmacognosy Communications. 3(4): 41-48. https://doi.org/10.5530/pc.2013.4.6
Grivennikov, S.I., Greten, F.R., and Karin, M. 2010. Immunity, inflammation, and cancer. Cell.140(6): 883-899. https://doi.org/10.1016/j.cell.2010.01.025
Halabi, M.F., and Sheikh, B.Y. 2014. Anti-proliferative effect and phytochemical analysis of Cymbopogon citratus extract. BioMed Research International. 2014: 906239. http://dx.doi.org/10.1155/2014/906239
Islam, B.U., Habib, S., Ahmad, P., Allarakha, S., Moinuddin, and Ali, A. 2015. Pathophysiological role of peroxynitrite induced DNA damage in human diseases: A special focus on poly (ADP-ribose) polymerase (PARP). Indian Journal of Clinical Biochemistry. 30(4): 368-385. https://doi.org/10.1007/s12291-014-0475-8
Joo, T., Sowndhararajan, K., Hong, S., Lee, J., Park, S.Y., Kim, S., and Jhoo, J.W. 2014. Inhibition of nitric oxide production in LPS-stimulated RAW
264.7 cells by stem bark of Ulmus pumila L. Saudi Journal of Biological
Sciences. 21(5): 427-435. https://doi.org/10.1016/j.sjbs.2014.04.003 Katsukawa, M., Nakata, R., Takizawa, Y., Hori, K., Takahashi, S., and Inoue, H.2010. Citral, a component of lemongrass oil, activates PPAR alpha and gamma and suppresses COX-2 expression. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 1801(11): 1214-1220. https://doi.org/10.1016/j.bbalip.2010.07.004
Kavanaugh, N.L., and Ribbeck, K. 2012. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Applied and Environmental Microbiology. 78(11): 4057-4061. https:// doi.org/10.1128/AEM.07499-11
Lee, C.W., Lee, S.H., Lee, J.W., Ban, J.O., Lee, S.Y., Yoo, H.S., and Hong, J.T. 2007. 2-Hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. Journal of Pharmacological Sciences.104(3): 284-284.
Lee, H.S., Kim, S.Y., Lee, C.H., and Ahn, Y.J. 2004. Cytotoxic and mutagenic effects of Cinnamomum cassia bark-derived materials. Journal of Microbiology and Biotechnology.14(6): 1176-1181.
Lee, S.H., Lee, S.Y., Son, D.J., Lee, H., Yoo, H.S., Song, S.G., and Hong, J.T.2005. Inhibitory effect of 2’-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-kappa B activation in RAW 264.7 cells. Biochemical Pharmacology. 69(5): 791-799.
Liakos, I.L., Abdellatif, M.H., Innocenti, C., Scarpellini, A., Carzino, R., Brunetti, V., and Pompa, P.P. 2016. Antimicrobial lemongrass essential oil-copper ferrite cellulose acetate nanocapsules. Molecules. 21(4): 520.https://doi.org/10.3390/molecules21040520
Lin, C.C., Wu, S.J., Chang, C.H., and Ng, L.T. 2003. Antioxidant activity of Cinnamomum cassia. Phytotherapy Research. 17(7): 726-730. https:// doi.org/10.1002/ptr.1190
Matan, N., Matan, N., and Ketsa, S. 2012. Effect of heat curing on antifungal activities of anise oil and garlic oil against Aspergillus niger on rubberwood. International Biodeterioration & Biodegradation. 75: 150-157. https://doi.org/10.1016/j.ibiod.2012.03.012
Okin, D., and Medzhitov, R. 2012. Evolution of inflammatory diseases. Current
Biology. 22(17): R733-740. https://doi.org/10.1016/j.cub.2012.07.029 Ooi, L.S. M., Li, Y.L., Kam, S.L., Wang, H., Wong, E.Y.L., and Ooi, V.E.C. 2006.
Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. American Journal of Chinese Medicine. 34(3): 511-522. https://doi.org/10.1142/ S0192415X06004041
Pannee, C., Chandhanee, I., and Wacharee, L. 2014. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. Journal of Advanced Pharmaceutical Technology & Research. 5(4):164-170. https://doi.org/10.4103/2231-4040.143034
Rahamooz-Haghighi, S., and Asadi, M. 2016. Anti-proliferative effect of the extracts and essential oil of Pimpinella anisum on gastric cancer cells. Journal of Herbmed Pharmacology. 5: 157-161.
Reuter, S., Gupta, S.C., Chaturvedi, M.M., and Aggarwal, B.B. 2010. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine. 49(11): 1603-1616. https://doi.org/10.1016/ j.freeradbiomed.2010.09.006
Swamy, M.K., Akhtar, M.S., and Sinniah, U.R. 2016. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evidence-Based Complementary and Alternative Medicine. 2016: Article ID 3012462. http://dx.doi.org/ 10.1155/2016/3012462
Tazawa, H., Kawaguchi, T., Kobayashi, T., Kuramitsu, Y., Wada, S., Satomi, Y., and Okada, F. 2013. Chronic inflammation-derived nitric oxide causes conversion of human colonic adenoma cells into adenocarcinoma cells. Experimental Cell Research. 319(18): 2835-2844. https://doi.org/ 10.1016/j.yexcr.2013.08.006
Wenzel, U., Kuntz, S., de Sousa, U.J., and Daniel, H. 2003. Nitric oxide suppresses apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. International Journal of Cancer. 106(5): 666-675. https://doi.org/10.1002/ijc.11294
Xuesheng, H., and Parker, T.L. 2017. Lemongrass (Cymbopogon flexuosus) essential oil demonstrated anti-inflammatory effect in pre-inflamed human dermal fibroblasts. Biochimie Open. 4: 107-111. https://doi.org/ 10.1016/j.biopen.2017.03.004
Yang, C.H., Li, R.X., and Chuang, L.Y. 2012. Antioxidant activity of various parts of cinnamomum cassia extracted with different extraction methods. Molecules. 17(6): 7294-7304. https://doi.org/10.3390/molecules 17067294
Yang, X.Q., Zheng, H., Ye, Q., Li, R.Y., and Chen, Y. 2015. Essential oil of Cinnamon exerts anti-cancer activity against head and neck squamous cell carcinoma via attenuating epidermal growth factor receptor-tyrosine kinase. Journal of the Balkan Union of Oncolo
Nakuntwalai Wisidsri*, Suradwadee Thungmungmee, and Warachate Khobjai
Department of Thai Traditional Medicine, Thai Traditional Medicine College, Rajamangala University of Technology Thanyaburi, Pathum Thani 12130, Thailand
*Corresponding author. E-mail: nakuntwalai_w@rmutt.ac.th
Total Article Views
Article history:
Received: September 4, 2018;
Revised: January 23, 2019;
Accepted: Febuary 1, 2019

