
Spa Product Containing Plumeria acuminataand and Pandanus amaryllifolius Absolute for Antioxidant and Anti-wrinkle Activity: In vitro and In vivo Testing
Kunthida Taisakun, Sunee Chansakaow, Worrapan Poomanee, and Pimporn Leelapornpisid *Published Date : May 14, 2024
DOI : https://doi.org/10.12982/NLSC.2024.033
Journal Issues : Number 3, July-September 2024
Abstract This study investigated the biological activities of Plumeria acuminata absolute (PLA), Pandan amaryllifolius absolute (PDA), and the essential oil blends (EOB2, EOB5) combining PLA, PDA, and other essential oils. It also aimed to identify the selected EOB for GC-MS analysis, develop this EOB into a cream, and determine its stability. Additionally, clinical evaluations were conducted to evaluate the cream’s efficacy on skin moisture, wrinkles, and irritation. PLA, PDA, and EOB2 exhibited potent anti-oxidants and anti-aging effects. They effectively showed free radicals scavenging (DPPH, ABTS), inhibiting lipid peroxidation, and suppressing hyaluronidase and collagenase activities. Due to its superior stability, EOB2 was selected for subsequent analysis. It demonstrated a potent inhibitory activity against MMP-2 enzymes. From GC-MS analysis, limonene was identified as the main component in EOB2. The 1.5%EOB cream maintained excellent stability under accelerated conditions. From the clinical study tested in 20 healthy volunteers after 8 weeks of twice diary application, it was found that the cream containing 1.5%EOB2 did not cause any skin irritation and significantly improved skin hydration. It also showed statistically significant decreases in wrinkle area, wrinkle depth, and skin roughness compared to the baseline. In conclusion, cream containing EOB could be a promising cosmeceutical or spa product for anti-aging purposes.
Keywords: Plumeria acuminata extract, Pandan amaryllifolius extract, Essential oil blend, Anti-oxidant, Anti-wrinkle, Spa product
Funding: The authors are grateful for the research funding provided by the Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
Citation: Taisakun, K., Chansakaow, S., Poomanee, W., and Leelapornpisid, P. 2024. Spa product containing Plumeria acuminata and Pandanus amaryllifolius absolute for antioxidant and anti-wrinkle activity: In vitro and in vivo testing. Natural and Life Sciences Communications. 23(3): e2024033.
INTRODUCTION
Thailand attracts numerous tourists yearly due to its diverse attractions, including natural and cultural tourism, as well as high-quality spa experiences. Both foreign and domestic tourists seek this unique Thai experience. In today’s fast-paced world, with high competition and exposure to pollution, people are experiencing higher levels of stress, premature aging, and wrinkles. As a result, spa services and spa products have become an important option for relaxation and rejuvenation, contributing to the significant growth of the spa industry in Thailand. Thailand is fortunate to possess an abundance of aromatic plants with immense potential for use in cosmetics and spa products. This study focuses on two such promising plants: pandan (Pandan amaryllifolius) and leelawadee (Plumeria acuminata). Both are widely available and easy to cultivate, making them ideal candidates for further exploration.
Pandan, a sprawling plant with long green leaves and a fragrant aroma, belongs to the Pandanaceae family. (Chauhan et al., 2014; Bhuyan and Sonowal, 2021) Beyond its delightful fragrance, pandan boasts a pleasant, sweet taste, this unique combination making it a popular natural flavoring agent in Southeast Asia countries like Malaysia, Indonesia, and Thailand. (Cheetangdee and Chaiseri, 2006; Zaki et. al., 2019) Many studies have been repeated on the biological properties and various components of P. amaryllifolius leaves. Studies reveal the extracts from P. amaryllifolius leaves exhibit anti-oxidant activity and contain multiple phenolic and flavonoid compounds. The exact composition can vary depending on the cultivation area and extraction solvent used. Interestingly, a 3% extract from P. amaryllifolius leaves has been successfully incorporated into an oil-in-water emulsion formula, demonstrating excellent stability even under accelerated conditions. (Jimtaisong and Krisdaphong, 2013; Ghasemzadeh and Jaafar, 2013) Furthermore, it has exhibited effectiveness in inhibiting collagenase and elastase enzymes, indicating its potential to reduce wrinkles. (Chaiyana et al., 2021)
Leelawadee flowers (Plumeria acuminata) grace the world as a perennial woody plant belonging to the Apocynaceae family. This plant is characterized by milky white latex and solitary leaves. It is also a pleasant fragrance. (Tohar et al., 2006; Malimart et al., 2018) In Thailand, the flower was originally imported from Cambodia. (Farooque et al., 2012; Shinde et al., 2014; Goswam et al., 2016) The oil of P. acuminata is primarily composed of ester, with benzyl salicylate and benzyl benzoate being the main components. (Tohar et al., 2006) Extracts from P. acuminata exhibit anti-oxidant properties, with the effectiveness varying depending on the amount of the extract used. These anti-oxidant properties were tested using DPPH radical, superoxide anion radical, nitro oxide radical, and hydroxyl radical scavenging assays. Significantly, patents have been developed for the application of P. acuminata extracts in cosmetics. These patents specifically highlight the extract’s beauty benefit, particularly targeting areas with wrinkles. The research behind these patents involves both in vitro and in vivo testing. (Gupta et al., 2007; Mei and Lyga, 2014)
Due to the promising biological activities of P. amaryllifolius and P. acuminata for cosmetic applications, this study investigates their potential further. The research extracts these plant essences and utilizes their aromatic properties. By blending them with other essential oils, the goal is to develop a unique and pleasant essential oil blend formulation. The potential of this blend to combat free radicals and delay aging will be assessed through in vitro and in vivo experiments. Additionally, the essential oil blend will be incorporated into a spa cream product to evaluate its skin irritation profiles and efficiency in human volunteers.
MATERIALS AND METHODS
Materials
2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH), 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Collagen from bovine Achilles tendon, Collagenase from Clostridium histolyticum, Gallic acid, Hyaluronidase from bovine testes, L-Ascorbic acid, Linoleic acid, Quercetin, and Tannic acid were purchased from Sigma-Aldrich (United States). Denature Ethanol (DEB 96) was purchased from United Chemical& Trading Co., LTD. (Thailand). Petroleum ether was purchased from ACI Lab-scan LTD (Ireland). Bovine serum albumin and Ferrous chloride (FeCl2) were purchased from Merck (Germany). Potassium persulfate (K2S2O8) was purchased from Uni-lab (Australia). A Finn Chamber® from SmartPractice, Phoenix, AZ (United States) was also used.
Plant collection and extraction
The leaves of P. amaryllifolius and flowers of P. acuminata were collected from Chiang Mai province. The fresh plant materials were identified and authenticated by the taxonomist,and kept the voucher specimens in (voucher no. 0023376 for P. acuminata and 0023377 for P. amaryllifolius) the Faculty of Pharmacy, Chiang Mai University. The plants were macerated with petroleum ether at room temperature for 24 hours and filtered. Then, the crude extract was evaporated by a rotary evaporator at 40°C to obtain a concrete containing wax, color, and fragrance. The concrete was treated with 95% denatured ethanol to solubilize only aromatic compounds, then evaporated out the ethanol to obtain an absolute. The absolutes were named P. amaryllifolius absolute (PDA) and P. acuminata absolute (PLA). Finally, they were stored in tightly closed containers in a refrigerator before further testing.
Blending of absolute extracts with essential oil
The PDA and PLA were blended with some different essential oils. All the essential oil blends were evaluated for scent satisfaction by 20 volunteers, and two formulas (EOB2 and EOB5) that presented the most acceptable scent were chosen to test their biological activities.
Determination of antioxidant activities
DPPH radical scavenging activity
The scavenging activity of PDA, PLA, and both essential oil blend on DPPH radicals were determined using the method of Kiattisin et al. (2006) with some modifications. The samples were dissolved in ethanol, and 20 µl of each dissolved sample was mixed with 180 µl of 120 mM DPPH in ethanol in a 96-well plate. The mixture was then incubated in the dark for 30 minutes, and the absorbance was measured at 520 nm using a SpectraMax M3 (SpectraMax M3, USA). Ascorbic and Trolox were used as positive control. The IC50 values were determined using 50% inhibition of DPPH radical. The percentage inhibition was calculated using the following equation:
%inhibition = [(Acontrol-Asample)/Acontrol]×100 (1)
Where Acontrol is the absorbance of DPPH- absorbance of solvent and Asample is the absorbance of the sample with DPPH-absorbance of the sample with solvent.
ABTS radical scavenging activity
The free radicals scavenging properties of PDA, PLA, and both essential oil blends were determined using the method of Poomanee et al. (2015). An ABTS stock solution was prepared by mixing 140 mM K2S2O8 in DI water and ABTS in distilled water and incubated at room temperature in the dark for 16 hours. After incubation, the ABTS stock solution was dissolved in ethanol. Then, 2 µl of each dissolved sample was mixed with 200 µl of the ABTS solution in a 96-well plate and kept at room temperature for 6 minutes. The absorbance was measured at 734 nm using a SpectraMax M3 (SpectraMax M3, USA). Ascorbic acid and Trolox were taken as positive controls. The IC50 and percentage inhibition were then calculated using the following equation:
%inhibition = [(Acontrol-Asample)/Acontrol]×100 (2)
where Acontrol and Asample are similar to the DPPH assay but change to ABTS.
Lipid peroxidation inhibition activity
The ability of each sample to inhibit linoleic acid peroxidation was determined using the method of Poomanee et al. (2015) with some modifications. The sample was dissolved in ethanol, and then, an aliquot of the sample (75 µl) was mixed with distilled water (175 µl), 20 mM Phosphate buffer pH 7.0 (350 µl), 1.3% linoleic acid in methanol (350 µl) and 46.35 mM AAPH (50 µl). The mixture was then incubated in a water bath at 45°C for 4 hours. The control was based on ethanol instead of a sample. The degree of lipid peroxidation was determined using the ferric-thiocyanate method. After incubation, the reaction mixture was mixed with 20 mM FeCl2 in 3.5% HCl (50 µl), 10% NH4SCN solution (50 µl), and 75% methanol (4.85 ml) at room temperature for 3 minutes in a test tube. The absorbance was measured at 500 nm using a UV-Vis spectrophotometer, UV-2600i (Shimadzu, Japan). Ascorbic acid and Trolox served as the positive controls. The IC50 was calculated from the regression curve of concentration at 50% inhibition using the equation for percentage inhibition as follows:
%inhibition = [(Acontrol-Asample)/Acontrol] ×100 (3)
where Acontrol and Asample are similar to the DPPH assay but change to lipid peroxidation.
Determination of hyaluronidase enzyme inhibition activity
The inhibitory effects of the samples on hyaluronidase enzyme were determined using the modified protocol of Nema et al. (2013). The assay consisted of hyaluronidase (100 µl) in 20 mM sodium phosphate with 77 mM sodium chloride and 0.01% Bovine Serum Albumin (BSA) (pH 7.0) mixed with the test sample in dimethylsulphoxide (50 µl) for 10 minutes at 37°C. Subsequently, 0.3% hyaluronic acid in 300 mM phosphate buffer pH 5.35 (100 µl) was added to the mixture and further incubated for 45 minutes at 37°C. Finally, the acid albumin solution, made up of 0.1% BSA in 24 mM sodium acetate with 79 mM acetic acid pH 3.75, was mixed with the mixture at room temperature for 10 minutes. The absorbance was measured at 600 nm using a SpectraMax M3 (SpectraMax M3, USA). Tannic acid was used as the reference standard. The anti-hyaluronidase activity of each sample was expressed as the percentage of hyaluronidase inhibition, which was calculated using the following equation:
%inhibition=[(Asample-Acontrol)/ Acontrol] ×100 (4)
Determination of collagenase enzyme inhibition activity
The inhibition of collagenase was determined using the methods of Yasmin et al. (2014) with some modifications. Each sample was dissolved in 20%w/w Tween 20. A mixture of samples (50 µl), 0.1 mg/ml collagenase in 50 mM tris buffer pH 7.5 (20 µl), 5 mM CaCl2 in 125 mM borate buffer pH 7.5 (100 µl), and distilled water (30 µl) was incubated in a water bath at 37°C for 10 minutes. Then, 1 mg/ml collagen in 0.5 M NaOH (20 µl) was added to the mixture and incubated in a water bath at 37°C for 60 minutes. After incubation, the enzymatic solution was mixed with 0.75 mM 3,4-DHPAA (250 µl), 125 mM sodium borate pH 8 (250 µl), and 1.25 mM NaIO4 (250 µl). The mixture was immediately reacted in a water bath at 37°C for 10 minutes and then kept in an ice water bath to stabilize the fluorophore. The fluorescence intensity was measured using a SpectraMax M3 (SpectraMax M3, USA) at excitation (375 nm) and emission (465 nm). The borate buffer was used instead of the collagenase solution as a blank, and 20% Tween 20 was used instead of samples as a control. Quercetin and gallic acid were taken as positive controls. The IC50 values were determined using the concentration of samples that reduced 50% of enzymatic activity. The percentage of inhibition was calculated using the following equation:
Inhibition = [(Fluorescence intensity control – Fluorescence intensity sample)/ Fluorescence intensity control] ×100 (5)
Testing the stability of EOB2 and EOB5 for selection in the O/W cream formulation.
Stability testing of the EOB2 and EOB5 was conducted using the heating-cooling method for a total of 6 cycles. The samples were stored at a temperature of 4°C for 24 hours and then at 45°C for another 24 hours, counting as 1 cycle and under different conditions (4°C, 45°C, RT in the dark and RT in the light) for 1 month. After the designated time, the samples were taken out for biological efficacy assessment using the DPPH and lipid peroxidation methods.
Determination of MMP-2 enzymatic activity
The essential oil blend selected for testing was evaluated for its inhibitory effects on the gelatinolytic activity of matrix metalloproteinase-2 (MMP-2) and compared to ascorbic acid using Manosroi et al. (2012) protocol. The EOB2 solution (1 mg/ml) and ascorbic acid (1 mg/ml) were dissolved in 10%v/v DMSO. Human skin fibroblasts were maintained in culture medium for 24 hours, treated with the sample and ascorbic acid, and then incubated for 48 hours. After incubation, the culture medium was sterilized by filtering through a 0.2-micron pore-size membrane, and MMP-2 inhibition was assessed by SDS-PAGE zymography. The displayed transparencies were measured using a Gel documentation system (Bio-Rad Laboratories, UK) and analyzed by Quantity 1-D analysis software.
Gas chromatography-Mass spectrometry analysis
GC/MS analyses of the essential oil were carried out using an Agilent 7890A GC system interfaced with an Agilent mass spectrometer detector. The system was equipped with a DB5-MS column (30 m x 0.25 mm i.d., 0.25 µm film thickness). The column temperature was programmed from 70°C to 80°C at 2°C/min, then to 90°C at 1°C/min, and finally to 250°C at 3°C/min. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The injection volume was 0.2 µL neat with a split ratio of 500:1. The mass scan range was 50-500 amu. The injector and detector temperatures were maintained at 250°C and 280°C, respectively. (Goswami et al., 2016)
Preparation of O/W emulsion containing essential oil blend
The spa product formulation was prepared using a cold process, and each product contained a different essential oil blend. The spa product consisting of jojoba oil, rice bran oil, cyclomethicone, sodium polyacrylate (and) C13-14 isoalkane (formerly C13-14 isoparaffin (and) laureth-7), propylene glycol, glycerin, tween 20, phenoxyethanol, distilled water, and essential oil blend (1.5%).
Clinical evaluation for skin irritation and performance testing
The study was conducted in accordance with standard ethical guidelines, including the Declaration of Helsinki, ICH GCP, and international ethical guidelines. Approval for the study was obtained from the Research Ethics Committee at Faculty of Pharmacy, Chiang Mai University, identified by the approval number 013/2022/E. All of the volunteers have completed the consent form.
The skin irritation test
A skin irritation test was first conducted on 20 healthy volunteers using patch tests (Finn Chamber®) on the backs of the participants. (Kamma et al., 2019) The patch was applied with 1.5% essential oil blend in propylene glycol, base cream, and a 1.5% EOB cream. A positive control of 1% sodium lauryl sulfate (SLS) was also included. After 48 hours of exposure, the patch was removed, and erythema, edema, or other skin irritations were observed and evaluated at 1 hour, 24 hours, and 7 days after patch removal following the Draize Scoring system. The PII (Primary Irritation Index) values were then calculated using the following equation: PII value = [(∑edema at 1 h/24h/7d) + (∑erythema at 1 h/24h/7d)]/ (number of the observations of treated sites) x (number of sample).
Performance test
Twenty healthy volunteers aged 30-60 years (4 males and 16 females) were enrolled in this study and provided a signed consent form. The performance test involved comparing a base cream and 1.5% EOB cream on the lower forearm of the volunteers. The cream base was applied to the right forearm, which was separated from the untreated area, while the 1.5% EOB cream was applied on the left forearm. Both products were applied twice a day (morning and evening) for 60 days. The skin moisturizer content of the 20 healthy volunteers was evaluated using a corneometer®, while the skin wrinkles was evaluated using a visiometer®. The percentage efficacy was calculated using the following equation: % efficacy = (Vd-V0) x 100/V0, where V0 is the value at the initial point (day 0), and Vd is the value at the measuring points (30 and 60 days). After 60 days of product application, the volunteers also completed a satisfaction questionnaire for both products. (Pueknang and Saewan, 2022)
Statistical analysis
All assays were done in triplicates, and the results were presented as the mean ± standard deviation (S.D.) One-way ANOVA with multiple comparisons was employed as statistical analysis. The paired sample t-test was conducted to compare differences in means of skin parameters from the performance testing. All statistical calculations were performed using the SPSS statistic 17.0 program. A P-value <0.05 was considered statistically significant.
RESULTS
The percentage yield of the absolute and essential oil blend formulations
The percentage yields of PDA and PLA were 0.14 ± 0.03 and 0.12 ± 0.01%, respectively. EOB2 and EOB5 received the highest satisfaction scores from a volunteer group of 20 individuals. The composition of these essential oil blends is shown in Table 1.
Table 1. The composition of EOB2 and EOB5.
|
Formulation |
Composition |
|
EOB2 |
Orange oil, Lemon oil, Bergamot oil, Citronella java, Rosemary oil, Cedarwood oil, Ylang ylang oil, PDA, PLA |
|
EOB5 |
Orange oil, Lavender oil, Cedarwood oil, Ylang ylang oil, PDA, PLA |
Antioxidant activities
DPPH radical scavenging activity
Based on the DPPH radical scavenging assay, the PDA and PLA had IC50 values of 0.12 ± 0.13 mg/ml and 0.63 ± 0.01 mg/ml, respectively. These values showed no significant difference (P >0.05) compared to the standards (Trolox and ascorbic acid). The IC50 values of the EOB2 and EOB5 were found to be 9.98 ± 1.46 mg/ml and 11.30 ± 0.51 mg/ml, respectively, as shown in Table 2.
ABTS radical scavenging activity
The analysis of ABTS radical scavenging activity of PDA and PLA revealed IC50 values of 0.11 ± 0.01 mg/ml and 0.11 ± 0.02 mg/ml, respectively. This suggested that PDA was more effective in inhibiting free radicals compared to PLA, although the difference was not statistically significant (P >0.05). As for the essential oil blend, EOB2 exhibited an IC50 value of 2.33 ± 0.19 mg/ml, which was found to be more efficient than EOB5 with an IC50 value of 2.94 ± 0.10 mg/ml. This difference between EOB2 and EOB5 is statistically significant (P <0.05) according to Table 2.
Lipid peroxidation inhibition assay
From the experimental results in Table 2, it was found that PDA exhibited the most efficient inhibition of linoleic acid peroxidation, with an IC50 value of 0.52 ± 0.05 mg/ml. There was no statistically significant difference (P >0.05) compared to the standard (Trolox and ascorbic acid). PLA demonstrated a significant inhibitory effect on linoleic acid peroxidation (P >0.05) comparable to EOB2, with IC50 values of 3.07 ± 0.25 mg/ml and 3.65 ± 0.28 mg/ml, respectively. EOB5 showed a significantly different effect (P <0.05) with an IC50 value of 7.09 ± 0.27 mg/ml when compared to all other samples.
Collagenase enzyme inhibition activity
PLA and PDA effectively inhibited collagenase with IC50 values of 0.40 ± 0.003 and 0.43 ± 0.09 mg/ml, respectively. Both mentioned aromatic compounds showed non-significant differences (P >0.05) from the standard compounds (gallic acid and quercetin), as shown in Table 2. In addition, EOB2 demonstrated a statistically significant higher efficiency in inhibiting collagenase compared with EOB5 (P <0.05).
Hyaluronidase enzyme inhibition activity
Inhibitory effects on hyaluronidase enzyme showed no significant difference in efficacy between EOB2 and EOB5 (P >0.05), with IC50 values of 6.005 ± 0.21mg/ml and 5.690 ± 0.18 mg/ml, respectively. However, there was a significant difference in efficacy between PDA and PLA, with IC50 values of 1.70 ± 0.41 mg/ml and 0.160 ± 0.02 mg/ml, respectively, as shown in Table 2.
Table 2. The IC50 value of anti-oxidant activities, anti-collagenase activity, and anti-hyaluronidase activity.
|
Sample |
IC50 value of anti-oxidant activities (mg/ml) |
IC50 value of anti-collagenase activity (mg/ml) |
IC50 value of anti-hyaluronidase activity (mg/ml) |
||
|
DPPH assay |
ABTS assay |
Lipid peroxidation |
|||
|
EOB2(fresh) |
9.98 ± 1.46b |
2.33 ± 0.19c |
3.65 ± 0.28b |
4.19 ± 0.13b |
6.00 ± 0.21d |
|
EOB5(relax) |
11.30 ± 0.51b |
2.94 ± 0.10d |
7.09 ± 0.27c |
6.52 ± 0.74c |
5.69 ± 0.18d |
|
PDA |
0.12 ± 0.13a |
0.11 ± 0.01ab |
0.52 ± 0.05a |
0.43 ± 0.09a |
0.16 ± 0.02a |
|
PLA |
0.63 ± 0.01a |
0.11 ± 0.02ab |
3.07 ± 0.25b |
0.40 ± 0.03a |
1.70 ± 0.41c |
|
Trolox |
0.07 ± 0.00a |
0.06 ± 0.00a |
0.07 ± 0.00a |
- |
- |
|
Ascorbic acid |
0.04 ± 0.00a |
0.03 ± 0.00b |
0.62 ± 0.11a |
- |
- |
|
Quercetin |
- |
- |
- |
0.01 ± 0.03a |
- |
|
Gallic acid |
- |
- |
- |
0.06 ± 0.01a |
- |
|
Tannic acid |
- |
- |
- |
- |
0.78 ± 0.02b |
|
Note: a,b,c, and d indicate significant differences (P <0.05) between samples analyzed by One-Way ANOVA with multiple comparisons using the Tukey test. |
|||||
The stability of EOB2 and EOB5 for formulation selection
EOB2 was selected for formulation due to its significantly stronger DPPH scavenging activity and higher lipid peroxidation compared to EOB5, as shown in Figure 1.
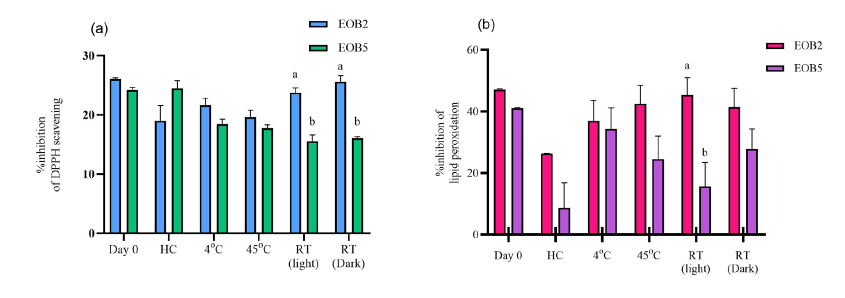
Figure 1. The %inhibition of the anti-oxidant activity of EOB2 compared to EOB5 after under accelerated conditions: (a) DPPH radical scavenging (b) lipid peroxidation inhibition. The different alphabetical superscripts indicate statistical differences (P <0.05) between samples under the same condition analyzed by the Independent-Sample T-test.
Testing the inhibitory effect of matrix metalloproteinase-2 (MMP-2) in human cells by EOB2
EOB2 at a concentration of 0.1 mg/ml demonstrated an inhibitory efficacy of 30.43 ± 1.06% on matrix metalloproteinase-2 (MMP-2) in human cells, while the same concentration of standard ascorbic acid exhibited an inhibitory efficiency of 28.49 ± 2.82% on MMP-2, as shown in Table 3.
Table 3. Comparison of the inhibitory ability of MMP-2 between EOB2 and ascorbic acid.
|
Sample |
Percentage MMP-2 activity inhibition |
|
0.1 mg/ml EOB2 |
30.43 ± 1.06 |
|
0.1 mg/ml Ascorbic acid |
28.49 ± 2.82 |
|
Note: The values are the results of three repeated experiments. |
|
Gas chromatography-Mass spectrometry (GC-MS) analysis
The composition of EOB2 was analyzed by GC-MS, revealing 33 components, as shown in Table 4. The most abundant component was Limonene. Eucalyptol was the second most abundant component.
Table 4. Composition of the EOB2 analyzed by GC-MS

Skin irritation test on human volunteers
Both EOB cream and Based cream had a PII value of 0, indicating non-irritation. Similarly, the 1.5% EOB2 in propylene glycol had a PII value of 0.38, also classified as non-irritating. In contrast, the positive control had a PII value of 0.99, indicating slight irritation.
Performance test of spa products on volunteers
Skin wrinkles
Based on the wrinkle area measurement on the forearms of 20 volunteers, the EOB cream (containing 1.5% EOB2) and the cream base (without active ingredients) showed significant reductions (P <0.05) compared to baseline (day 0) after 30 days and 60 days, shown by the Table 5. At 30 days, the EOB cream achieved a wrinkle reduction efficiency of -6.02 ± 2.61% compared to other areas (P >0.05). However, this effect became statistically significant (P <0.05) by day 60 compared to the untreated area, with an efficiency of -14.34%, as shown in Figure 2A.
The application of EOB cream in the forearm region resulted in a statistically significant reduction in wrinkle depth from baseline (P <0.05), according to Table 5. After 30 and 60 days of product use, EOB cream showed a trend of being more effective in reducing wrinkle depth compared to other areas, although this difference was not statistically significant (P >0.05), as indicated in Figure 2B.
EOB cream and cream based both reduced average roughness (Roughness, R3) in the forearm region, as detailed in Table 5. Based on the statistical analysis of %efficiency changes in Figure 2C, all tested areas had a reduction in skin roughness after 30 days. In comparison, EOB cream showed the greatest reduction at -9.00 ± 2.54% compared to other areas (P >0.05). After 60 days, the area treated with EOB cream showed a statistically significant reduction in roughness (P <0.05) at -18.66 ± 2.59% compared to untreated areas.
EOB cream and cream base significantly reduced the arithmetic average roughness (Roughness, R5) on the forearm, presented in Table 5. EOB cream showed the highest %efficiency in reducing skin roughness after 30 and 60 days, with no statistically significant difference compared with all areas, as shown in Figure 2D. Changes in skin texture can be observed in Figure 4, which shows images captured using Visioscan® to illustrate the differences in skin appearance. Notably, the skin area treated with EOB cream exhibited shallower wrinkles and a reduced wrinkle area on day 60.
Table 5. Skin wrinkle parameters before and after application in 30 and 60 days of the cream containing 1.5%EOB2, cream base, and untreated area.
|
Testing area |
Times |
Surface (%) |
Volume (mm3) |
Roughness, R3 |
Roughness, R5 |
|
Untreated A |
Before |
441.92 ± 64.67 |
60.55 ± 22.30 |
41.95 ± 10.51 |
8.45 ± 1.79 |
|
After 30 days |
428.43 ± 46.90 |
58.03 ± 27.36 |
40.38 ± 8.94 |
8.17 ± 1.56 |
|
|
After 60 days |
422.65 ± 73.31* |
58.88 ± 23.96 |
40.38 ± 11.97* |
7.97 ± 1.94* |
|
|
EOB cream |
Before |
483.20 ± 69.97 |
64.68 ± 30.88 |
41.22 ± 7.55 |
8.57 ± 1.62 |
|
After 30 days |
447.66 ± 34.98* |
53.97 ± 24.19* |
37.08 ± 5.92* |
7.75 ± 0.92* |
|
|
After 60 days |
408.08 ± 35.68* |
48.95 ± 15.36* |
33.02 ± 4.93* |
7.18 ± 0.97* |
|
|
Untreated B |
Before |
439.91 ± 53.55 |
55.90 ± 19.89 |
41.22 ± 9.24 |
8.40 ± 1.55 |
|
After 30 days |
433.67 ± 46.32 |
52.70 ± 18.76 |
40.33 ± 8.52 |
8.15 ± 1.42 |
|
|
After 60 days |
421.30 ± 63.97* |
55.92 ± 27.18 |
39.63 ± 10.78* |
7.90 ± 1.82* |
|
|
Cream base |
Before |
500.54 ± 80.15 |
61.03 ± 21.33 |
42.07 ± 8.36 |
8.83 ± 1.73 |
|
After 30 days |
472.71 ± 43.20* |
55.37 ± 24.02 |
39.53 ± 4.80* |
8.20 ± 1.39* |
|
|
After 60 days |
464.22 ± 95.95* |
55.32 ± 21.43 |
38.27 ± 9.64* |
8.05 ± 2.01* |
Note: * Indicates significant differences (P <0.05) and uses standard error (SE) definition between before and after application analyzed by Pair T-test.
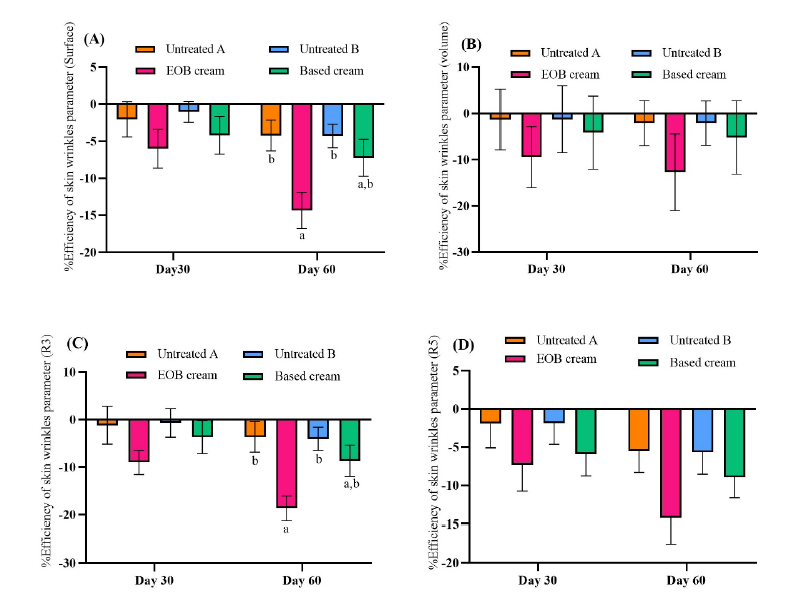
Figure 2. The %efficiency of skin wrinkle parameters after application of the cream containing 1.5%EOB2 and cream base: (A) Surface, (B) Volume, (C) Roughness; R3, (D) Roughness; R5. The different alphabetical superscripts indicate statistical differences (P <0.05) between different areas analyzed by One-Way ANOVA with multiple comparisons using the Tukey test.
Skin moisturizing
Based on the statistical analysis, both EOB cream and cream base increase skin moisture after 30 and 60 days compared to baseline (P <0.05), according to Table 5. EOB cream exhibited a higher %efficiency in moisture increase than other areas, with statistically significant (P <0.05) results compared to the untreated areas at day 30 and day 60, reaching 22.96% and 38.96%, as shown in Figure 3.
Table 6. Skin moisturizing values after applying the cream containing 1.5%EOB2, cream base, and untreated areas in 30 and 60 days.
|
Testing area |
Skin moisturizing value (Corneometer® arbitrary units) |
||
|
Before |
After 30 days |
After 60 days |
|
|
Untreated A |
49.38 ± 6.01 |
49.89 ± 6.70 |
47.72 ± 6.62 |
|
EOB cream |
46.26 ± 7.56 |
56.14 ± 9.12* |
63.43 ± 10.71* |
|
Untreated B |
47.90 ± 4.63 |
49.14 ± 7.52 |
48.89 ± 6.87 |
|
Based cream |
43.70 ± 8.91 |
50.80 ± 8.91* |
54.78 ± 8.69* |
Note: * Indicates significant differences (P <0.05) and uses standard error (SE) definition between before and after application analyzed by Pair T-test. a.u. arbitrary Corneometer® units
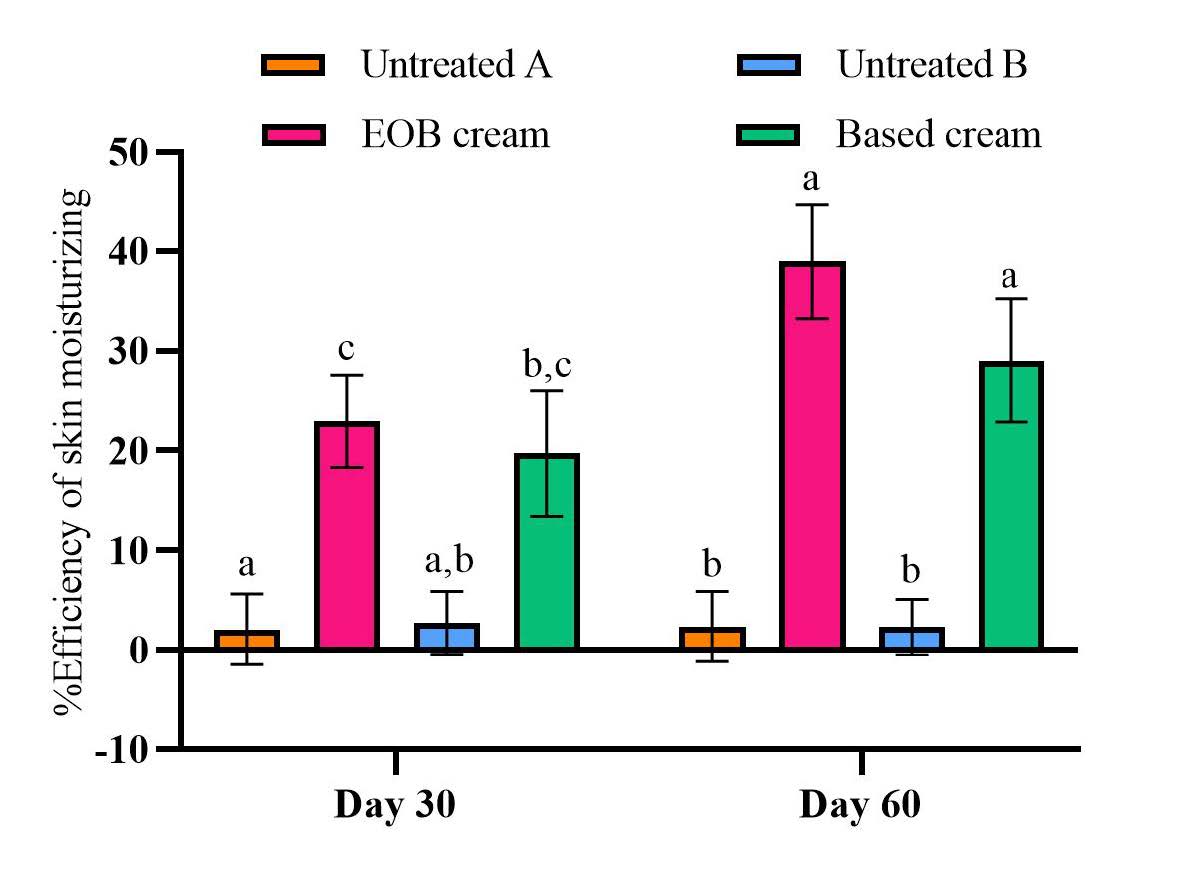
Figure 3. The %efficiency of skin moisturizing after applying the cream containing 1.5%EOB2 and cream base. The different alphabetical superscripts indicate statistically differences (P <0.05) between different area analyzed by One-Way ANOVA with multiple comparison using Tukey test.
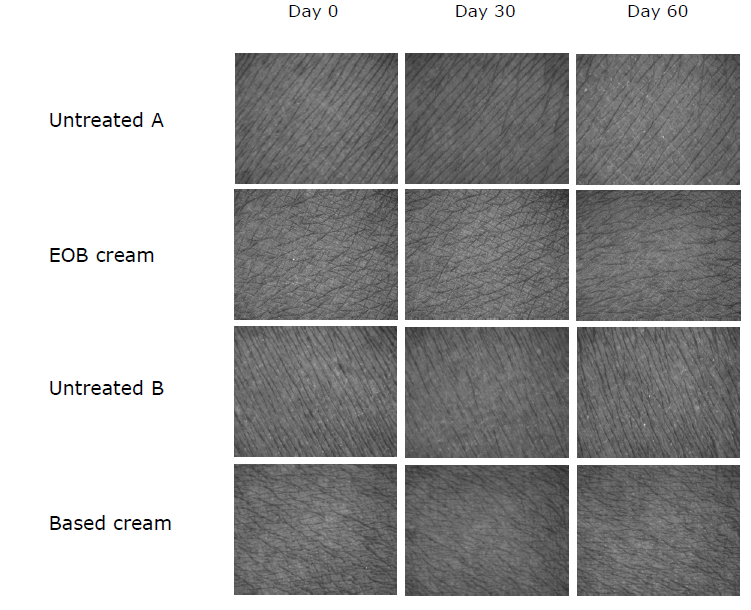
Figure 4. Depth level of skin roughness and wrinkles of human skin volunteers on untreated skin and skin after treatment with the EOB cream and cream base for 0, 30, and 60 days by Visiometer®
Satisfaction test
The survey results showed that the overall satisfaction scores given by the volunteers for EOB cream and based cream were 4.23 and 4.03, respectively. The overall satisfaction scores for product characteristics, such as texture and scent, were 4.28 and 4.13 for EOB cream and based cream, respectively.
DISCUSSION
PDA and PLA were obtained through maceration extraction using petroleum ether and absolute ethanol. Absolute emitted a specific aroma of the respective plant. The color of PDA absolute was dark brown, while that of PLA was yellow. Similar to the preceding experiment using this extraction method, the absolute from flowers of M. alba yielded 0.0497 and 0.0505% from 150 and 200 g/L, respectively. The physical appearance of the absolute is brown, with a liquid at room temperature, and it emits an odor reminiscent of fresh flowers. (Punjee et al., 2009) The scent satisfaction of the EOB formulations with PDA and PLA were tested in 20 volunteers, who rated the scent levels through sniffing using filter paper and smelling through a candle lamp diffuser. The results showed high satisfaction with two out of five formulations. The essential oil chosen for blending is from a laboratory collection. EOB2 consists of PDA and PLA blended with orange oil, lemon oil, bergamot oil, citronella oil, rosemary oil, cedarwood oil, and ylang-ylang oil, which elicited a fresh feeling, while EOB5 blended with orange oil, lavender oil, cedarwood oil, and ylang-ylang oil, induced a sense of relaxation. The blending of essential oils is done to enhance the scent, as using a PDA and PLA can result in an overly strong and intense smell. It may contribute to synergistic effects when combined together. (Harris R., 2002)
The results from the tests for anti-oxidant activity using DPPH assay, ABTS assay, and lipid peroxidation assay confirmed that PDA, PLA, EOB2, and EOB5 had anti-oxidant activity. This differs from related research that compared the extract from fresh and dried leaves of P. amaryllifolius, where the fresh leaves of P. amaryllifolius did not exhibit anti-oxidant activity. This may have been due to variations in the extraction methods, and the observed differences in anti-oxidant activities could be attributed to the different sampling locations. (Ghasemzadeh and Jaafar, 2013; Yan and Asmah, 2010) Regarding Plumeria, various studies have investigated the anti-oxidant properties of different varieties of Plumeria spp. They exhibited similar anti-oxidant activities. (Goswami et al., 2016; Sirisha et al., 2013) However, when PDA and PLA were mixed into essential oil blends (EOB2 and EOB5), their anti-oxidant activities decreased significantly compared with the PDA and PLA (P <0.05). This may be due to the dilution effect and some essential oils lacking anti-oxidant properties. Because of the inclusion of citrus oil in this blended essential oil, its effects are similar to those observed in previous research that investigated the changes in free fatty acid content, peroxidation value, and iodine value in corn oil using orange peel extracts. The study revealed that the blended essential oil exhibited significant inhibitory properties against the oxidation of corn oil, particularly at concentrations of 1,600 and 2,000 ppm. (Rehman Z., 2005) The absolute and EOB increased collagen production in vitro. These findings are consistent with related studies comparing the effectiveness of various plant species and indicating that boiled Pandan extract exhibited good inhibition of collagenase activity. (Chaiyana et al., 2021) Research studies have also been conducted on P. acuminata extracted obtained using water/ethanol, demonstrating their effectiveness in reducing wrinkles through collagenase inhibition activity. (Gupta et al., 2007)
From the stability testing of the essential oil blend under freeze-thaw cycling conditions and different conditions for 1 month, it was observed that EOB2 and EOB5 maintained their characteristics, including scent and color, unchanged from before the testing. Furthermore, EOB2 had a greater ability to scavenge free radical DPPH and exhibited lipid peroxidation than EOB5 (P <0.05, analyzed by one-way ANOVA). Based on these findings, EOB2 was selected for formulation and MMP-2 testing. EOB2 demonstrated greater stability than EOB5, likely due to incompatible blending of essential oil and absolute. This incompatibility may lead to a reduced anti-oxidant effect in EOB5.
MMPs (Matrix Metalloproteinases) are enzymes in the body that break down extracellular matrix components, including collagen, elastin, and glycosaminoglycans. Calcium and zinc serve as co-factors, accelerating the activity of these enzymes. Various types of MMPs exist in the human body, but those specifically involved in degrading extracellular matrix components outside the cells include MMP-2, also known as gelatinase-A. This enzyme breaks down collagenase and is located at the dermal-epidermal junction. (Quan et al., 2009; Wen et al., 2010) EOB2 has a 1.07-fold higher ability to inhibit MMP-2 compared to ascorbic acid.
GC-MS analysis of EOB2 revealed that limonene and eucalyptol are the most prominent components. It seems that EOB2 may have anti-oxidant and anti-aging properties based on the presence of limonene and eucalyptol. This aligns with previous research that studied the seasonal variations in the essential oil of Callistemon subulatus. The research found that α-pinene and eucalyptol were the major components and exhibited potential anti-aging properties. (Rabie et al., 2023) Furthermore, a distinct study conducted on HaCaT cells that were induced to age prematurely revealed the advantageous impacts of limonene on skin aging caused by hydrogen peroxide. (Secerli et al., 2023)
The skin irritation testing of 20 healthy volunteers revealed the following products: EOB cream A (containing 1.5% EOB2), Based cream (cream without active ingredients), and 1.5% EOB2 in propylene glycol. The positive control used was a 1% (w/v) sodium lauryl sulfate, while untreated skin (Blank) served as a negative control. The tests were conducted according to the criteria set by the Draize model and OECD guidelines for primary dermal irritation/corrosion (2015). It was observed that neither EOB cream nor Based cream showed any erythema on the test site. In contrast, the positive control showed erythema, while the 1.5% EOB2 exhibited slight erythema at 1 hour and 24 hours. All three samples do not cause skin irritation.
The performance test of EOB cream on 20 volunteers revealed that EOB cream was effective in reduction of skin wrinkles, as measured by Visiometer®. It also significantly increases skin moisture, as measured by the Corneometer®, when compared both baseline and untreated areas over a 60-day period. Similar to the related research findings, essential oil blends for the clinical study could reveal that the four essential oils under investigation, namely, tea tree, tangerine, eucalyptus, and lavender, exhibited a synergistic effect in enhancing skin hydrolipidic characteristics, leading to improved morphologic properties and skin barrier function. (Infante et al., 2022)
Based on the satisfaction results of EOB cream and based cream, both products had satisfaction scores within the same range. Volunteers indicated a higher preference for using EOB cream over based cream and provided feedback suggesting a slight adjustment to the essential oil of EOB cream to make it more pleasing for product use.
CONCLUSION
PLA and PDA mixed with some essential oil blends exhibited promising biological activities in terms of anti-oxidant and anti-wrinkle properties. They also possessed a pleasing scent, as reported by 20 participants. The cream containing 1.5%EOB maintained stability under accelerated conditions in both physical and biological attributes. In the case of clinical testing in 20 healthy volunteers, the cream containing 1.5%EOB did not cause any skin irritation and increased skin hydration measured by a Corneometer®. It also reduced surface area, volume, and skin roughness measured using a Visiometer® and demonstrated statistically significant differences when compared with baseline measurements (P <0.05). The volunteers expressed satisfaction with the product. This research may be valuable for those interested in studying the extraction of various absolutes for testing biological activity and may also have potential future cosmeceutical and spa product applications.
ACKNOWLEDGEMENTS
The authors express their sincere gratitude to the Faculty of Pharmacy, Chiang Mai University, for its generous research support funding and for providing access to all facilities. We are grateful to Mr. Thomas McManamon for his assistance with the English proofreading and corrections.
AUTHOR CONTRIBUTIONS
Kunthida Taisakun assisted in conducting the experiments, performed the statistical analysis, and wrote the manuscript. Pimporn Leelapornpisid, Sunee Chansakaow, and Worrapan Poomanee contributed by creating the data visualizations, designing the experiments and revised the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors assert that they have no conflicts of interest in relation to this work.
REFERENCES
Bhuyan, B., and Sonowal, R., 2021. An overview of Pandanus amaryllifolius Roxb. exlindl. and its potential impact on health. Current Trends Pharmaceutical Research. 8 (1): 138-157.
Chaiyana, W., Charoensup, W., Sriyab, S., Punyoyai, C., and Neimkhum, W. 2021. Herbal extracts as potential antioxidant, anti-aging, anti-inflammatory, and whitening cosmeceutical ingredients. Chemistry & Biodiversity. 18(7): 1-14.
Chauhan, A., Goyal, M. K., and Chauhan, P., 2014. GC-MS technique and its analytical applications in science and technology. Journal of Analytical& Bioanalytical Techniques. 5(6): 1-5.
Cheetangdee, V., and Chaiseri, S. 2006. Free amino acid and reducing sugar composition of pandan (Pandanus amaryllifolius) leaves. Kasetasart Joournal. (Natural Science). 40(Suppl.): 67-74.
Farooque, A. MD., Mazumder, A., Shambhawee, S., and Mazumder, R. 2012. Review on Plumeria acuminata. International Journal of Research in Pharmacy and Chemistry. 2(2): 467-469.
Ghasemzadeh, A., and Jaafar, H. ZE. 2013. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement and Alternative Medicine. 13(341).
Goswami, P., Chauhan, A., Verma, R.S., and Padalia, R.C. 2016. Chemical constituents of floral volatiles of Plumeria rubra L. from India. Medicinal & Aromatic Plants. S3: 1-5.
Gupta, M., Mazumder, U. K., and Gomathi, P. 2007. Evaluation of antioxidant and free radical scavenging activities of Plumeria acuminata leaves. Journal of Biological Sciences. 7(8): 1361-1367.
Harris, R. 2002. Synergism in the essential oil world. The International Journal Aromatherapy. 12(4): 179-186.
Infante V. H. P., Maia Campos P. M. B. G., Gaspar L. R., Darvin M. E., Schleusener J., Rangel K. C., Meinke M. C., and Lademann J. 2022. Safety and efficacy of combined essential oils for the skin barrier properties: In vitro, ex vivo and clinical studies. International Journal of Cosmetic Science. 44(1): 118-130.
Jimtaisong, A., and Krisdaphong, P. 2013. Antioxidant activity of Pandanus amaryllifolius leaf and root extract and its application in topical emulsion. Tropical Journal of Pharmaceutical Research. 12(3): 425-431.
Kamma, M., Lin, W. C., Lau, S., Chansakaow, S., and Leelapornpisid, P. 2019. Anti-aging cosmeceutical product containing of Nymphaea rubra Roxb. ex Andrews extract. Chiang Mai Journal of Science. 46(6): 1143-1160.
Kiattisin, K., Nantarat, T., and Leelapornpisid, P. 2016. Evaluation of antioxidant and anti-tyrosinase activities as well as stability of green and roasted coffee bean extracts from Coffea arabica and Coffea canephora grown in Thailand. Journal of Pharmacognosy and Phytotherapy. 8(10): 182-192.
Malimart, K., Saensouk, P., and Thongphairoj, U. 2018. Anatomy of some Apocynaceae in Thailand. Journal of Science and Technology Mahasarakham University. 37(1): 51-64.
Mei B.C., and Lyga J. W. 2014. Plumeria acuminata extracts and methods of use. International application published under the patent cooperation treaty. No. WO2014/163960A1.
Manosroi, A., Kumguan, K., Chankhampan, C., Manosroi, W., and Manosroi, J. 2012. Nanoscale gelatinase A (MMP-2) inhibition on human skin fibroblasts of Longkong (Lansium domesticum Correa) leaf extracts for anti-aging. Journal of Nanoscience and Nanotechnology. 12(9): 7187 -7197.
Nema, N. K., Maity, N., Sarkar, B. K., and Mukherjee, P. K., 2013. Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica. Pharmaceutical Biology. 51(9): 1182-1187.
OECD. 2015. Test No. 404: Acute dermal irritation/corrosion. OECD Guidelines for the Testing of Chemicals. Section4. Paris.
Punjee, P., Dilokkunanant, U., Sukkatta, U., Vajrodaya, S., Haruethaitanasan, V., Pitpiangchan, P., and Rakthaworn, P. 2009. Scented extracts and essential oil extraction from Michelia alba D.C. Kasetsart Journal - Natural Science. 43: 197-203.
Poomanee, W., Chaiyana, W., Intasai, N., and Leelapornpisid, P. 2015. Biological activities and characterization of the pod extracts from sompoi (Acacia concinna Linn) grown in northern Thailand. International Journal of Pharmacy and Pharmaceutical Sciences. 7(5): 237-241.
Pueknang, J., and Saewan, N. 2022. Stability and anti-aging of encapsulated ferulic acid in phosphorylated rice starch. Molecules. 27(11).
Quan, T., Qin, Z., Xia, W., Shao, Y., Voorhees, J., and Fisher, G. 2009. Matrix-degrading metalloproteinases in photoaging. Journal of Investigative Dermatology Symposium Proceedings. 14(1): 20-24.
Rabie, O., El-Nashar, H. A. S., Majrashi, T. A., Al-Warhi, T., El Hassab, M. A., Eldehna, W. M., and Mostafa, N. M. 2023. Chemical composition, seasonal variation, and antiaging activities of essential oil from Callistemon subulatus leaves growing in Egypt. Journal of Enzyme Inhibition and Medicinal Chemistry. 38(1): 1-7.
Rehman, Z. 2006. Citrus peel extract – A natural source of antioxidant. Food Chemistry. 99: 450-454.
Secerli, J., Erdem, O., and Bacanl, M. 2023. Antiaging effects of limonene in ageing-induced HaCaT cells. Institute for Genetic Engineering and Biotechnology University of Sarajevo. 7(1): 1-8.
Sirisha, K., Rajendra, Y., Gomathi, P., Soujanya, K., and Yasmeen, N. 2013. Antioxidant and anti-inflammatory activities of flowers of Plumeria rubra L. F. rubra and Plumeria rubra F. lutea: A Comparative study. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 4(4): 743-756.
Shinde, P. R., Patil, P. S., and Bairagi, V. A. 2014. Phytopharmacological review of Plumeria species. Scholars Academic Journal of Pharmacy. 3(2): 217-227.
Tohar, N., Awang, K., Mohd, M. A., and Jantan, I. 2006. Journal of Essential Oil Research. 18(6): 613-617.
Tohar, N., Mohd, M. A., Jantan, I., and Awang, K. 2006. A comparative study of the essential oils of the genus Plumeria Linn. From Malaysia. Flavor and Fragrance Journal. 21: 859-863.
Wen, K. Shih, C., Hu, J., Liao, S., Su, T., and Chiang, H. 2010. Inhibitory effects of Terminalia catappa on UVB-induced photodamage in fibroblast cell line. Evidence-Based Complementary and Alternative Medicine. 2011(904532).
Yan, S.W., and Asmah, R. 2010. Comparison of total phenolic contents and antioxidant activities of turmeric leaf, pandan leaf and torch ginger flower. International Food Research Journal. 17: 417-423.
Yasmin, H., Kabashima, T., Rahman, M. S., Shibata, T., and Kai, M. 2014. Amplified and selective assay of collagens by enzymatic and fluorescent reactions. Scientific Reports. 4(4950).
Zaki, N. A. M., Hashib, S. A., Ibrahim, U. K., and Bakhtiar, P. A. N. A. 2020. Total phenolic content and antioxidant activity of Pandanus amaryllifolius by soaking and microwave-assisted extraction. IOP Conference. Series: Materials Science and Engineering. 778(1).
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kunthida Taisakun, Sunee Chansakaow, Worrapan Poomanee, and Pimporn Leelapornpisid *
Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Pimporn Leelapornpisid, E-mail: pim_leela@hotmail.com
Total Article Views
Editor: Nisit Kittipongpattana,
Chiang Mai University, Thailand
Article history:
Received: December 16, 2023;
Revised: April 25, 2024;
Accepted: May 3, 2024;
Online First: May 14, 2024

