
Enhancement of Biological Activities and Phytochemical Compounds of Oryza sativa L. Extract through Probiotic Fermentation
Panipak Pothirat, Isara Wattananapakasem, Pimporn Leelapornpisid, Pimpisid Koonyosying, Nara Yaowiwat, Siriwan Nawong, and Worrapan Poomanee*Published Date : April 25, 2024
DOI : https://doi.org/10.12982/NLSC.2024.029
Journal Issues : Number 3, July-September 2024
Abstract This study aimed to enhance the biological effects and phytochemical content of probiotic fermented red jasmine rice (Oryza sativa L.) extract to apply as a functional ingredient in anti-aging application. In addition, safety evaluation in terms of cytotoxicity and irritation profile were explored. Lactobacillus plantarum and Lactobacillus rhamnosus were used to ferment the aqueous extract of red jasmine rice to produce fermented extracts as BG and LRB, respectively. The biological potentials in terms of antioxidation, anti-enzymatic effects and anti-inflammation with cytotoxicity of fermented extracts were evaluated and compared with nonfermented extract. In addition, alteration in phytochemical constituents of the probiotic-fermented extracts from nonfermented extract were illustrated by synchrotron radiation–based–FTIR microspectroscopy (SR-FT-IR). The results revealed that BG and LRB demonstrated improved antioxidant and anti-tyrosinase effects compared to the control. Additionally, BG and LRB significantly increased the total phenolic contents corresponding to the enhanced biofunctional effects confirmed by SR-FT-IR. Moreover, BG showed a satisfactory safety in human skin cell lines and Hen's egg chorioallantoic membrane. This study implied augmentative effect of probiotic fermentation on the bioactive efficacy of red jasmine rice. Additionally, the safety investigation confirmed that this fermented extract could be used as a functional novel ingredient for anti-aging nutraceutical and cosmetic applications.
Keywords: Nutraceutical, Fermented plant extract, Probiotic, Red jasmine rice, Whitening
Funding: The authors are grateful for the research funding provided by the TA/RA Scholarship from the Graduate School, Chiang Mai University.
Citation: Pothirat, P., Wattananapakasem, I., Leelapornpisid, P., Koonyosying, P., Yaowiwat, N., Nawong, S., and Poomanee, W. 2024. Enhancement of biological activities and phytochemical compounds of Oryza sativa L. extract through probiotic fermentation. Natural and Life Sciences Communications. 23(3): e2024029.
INTRODUCTION
Rice (Oryza sativa L.), a principal food for more than one half of the world’s population, have several beneficial nutrients. The USDA National Small Grains Collection classifies rice in six groups: purple, variable purple, red, brown, speckled brown, light brown and white rice (Francavilla and Joye, 2020). The most common rice consumed by humans is white rice. However, colored rice is currently of greater interest because of its potential health benefits beyond white rice. Red rice, called as Khaw Dang in Thai, is a highly nutritious rice containing flavones, tannin, phenolics, sterols, tocols, γ-oryzanols, amino acid and essential oils. Yodkeeree et al. (2018) reported that red rice extract could prevent UV-induced collagen and hyaluronic acid degradation through inhibiting collagenase and matrix metalloproteinase-2 activities. In addition, the active compounds of the ethanolic extract potentially reduces the melanin content through inhibiting tyrosinase activity. Therefore, red rice is gaining greater attention in the cosmetic industry such as red jasmine rice owing to its anti-aging and anti-tyrosinase potentials.
One of the novel cosmetic technologies for enhancing desirable biological activities and diminishing the toxicity of the natural resources is probiotic fermentation. The World Health Organization defines probiotics as live microorganisms, which when administered in adequate amounts confer a health benefit on the host (Mack, 2005). Probiotics have been widely reported to modify diseases of the digestive tract such as diarrhea, inflammatory bowel disease and irritable bowel syndrome (Lew and Liong, 2013). Furthermore, direct consumption of probiotics as oral supplement has been demonstrated to effectively treat topical skin conditions such as improving atopic dermatitis, refining rosacea and also reducing acne (Knackstedt et al., 2020). Interestingly, probiotics for skin benefits can be consumed through the oral route and via topical application. The probiotics cosmetic market has grown in recent years because the increasing consumer realization of the advantages of natural cosmetic probiotics. From the 10-year-forecast, the market for probiotic cosmetics market will keep expanding and compound annual growth rate may rise to 12%. (Fact. MR, 2022). Lactobacillus spp. is one of main probiotics producing lactic acid that rendering various dermal benefits. Noticeably, several reports have widely indicated that the biological potential of plant extracts fermented with Lactobacillus were potentially enhanced in terms of antioxidant, anti-inflammatory, and antiphotoaging potentials as well as augmented phytochemical contents (Jin et al., 2019; Park et al., 2021; Williams and Hekmat, 2017; Zubaidah et al., 2012). There are many species of Lactobacillus spp. such as L. aciophilus, L. casei, L. plantarum and L. rhamnosus. Different species can be found in different environments because of their survival conditions. Moreover, it correlates to probiotic biological activities (Zhong et al., 1998). Christensen et al. (2002) confirmed that various Lactobacillus spp. have different mechanism activating dendritic cells. Accordingly, probiotics fermentation can promote processed plants and add value to an economic crop providing superior advantages over nonfermented samples. Nevertheless, no report is available Lactobacillus fermentation to enhance the biological potential and phytochemical contents of the red jasmine rice for nutraceutical and cosmetic applications. In this study, enhancing the biological properties of red jasmine rice extract (RJE) related to anti-aging purpose along with the augmenting its phytochemical profile through using Lactobacillus fermentation were thus investigated for the first time. In addition, to ensure the safety of the extract, irritation and cytotoxicity tests were executed using Hen's egg chorioallantoic membrane (HET-CAM) and human skin cell lines, respectively. The findings demonstrated that the Lactobacillus fermented RJE could constitute a novel functional bioactive ingredient for nutraceutical and cosmetic applications.
MATERIAL AND METHODS
Chemical materials
Ammonium thiocyanate, borate, calcium chloride dihydrate, ferrous sulfate (FeSO4), Folin-Ciocalteu reagent, potassium chloride (KCl), potassium persulfate, sodium acetate (CH3CO2Na) and sodium carbonate were obtained from Loba Chemie. Ascorbic acid, beta-arbutin, collagen, collagenase, gallic acid, kojic acid, L-DOPA, L-tyrosinase, linoleic acid, lipopolysaccharide (LPS), mushroom tyrosinase, nitric oxide (NO), sodium periodate, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), Trolox and 3,4-dihydroxyphenylcetic acid were acquired from Sigma Aldrich. Dimethyl sulfoxide (DMSO), ethanol, formic acid, Griess reagent, hydrochloric acid (HCl) and methanol were attained from RCI Labscan Ltd. Iron (II) chloride was obtained from Merck. Dulbecco's Modified Eagle Medium (DMEM) was purchased from Gibco. While sodium lauryl sulfate (SLS) was purchased from Namsiang, Chiang Mai, Thailand.
Plant materials and probiotics
Red jasmine rice was obtained from Phrae Province, Thailand. The stock solutions of probiotics including Lactobacillus plantarum BG112 and Lactobacillus rhamnosus (Lyofast LRB) were purchased as a lyophilized pellet (Sacco s.r.l, Cadorago, Como, Italy). The cultures were prepared to produce a final concentration of 109 CFU/ml.
Extract preparation and probiotic fermentation
The decoction method was used to produce the extracts. Red jasmine rice was soak in deionized water (DI water) in ratio 1:2 followed by heating at 80°C for 10 min while stirring continuously. Then the sample was filtered. The RJE solution was divided into three groups; RJE, served as a negative control; the second group was fermented with L. plantarum and the third was fermented with L. rhamnosus.
The two sample groups of probiotic fermented-red rice extract were prepared following the modified method of Wattananapakasem et al. (2021). Briefly, the RJE solution was individually mixed with 2% w/w of each type of probiotics. The mixture was incubated at 37°C for 24 h; then the fermented sample extracts were collected using centrifugation. Finally, the supernatant solutions of all groups were freeze-dried at 0.2 mbar and -40°C for two days by freeze dryer (Christ, Germany) while maltodextrin served as a carrier. The obtained extracts in powder form were stored at -20°C until used.
Determination of antioxidant activity
DPPH scavenging assay
DPPH scavenging activity of each extract was assayed following Poomanee et al. (2015) with modifications. The aqueous sample solution was mixed with 167 μM DPPH dissolved in ethanol and stored in the dark for 30 min. Then, the absorbance of the test solution was measured at 520 nm using microplate reader (SPECTROstar Nano, BMG Labtech, Germany).
The percentage of inhibition was calculated according to the formula:
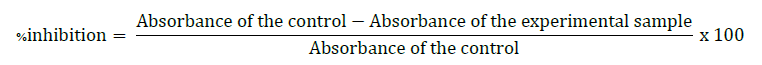
Ascorbic acid and Trolox served as positive controls. The concentration that reduced the absorbance by 50% (IC50) was also calculated from the regression curve.
ABTS scavenging assay
ABTS scavenging activity of each extract was performed using the method of Maneechai et al. (2023) with modifications. The ABTS stock solution, consisting of ABTS radical cation was prepared by reacting 7 mM ABTS in DI water with 140 mM potassium persulfate in DI water and stored in the dark up to 16 h. The stock solution was then diluted using DI water. Each aqueous solution of the extract or positive controls were allowed to react with the ABTS working solution. The mixture was then measured using a microplate reader (SPECTROstar Nano, BMG Labtech, Germany) at a wavelength of 734 nm after storing in the dark for 6 min. The percentage of inhibition was calculated using the aforementioned formula. Ascorbic acid and Trolox served as positive controls. ABTS scavenging activity in terms of IC50 and Trolox equivalent antioxidant concentration (TEAC; mg of Trolox/g of sample) were reported.
Linoleic acid peroxidation assay
The inhibition of linoleic acid peroxidation of each extract was assayed according to Maneechai et al. (2023). The reaction mixture contained aqueous solution of each sample, 1.3% w/v linoleic acid in methanol, phosphate buffer saline (PBS) pH 7.0 and DI water. After that, APPH in PBS solution in the concentration of 46.35 mM was added to start the lipid peroxidation reaction and the mixture was then incubated in the dark at 45 to 50°C for 4 h. The oxidation status was determined using the ferric-thiocyanate method. The percentage of inhibition was calculated according to the aforementioned formula. Ascorbic acid and Trolox served as positive controls. Percent inhibition was plotted as a function of the concentration and IC50 of each extract was calculated.
Ferric reducing antioxidant power (FRAP) assay
The experimentation was performed following the method of Otang-Mbeng and Sagbo (2020) with modifications. The working FRAP reagent was freshly prepared by mixing 30 mM CH3CO2Na buffer pH 3.6, 20 mM ferric chloride solution, 10 mM TPTZ in 40 mM HCl and DI water. FeSO4 was used as a standard. Each extract aqueous solution was mixed with FRAP reagent and incubated at 37°C for 30 min. The absorbance of the colored complex was measured at 593 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, Germany). The antioxidant potential was calculated from the FeSO4 calibration curve and presented as FRAP value (mg of Fe2+/g of sample) and equivalent concentration of 1 mM standard (EC1), namely, the concentration of sample having the same absorbance with 1 mM of standard.
Determination of anti-enzymatic activity related to skin problem
Evaluation of tyrosinase inhibition potential
The inhibitory effect of tyrosinase of each extract was determined in a 96-well microplate following Poomanee et al. (2015) with slight modifications. Tested extract solution was mixed PBS pH 6.8 and 1.66 mM mushroom tyrosinase in PBS in equal volumes. Next, 0.85 mM of L-tyrosinase or L-DOPA substrates was added into the reaction and then incubated at room temperature for 20 min. The absorbance was finally measured using a microplate reader (SPECTROstar Nano, BMG Labtech, Germany) at 492 nm. Percentage inhibition was calculated using the aforementioned equation. Beta-arbutin, kojic acid and ascorbic acid were positive controls in this assay. The IC50 value was also calculated from the calibration curve of the concentration versus %inhibition.
Evaluation of anti-collagenase potential
Aqueous solution of each extract was mixed with 5 mM calcium chloride dihydrate, collagenase solution (0.1 mg/ml) and DI water in an Eppendorf tube. Then, collagen solution (1 mg/ml) was added. The mixture was incubated at 37°C. After 1 h, 125 mM borate buffer pH 7.5, 0.75 mM 3,4-dihydroxyphenylcetic acid and 1.25 mM sodium periodate were added. The mixture was incubated at 37°C for 10 min. Fluorescence of the tested sample was measured using a fluorescence microplate reader (SpectraMax M3, Molecular Devices, USA) at an excitation maximum of 545 and emission maximum of 612 nm. Percentage inhibition was calculated according to the aforementioned equation, while ascorbic acid served as a positive control. The IC50 value was also calculated.
Determination of phytochemical compounds
Total phenolic content
Total phenolic contents (TPC) of all samples were determined using the Folin-Ciocalteu method following Khammitham et al. (2023). Briefly, each aqueous solution extract was mixed with diluted Folin-Ciocalteu reagent in DI water at a ratio of 1:10, followed by adding 7.5% w/v sodium carbonate solution. The reaction mixture was then left at room temperature for 30 min. The absorbance was then measured at 765 nm using a UV-VIS spectrophotometer (Shimadzu UV-2600i, Japan). Gallic acid was used as a standard and the TPC of each sample was expressed as mg of gallic acid equivalent/g of the extract.
Total anthocyanin content
Total anthocyanin content (TAC) of all samples was determined using the pH differential method (Lee et al., 2005). Aqueous solutions of each extract were added into each buffer including 0.025 M KCl buffer pH 1.0 and 0.4 M CH3CO2Na buffer pH 4.5. The absorbance of mixture solutions was measured at 520 nm using a UV-VIS spectrophotometer (Shimadzu UV-2600i, Japan) and should be between 0.2 and 1.0. Then the appropriate dilution factor of tested sample with KCl buffer pH 1.0 and CH3CO2Na buffer pH 4.5 was measured at both 520 and 700 nm. TAC was calculated according to the equation:

where A = (A520 - A700) pH1.0 - (A520 - A700) pH4.5
MW = 449.2 g/mol for cyanidin-3-glucoside
DF = dilution factor of sample and buffer
ε = 26,900 M
1 = pathlength in cm
103 = factor for conversion from g to mg
After anthocyanin pigment was calculated, cyanidin-3-glucoside equivalent in mg/L was changed to mg of cyanidin-3-glucoside equivalent/g of the extract. The result was reported as monomeric anthocyanin pigment.
SR-FT-IR microspectroscopy
To evaluate the alteration of phytochemical constituents of the sample after fermentation, SR-FT-IR was carried out to investigate the functional group using IR Beamline BL4.1 (IR Spectroscopy & Imaging) by IR spectrometer (Vertex70, Bruker Optics). Accessories - Diamond EX’Press Compression cell 1.6 mm STJ-0169 was attached to an FT-IR Microscope (Hyperion 2000, Bruker Optics) having the measurement system-Mercury Cadmium Telluride (aarrow band MCT). It could measure infrared light absorption ranging from 4,000 to 700 cm-1. Using OPUS 7.5 (Bruker Optics, Germany) could set spectral measurement of transmittance type (resolution 4 cm-1 value was at 64 scans). Samples and negative control were left in desiccators for 3 h to actual remove moisture and to equilibrate to room temperature before scanning. The sample was placed on a clean diamond cell. The beam was emitted from the source, and the spectrum was collected. Data preprocessing was conducted using OPUS Program, Version 7.5. Intensity measurements were determined on the recording the height of the absorbance bands of deconvoluted spectra from baseline.
Determination of anti-inflammatory effect
The selected fermented extract showing the greatest biological properties was determined, comparing with RJE, through the inducible NO in LPS-activated RAW 264.7 macrophage cell lines following Torres-Rodríguez et al. (2016). Briefly, RAW 264.7 macrophage cells (ATCC, USA) were seeded in 6-well plate for 2x105 cells/well and incubated in 5% of CO2 at 37°C. After 48 h of incubating, cells were stimulated by adding 1 µg/mL LPS and incubated for 24 h. LPS-activated RAW 264.7 macrophage cell lines were plated in 96-well plate for 1x104 cells/well in DMEM media and treated with tested extracts or triamcinolone acetonide, served as a positive control. Then, the treated cells were incubated for 24 h. NO production was analyzed using Griess reaction. Griess reagent was added with an equal volume of spent media. Then, the plate was incubated at room temperature for 5 min and the absorbance was read at 515 nm in a microplate reader (SPECTROstar Nano, BMG Labtech, Germany). Percentage of inducible NO inhibition was calculated using the aforementioned formula.
Determination of cytotoxic effect
Human derived keratinocyte (HaCaT) and human skin fibroblast (Detroit 551) cells were employed in MTT assay to elucidate the cytotoxicity of the extract on skin cells following Poomanee et al. (2015) with slight modifications. Cells were plated in 96-well plate for 1x104 cells/well in DMEM media and cultured for 24 h. Adherent cells were mixed with samples, followed by incubating with 5% of CO2 at 37°C. After 24 and 48 h, the medium was removed and then investigated cytotoxicity. MMT solution was added to each well and incubated for 4 h at 37°C, and the supernatant was removed. DMSO was then added to dissolve the formazan crystal. The absorbance was detected at 540 nm and 630 nm using a multimode reader (Bio-TEK Synergy H4, Aligant, USA). Percentage of cell viability was calculated by the following formula:
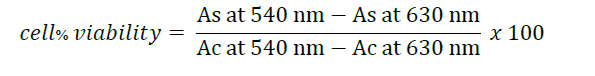
where As is absorbance of the sample and Ac is absorbance of the control. Control refers to untreated cells, while ascorbic acid serves as a positive control.
Determination of irritation test
The selected extract was investigated for its irritation profile using the HET-CAM method. Briefly, 30 μl of the extract was exposed to the HET-CAM. The reaction was observed after 5 min and 60 min under a microscope. Moreover, the time for the appearances of endpoint; including vascular hemorrhage, vascular lysis and vascular coagulation; were observed and recorded in sec over a period of 5 min. Then, irritation score (IS) was calculated according to the formula:
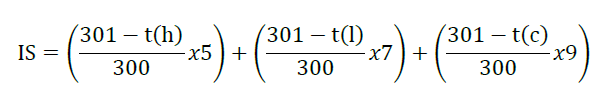
where t(h), t(l), and t(c) are the time (sec) of the action of vascular hemorrhage, vascular lysis, and vascular coagulation within 300 sec after dropping the extract, with 1% w/w SLS as a positive control. 9% v/v Normal saline solution served as negative control and vehicle. The experiment and IS was determined according to the method descripted by Chaiyana et al. (2017).
Statistical analysis
SPSS Version 23 was used for data analysis. All results were expressed as mean ± SD (each performed in triplicate). Tukey’s of one-way ANOVA was used to analyze. Statistically significant differences are shown by P value < 0.05.
RESULTS
Extraction and fermentation
RJE after freeze drying was a dry, light reddish powder with a pH of 6.91. While L. plantarum fermented RJE (BG) and L. rhamnosus fermented RJE (LRB) were dry, light brownish powder with a pH of 4.63 and 4.50, respectively. Percentage yield of RJE, BG and LRB were 3.14 ± 0.17, 1.95 ± 0.15, and 1.90 ± 0.17% w/w, respectively.
Enhancement of antioxidant activities
Figure 1 revealed DPPH radical scavenging effects of RJE, BG and LRB in terms of IC50 which were 0.17 ± 0.01, 0.12 ± 0.03 and 0.14 ± 0.01 mg/ml, respectively. However, there did not significantly differ. Similar outcomes were observed regarding ABTS activity. The IC50 values of RJE, BG and LRB was 0.80 ± 0.11, 0.60 ± 0.06 and 0.64 ± 0.02 mg/ml, respectively. Correspondingly, TEAC value of the samples was 33.12 ± 7.27, 39.75 ± 9.97 and 38.84 ± 1.63, respectively, as demonstrated in Figure 1B. Noticeably, our result in Figure 1C showed a significant difference between three samples in terms of inhibitory effect against linoleic acid peroxidation through IC50 values of RJE, BG and LRB which were 11.56 ± 0.62, 1.38 ± 0.45 and 5.80 ± 0.33 mg/ml, respectively. The FRAP assay (Figure 1D) found that LRB exhibited 2.20 ± 0.20 mg/ml of EC1 and 0.48 ± 0.01 mM FeSO4/mg extract of FRAP values which indicated the enhancement of the reducing power of the extract. On the other hands, the EC1 of LRB did not significantly differ from that of RJE’s (2.58 ± 0.16 mg/ml) and the FRAP value of LRB also did not significantly different from BG, which was 0.45 ± 0.01 mM FeSO4/mg extract.
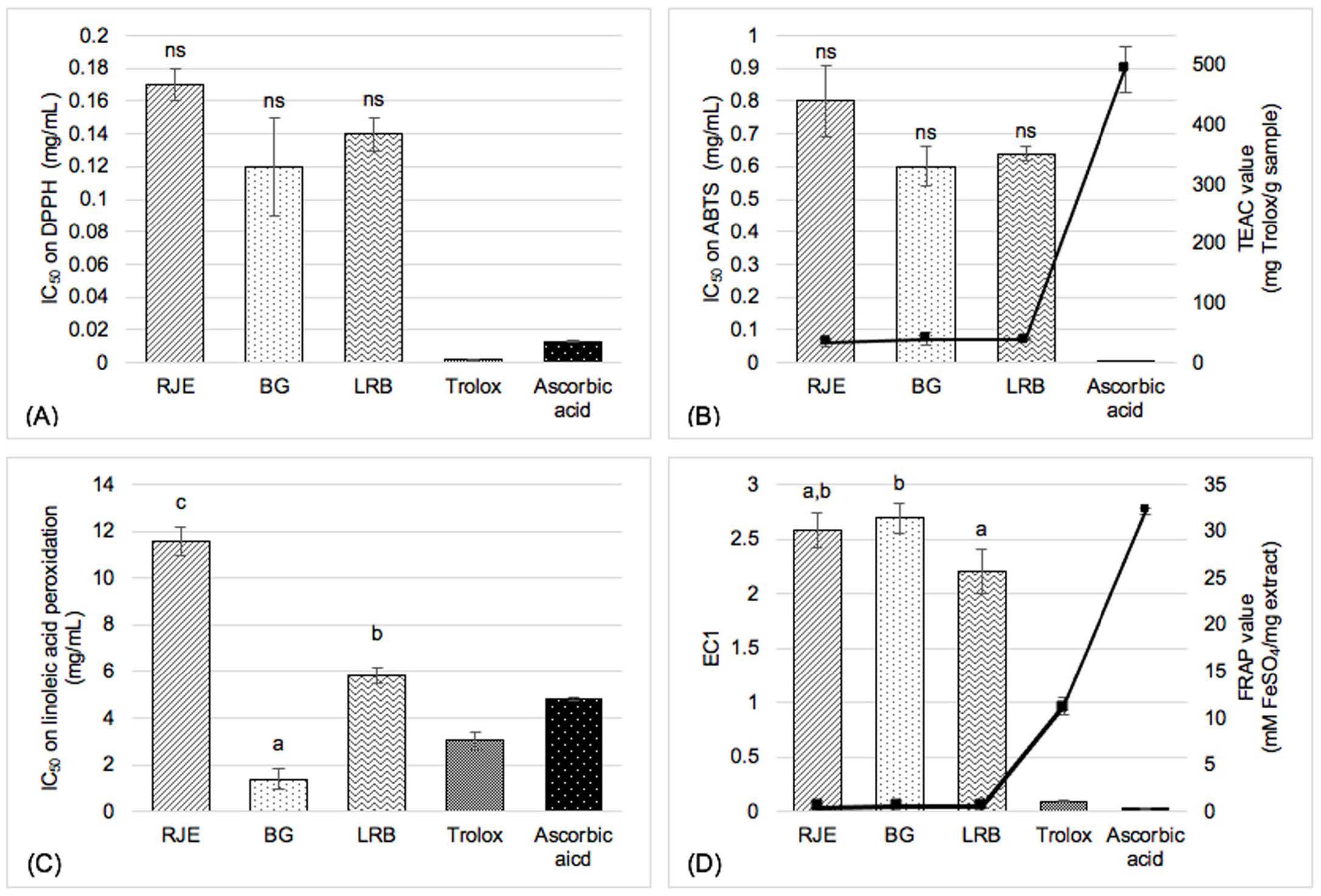
Figure 1. Potency of antioxidant activity evaluated by (A) DPPH (B) ABTS (C) linoleic peroxidation and (D) FRAP assay. Values are expressed as mean (n = 3) ± SD; RJE – Red jasmine rice extract, BG – L. plantarum BG112 fermented red jasmine rice extract, LRB – L. rhamnosus fermented red jasmine rice extract; Different superscripted letters in each column (a>b>c) present statistically significant differences between the three extracts (P<0.05) and ns is not significant difference (P>0.05).
Enhancement of anti-enzymatic effects related to skin aging
From the results, RJE had no capacity inhibiting hydroxylation of both L-tyrosine and L-DOPA as illustrated in Figure 2A while the whitening effects was shown apparently in BG and LRB. Moreover, the effects of BG and LRB through L-tyrosine hydroxylation process revealed no significant difference, with IC50 values of 0.45 ± 0.01 and 0.47 ± 0.11 mg/ml, respectively; whereas the IC50 of BG and LRB inhibiting tyrosinase enzyme with L-DOPA substrate was 0.47 ± 0.02 and 0.67 ± 0.03 mg/ml, respectively, as shown in Figure 2B. Figure 2C demonstrated no significantly different effect of inhibiting collagenase enzyme among the three extracts.
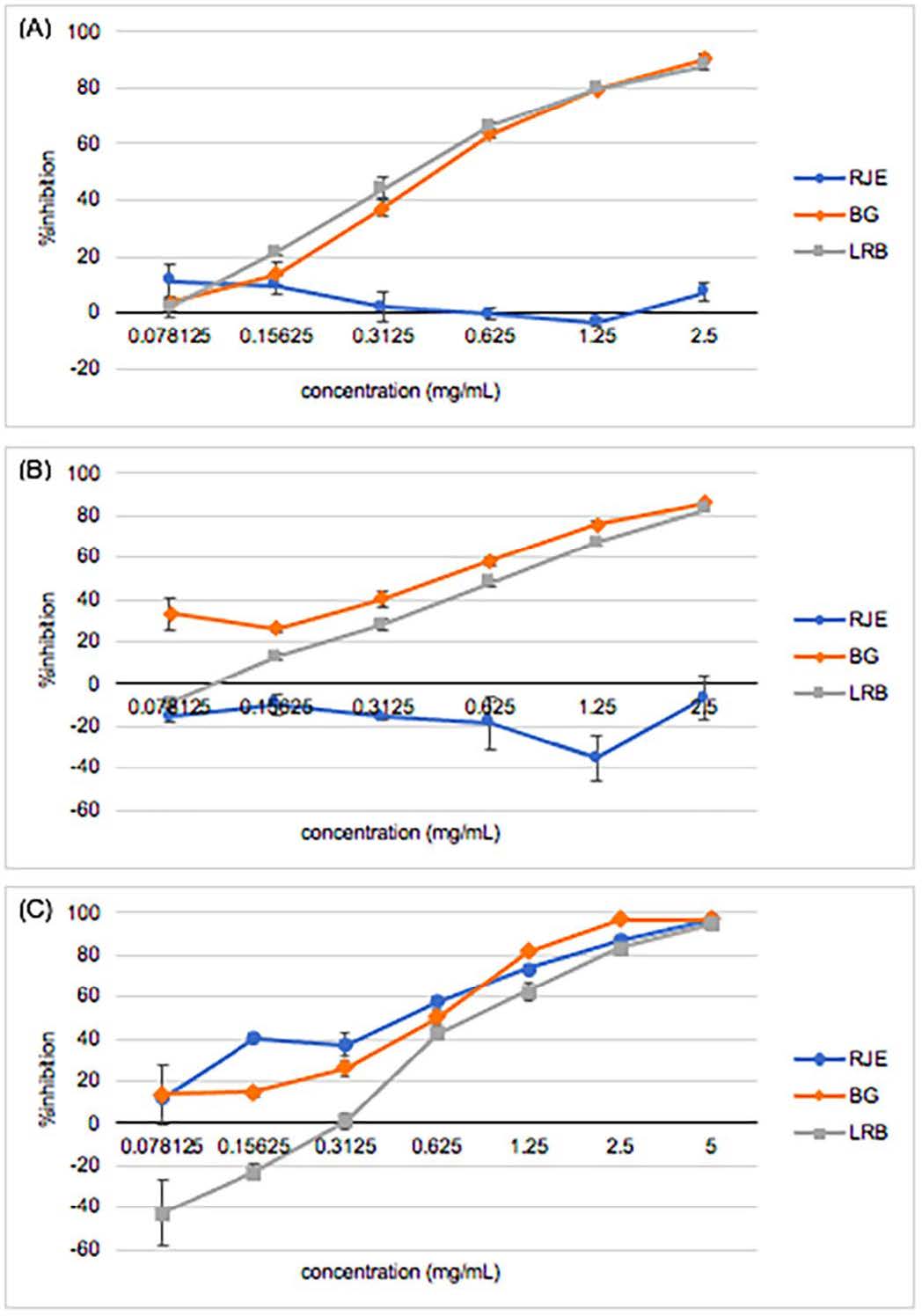
Figure 2. Ability of RJE – Red jasmine rice extract, BG – L. plantarum BG112 fermented red jasmine rice extract, and LRB – L. rhamnosus fermented red jasmine rice extract inhibiting (A) tyrosinase enzyme as L-tyrosine and (B) L-DOPA were substrate and (C) collagenase enzyme.
Augmentation of phytochemical compounds
The contents of phytochemical compounds including TPC and TAC were investigated. TPC of RJE, BG and LRB were 32.66 ± 0.24, 46.72 ± 0.21 and 46.79 ± 0.12 mg gallic acid/g sample, respectively. Additionally, the TPC exhibited no difference between BG and LRB. According to TAC, RJE had 1.93 ± 0.68 mg cyanidin-3-glucoside/g sample, whereas BG and LRB had -3.56 ± 1.54 and -5.79 ± 1.55 mg cyanidin-3-glucoside/g sample, respectively, indicating no anthocyanins in the fermented samples.
SR-FT-IR
Alteration in functional groups of the probiotic-fermented extracts and nonfermented extracts were illustrated by SR-FTIR. The result illustrated in Figure 3. In our study, PCA was employed to separate each biochemical composition to investigate its correlation with different types of samples. PCA score plots of PC1 (54%) and PC2 (11%) explain the correlation between the functional groups of the phytochemical compositions within different probiotic-fermented extracts and those of the RJE as a control treatment (Figure 3A). Differences in the functional groups found in the RJE were separated as shown in Figure 3B containing 1,687, 1,610, 1,515, 1,436, 1,280, 1,164, 1,147, 1,108, 1,072, 1,014 and 917 cm-1, whereas PC2 as shown in Figure 3C consisted of 1,143, 1,022 and 983 cm-1. Different types of fermented extracts could be divided using PC2 loading. The wavenumber at approximately 1,690 cm-1, related to C=O stretching vibration that is H-bonded and around 1,600 cm-1 was related to stretching vibrations of aromatic -C=O and -C=C bonds in carboxylic acid anions (Fatchiyah et al., 2020; Pompeu et al., 2018). The peak at 1,460-1,438 cm-1 is corresponds to C-H bending; alkane, methyl group; O-H bending; carboxylic acid. The bands in the range of 1,280 to 1,020 cm-1 which correspond to the C-O stretching vibrations in glycosidic bonds (Fatchiyah et al., 2020; Schulz and Baranska, 2007; Wongsa et al., 2022). These results showed that the peaks at specific wavenumbers were associated with phenolic structure. Generally, the region ranges of 1,755-1,400 cm-1 and 1,000-870 cm-1 are considered as the distinguishing wavelengths for phenolic compounds (Abbas et al., 2017). The results corresponded with Samyor et al. (2016)’s study reporting the presence of carbonyl (C=O) linkages were identified as the peaks at 1076 and 1,162 cm-1. The FT-IR band of C=O stretching is chiefly due to the presence of the carboxyl (–C=O) group of phenolic compounds of pigmented rice cultivars. Additionally, as shown in Figure 3D, at the peak of 1027 and 1,010 cm-1, BG and LRB showed the higher peaks indicating the increase in the carboxylic acid functional groups. Also, BG illustrated the higher peak.
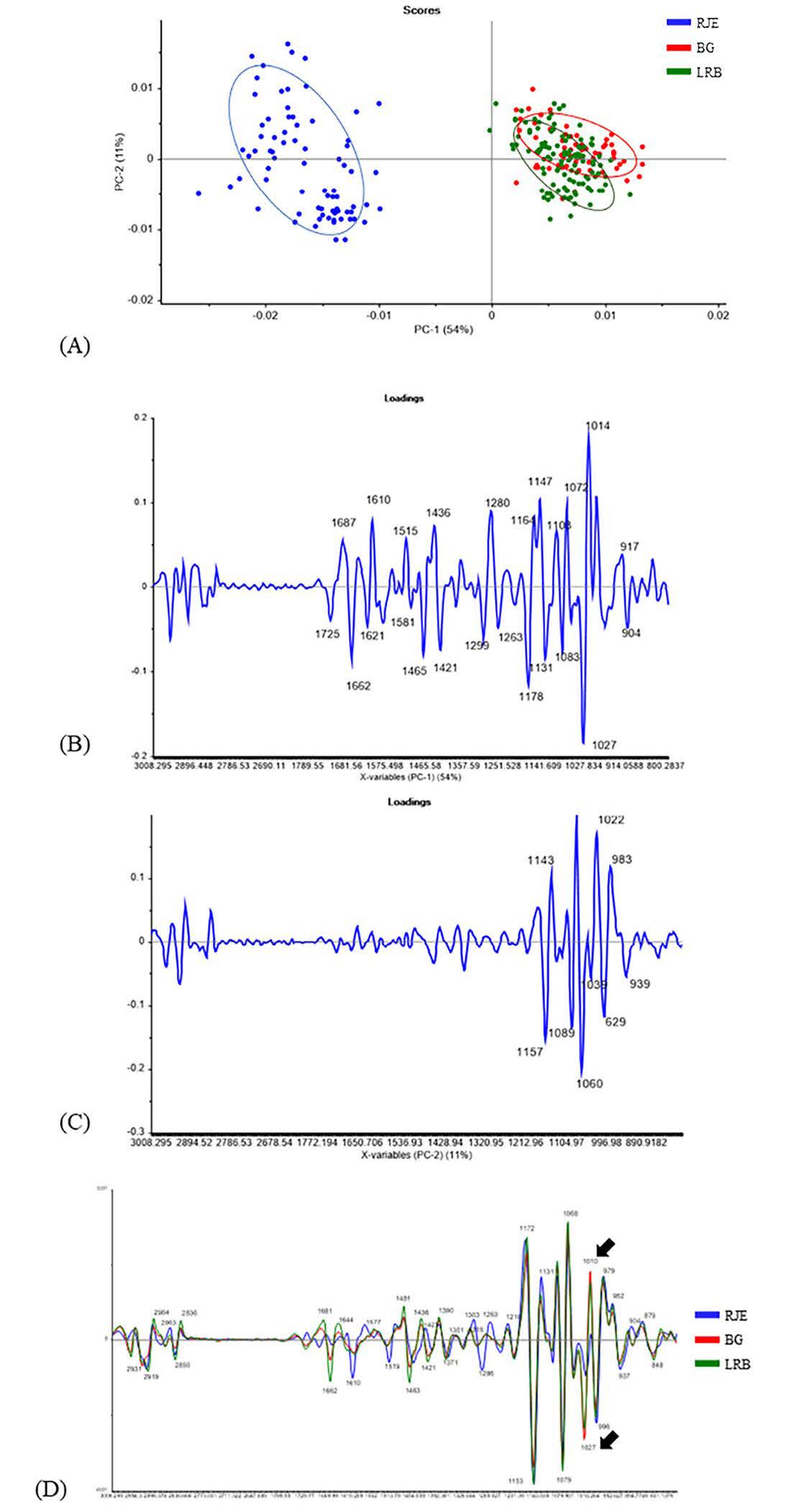
Figure 3. (A) PCA of RJE – Red jasmine rice extract, BG – L. plantarum BG112 fermented red jasmine rice extract, and LRB – L. rhamnosus fermented red jasmine rice extract; (B) PCA score plots of PC1 (54%); (C) PCA score plots of PC2 (11%); (D) PCA score plots of all samples.
Enrichment of anti-inflammatory effect
Percent inhibition of producing NO was shown in Figure 4. At the concentration of 0.5 mg/ml, RJE and BG showed anti-inflammation in LPS-activated RAW 264.7 macrophages that was 0.80 and 0.86 times of the anti-inflammatory drug, triamcinolone acetonide, respectively.
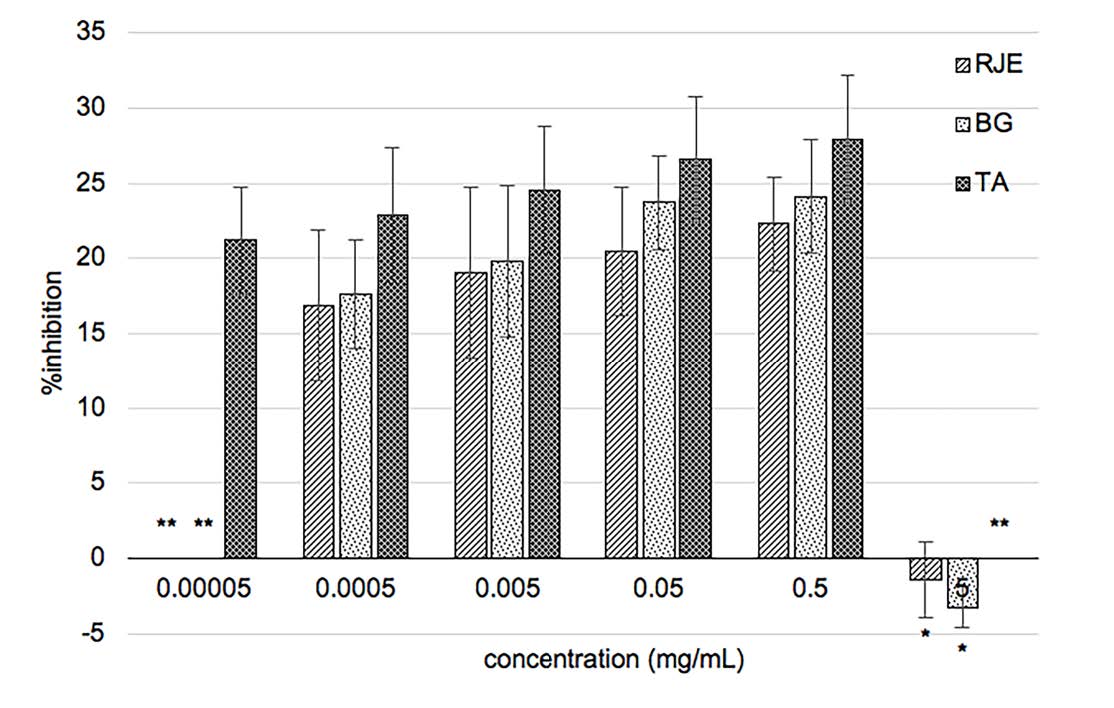
Figure 4. Percent inhibition of nitric oxide production of RJE – Red jasmine rice extract, BG – L. plantarum BG112 fermented red jasmine rice extract, and TA –triamcinolone acetonide; * means the color of extract interferes with the absorbance; ** stands for not analyzed.
Cytotoxic effects of the extracts
The viability of human skin cell after exposed with RJE and BG shown in Figure 5. In general, the percentage of cell viability was more than 80%, implying that the extract was nontoxic; within 60 to 80% meant weak; 40 to 60% was moderate and below 40% indicated strong cytotoxicity (Lopez-Garcia et al., 2014). When exposed to <0.25 mg/ml of RJE and BG for 24 h, HaCaT cell viability was more than 80%. While in 48 h, the concentration of 0.125 mg/ml and below was nontoxic. At 0.016 mg/ml of BG didn’t damage HaCaT cells; contrariwise, it could induce cell proliferation as expressed in Figure 5A and 5B. Additionally, it was statistically significant difference (P <0.05) between BG and RJE after 24 h and 48 h of exposure. In Figure 5C and 5D showed that RJE and BG were not toxic to Detroit 551 cells; nonetheless, Detroit 551 viability exhibited a time-dependent effect.
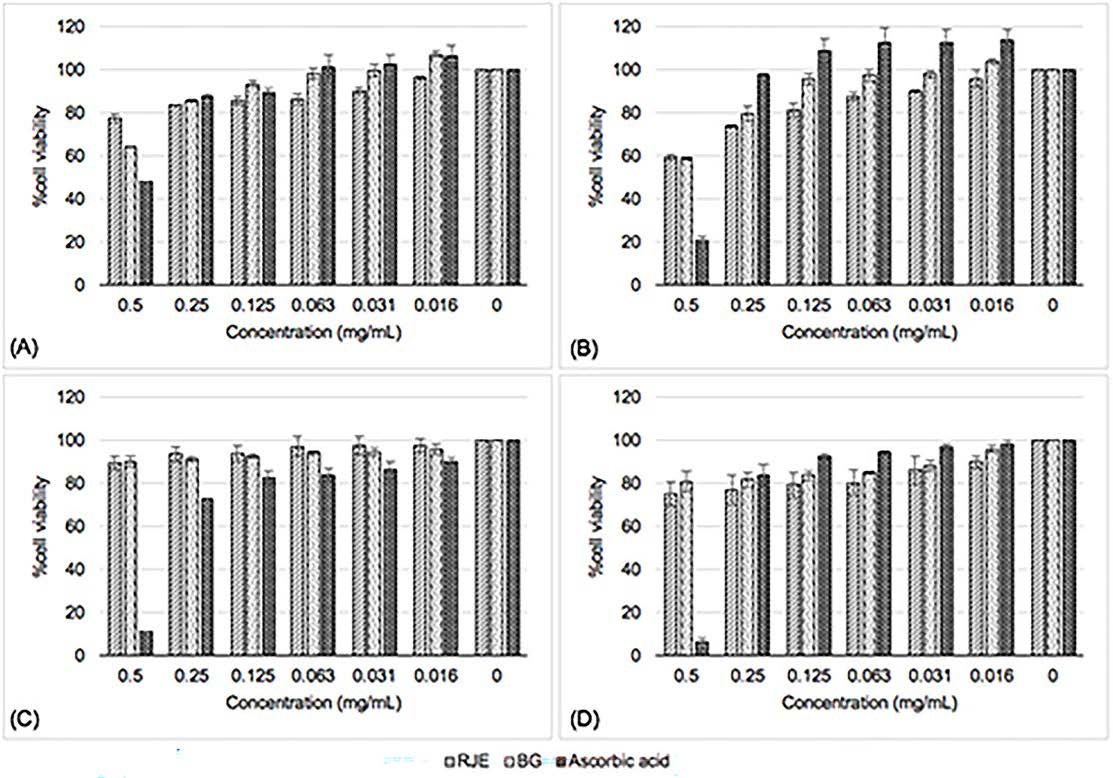
Figure 5. Cytotoxicity of RJE – Red jasmine rice extract, BG – L. plantarum BG112 fermented red jasmine rice extract and ascorbic acid on (A) HaCaT cells after 24 h (B) HaCaT cells after 48 h (C) Detroit 551 cells after 24 h and (D) Detroit 551 cells after 48 h.
In vivo irritation test
As shown in Figure 6, no vascular hemorrhage, lysis and coagulation were noted after being exposed to 5 mg/ml of BG and normal saline solution after 60 min of experimenting. In contrast, 1% w/w SLS as a positive control produced an obvious vessel lysis after 60 min with 12.8 ± 0.2of IS as shown in Figure 6(A)-below.
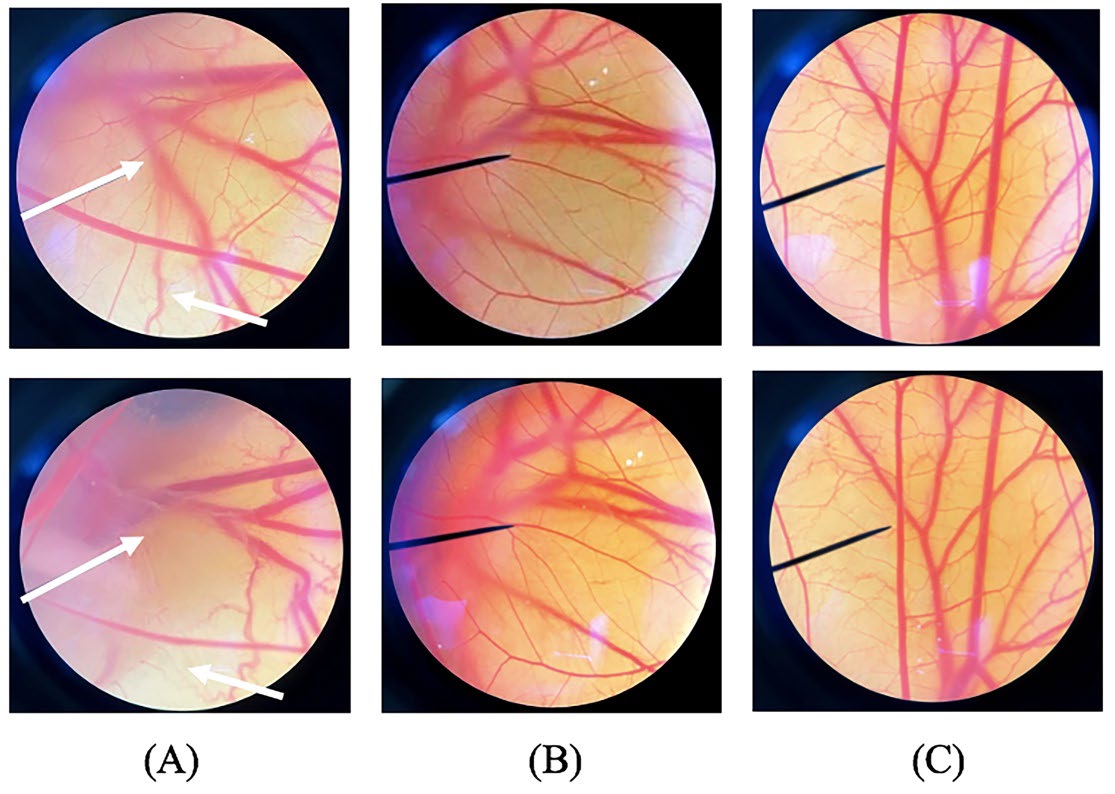
Figure 6. HET-CAM photograph under microscopy before applying (upper) and at 60 min (below) of exposed to (A) 1% w/w SLS, (B) normal saline solution, and (C) 5 mg/ml of L. plantarum BG112 fermented red jasmine rice extract.
DISCUSSION
Numerous studies have shown that Lactobacillus fermentation potentially improved the biological potential, including antioxidant, anti-inflammatory, and anti-photoaging properties, as well as phytochemical contents of the natural extracts which can enhance the value of processed plants, offering significant benefits over non-fermented ones, both in health aspects and economic value (Zubaidah et al., 2012; Williams and Hekmat, 2017; Jin et al., 2019; Park et al., 2021). From the result, pH of the extract declined through the fermentation process. Zubaidah et al. (2012) reported that lactic acid bacteria converted glucose in rice extract to organic acids, for example lactate and acetate. Therefore, the accumulation of lactic acid from bacteria also decreased the pH of the solution extract. However, selecting the appropriate strain of probiotics to be fermented with plant should be concerned properly (Shin et al., 2018). Herein, two Lactobacillis spp. were chosen to be studied including L. plantarum and L. rhamnosus. Both species can cultivate with red rice, furthermore it can enhance phytochemical compounds and its bioactivities. (Saman et al., 2011; Williams and Hekmat, 2017; Zubaidah et al., 2012) L. plantarum has been widely applied in pharmaceutical industries because of its antioxidation and anti-inflammation abilities (Arasu et al., 2016). However, L. rhamnosus is popularly used owing to its abilities to promote wound healing, reduce skin inflammation and delay UV-induced skin tumor development (Ho et al., 2021). Our study found that BG and LRB exerted higher DPPH and ABTS radical scavenging effects than RJE with no significant difference. Moreover, BG presented the highest inhibitory effect against linoleic acid peroxidation followed by LRB and RJE. This finding indicates that probiotic fermentation could enhance the ability of terminating lipid peroxidation. Zubaidah et al. (2012) found that L. plantarum and L. rhamnosus have the highest viability when fermented with rice which can also enhance the plant’s potential. Previous report found that black rice extract fermented with Lactobacillus spp. had higher free radical scavenging activity than nonfermented black rice extract indicating that probiotic fermentation increased the number of antioxidant components, specifically polyphenol compounds, which could improve its radical-scavenging ability (Wattananapakasem et al., 2021). However, one study reported that Lactobacillus spp. fermented plant extract exhibited weak anti-radical activity and did not significantly differ compared with plant extract (Shin et al., 2018). Our FRAP assay finding corresponded to that of the study of Jin et al. (2019) in which L. plantarum fermented with mango pulp increased FRAP value which may have been related to the higher TPC and was able to reduce the TPTZ-Fe3+ complex. All of antioxidant results indicated that three samples had the same free radical scavenging ability, yet probiotic-fermented samples indicated stronger lipid peroxidation inhibition and greater reducing power.
Anti-enzymatic effects related to skin aging of samples were investigated including tyrosinase and collagenase. Tyrosinase is the key enzyme and rate-limiting enzyme for melanogenesis involving melasma, dark skin and age spots. RJE couldn’t inhibit tyrosinase enzyme. Nevertheless, fermenting RJE with probiotics apparently improved inhibitory effect against tyrosinase enzyme. In addition, BG had significantly stronger tyrosinase inhibition than LRB. The obvious stronger effect of the fermented extracts might have resulted from the generated acid compounds of the fermented samples owing to the decreased pH compared with that of RJE. Sangkaew et al. (2023) explained that fermented rice could generate several substrates inhibiting melanogenesis such as amino acids and fatty acids. However, ascorbic acid, kojic acid and β-Arbutin offered better activity than extracts. Collagen is the main structural connective tissue in the dermis skin playing a role in skin firmness. Collagenase is a protease enzyme breaking down the peptide bind in collagen and elevating the presence of wrinkles. Our result showed all extracts had no ability inhibiting collagenase enzyme. No studied has compared the fermented red rice extract with red rice extract on collagenase inhibition until now. By the way, Seo et al. (2010) found that fermented rice could further increase the collagen in fibroblast skin by increasing the synthesis of pro-collagen.
According to phytochemical analysis, probiotics fermentation significantly increased the TPC of RJE. The augmented inhibitory effect on lipid peroxidation, reducing power and tyrosinase inhibition ability might be attributed to increased content of phenolic compounds which was also reported in several related studies (Jin et al., 2019; Wattananapakasem et al., 2021). RJE presented low TAC and was not observed in BG and LRB. The result was consistent with Sompong et al. (2011) reporting that red rice varieties were devoid of anthocyanin content. The color of kernels in purple rice mainly from cyanidin-3-glucoside and peonidin-3-glucoside, which were categorized as anthocyanins. These are found in abundance in dark purple rice, known as black rice. On the other hand, red rice varieties are rich in phenolic compounds. Ferulic acid was found as the highest bound phenolic acid identified in red rice varieties followed by ρ-coumaric and vanillic acid (Deng et al., 2013).
Alteration in functional groups of the probiotic-fermented extracts and nonfermented extracts were illustrated by SR-FTIR. From the results, it could be concluded that BG and LRB showed the increase in the carboxylic acid functional groups compared to RJE, thereby augmenting the number of phenolic acids or organic acids of which BG illustrated the higher peaks at 1027 and 1010 cm-1. This finding was in correspondence with the stronger antioxidant and anti-enzymatic potentials and higher TPC of probiotic-fermented extract compared with nonfermented extract as shown in Figure 1 and 2. These could be explained that the enhancement of phenolic contents in cereal grains resulted from the action of both endogenous and bacterial enzymes which from probiotics fermentation, including esterases, xylanases and phenoloxidases, thereby affecting their chemical structures, bioactivity, and bioavailability (Hole et al., 2012). To be more precise, future research should be found out the precise nutrients that increase following probiotics fermentation by HPLC analysis.
Since BG presented the strongest effect in terms of lipid peroxidation inhibition; therefore, BG was chosen to be studied for its anti-inflammation and safety evaluation compared with RJE. External stimuli such as UV and heat, induce a high level of NO in human skin. NO produces inflammatory cytokines blocking the defense of UV-inducing erythema and can provoke the melanogenesis of the skin (Cals-Grierson and Ormerod, 2004). BG exhibited greater potential inhibiting the production of NO than RJE in every concentration. A previous study demonstrated that ferulic acid, a phenolic acid compound found in red rice, showed inhibitory power on the release of pro-inflammatory factors such as NO (Seo et al., 2010) and probiotics fermented aqueous rice extract offered a product maintaining the inflammatory effect (Li et al., 2019).
To evaluate the safety of extracts, their cytotoxicity was determined on HaCaT and Detroit 551 cells, represented as human derived keratinocyte mainly found in epidermis and human skin fibroblast mainly found in dermis, respectively. The results indicated that a low concentration of BG could promote cell growth and be toxic at the high concentration, which was a similar trend to ascorbic acid’s effect. The previous study also reported that a large dose of ascorbic acid was toxic to skin cells but at a low amount activated skin cell proliferation (Wang, Yin, and Wang, 2016). The consequence of ascorbic acid on Detroit 551 cells was similar to keratinocytes as reported by Wang et al. (2016).
The HET-CAM test is an alternative method to detect ocular corrosive and severe irritation. HET-CAM is the membrane surrounding the placenta of a chick embryo, containing various blood vessels. It indicated that 5 mg/ml of BG was safe and would not cause any irritation. Though 1% w/w SLS, a positive control, produced severe irritation.
CONCLUSION
This study has proven that probiotic fermentation could raise biofunctional and bioactive phenolic compounds of red jasmine rice for which L. plantarum was an appropriate probiotic strain. Antioxidant in terms of lipid peroxidation inhibition and anti-tyrosinase effects showed better potential after probiotic fermentation. These potentials also correlated to increased total phenolic content. To clarify, SR-FT-IR showed that the alteration in functional groups of probiotics-fermented extracts at phenolic wavenumbers showed a higher peak than nonfermented extracts as shown in the PCA score plot. Fermented-red jasmine rice extract also enhanced the inhibitory effect on NO production indicating an augmented inflammatory potential. Additionally, the extract was safe to human skin cells in both epidermis and dermis layers; plus, it did not produce any irritation. Nevertheless, future research may need to investigate the safety of L. plantarum-red jasmine rice extract fermentation in human volunteers and develop cosmeceutical and nutraceutical products providing economical choices and value-added natural ingredients.
ACKNOWLEDGEMENTS
The authors truly appreciated the TA/RA Scholarship from the Graduate School, Chiang Mai University and Faculty of Pharmacy, Chiang Mai University for financially supporting. The authors would like to thank the Department of Biochemistry, Faculty of Medicine, Chiang Mai University for microbiology laboratory facilities and sincerely thanks Dr. Narisara Paradee and Dr. Adchara Prommaban for guiding, advising and counseling on cytotoxic analysis.
AUTHOR CONTRIBUTIONS
Panipak Pothirat was involved in data collecting and formal analysis and writing original draft. Pimporn Leelapornpisid, Worrapan Poomanee and Isara Wattananapakasem were involved in conceptualization, lab facilities, supervision, resources, funding and editing manuscript. Nara Yaowiwat was involved in resources. Isara Wattananapakasem was responsible for probiotic fermentation. Pimpisid Koonyosying supervised the cytotoxicity evaluation. Siriwan Nawong and Isara Wattananapakasem examined and summarized the IR experimental section.
REFERENCES
Abbas, O., Compère, G., Larondelle, Y., Pompeu, D., Rogez, H., and Baeten, V. 2017. Phenolic compound explorer: A mid-infrared spectroscopy database. Vibrational Spectroscopy. 92: 111-118.
Arasu, M.V., Al-Dhabi, N.A., Ilavenil, S., Choi, K.C., and Srigopalram, S. 2016. In vitro importance of probiotic Lactobacillus plantarum related to medical field. Saudi Journal of Biological Sciences. 23(1): S6-S10.
Cals-Grierson, M.M., and Ormerod, A.D. 2004. Nitric oxide function in the skin. Nitric Oxide. 10(4): 179-193.
Chaiyana, W., Punyoyai, C., Somwongin, S., et al. 2017. Inhibition of 5 alpha-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex Vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 9(10): 1105.
Christensen, H.R., Frokiaer, H., and Pestka, J.J. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of Immunology. 168(1): 171-178.
Deng, G.F., Xu, X.R., Zhang, Y., Li, D., Gan, R.Y., and Li, H.B. 2013. Phenolic compounds and bioactivities of pigmented rice. Critical Reviews in Food Science and Nutrition. 53(3): 296-306.
Probiotic Cosmetic Product Market [internet]. 2022 Mar. Rockville (MD): Fact.MR; [cited 2024 Mar 20]. Available from: https://www.factmr.com/report/4188/probiotic-cosmetic-products-market
Fatchiyah, F., Sari, D.R.T., Safitri, A., and Cairns, J.R. 2020. Phytochemical compound-and nutritional value in black rice from Java Island, Indonesia. Systematic Reviews in Pharmacy. 11(7): 414-421.
Francavilla, A., and Joye, I.J. 2020. Anthocyanins in whole grain cereals and their potential effect on health. Nutrients. 12(10): 2922-2942.
Ho, C.C., Ng, S.C., Chuang, H.L., et al. 2021. Seven traditional Chinese herbal extracts fermented by Lactobacillus rhamnosus provide anti-pigmentation effects by regulating the CREB/MITF/tyrosinase pathway. Environmental Toxicology. 36(4): 654-664.
Hole, A.S., Rud, I., Grimmer, S., Sigl, S., Narvhus, J., and Sahlstrom, S. 2012. Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus , Lactobacillus johnsonii , and Lactobacillus reuteri. Journal of Agricultural and Food Chemistry. 60(25): 6369-6375.
Jin, X., Chen, W., Chen, H., Chen, W., and Zhong, Q. 2019. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as starter culture to produce mango slurry: Microbiological, chemical parameters and antioxidant activity. Molecules. 24(23): 4394-4408.
Khammitham, T., Leelapornpisid, P., Chansakaow, S., Leelapornpisid, W., and Poomanee, W. 2023. Cleistocalyx nervosum var. paniala extract: Anti-acne, anti-oxidant, anti-inflammatory activities and safety for cosmeceutical applications. Natural and Life Sciences Communications. 22(4): e2023064.
Knackstedt, R., Knackstedt, T., and Gatherwright, J. 2020. The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Experimental Dermatology. 29(1): 15-21.
Lee, J., Durst, R.W., and Wrolstad, R.E. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International. 88(5): 1269-1278.
Lew, L.C., and Liong, M.T. 2013. Bioactives from probiotics for dermal health: functions and benefits. Journal of Applied Microbiology. 114(5):1241-1253.
Li, S.C., Lin, H.P., Chang, J.S., and Shih, C.K. 2019. Lactobacillus acidophilus-fermented germinated brown rice suppresses preneoplastic lesions of the colon in rats. Nutrients. 11(11): 2718-2734.
Lopez-Garcia, J., Lehocky, M., Humpolicek, P., and Saha, P. 2014. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. Journal of Functional Biomaterials. 5(2): 43-57.
Mack, D.R. 2005. Probiotics-mixed messages. Canadian family physician Medecin de Famille Canadien. 51(11): 1455-1464.
Maneechai, P., Leelapornpisid, P., and Poomanee, W. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Natural and Life Sciences Communications. 22(2): e2023029.
Otang-Mbeng, W. and Sagbo, I.J. 2020. Anti-melanogenesis, antioxidant and anti-tyrosinase activities of Scabiosa columbaria L. Processes. 8(2): 236-246.
Park, S.H., Kim, J.G., Jang, Y.A., Bayazid, A.B., and Ou Lim, B. 2021. Fermented black rice and blueberry with Lactobacillus plantarum MG4221 improve UVB-induced skin injury. Food and Agricultural Immunology. 32(1): 499-515.
Pompeu, D., Larondelle, Y., Rogez, H., Abbas, O., Pierna, J., and Baeten, V. 2018. Characterization and discrimination of phenolic compounds using fourier transform raman spectroscopy and chemometric tools. Biotechnology, Agronomy, Society and Environment. 22(1): 13-28.
Poomanee, W., Chaiyana, W., Intasai, N., and Leelapornpisid, P. 2015. Biological activities and characterization of the pod extracts from sompoi (Acacia concinna Linn) grown in northern Thailand. International Journal of Pharmacy and Pharmaceutical Sciences. 7(5): 237-241.
Saman, P., Fuciños, P., Vázquez, J.A., Pandiella, S.S. 2011. Fermentability of brown rice and rice bran for growth of human Lactobacillus plantarum NCIMB 8826. Food Technology and Biotechnology. 49: 128-132.
Samyor, D., Deka, S., and Das, A. 2016. Phytochemical and antioxidant profile of pigmented and non-pigmented rice cultivars of Arunachal Pradesh, India. International Journal of Food Properties. 19: 1104-1114.
Sangkaew, O., Prombutara, P., Roytrakul, S., and Yompakdee, C. 2023. Metatranscriptomics reveals sequential expression of genes involved in the production of melanogenesis inhibitors by the defined microbial species infermented unpolished black rice. Microbiology Spectrum. 11(2): 1-17.
Schulz, H., and Baranska, M. 2007. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vibrational Spectroscopy. 43(1): 13-25.
Seo, Y.K., Jung, S.H., Song, K.Y., Park, J.K., and Park, C.S. 2010. Anti-photoaging effect of fermented rice bran extract on UV-induced normal skin fibroblasts. European Food Research and Technology. 231(2): 163-169.
Shin, D., Lee, Y., Huang, Y.H., et al. 2018. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complementary Medicine and Therapies. 18(1): 196-206.
Sompong, R., Siebenhandl-Ehn, S., Linsberger-Martin, G., and Berghofer, E. 2011. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chemistry. 124(1): 132-140.
Torres-Rodríguez, M.L., García-Chávez, E., Berhow, M., and De Mejia, E.G. 2016. Anti-inflammatory and anti-oxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated RAW 264.7 macrophages. Journal of Ethnopharmacology. 188: 266-274.
Wang, G., Yin, T., and Wang, Y. 2016. In vitro and in vivo assessment of high-dose vitamin C against murine tumors. Experimental and Therapeutic Medicine. 12(5): 3058-3062.
Wattananapakasem, I., Penjumras, P., Malaithong, W., Nawong, S., Poomanee, W., and Kinoshita, H. 2021. Effect of heat-moisture treatment of germinated black rice on the physicochemical properties and its utilization by lactic acid bacteria. Journal of Food Science and Technology. 58(12): 4636-4645.
Williams, M., and Hekmat, S. 2017. Lactobacillus rhamnosus GR-1 in fermented rice pudding supplemented with short chain inulin, long chain inulin, and oat as a novel functional food. Fermentation. 3(4): 55-67.
Wongsa, P., Phatikulrungsun, P., and Prathumthong, S. 2022. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Scientific Reports. 12(1): 6631-6642.
Yodkeeree, S., Thippraphan, P., Punfa, W., Srisomboon, J., and Limtrakul, P. 2018. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Natural Product Communications. 13(8): 967-972.
Zhong, W., Millsap, K., Hobrzanska, H.B., and Reid G. 1998. Differentiation of Lactobacillus species by molecular typing. Applied and Environmental Microbiology. 46(7): 2418-2423.
Zubaidah, E., Nurcholis, M., Wulan, S.N., and Kusuma, A. 2012. Comparative study on synbiotic effect of fermented rice bran by probiotic lactic acid bacteria Lactobacillus casei and newly isolated Lactobacillus plantarum B2 in wistar rats. APCBEE Procedia. 2: 170-177.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Panipak Pothirat1, Isara Wattananapakasem2, Pimporn Leelapornpisid1, Pimpisid Koonyosying3, Nara Yaowiwat4, Siriwan Nawong5, and Worrapan Poomanee1, 6, *
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, 50200, Thailand.
2 Program of Food Technology, Maejo University-Phrae Campus, Phrae, 54140, Thailand.
3 Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand.
4 School of Cosmetic Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand.
5 Synchrotron Light Research Inst. (SLRI), Nakhon Ratchasima, 30000, Thailand.
6 Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, 50200, Thailand.
Corresponding author: Worrapan Poomanee, E-mail: worrapan.p@cmu.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: February 1, 2024;
Revised: April 4, 2024;
Accepted: April 22, 2024;
Online First: April 25, 2024

