
Population Structure and Functional Diversity of Rice Landraces: A Review
Tonapha Pusadee, Sansanee Jamjod, Chanakan Prom-u-thai, Pennapa Jaksomsak, and Benjavan Rerkasem*Published Date : April 9, 2024
DOI : https://doi.org/10.12982/NLSC.2024.027
Journal Issues : Number 2, April-June 2024
Abstract Rice landraces or local varieties that have remained in cultivation on-farm are embedded within their agroecological and cultural environment. Rice landraces are genetically diverse, but with a variation that can be well structured, not random. This review examines the population structure and functional diversity revealed in the rice germplasm acquired from farmers in recent decades. Sizable areas of local varieties are found in parts of Asia with distinctive agroecological environment and cultural heritage. A tally of variety names provides a first approximation of diversity, a frequency distribution of the varieties describes a basic structure of diversity in an agroecosystem. Genetic variation is detectable by microsatellites analysis or expressed in specific functional traits, at various organizational levels of the population, even among those exhibiting uniform appearances. The population dynamics of genetically diverse landraces perpetuated by many farmers are shaped by variations in the bio-physical environment and management practices, but those relying on one or two farmers for their maintenance face a risk of extinction. Diversity in widely grown varieties may either be within the farmer’s seed cache, or among populations of the same variety maintained by different farmers. Local rice varieties or landraces continue to play a crucial role in rice farming, the economic value of some is enhanced by their unique grain quality features. Genetically diverse rice landraces that either contribute towards meeting the household’s rice requirement or those that have become commercially successful, also provide an important service in the in situ conservation of germplasm.
Keywords: Rice, Molecular analysis, Diversity, Adaptation
Funding: Partial support for this research from Chiang Mai University and Lanna Rice Research is gratefully acknowledged.
Citation: Pusadee, T., Jamjod, S., Prom-u-thai, C., Jaksomsak, P., and Rerkasem, B. 2024. Population Structure and Functional Diversity of Rice Landraces: A Review. Natural and Life Sciences Communications. 23(2): e2024027.
INTRODUCTION
Rice (Oryza sativa L.), the main source of the food energy for most people in Asia, is embedded in the continent’s numerous cultures and traditions. Local rice varieties or landraces, are recognized by their grain quality features (Rerkasem and Rerkasem, 2002; Appa Rao et al., 2006a; Calingacion et al., 2014), along with their unique morphology and well-established local names that are generally used to identify local crop varieties or landraces (Brown, 1978; Harlan, 1992). Excluding the seed preserved in cold storage in gene banks, management of the local rice germplasm, the key input in rice farming for most of the history of rice cultivation, is in the hand of a vast number of farmers, in contrast to the centralized breeding and seed system for the development and maintenance of modern and other major rice varieties. Selection for traits deemed useful in rice cultivation and consumption was taking place long before the arrival of rice science. Double cropping of rice (two rice crops on the same land in one year) was already in practice in China’s southern provinces some time before 1000 AD, centuries before the understanding of the photo-thermo control of flowering in plants, with germplasm introduced from Indochina (Ho, 1956; Dalrymple, 1986). Japan’s rono varieties were selected in the 19th century for responsiveness to nitrogen fertilizer, available in an organic form as soybean cake from Manchuria (Hayami and Ruttan, 1985). The area under local rice varieties has declined since the final decades of the 20th century, when their replacement by a limited number of economically more viable mega-varieties has accelerated, as exemplified in India (Hammer et al., 1996), Laos (Appa Rao et al., 2006b) and Thailand (Rerkasem, 2015). The germplasm preserved in national and international gene banks around the world provides a safeguard against genetic losses (Lee et al., 2020). In the meantime, local rice varieties that remain important to rice farming can still be found in many parts of Asia, e.g. in Laos (Appa Rao et al., 2006b), India (Roy et al., 2014), Thailand (Rerkasem, 2015) and Indonesia (Thomson et al., 2009). Interests in rice landraces often revolves around traits for deployment in breeding programmes. This review offers an analysis of the diverse ways genetic variation occurs in on-farm rice germplasm across various parts of Asia. This variation is influenced by the local environment and farmers, who are in turn provided with a readily available resource.
Local rice varieties are well known for their genetic diversity, but with a variation that can be well structured and not random or haphazard. This paper examines the ways in which local rice varieties are distributed, and the various patterns of diversity identified by molecular analyses of microsatellite (simple sequence repeats) markers, and functional traits. The review begins with reports of the recent prevalence of local rice varieties, in different regions in Thailand and Laos, to demonstrate their eco-geographical and cultural contexts. The frequency distribution of the rice varieties in upland villages in Laos and a flood prone agroecosystem in Thailand is used to highlight their uneven distribution. Population structure and functional diversity of local rice varieties, originating from farmers’ fields in recent decades, were described by the pattern of variation observed in selected varieties extensively grown across villages, provinces and sometimes nationally. Variations among individual seeds in a seed cache or among plants growing together in a field identified by molecular analyses and in heritable traits are examined. Population structure of the widely grown varieties is elucidated by analysis of the molecular variance. A loss of diversity in the landraces that have become improved local varieties by pure line selection is described, and changes in their adaptability highlighted. Awareness of the possible structure of diversity within and among local rice varieties should be beneficial in germplasm management, utilization and conservation.
ON-FARM DISTRIBUTION OF LOCAL RICE VARIETIES
Safeguards against genetic losses from the replacement of local crop varieties in farmers’ field by modern varieties (Harlan, 1975) has been provided by the preservation of germplasm in national and international gene banks (Lee et al., 2020). The local rice varieties that may still be found on-farm in most rice growing countries of Asia in the twenty-first century (e.g. Appa Rao et al., 2006b; Bajracharya et al., 2006; Fukuoka et al., 2006; Thomson et al., 2009; Roy et al., 2014; Jamjod et al., 2017; Xiongsiyee et al., 2018), provide farmers with a readily available resource as well as enable the structure of their variation and their agroecological context to be studied. In Thailand, local rice varieties were planted in less than 3% of country’s rice area in the wet season of 2019, they nevertheless accounted for a sizable area in some provinces (Figure 1). The highest concentration of local rice varieties were found in regions with distinctive eco-geographical environment and cultural heritage, i.e. the mountainous northern provinces, areas prone flooding in the Central Plain and the South. The northern highlands, defined as areas at 500 m or higher in elevation and lower lying areas between the mountains (HDRI, 1999), are populated by ethnic minorities, each with their own distinctive culture and unique rice germplasm. Landraces that are adapted to the various regimes of flooding, i.e. with different flood depth, duration and the rate of water rise and fall, are found in the flood prone regions (Sommut, 2003). Local rice varieties in the South, under the influence of the Northeast as well as the Southwest monsoon, differ from varieties in the rest of the country with the growing season that commences 4-8 weeks later. Thai Muslims and ethnic Malays also make up a large proportion of the population in some southern provinces. Rice cultivation in Laos, with its unique germplasm of glutinous rice, still relies on local rice varieties to a large extent even though the area under modern and improved varieties has expanded (Linquist et al., 2006a). The richest diversity of local rice varieties were found in the northern region, populated by a diversity of ethnic groups who depend largely on upland rice for their subsistence (Appa Rao et al., 2006c). Eighty-five percent of the seed samples collected at the turn of the millennium were identified as glutinous by farmers, with an amylose content ranging from 0-15% (Schiller et al., 2006). Modern and improved rice varieties are defined as glutinous with an amylose content of 0-2% (Juliano and Villareal, 1993; Dela Cruz and Khush, 2000; TRKB, 2024a). Local rice varieties, on the other hand, are generally judged as glutinous by the opaque endosperm that contrasts with the translucent endosperm of non-glutinous rice, and confirmed by the glue-like stickiness of the cooked grain.
A first approximation of the diversity of local rice varieties in a location is provided by a simple tally of variety names, e.g. 15-30 varieties per village and 2-5 per household found in highland villages in Laos (Pandey and Sanamongkhoun 1998; Phanthaboun, 2009; Xiongsiyee et al., 2018) and Thailand (Dennis, 1987; Unthong et al., 2007), and 118 varieties in half a million hectare of flood prone rice land in Thailand (Sommut 2006). Where rice is produced for subsistence, several varieties are normally grown by each of the farming households, to meet a range of culinary, environmental and management requirements. For example, a glutinous variety is commonly grown for use in festive and ceremonial occasions and for brewing alcohol, by those who consume non-glutinous rice as the staple. An early maturing variety, identified by a prefix or suffix denoting earliness in the name (Rerkasem and Rerkasem. 1984; Appa Rao et al., 2006a), evens out labour demand at harvesting and ensures an early replenishment of the household rice supply. Adapted varieties, e.g. to a different temperature regime or water supply, provide solutions to variation in the physical environment. The rice crop is under an especially diverse soil condition under shifting cultivation (Kunstadter, 1978; Zinke et al., 1978; Yimyam, 2006). Cropping is moved regularly to a new field within the rotation cycle, while the burning of vegetation in the slash and burn cultivation causes changes in soil acidity and fertility, with an uneven distribution of the ash from burning contributing to even more variability. The basic structure of the population of rice landraces in a location as described by the frequency distribution of the variety names is demonstrated by a survey of 4 small villages in Luang Prabang province of Laos in 2007 (Table 1).
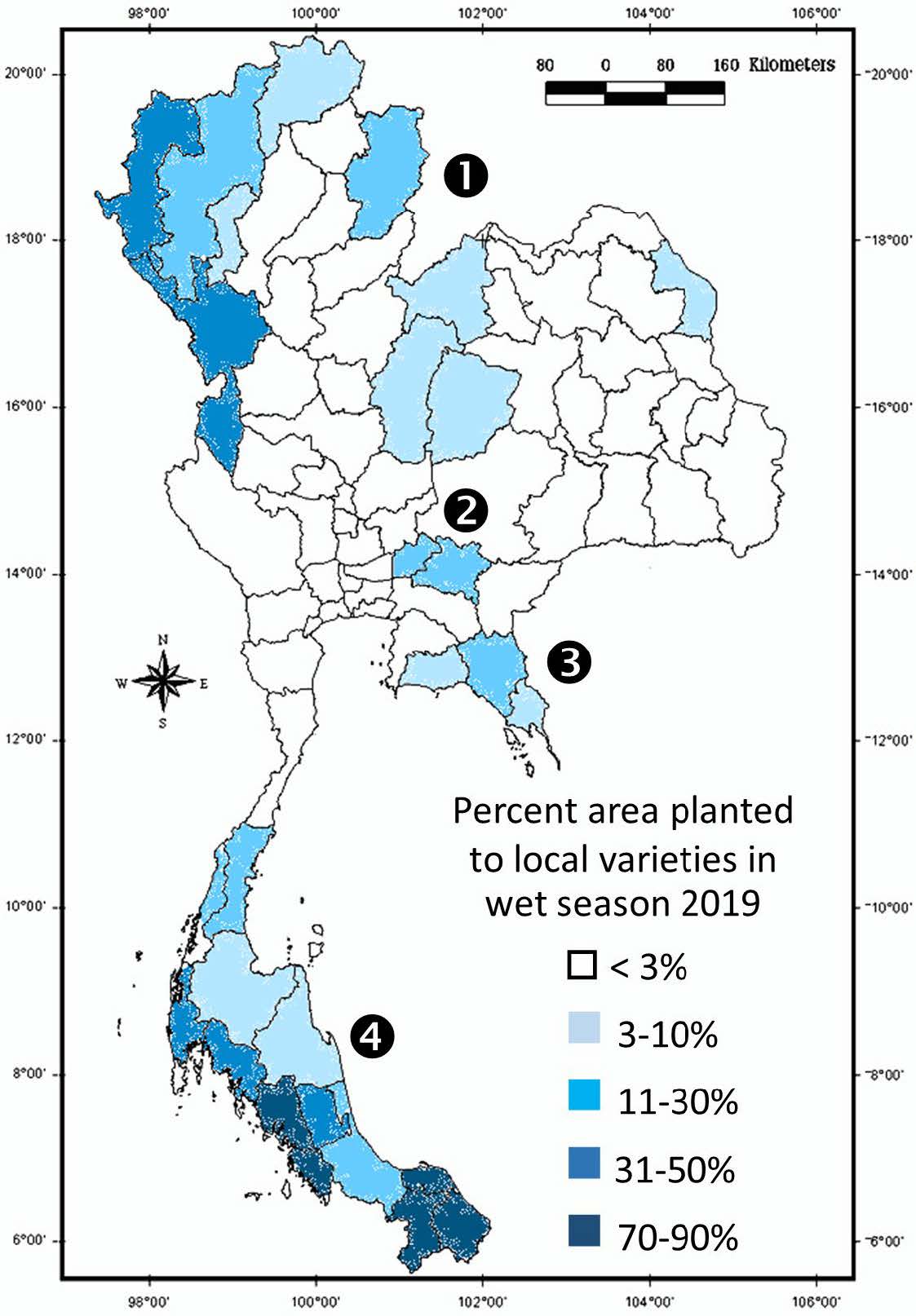
Figure 1. Distribution of area planted to local rice varieties in Thailand in the wet season 2019, by province and region ( 1 , mountainous northern; 2 ,flood prone; 3 , maritime provinces; 4 , Southern region)
Source: drawn from data in OAE (2024)
Table 1. A survey of local rice varieties in two villages in Phonxay district and two in Pak Ou district of Luang Prabang province of Laos in 2007.
|
Village |
Houayman |
Thapho |
Ladthahea |
Houayleung |
|
District |
Phonxay |
|
Pak Ou |
|
|
Number of households (HH) |
50 |
68 |
95 |
60 |
|
Population |
289 |
377 |
497 |
336 |
|
Ethnicity |
Khamu |
Khamu+Lao |
Tai Lue |
Khamu |
|
Number of rice varieties |
|
|
|
|
|
- All |
17 |
13 |
16 |
17 |
|
- Planting frequency1 |
66 |
48 |
72 |
64 |
|
- Planted by majorities of HHs2 |
3(76-94%) |
2(100%) |
1(100%)3 |
1(88%)3 |
|
- Planted by one HH in village |
7 |
7 |
5 |
3 |
|
- With glutinous endosperm |
16 |
12 |
15 |
15 |
|
Diversity index, H' |
2.248 |
2.060 |
2.321 |
2.419 |
1 Total number of times all of the varieties were planted in the village
2 Percent of households planting the varieties in brackets
3 Same variety named Phae Pee
Source: Compiled from data in Phanthaboun (2009)
As different rice varieties were cultivated in several fields managed by each household, the number of times the varieties were planted totalled several times the number of the varieties found in each village. The Shannon-Weaver diversity index (H’), in which the frequency distribution of the varieties is accounted for (Powers and McSorley, 2000), identifies the village with the highest diversity with the highest H’ value. In each village dominant varieties, i.e. planted by the majority of the village households, and rare varieties were identified. The variety Phae Pee was planted by nearly every household in both Pak Ou villages, while 30-50% of the varieties were planted by single households. Almost all of the varieties found had glutinous endosperm, in accordance with the glutinous rice consuming habit of the ethnicity of the village population. The frequency distribution of landraces in the entirety of the agroecosystem, as in a flood prone region in the lower Chao Phraya Plain in Thailand (Figure 2) can affect their population dynamics by variations in the environment and farmers’ seed management, with a risk of extinction for those rare landraces that depend on just one or two farmers for their maintenance. In a highly decentralized system of classification, a source of error in the tally of names may come from the same names having been given to different genotypes or different names to the same rice, as can be frequently seen in accessions of rice seed listed under the same names but with obviously different morphologies in gene banks (Titiprasert et al., 2001; Appa Rao et al., 2006a). Thirty-four accessions of Pingaew, a local floating rice variety from Thailand’s Central Plain were found to be differentiated by more than 50% by a set of molecular markers (Vanavichit et al., 2001). A confusion arising from a reliance on local rice names as a sole classification criterion is exemplified by Myanmar’s high quality rice that was awarded the World Best Rice in 2010 under the name ‘Paw Son’ (The Rice Traders Association, 2010), exported as Pearl Paw San for its short round grain, and identified by several different local names, listed under a group known either as Meedon (Thein et al., 2012) or Paw San or Paw San Hmwe (Oo et al., 2015).
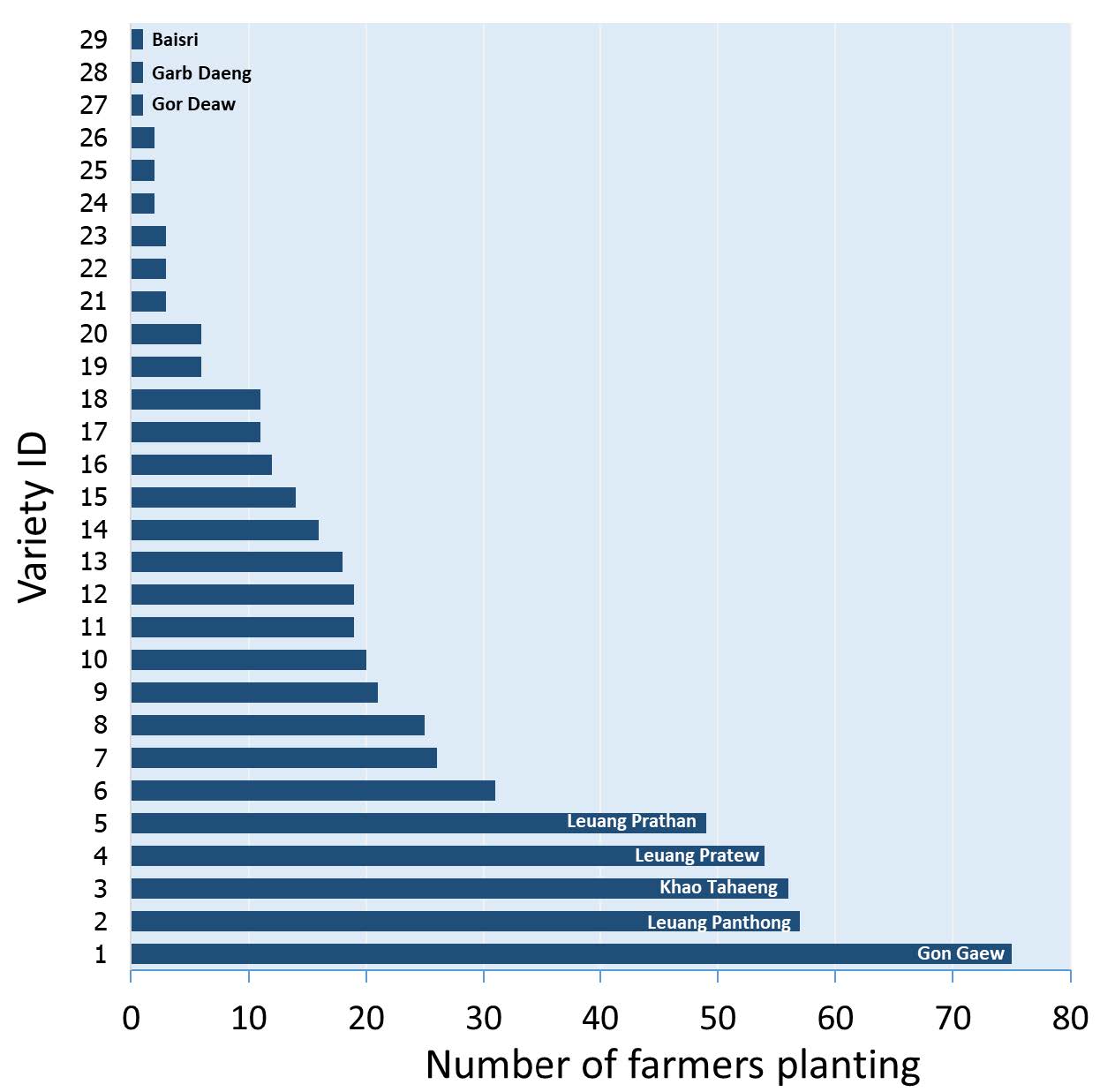
Figure 2. Frequency distribution of 29 local rice varieties in Thailand’s flood prone region (location 2 in Figure 1), with the five most commonly planted and rare varieties identified by name.
Source: drawn from data in Sommut (2003)
Diversity of local rice varieties appears to be associated with ethnic diversity among the local population, with the rice germplasm of different ethnic groups identified by names in different languages. In Laos where local rice varieties are still important to rice production in many areas, for example, there are 48 recognized ethnic groups (ADB, 2001). Recording the local rice variety names in the languages of the various ethnicities proved to be extremely challenging (Appa Rao et al., 2006c). In the highlands of northern Thailand the largest set of local rice germplasm was found among the Karen (Unthong et al., 2007; Jamjod et al. 2017), who has a long history of wetland and upland rice cultivation as well as the largest population among the minority groups in the country. Karen, Khamu, Lua, Thai, and Shan are the traditional rice-growing groups. The Hmong, Lahu, Akha, Mien, and Lisu, who formerly depended on opium as a cash crop, also have their own rice varieties. Little is known about the uniqueness or genetic distances among the local rice germplasms maintained by different ethnic groups, although exchanges of rice varieties, seed and information are known to take place among different groups. For example, Akha rice has a reputation of being well adapted to poor soils, while former opium-growing shifting cultivators settled down to grow rice by buying the rice land, and transferring the rice technology, including the rice seed and varieties to skills and irrigation management, from groups with the expertise such as the Karen (Rerkasem et al., 2002, 2009).
POPULATION STRUCTURE OF SELECTED LANDRACES
Phka Malis, also known locally as Neang Malis or KDM (Khao Dok Mali), is a rice landrace that has become established as Cambodia’s economically important variety for its aromatic grain with premium quality and price (IFC, 2015; Cramb et al., 2020). Similarly in Thailand, local landraces of aromatic jasmine rice are listed under the name of Dawk Mali or Khao Dawk Mali for whiteness of the milled grain and the aroma in the rice germplasm database (Titiprasert et al., 2001). However, a pure line selection of the rice seed sourced from a farmer in Bangkhla (near Bangkok) in 1950s produced Khao Dawk Mali 105 (KDML105, descendants of panicle number 105 from some 200 panicles evaluated) (TRKB, 2024b). Photoperiod insensitive, high yielding, modern varieties began to be released in 1970s, and by the first decades of the 21st century accounted for half of Thailand’s annual rice production (Prom-u-thai and Rerkasem, 2020). Pure line selection of landraces, that has given rise to locally improved varieties (Dennis, 1987) or improved local varieties, has nevertheless been an important strategy in rice breeding and improvement in Thailand, largely targeting the lowland rainfed agroecosystem. Several other common local varieties have also undergone the pure line selection process, in addition to KDML105, e.g. Hahng Yi 71, Pingaew 56, Nahng Mon 4, Khao Tah Haeng 17, Leuang Pratew 123, Muey Nawng became Muey Nawng 62M, etc. (TRKB, 2024b), with the number added to distinguish them from the original population from farmers. The local rice varieties that have undergone a pure line selection require maintenance to preserve their genetic homogeneity, integrity and purity (Zeven, 1998). Support from a robust public rice breeding and seed system has contributed to the expansion of area under KDML105 to 4.2 million hectares (more than 40% of Thailand main season rice crop) in 2021 (computed from OAE, 2024). In addition to its direct use for sowing by farmers, KDML105 was employed in breeding programmes for aromatic jasmine rice, by being used as a parent and providing a blue print for quality characteristics, e.g. IR841-85 was bred at the International Rice Research Institute and released in the US as Jasmine 85 (Marchetti et al., 1998), and in other countries under various names (Khush and Virk, 2005). Quality characteristics of KDML105 is the benchmark on which new aromatic jasmine rice varieties are judged (e.g. Sangtong, 2020; and see description of grain quality of new varieties, RD33, Hawm Khlong Luang 1, Hawm Suphan Buri, in TRKB, 2024b). Similarly, the premium quality and priced Basmati rice of India and Pakistan, which originated as landraces in the Himalayan foothills (Bhattacharjee et al., 2002), have provided the key genetic traits and the template for its unique grain quality features in breeding and varietal improvement (Mahajan et al., 2018; Singh et al., 2018).
Knowledge about the population structure of genetically diverse rice landraces has been greatly expanded from detail studies of multiple accessions of landraces from Thailand, Cambodia, Laos, Myanmar and Nepal (Table 2). These local varieties were employed in small scale production for subsistence and local consumption as well as those that have become commercialized for domestic and export market. Molecular diversity analyses, by variation in the microsatellites (simple sequence repeats) of the DNA, has brought about a better understanding of the genetic structure of local rice landraces. Molecular diversity and variation in some functional traits have been established within the landrace populations (e.g. Pintasen et al., 2007; Bounphanousay, 2007; Pusadee et al., 2009; Jaksomsak et al., 2015; Laenoi et al., 2015; Sreethong et al., 2020; Fongfon et al., 2021). Partitioning of the molecular variance allows the variation at different organizational levels of the population to be compared (Excoffier et al., 1992), i.e. among plants in the same field versus those growing in different fields and villages, or within and among the seed caches of different farmers. Two patterns of molecular genetic structure, in relative diversity within and among accessions of each landrace (i.e. populations of same variety maintained by individual farmers), have emerged from widely grown landraces so far studied. Some landraces have a greater variation among individuals in each of its accessions, and less differentiation among accessions of the same landrace, while others are more highly differentiated among accession, and less genetically diverse within accession. Compared with the microsatellite diversity of Oryza rufipogon, the ancestral wild rice (Pusadee et al., 2016), considerable molecular variations have generally been encountered in single landrace populations, e.g. those from East Kalimantan in Indonesia (Thomson et al., 2009), China (Zhang et al., 2013) and East and North-east India (Das et al., 2013; Roy et al., 2014). The landraces with the variation within the individual accessions accounting for most of their diversity are represented by Bue Chomee, commonly grown as wetland rice at about 600-700 m elevation in Karen villages ranging over some 350 km in the mountain ranges west of Chiang Mai in Thailand, with 68% of its molecular variance found among individuals in a farmer’s seed cache or field (Pusadee et al., 2009).
The landraces with most of their diversity represented by variation among accessions of the same variety are those that have been studied in details due to their special quality. For instance, Kai Noi, a glutinous rice with globulus round grain and exceptional cooking quality from northern Laos, was found to have 79% of its molecular variance accounted for by differentiation among accessions and only 6% within accessions (Vilayheuang et al., 2016). Similarly, 80% of the molecular variance in Khao Kam, a pigmented rice, from the north and northeast of Thailand was among accessions and only 16% within accessions (Pusadee et al., 2019). Low diversity is often associated with the bottleneck effect in a population descended from a limited number of individuals (Gravuer et al., 2005). Genetic diversity of the original germplasm, its life history and management are all likely to contribute to the level and structure of genetic diversity of rice landraces. The low diversity of Khao Kam was explained by its customary cultivation in very small area for occasional usages (Pusadee et al., 2019), similar to the recognition in wildlife conservation that species diversity may be limited by the small size of the population (Frankham, 1996). Selection pressure in the presence of an insect pest was suggested by lower molecular diversity associated with greater resistance to the rice gall midge (Oupkaew et al., 2011; Pusadee et al., 2014). An even more limited molecular variation among accessions of a cold-tolerant landrace from Jumla in Nepal was attributed to an extremely strong selection pressures for adaptation to an extreme altitudes at 2,200–3,000 m, combined with a possibly common origin of a single introduction (Bajracharya et al., 2006).
In areas where wild rice is prevalent, e.g. in Cambodia, Laos, Myanmar and Thailand into the 21st century (Pusadee et al. 2016), the diversity of the cultivated rice continues to be added to by introgression of genetic materials from wild rice (Oryza rufipogon) and weedy rice (O. sativa f. spontanea) (Wongtamee et al., 2017). Evidence of gene flow in the opposite direction was indicated by the presence of cultivated rice traits in the wild population (Oka, 1988). Farmers’ management practices affect the diversity of local rice varieties through the selection and dispersal of seeds. While some farmers meticulously examine thousands of seed panicles to select desired types and discard undesirables, others exercised vigilance in culling off-types in the seed crop (Figure 3). Many simply keep a portion of the harvested crop for use in planting in the next season (Oupkaew et al., 2011; Pusadee et al., 2014; Rerkasem, 2016). A system of seed sharing and exchange is the customary process by which the rice seed is distributed among farmers and villages. New varieties may be introduced into a village by new members, e.g. those immigrating from another village due to a marriage custom (Sirabanchongkran et al., 2004). Within a seed exchange network, differences in the management by individual farmers, e.g. from whom the seed is sourced and for how long the seed-lot has been cultivated by each farmer, as well as the way in which seed selection is practiced (Oupkaew et al., 2011; Pusadee et al., 2014), are likely to contribute to genetic diversity among different accessions of each local rice variety.

Figure 3. Selecting of grain quality by breaking the husk off a few grains on the panicle to examine the kernel inside ((A) – Lek, (B) – Pued, in Nakorn Nayok) or culling off-type panicles from sheaves harvested for seed ((C) – Boonma, in Chiang Mai).
Sources: photos by Chanya Maneechote and Utumporn Chaiwong.
WITHIN VARIETY FUNCTIONAL DIVERSITY
Functional diversity in local varieties of rice includes traits that are recognized and used, with adaptation and grain quality characteristics being among the most valued, as well as those unknown to farmers and consumers. Individual landraces are well adapted to specific eco-geographical environments and management needs (McCouch, 2004). A rich diversity of traits for adaptation to different flood regimes, for example, has been identified among the rice landraces of the flood prone regions of Bangladesh (Bashar et al., 2004), Cambodia (Javier, 1997), India (Singh et al., 2004; Goswami et al., 2015), and Thailand (Sommut, 2003). In the highlands, different varieties are grown at different altitudes and micro-environments (e.g. Bajracharya et al., 2010; Jamjod et al., 2017), with cold tolerance an important trait for rice at higher altitudes (Bajracharya et al., 2006; Zeng et al., 2006). Variation in the seasonal adaptation of local rice varieties is governed by their photo-thermo response under the control of water. In monsoonal Asia, adaptation can mean a phenology matching rainfall pattern or water availability, or an escape from the floods. Early or late- maturing varieties are differentiated from the main season varieties by a prefix or suffix denoting earliness or lateness in the variety names (Titiprasert et al., 2001; Appa Rao et al., 2006a).
The name Muey Nawng (Table 2) is commonly associated with resistance to the rice gall midge (Orseolia oryzae, Wood-Mason) in Northern Thailand, where it has long been a constraint to rice production in the foothill valleys (Supamongkol, 2006), and also in Laos (P. Inthapanya, K. Phanthaboun, pers. Comm). The leaves that have been transformed into tube-like galls, somewhat like onion leaves, by the feeding gall midge larvae (Rajamani et al., 2004), are commonly recognized by the local name of Bua (spring onion), the absence of which identifies resistant varieties (Tayathum et al., 2004; Supamongkol, 2006). Other variable functional traits in Muey Nawng are the endosperm starch type that is easily and readily detected by farmers, with the opaque glutinous (waxy) and translucent non-glutinous grain, and flowering time that corresponds with the crop earliness or lateness in maturity. The presence of non-glutinous grains in accessions of Muey Nawng, a glutinous rice, confirmed by iodine (I2/KI) staining, has been found to range from nil, fewer than one in five grains, to more than 60% (Supamongkol, 2006). Muey Nawng Khao Chao (non-glutinous Muey Nawng) is found in ethnic minority villages where non-glutinous rice is the staple, e.g. among the Karen (Pusadee et al., 2014). Variation in maturity among 82 accession of Muey Nawng was indicated the flowering time that ranged from 86 to 107 days from transplanting, compared with 105 days in Muey Nawng 62M (Supamongkol, 2006). The flexibility afforded by variation in the gall midge resistance, endosperm starch type and maturity of the landrace Muey Nawng is no longer present in Muey Nawng 62M, the ‘improved’ local variety developed by pure-line selection.
Knowledge about certain functional traits can be limited and incomplete without controlled experimentation, especially those involving disease or insect pest resistance. Although accessions of Bue Polo have been found to range from moderately resistant to resistant to the blast disease (caused by Magnaporthe grisea) in laboratory testing (Naruebal, 2009), there is no report of highland farmers being aware of the resistance. Susceptibility and resistance to gall midge of rice varieties have been reported to depend on the insect population (i.e. interaction effects of rice variety and insect population) (Oupkaew et al., 2011). It is unlikely that farmers are informed of this, or even of the existence different versions of the gall midge. Variations in the intensity of infestation are generally attributed to climatic fluctuations.
Throughout Asia grain quality can sometimes be more important to rice growers and consumers than yield or pest and disease resistance (Prom-u-thai and Rerkasem, 2020). Dimension of the un-husked paddy grain is a distinctive feature of rice varieties that can be used to measure varietal purity of a sample of rice grain (IRRI, 2024). Paddy grain length and width are distinctive features specific to rice varieties, which are commonly documented in publications (e.g. see Khush and Virk, 2005; TRKB, 2024b). New, modern upland rice varieties were rejected by farmers in the highlands despite their higher yield, because of their ‘wrong’ grain size and shape (Heidhues and Rerkasem, 2006). On the other hand, thousand grain weight is unreliable as an indicator of the grain size typical of individual varieties. Although the maximum grain size is fixed by the rigid husk (Chen et al., 1994; Zeng and Shannon, 2000), significant genotype and environment interaction effects on 1000 grain weight (e.g. Shrestha et al., 2012; Tejakum et al., 2019), are likely outcomes of incomplete filling in a portion of the grain under sub-optimum conditions (Laenoi et al., 2018).
Table 2. Some local rice varieties with multiple accessions maintained and grown by different farmers in Thailand, Cambodia, Laos, Myanmar and Nepal.
|
Varietal type and agroecosystem details |
Variety name(s) (references in brackets) |
|
|
||
|
|
Thailand: wetland rice in the highlands |
Bue Chomee (Meesin, 2004); Bue Polo (Naruebal, 2009) |
|
|
Thailand: flood prone and deep-water area in the Central Plain |
Gon Gaew; Leuang Panthong; Khao Tah Haeng; Leuang Pratew (Sommut, 2003) |
|
|
Thailand: gall midge resistant wetland rice with glutinous grain with some non-glutinous version in foothill valleys with serious gall midge problem |
Muey Nawng (Supamongkol, 2006; Oupkaew et al., 2011; Pusadee et al., 2014) |
|
|
Laos: upland rice for subsistence production in the highlands |
Laboun; Nok; Makhinsoung (Linquist et al., 2006b; Xangsayasane, 2015) |
|
|
Nepal: wetland rice at extreme altitudes, (2200–3000 m) at Jumla |
Seto Marshi; Rato Marshi; Kalo Marshi (Bajracharya et al., 2006) |
|
||
|
|
Cambodia: premium quality and priced jasmine type grain grown as wetland rice in lowland rainfed environment |
Phka Malis; Neang Malis or KDM (Khao Dok Mali) (IFC, 2015; Cramb et al., 2020) |
|
|
Laos: special quality glutinous variety, with small globular grain, grown as wetland rice in rainfed environment in northern provinces of Houaphanh and Xiangkhouang |
Kai Noi (Appa Rao et al., 2006b; Vilayheuang et al., 2016) |
|
|
Myanmar: special quality rice recognized under different local names, the grain elongates when cooked (like Basmati rice), grown as wetland rice in lowland rainfed environment mostly in the Ayeyarwady Division |
Paw San Hmwe, Paw Son; Pearl Paw San; or Meedon (Oo et al., 2015) |
|
|
Thailand: special quality variety with red pericarp and long slender grain, grown in small scale commercial production in wetland environment in Songkhla Lake Basin in the south |
Sang Yod (Panomjan et al., 2016) |
|
|
Laos/Thailand: rice with purple pericarp, mostly with glutinous endosperm, known by the generic name Khao Kam, Khao Dam or Khao Niaw Dam |
Khao Kam, Khao Dam (purple or black rice in the literature : Appa Rao et al., 2006d; Bounphanousay, 2007; Pusadee et al. 2019; Fongfon et al., 2020) |
The rice grain type is identified as large, slender or round grain by a scheme proposed by Matsuo, with a classification based on a plot of their un-husked paddy grain length and width (Matsuo, 1952; Oka, 1988). Local rice varieties in the highlands of Laos and Thailand have thus been shown to be mainly large-grain (Phanthaboun, 2009; Jamjod et al., 2017; Xiongsiyee et al., 2018). The uniformity or variability in grain type is illustrated by the Matsuo plot of selected local rice varieties from Thailand, and Paw San Hmwe, a special quality rice from Myanmar (Figure 4). Muey Nawng was identified as uniformly large grain, and Bue Chomee as slender grain except for two accessions with a large grain off-type. A descriptor - large, medium or small - commonly affixed to the name Bue Polo by farmers in its range of distribution in Mae Hong Son (Naruebal, 2009) is a reflection of a large variation among accessions in their grain length and width, although all were identified as large grain. Variation in grain size and shape was even greater in Khao Kam in the Matsuo classification, and among a larger collection in Laos (Appa Rao et al., 2006d). Accessions of Paw San Hmwe from Myanmar, were also highly variable, with accessions distributed in the borderline area among the different grain types in the Matsuo plot, and the majority identified as large grain.
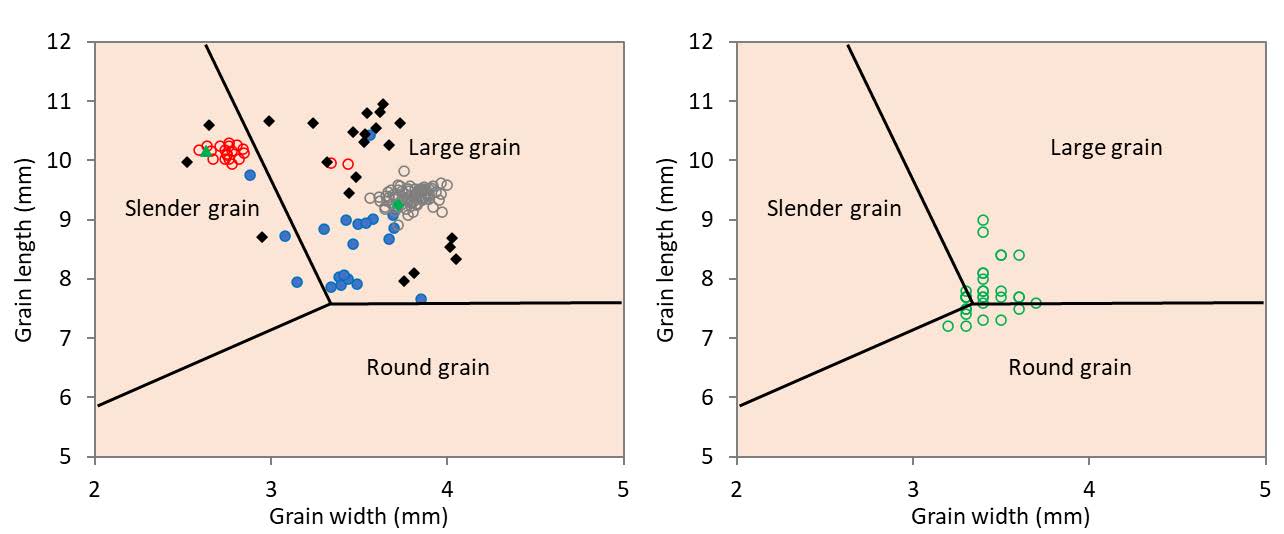
Figure 4. Grain size and shape distribution of Bue Chomee1 (o, n = 20 ), Bue Polo2 (•, n = 20), Muey Nawng3 (o, n = 82), and Khao Kam2 (♦, n = 22), from Thailand (A), and Paw San Hmwe4 (o, n = 31) from Myanmar (B), by the length and width of unhusked seed by Matsuo’s scheme (Oka, 1988), with RD6 (a mega variety, ►) and MN62M (a pure line selection of Muey Nawng, ♦) as references, (n = number of accessions for each variety).
Sources: drawn with data from 1Meesin (2004), 2Sansanee Jamjod (unpublished), 3Supamongkol (2006, 4Oo et al. (2015).
Basmati from India and Pakistan and Khao Dawk Mali from Thailand were landraces that have evolved into mega varieties on the strength of their distinctive and unique grain quality characteristics (Bhattacharjee et al., 2002; Prom-u-Thai and Rerkasem, 2020). Other landraces are gaining in popularity and recognition as special quality or novelty rice among widening circles of consumers in domestic and export markets. The commercialization of the local rice varieties is stimulated by their premium prices, and facilitated by development of small and medium size modern rice mills, and the ever expanding internet and social media marketplace. Examples of rice landraces that are now being grown for the market include Phka Malis in Cambodia, Kai Noi in Laos, Paw San Hmwe in Myanmar, Sang Yod in Thailand and Kao Kam in Laos and Thailand (Table 2). An attempt to differentiate among landrace-derived jasmine rice varieties by 15 microsatellite markers found Cambodia’s Phka Rumdeng and Phka Rumduol to be separated from Thai Hom Mali (Khao Dawk Mali 105 or RD15, unspecified) at only one marker, while Thai Hom Mali and Phka Romeat were completely identical (Nader et al., 2020). It is suspected that Phka Malis (also known in Cambodia as Neang Malis or KDM (Khao Dok Mali) (IFC, 2015; Cramb et al., 2020), which was not included in the study, could also be genetically identical Khao Dawk Mali 105. Besides Khao Dok Mali, Bue Ner Moo, an aromatic, large-grain landrace is grown as wetland rice in the highlands in Thailand by some Karen farmers. Variation in the intensity of the aroma among Bue Ner Moo accessions has been shown to be associated with the molecular variation in the aroma-controlling gene Badh2 (Chan-in et al., 2019, 2020). Laos’ Kai Noi is differentiated into sub-groups by the husk colouring, with words such as yellow, red, black, striped, etc. appended to the name. In spite of the husk colour, Kai Noi accessions were rather uniform genetically, with variation among the sub-groups accounted for only 6% of its molecular variance (Vilayheuang et al., 2016). After the removal of the husk, the brown rice (endosperm with intact pericarp and embryo) of different Kai Noi sub-groups were also generally indistinguishable (Appa Rao et al., 2006b). Variation in the intensity of the pigmentation and anthocyanin concentration is a common feature among rice varieties with pigmented pericarp (Yodmanee et al., 2011; Rerkasem et al., 2015; Bhat et al., 2020). Genotypic variation among accessions of Sang Yod, a red pigmented rice from Southern Thailand with uniform grain size and shape, was indicated by variation in the intensity of their pericarp colour when grown together at one site (Panomjan et al., 2016). On-farm diversity of Muey Nawng and Sang Yod suggests that neither landrace has been entirely displaced by the pure line selected Muey Nawng 62M (released 1959) and Sang Yod Phattalung (released 2007) (TRKB, 2024b).
In the case of Myanmar’s Paw San Hmwe, the emergence of several different groupings from 31 accessions by a cluster analysis based on 7 grain quality markers and 19 microsatellite markers (Oo et al., 2015) suggests that the rice is likely a collection of landraces instead of a single variety. Functional diversity in individual rice landraces may have positive or negative effect on their economic value, depending on the traits. Variation in adaptation provides a flexibility for coping with variation in the agroecological environment, but variation in grain quality may be an obstacle to utilization and commercialization. Myanmar’s Paw San Hmwe exemplifies the later situation, with variation in key grain quality features, such as aroma, texture and elongation of the cooked grain, and most notably the grain size and shape (Figure 4). While much interest has been generated in Myanmar by Pearl Paw Son winning the World Best Rice Award in 2011 (Myint and Napasintuwong, 2016; Myint and San, 2019), only a minority of accessions had the globular round grain (5 mm milled grain length) distinctive of the award winning rice (Oo et al., 2015), while the majority were identified as large grain.
Rice with a purple pigmentation in the pericarp is well known throughout Asia (Appa Rao et al., 2006d). Commonly identified by their generic name of Khao Kam or Khao Dam (purple or black rice in the literature), the local landraces with purple pericarp of Laos and Thailand are highly diverse in their morphologies, adaptation and grain quality features, including grain size and shape (Figure 4, B; Bounphanousay, 2007; Fongfon et al. 2021). Landraces vary in the plant parts (i.e. lemma and palea, sterile lemma, apiculus, stigma, ligule, auricle, collar, internodes, leaf blade, or leaf sheath), that are pigmented in addition to the pericarp (Figure 5). The ubiquitous prevalence of Khao Kam in traditional farming systems is demonstrated by its distribution in all 136 districts of Laos’ 17 provinces, found in rice germplasm collecting effort in 1995-2000 (Inthapanya et al., 2003; Appa Rao et al., 2004, 2006d). Variations have also been reported in Khao Kam in its contents of the micronutrients iron (Fe) and zinc (Zn), and bioactive compounds with nutritional and health benefits such as anthocyanin and γ-oryzanol (Bounphanousay, 2007; Boonsit et al., 2010; Yodmanee et al., 2011; Jamjod et al., 2017).
Functional diversity in non-obvious traits is illustrated by variation in the tolerance to soil acidity in local rice varieties, which might be expected among those from areas where the crop has been subjected to the stress of soil acidity, such as upland rice on acidic soils and wetland rice on acid sulphate soils. Reaction to aluminium ion (Al3+), the key stress factor in acid soils, of individual seedlings allows the variation to be detected, including within a farmer’s seed cache (Figure 6). Significant variation in the tolerance to acidity have been found among different rice varieties, and also among accessions of the same variety and among individuals in an accession (Phattarakul, 2008; Laenoi et al., 2015). Variation in the content of nutrients and useful compounds in local rice varieties is often masked, by being averaged out in chemical analyses in which a sample is processed by blending together some 50-100 seeds. The grain iron (Fe), zinc (Zn), anthocyanin and phenol contents and anti-oxidative capacity of single seed descent lines of some landraces from the highlands of Northern Thailand were thus identified as having significantly surpassed the bulk contents of their own parental populations (Sreethong et al., 2020). Variation in the Fe and Zn content among individual seeds in a farmer’s seed cache can be determined by a staining specific to each element, i.e. Perl’s Prussian blue for Fe (Prom-u-thai et al., 2003; Pintasen et al., 2007) and dithizone for Zn (Jaksomsak et al., 2015).

Figure 5. Green leaf blades and leaf sheath, purple panicle (A), unhusked paddy (B) and purple unpolished grain (C) of Kam Hom CMU, an aromatic purple rice variety selected from Ja Nae Nae, a landrace from a Lisu farmer by Chanakarn Prom-u-thai
Sources: photos by Sunisa Niruntrayakul and Pennapa Jaksomsak

Figure 6. Variation in sensitivity to soil acidity, expressed as root growth restriction by aluminium (Al3+), in two accessions of Leuang Yai, a local rice variety from an area with acid sulphate soil in Central Thailand.
Source: photos by Nattinee Phattarakul
CONCLUSIONS AND IMPLICATIONS FOR UTILIZATION AND CONSERVATION
Local rice varieties or landraces have remained important to rice farming in many areas of Asia. They are an essential input for a subsistence production of rice in areas unreachable by modern or other improved varieties for various ecological and socio-economic reasons. Key findings of the review are as follows. First, the population dynamics of genetically diverse local rice varieties are shaped by their uneven distribution within the agroecosystem, with a risk of extinction for the varieties that rely on just one or two farmers for their maintenance. Second, diversity of each widely cultivated variety is influenced by the variation in the bio-physical environment and farmers’ management and sharing of rice seeds. Third, the production of some local rice varieties are being commercialized on the strength of their value enhancing, novel and unique grain quality features, a success achieved in spite of an absence of the centralized genetic control and seed system support provided to modern and improved varieties. Fourth, local rice varieties exhibit a greater diversity than might appear from a simple tally of names, with variations in functional traits and at the molecular level revealed by microsatellite analysis, even among those with seemingly uniform morphologies. Diversity can be predominantly among plants growing in the same farmer’s field and within a seed cache. It can also exist among different seed-lots of the same landrace grown by different farmers, in different villages and provinces. A common generic name may apply to a group of local rice varieties sharing certain common characteristics, but differing in other aspects. All these genetic variations need to be considered in germplasm management, utilization and conservation. Finally, adaptation flexibility is lost when local rice varieties are ‘improved’ through pure line selection. Local rice varieties that have retained their genetic diversity while being cultivated, either for subsistence or commercially, also play a crucial role in the in situ conservation of germplasm.
ACKNOWLEDGEMENTS
The authors wish to thank the farmers for generously sharing seed and information. Partial support for this research from Chiang Mai University and Lanna Rice Research is gratefully acknowledged.
AUTHOR CONTRIBUTIONS
Tonapha Pusadee was responsible for the review of molecular diversity, Sansanee Jamjod for genetics, Chanakan Prom-u-thai and Pennapa Jaksomsak for nutritive quality, Benjavan Rerkasem for overall synthesis, interpretation and preparation of manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
ADB. 2001. Participatory poverty assessment: Lao People’s Democratic Republic. Asian Development Bank, Manila, Philippines.
Appa Rao, S., Schiller, J.M., Bounphanousay, C., Alcantara, A.P., and Jackson, M.T. 2006a. Naming of traditional rice varieties by the farmers of Laos. p. 141-157. In: Schiller, J.M., Chanphengxay, M.B., Linquist, B., Appa Rao, S. (eds), Rice in Laos, International Rice Research Institute, Los Baños, Philippines.
Appa Rao, S., Schiller, J.M., Bounphanousay, C., and Jackson, M.T. 2006b. Development of traditional rice varieties and on-farm management of varietal diversity in Laos. p. 187-196. In: Schiller, J.M., Chanphengxay, M.B., Linquist, B., Appa Rao, S. (eds), Rice in Laos, International Rice Research Institute, Los Baños, Philippines.
Appa Rao, S., Schiller, J.M., Bounphanousay, C., and Jackson, M.T. 2006c. Diversity within the traditional rice varieties of Laos. p. 123-140. In: Schiller, J.M., Chanphengxay, M.B., Linquist, B., Appa Rao, S. (eds), Rice in Laos. International Rice Research Institute, Los Baños, Philippines.
Appa Rao, S., Schiller, J.M., Bounphanousay, C., Inthapanya, P., and Jackson, M.T. 2006d. The coloured pericarp (black) rice of Laos. p. 175-186. In: Schiller, J.M., Chanphengxay, M.B., Linquist, B., Appa Rao, S. (eds), Rice in Laos. International Rice Research Institute, Los Baños, Philippines.
Appa Rao, S., Bounphanousay, C., and Inthapanya, P. 2004. Collection, classification and characterization of traditional black rice varieties of the Lao PDR. Lao Journal of Agriculture and Forestry. 7: 27-34.
Bajracharya, J., Steele, K.A., Jarvis, D.I., Sthapit, B.R., and Witcombe, J.R. 2006. Rice landrace diversity in Nepal: Variability of agro-morphological traits and SSR markers in landraces from a high-altitude site. Field Crops Research. 95(2, 3): 327–335.
Bajracharya, J., Rana, R.B., Gauchan, D., Sthapit, B.R., Jarvis, D.I., and Witcombe, J.R. 2010. Rice landrace diversity in Nepal. Socio-economic and ecological factors determining rice landrace diversity in three agro-ecozones of Nepal based on farm surveys. Genetic Resources and Crop Evolution. 57(7): 1013-1022.
Bashar, M.K., Haque, M.M., and Zaman, S.M.H. 2004. Rice biodiversity and genetic wealth of flood-prone environments of Bangladesh. p. 129-141. In: Bhuiyan, S.I., Abedin, M.Z., Singh, V.P., and Hardy, B. (eds), Rice research and development in the flood-prone ecosystem, International Rice Research Institute, Los Baños, Philippines.
Bhat, F.M., Sommano, S.R., Riar, C.S., Seesuriyachan, P., Chaiyaso, T., and Prom-u-Thai, C. 2020. Status of bioactive compounds from bran of pigmented traditional rice varieties and their scope in production of medicinal food with nutraceutical importance. Agronomy. 10:1817.
Bhattacharjee, P., Singhal, R.S., and Kulkarni, P.R. 2002. Basmati rice: a review. International Journal of Food Science and Technology 37: 1-12.
Boonsit, P., Pongpiachan, P., Julsrigival, S., and Karladee, D. 2010. Gamma oryzanol content in glutinous purple rice landrace varieties. Chiang Mai University Journal of Natural Science. 9(1): 151-157.
Bounphanousay, C. 2007. Use of phenotypic characters and DNA profiling for classification of the genetic diversity in black glutinous rice of the Lao PDR. Ph.D. Thesis. Khon Kaen University, Khon Kaen, Thailand. http://lad.nafri.org.la/fulltext/2936-0.pdf
Brown, A.H.D. 1978. Isozymes, plant population genetics structure and genetic conservation. Theoretical and Applied Genetics. 52: 145–157.
Calingacion, M., Laborte, A., Nelson, A., Resurreccion, A., Concepcion, J.C., et al. 2014. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS One. 9(1): e85106.
Chan-in, P., Jamjod, S., Yimyam, N., and Pusadee, T. 2019. Diversity of BADH2 alleles and microsatellite molecules in highland fragrant rice landraces. Journal of Agriculture. 35(1): 23–35. (in Thai)
Chan-in, P., Jamjod, S., Yimyam, N., Rerkasem, B., Pusadee, T., 2020. Grain quality and allelic variation of the Badh2 gene in Thai fragrant rice landraces. Agronomy 10: 779.
Chen, C.L., Li, C.C., and Sung, J.M., 1994. Carbohydrate metabolism enzymes in CO2-enriched developing rice grains of cultivar varying in grain size. Physiologia Plantarum. 90: 79–85.
Cramb, R., Sareth, C., and Vuthy, T. 2020. The commercialisation of rice farming in Cambodia. In: Cramb, R. [ed], White gold: the commercialisation of rice farming in the Lower Mekong Basin, Springer Nature, Singapore. pp 227-244.
Dalrymple, D.G. 1986. Development and spread of high-yielding rice varieties in developing countries. U.S. Department of Agriculture in cooperation with the U.S. Agency for International Development.
Das, B., Sengupta, S., Parida, S.K., Roy, B., Ghose, M., Prasad, M., and Ghose, T.K. 2013. Genetic diversity and population structure of rice landraces from Eastern and North Eastern States of India. BMC Genetics 14: 71. http://www.biomedcentral.com/1471-2156/14/71
Dela Cruz, N., and Khush, G.S. 2000. Rice grain quality evaluation procedures. p 15-28. In: Singh, R.K., Singh, U.S., and Khush, G.S. (eds), Aromatic rices. Oxford and IBH Publishing Co., New Delhi (India).
Dennis, J.V. 1987. Farmer management of rice variety diversity in Northern Thailand. Ph.D. Dissertation. Cornell University, Cornell, New York, USA.
Excoffier, L., Smouse, E.P., and Quattro, M.J. 1992. Analysis of molecular variance inferred from metric distance among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131(2): 479–491.
Fongfon, S., Pusadee, T., Prom-u-thai, C., Rerkasem, B., and Jamjod, S. 2021 Diversity of purple rice (Oryza sativa L.) landraces in Northern Thailand. Agronomy. 11: 2029.
Frankham, R. 1996. Relationship of genetic variation to population size in wildlife. Conservation Biology. 10(6): 1500–1508.
Fukuoka, S., Suu, T.D., Ebana, K., Trinh, L.N., Nagamine, T., Okuno, K. 2006. Diversity in phenotypic profiles in landrace populations of Vietnamese rice: A case study of agronomic characters for conserving crop genetic diversity on farm. Genetic Resources and Crop Evolution. 53: 753–761.
Goswami, S., Labar, R., Paul, A., Adak, M.K., and Dey, N. 2015. Physio-biochemical and genetic exploration for submergence tolerance in rice (Oryza sativa L.) landraces with special references to Sub1 loci. American Journal of Plant Sciences. 6(12): 1893-1904.
Gravuer, K., von Wettberg, E., and Schmitt, J. 2005. Population differentiation and genetic variation inform translocation decisions for Liatris scariosa var. novae-angliae, a rare New England grassland perennial. Biological Conservation. 124(2): 155–167.
Hammer, K., Knüpffer, H., Xhuveli, L., Perrino P. 1996. Estimating genetic erosion in landraces — two case studies. Genet Resources and Crop Evolution. 43: 329-336.
Harlan, J.R. 1975. Our vanishing genetic resources. Science. 188: 618-621.
Harlan, J.R. 1992. Crops and Man. American Society of Agronomy; Crop Science Society of America, Madison, WI.
Hayami, Y., and Ruttan, V.W. 1985. Agricultural development. Johns Hopkins University Press, Baltimore, MD, USA.
Heidhues, F., and Rerkasem, B. 2006. IRRI’s upland rice research follow-up review CGIAR Science Council. https://cgspace.cgiar.org/handle/10947/4245
Ho, P. 1956. Early ripening rice in Chinese history. Economic History Review. 9: 200-216.
HRDI. 2511. In-depth highland population data in 20 provinces. https://web2012.hrdi.or.th/about_us/page/about
IFC. 2015. Cambodia rice export potential and strategies. International Finance Corporation, Cambodia Agribusiness Series Number 4.
Inthapanya, P., Bounphanousay, C., and Voladeth, S. 2003. Lowland black rice (Khao kam). Lao Journal of Agriculture and Forestry. 7: 17-25.
IRRI. 2024. Measuring varietal purity. Rice Knowledge Bank, International Rice Research Institute, Los Baños, Philippines. http://www.knowledgebank.irri.org/training/fact-sheets/postharvest-management/rice-quality-fact-sheet-category/measuring-varietal-purity-fact-sheet
Jamjod, S., Yimyam, N., Lordkaew, S., Prom-u-thai, C., and Rerkasem, B. 2017. Characterization of on-farm rice germplasm in an area of the crop’s center of diversity. Chiang Mai University Journal of Natural Science. 16(2): 85-98.
Jaksomsak, P., Yimyam, N., Dell, B., Prom-u-thai, C., and Rerkasem B. 2015. Variation of seed zinc in a local upland rice germplasm from Thailand. Plant Genetic Resources. 13(2): 168-175.
Javier, E.L. 1997. Rice ecosystems and varieties. p. 39-81. In: Nesbitt, H.J. (ed), Rice Production in Cambodia, International Rice Research Institute, Los Baños, Philippines.
Juliano, B.O., and Villareal, C.M. 1993. Grain quality evaluation of world rices. International Rice Research Institute, Los Baños, Philippines
Khush, G.S., and Virk, P.S. 2005. IR varieties and their impact. International Rice Research Institute, Los Baños, Philippines.
Kunstadter, P. 1978. Subsistence agricultural economics of Lua' and Karen hill farmers, Mae Sariang District, Northwestern Thailand. p. 74 – 133. In: Kunstadter, P., Chapman, E.C., and Sabhasri, S. (eds), Farmers in the forest. The University Press of Hawaii, Honolulu, Hawaii, USA.
Laenoi, S., Phattarakul, N., Jamjod, S., Yimyam, N., Dell, B., and Rerkasem, B. 2015. Genotypic variation in adaptation to soil acidity in local upland rice varieties. Plant Genetic Resources. 13(3): 206–212.
Laenoi S, Rerkasem B, Lordkaew S, Prom-u-thai C. 2018. Seasonal variation in grain yield and quality in different rice varieties. Field Crops Research. 221: 350–357.
Lee, J.S., Chebotarov, D., Platten, J.D., McNally, K., and Kohli, A. 2020. Advanced strategic research to promote the use of rice genetic resources. Agronomy. 10(11): 1629.
Linquist, B., Kazuki, S., Keoboualapha, B., Phengchan, and S., Phanthaboon, K. 2006b. Improving upland rice-based cropping systems in Laos. p 197-214. In: Schiller, J.M., Chanphengxay, M.B., Linquist B, Appa Rao, S. (eds) Rice in Laos, International Rice Research Institute, Los Baños, Philippines.
Linquist, B., Keoboualapha, Sipaseuth, and Inthapanya, P. 2006a. Rice production systems of Laos. p 29–45. In: Schiller, J.M., Chanphengxay, M.B., Linquist B, Appa Rao, S. (eds) Rice in Laos, International Rice Research Institute, Los Baños, Philippines.
Mahajan, G., Matloob, A., Singh, R., Singh, V.P., Chauhan, BS. 2018. Basmati rice in the Indian subcontinent: Strategies to boost production and quality traits. Advances in Agronomy. 151: 159-212.
Marchetti, M.A., Bollich, C.N., Webb, B.D., Jackson, B.R., McClung, A.M., Scott, J.E., Hung, H.H. 1998. Registration of 'Jasmine 85' rice. Crop Science. 38: 896.
Matsuo, T. 1952. Genecological studies on cultivated rice. Bulletin of the National Institute of Agricultural Sciences, Nishigahara. Series D, Plant Physiology, Genetics and Crops in General. Tokyo. 1-11.
McCouch, S. 2004. Diversifying selection in plant breeding. PLoS Biology. 2(10): 1507–1512.
Meesin, S. 2004. The structure of genetic diversity in a local rice germplasm. MS Thesis (Agronomy), Chiang Mai University, Thailand.
Myint, P.L., and Napasintuwong, O. 2016. Economic analysis of Paw San rice adoption in Myanmar. Asian Journal of Agricultural Research. 10(5): 175-184.
Myint, T., San, A.M. 2019. Export potential of Myanmar Paw San Hmwe rice: Policy analysis through comparative advantage. Food and Fertilizer Technology Centre for the Asian and Pacific Region. https://ap.fftc.org.tw/article/1380
Nader, W., Ouk, M., Elsner, J., Brendel, T., and Schubbert, R. 2020. The DNA fingerprint in food forensics part II: The Jasmine rice case. Agro Food Industry Hi Tech. 31(1): 4-8.
Naruebal, S. 2009. Varietal diversity of highland rice Bue Polo variety in Mae Hong Son province. MSc (Geosocial Based Sustainable Development), Graduate School, Maejo University.
OAE. 2024. Rice growing season 2562/2563, by variety. Office of Agricultural Economics, Thai Ministry of Agriculture and Cooperatives.
Oka, H.L. 1988. Origin of cultivated rice. Japan Scientific Societies Press and Elsevier.
Oo, K.S., Kongjaimun, A., Khanthong, S., Yi, M., Myint, T.T., Korinsak, S., Siangliw, J.L., Myint, K.M., Vanavichit, A., Malumpong, C., and Toojinda, T. 2015. Characterization of Myanmar Paw San Hmwe accessions using functional genetic markers. Rice Science. 22(2): 53-64.
Oupkaew, P., Pusadee, T., Sirabanchongkran, A., Rerkasem, K., Jamjod, S., and Rerkasem, B. 2011. Complexity and adaptability of a traditional agricultural system: Case study of a gall midge resistant rice landrace from northern Thailand. Genetic Resources and Crop Evolution. 58: 361-372.
Pandey, S., and Sanamongkhoun M. 1998. Rainfed lowland rice in Laos: A socio-economic benchmark study. International Rice Research Institute, Los Baños, Philippines.
Panomjan, N., Jamjod, S., Rerkasem, B., Dell, B., and Prom-u-thai, C. 2016. Variation in seed morphology of Sang Yod rice variety from southern Thailand. Khon Kaen Agriculture Journal. 44(1): 83-94.
Phanthaboun, K. 2009. Genetic diversity of local rice varieties in Luang Prabang, Lao PDR. M.Sc. Thesis (Agronomy), Chiang Mai University, Chiang Mai, Thailand.
Phattarakul, N. 2008. Genotypic variation in tolerance to acid soil in local upland rice varieties. Ph.D. Thesis (Agronomy), Chiang Mai University, Chiang Mai, Thailand.
Pintasen, S., Prom-u-thai, C., Jamjod, S., Yimyam, N., and Rerkasem, B. 2007. Variation of grain iron content in a local upland rice germplasm from the village of Huai Tee Cha in northern Thailand. Euphytica. 158: 27–34.
Powers, L.E., and McSorley, R. 2000. Ecological principles of agriculture. Delmar. Thomson Learning.
Prom-u-thai, C., Dell, B., Thompson, G., and Rerkasem, B. 2003. Easy and rapid detection of iron in rice grain. ScienceAsia. 29: 213-217.
Prom-u-Thai, C., and Rerkasem, B. 2020. Rice quality improvement. A review. Agronomy for Sustainable Development. 40: 28.
Pusadee, T., Jamjod, S., Chiang, Y., Rerkasem, B., and Schaal, B.A. 2009. Genetic structure and isolation by distance in a landrace of Thai rice. Proceedings of the National Academy of Sciences of the USA. 106(33): 13880–13885.
Pusadee, T., Oupkaew, P., Rerkasem, B., Jamjod, S., and Schaal, B.A. 2014. Natural and human-mediated selection in a landrace of Thai rice (Oryza sativa) Annals of Applied Biology. 165: 280-292.
Pusadee, T., Rerkasem, B., Jamjod, S., and Schaal, B.A. 2016. Life-history traits, and geographical divergence in wild rice (Oryza rufipogon Griff.) gene pool in Indochina Peninsula region. Annals of Applied Biology. 168(1): 52-65.
Pusadee, T., Wongtamee, A., Rerkasem, B., Olsen, K.M., and Jamjod, S. 2019. Farmers drive genetic diversity of Thai purple rice (Oryza sativa L.) landraces. Economic Botany 73: 76-85.
Rajamani, S., Pasalu, I.C., Mathur, K.C., Sain, M. 2004. Biology and ecology of rice gall midge. p. 17-22. In: Bennett, J., Bentur, J.S., Pasulu, I.C., and Krishnaiah, K. (eds), New approaches to gall midge resistance in rice. International Rice Research Institute, Los Baños, Philippines, and Indian Council of Agricultural Research, New Delhi, India.
Rerkasem, B. 2015. The agroecosystem of Thai rice: A review. Chaing mai University Journal of Natural Science. 14(1): 1-21.
Rerkasem, B. 2016. Thai rice. Chiang Mai University Press. 150 p.
Rerkasem, B., Jumrus, S., Yimyam, N., and Prom-u-thai, C. 2015. Variation of grain nutritional quality among Thai purple rice genotypes grown at two different altitudes. Science Asia. 41: 377–385.
Rerkasem, B., and Rerkasem, K. 1984. The agroecological niche and farmer selection of rice varieties in the Chiangmai Valley, Thailand. p. 303-311. In: Rambo, A.T., and Sajise, P.E. [eds], An introduction to human ecology research on agricultural systems in Southeast Asia. University of the Philippines at Los Baños, Philippines.
Rerkasem, K., Yimyam, N., Korsamphan, C., Thong-ngam, C., and Rerkasem, B. 2002. Agrodiversity lessons in mountain land management. Mountain Research and Development. 22: 4-9
Rerkasem, K., Yimyam, N., and Rerkasem, B. 2009. Land use transformation in the mountainous mainland Southeast Asia region and the role of indigenous knowledge and skills in forest management. Forest Ecology and Management. 257: 2035–2043.
Roy, S., Banerjee, A., Pattanayak, A., Roy, S.S., Rathi, R.S., Misra, A.K., Ngachan, S.V., and Bansal, K.C. 2014. Chakhao (delicious) rice landraces (Oryza sativa L.) of North-east India: Collection, conservation and characterization of genetic diversity. Plant Genetic Resources. 12(3): 264-272.
Sangtong, V. 2020. Aromatic genes in rice. Maejo Press, Maejo University, Chiang Mai, Thailand.
Schiller, J.M., Appa Rao, S., Inthapanya, P., and Hatsadong. 2006. Glutinous rice in Laos. p 197-214. In: Schiller, J.M., Chanphengxay, M.B., Linquist, B., Appa Rao, S. (eds), Rice in Laos. International Rice Research Institute, Los Baños, Philippines.
Shrestha, S., Asch, F., Dusserre, J., Ramanantsoanirina, A., and Brueck, H. 2012. Climate effects on yield components as affected by genotypic responses to variable environmental conditions in upland rice systems at different altitudes. Field Crops Research. 134: 216-228.
Singh, R.K., Dwivedi, J.L., Thakur, R., Mallik, S., and Ahmed, T. 2004. Rice biodiversity and genetic wealth of the flood-prone environment in eastern India. In: Bhuiyan, S.I., Abedin, M.Z., Singh, V.P., and Hardy, B. [eds], Rice Research and Development in the Flood-Prone Ecosystem, International Rice Research Institute, Los Baños, Philippines, pp 115-128.
Singh, V., Singh, A.K., Mohapatra, T., Krishnan, G., and Ellur, R.K. 2018. PusaBasmati 1121 – a rice variety with exceptional kernel elongation and volume expansion after cooking. Rice 11:19.
Sirabanchongkran, A., Rerkasem, K., Yimyam, N., Boonma,W., Coffey, K., Pinedo-Vasquez, M., and Padoch, C. 2004. Varietal turnover and seed exchange: Implications for conservation of rice genetic diversity on-farm. International Rice Research Notes. 29: 18-20.
Sommut, W. 2003. Changes in flood-prone rice ecosystems in Thailand, crop year 2000-2001. Department of Agriculture, Thailand.
Sreethong, T., Prom-u-thai, C.T., Rerkasem, B., Dell, B., and Jamjod, S. 2020. Identifying rice grains with premium nutritional quality among on-farm germplasm in the highlands of Northern Thailand. Quality Assurance and Safety of Crops and Foods. 12(3): 12-23.
Supamongkol, P. 2006. Genetic diversity of local rice cv. Muey Nawng. M.Sc. Thesis (Agronomy), Chiang Mai University, Chiang Mai, Thailand.
Tayathum, C., Attathom, T., Thongphak, D., and Sripongpankul, K. 2004. Rice gall midge in Thailand: current status and biotype characterization. In: Bennett, J., Bentur, J.S., Pasulu, I.C., and Krishnaiah, K. [eds], New approaches to gall midge resistance in rice. International Rice Research Institute, Los Baños, Philippines, and Indian Council of Agricultural Research, pp 89-97.
Tejakum, P., Khumto, S., Jamjod, S., Yimyam, N., Pusadee, T. 2019. Yield, grain quality and fragrance of a highland fragrant rice landrace variety, Bue Ner Moo. Khon Kaen Agriculture Journal. 47(2): 317-326.
The Rice Traders Association. 2010. World’s best rice. https://thericetrader.com/conferences/2019-wrc-manila/worlds-best-rice/ (Accessed 25 January 2021)
Thein, M.S., Lee, G.A., Cho, G.T., Sung, J.S., Jeong, J.W., Park, J.H., and Baek, H.J. 2012. Assessment of genetic diversity in Meedon rice (Oryza sativa L.) germplasm. Journal of the Korean Society for International Agriculture. 24(2): 232–240.
Thomson, M.J., Polato, N.R., Prasetiyono, J., Trijatmiko, K.R., Silitonga, T.S., and McCouch, S.R. 2009. Genetic diversity of isolated populations of Indonesian landraces of rice (Oryza sativa L.) collected in East Kalimantan on the island of Borneo. Rice 2: 80–92.
Titiprasert, V., Vuthiyano, C., Jaichagun, M., Marklua, T., Tongjuepet, P., Raksasilp, S. and Deeman, A. 2001. Plant Germplasm Database: Rice. Department of Agriculture, Bangkok.
TRKB, 2024a. Pre- and post-harvest management: Rice quality. Thailand Rice Knowledge Bank, Division of Rice Research and Development. https://newwebs2.ricethailand.go.th/webmain/rkb3/title-index.php-file=content.php&id=6-2.htm
TRKB, 2024b. Rice varieties. Thailand Rice Knowledge Bank, Division of Rice Research and Development. http://www.ricethailand.go.th/rkb3/Varieties.htm.
Unthong, A., Kaosa-ard, M. and Punkiew, N. 2007. Factors affecting upland farmers’ choice of local rice varieties in Thailand. Economic Journal (Kasetsart University). 14(2): 70-85.
Vanavichit, A., Tragoolrung, S., and Toojinda, T. 2001. Biotechnology and rice improvement. In: Science, Technology and Thai Rice. BIOTEC and NSTDA, Bangkok, pp 79-121.
Vilayheuang, K., Machida-Hirano, R., Bounphanousay, C., and Watanabe, K.N. 2016. Genetic diversity and population structure of ‘khao kai noi’, a Lao rice (Oryza sativa L.) landrace, revealed by microsatellite DNA markers. Breeding Science. 66(2): 204-212.
Wongtamee, A., Maneechote, C., Pusadee, T., Rerkasem, B., and Jamjod, S. 2017. The dynamics of spatial and temporal population genetic structure of weedy rice (Oryza sativa f. spontanea Baker). Genetic Resources and Crop Evolution. 64: 23-39.
Xiongsiyee, V., Rerkasem, B., Veeradittakit, J., Saenchai, C., Lordkaew, S., Prom-u-thai, C.T. 2018. Variation in grain quality of upland rice from Luang Prabang Province, Lao PDR. Rice Science. 25: 94-102.
Yimyam, N. 2006. Fallow regeneration and upland rice yield variation in a system of shifting cultivation with PADA (Macaranga denticulata (BL.) Muell.Arg) as the fallow enriching species in Northern Thailand. Ph.D. Thesis (Agronomy), Chiang Mai University, Chiang Mai, Thailand.
Yodmanee, S., Karrila, T.T., and Pakdeechanuan, P. 2011. Physical, chemical and antioxidant properties of pigmented rice grown in Southern Thailand. International Food Research Journal. 18(3): 901-906.
Zeng, Y., Li, S., Pu, X., Du, J., Yang, S., Liu, K., Gui, M., and Zhang, H. 2006. Ecological difference and correlation among cold tolerance traits at the booting stage for core collection of rice landrace in Yunnan, China. Chinese Journal of Rice Science. 20(3): 265–271.
Zeng, L., and Shannon, M.C., 2000. Salinity effects on seedling growth and yield components of rice. Crop Science. 40: 996–1003.
Zeven, A.C. 1998. Landraces: A review of definitions and classifications. Euphytica. 104: 127–139.
Zhang L., Cao, G., and Han, L. 2013. Genetic diversity of rice landraces from lowland and upland accessions of China. Rice Science. 20(4): 259-266.
Zinke, P.J., Sabhasri, S., and Kunstadter, P. 1978. Soil fertility aspects of the Lua’ forest fallow system of shifting cultivation. Kunstadter, P., Chapman, E.C., and Sabhasri, S. [eds], Farmers in the forest. The University Press of Hawaii, Honolulu, pp 134-159.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tonapha Pusadee1, 3, Sansanee Jamjod1, 2, Chanakan Prom-u-thai1, 2, Pennapa Jaksomsak1, 2, and Benjavan Rerkasem4, *
1 Department of Plant and Soil Science, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Lanna Rice Research Centre, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Agrobiodiversity in Highland and Sustainable Utilization Research Group, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand.
4 Plant Genetic Resource and Nutrition Laboratory, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Benjavan Rerkasem, E-mail: benjavan.r@cmu.ac.th
.
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: February 9, 2024;
Revised: March 29, 2024;
Accepted: March 29, 2024;
Online First: April 9, 2024

