
Terminalia bellirica Extract Suppresses SARS-Cov-2 Nucleocapsid-Induced Inflammation in A549 Cells
Peeranut Winidmanokul, Suthida Panwong, and Aussara Panya*Published Date : April 9, 2024
DOI : https://doi.org/10.12982/NLSC.2024.026
Journal Issues : Number 2, April-June 2024
Abstract SARS-CoV-2 infection triggers a host-immune response via cytokine release that, when excessive, leads to severe inflammation and life-threatening complications. To reduce the risks associated with cytokine storms, alternative approaches are needed. Traditional Thai herbal extracts are recognized for their potential as safe and effective anti-inflammatory agents against various diseases. Hence, this study aims to examine the anti-inflammatory effects of ethanolic extracts from Terminalia chebula, Terminalia bellirica, Phyllanthus emblica, and Andrographis paniculata in reducing inflammation in the A549 alveolar basal epithelial cell lines. We conducted a comparative analysis of the anti-inflammation efficacy of four extracts in reducing the COX-2 upregulation induced by TNF-α stimulation in A549 cells. Among them, T. bellirica exhibited the highest effectiveness in reducing COX-2 levels to 0.38-fold. Furthermore, we validated the anti-inflammation properties of T. bellirica in diminishing inflammation-induced SARS-CoV-2 nucleocapsid. Lentivirus transduction expressing SARS-CoV-2 nucleocapsid demonstrated a dose-dependent increase in the expression of pro-inflammatory cytokines and mediators including TNF-α, IL-8, CXCL-10, and COX-2. Interestingly, treatment with sublethal doses of T. bellirica (30 and 60 μg/mL) led to a significant reduction in COX-2 expression by 30% and 70%, TNF-α by 46% and 75%, IL-8 by 39% and 48%, and CXCL-10 by 46% and 80%, respectively. These findings confirm the potent anti-inflammatory effects of T. bellirica, highlighting its potential as a novel treatment for alleviating the severity of cytokine storms in SARS-CoV-2 and related diseases.
Keywords: Anti-inflammatory effects, Thai traditional herbs, SARS-CoV-2, Cytokine storm, Terminalia bellirica
Funding: This research project was supported by the CMU Junior Research Fellowship Program, Chiang Mai University 2020. Peeranut Winidmanokul was supported by a Development and Promotion of Science and Technology Talents Project (DPST), Royal Government of Thailand Scholarship.
Citation: Winidmanokul, P., Panwong, S., and Panya, A. 2024. Terminalia bellirica extract suppresses SARS-Cov-2 nucleocapsid-induced inflammation in A549 cells. Natural and Life Sciences Communications. 23(2): e2024026.
INTRODUCTION
The global COVID-19 pandemic, caused by SARS-CoV-2, has emerged as a significant health crisis, primarily affecting the respiratory tract, and manifesting a wide range of symptoms. These symptoms involve increased levels of cytokine and chemokine, causing a cytokine storm that contributes to the severity of the disease, multi-organ failure, and lethality (Dong et al., 2020; Garciá et al., 2021). Infection with SARS-CoV-2 triggers a host's immune response, causing cytokine storm, and inflammation. The spike and nucleocapsid proteins of SARS-CoV-2 induce inflammation via toll-like receptors (TLR) 2-mediated activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling pathway (Nie et al., 2023). Furthermore, the nucleocapsid protein of both SARS-CoV and SARS-CoV-2 have been reported to play a role in promoting cytokine overexpression through the NFκB cascade (Zhang et al., 2007; Su et al., 2021; Xia et al., 2021; Gudowska-Sawczuk & Mroczko, 2022).
Triggering the NFκB signaling can upregulate the production of pro-inflammatory mediators to combat abnormal stages such as cell damage and infection, including IL-6, and TNF-α, chemokine, like IL-8 and CXC-motif chemokine ligand (CXCL)-10, and inflammatory-related enzymes, like cyclooxygenase (COX)-1 and -2 (Poligone & Baldwin, 2001; Baghaki et al., 2020; Lu et al., 2023). Elevated levels of these mediators lead to activation and recruitment of other leukocytes to the site of infection, resulting in localized inflammation (Morris et al., 2021; Rabaan et al., 2021). Previously, the association between cytokine storm and the severity of COVID-19 has been extensively documented, with severe and critically severe COVID-19 patients exhibiting a significant increase in IL-1β, TNF-α, IL-6, and CXCL-10 in the serum emphasizing the contribution of NFκB signaling during SARS-CoV-2 infection. TNF-α has been recognized for its pivotal role in COVID-19. As one of the primary pro-inflammatory cytokines in the innate immune response, TNF-α is frequently upregulated in acute lung injury, triggering cytokine release syndrome. The elevation of TNF-α levels is associated with disease severity, as evidenced by the discrimination between patients with Multisystem Inflammatory Syndrome in Children (MIS-C) and severe COVID-19 (Diorio et al., 2020). Additionally, TNF-α plays a significant role in virus infection, facilitating the interaction of SARS-CoV with angiotensin-converting enzyme 2 (ACE2), thereby expediting viral entry into infected cells (Haga et al., 2008). This mechanism may contribute to the significant induction of cytokine storm observed in COVID-19.
The nucleocapsid and spike protein of SARS-CoV has been demonstrated to induce COX-2 expression in mammalian cells (Liu et al., 2006; Yan et al., 2006) which may contribute to lung inflammation during infection. This induction of COX-2 is implicated in the development of virally induced pulmonary alveolar, interstitial, and systemic inflammation. COX-2 production promotes tissue inflammation by converting arachidonic acid into prostaglandin E2 (PGE2), promoting local inflammation that leads to vasodilation, increased blood pressure, and recruitment of inflammatory elements (Ricciotti & Fitzgerald, 2011; Filep, 2022; Rabaan et al., 2021). If the virus spreads continuously, inflammation as well as uncontrolled release of cytokine may become systemic which can exacerbate the pathogenesis of COVID-19 (Karwaciak et al., 2021; Morris et al., 2021).
These increased cytokine levels have been linked to the progression of conditions like acute respiratory distress syndrome (ARDS), multiple organ failure, and even a fatal outcome (Nie et al., 2023). Consequently, a therapeutic strategy aimed at suppressing or modulating the excessive inflammation observed in COVID-19 remains warranted. These strategies should complement vaccination efforts designed to prevent intensive viral infection (Jackson et al., 2022).
Nonsteroidal anti-inflammatory drugs (NSAIDs), typically used to alleviate pain and inflammation in a variety of conditions, have also been used in the treatment of COVID-19 patients (Fajgenbaum & June, 2020; Micallef et al., 2020; Reese et al., 2022). While previous studies demonstrated the safety of NSAIDs in COVID-19 treatment, there has been a controversial issue regarding NSAIDs utility. Some concerns emerged suggesting that NSAIDs, particularly ibuprofen (an NSAID drug), may potentially exacerbate COVID-19 symptoms. This concern arises from the observations that ibuprofen treatment can lead to an increase in the expression of the ACE2 receptor, which serves as a cognate receptor of SARS-CoV-2. This revelation has raised questions about the revealed the administration of NSAIDs in COVID-19 patients. On the other hand, the use of traditional herb extracts is currently under investigation as a potential alternative therapeutic approach for COVID-19 (Chen et al., 2021; Sa-Ngiamsuntorn et al., 2021). Previous studies have documented the anti-inflammatory properties of Thai traditional herbs in various disease and infection models (Jayesh et al., 2017), suggesting their potential utility in addressing inflammation-related complications associated with COVID-19. Terminalia chebula (Ekambaram et al., 2022), Terminalia bellirica (Jayesh et al., 2017), Phyllanthus emblica (Sripanidkulchai & Junlatat, 2014), and Andrographis paniculata (Li et al., 2017) have received national recognition for their medicinal properties, confirming their efficacy and safety (Urumarudappa et al., 2020). However, none of those have studied their effect in inhibiting cytokine production and inflammation response. Therefore, we aimed to investigate and compare the selected Thai anti-inflammatory herbs in reducing cytokine storm in virus-induced inflammation conditions using TNF-α and transduction of SARS-CoV-2 nucleocapsid. Our finding may provide valuable alternative treatments against SARS-CoV-2 infection or other diseases related to cytokine storms.
MATERIAL AND METHODS
Cell cultures
The human non-small cell lung cancer cell line, A549 (ATCC No.: CCL-185), established from alveolar epithelial cells of pulmonary carcinoma patients was purchased from ATCC (American Type Culture Collection, VA, USA). The cells were cultured in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) The highly efficient protein-synthesized cell line, HEK293T (ATCC No.: CRL-11268), was purchased from ATCC (American Type Culture Collection, VA, USA) and cultured in the Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). The media was supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), penicillin-streptomycin, and L-glutamine (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Cells were incubated at 37°C and 5% CO2 incubator.
Herb extraction
Four Thai traditional herbs, Terminalia chebula, Terminalia bellirica, Phyllanthus emblica and Andrographis paniculata, were gifted from Asst.Prof.Dr. Hataichanok Pandith and identified by Asst.Prof.Dr. Narin Printarakul, plant taxonomist, Department of Biology, Faculty of Science, Chiang Mai University. The dried samples, fruits of T. chebula, T. bellirica and P. emblica, and leaves of A. paniculata, were extracted by 70% ethanol (EtOH) on an orbital shaker at a speed of 160 rpm at room temperature for 24 hours. The extracted solutions were filtrated through Whatman® filter paper No.1 (Whatman Little Chalfont, Buckinghamshire, United Kingdom) and concentrated under reduced pressure using a rotary evaporator to produce the crude EtOH dried extract. The extracts were stored at -20°C until used. The working stock of herb extracts was dissolved in DMSO.
Cell viability assay
The cytotoxicity of each herb extract was investigated by PrestoblueTM cell viability reagent (Invitrogen, Carlsbad, CA, USA). A549 were plated on 96-well plates (1.0 x 104 cells per well) and incubated at 37 ºC and 5% CO2. After 24 hours of incubation, the cells were treated with ethanolic herb extracts at a concentration ranging from 0.16 – 500 μg/mL and incubated at 37°C and 5% CO2 for 24 and 48 hours. After the incubation, the cells were washed with phosphate buffer saline (PBS). PrestoblueTM cell reagent was added to the cells to measure the reducing capacity of living cells. The plate was measured the absorbance at 570 and 595 nm by using NS-100 NanoScan Microplate Reader (Hercuvan Lab System, Cambridge, UK). The absorbances of herb-treated groups were compared to the non-treated control (set as 100%), to calculate the percentage of cell viability as the following equation.
% Cell viability = [(OD570-OD595) treated cells/(OD570-OD595) non-treated cells] X 100
The half-maximal cytotoxicity concentration (CC50) of each herb at each concentration was analyzed using non-linear regression in GraphPad Prism Software version 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Construction of SARS-CoV-2 nucleocapsid-expressing lentivirus vector
Full-length coding sequencing of SARS-CoV-2 nucleocapsid gene was amplified using SARS-CoV-2 nucleocapsid control (IDT, Coralville, IA, USA) as the template. The coding sequence was cloned into a pCDH lentivirus vector with MYC tag to produce pCDH-nucleocapsid vector. The ligated product was transformed into Escherichia coli strain DH5α. The positive clones were screened using colony-PCR and confirmed the sequence correction using the DNA sequencing technique. The expression of nucleocapsid was confirmed by Western blot using the anti-MYC tag antibody (Clone 9E10, Santa Cruz Biotechnology, CA, USA).
Lentivirus production
Lentivirus packaging was performed to produce lentivirus harboring SARS-CoV-2 nucleocapsid gene. The HEK293T cells were plated in a 6-well plate with DMEM medium supplemented with 10% fetal bovine serum, Pen Strep, and L-glutamine (all reagents from Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and incubated at 37°C and 5% CO2 for 24 hours. After incubation, the cultured medium was replaced with reduced-serum Opti-MEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Then, the cells were transfected with a pCDH-nucleocapsid vector with the packaging (psPAX2) and envelope (pMD2.G) plasmid vectors using LipofectamineTM 2000 (Thermo Fisher Scientific, Waltham, MA, USA) follows the manufacturer’s instruction. After 72 hours of incubation, the lentivirus harboring SARS-CoV-2 nucleocapsid was harvested and kept at 80°C until use.
Inflammation induction in A549
The recombinant TNF-α (ImmunoTools, Friesoythe, Germany) was used to generate inflammation model in A549. This condition was used to determine the anti-inflammatory effect of herb extracts and compare their effectiveness. Briefly, the cells were plated in a 12-well plate with approximately 1.5 x 105 cells per well a day before the experiment. The TNF-α at concentrations of 10, 100, and 1,000 ng/mL were added to the cells and incubated for 24 hours. After incubation, the cells were collected to measure the mRNA expression of proinflammatory cytokine using quantitative reverse transcription polymerase chain reaction (qRT-PCR).
To evaluate the anti-inflammatory effect of the candidate herb, the lentivirus harboring SARS-CoV-2 nucleocapsid was transduced into A549 to induce inflammation. A549 cells were plated in a 12-well plate with approximately 1.0 x 105 cells per well a day before the experiment. The lentivirus (MOI = 0.5, 1, and 2) was added to the cells and incubated for 24 hours. After incubation, the cells were collected to measure the mRNA expression of proinflammatory cytokine using qRT-PCR.
Anti-inflammatory effect of herb extracts
The effects of the herb in reducing cytokine production in TNF-α treated or lentivirus-transduced A549 cells were determined. The extracts of T. chebula, T. bellirica, P. emblica, and A. paniculata at the same concentration (30 µg/mL) that is non-toxic to A549, were added to TNF-α treated or lentivirus-transduced A549 cells. The cells were harvested at 24 hours after incubation and measured for the level of mRNA expression using qRT-PCR.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
The total RNA was isolated using TRIzolTM reagent (Invitrogen, Carlsbad, CA, USA). The qRT-PCR was performed using SensiFAST SYBR No-ROX One-step Kit (Meridian Bioscience, Memphis, TN, USA) to monitor the alteration of gene expression, composed of pro-inflammatory cytokines; TNF-α, chemokines; IL-8 and CXCL-10 and inflammation-related enzyme; COX-2. The reaction was performed in qTOWER³ Real-time PCR, (Analytik Jena GmbH+Co. KG, Jena, Germany) The GAPDH was used as a control housekeeping gene. The primers of each gene are shown in Supplementary table 1.
RESULTS
Cytotoxicity of ethanolic herb extracts on A549 lung epithelial cells
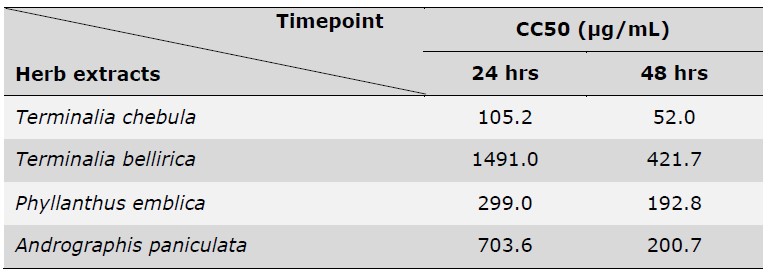
In total, four herbs including Terminalia chebula, Terminalia bellirica, Phyllanthus emblica, and Andrographis paniculata were selected from the NLEM list of Thailand based on their reported anti-inflammation capacity in both in vitro (Ekambaram et al., 2022; Li et al., 2017; Sripanidkulchai & Junlatat, 2014; M. Tanaka et al., 2018) and in vivo (Jayesh et al., 2017; Kim et al., 2022; C. C. Wang et al., 2017; Zou et al., 2016) models. The ethanolic extracts of four herbs were determined for their effects on the cell viability of A549 lung epithelial cell lines. The results showed that herb extract exhibited the cytotoxic effect in a dose-dependent manner (Table 1 and Figure 1). The half-maximal cytotoxic concentration (CC50) was calculated, revealing CC50 values of 105.2 μg/mL (24 hours) and 52.0 μg/mL (48 hours) for T. chebula, 1491 μg/mL (24 hours) and 421.7 μg/mL (48 hours) for P. emblica, 299 μg/mL (24 hours) and 192.8 μg/mL (48 hours) for T. bellirica, 703.6 μg/mL (24 hours) and 200.7 μg/mL (48 hours) for A. paniculata, respectively (Table 1).
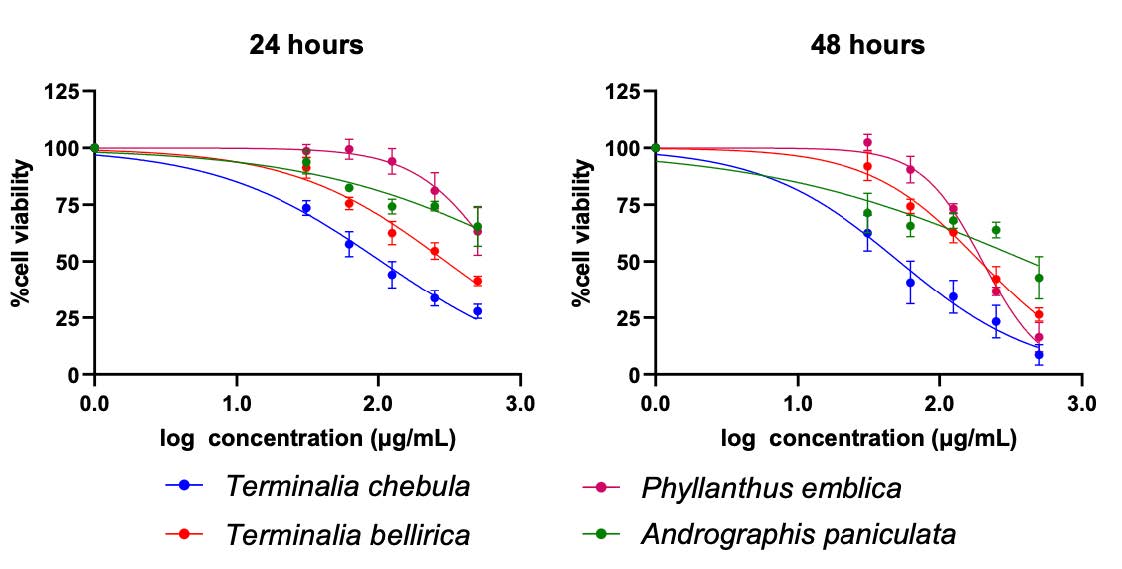
Figure 1. Cytotoxicity of herb extracts on A549 lung epithelial cells. A549 was treated with various concentration (0.16-500 µg/mL) of herb extracts for 24 (left) and 48 hours (right). Cytotoxicity of the herb extracts were determined by cell viability assay which represents in percentage of cell viability relative to that of non-treatment control.
Table 1. The CC50 values of Terminalia chebula, Terminalia bellirica, Phyllanthus emblica, and Andrographis paniculata ethanolic extracts on A549 at 24 hours and 48 hours.
TNF-α promoted inflammation in A549 by increasing inflammatory gene expression.
Recombinant TNF-α was used to induce the inflammation condition in A549 cells. After 24 hours of TNF-α treatment, the expression level of IL-8, CXCL-10, TNF-α, and COX-2 were detected by qRT-PCR. The results indicated that treatment of TNF-α at the concentrations of 10, 100, and 1000 ng/mL increased the expression of IL-8, CXCL-10, TNF-α, and COX-2 in a dose-dependent fashion (Figure 2). Treatment with 10, 100 and 1000 ng/mL augment the mRNA expression to 11.27-, 58.89-, and 115.1-fold for IL-8; 346.3-, 775.6-, and 1987-fold for CXCL-10; 12.27-, 32.97-, and 179.3-fold for TNF-α; 12856-, 19633-, and 77813-fold for COX-2, respectively. The upregulation of these cytokine expressions confirmed the inflammation induction in A549 caused by TNF-α.
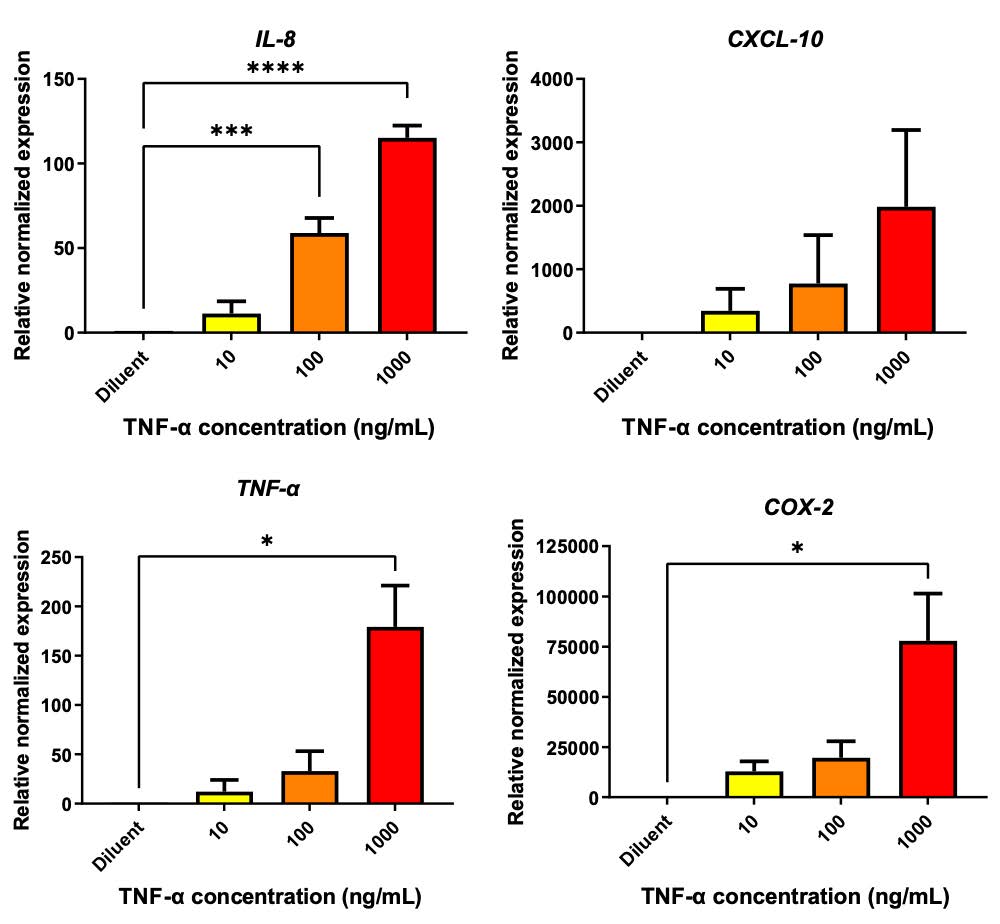
Figure 2 TNF-α triggered the inflammation response in A549. TNF-α at the concentrations of 10, 100 and 1000 ng/mL was treated to A549 cells for 24 hours. The treated cells were collected and evaluated the gene expression of IL-8, CXCL-10, TNF-α and COX-2 using qRT-PCR. The data was normalized with GAPDH and represented relative normalized expression where non-treated control was set as 1.0. (* P < 0.05, ** P < 0.01, *** P < 0.001).
Terminalia bellirica ethanolic extract potentially exhibited anti-inflammatory effects against TNF-α-induction.
The sublethal dose of each herb extract was used to evaluate their anti-inflammatory effects on TNF-α treated A549. Herb extracts at the equal concentration (30 µg/mL) was added to A549 cells upon TNF-α stimulation (1,000 ng/mL). The efficiency of the extract on lowering inflammation was compared based on their ability to reduce the COX-2 expression assessed by qRT-PCR. The result showed that T. bellirica exhibited the most potential by reducing the COX-2 expression to 0.38-fold, followed by P. emblica, and A. paniculata, which decreased the expression to 0.47, and 0.40-fold respectively, compared to non-treated control. In contrast, treatment of T. chebula failed to inhibit COX-2 expression (Figure 3)
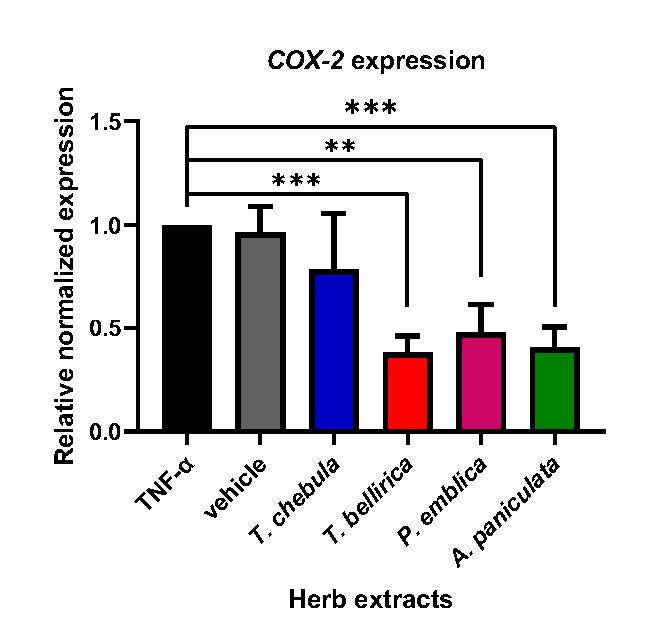
Figure 3. Effects of herb ethanolic extracts on COX-2 expression in TNF-α-induced A549. A549 were treated with sublethal doses of herb extracts (30 µg/mL) in the presence of high-dose TNF-α (1,000 ng/mL) for 24 hours. The COX-2 gene expression was determined using qRT-PCR. The graph showed the data in relative expression of normalized to GAPDH (* P < 0.05, ** P < 0.01, *** P < 0.001).
SARS-CoV-2 nucleocapsid induced inflammation in A549.
Previously, the role of SARS-CoV-2 nucleocapsid in inducing cytokine storms has been reported (Karwaciak et al., 2021). To confirm the effect of the SARS-CoV-2 nucleocapsid on inducing inflammation response, we assessed alterations in the gene expression of pro-inflammatory cytokines upon introducing nucleocapsid in A549 cells. The lentivirus harboring SARS-CoV-2 nucleocapsid encoding gene was generated according to the scheme depicted in Figure 4A and used to transduce A549 cells. The expression of SARS-CoV-2 nucleocapsid was observed using an immunofluorescence assay (IFA). The result demonstrated that the majority of nucleocapsid protein was localized in the cytoplasm (Figure 4B). Additionally, a western blot analysis confirmed the expression of nucleocapsid protein, showing a band at approximately 43.33 kDa (Figure 4C).
To determine the inflammation response, the qRT-PCR was performed after 48 hours of lentivirus transduction. The result demonstrated that transduction with lentivirus at the MOI of 2 remarkably elevated the gene expression of IL-8, CXCL-10, TNF-α, and COX-2 (Figure 5), upregulating their expression levels to 2.173-fold for IL-8; 2.343-fold for TNF-α; 3.108-fold for CXCL-10; and 1.670-fold for COX-2. (Figure 5). These findings underscore the potential role of the nucleocapsid in promoting inflammation in A549 cells.
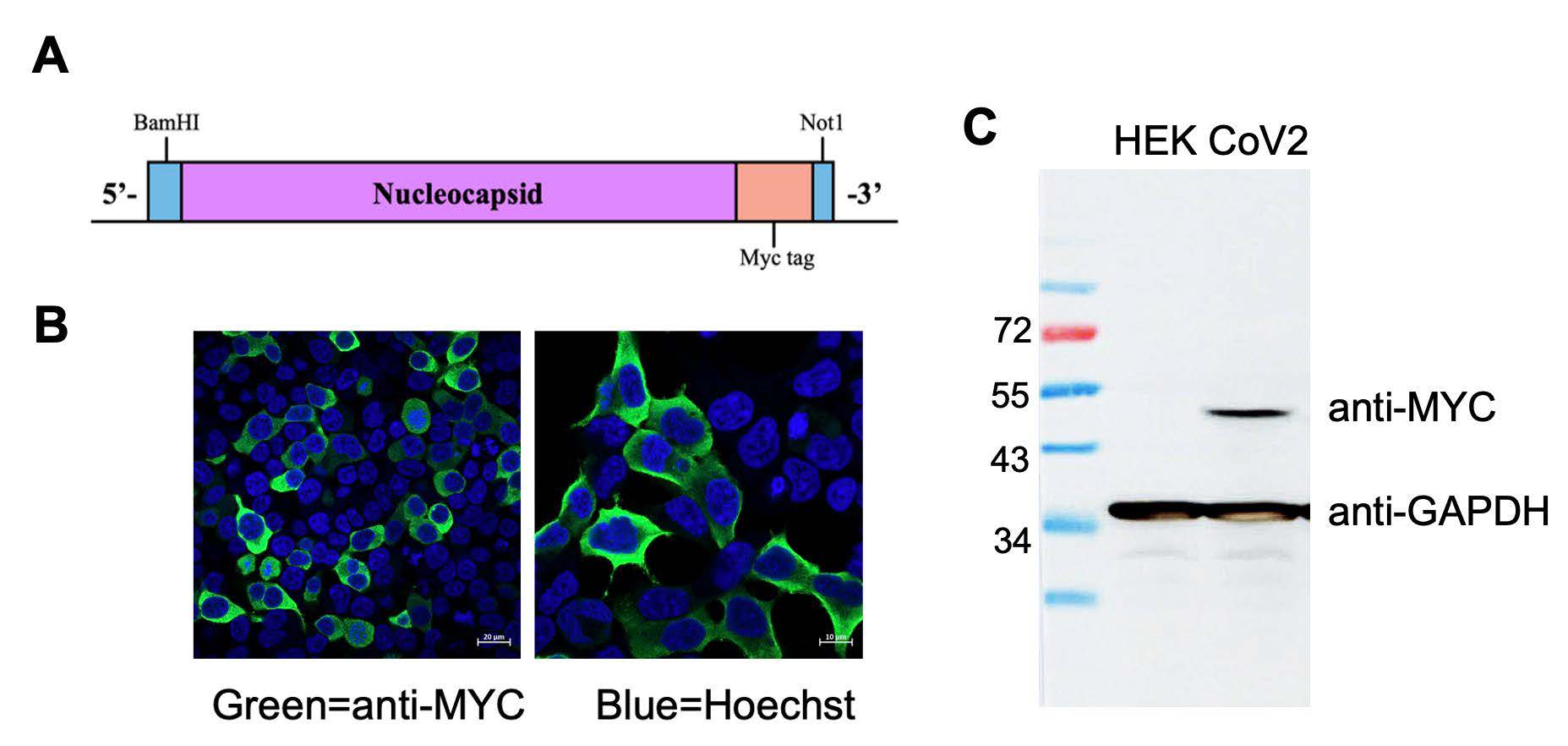
Figure 4. Lentivirus harboring SARS-CoV-2 nucleocapsid gene and expression. (A) A construct map of nucleocapsid gene with myc tag enclosed by BamHI and NotI restriction site. (B) SARS-CoV-2 nucleocapsid expression and localization were determined by IFA and confocal microscope. (C) Western blot analysis confirmed the expression of nucleocapsid with 46 kDa in size.
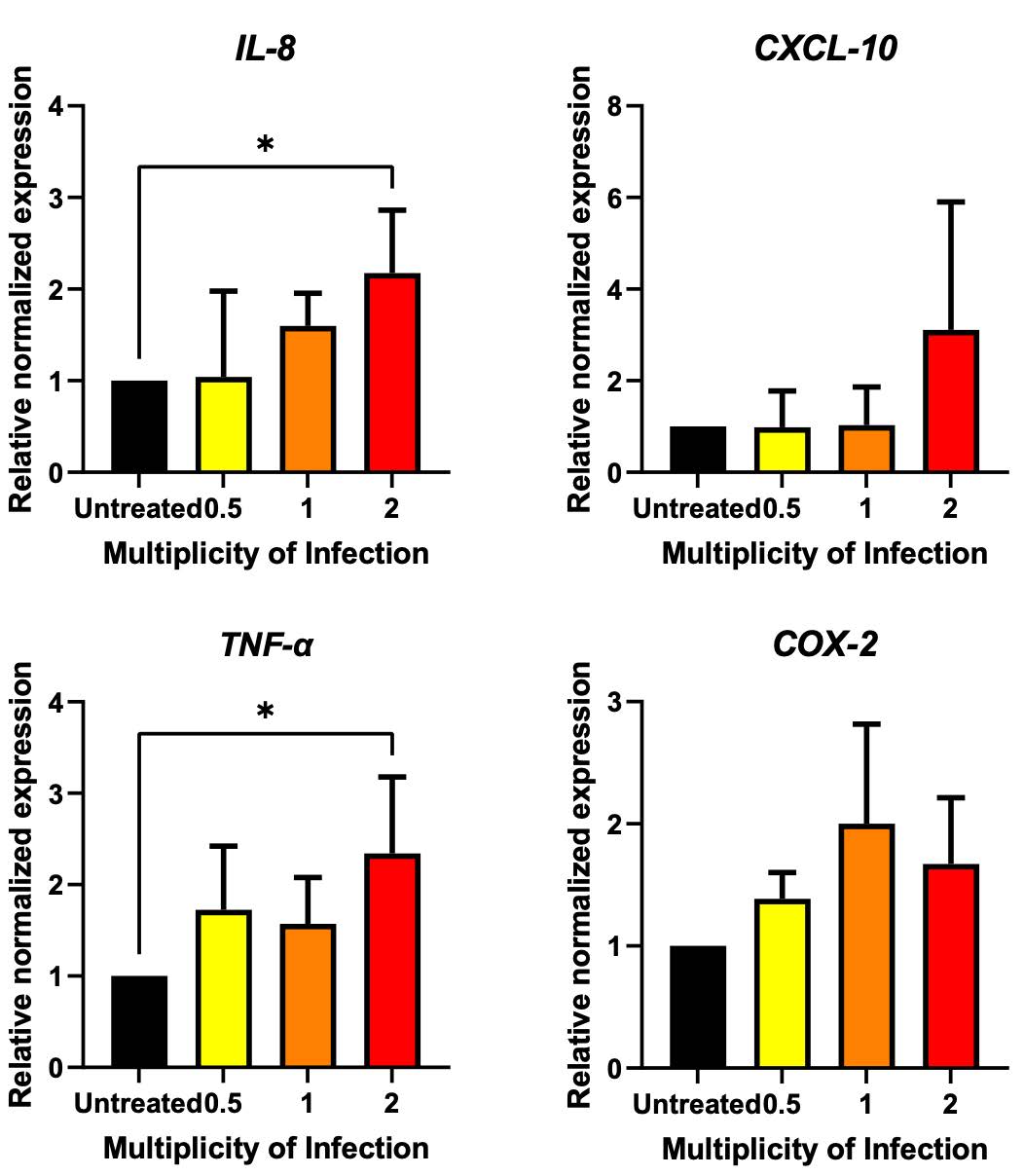
Figure 5. Effect of SARS-CoV-2 nucleocapsid on inflammation stimulation in A549 cells. A549 cells were transduced with nucleocapsid-expressing lentivirus at the MOI of 0.5, 1 and 2. The changes in gene expression were determined after 48 hours of treatment using qRT-PCR. The expression level was normalized to GAPDH and represented as a relative normalized expression (* P < 0.05, ** P < 0.01, *** P < 0.001).
Terminalia bellirica extract reduced inflammatory gene expression in nucleocapsid-transduced A549 cells.
The anti-inflammatory effects of T. bellirica extract were investigated against SARS-CoV-2 nucleocapsid in A549 cells. A549 cells were transduced with lentivirus containing nucleocapsid gene at the MOI of 2 for 48 hours, followed by treatment with T. bellirica extract at the sub-lethal concentration of 30 and 60 µg/mL which have been already confirmed their low cytotoxic to A549. Alterations in the expression of IL-8, CXCL-10, TNF-α, and COX-2 expression were assessed at 24 hours after extract treatment using qRT-PCR. The results demonstrated that T. bellirica extract significantly reduced the expression of inflammatory genes in a dose-dependent manner. At a concentration of 60 µg/mL, T. bellirica extract showed a significant reduction in TNF-α and COX-2 expression to 0.25 and 0.30, respectively. Similarly, at a concentration of 30 µg/mL, T. bellirica extract significantly decreased the expression of CXCL-10 and TNF-α to 0.54 and 0.54 respectively. These findings highlight the potential anti-inflammatory properties of T. bellirica extract and its ability to modulate the expression of key inflammatory genes in a dose-dependent manner (Figure 6).
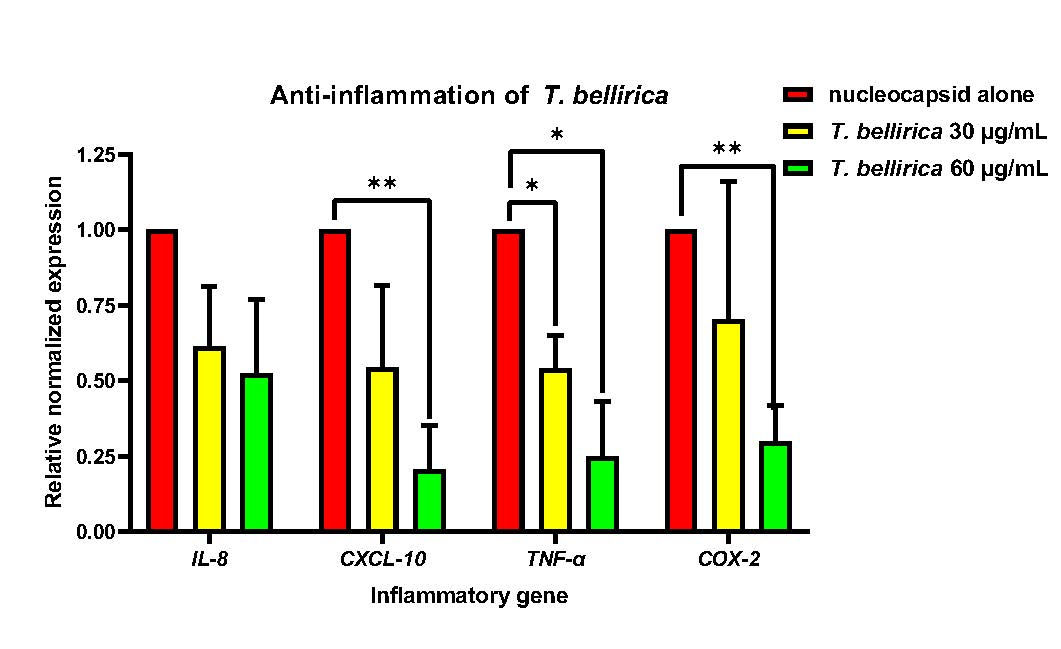
Figure 6. Anti-inflammatory effect of T. bellirica in reducing the overexpression of IL-8, CXCL-10, TNF-α, and COX-2 in nucleocapsid-induced A549 cells. A549 cells were transduced with nucleocapsid-expressing lentivirus for 48 hours. Then, sublethal doses of T. bellirica extracts (60 and 30 µg/mL) were treated in the transduced cells. The changes in gene expression were determined after 24 hours of treatment using qRT-PCR. The expression level was normalized to GAPDH and represented as a relative normalized expression (* P < 0.05, ** P < 0.01, *** P < 0.001).
DISCUSSION
COVID-19, caused by SARS-CoV-2, can induce a potentially life-threatening cytokine storm characterized by excessive production of pro-inflammatory cytokines, remarkably TNF-α and IL-6 (Dong et al., 2020; Garciá et al., 2021; Morris et al., 2021; Rabaan et al., 2021). Severe COVID-19 cases are often associated with cytokine storms, and recent research has highlighted the nucleocapsid protein of SARS-CoV-2 as a primary instigator (Karwaciak et al., 2021). Cytokine storms can lead to acute respiratory distress syndrome and multiple organ failure (Rabaan et al., 2021; Soy et al., 2020). Besides cytokines, enzymes like Janus kinase and cyclooxygenase-2 play significant roles, with current treatments often involving nonsteroidal anti-inflammatory drugs (NSAIDs) (Baghaki et al., 2020; Capuano et al., 2020; Gajjela & Zhou, 2022). Notably, controversy exists regarding the association of NSAID usage and potential complications in COVID-19 patients, prompting increased interest in traditional plant-based remedies as an alternative approach. This study thus aimed to evaluate the anti-inflammatory effects of T. chebula, T. bellirica, P. emblica, and A. paniculata in diminishing inflammation-induced SARS-CoV-2 nucleocapsid.
In this study, the ethanolic extracts were prepared as described in the former protocol (Thongyim et al., 2023). TNF-α has been identified as a pivotal inflammatory cytokine associated with various inflammatory processes (Tanaka et al., 2016). It plays a significant role in directly stimulating inflammation within target cells, recruit innate immune cells and activating essential inflammatory mediators such as cyclooxygenase (COX)-2 which stimulate vasodilation to allow other immune cells to the site of inflammation (Medeiros et al., 2010; Poligone & Baldwin, 2001). This activation initiates the initial stages of the inflammatory response and can subsequently escalate inflammation to more severe stages. Additionally, this study focused on in vitro inflammation model in A549 lung epithelial cells alone, so, TNF-α was more specific and notably suitable than IL-6 which more remarkably orchestrates the recruitment and activation of various immune cells, especially potent cytokine releasing cells in adaptive immunity, in response to inflammation (T. Tanaka et al., 2014; X. Wang et al., 2022).
Therefore, TNF-α was chosen to induce the inflammation in A549 cells over other cytokines. The anti-inflammatory effects of four herb extracts were judged by their efficiencies to reduce the overexpression of COX-2 gene which has a direct relation to both inflammation induction and inflammation response (Baghaki et al., 2020; Conti et al., 2020) and also exhibited the highest upregulation after overexposure of TNF-α in this study (Figure 2). The result showed that T. bellirica illustrated the highest potential in anti-inflammation activity against TNF-α-induced inflammation in A549 (Figure 3). The ability of T. bellirica to suppress the inflammation was previously reported in LPS-induced inflammation model which can diminish inflammatory gene expression via NFκB pathway, resulting in the downregulation of several proinflammatory mediators, including TNF-α and COX-2 (Figure 7) (Jayesh et al., 2017; M. Tanaka et al., 2018). Recently, our research group reported the anti-viral effects of T. bellirica extract on lowering the dengue virus infection and its potential to reduce the inflammation caused by virus infection. The results suggested that the anti-inflammation of T. bellirica extract might be possibly through the classical inflammation pathway shared among virus infection (Panya et al., 2021; Jantakee et al., 2023). Importantly, the anti-inflammatory effect of T. bellirica may related to their bioactive compound, gallic acid, which has been previously reported for anti-inflammatory effect (M. Tanaka et al., 2016) and illustrated low cytotoxicity effect in vivo studies (Prabhu et al., 2012), and ellagic acid, which also has been described their anti-inflammatory effect in vivo study (Gupta et al., 2021). However, both T. chebula and P. emblica also contain gallic acid, along with other phenolic compounds such as chebulagic acid and ascorbic acid, albeit in different ratios. These variations in composition may influence their synergistic mechanism of their bioactive compounds themselves and overall effectiveness in reducing inflammation (Mahdi Vazirian et al., 2011; Panya et al., 2021; Patel et al., 2022). On the other hand, A. paniculata exhibited distinctly separated bioactive compound, mainly andrographolide (Sa-Ngiamsuntorn et al., 2021).
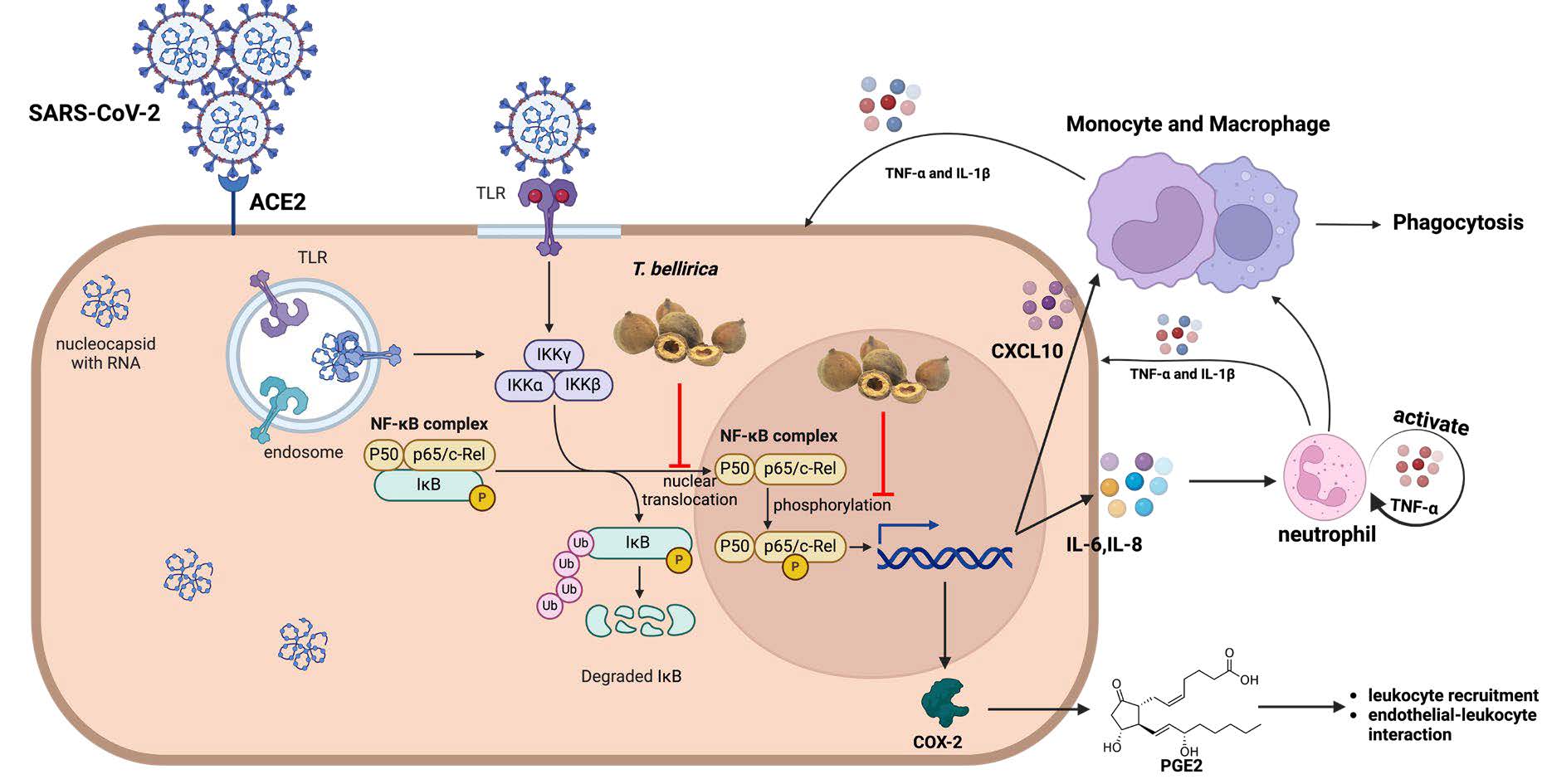
Figure 7. Inhibition pathway of T. bellirica against inflammatory response. Crude ethanolic T. bellirica extracts, which has combination of several bioactive compounds, can suppress the expression inflammatory mediators through inhibition of nuclear translocation of p65 and phosphorylation of p65 in nucleus. Resulting in decreased expression of inflammatory cytokines, chemokines, and enzymes. (Created with BioRender.com)
We selected T. bellirica for the following experiments to confirm its anti-inflammatory effect in nucleocapsid-induced inflammation. Nucleocapsid transduction significantly induced the upregulation of inflammatory gene expression (IL-8, CXCL-10, TNF-α, and COX-2) (Figure 5), which were consistent with previous reports in COVID-19 patients, especially in mild and severe patients (Morris et al., 2021; Rabaan et al., 2021). Interestingly, treatment of T. bellirica could inhibit the IL-8, CXCL-10, TNF-α, and COX-2 expression in nucleocapsid-transduced A549 cells (Figure 6) demonstrating that the anti-inflammation potential of T. bellirica to prevent cytokine storm related to NFκB pathway which is significant upstream regulation of those genes. Consistently, T. bellirica extract has been previously reported their effect on NFκB pathway (M. Tanaka et al., 2018).
In vivo studies reported the safety of T. bellirica in animal models. Prabhu et al. (2012) investigated the effects of an ethanolic extract of T. bellirica, specifically examining orally administered doses of up to 2000 mg/kg body weight in mice (Prabhu et al., 2012). Their findings provided crucial insights into the tolerability of this extract, reinforcing its potential for therapeutic use. Similarly, Jayesh et al. (2017) explored the impact of an aqueous acetone extract of T. bellirica on liver function in rats, with the highest dose tested being 2000 mg/kg body weight. The study revealed no significant toxicity in treated mice compared to the control group supporting the safety profile of T. bellirica (Jayesh et al., 2017). These investigations into the safety and tolerability of T. bellirica extracts lay the foundation for considering its potential application in clinical models. Notably, the effective doses in our experiment (30 and 60 µg/mL) were much lower than the concentration used in an animal model highlighting the potential for the clinical application of T. bellirica extract in preventing cytokine storms. Our findings support that T. bellirica extract could potentially relieve the risk of cytokine storm induced by SARS-CoV-2 nucleocapsid in lung cells. However, further studies encompassing cellular pathways, in vivo investigations, and clinical trials are warranted to comprehensively confirm and clarify the efficacy and safety of this ethanolic extract.
CONCLUSION
This study focuses on anti-inflammatory herbs from Thai National List of Essential Medicines (NLEMs) against inflammation induced by SARS-CoV-2 nucleocapsid in vitro lung model. The screening experiments illustrated the most effective herb extracts, Terminalia bellirica, over the others. We confirmed the anti-inflammatory efficacy of T. bellirica, which notably reduced the expression levels of CXCL-10, TNF-α, and COX-2 in SARS-CoV-2 nucleocapsid induced inflammation in A549. These results provided valuable insights into the therapeutic potential of Thai traditional herbs, particularly T. bellirica, in reducing the inflammatory response induced by SARS-CoV-2. Their anti-inflammatory properties could serve as a promising approach to relieving cytokine storm and its associated complications in COVID-19 or related diseases. However, further in vivo studies are still required to investigate its potentials and implications in more advanced model of study.
ACKNOWLEDGEMENTS
The author would like to thank Prof. Pa-thai Yenchitsomanus, Division of Molecular Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand, for the lentivirus construct backbone used in this study and Asst. Prof. Hathaichanok Pandith, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, for Terminalia chebula, Terminalia bellirica, Phyllanthus emblica and Andrographis paniculate extracts. The research was funded by CMU Junior Research Fellowship Program, Chiang Mai University.
AUTHOR CONTRIBUTIONS
A.P. performed the conceptualization. P.W. A.P. and S.P. designed the study methodology. P.W., A.P. and S.P. performed the experiments. P.W. and A.P. performed the statistical analyses. P.W. prepared the draft of the manuscript. A.P. edited the manuscript for accuracy and important intellectual content. All authors have contributed to the final revision of the manuscript and approved submission for journal publication.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Baghaki, S., Yalcin, C. E., Baghaki, H. S., Aydin, S. Y., Daghan, B., and Yavuz, E. 2020. COX2 inhibition in the treatment of COVID-19: Review of literature to propose repositioning of celecoxib for randomized controlled studies. In International Journal of Infectious Diseases (Vol. 101, pp. 29–32). Elsevier B.V.
Capuano, A., Scavone, C., Racagni, G., and Scaglione, F. 2020. NSAIDs in patients with viral infections, including Covid-19: Victims or perpetrators? Pharmacological Research. 157: 104849.
Chen, J. S., Alfajaro, M. M., Chow, R. D., Wei, J., Filler, R. B., Eisenbarth, S. C., and Wilen, C. B. 2021. Nonsteroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. Journal of Virology. 95(7): e00014-e00021.
Conti, P, Caraffa, A., Gallenga, C. E., Ross, R., Kritas, S. K., Frydas, I., Younes, A., and Ronconi, G. 2020. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. Journal of Biological Regulators & Homeostatic Agents. 34(6): 1971-1975.
Diorio, C., Henrickson, S. E., Vella, L. A., McNerney, K. O., Chase, J., Burudpakdee, C., Lee, J. H., Jasen, C., Balamuth, F., Barrett, D. M., Banwell, B. L., Bernt, K. M., Blatz, A. M., Chiotos, K., Fisher, B. T., Fitzgerald, J. C., Gerber, J. S., Gollomp, K., Gray, C., Grupp, S. A., … Bassiri, H. 2020. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. The Journal of clinical investigation. 130(11): 5967–5975.
Dong, E., Du, H., and Gardner, L. 2020. An interactive web-based dashboard to track COVID-19 in real time. In The Lancet Infectious Diseases. 20(5): 533-534.
Ekambaram, S. P., Aruldhas, J., Srinivasan, A., & Erusappan, T. (2022). Modulation of NF-κB and MAPK signalling pathways by hydrolysable tannin fraction from Terminalia chebula fruits contributes to its anti-inflammatory action in RAW 264.7 cells. The Journal of Pharmacy and Pharmacology. 74(5): 718–729.
Fajgenbaum, D. C., & June, C. H. (2020). Cytokine Storm. New England Journal of Medicine. 383(23): 2255–2273.
Filep, J. G. (2022). Targeting neutrophils for promoting the resolution of inflammation. Frontiers in Immunology.13: 866747.
Gajjela, B. K. and Zhou, M. M. 2022. Calming the cytokine storm of COVID-19 through inhibition of JAK2/STAT3 signaling. In Drug Discovery Today (Vol. 27, Issue 2, pp. 390–400). Elsevier Ltd.
Garciá, M. M. A., Mancilla-Galindo, J., Paredes-Paredes, M., Tiburcio, Á. Z., & Ávila-Vanzzini, N. (2021). Mechanisms of infection by SARS-CoV-2, inflammation and potential links with the microbiome. Future Virology. 16(1): 43-57.
Gudowska-Sawczuk, M. and Mroczko, B. 2022. The Role of Nuclear Factor Kappa B (NF-κB) in Development and Treatment of COVID-19: Review. International Journal of Molecular Sciences. 23(9): 5283.
Gupta, A., Kumar, R., Ganguly, R., Singh, A. K., Rana, H. K., and Pandey, A. K. 2021. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicology Reports. 8: 44–52.
Haga, S., Yamamoto, N., Nakai-Murakami, C., Osawa, Y., Tokunaga, K., Sata, T., Yamamoto, N., Sasazuki, T., and Ishizaka, Y. 2008. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proceedings of the National Academy of Sciences of the United States of America. 105(22): 7809–7814.
Jackson, C. B., Farzan, M., Chen, B., & Choe, H. (2022). Mechanisms of SARS-CoV-2 entry into cells. In Nature Reviews Molecular Cell Biology. 23(1): 3-20.
Jantakee, K., Panwong, S., Pandith, H., Tragoolpua, Y., and Panya, A. 2023. Stability of Terminalia bellirica extract and its combination effect with alpha-mangostin in inhibiting dengue virus infection. Chiang Mai Journal of Science. 50(2): 1–14.
Jayesh, K., Helen, L. R., Vysakh, A., Binil, E., and Latha, M. S. 2017. Ethyl acetate fraction of Terminalia bellirica (Gaertn.) Roxb. fruits inhibits proinflammatory mediators via down regulating nuclear factor-κB in LPS stimulated Raw 264.7 cells. Biomedicine and Pharmacotherapy. 95: 1654–1660.
Karwaciak, I., Sałkowska, A., Karaś, K., Dastych, J., & Ratajewski, M. (2021). Nucleocapsid and spike proteins of the coronavirus sars-cov-2 induce il6 in monocytes and macrophages—potential implications for cytokine storm syndrome. Vaccines. 9(1): 54.
Kim, H. J., Song, H. K., Park, S. H., Jang, S., Park, K. S., Song, K. H., Lee, S. K., & Kim, T. (2022). Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-ĸB signaling in vitro. Phytomedicine. 104.
Li, Y., He, S., Tang, J., Ding, N., Chu, X., Cheng, L., Ding, X., Liang, T., Feng, S., Rahman, S. U., Wang, X., and Wu, J. 2017. Andrographolide inhibits inflammatory cytokines secretion in LPS-stimulated RAW264.7 cells through suppression of NF-κB/MAPK signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2017: 8248142.
Liu, M., Gu, C., Wu, J. et al. 2006. Amino acids 1 to 422 of the spike protein of SARS associated coronavirus are required for induction of cyclooxygenase-2. Virus Genes 33: 309–317.
Lu, Y., Ye, Z., Liu, X., Zhou, L., Ding, X., & Hou, Y. (2023). Role of SARS‑CoV‑2 nucleocapsid protein in affecting immune cells and insights on its molecular mechanisms. Experimental and Therapeutic Medicine, 26, 504.
Medeiros, R., Figueiredo, C. P., Pandolfo, P., Duarte, F. S., Prediger, R. D. S., Passos, G. F., and Calixto, J. B. 2010. The role of TNF-α signaling pathway on COX-2 upregulation and cognitive decline induced by β-amyloid peptide. Behavioural Brain Research. 209(1): 165–173.
Micallef, J. Soeiro, T. and Jonville-Béra, A-P. 2020. Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapies. 75(4): 355-362.
Morris, G., Bortolasci, C. C., Puri, B. K., Marx, W., O’Neil, A., Athan, E., Walder, K., Berk, M., Olive, L., Carvalho, A. F., and Maes, M. 2021. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all?. Cytokine. 144: 155593.
Nie, Y., Mou, L., Long, Q., Deng, D., Hu, R., Cheng, J., and Wu, J. 2023. SARS-CoV-2 ORF3a positively regulates NF-κB activity by enhancing IKKβ-NEMO interaction. Virus Research. 328: 199086.
Panya, A., Jantakee, K., Punwong, S., Thongyim, S., Kaewkod, T., Yenchitsomanus, P. T., Tragoolpua, Y., and Pandith, H. 2021. Triphala in traditional ayurvedic medicine inhibits dengue virus infection in huh7 hepatoma cells. Pharmaceuticals. 14: 1236.
Patel, S. K., Shutter, A. K., Patil, R., Desangi, A., Malali, V., Patil, J., Patil, S., Das, K. K., and Parvatikar, P. P. 2022. In-Vitro antioxidant, anti-inflammatory and cytotoxic effects of different solvent extraction Terminalia chebula, Terminalia billerica, Phyllanthus emblica. Research Journal of Pharmacy and Technology. 15(7): 2940–2944.
Poligone, B. and Baldwin, A. S. 2001. Positive and negative regulation of NF-κB by COX-2. Roles of different prostaglandins. Journal of Biological Chemistry. 276(42): 38658–38664.
Prabhu, V., Chidambaranathan, N., and Gopal, V. 2012. Evaluation and quantification of angiogenesis activity of Terminalia bellirica roxb, by mice sponge implantation method. Journal of Young Pharmacists. 4(1): 22–27.
Rabaan, A. A., Al-Ahmed, S. H., Muhammad, J., Khan, A., Sule, A. A., Tirupathi, R., Mutair, A. Al, Alhumaid, S., Al-Omari, A., Dhawan, M., Tiwari, R., Sharun, K., Mohapatra, R. K., Mitra, S., Bilal, M., Alyami, S. A., Emran, T. Bin, Moni, M. A., and Dhama, K. 2021. Role of inflammatory cytokines in covid-19 patients: A review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 9: 436.
Reese, J. T., Coleman, B., Chan, L., Blau, H., Callahan, T. J., Cappelletti, L., Fontana, T., Bradwell, K. R., Harris, N. L., Casiraghi, E., Valentini, G., Karlebach, G., Deer, R., McMurry, J. A., Haendel, M. A., Chute, C. G., Pfaff, E., Moffitt, R., Spratt, H., … Robinson, P. N. 2022. NSAID use and clinical outcomes in COVID-19 patients: a 38-center retrospective cohort study. Virology Journal. 19: 84.
Ricciotti, E., & Fitzgerald, G. A. (2011). Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(5), 986–1000.
Sa-Ngiamsuntorn, K., Suksatu, A., Pewkliang, Y., Thongsri, P., Kanjanasirirat, P., Manopwisedjaroen, S., Charoensutthivarakul, S., Wongtrakoongate, P., Pitiporn, S., Chaopreecha, J., Kongsomros, S., Jearawuttanakul, K., Wannalo, W., Khemawoot, P., Chutipongtanate, S., Borwornpinyo, S., Thitithanyanont, A., and Hongeng, S. 2021. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. Journal of Natural Products. 84(4): 1261–1270.
Soy, M., Keser, G., Atagündüz, P., Tabak, F., Atagündüz, I., and Kayhan, S. 2020. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clinical Rheumatology.39(7): 2085–2094.
Sripanidkulchai, B. and Junlatat, J. 2014. Bioactivities of alcohol based extracts of Phyllanthus emblica branches: Antioxidation, antimelanogenesis and anti-inflammation. Journal of Natural Medicines. 68(3): 615–622.
Su, C. M., Wang, L., and Yoo, D. 2021. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Scientific Reports. 11(1): 13464.
Tanaka, M., Kishimoto, Y., Saita, E., Suzuki-Sugihara, N., Kamiya, T., Taguchi, C., Iida, K., and Kondo, K. 2016. Terminalia bellirica extract inhibits low-density lipoprotein oxidation and macrophage inflammatory response in vitro. Antioxidants. 5: 20.
Tanaka, M., Kishimoto, Y., Sasaki, M., Sato, A., Kamiya, T., Kondo, K., and Iida, K. 2018. Terminalia bellirica (Gaertn.) Roxb. extract and gallic acid attenuate LPS-induced inflammation and oxidative stress via MAPK/NF- κ B and Akt/AMPK/Nrf2 pathways. Oxidative Medicine and Cellular Longevity. 2018: 9364364.
Tanaka, T., Narazaki, M., and Kishimoto, T. 2014. Il-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. 6(10): a016295.
Thongyim, S., Chiangchin, S., Pandith, H., Tragoolpua, Y., Jangsutthivorawat, S., and Panya, A. 2023. Anti-inflammatory activity of glyceryl 1,3-distearate identified from Clinacanthus nutans Extract against bovine mastitis pathogens. Antibiotics. 12(3): 549.
Urumarudappa, S. K. J., Tungphatthong, C., Prombutara, P., and Sukrong, S. 2020. DNA metabarcoding to unravel plant species composition in selected herbal medicines on the National List of Essential Medicines (NLEM) of Thailand. Scientific Reports. 10: 18259.
Vazirian, M., Khanavi, M., Amanzadeh, Y., and Hajimehdipoorb, H. 2011. Gallic Triphala. Iranian Journal of Pharmaceutical Research. 10(2): 233–236.
Wang, C. C., Yuan, J. R., Wang, C. F., Yang, N., Chen, J., Liu, D., Song, J., Feng, L., Tan, X. Bin, and Jia, X. Bin. 2017. Anti-inflammatory effects of Phyllanthus emblica L on benzopyrene-induced precancerous lung lesion by regulating the IL-1β/miR-101/Lin28B signaling pathway. Integrative Cancer Therapies. 16(4): 505–515.
Wang, X., Tang, G., Liu, Y., Zhang, L., Chen, B., Han, Y., Fu, Z., Wang, L., Hu, G., Ma, Q., Sheng, S., Wang, J., Hu, X., and Shao, S. 2022. The role of IL-6 in coronavirus, especially in COVID-19. Frontiers in Pharmacology. 13: 1033674.
Xia J, Tang W, Wang J, Lai D, Xu Q, Huang R, Hu Y, Gong X, Fan J, Shu Q and Xu J 2021. SARS-CoV-2 N protein induces acute lung injury in mice via NF-ĸB activation. Frontiers in Immunology. 12: 791753.
Yan, X., Hao, Q., Mu, Y., Timani, K. A., Ye, L., Zhu, Y., and Wu, J. 2006. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. International Journal of Biochemistry & Cell Biology. 38(8): 1417–1428.
Zhang, X., Wu, K., Wang, D., Yue, X., Song, D., Zhu, Y., and Wu, J. 2007. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-κB. Virology. 365(2): 324–335.
Zou, W., Xiao, Z., Wen, X., Luo, J., Chen, S., Cheng, Z., Xiang, D., Hu, J., and He, J. 2016. The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-ΚB pathway. BMC Complementary and Alternative Medicine. 16: 483.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Peeranut Winidmanokul1 Suthida Panwong2 and Aussara Panya3, *
1 Master of Science Program in Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Doctoral Program in Applied Microbiology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Department of Biology Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Aussara Panya, E-mail: aussara.pan@cmu.ac.th
Total Article Views
Editor: Patchara Sattayawat,
Chiang Mai University, Thailand
Article history:
Received: November 29, 2023;
Revised: March 12, 2024;
Accepted: March 29, 2024;
Online First: April 9, 2024

