
Comparative Study on the Effect of Hot Air and Vacuum Drying on Physiochemical Properties and antioxidant Activities of Red Dragon Fruit (Hylocereus polyrhizus) Peel
Tran Hong Quan*, Tran Tieu Yen, Giap Pham Ngoc Tram, Nguyen Phung Tien, Supatra Karnjanapratum, and Saroat RawdkuenPublished Date : February 22, 2024
DOI : https://doi.org/10.12982/NLSC.2024.023
Journal Issues : Number 2, April-June 2024
Abstract The red dragon fruit (Hylocereus polyrhizus) peel was subjected to freeze drying (FD), hot air drying (at 50 – 65°C) (HAD), and vacuum drying (at 50 – 65°C) (VD), in which physical properties (color, water activity, water absorption index-WAI, and microstructure), bioactive compounds (ascorbic acid, betacyanin, polyphenol, and flavonoid), and antioxidant activities (DPPH and metal chelating activities) were monitored. The results showed that the lightness (L*) of red dragon fruit peel (RDFP) decreased as HAD and VD were applied especially at higher drying temperatures. Whereas the water absorption index (WAI) of the RDFP was not affected by drying methods. The microstructure surfaces of FD-dried RDFP were smoother than those of samples dried by HAD and VD-dried RDFP. After the drying processes, the bioactive compounds and antioxidative properties of the RDFP powder decreased significantly. However, higher bioactive compounds and antioxidant activities in RDFP dried by VD compared to the samples dried by HAD at the same drying temperatures. The RDFP powder dried by VD at 55°C retaining high physiochemical and antioxidative properties could be utilized as a colorant and nutraceutical ingredient in several food products.
Keywords: Red dragon fruit peel, Freeze drying, Vacuum drying, Hot air drying, Bioactive compounds, Antioxidant activity
Funding: The authors are grateful for the research funding provided by Vinh Long University of Technology Education.
Citation: Quan, T.H., Yen, T.T., Tram, G.P.N., Tien, N.P., Karnjanapratum, S., and Rawdkuen, S. 2024. Comparative study on the effect of hot air and vacuum drying on physiochemical properties and antioxidant activities of red dragon fruit (Hylocereus polyrhizus) peel. Natural and Life Sciences Communications. 23(2): e2024023.
INTRODUCTION
Red dragon fruit also known as Pitaya (Hylocereus polyrhizus) is a promising tropical fruit because it is rich in nutritional compositions including carbohydrate, protein, fiber, vitamins (vitamin C, B1, B2, and, B3), and minerals (calcium, potassium, and magnesium) found in the flesh, peel, and seeds (Mahayothee et al., 2019; Yen et al., 2022). In addition, a variety of bioactive compounds such as betalains, carotenoids, flavonoids, and polyphenols are also present in the fruit peel and pulp (Mahayothee et al., 2019). These compounds may give protection against certain oxidative stress-related damages (Sengkhamparn et al., 2013). Thus, red dragon fruit has been widely consumed fresh or processed into a variety of products such as yogurts, ice creams, jams, jellies pastries, and wines (Jalgaonkar et al., 2022). However, a high increase in dragon fruit processing resulted in high amounts of waste materials such as peels and seeds. Since this amount of waste can cause a negative impact on the environment, utilizing these wastes into value-added products must be given more attention. Various studies have shown that dragon fruit peel is a potential source of natural functional food ingredients, including betalains, carotenoids, flavonoids, and polyphenols (Sengkhamparn et al., 2013; Pichayajittipong and Thaiudom, 2014; Jalgaonkar et al., 2022). These compounds may give protection against certain oxidative stress-related damages (Mahayothee et al., 2019). Red dragon fruit peel (RDFP) mainly consists of betacyanin (150.46 mg/100g), pectin (10.79%), and 69.30% total dietary fiber (Chia and Chong, 2015). Moreover, betacyanins exhibited the highest antioxidant activities in both the ferric reduction activity potential (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays, and their activities were almost 10 times higher in peels than in the flesh of red dragon fruit (Sengkhamparn et al., 2013). However, the fresh peels are perishable and difficult to use directly in food products due to high moisture content (84-91%) (Jalgaonkar et al., 2022). Therefore, dragon fruit peel powder has been as an ingredient to improve the nutritional value of food products with high consumer palatability and acceptability, such as noodles and steamed bread (Shiau et al., 2020; Chumroenvidhayakul et al., 2022).
The drying process, a famous technique to produce dietary fiber powder, affected the bioactive compounds and antioxidant activity of plant powder products (Sengkhamparn et al., 2013). Hot air drying has been considered a low-cost operation and a simple method, in which the raw material is directly exposed to the hot air in the drying chamber (Ruttarattanamongkol et al., 2016). Nevertheless, it has shown several drawbacks found in dried products including lower antioxidative activity, higher darkness, and shrinkage compared to the fresh materials (Balzarini et al., 2018). Vacuum drying is an alternative drying process, in which moist material is dried under low atmospheric pressure. Thus, it is more suitable for thermal and oxygen-sensitive components especially bioactive compounds contained in fruits and vegetables (Orikasa et al., 2014). However, a lack of information on the impacts of different drying processes on bioactive compounds and antioxidative activities of the peels of the red dragon fruits has existed. The main objective of this study was to evaluate the effects of hot air and vacuum drying on the physiochemical compositions and antioxidant properties of RDFP. Antioxidation potentials were determined colorimetrically for scavenging activities against DPPH. Metal chelating activities were also determined, as excess free metals have been implicated in the induction and formation of free radicals. Therefore, this study will provide the appropriate drying conditions for this economic potential by-product.
MATERIALS AND METHODS
Materials and chemicals
Red dragon fruits (Hylocereus polyrhizus) were purchased from Global G.A.P. farms in Tien Giang province, Viet Nam.
All chemicals used in the experiments were analytical grade and purchased from Merck KGAA Co. (Darmstadt, Germany) including Folin-ciocalteu, FeSO4, catechol, Ferrozine, and 2,2-diphenyl-1-picrylhydrazyl (DPPH).
Drying processes
The red dragon fruits were washed under running tap water and wiped to dry. The fresh peel was separated manually and cut into small pieces (3 x 3 cm). Then, the peels were subjected to a hot air drier (DRC - 16T, Viet Nam) with airflow rates of 1.5 m/s using a tray dryer at different temperatures (50, 55, 60 and 65°C for 12 h, 8 h, 7h, and 5 h, respectively) and vacuum dryer (Cryste Puriven Vacuum 64, Korea) under pressure of -80 kbar and 140 l/min flow rate of vacuum pump (140RND Oil-Less Piston Pumps, Korea) at different temperatures (50, 55, 60 and 65°C for 27 h, 24 h, 20 h, and 17 h, respectively). The drying process was performed until constant moisture content. The moisture content of the sample was determined using a moisture analyzer (MOC-63U Shimadzu, Japan). Along with hot air and vacuum drying, the peel samples were frozen at -40°C for 8 h. The frozen samples were subjected to sublimation using a freeze-dryer (CoolSafe Touch 55-15, Denmark) at -50°C for 25 h. The dried red dragon fruit peels were ground using a blender (Philips Blender HR2221, China). The powder was sieved through a size 16 mesh screen, packed in polyethylene bags and then placed in the freezer (-18°C) until analysis was completed.
Analysis
Determination of color (L*, a*, and b* values), water activity (aw), and water absorption index (WAI) of red dragon fruit peel (RDFP)
The colors of RDFP were measured using a colorimeter (MSEZ-4500 L, HunterLab, USA) and expressed in the CIE system. L*, b*, and a* values represent brightness, yellowness, and redness, respectively. The aw of RDFP was determined by a Water Activity Meter Set (Rotronic HP23-AW-40, Switzerland).
The water absorption index (WAI) of RDFP was determined following the method of Yousf et al. (2017). Generally, the powder was finely ground and suspended in distilled water at room temperature for 30 min, gently stirred during this period, and then centrifuge at 3000 rpm for 15 min. The supernatant liquid was poured carefully into a tared evaporating dish. The remaining solid part was weighed. The WAI was calculated using the following formula:
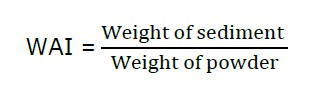
Determination of betacyanin, total titratable acidity, ascorbic acid, total phenolic and flavonoid contents of red dragon fruit peel (RDFP)
Betacyanin content (BC) of RDFP was performed following the protocol of Yen et al. (2022). Basically, powder (1 g) was mixed in 0.2 M sodium phosphate buffer (70 mL, pH 6,5) and 0.1 M acid citric (30 mL). The mixture was subjected to a spectrophotometer (2602 Labomed, USA) to record the absorbance at a wavelength of 537 nm (A537). The BC was calculated as the following formula:
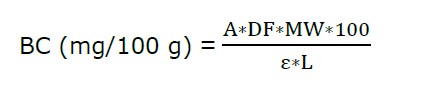
where A is the A537; DF is dilution factor; Ɛ is molar extinction coefficient of betanin (60,000 L/mol.cm); L is path length of the cuvette (1 cm); and MW is molecular weight of betacyanin (550 g/mol).
Total titratable acidity (TTA) and ascorbic acid content (AA) of RDFP was analyzed as per the description of Freitas and Mitcham (2013) and Choo and Yong (2011) using the iodine titration method, respectively.
The total phenolic content (TPC) and flavonoid content (FC) of the RDFP were analyzed as described by the methods of Piskov et al. (2020). To extract phenolic and flavonoid compounds, 1 g sample was mixed with 50 mL absolute methanol. After extracting for 24 h using a shaker at 150 rpm in the dark, the mixture was centrifuged at 4,000 rpm for 20 min and decanted. The liquid extract was then evaporated until dry by nitrogen gas at 40°C. The residue was dissolved again in absolute methanol to a concentration of 50 mg/mL and kept at 4°C.
For the TPC assay, the Folin-Ciocalteau reagent procedure was used. Briefly, 1.8 mL of ten-fold diluted Folin-Ciocalteau reagent was mixed with 40 µL of the RDFP extract in an aluminum foil-covered test tube for 5 min. Thereafter, 1.2 mL of sodium bicarbonate solution (7.5% w/v) was added and thoroughly vortexed. The reaction was kept in the dark for 1 h, and the absorbance of the solution was measured at 765 nm by a spectrophotometer. The TPC of the samples was expressed as mg gallic acid equivalents per 1 g RDFP per dry weight (mg GAE/g).
For the FC procedure, the RDFP extract (0.5 mL) was mixed with 10% AlCl3 (0.1 mL), 1 mol/L CH3COONa (0.1 mL), and distilled water (2.8 mL). Then, the mixture was kept at ambient temperature in the dark for 30 min. The absorbance (A415 nm) of the reaction mixture was recorded using a spectrophotometer. FC was presented as mg quercetin equivalents per 1 g RDFP per dry weight (mg QE/g).
Determination of antioxidant activities of red dragon fruit peel powder (RDFP)
DPPH radical scavenging activity
The DPPH radical scavenging activity (%) of the RDFP was conducted as per the protocol of Elmastas et al. (2007). For methanol extraction, the samples (10 g) were added into absolute methanol (100 mL) and shaken at 150 rpm for 24 h at room temperature. This step was repeated until the extracting solvent became uncolored. The obtained extract was filtered through filter paper Whatman no. 2. The filtrate was then removed methanol using a vacuum rotary evaporator (WEV-1010, Daihan, Korea) at 40°C. The dried extract was kept in an amber bottle and stored at 4°C until analysis to inhibit oxidation. To analyze DPPH radical scavenging activity, the dried extract (10 mg) was dissolved in absolute methanol (10 mL). One milliliter of 0.1 mM DPPH freshly prepared in absolute methanol was then mixed with 3 mL of the dried RDFP extract. The mixture was stirred well and kept for 30 min at room temperature. The absorbance (A517 nm) was recorded in a spectrophotometer. The activity was calculated using the following formula:
DPPH radical scavenging activity (%) = [(A0 - A1)/A0] ×100
where A0 is A517 nm of the blank (without sample extract), and A1 is A517 nm of sample extract.
Metal chelating activity
The metal chelating activity (%) of the RDFP was described by Wong et al. (2014). Briefly, 0.2 mL of 0.1 mM FeSO4 and 0.4 mL of 0.25 mM ferrozine were thoroughly mixed with 0.2 mL of the RDFP extract. After incubation in the dark at room temperature for 30 min, the absorbance of the mixture was recorded at 562 nm using a spectrophotometer. The metal chelating activity was calculated according to the following formula:
Metal chelating activity (%) = [(A0 - A1)/A0] ×100
where A0 is absorbance of the control (without sample extract), and A1 is absorbance of sample extract.
Scanning electron micrograph (SEM)
Microstructure of the selected RDFP was visualized using a scanning electron microscope (Quanta400, FEI, Czech Republic) at an accelerating voltage of 20 kV.
Statistical analysis
All the experimental results of triplicate measurements were shown as mean ± standard deviation (STD). Data were compared variances across the means of different groups using analysis of variance (ANOVA) and Duncan’s multiple range test (Steel and Torrie, 1980). For pair comparison, T-test was used. Statistical analysis was conducted using the Statistical Package for Social Science for windows (SPSS 11.0, SPSS Inc., Chicago, IL, USA).
RESULTS
The color (L*, a*, and b* values), water absorption index (WAI), and water activity (aw) of RDFP as affected by different drying conditions
Table 1 represents the effect of hot air drying (HAD) and vacuum drying (VD) temperatures on the color of the red dragon fruit peel (RDFP). It was observed that there was no significant difference between the RDFP dried by freeze-drying (FD) and VD at 50-55°C (P > 0.05). HAD caused the decreased lightness (L*) of RDFP especially at higher drying temperatures (P <0.05). The lowest L* value (36.52 ± 2.89) was recorded in the RDFP dried by HAD at 65°C. It was the same trend when the RDFP was subjected to VD at drying temperatures higher than 60°C. The increase in the higher temperature (65°C) yielded a decrease in the L* values of the RDFP (P < 0.05). The L* values of samples dried by VD were higher than those of samples dried by HAD at the same temperatures. However, there was no significant difference in the L* value of the peels dried by VD and HAD at drying temperatures higher than 50°C (P > 0.05). The change of L* value was in line with the decreased a* and increased b* values of the RDFP as a function of drying temperatures. For a* value, the highest value was recorded in the powder dried by FD (42.55±0.79). Whereas, HAD caused a decrease in the redness of the powder, especially at the high drying temperatures. The lowest value was noted in the powder dried by HAD at 65°C (30.17 ± 1.59), while the RDFP dried at 55°C possessed the highest a* value, compared to the other drying temperatures (P < 0.05). Nevertheless, the yellowness (b* value) of the RDFP was dramatically increased as the high drying temperatures were applied. The highest b* value was observed in the RDFP dried at 65°C (0.56 ± 0.06). The FD yielded the RDFP with the lowest value (-8.18 ± 0.15). The decreased a* and increased b* values were also recorded in RDFP dried by VD. However, higher a* and lower b* values could be observed in RDFP dried by VD than those of peel dried by HAD at drying temperatures 50-55°C (P < 0.05).
The WAI of dried RDFP at different drying conditions is shown in Table 1. The WAI of the RDFP ranged from 13.83 to 14.39. There was no statistically significant difference between all drying conditions (P > 0.05). This result indicated that the dried RDFP still retained the ability to bind with water molecules. Therefore, the RDFP dried by HAD or VD at drying temperatures of 50-65°C had good swelling ability and water absorption. Thus, the RDFP can be easily applied or mixed into different food products.
The water activity (aw) of RDFP dried at different temperatures and methods is presented in Table 1. The aw of the dried RDFP tends to decrease with increasing drying temperatures by both HAD and VD (P < 0.05). For HAD, the RDFP dried at 65°C had the lowest aw (0.24) compared with other temperatures. The aw of the freeze-dried sample was 0.22. The higher the drying temperatures, the more free water molecules could be effectively removed, contributing to a reduction in water activity. Moreover, VD reduced the aw of the RDFP as a function of drying temperatures (P < 0.05). Specifically, the aw decreased from 0.25 to 0.18 when the drying temperatures increased from 50 to 65°C, respectively. Additionally, when comparing the two drying methods at the same temperature, the VD had a lower aw than that of HAD (P < 0.05). Typically, the moisture content in the vacuum-dried sample was 4.79%, which was lower than that of the hot air-dried sample (5.67%) at the temperature of 60°C. Moreover, the peels dried by VD had lower moisture content as compared to those dried by HAD at the same drying temperature (P < 0.05) (Table 1).
Table 1. Effect of different drying conditions on color values (L*, a*, and b*), water absorption index (WAI), water activity (aw), and moisture content (%) of red dragon fruit peel.
|
Drying conditions |
L* |
a* |
b* |
Water absorption index (WAI) |
Water activity (aw) |
Moisture content (%) |
|
FD (Control) |
47.78au ± 1.88* |
42.55au ± 0.79 |
-8.18ey ± 0.15 |
14.39au ±0.53 |
0.22dv ± 0.00 |
4.79duv ± 0.19 |
|
HAD 50 oC 55 oC 60 oC 65 oC |
45.90bB ± 0.73 45.68bA ± 1.99 40.05cA ± 2.06 36.52dA ± 2.89 |
32.25cdB ± 1.88 35.49bB ± 0.45 33.31cA ± 0.42 30.17dA ± 1.59 |
-2.50dA ± 0.28 -0.16bA ± 0.05 -1.86cA ± 0.09 0.56aA ± 0.06 |
13.83aA ± 0.37 13.90aA ± 0.42 13.98aA ± 0.45 14.24aA ± 0.70 |
0.41aA ± 0.00 0.27bA ± 0.01 0.27bA ± 0.00 0.24cA ± 0.00 |
5.91aA ± 0.09 5.73bA ± 0.05 5.67bA ± 0.02 5.39cA ± 0.07 |
|
VD 50 oC 55 oC 60 oC 65 oC |
48.5uA ± 2.82 47.70uA ± 0.44 40.74vA ± 3.55 37.50vA ± 0.52 |
35.55wA ± 0.24 37.77vA ± 0.17 33.55xA ± 0.14 32.50xA ± 0.52 |
-4.60uB ± 0.42 -5.52xB ± 0.02 -5.07wB ± 0.33 0.62vA ± 0.03 |
14.26uA ± 0.37 14.18uA ± 0.32 13.98uA ± 0.45 14.37uA ± 0.11 |
0.25uB ± 0.00 0.24uB ± 0.01 0.23uvB ± 0.01 0.18wB ± 0.00 |
4.93uB ± 0.03 4.86uB ± 0.05 4.69vB ± 0.06 4.23wB ± 0.02 |
Note: * Different lowercase superscripts (a-e) and (u-y) in the same column under the same drying conditions including the control indicate significant differences (P < 0.05). Different uppercase superscripts (A-B) in the same column under the same temperature of two drying conditions indicate significant differences (P < 0.05).
The total titratable acidity, ascorbic acid, and betacyanin of RDFP as affected by different drying conditions
The effects of temperature and drying methods on total titratable acidity (TTA, %) and ascorbic acid content (AA, %) in RDFP are documented in Table 2. The TTA of the RDFP ranged from 3.10% to 3.35%. There was no significant difference in TTA between drying temperatures and drying methods (P > 0.05). By contrast, the AA of RDFP decreased as the high drying temperatures were applied (P < 0.05), which was specially recorded in HAD-RDFP. The highest AA was noted in the peels dried by FD (0.36%). The lowered values were observed in the RDFP dried by HAD and VD. Nevertheless, more retention of AA in RDFP was dried by VD as compared to the one dried by HAD at the same drying temperature (P < 0.05). Except for the sample dried at 65°C, no significant difference was observed between the two drying methods (P > 0.05).
Table 2 presents the betacyanin content (BC, mg/100g) of RDFP affected by different drying conditions. The fresh RDFP contained 299.43 ± 20.33 mg/100g BC. This component decreased significantly under drying processes. The highest amount of BC was noted in FD-RDFP (261.04 ± 0.93 mg/100g). The BC value was higher in the FD samples than in other drying methods, except the sample dried by VD at 50°C, mainly due to the lower temperatures of FD, which contribute to the preservation of betacyanin compounds. Lowered values were obtained in the RDFP dried by HAD and VD (P < 0.05). Additionally, the increase in drying temperatures caused the decrease of BC in dried RDFP. However, the BC of HAD-RDFP was not significantly different from that of VD-RDFP at the same drying temperatures (P > 0.05).
Table 2. Effect of different drying conditions on total titratable acidity, ascorbic acid, betacyanin, flavonoid, and total phenolic contents of red dragon fruit peel.
|
Drying conditions |
Total titratable acidity (TTA, %) |
Ascorbic acid (AA, %) |
Betacyanin (BC, mg/100g) |
Flavonoid (FC, mg QE/g) |
Total phenolic content (TPC, mg GAE/g) |
|
Fresh peel |
3.18auv ± 0.14* |
0.84au ± 0.00 |
299.43au ± 20.33 |
88.18au ± 0.77 |
2.99au ± 0.22 |
|
FD (Control) |
3.24auv ± 0.00 |
0.36bv ± 0.00 |
261.04bv ± 0.93 |
80.35bv ± 4.53 |
0.40bv ± 0.02 |
|
HAD 50 oC 55 oC 60 oC 65 oC |
3.19aA ± 0.04 3.27aA ± 0.04 3.21aA ± 0.04 3.23aA ± 0.08 |
0.29cB ± 0.00 0.25dB ± 0.00 0.24eB ± 0.00 0.23fA ± 0.00 |
233.42cA ± 10.75 180.57dA ± 14.99 160.31deA ± 9.30 152.08eA ± 13.93 |
67.64cA ± 2.16 52.56dA ± 3.15 22.90eB ± 1.83 23.01eB ± 1.39 |
0.34cB ± 0.01 0.33cB ± 0.00 0.29dA ± 0.02 0.26eA ± 0.01 |
|
VD 50 oC 55 oC 60 oC 65 oC |
3.26uvA ± 0.04 3.35uA ± 0.12 3.24uvA ± 0.10 3.10vA ± 0.20 |
0.32wA ± 0.00 0.29xA ± 0.00 0.26yA ± 0.00 0.24zA ± 0.00 |
255.42vA ± 0.77 203.36wA ± 5.05 170.32xA ± 8.81 167.18xA ± 2.33 |
73.24wA ± 3.29 62.30xA ± 5.30 52.38yA ± 1.75 33.64zA ± 2.71 |
0.37wA ± 0.01 0.36wA ± 0.01 0.31xA ± 0.01 0.27yA ± 0.01 |
Note: * Different lowercase superscripts (a-f) and (u-z) in the same column under the same drying conditions including the control indicate significant differences (P < 0.05). Different uppercase superscripts (A-B) in the same column under the same temperature of two drying conditions indicate significant differences (P < 0.05).
The flavonoid and total phenolic contents of RDFP as affected by different drying conditions
The effects of different drying conditions on the total phenolic content (TPC) and flavonoid content (FC) of RDFP were studied and the results are shown in Table 2. The fresh RDFP contained 2.99 ± 0.22 mg GAE/g and 88.18 ± 0.77 mg QE/g (Table 2). After the drying processes, these components decreased significantly (P < 0.05). The TPC and FC of FD-RDFP were the highest levels (0.40 ± 0.02 mg GAE/g and 80.35 ± 4.53 mg QE/g, respectively). The TPC and FC of the RDFP dried by HAD at 60 and 65°C decreased approximately ten and four times, respectively, compared to the fresh peels. However, higher content of total phenolic and flavonoid had been found in the samples dried by VD at the same drying temperatures (P < 0.05). There was no significant difference in the TPC of the peels dried by HAD and VD at 65°C (P > 0.05). In addition, the increases in drying temperatures yielded decreases in TPC and FC (P < 0.05). This proved that higher temperatures degraded these heat-sensitive components. Thus, VD at 55°C could be applied for retaining the TPC and FC of the RDFP.
Antioxidant activities of RDFP as affected by different drying conditions
The antioxidant activities of dried RDFP in terms of DPPH radical scavenging activity (DPPH RSA, %) and metal chelating activity (MCA, %) affected by different conditions are presented in Figure 1. The fresh RDFP possessed high DPPH RSA and MCA, which were 90.54 ± 5.10 (%) and 90.01 ± 9.13 (%), respectively (Figure 1). The drying processes decreased the antioxidant activities of the RDFP. FD caused approximately 40% and 20% loss of DPPH RSA and MCA, respectively, compared to the fresh material. However, the lowest antioxidant capacity was determined in HAD technique at 65°C both in DPPH RSA (13.18 ± 2.15%) and MCA (31.84 ± 2.29%). Compared to HAD at the same drying temperatures, the VD could retain higher antioxidant capacity (P < 0.05). In summary, the VD at 55°C was an appropriate drying method for protecting the bioactive compounds and antioxidant activities of the RDFP because of retaining higher values of phytochemicals (AA, BC, FC, TPC) and antioxidative activities (DPPH RSA and MCA) than other drying conditions.
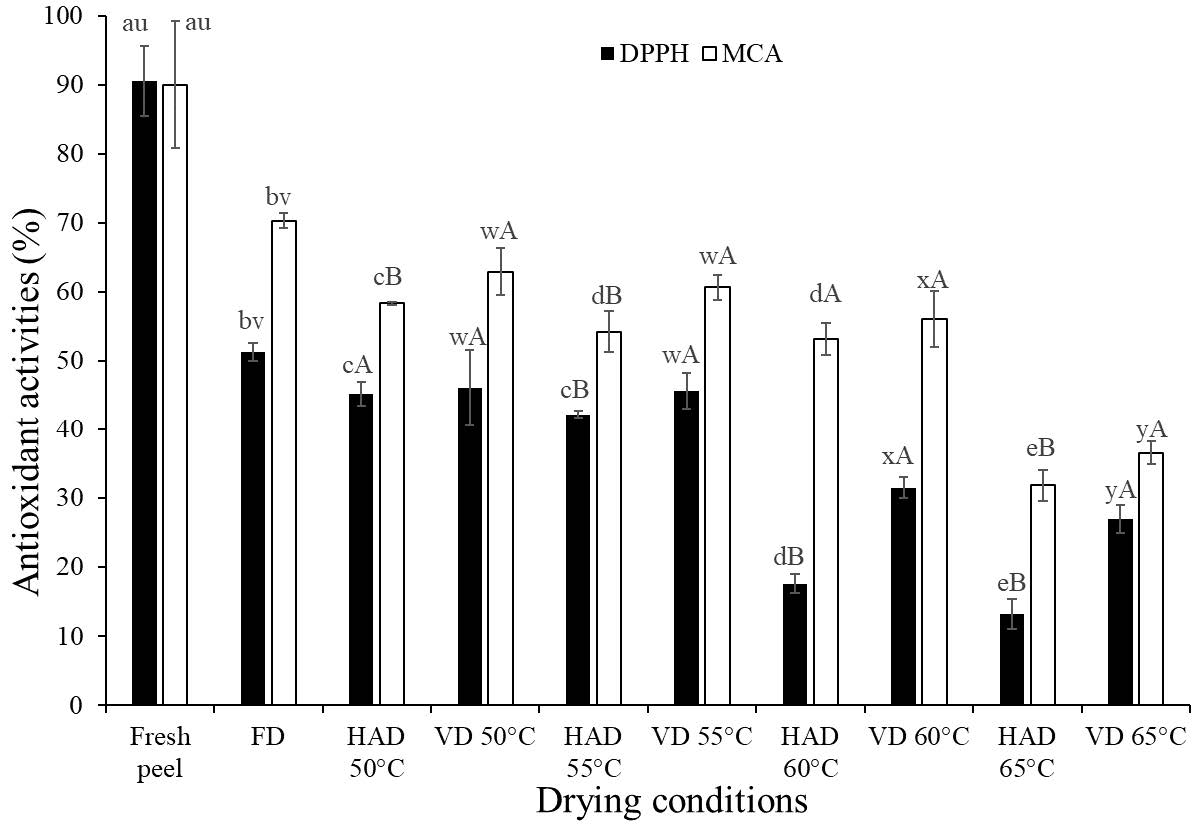
Figure 1. Antioxidant activities (%): DPPH radical scavenging (DPPH RSA) and metal chelating (MCA) activities of the fresh peels and RDFP dried by different drying conditions. FD: freeze drying, HAD: hot air drying, VD: Vacuum drying.
Microstructure
Scanning electron micrographs (SEM) of RDFP powder produced by FD, VD at 55°C, and HAD at 55°C are illustrated in Figure 2. In general, the drying process resulted in the rupturing of the cell wall. However, the FD-RDFP powder had a smoother surface than the samples dried by HAD and VD. This is because the removal of water during the freeze-drying process occurred by sublimation from frozen substances with the simultaneous effect of the vacuum (Ren et al., 2021). Nevertheless, there was no difference in the surface characteristic of the RDFP dried by HAD and VD at 55°C.

Figure 2. Scanning electron microscopic photograph of RDFP powder prepared by freeze drying, vacuum drying, and hot air drying at 55°C. Magnification: 5000 x, Scale bar = 5 µm.
DISCUSSION
It was reported that the color of RDFP was brighter under VD compared to that of RDFP dried by HAD. The decrease in a* (redness) and increase in b* (yellowness) values were related to the degradation of BC in the dried RDFP under the HAD condition. Furthermore, the Maillard and enzymatic reactions were responsible for browning formation, which lowered under freeze-drying conditions (Jia et al., 2019). Moreover, Balzarini et al. (2018) noted that VD reduces browning reactions due to its contribution to oxygen separation. The water absorption index (WAI) of the RDFP is a criterion used to measure the water absorption and swelling capacity of polymeric components such as starch or cellulose (Patria et al., 2020). The results showed that the WAI of RDFP was not significantly affected by drying methods and temperatures. It noted that the aw of the dried RDFP produced by HAD and VD was in the range of 0.18-0.41. Quan et al. (2023) reported that the aw was less than 0.6, which was stable with storage and appropriate for application in many kinds of food products. The higher drying temperatures could remove more free water in the samples, thus lower aw could be observed. Moreover, an increase in vapor pressure gradient inside samples at higher temperatures leads to higher heat transfer rates (Sehrawat et al., 2018). The lower aw could be recorded in VD-dried RDFP than that of HAD-dried RDFP. This result is because VD could remove the free water more effectively than atmospheric conditions. Bozkir (2020) found that the drying rate and effective water diffusivity of orange slices dried by VD were higher than those dried by HAD at the same drying temperatures.
The TTA in RDFP was not affected by drying methods and temperatures. There was no significant difference between the fresh and the dried peels. It might be due to malic acid, which is commonly used to calculate TTA in red dragon fruit, being stable during the drying process. Pu et al., (2018) reported that the TTA of the fresh jujube fruits (Zizyphus jujuba cv. Junzao) calculated based on malic acid equivalent was no significant difference with the fruits dried by hot air dryer at 55°C for 24 h. Moreover, Farooq et al., (2020) also revealed that no significant difference between tomato powder dried by FD and HAD could be observed. Nevertheless, higher bioactive compounds were found in RDFP dried under VD conditions than those of peel dried by HAD. However, the highest contents of bioactive compounds were recorded in RDFP dried by FD. Ascorbic acid, phenolic compounds, and betacyanins have been associated with antioxidant activity. They are unstable compounds that are easily degraded during thermal treatment (Jakkranuhwat and Kunchansombat, 2020). The AA is oxidized to dehydroascorbic acid under aerobic conditions, followed by hydrolysis and further oxidation. AA degradation followed a first-order reaction. A higher degradation rate was observed in HAD than in FD and VD (Santos and Silva, 2008). Orikasa et al. (2014) also found that the residual ratio of L-AA of kiwi slices after VD is higher than that after HAD at all tested temperatures. The AA in fruits and vegetables was decomposed by a non-enzyme or by enzyme oxidation, which was accelerated more at higher temperatures. Moreover, the VD process with a small amount of atmospheric oxygen might inhibit the non-enzymatic decomposition of AA in the samples (Orikasa et al., 2014). This result also proved that betacyanin is more decomposition under high temperatures than in the presence of atmospheric oxygen. Wong and Siow (2015) revealed that heat, oxygen, light, pH, and moisture are the primary factors causing for discoloration of betacyanin. Therefore, VD was a proper drying method for maintaining ascorbic acid and betacyanin content in the RDFP.
The reduction in TPC of the dried dragon fruit products could be due to the degradation (oxidation) of phenolic compounds during thermal treatment, especially in the HAD and VD samples because of the longer drying time (Dadhaneeya et al., 2023). Antony and Farid (2022) used three mechanisms to explain the behavior of polyphenols at high temperatures. First, the insoluble phenolic compounds may be released when the lignin bonds to phenolic acids are broken. Secondly, lignin itself may be degraded at high temperatures. Lastly, at high temperatures, thermal degradation of the polyphenols may occur. Thermal degradation is the most common mechanism used to explain the fall in polyphenol yield during high-temperature drying. Ozcan-Sinir et al. (2019) documented that the VD technique allows minimum degradation in phenolic content compared to HAD. This result was in line with that of Balzarini et al. (2018) who presented that the polyphenol content of chicory roots dried by VD was higher than that of dried by HAD at the same drying conditions. Papoutsis et al. (2017) reported that high temperatures applied for drying resulted in the degradation of some flavonoid compounds of immature calamondin peels. In general, phenolic compounds can be degraded by enzymes and through other oxidation reactions, which could help explain their decrease when observed. In addition, phenolic compounds could bind to other molecules, making their quantification difficult in some cases and leading to a decrease in the measured values. FD is also responsible for the increases in TPC and different metabolic pathways leading to the conversion of other compounds into phenolic compounds and their accumulation (Boateng et al., 2021).
Due to the higher retention of bioactive compounds (AA, BC, FC, TPC) in RDFP dried by FD and VD compared to HAD, higher antioxidant activities including DPPH radical scavenging activity and MCA were detected in the powders. It was also observed that trends in antioxidant activities align with color changes. The higher the a* value, the higher BC, which is correlated with higher antioxidant activities. The term antioxidant is attributed to hundreds of compounds that can delay the onset, or slow the rate, of oxidation of materials, including phenols and vitamins. It was postulated that they inhibit chain reactions as hydrogen donors or acceptors for free radicals. Antioxidants can be sensible to high temperatures and many degradation compounds may arise (Harguindeguy and Fissore, 2020). Madane et al. (2020) found that dragon fruit (Hylocereus undatus) peel had radical scavenging activity and chelating activity on Fe2+. The DPPH radical scavenging activity of the Hylocereus undatus peel powder extract with a concentration of 1.0 mg/mL was 68.57% (Madane et al., 2020). The results were in line with Kayacan et al. (2018) who reported that bee pollen dried by VD had higher total antioxidant capacity determined by both DPPH and copper-reducing antioxidant capacity methods than that of HAD at the same drying condition. The decreases in the antioxidant activities were in agreement with the decreases in bioactive compounds including ascorbic acid, betacyanin, and phenolic compounds in the dried RDFP. The phenolic compounds are responsible for their antioxidative activity due to their redox properties and ability to act as hydrogen donors and singlet oxygen quenchers (Asamoa et al., 2018). Moreover, phenols are important plant constituents because of their scavenging ability due to their hydroxyl groups (Elmastas et al., 2007). Thus, VD at 55°C could be selected as a proper drying condition for RDFP to produce powder with high bioactive compounds and antioxidative activities.
The drying process significantly affected the material cells and structure. Previous research had reported that low-pressure superheated steam drying provided better quality dried products in terms of less shrinkage and higher porosity and rehydration (Tawatsinlapasorn et al., 2017). Therdthai and Zhou (2009) showed that microwave vacuum-dried mint leaves were more porous and open than hot air-dried ones. This result was also similar to that of Ren et al. (2021) who illustrated that ginger dried by FD had slight damage to the cell wall, and a good retention of intact starch granules in cells than that of samples dried by HAD and VD. Zannou et al., (2021) documented that utilizing high-temperature air for drying induces significant tissue shrinkage and collapse. It has been also noted that the drying process under high temperatures can result in notable deterioration, deformation, and folding of cellular structures, possibly attributed to thermal degradation, loss of moisture, and denaturation of specific chemical constituents, particularly bioactive compounds (Pashazadeh et al., 2024). The changes in microstructural properties could be explained by the glass transition theory. This theory describes the shrinking during the heat-related phenomenon. Per this concept, fewer collapses or more pores are found throughout the material if exposed to temperatures below the glass transition's temperature (Boateng et al., 2021). The resulting shrinkage was relatively low in freeze-dried RDFP, where the drying temperature was below the glass transition, and the dried RDFP had a smooth structure. The high drying temperatures in heat drying (VD and HAD) were above the glass transition temperature, and thus the shrinkages were higher in the heat-dried RDFP than the freeze-dried one.
Although FD and VD, which are widely used in food manufacturing, have always been considered as one of the best drying methods for foods, the major disadvantage of FD and VD is the high production cost and thus reduces economic competitiveness to HAD. For example, to achieve the same moisture content in products, the production cost of FD and VD can be as much as 200–500% higher than that of HAD (Jiang et al., 2014). Thus, the optimal drying conditions selected in this research should be further studied on a commercial scale and consider the economic aspects.
CONCLUSIONS
The physiochemical properties and antioxidant activities of red dragon fruit (Hylocereus polyrhizus) peel were affected by drying temperatures and drying methods. The drying process decreased the color, bioactive compounds including ascorbic acid, betacyanin, polyphenol, and flavonoid, and antioxidant activities of the RDFP. Freeze-dried RDFP had better product quality, in which the highest physiochemical and antioxidant properties were recorded, compared to the samples dried by both HAD and VD. From the SEM result, the FD-dried RDFP had a highly smooth surface whereas the HAD and VD-dried RDFP had rough and damaged surfaces. Compared to HAD, the VD-dried samples could retain more phytochemicals to a large extent, thus antioxidant properties improved. Therefore, the dried RDFP powder produced by VD at 55°C with excellent physiochemical and bioactive compounds could be used as a potential ingredient in several food products.
ACKNOWLEDGEMENTS
The authors thank Vinh Long University of Technology Education for providing instruments.
AUTHOR CONTRIBUTIONS
Dr. Tran Hong Quan designed the study. Giap Pham Ngoc Tram, Tran Tieu Yen, and Nguyen Phung Tien conducted the experiments. Dr. Tran Hong Quan supervised the study. Dr. Tran Hong Quan and Tran Tieu Yen contributed to the manuscript preparation, reading and approval for publication. Assoc. Prof. Dr. Supatra Karnjanapratum and Assoc. Prof. Dr. Saroat Rawdkuen revised the manuscript and gave valuable suggestion.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Antony, A. and Farid, M. 2022. Effect of temperatures on polyphenols during extraction. Applied Sciences. 12(4): 2107.
Asamoa, A.A., Essel, E.A., Agbenorhevi, J.K., and Oduro, I.N. 2018. Effect of processing methods on the proximate composition, total phenols and antioxidant properties of two mushroom varieties. American Journal of Food and Nutrition. 6(2): 55-59.
Balzarini, M.F., Reinheimer, M.A., Ciappini, M.C., and Scenna, N.J. 2018. Comparative study of hot air and vacuum drying on the drying kinetics and physicochemical properties of chicory roots. Journal of Food Science and Technology. 55(10): 4067-4078.
Boateng, I. D., Soetanto, D. A., Yang, X. M., Zhou, C., Saalia, F. K., and Li, F. 2021. Effect of pulsed-vacuum, hot-air, infrared, and freeze-drying on drying kinetics, energy efficiency, and physicochemical properties of Ginkgo biloba L. seed. Journal of Food Process Engineering. 44(4): e13655.
Bozkir, H. 2020. Effects of hot air, vacuum infrared, and vacuum microwave dryers on the drying kinetics and quality characteristics of orange slices. Journal of Food Process Engineering, 43(10): e13485.
Chia, S. and Chong, G. 2015. Effect of drum drying on physico-chemical characteristics of dragon fruit peel (Hylocereus polyrhizus). International Journal of Food Engineering. 11(2): 285-293.
Choo, W.S., Yong, and W.K. 2011. Antioxidant properties of two species of Hylocereus fruits. Advances in Applied Science Research. 2(3): 418-425.
Chumroenvidhayakul, S., Thilavech, T., Abeywardena, M., and Adisakwattana, S. 2022. Investigating the impact of dragon fruit peel waste on starch digestibility, pasting, and thermal properties of flours used in Asia. Foods. 11(14): 2031.
Dadhaneeya, H., Kesavan, R.K., Inbaraj, B.S., Sharma, M., Kamma, S., Nayak, P.K., and Sridhar, K. 2023. Impact of different drying methods on the phenolic composition, in vitro antioxidant activity, and quality attributes of dragon fruit slices and pulp. Foods. 12(7): 1387.
Elmastas, M., Isildak, O., Turkekul, I., and Temur, N. 2007. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. Journal of Food Composition and Analysis. 20(3-4): 337-345.
Farooq, S., A. Rather, S., Gull, A., Ahmad Ganai, S., Masoodi, F. A., Mohd Wani, S., and Ganaie, T. A. 2020. Physicochemical and nutraceutical properties of tomato powder as affected by pretreatments, drying methods, and storage period. International Journal of Food Properties. 23(1): 797-808.
Freitas, S.T.D. and Mitcham, E.J. 2013. Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Scientia Agricola. 70: 257-262.
Harguindeguy, M. and Fissore, D. 2020. On the effects of freeze-drying processes on the nutritional properties of foodstuff: A review. Drying Technology. 38(7): 846-868.
Jakkranuhwat, N. and Kunchansombat, N. 2020. Effect of foam-mat drying conditions on antioxidant activity, total phenolic compound, anthocyanin content and color of purple-fleshed sweet potato powder. Chiang Mai University Journal of Natural Sciences. 20(2): e2021045.
Jalgaonkar, K., Mahawar, M.K., Bibwe, B., and Kannaujia, P. 2022. Postharvest profile, processing and waste utilization of dragon fruit (Hylocereus Spp.): A review. Food Reviews International. 38(4): 733-759.
Jia, Y., Khalifa, I., Hu, L., Zhu, W., Li, J., Li, K., and Li, C. 2019. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food and Bioproducts Processing. 118: 67-76.
Jiang, H., Zhang, M., Mujumdar, A. S., and Lim, R. X. 2014. Comparison of drying characteristic and uniformity of banana cubes dried by pulse‐spouted microwave vacuum drying, freeze drying and microwave freeze drying. Journal of the Science of Food and Agriculture. 94(9): 1827-1834.
Kayacan, S., Sagdic, O., and Doymaz, I. 2018. Effects of hot-air and vacuum drying on drying kinetics, bioactive compounds and color of bee pollen. Journal of Food Measurement and Characterization. 12(2): 1274-1283.
Madane, P., Das, A.K., Nanda, P.K., Bandyopadhyay, S., Jagtap, P., Shewalkar, A., and Maity, B. 2020. Dragon fruit (Hylocereus undatus) peel as antioxidant dietary fibre on quality and lipid oxidation of chicken nuggets. Journal of Food Science and Technology. 57(4): 1449-1461.
Mahayothee, B., Komonsing, N., Khuwijitjaru, P., Nagle, M., and Müller, J. 2019. Influence of drying conditions on colour, betacyanin content and antioxidant capacities in dried red-fleshed dragon fruit (Hylocereus polyrhizus). International Journal of Food Science & Technology. 54(2): 460-470.
Orikasa, T., Koide, S., Okamoto, S., Imaizumi, T., Muramatsu, Y., Takeda, J.-i., Shiina, T., and Tagawa, A. 2014. Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. Journal of Food Engineering. 125: 51-58.
Ozcan-Sinir, G., Ozkan-Karabacak, A., Tamer, C.E., and Copur, O.U., 2019. The effect of hot air, vacuum and microwave drying on drying characteristics, rehydration capacity, color, total phenolic content and antioxidant capacity of Kumquat (Citrus japonica). Food Science and Technology 39, 475-484.
Papoutsis, K., Pristijono, P., Golding, J.B., Stathopoulos, C.E., Bowyer, M.C., Scarlett, C.J., and Vuong, Q.V. 2017. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. International Journal of Food Science & Technology. 52(4): 880-887.
Pashazadeh, H., Redha, A.A., and Koca, I. 2024. Effect of convective drying on phenolic acid, flavonoid and anthocyanin content, texture and microstructure of black rosehip fruit. Journal of Food Composition and Analysis. 125: 105738.
Patria, D., Sutrisno, A., Hsu, J., and Lin, J. 2020. Physical properties and cooking quality of extruded restructured rice: Impact of water temperature and water level. Food Research. 4(5): 1616-1622.
Pichayajittipong, P. and Thaiudom, S. 2014. Optimum condition of beta-cyanin colorant production from red dragon fruit (Hylocercus polyrhizus) peels using response surface methodology. Chiang Mai University Journal of Natural Sciences. 13(1): 483-496.
Piskov, S., Timchenko, L., Grimm, W.-D., Rzhepakovsky, I., Avanesyan, S., Sizonenko, M., and Kurchenko, V. 2020. Effects of various drying methods on some physico-chemical properties and the antioxidant profile and ACE inhibition activity of oyster mushrooms (Pleurotus ostreatus). Foods. 9(2): 160.
Pu, Y., Ding, T., Wang, W., Xiang, Y., Ye, X., Li, M., and Liu, D. 2018. Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). Journal of the Science of Food and Agriculture. 98(2): 628-634.
Quan, T.H., Tram, G.P.N., Yen, T.T., Tien, N.P., Kaewthong, P., and Karnjanapratum, S. 2023. The effect of golden oyster mushroom (Pleurotus citrinopileatus) powder on the physiochemical, antioxidative, and sensory properties of noodles. Acta Scientiarum Polonorum Technologia Alimentaria. 22(3): 351-360.
Ren, Z., Yu, X., Yagoub, A.E.A., Fakayode, O.A., Ma, H., Sun, Y., and Zhou, C. 2021. Combinative effect of cutting orientation and drying techniques (hot air, vacuum, freeze and catalytic infrared drying) on the physicochemical properties of ginger (Zingiber officinale Roscoe). LWT. 144: 111238.
Ruttarattanamongkol, K., Chittrakorn, S., Weerawatanakorn, M., and Dangpium, N. 2016. Effect of drying conditions on properties, pigments and antioxidant activity retentions of pretreated orange and purple-fleshed sweet potato flours. Journal of Food Science and Technology. 53(4): 1811-1822.
Santos, P.H.S. and Silva, M.A. 2008. Retention of vitamin C in drying processes of fruits and vegetables-A review. Drying Technology. 26(12): 1421-1437.
Sehrawat, R., Nema, P.K., and Kaur, B.P. 2018. Quality evaluation and drying characteristics of mango cubes dried using low-pressure superheated steam, vacuum and hot air drying methods. LWT. 92: 548-555.
Sengkhamparn, N., Chanshotikul, N., Assawajitpukdee, C., and Khamjae, T. 2013. Effects of blanching and drying on fiber rich powder from pitaya (Hylocereus undatus) peel. International Food Research Journal. 20(4): 1595.
Shiau, S.-Y., Li, G.-H., Pan, W.-C., and Xiong, C. 2020. Effect of pitaya peel powder addition on the phytochemical and textural properties and sensory acceptability of dried and cooked noodles. Journal of Food Processing and Preservation. 44(7): e14491.
Steel, R.G.D. and Torrie, J.H. 1980. Principles and procedures of statistics, a biometrical approach. McGraw-Hill Kogakusha, Ltd.
Tawatsinlapasorn, N., Kuljarachanan, T., Chiewchan, N., and Devahastin, S. 2017. Effects of drying techniques on selected functional properties and bioactive compounds of dietary fiber from the outer leaves of cabbage. Chiang Mai University Journal of Natural Sciences. 16(1):19-30.
Therdthai, N. and Zhou, W. 2009. Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). Journal of Food Engineering. 91(3): 482-489.
Wong, F.-C., Yong, A.-L., Ting, E.P.-S., Khoo, S.-C., Ong, H.-C., and Chai, T.-T. 2014. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research. 13(4): 1409.
Wong, Y.-M. and Siow, L.-F. 2015. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate as models. Journal of Food Science and Technology. 52: 3086-3092.
Yen, T.T., Quan, T.H., Nhung, H.T.H., Tram, G.P.N., Karnjanapratum, S., and Benjakul, S. 2022. Development of antioxidative red dragon fruit bar by using response surface methodology for formulation optimization. Applied Food Research. 2(2): 100173.
Yousf, N., Nazir, F., Salim, R., Ahsan, H., and Sirwal, A. 2017. Water solubility index and water absorption index of extruded product from rice and carrot blend. Journal of Pharmacognosy and Phytochemistry. 6(6): 2165-2168.
Zannou, O., Pashazadeh, H., Ghellam, M., Hassan, A. M., and Koca, I. 2021. Optimization of drying temperature for the assessment of functional and physical characteristics of autumn olive berries. Journal of Food Processing and Preservation. 45(9): e15658.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tran Hong Quan1, *, Tran Tieu Yen1, Giap Pham Ngoc Tram1, Nguyen Phung Tien1, Supatra Karnjanapratum2, and Saroat Rawdkuen3
1 Department of Food Technology, Faculty of Applied Biological Sciences, Vinh Long University of Technology Education, Vinh Long, 85110, Vietnam.
2 Division of Marine Product Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai, 50100, Thailand.
3 Unit of Innovative Food Packaging and Biomaterials School of Agro-Industry, Mae Fah Luang University, 333 Moo 1, Thasud, Muang, Chiang Rai, 57100 Thailand.
Corresponding author: Tran Hong Quan, E-mail: quanth@vlute.edu.vn
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 17, 2023;
Revised: February 19, 2024;
Accepted: February 20, 2024;
Online First: February 22, 2024

