
Biological Properties of Propolis from Tetragonula sp. Against Subgingival Bacteria Proteolytic Activity in Periodontitis
Devina Nurul Isnaini, Dias Bintang Rakasiwi, Fahreza Naufaldi Putra Nawawi, Ikhsan Maulana, Ika Andriani, and Arya Adiningrat*Published Date : February 21, 2024
DOI : https://doi.org/10.12982/NLSC.2024.020
Journal Issues : Number 2, April-June 2024
Abtract Periodontitis refers to the damaged periodontal tissues as a manifest of mixed bacterial infection and pathogenic activities. This bacterial complex produces critical virulence factors which are responsible for disease development. Some commercially available antimicrobial agents have been reported in generating cytotoxicity effects towards the oral tissues. Propolis has alternatively been reported to also have an inhibitory effect on the oral pathogenic bacteria. This study aimed to evaluate the ethanolic extract of propolis (EEP) properties through quercetin levels and antioxidant capacity analysis, while the functional effectiveness was observed by its anti-proteolytic activity. The quercetin level and antioxidant capacity in EEP were found to increase gradually as the increased extract concentration. Furthermore, bacteria proteolytic activity was also correspondingly inhibited.
Keywords: Phenolic compound, Antioxidant activity, Antibacterial, Quercetin
Citation: Isnaini, D.N., Rakasiwi, D.B., Nawawi, F.N.P., Maulana, I., Andriani, I., Adiningrat, A. 2024. Biological properties of propolis from Tetragonula sp. against subgingival bacteria proteolytic activity in periodontitis. Natural and Life Sciences Communications. 23(2): e2024020.
INTRODUCTION
Periodontitis is clinically defined as an inflammatory disease on periodontal tissue associated with putative periodontal pathogens. The irreversible damage on surrounding tissues and alveolar bone loss belong to the key features of periodontitis. It is also strongly associated with microbiome dysbiosis in the subgingival environment. In the progressing periodontitis, the anaerobic, gram-negative, proteases-producing pathogenic bacteria are enriched due to the environment alteration that came from tissue breakdown. Oral pathogens commonly produce several virulence factors to better support the disease and further tissue damage (Tonetti et al. 2018; Van Dyke et al. 2020). One of the virulence factors may exhibit in the form of proteolytic agents that alter the protein-related component of the surrounding oral (Neilands et al. 2015; Dahlen et al. 2019; Cleaver et al. 2023).
The optimum pathogen eradication in developing periodontitis needs to be performed as an action to prevent both irreversible soft and hard tissues damages. This can be applied with common antibacterial agents utilization such as chlorhexidine (CHX) and sodium hypochlorite (NaOCl) (Hussain et al. 2022). They have effective bacterial dissolution ability; however, the cytotoxicity effects are concerning. These agents show a pronounced toxic effect on human cells (Patel and Gangadin 2017; Bhavikatti et al. 2021). Addressing this issue, formulating an alternative antimicrobial agent that is effective against pathogenic bacteria and, yet, does not confer toxicity, could be beneficial for further development in clinical purposes prior utilization.
Many researchers reported that propolis has bioactive compounds associated with antibacterial activity against various pathogens. It is a mixture of natural resins from plants and bee’s metabolic substances which naturally serves as materials for hive structure and protection (Chen et al. 2018; Rufatto et al. 2018; Touzani et al. 2019). Propolis is also considered as an alternative antimicrobial agent since it exhibits less cytotoxicity effects towards human cells (Campoccia et al. 2021; Adiningrat, Maulana, et al. 2023). Moreover, one other study also indicates a better clinical improvement in the treatment of periodontitis using propolis compared to CHX (Eghbali Zarch et al. 2021). Propolis as a local and natural product contains both flavonoid and terpenoid compounds, which are biologically active substances as thought to have both effective antibacterial and antioxidant ability (Stähli et al. 2021). However, the actual content of propolis which may reflect its effectiveness varies among regions, plantations, and seasons (Fikri et al. 2019; Iqbal et al. 2019). Previous study indicates that both flavonoid and terpenoid in our local propolis samples reaches 91.77% of bioactive substances (Oktaweni et al. 2022). Therefore, this study aims to observe the profile of our local propolis through flavonoid compounds, antioxidant capacity, and subgingival bacteria proteolytic activity in the disease environment.
MATERIAL AND METHODS
Ethical Clearance
The research methodology was approved by the Ethical Committees of the Faculty of Medicine and Health Sciences (FKIK) Universitas Muhammadiyah Yogyakarta (No. 005/EC-EXEM-KEPK FKIK UMY/IX/2019).
Bacterial Culture
Isolated clinical bacteria sample was kindly provided by the Molecular Medicine and Therapy (MMT) Laboratory. It was previously collected as pocket periodontal swab using paper point from periodontitis condition, which had been clinically approved by academic dental hospital periodontics. Bacteria sample was cultured in tryptose phosphate broth (TPB) media for 24h under anaerobic condition at 37°C, prior to the analysis. All the procedures were conducted at MMT Laboratory, Universitas Muhammadiyah Yogyakarta.
Ethanolic Extract of Propolis
Raw propolis material from Tetragonula sp. were obtained from a local apiary in Nglipar, Gunung Kidul, Yogyakarta. The ethanolic extracts were prepared according to previous study (Adiningrat, Kusnadi, et al. 2023). Briefly, the propolis were diluted in 40% ethanol (Chew et al. 2011) and filtered. The solvent was fully evaporated using a drying oven (Biobase, China). Ethanolic extracts of propolis (EEP) were diluted in TPB media following the indicated concentrations.
Gelatin Liquefaction
The procedure was performed according to (Elavarashi et al. 2017). The bacteria were cultured in growth media containing EEPs or 2% CHX for 48h under anaerobic condition at 37°C, with triplicate for each treatment. We included the 0.8% and 10% EEPs from our previous studies that confer optimum inhibitory activity against several bacteria (Fauzi et al. 2018; Adiningrat et al. 2022). Each culture was centrifuged at 5,000rpm (Biocen, Ortho Alresa, Spain) for 10 min. The supernatant was injected to gelatine media and incubated anaerobically for 48h at 37°C. Post-incubated gelatine was chilled at 4°C for 60 min. Volumes of the frozen and unfrozen gelatine were measured for further liquefaction calculation.
-Δ Liquefaction Volume = unfrozen gelatine (sample) - unfrozen gelatine (untreated)
Total Quercetin Analysis
Total quercetin content was analyzed using aluminium chloride colorimetric method (Mammen and Daniel 2012). Quercetin (Thermo Fisher Scientific, USA) was used as standard to make the calculation curve. 100 mg of quercetin was dissolved in 1 ml DMSO and diluted to 0 µg/ml, 20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml, 100 µg/ml using 40% ethanol (R2 = 0.9851, y =3.9726x2– 9.3478x + 2.1724). Ethanolic propolis was diluted into several concentrations with triplication: 0.008%, 0.016%, 0.8%, and 10%. 1 ml of each EEP was added with 0.05 ml AlCl3 10% and 0.05 ml 0.1 mM C2H3KO2. The obtained mixture was then vortexed and incubated for 30 minutes at room temperature. The absorbance was measured at 415nm using a UV-vis spectrophotometer (Halo RB-10, Dynamics, UK).
DPPH Assay
The antioxidant assay was carried out according to (Mishra et al. 2012; Meziane et al. 2023) with modifications. A concentration of 0.3 mM DPPH in 40% ethanol was prepared prior to the assay. It was mixed with the EEP following the indicated concentration (v/v) 0.002%, 0.004%, 0.006%, 0.008%, 0.010%, 0.012%, 0.014%, and 0.016%, with triplication. The mixture was then incubated in the dark room for 20min and was measured at 517 nm. The inhibition activity of the EEP was calculated according the following formula:
%DPPH free radical inhibition = [(A0-A1)/A0] x 100
A0 is the absorbance of untreated DPPH, while A1 is the absorbance of a mixture of DPPH and EEP.
Statistical Analysis
Data were analyzed using GraphPad Prism 9 (GraphPad Software, USA). The normality test was performed using Shapiro-Wilk, followed by Student’s t-test or Mann-Whitney U test due to data normality.
RESULTS
Proteolytic Activity
We determined the proteolytic activity of the bacteria using –Δ liquefaction volume as the value of post treatment solidified gelatine to the untreated. Figure 1 showed that gelatine was not solidified after subgingival bacteria treatment without any EEP or CHX intervention. CHX showed the strongest inhibitory capacity (77.19 ± 2.41 µL gelatine solidified) as the positive control, whereas 10% EEP (36.20 ± 4.17 µL) as the targeted treatment also showed significant inhibition properties against the proteolytic activity of the subgingival bacteria.
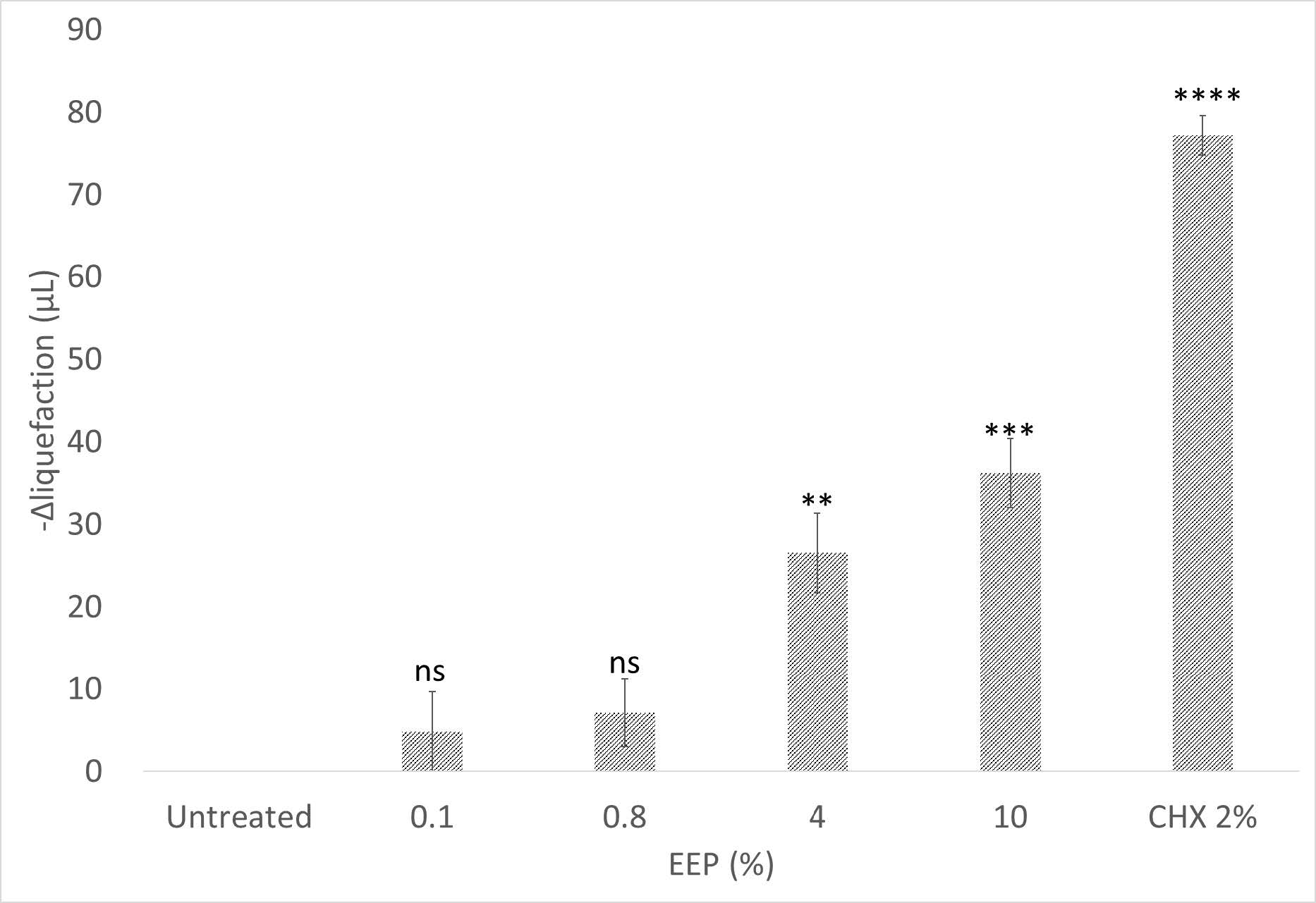
Figure 1. Subgingival bacteria proteolytic activity of each treatment. Solidified gelatine at lower temperature after treatments were measured as –Δ liquefaction volume value. The bars were constructed using average and standard error of mean (SEM) values of each treatment. n=3, ****P <0.0001, ***P <0.001, **P <0.01, ns=non-significant.
Quercetin Content of Propolis
The quantitative level of quercetin in EEP was shown in Figure 2. The level of quercetin was in accordance with the increasing EEP concentration. The highest level of quercetin was exhibited by 10% EEP (196.7 ± 4.468 µg/mL).
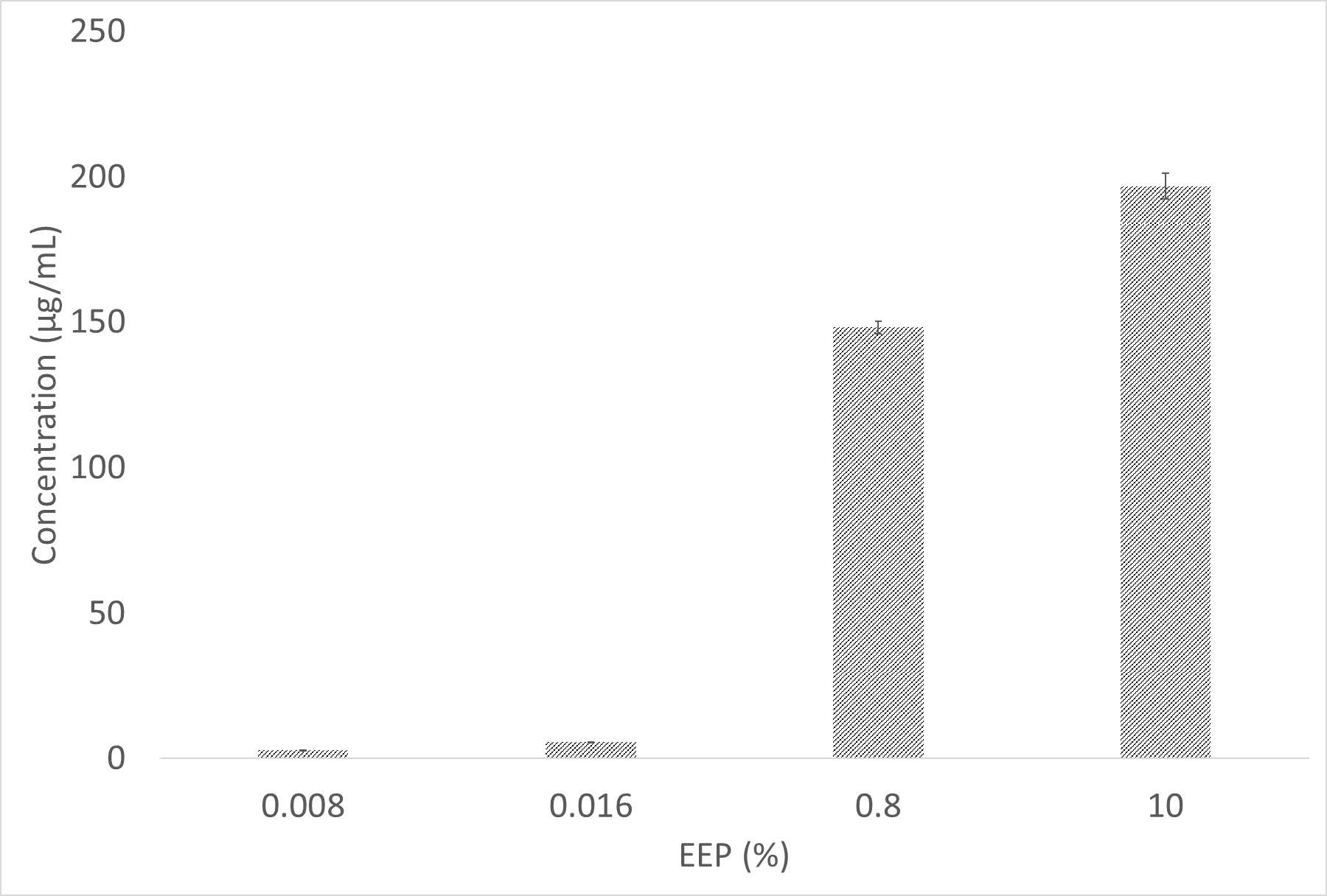
Figure 2. Quercetin content of several concentrations of EEP. The optical density of the product formed after colorimetric reaction was used for determining the concentration (n=3).
Antioxidant Capacity
The free radical scavenging activity of several concentrations of EEP was evaluated through DPPH assay. The antioxidant activity increased in a positive correlation corresponding to the increased EEP concentration, with the highest concentration being shown by 0.016% EEP reaching up to 37.07±3.33% inhibitory ability (Figure 3).
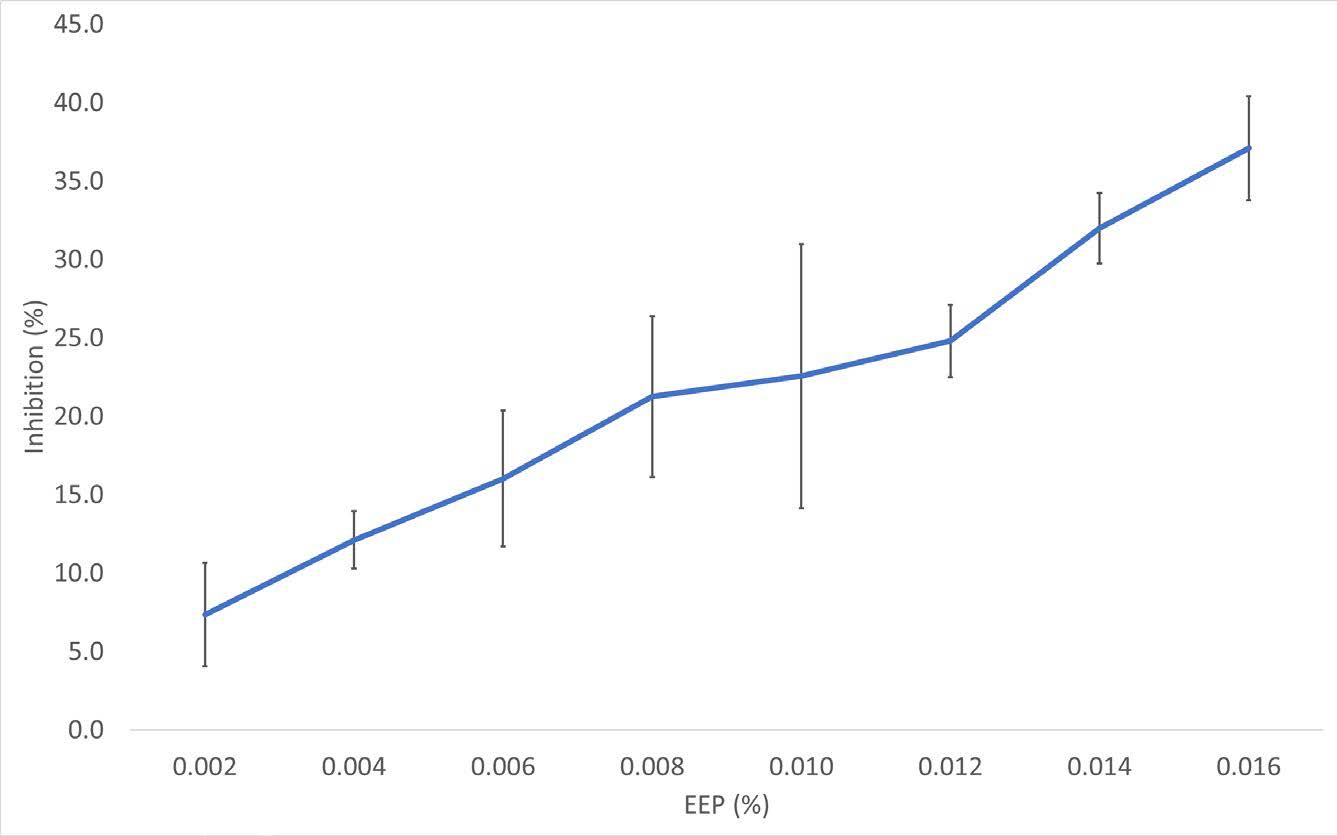
Figure 3. Antioxidant capacity of EEP. Ability of free radical inhibition value was obtained from the absorbance of the control and treatments. The lines were constructed using the average values and SEM of each treatment (n=3).
DISCUSSION
In this study, we demonstrated the proteolytic inhibitory capacity of propolis against the subgingival bacteria which were indicating two things. First, it was confirmed that the subgingival bacteria tend to exhibit proteolytic activity toward gelatine structure, as similar to the previously reported (Davies et al. 2021). Second, bacterial gelatinase activity decreased gradually along with the increased concentration of EEP treatments. This result was in accordance with the previous study which is also suggesting the inhibitory capacity of propolis against proteolytic activity but in the Enterococcus faecalis bacteria (Adiningrat et al. 2022). The inhibitory capacity of propolis extract against bacterial proteolytic activity could be related to the flavonoid substance of the propolis extract. This active compound of propolis may interact with the gelatinase enzyme active site of the bacteria and inhibit its interaction with the substrate, resulting in altered proteolytic activity (Cirkovic Velickovic and Stanic-Vucinic 2018).
We measured the total flavonoid component of EEP through quercetin level, since it is a commonly used flavonoid derivative as the flavonoid standard (Shu et al. 2011). Our data showed a positive relationship between quercetin level and the propolis extract concentration. This result was in accordance with the previous studies, which also indicated the high quercetin level in propolis (Oroian et al. 2020; Ding et al. 2021). The inhibitory capacity against proteolytic activity of our local propolis flavonoid compound tended to have lesser concentration than previous studies in order to reach its optimum inhibition capacity. It seemed that the optimal inhibitory activity of our local propolis extract required approximately 150-200µg/mL of flavonoid, while the optimum inhibitory concentration of flavonoid compounds against bacteria varied in several studies. Most studies suggest more than 600µg/mL flavonoid extract to achieve the desired effect (Shu et al. 2011; Touzani et al. 2021), hence, in this finding, EEP was considered as a potential antibacterial agent with minimum required concentration. Flavonoids may bind to the bacterial protein through non-specific hydrogen bonds, hydrophobic effects, and the formation of covalent bonds as well. These backbone and side chain interactions may result in the inhibition of bacterial enzymatic proteolytic activity (Kumar and Pandey 2013).
It is also suggested by our study that propolis did not only exhibit antibacterial potency but also confer other beneficial effects on oral tissue through its free radical scavenging activity. Our results indicated that free radical activity was altered by EEP treatment up to 37%, in a concentration-dependent manner. This ability could protect the surrounding cellular environment from oxidative stress in defected oral tissues (Rivera-Yañez et al. 2018). Antioxidant capacity of propolis is mainly attributed to its flavonoids content (Nichitoi et al. 2021). It was also reported that flavonoids might alter the free radicals by transferring its charged molecule and delocalizing the unpaired electron, and also protecting the surrounding tissue as well (Fernandez-Panchon et al. 2008).
Our local propolis from stingless bee (Tetragonula sp.) may have different and unique component compared to previous studies, and this could be relied on the species differences and plantation diversity surrounding the apiary. The propolis showed both potencies in antibacterial and antioxidant properties through its extracted formulation. This study suggested a mechanism of previously described bacterial growth inhibitory activity of propolis. The findings may lead to further analysis on more advanced characterization of propolis extract and the detailed mechanism of action related to its optimum concentration for its health beneficial properties and for periodontal disease supporting remedy.
CONCLUSION
Overall, the EEP from our local propolis showed pronounced inhibitory capacity against subgingival bacterial proteolytic activity, and it also showed health beneficial properties through exhibiting antioxidant capacity.
ACKNOWLEDGEMENTS
We thank the Molecular Medicine and Therapy Laboratory Universitas Muhammadiyah Yogyakarta for providing worthwhile assistance and research facilities.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interests.
REFERENCES
Adiningrat, A., Kusnadi, R.A., Allam, A.S., Sofiani, E., Maulana, I., and Yumoto, H. 2023. The effect of probiotic Lactobacillus acidophilus and ethanolic propolis compound toward nucleic acid deposition in the extracellular polymeric substance of root canal bacteria. European Journal of Dentistry. 17(02): 418–423.
Adiningrat, A., Maulana, I., Fadhlurrahman, A.G., Kurniawan, M.F., and Seno Aji, N.R.A. 2023. Evaluation of bio-compatibility and effectiveness of propolis Tetragonula sp. as dental anti-microbial agent. Journal of stomatology (Czas Stomatol). 76(2): 94–100.
Adiningrat, A., Prabowo, R.A.W., Kurnia, R., Septianti, N.F.F., Maulana, I., and Sofiani, E. 2022. Metabolism-independent phenomenon in ethanolic propolis inhibitory capacity towards enterococcus spp proteolytic activity. Odonto : Dental Journal. 9(2): 206–214.
Bhavikatti, S.K., Karobari, M.I., Zainuddin, S.L.A., Marya, A., Nadaf, S.J., Sawant, V.J., Patil, S.B., Venugopal, A., Messina, P., and Scardina, G.A. 2021. Investigating the antioxidant and cytocompatibility of Mimusops elengi Linn extract over human gingival fibroblast cells. International Journal of Environmental Research and Public Health. 18(13): 7162.
Campoccia, D., Ravaioli, S., Santi, S., Mariani, V., Santarcangelo, C., De Filippis, A., Montanaro, L., Arciola, C.R., and Daglia, M. 2021. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomedicine & Pharmacotherapy. 141: 111895.
Chen, Y.-W., Ye, S.-R., Ting, C., and Yu, Y.-H. 2018. Antibacterial activity of propolins from Taiwanese green propolis. Journal of Food and Drug Analysis. 26(2): 761–768.
Chew, K.K., Ng, S.Y., Thoo, Y.Y., Khoo, M.Z., Wan Aida, W.M., and Ho, C.W. 2011. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. International Food Research Journal. 18: 571–578.
Cirkovic Velickovic, T.D. and Stanic-Vucinic, D.J. 2018. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Comprehensive Reviews in Food Science and Food Safety. 17(1): 82–103.
Cleaver, L.M., Carda-Diéguez, M., Moazzez, R., and Carpenter, G.H. 2023. Novel bacterial proteolytic and metabolic activity associated with dental erosion-induced oral dysbiosis. Microbiome. 11(1): 69.
Dahlen, G., Basic, A., and Bylund, J. 2019. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. Journal of Clinical Medicine. 8(9): 1339.
Davies, J.R., Kad, T., Neilands, J., Kinnby, B., Prgomet, Z., Bengtsson, T., Khalaf, H., and Svensäter, G. 2021. Polymicrobial synergy stimulates Porphyromonas gingivalis survival and gingipain expression in a multi-species subgingival community. BMC Oral Health. 21(1): 639.
Ding, Q., Sheikh, A.R., Gu, X., Li ,J., Xia, K., Sun, N., Wu, R.A., Luo, L., Zhang, Y., and Ma, H. 2021. Chinese propolis: Ultrasound‐assisted enhanced ethanolic extraction, volatile components analysis, antioxidant and antibacterial activity comparison. International Journal of Food Sciences and Nutrition. 9(1): 313–330.
Eghbali Zarch, R., Askari, M., boostani, H., and Mirzaii-Dizgah, I. 2021. Effect of propolis extract on clinical parameters and salivary level of matrix metalloproteinase 8 in periodontitis patients: A randomized controlled clinical trial. Journal of Advanced Periodontology & Implant Dentistry. 13(2): 84–90.
Elavarashi, E., Kindo, A.J., and Rangarajan, S. 2017. Enzymatic and non-enzymatic virulence activities of dermatophytes on solid media. Journal of Clinical and Diagnostic Research. 11(2): DC23–DC25.
Fauzi, A.F., Indiana, S.K., Wicaksono, R.H., and Adiningrat, A. 2018. A challenge in ethanolic propolis utilization from Apis trigona as an oral antimicrobial agent. Journal of International Dental and Medical Research. 11(2): 682–686.
Fernandez-Panchon, M.S., Villano, D., Troncoso, A.M., and Garcia-Parrilla, M.C. 2008. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Critical Reviews in Food Science and Nutrition. 48(7): 649–671.
Fikri, A.M., Sulaeman, A., Marliyati, S.A., and Fahrudin, M. 2019. Antioxidant activity and total phenolic content of stingless bee propolis from Indonesia. Journal of Apicultural Science. 63(1): 139–147.
Hussain, A.M., Weijden, G.A. (Fridus), and Slot, D.E. 2022. Effect of a sodium hypochlorite mouthwash on plaque and clinical parameters of periodontal disease‐a systematic review. International Journal of Dental Hygiene. 20(1): 40–52.
Iqbal, M., Fan, T., Watson, D., Alenezi, S., Saleh, K., and Sahlan, M. 2019. Preliminary studies: The potential anti-angiogenic activities of two Sulawesi Island (Indonesia) propolis and their chemical characterization. Heliyon. 5(7): e01978.
Kumar, S. and Pandey, A.K. 2013. Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal. 2013: 162750.
Mammen, D. and Daniel, M. 2012. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chemistry. 135(3): 1365–1368.
Meziane, Y., Megateli, S., and Chaouia, C. 2023. In vitro assessment of total bioactive contents and antioxidant capacity of grape juices extracts of table and wine varieties from algeria and their correlations. Natural and Life Sciences Communication. 22(3): e2023050.
Mishra, K., Ojha, H., and Chaudhury, N.K. 2012. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chemistry. 130(4): 1036–1043.
Neilands, J., Wickström, C., Kinnby, B., Davies, J.R., Hall, J., Friberg, B., and Svensäter, G. 2015. Bacterial profiles and proteolytic activity in peri-implantitis versus healthy sites. Anaerobe. 35: 28–34.
Nichitoi, M.M., Josceanu, A.M., Isopescu, R.D., Isopencu, G.O., Geana, E.-I., Ciucure, C.T., and Lavric, V. 2021. Polyphenolics profile effects upon the antioxidant and antimicrobial activity of propolis extracts. Scientific Reports. 11(1): 20113.
Oktaweni, F., Sutikno, S., and Sudaryadi, I. 2022. Pollen diversity and propolis’s bioactive compounds of stingless bees (Tetragonula laeviceps Smith 1857) From Kedungpoh Meliponiculture, Gunungkidul, Yogyakarta.: Yogyakarta, Indonesia. [accessed 2022 Jun 26]. https://www.atlantis-press.com/article/125974055.
Oroian, M., Ursachi, F., and Dranca, F. 2020. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrasonics Sonochemistry. 64: 105021.
Patel, E. and Gangadin, M. 2017. Managing sodium hypochlorite accidents: The reality of toxicity. South African Dental Journal. 72(6):a5.
Rivera-Yañez, N., Rodriguez-Canales, M., Nieto-Yañez, O., Jimenez-Estrada, M., Ibarra-Barajas, M., Canales-Martinez, M.M., and Rodriguez-Monroy, MA. 2018. Hypoglycaemic and antioxidant effects of propolis of chihuahua in a model of experimental diabetes. Evidence-Based Complementary and Alternative Medicine. 2018: 4360356.
Rufatto, L.C., Luchtenberg, P., Garcia, C., Thomassigny, C., Bouttier, S., Henriques, J.A.P., Roesch-Ely, M., Dumas, F., and Moura, S. 2018. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiological Research. 214: 74–82.
Shu, Y., Liu, Y., Li, L., Feng, J., Lou, B., Zhou, X., and Wu, H. 2011. Antibacterial activity of quercetin on oral infectious pathogens. African Journal of Microbiology Research. 5(30): 5358-5361.
Stähli, A., Schröter, H., Bullitta, S., Serralutzu ,F., Dore, A., Nietzsche, S., Milia, E., Sculean, A., and Eick, S. 2021. In Vitro activity of propolis on oral microorganisms and biofilms. Antibiotics. 10(9): 1045.
Tonetti, M.S., Greenwell, H., and Kornman, K.S. 2018. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Periodontology. 89: S159–S172.
Touzani, S., Embaslat, W., Imtara, H., Kmail, A., Kadan, S., Zaid, H., ElArabi, I., Badiaa, L., and Saad, B. 2019. In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: Physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. BioMed Research International. 2019: 4836378.
Touzani, S., Imtara, H., Katekhaye, S., Mechchate, H., Ouassou, H., Alqahtani, A.S., Noman, O.M., Nasr, F.A., Fearnley, H., Fearnley, J., et al. 2021. Determination of phenolic compounds in various propolis samples collected from an African and an Asian region and their impact on antioxidant and antibacterial activities. Molecules. 26(15): 4589.
Van Dyke, T.E., Bartold, P.M., and Reynolds, E.C. 2020. The nexus between periodontal inflammation and dysbiosis. Frontiers in Immunology. 11: 511.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Devina Nurul Isnaini1, Dias Bintang Rakasiwi1, Fahreza Naufaldi Putra Nawawi1, Ikhsan Maulana3, Ika Andriani2, and Arya Adiningrat3, 4, *
1 Clinical Program, Faculty of Dentistry, Universitas Muhammadiyah Yogyakarta, Indonesia.
2 Department of Periodontology, Faculty of Dentistry, Universitas Muhammadiyah Yogyakarta, Indonesia.
3 Molecular Medicine and Therapy Laboratory, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta, Indonesia.
4 Department of Oral Biology and Biomedical Sciences, Faculty of Dentistry, Universitas Muhammadiyah Yogyakarta, Indonesia.
Corresponding author: Arya Adiningrat, E-mail: adiningrat@umy.ac.id
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: September 23, 2023;
Revised: January 8, 2024;
Accepted: February 13, 2024;
Published online: February 21, 2024

