
Cosmeceutical Activities of Essential Oils from the Rhizomes of Plants in the Zingiberaceae Family
Pattiya Tammasorn, Watchara Kanjanakawinkul, and Wantida Chaiyana*Published Date : February 20, 2023
DOI : https://doi.org/10.12982/NLSC.2024.021
Journal Issues : Number 2, April-June 2024
Abstract The plants in the Zingiberaceae family are often rhizomatous annual or perennial herbs with considerable economic value. This study aimed to investigate the cosmeceutical activities of essential oils from the rhizomes of Hedychium coronarium J. Koenig, Curcuma caesia Roxb., and Curcuma longa L. The essential oils were obtained from hydrodistillation and subjected to investigations of antioxidant activities by 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power, and ferric thiocyanate assay. Furthermore, the whitening properties were investigated in terms of anti-tyrosinase inhibition, whereas the anti-skin aging activities were investigated through collagenase, elastase, and hyaluronidase inhibition. The results showed that all essential oils exhibited low antioxidant, anti-elastase, and anti-collagenase activities. On the other hand, H. coronarium oil was the only essential oil in the present study, which was found to possess anti-tyrosinase activity, with the inhibition of 98.4 ± 5.5% and 30.2 ± 1.7% at the final concentration of 0.1 mg/ml, when the substrate were L-tyrosine and L-DOPA, respectively. Interestingly, it was highlighted that the anti-tyrosinase activity of H. coronarium oil was stronger than that of kojic acid, a well-known whitening agent, when the substrate was L-tyrosine. Besides, H. coronarium oil and C. longa oil exhibited promising anti-hyluronidase activities with inhibition of 83.6 ± 1.0% and 82.9 ± 2.2%, respectively, which were comparable to that of epigallocatechin gallate (100.0 ± 0.0%). All essential oil induced irritations in the HET-CAM test at the tested concentration of 5 mg/ml in 10% dimethyl sulfoxide in olive oil. Therefore, H. coronarium oil was suggested as an attractive ingredient in cosmeceutical products for whitening and anti-aging.
Keywords: Anti-aging, Curcuma caesia, Curcuma longa, Hedychium coronarium, Whitening
Funding: The authors are appreciative of the support for their research received from the Graduate school, Chiang Mai University and the Master’s degree program in Cosmetic Science, Chiang Mai University.
Citation: Tammasorn, P., Kanjanakawinkul, W., and Chaiyana, W. 2024. Cosmeceutical activities of essential oils from the rhizomes of plants in the zingiberaceae family. Natural and Life Sciences Communications. 23(2): e2024021.
INTRODUCTION
Plants in the Zingiberaceae family are known for their rhizomatous growth habit and are commonly cultivated as annual or perennial herbs. Apart from being utilized as vegetables, spices, sauces, flavorings, and medications, these plants possess significant quantities of essential oils in their rhizomes. In the past few years, there has been a lot of research on the bioactive compounds in essential oils. In addition, essential oils play a versatile role in cosmetics, serving as preservatives, active agents, and beneficial additions to the skin, beyond their aromatic contributions (Sharma et al., 2023). The increasing preference for natural components has played a substantial role in rekindling interest in plant extracts, specifically essential oils, within the cosmetics and healthcare industries (Sharma et al., 2023). Many plants essential oils have important biological properties, such as anti-inflammatory, antioxidants, antimicrobial, etc. (Raut and Karuppayil, 2014). Essential oils with low molecular weight are obtained by hydrodistillation, steam distillation, supercritical fluid extraction, and solvent extraction (Nakatsu et al., 2000). These findings highlight the diverse applications and potential benefits associated with essential oils derived from plants in the Zingiberaceae family.
Curcuma longa L., commonly known as turmeric, is a native plant in Southeast Asia belonging to the family Zingiberaceae that has been known since ancient times and is used in many different ways throughout the world (Fernández et al., 2021). It possesses effective anti-inflammatory, antifungal, and anti-mycotoxigenic properties (Avanco et al., 2017). Therefore, it has been used in traditional medicine systems like Ayurveda and traditional Chinese medicine for treating a wide range of inflammatory conditions. The essential oil extracted from the rhizome of C. longa has been the subject of scientific investigation, revealing its key chemical constituents. Among these constituents, 1,8-cineole (11.2%), α-turmerone (11.1%), β-caryophyllene (9.8%), a-turmerone (7.3%), and β-sesquiphellandrene (7.1%) have been prominently identified and quantified (Raina et al., 2002).
Curcuma caesia Roxb., known in India as black turmeric, belongs to the Zingiberaceae family and is underutilized compared to C. longa, endangered in Southeast Asia (Liu et al., 2013). C. caesia is considered a unique and rare species of turmeric with its distinct black or bluish-black color. Essential oil from the rhizomes of C. caesia exhibits strong antibacterial, anti-inflammatory, and antioxidant properties (Paw et al., 2020). It has been reported that the essential oil of C. caesia rhizome is predominantly composed of tropolone (15.86%), along with minor constituents including ledol (3.27%), β-elemenone (3.03%), α-bulnesene (3.02%), and spathulenol (2.42%) (Mukunthan et al., 2014). (Mukunthan et al., 2014).
Similarly, H. coronarium, also known as white ginger lily, has also been overlooked despite its potential, particularly its rhizome part. Regarding the utilization of H. coronarium flowers in the perfume industry, the other parts of the plants have been disposed of every year. However, the rhizomes of H. coronarium have been reported to contain more plentiful amounts of volatile oils compared to the other parts of the plant (da Silva et al., 2017) and widely acknowledged to have a variety of biological activities, including antibacterial, antioxidant, larvicidal, phytotoxic, anthelmintic, and anti-inflammatory activities (Matsumoto et al., 1993; Gao et al., 2008; Ray et al., 2018). The essential oil derived from both fresh and dried H. coronarium rhizome was found to contain significant amounts of 1,8-cineole (41.42% in fresh, 37.44% in dried), β-pinene (10.39% in fresh, 17.4% in dried), and α-terpineol (8.8% in fresh, 6.7% in dried), representing the major components (Joy et al., 2007).
Therefore, the rhizomes of H. coronarium and C. caesia which are underutilized and discarded as waste from the perfume industry, could have the potential to be used as active ingredients in the cosmetic and cosmeceutical fields. However, the biological actions on the prevention of skin aging or whitening effect of C. longa, C. caesia, and H. coronarium have not been documented. For these reasons, this research aimed to extract the essential oil and investigate their biological activities of C. longa, C. caesia, and H. coronarium related to anti-aging and whitening properties, which could add value to the agricultural waste from the industry. This study sought to explore the value that these essential oils. By utilizing agricultural waste as the source for extracting the essential oils, it provides a unique opportunity to repurpose and transform the waste into valuable resources. This process not only increases the value of the waste material but also unlocks the potential for creating value-added products.
MATERIAL AND METHODS
Materials
The rhizomes of H. coronarium, obtained from Sattahip, Chonburi, Thailand, during October - November 2022, were provided as a gift from Chulabhorn Royal Pharmaceutical Manufacturing Facility, Thailand. Each plant material was collected and dried for 72 h using the hot air oven at 45°C. Subsequently, the dried plant material was ground and collected in a tightly closed container for further experiment. Furthermore, C. caesia oil and C. longa oil were received as gifts by Ban Me Thuot Honey Bee JSC, Vietnam.
Essential oil hydrodistillation
The rhizome powder of H. coronarium was performed hydrodistillation for 2 h. In brief, 100 g of dried plant powder was subjected to a hydrodistillation process using a water volume of 1 L. The essential oil was collected from the Clevenger apparatus, and subsequently, After that, anhydrous sodium sulfate was used to eliminate any remaining water. After cooling, the essential oil was kept until usage in an opaque container at 4°C. The following equation calculated the yield of essential oil:
% Yield = (WA/WB) × 100,
where WA was the amount of the essential oil and WB was the weight of plant powder utilized in the hydrodistillation.
Antioxidant activities determination
2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
Each essential oil was evaluated for capacity to DPPH free radical scavenge using the technique of Chaiyana et al. (2019) and Brem et al. (2004). A volume ratio of 1:9 was used to mix the 167 µM DPPH solution with the sample solution, then kept in the dark for another 30 min. The absorbance was admeasured at 520 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation computed DPPH inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil, and ODb representsrepresented the absorbance of the combination with essential oil. Ascorbic acid, at the same final concentration as the tested samples of 0.1 mg/ml, served as the positive control. The examination was repeated three times.
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
Each essential oil was evaluated for capacity to ABTS free radical scavenging using the technique of Saeio et al. (2011). The ABTS free radical solution was made by combining ABTS with potassium persulphate (K2S2O8) at a volume ratio 2:3 for 16 h, incubated in the dark. After incubation, the ABTS solution was 20-fold ethanol diluted. Subsequently, mixed each sample solution was with ABTS solution in a ratio of 1:9 and waited for 5 min at room temperature. The absorbance was admeasured at 750 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The Trolox equivalent antioxidant capacityactivity (TEAC) of the essential oil was calculated after using Trolox to create a calibration curve (R2 = 0.997). The positive control was ascorbic acid, which was used at the same final concentration as the tested samples of 0.1 mg/ml. The examination was repeated three times.
Ferric reducing antioxidant power (FRAP) assay
Each essential oil was evaluated for capacity to ferric reducing antioxidant power using the technique of Saeio et al. (2011). FRAP solution was made by combining TPTZ solution in HCl, ferric chloride and acetate buffer (pH 3.6) in the ratio of 1:1:10. After that, the mixture of each sample solution and FRAP solution was prepared into radio 2:8 and waited for 5 min. The absorbance was admeasured at 595 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The equivalent concentrations (EC1) of the sample were calculated after using ferrous sulfate to create a calibration curve (R2 = 0.9846). Ascorbic acid, at the same final concentration as the tested samples of 0.1 mg/ml, served as the positive control. The examination was repeated three times.
Lipid Peroxidation by ferric thiocyanate (FTC) assay
Each essential oil was evaluated for capacity to lipid peroxidation by a ferric-thiocyanate assay using the technique of Chaiyana et al. (2017).Saeio et al. (2011). Firstly, the sample contained 10% NH4SCN solution, 2 mM FeCl2 solution, and 50% linoleic acid in DMSO was included in the mixes to initiate the reaction and held at 37°C for 60 min. The absorbance was admeasured at 500595 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation calculated lipid peroxidation inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil, and ODb representsrepresented the absorbance of the combination with essential oil. Ascorbic acid, at the same final concentration as the tested samples of 0.1 mg/ml, served as the positive control. The examination was repeated three times.
Anti-aging activities determination
Collagenase inhibitory activity determination
Each essential oil was evaluated for capacity to anti-collagenase activity using the technique of Thring et al. (2009). Each experiment started with determining the enzyme activity of collagenase and employed only more than 90% enzyme activity. A ratio 1:2 mixture of the sample with collagenase solution and 15 min of incubation. FALGPA in tricine buffer was added to the mixtures to start the reaction and monitored continuously for 20 min at 340 nm using a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation calculated collagenase inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil, and ODb representsrepresented the absorbance of the combination with essential oil. Epigallocatechin gallate (EGCG), at the same final concentration as the tested samples of 0.1 mg/ml, served as the positive control.
Elastase inhibitory activity determination
Each essential oil was evaluated for capacity to anti-elastase activity using the technique of Thring et al. (2009). Each experiment started with determining the enzyme activity of elastase and employed only more than 90% enzyme activity. A ratio 1:4 mixture of the sample with elastase solution and 15 min of incubation. The mixtures were combined with AAAPVN solution in Tris-HCl buffer to start the reaction and continuously representsadmeasured for 20 min at a wavelength of 410 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation calculated elastase inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil, and ODb is the absorbance of the combination with essential oil. The positive control was oleanolic acid, which was used at the same final concentration as the tested samples of 0.1 mg/ml.
Hyaluronidase inhibitory activity determination
Each essential oil was evaluated for capacity to anti-hyaluronidase activity using the technique of Nema et al. (2011). Only more than 90% of the hyaluronidase enzyme activity was used in each experiment. A ratio 1:5 mixture of the sample with hyaluronidase solution was incubated for 10 min at 37°C. After incubation, added hyaluronic acid solution and incubated again for 45 min at 37°C. Subsequently, added an acidic albumin solution and wait for 10 min at room temperature. The absorbance was admeasured at 600 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation computed hyaluronidase inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil, and ODb representsrepresented the absorbance of the combination with essential oil. The positive control was EGCG, which was used at the same final concentration as the tested samples of 0.1 mg/ml. The experiments were repeated three times.
Anti-tyrosinase activity determination
The modified method from Manosroi et al. (2010) and Saeio et al. (2011) was used to assess the anti-tyrosinase activity of each essential oil. Each experiment started with determining the enzyme activity of tyrosinase and employed only more than 90% enzyme activity. Briefly, 10 µL of the sample, 30 µL of tyrosinase (315 units/ml), and 60 µL phosphate buffer (pH 6.5) were combined and incubated at room temperature for 5 min. After incubation, added L-tyrosine or L-DOPA and incubated for 30 min at room temperature. Eventually, the final concentration of each sample was made up to 0.1 mg/ml. The absorbance was admeasured at 492 nm with a multimode microplate reader (BMG Labtech GmbH, Germany). The following equation calculated hyaluronidase inhibition:
% inhibition = [(ODa-ODb)/ODa] × 100,
where ODa representsrepresented the absorbance of the combination without essential oil and ODb representsrepresented the absorbance of the combination with essential oil. The positive control was kojic acid, which was used at the same final concentration as the tested samples of 0.1 mg/ml. The experiments were repeated three times.
Hen’s egg-chorioallantoic membrane test (HET-CAM) test)
The irritation study using the HET-CAM test of each essential oil was assessed using the technique of Chaiyana et al. (2019). After removing the eggshell and egg membrane, 30 μl of the sample at a concentration of 5 mg/ml, dissolved in 10 % DMSO in olive oil, was dropped into the CAM. Irritation was noted immediately following exposures and was kept under observation for 5 min. After 60 minutes, the irritation was re-observed to assess long-term irritation. Meanwhile, the positive and negative controls for the HET-CAM assay were sodium lauryl sulfate (1%, w/v) and normal saline solution (0.9%, w/v), respectively. Meanwhile, the HET-CAM assay's positive and negative control was 1% w/v sodium lauryl sulfate and 0.9% w/v normal saline solution, respectively. The following equation calculated the irritation score (IS):
Irritation score (IS) = [(301 − c(h)/300 × 5] + [(301 − c(l))/300 × 7] + [(301 − c(c))/300 × 9],
where the time when first vascular hemorrhage, first vascular lysis, and the first vascular coagulation was detected of c(h), c(l), and c(c), respectively. The irritation score (IS) was then assessed on a scale of 0.0 to 0.9 for no irritation, 1.0 to 4.9 for mild irritation, 5.0 to 8.9 for moderate irritation, and 9 to 21 for severe irritation. (Freire et al., 2015).
Statistical analysis
The data was shown as mean and standard deviation (SD). Differences among essential oil were analyzed by one-way ANOVA using GraphPad Prism program version 8.0.2, then Tukey's post-hoc analysis. With P < 0.05, differences were deemed significant.
RESULTS
H. coronarium essential oil
The essential oil was successfully extracted from the H. coronarium rhizome through a hydrodistillation process, yielding 0.25% w/w based on the dried H. coronarium powder. The oil was light yellowish liquid with a characteristic odor of H. coronarium rhizome.
Antioxidant activities of essential oils
Antioxidant activities are essential for counteracting oxidative stress by neutralizing excess reactive oxygen species (ROS) and protecting cells from damage (He et al., 2017). Since oxidative stress is characterized by an imbalance between ROS and the defense mechanisms of antioxidants, the prominence of ROS accelerates oxidative damage, leading to skin aging and contributing to changes in skin tone uniformity, the development of wrinkles, sagging, dryness, and roughness (Chaiyana et al., 2023). Therefore, antioxidants are recognized as an effective strategy to counteract the adverse effects of skin aging. The antioxidant activities of H. coronarium extracts were determined by four4 different methods. The DPPH and ABTS assays were applied to evaluate the potential of extracts to scavenge free radicals, which are associated with the electron transfer process (Abramovič et al., 2018). Besides, the ability of the extracts to reduce ferric ion (Fe3+) to ferrous ion (Fe2+) was demonstrated using a FRAP test (Benzie et al., 1996). In addition, the prevention of lipid peroxidation by the extracts was investigated using the ferric thiocyanate method (Gülçin et al.,2012). Antioxidant activities of each essential oil are shown in Figure 1. According to the findings, C. longa, C. caesia, and H. Coronarium oil showed promising antioxidant properties through their ability to reduce ferric and scavenge ABTS radicals. The EC1 values of C. longa and C. caesia oil were significantly higher (0.84 ± 0.01 and 0.82 ± 0.03 μg FeSO4 per μg extract, respectively) compared to H. coronarium oil (EC1 = 0.65 ± 0.01 μg FeSO4 per μg extract), indicating their superior ferric-reducing ability (P < 0.05). Notably, the ferric-reducing capacity of all essential oils surpassed that of ascorbic acid (EC1 = 0.43 ± 0.03 μg FeSO4 per μg extract), emphasizing their potential as potent antioxidants (P < 0.05). On the other hand, the ABTS radical scavenging activity of C. longa, C. caesia, and H. coronarium oils, with TEAC values of 1.4 ± 0.2, 1.2 ± 0.1, and 1.1 ± 0.1 µg Trolox/µg extract, respectively, approached that of ascorbic acid, with a TEAC value of 2.2 ± 0.1 µg Trolox/µg extract. Still, they were only negligiblymoderately effective at scavenging DPPH radicals and inhibiting lipid peroxidation. While ascorbic acid displayed the highest DPPH radical scavenging activity (96.6 ± 0.3%), the essential oils showed lower activities of 5.9 ± 1.8%, 5.8 ± 1.8%, and 13.1 ± 3.4% for C. longa, C. caesia, and H. coronarium, respectively. Regarding lipid peroxidation, ascorbic acid demonstrated the highest inhibition of 77.7 ± 4.2%, while the essential oils showed lower activities of 10.6 ± 1.3%, 0.0 ± 3.7%, and 11.7 ± 0.7% for C. longa, C. caesia, and H. coronarium, respectively. All essential oils exhibited comparable antioxidant activities in all assays tested in the present study. Interestingly, the ferric reducing ability of these essential oils were significantly more potent than that of ascorbic acid (P < 0.05). The findings were remarkable in that all essential oils had considerably lower ABTS and DPPH radical scavenging capabilities than ferric reduction capacity. The chemical nature of the oils is the most plausible explanation, as essential oils are complicated combinations of numerous aromatic molecules. The degree of hydroxylation and conjugation of the bonds has been associated with reducing capacity, whereas conjugated double bonds have been attributed to free radical scavenging ability (Zhao et al., 2023).
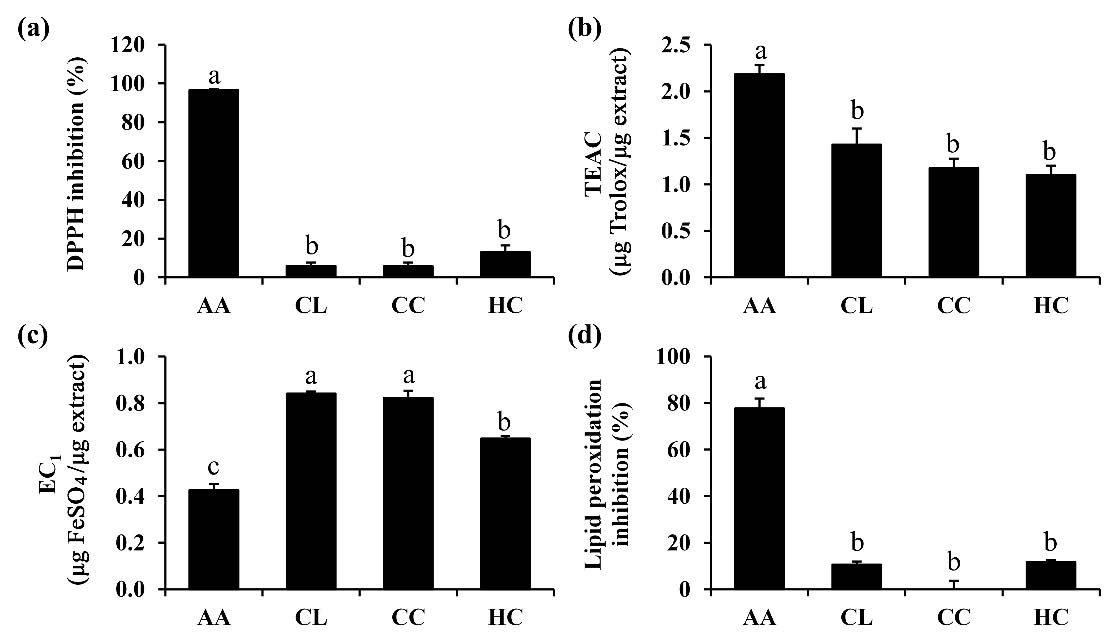
Figure 1. DPPH inhibition (a), Trolox equivalent antioxidant capacity (TEAC) from ABTS assay (b), equivalent concentration (EC1) from FRAP assay (c), and lipid peroxidation inhibition (d) of ascorbic acid (AA), C. longa oil (CL), C. caesia oil (CC), and H. coronarium oil (HC). The final concentration of all tested sample was 0.1 mg/ml. The letter a, b, and c signify statistically significant differences in the antioxidant activities among the essential oils, as determined by Tukey's post-hoc tests following ANOVA analysis. These letters indicate distinct groups with varying levels of antioxidant activity (P < 0.05).
Anti-aging activities of essential oils
The skin, the biggest organ in the body, provides crucial and significant protective functions, which deteriorate over time due to intrinsic and extrinsic aging processes (Low et al., 2021). Decreased extracellular matrix (ECM) is formed when photoaging damages fibroblasts, resulting in visible skin changes, including wrinkles, pigmentation, changes in skin thickness, etc. (Thring et al.,2009). In addition to a decline in ECM production, skin aging also involves ECM breakdown. ECM, which are mainly collagen fibers, elastin fibers, and hyaluronan, are degraded by matrix metalloproteinase-1 (MMP-1), elastase, and hyaluronidase, respectively (Baumann, 2007). The anti-collagenase, anti-elastase, and anti-hyaluronidase activities of each essential oil are shown in Figure 2. C. longa and C. caesia exhibited no significant difference in anti-collagen activity, with the inhibition of 42.8 ± 3.1% and 47.8 ± 10.2 %, respectively. Meanwhile, H. coronarium oil exhibited a low scavenging activity of 13.0 ± 4.1% compared with EGCG of 76.0 ± 1.2%, which are statistically significant differences (P < 0.05). H. coronarium oil and C. longa oil exhibited promising anti-hyaluronidase activities with inhibition of 83.6 ± 1.0% and 82.9 ± 2.2%, respectively, which were comparable to that of EGCG (100.0 ± 0.0%). However, the anti-elastase activities of all essential oil were considered negligible, the C. longa were suggested for further use as an anti-aging extract due to their potent collagenase and anti-hyaluronidase activities.
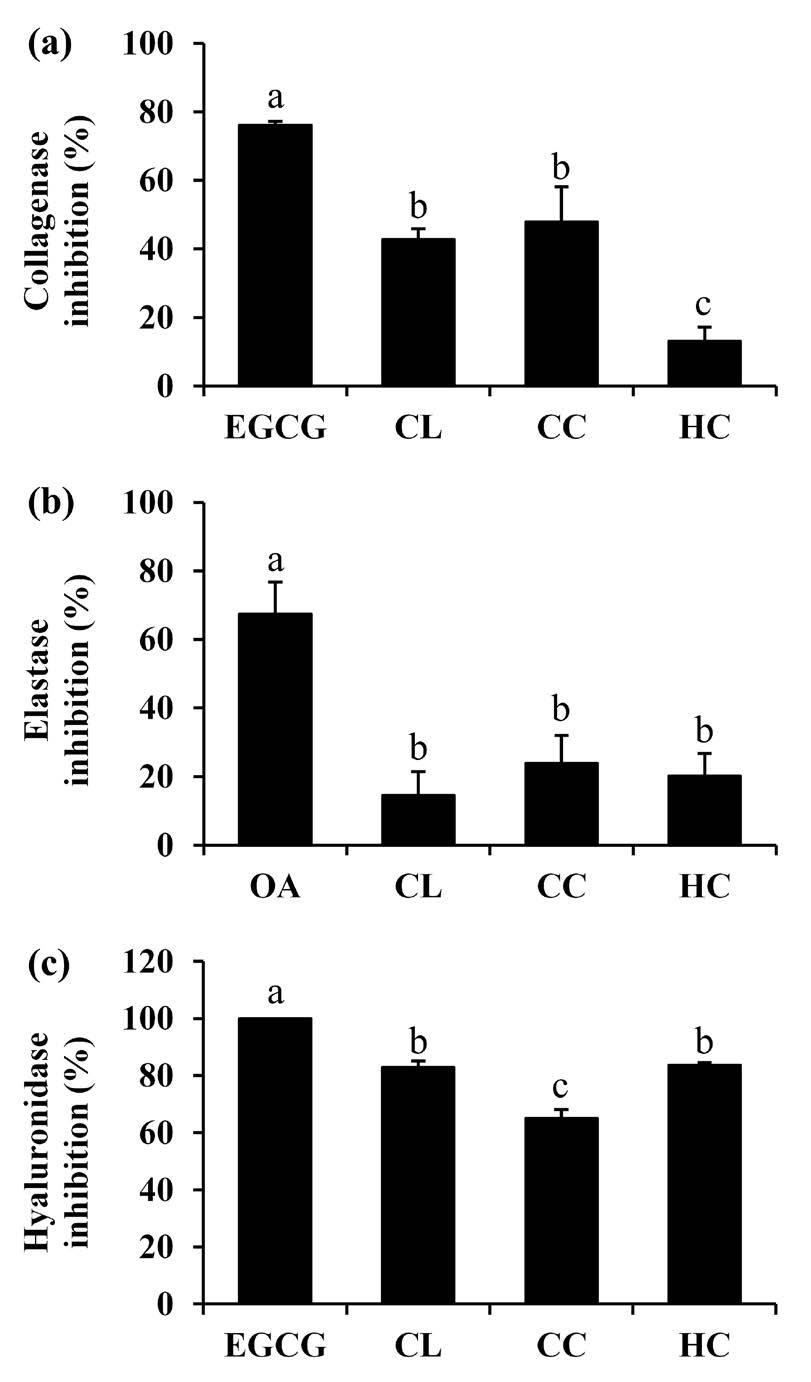
Figure 2. Collagenase inhibition (a), elastase inhibition (b), and hyaluronidase inhibition (c) of epigallocatechin gallate (EGCG), oleanolic acid (OA), C. longa oil (CL), C. caesia oil (CC), and H. coronarium oil (HC). The final concentration of all tested sample was 0.1 mg/ml. The letter a, b, and c signify statistically significant differences in the anti-aginganti-ageing activities among the essential oils, as determined by Tukey's post-hoc tests following ANOVA analysis. These letters indicate distinct groups with varying levels of anti-aginganti-ageing activity (P < 0.05).
Anti-tyrosinase activities of essential oils
Various skin conditions, such as sun melanosis, ephelides, melasma, senile lentigo, and post-inflammatory hyperpigmentation, involve excessive melanin synthesis and accumulation (Ortonne et al., 2008). Tyrosinase is a crucial enzyme in the first two stages of melanin production since it catalyzes the hydroxylation of L-tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and the oxidation of DOPA to dopaquinone (Di et al.,2016). Inhibition of tyrosinase would be another strategy to diminish melanin production, which would result in skin whitening. Anti-tyrosinase activities of each essential oil are shown in Figure 3. Final concentration of each essential oil was 0.1 mg/mlmL/min. H. coronarium oil exhibited the promising anti-tyrosinase activities, with the inhibition of 98.4 ± 5.5% and 30.2 ± 1.7%, when the substrate were L-tyrosine and L-DOPA, respectively. Interestingly, it was highlighted that at the same final concentration of 0.1 mg/ml, the anti-tyrosinase activity of H. coronarium oil was stronger than that of kojic acid (61.2 ± 2.1%), a well-known whitening agent, when the substrate was L-tyrosine. On the other hand, C. caesia and C. longa cannot inhibit tyrosinase enzyme. Therefore, H. coronarium oil was suggested for further use as an anti-tyrosinase.
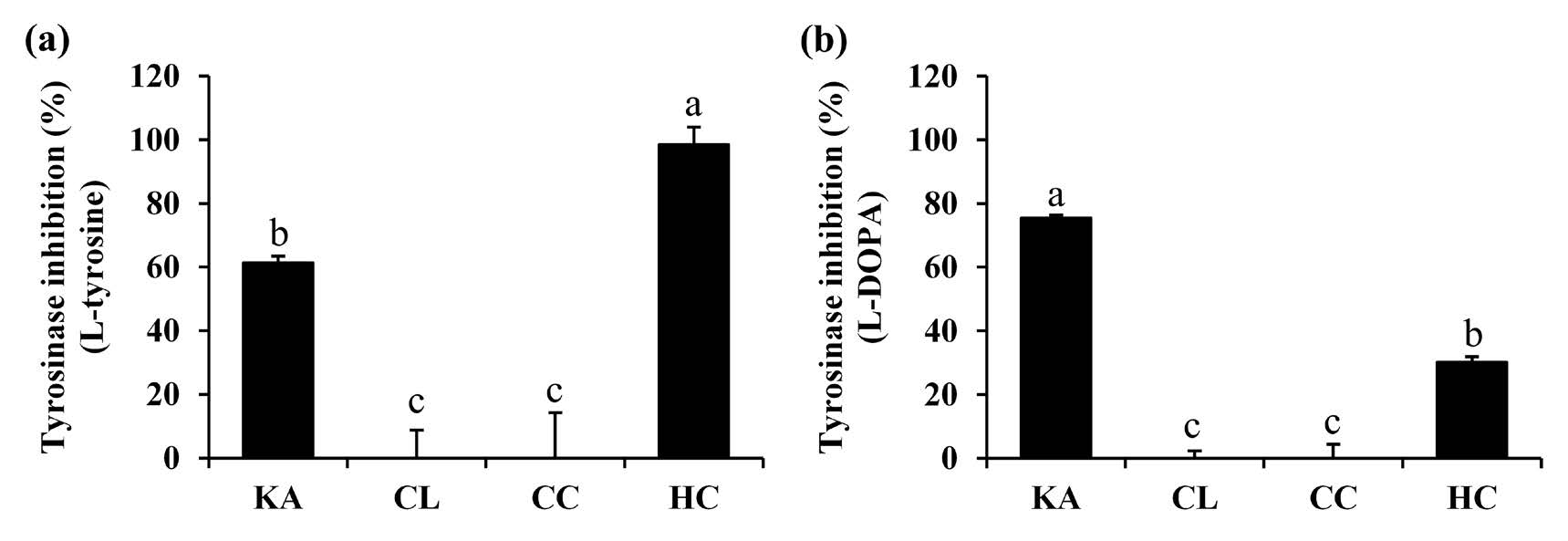
Figure 3. Tyrosinase inhibition (L-Tyrosine) (a), Tyrosinase inhibition (L-DOPA) (b) of kojic acid (KA), C. longa oil (CL), C. caesia oil (CC), and H. coronarium oil (HC). The final concentration of all tested sample was 0.1 mg/ml. The letter a, b, and c signify statistically significant differences in the anti-tyrosinase activities among the essential oils, as determined by Tukey's post-hoc tests following ANOVA analysis. These letters indicate distinct groups with varying levels of anti-tyrosinase activity (P < 0.05).
In vitro irritation properties of essential oils
The irritation test was another crucial evaluation to confirm the safety of topical products. The HET-CAM test has been extensively applied as an appropriate in vitro test throughout the global cosmetics industry during the last decade (Steiling et al., 1999). HET-CAM test was hence used in the present study to investigate the irritation effects of H. coronarium extracts. The data as shown in Table 1 noted that a positive control, 1% w/v SLS solution, causes severe irritation with an irritation score of 11.6 ± 0.0. The irritation signs of hemorrhage and vascular lysis were detected within 5 min of exposure. It was consistent with the previous study, which found that 1% w/v SLS solution caused severe irritation with an IS of 11.94 ± 0.01 (Somwongin et al., 2023). On other hand, the negative control, 0.9% w/v NaCl solution, showed non-irritation.
In the present study all the H. coronarium extracts were dissolved in 10% v/v dimethyl sulfoxide (DMSO) in olive oil at a concentration of 5 mg/ml. The results as shown in Table 1 noted that all extracts induced irritations in the HET-CAM test. The likely explanation could be due to the irritation potency of DMSO. Even at the concentration of 10% v/v DMSO in olive oil, it exhibited mild irritation with the IS of 4.8 ± 0.0. The results were in line with the previous study, which reported that more than 1% of DMSO are toxic for most mammalian cell types (Singh et al., 2017). However, all essential oils had the potential to be irritating since the essential oils exhibited a higher level of irritation compared to the vesicle control. This suggests that these essential oils possess irritant properties, potentially causing irritation or adverse reactions when applied. After 60 min of exposure, C. longa oil caused severe irritation, including bleeding, coagulation, and vascular lysis. Other irritation tests, such as the human patch test, were suggested to confirm the safety of C. longa, C. caesia, and H. Coronarium oil.
Table 1. Irritation score (IS) of essential oils.
|
Sample |
IS |
Irritation level |
|
Positive control |
11.6 ± 0.0a |
Severe irritation |
|
Negative control |
0.0 ± 0.0c |
No irritation |
|
Vehicle control |
4.8 ± 0.0b |
Mild irritation |
|
H. coronarium oil |
5.8 ± 1.4b |
Moderate irritation |
|
C. caesia oil |
8.8 ± 2.8a,b |
Moderate irritation |
|
C. longa oil |
10.1 ± 0.5a |
Severe irritation |
NOTE: Positive control: 1% w/v SLS solution; Negative control: 0.9% w/v NaCl aqueous solution; Vehicle control: 10% v/v DMSO in olive oil; The letter a, b, and c denote significant differences among the irritation of each essential oils (P< 0.05)
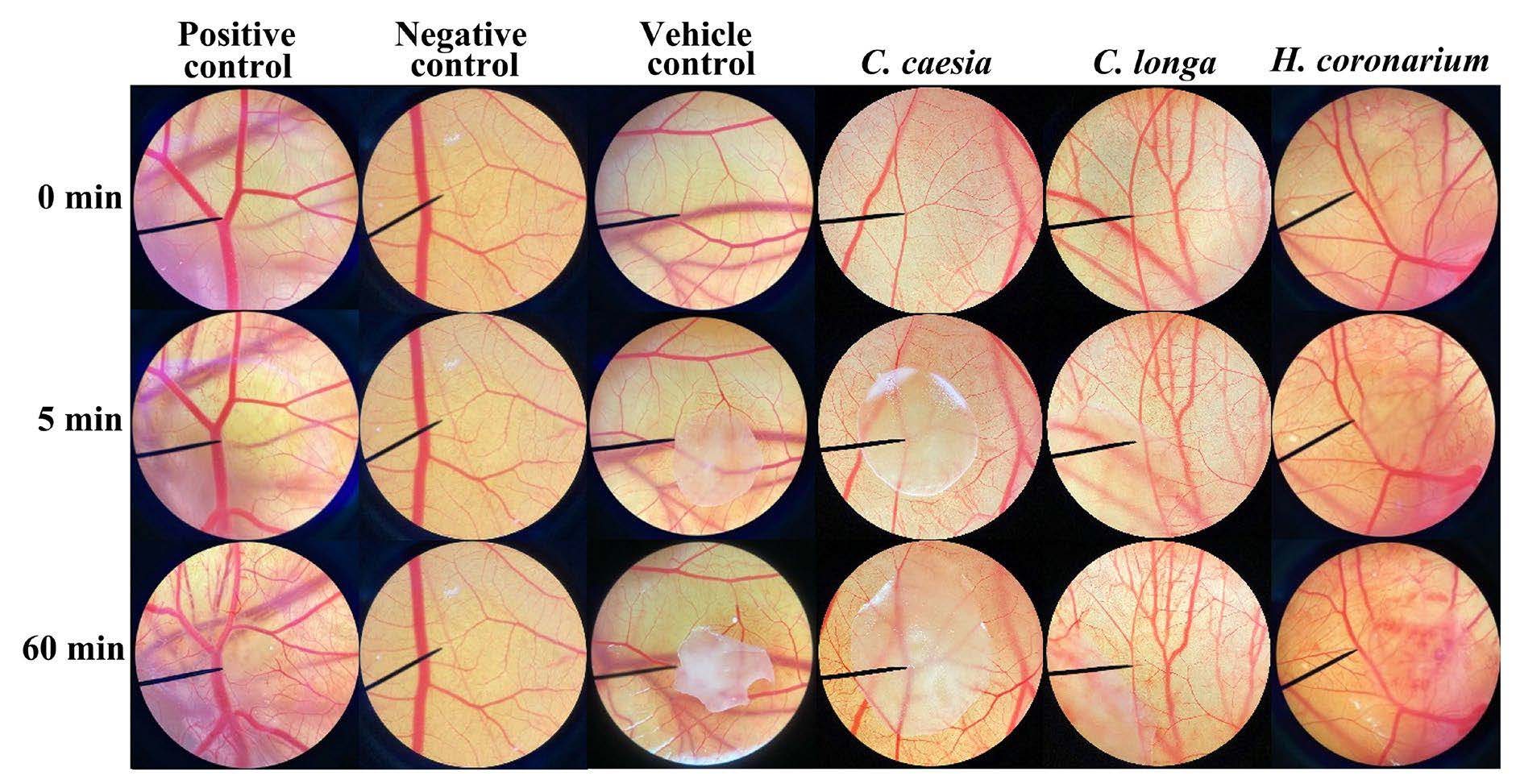
Figure 4. Chorioallantoic membrane after being exposed to a positive control, negative control, vehicle control, C. caesia oil, C. longa, and H. coronarium at 0, 5, and 60 min.
DISCUSSION
Among three different essential oils from the rhizomes of plants in the Zingiberaceae family, H. coronarium oil were interesting due to their potential in anti-hyaluronidase and anti-tyrosinase activities. H. coronarium oil exhibited remarkable anti-tyrosinase activity, demonstrating an inhibitory effect of 98.4 ± 5.5% when L-tyrosine was utilized as the substrate, suggesting its potential as a whitening agent. Additionally, H. coronarium oil exhibited promising anti-hyaluronidase activity of 83.6 ± 1.0%, which was comparable to EGCG, suggesting its potential as an anti-skin aging agent. Therefore, H. coronarium oil was suggested as an attractive natural source for cosmeceutical active ingredients. Further applications in the cosmeceutical area of H. coronarium rhizomes would be a great way to utilize and enhance the value of agricultural waste or byproducts. Since different forms of the extraction resulted in different cosmeceutical effects, H. coronarium oil could be used for further production of concrete, absolute, and essential oil. However, all aromatic products from H. coronarium oil might cause irritation. Therefore, further development of delivery systems for the encapsulation of H. coronarium oil was suggested for the reduction of the irritation. Further investigation into the chemical profiles of each essential oil is highly recommended to ascertain the predominant bioactive constituents, thereby facilitating the establishment of reliable markers for quality control purposes. The potential synergistic effects of combining different essential oils or incorporating them with other cosmeceutical ingredients to enhance their efficacy were also suggested.
CONCLUSION
H. coronarium rhizome oil outperformed kojic acid in inhibiting tyrosinase and exhibited promising anti-aging effects comparable to EGCG, suggesting its potential as an effective addition to skincare products for brightening and anti-skin aging. The findings remarked the significance of utilizing agricultural waste as a principal resource for extracting essential oils, presenting a unique opportunity to repurpose and transform such waste into valuable resources. This approach not only amplifies the inherent value of the discarded material but also unlocks the potential for the creation of value-added products.
ACKNOWLEDGEMENTS
The authors are thankful for H. coronarium materials supported by Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy, Chon Buri and C. caesia oil and C. longa oil received as gifts from Ban Me Thuot Honey Bee JSC, Vietnam. The authors also thank Dr. Saranya Juntrapirom and Mr. Anurak Bunrod from Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy for assistance in the HPLC analysis.
AUTHOR CONTRIBUTIONS
Pattiya Tammasorn was in charge of the experimental design, carried out all of the tests and statistical analysis, as well as drafted the manuscript. Watchara Kanjanakawinkul and Wantida Chaiyana assisted in planning the experiment and validated the experimental process during the research. Wantida Chaiyana confirmed the accuracy of all analysis, as well as drafting, revision, and editing of the manuscript. The final manuscript was read and approved by all authors.
CONFLICT OF INTEREST
All authors confirm that they don't have any interests in conflict.
REFERENCES
Abramovič, H., Grobin, B., Poklar Ulrih, N., and Cigić, B. 2018. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. Journal of Chemistry. 2018: 4608405.
Avanço, G.B., Ferreira, F.D., Bomfim, N.S., PASR, Peralta, R.M., Brugnari, T., Mallmann, C.A., Filho, B.A.A., Mikcha, J.M.G., Machinski, M., Peralta, R.M., Brugnari, T., Mallmann, C.A., et al. 2017. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 73: 806-813.
Baumann, L. 2007. Skin ageing and its treatment. The Journal of Pathology.: A Journal of the Pathological Society of Great Britain and Ireland. 211(2): 241-251.
Benzie, I.F. and Strain, J.J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 239(1): 70-76.
Brem, B., Seger, C., Pacher, T., Hartl, M., Hadacek, F., Hofer, O., Vajrodaya, S., Greger, H. et al. 2004. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry. 65(19): 2719-2729.
da Silva, C.F., Petró, R.R., Almeida, R.N., Cassel, E., and Vargas, R.M. 2021. On the production and release of Hedychium coronarium essential oil from nanoformulations. Industrial Crops and Products. 171: 113984.
Chaiyana, W., Jiamphun, S., Bezuidenhout, S., Yeerong, K., Krueathanasing, N., Thammasorn, P., Jittasai, P., Tanakitvanicharoen, S., Tima, S., and Anuchapreeda, S. 2023. Enhanced cosmeceutical potentials of the oil from Gryllus bimaculatus de Geer by nanoemulsions. International Journal of Nanomedicine. 2023(18): 2955-2972.
Chaiyana, W., Punyoyai, C., Somwongin, S., Leelapornpisid, P., Ingkaninan, K., Waranuch, N., Srivilai, J., Thitipramote, N., Wisuitiprot, W., Schuster, R., and Viernstein, H. 2019. Inhibition of 5 α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. Ex vaucher Vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 9(10): 1105.
da Silva, C.F., Petró, R.R., Almeida, R.N., Cassel, E., and Vargas, R.M. 2021. On the production and release of Hedychium coronarium essential oil from nanoformulations. Industrial Crops and Products. 171: 113984.
Di Petrillo, A., González-Paramás, A.M., Era, B., Medda, R., Pintus, F., Santos-Buelga, C., Fais, A. et al. 2016. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complementary and Alternative Medicine. 16(1): 453.
Fernández-Marín, R., Fernandes, S.C.M., Andrés, M.A., and Labidi, J. 2021. Microwave-assisted extraction of Curcuma longa L. oil: Optimization, chemical structure and composition, antioxidant activity and comparison with conventional soxhlet extraction. Molecules. 26(6): 1516.
Freire, P.L.L., Stamford, T.C.M., Albuquerque, A.J.R., Sampaio, F.C., Cavalcante, H.M.M., Macedo, R.O., Galembeck, A., Flores, M.A.P., Rosenblatt, A., Freire, P.L., Stamford, T.C., Albuquerque, A.J. et al. 2015. Action of silver nanoparticles towards biological systems: Cytotoxicity evaluation using hen's egg test and inhibition of Streptococcus mutans biofilm formation. International Journal of Antimicrobial Agents. 45(2): 183-187.
Gao, L., Liu, N., Huang, B., and Hu, X. 2008. Phylogenetic analysis and genetic mapping of Chinese Hedychium using SRAP markers. Scientia Horticulturae. 117(4): 369-377.
Gülçin, İ. 2012. Antioxidant activity of food constituents: An overview. Archives of Toxicology. 86(3): 345-391.
He, L., He, T., Farrar, S., Ji, L., Liu, T., and Ma, X. 2017. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 44(2): 532-553.
Joy, B., Rajan, A., and Abraham, E. 2007. Antimicrobial activity and chemical composition of essential oil from Hedychium coronarium. Phytotherapy Research. 21(5): 439-443.
Liu, Y., Roy, S.S., Nebie, R.H.C., Zhang, Y., and Nair, M.G. 2013. Functional food quality of Curcuma caesia, Curcuma zedoaria and Curcuma aeruginosa endemic to northeastern India. Plant Foods for Human Nutrition. 68(1): 72-77.
Low, E., Alimohammadiha, G., Smith, L.A., Costello, L.F., Przyborski, S.A., von Zglinicki, T., and Miwa, S. 2021. How good is the evidence that cellular senescence causes skin ageing? Ageing Research Reviews. 71: 101456.
Manosroi, A., Jantrawut, P., Akihisa, T., Manosroi, W., and Manosroi, J. 2010. In vitro anti-aging activities of Terminalia chebula gall extract. Pharmaceutical Biology. 48(4): 469-481.
Matsumoto, F., Idetsuki, H., Harada, K., Nohara, I., and Toyoda, T. 1993. Volatile components of Hedychium coronarium Koenig flowers. Journal of Essential Oil Research. 5(2): 123-133.
Mukunthan, K.S., Anil Kumar, N.V., Balaji, S., and Trupti, N.P. 2014. Analysis of essential oil constituents in rhizome of Curcuma caesia Roxb. from South India. Journal of Essential Oil Bearing Plants. 17(4): 647-651.
Nakatsu, T., Lupo, Jr. A.T., Chinn, Jr. J.W., and Kang, R.K. 2000. Biological activity of essential oils and their constituents. Studies in Natural Products Chemistry. 21: 571-631.
Nema, N.K., Maity, N., Sarkar, B., and Mukherjee, P.K. 2011. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Archives of Dermatological Research. 303: 247-252.
Ortonne, J.P. and Bissett, D.L. 2008. Latest insights into skin hyperpigmentation. Journal of Investigative Dermatology Symposium Proceedings. 13(1): 10-14.
Paw, M., Gogoi, R., Sarma, N., Pandey, S.K., Borah, A., Begum, T., and Lal, M. 2020. Study of antioxidant, anti-inflammatory, genotoxicity, and antimicrobial activities and analysis of different constituents found in rhizome essential oil of Curcuma caesia Roxb., collected from Northeast India. Current Pharmaceutical Biotechnology. 21(5): 403-413.
Raina, V.K., Srivastava, S.K., Jain, N., Ahmad, A., Syamasundar, K.V, and Aggarwal, K.K. 2002. Essential oil composition of Curcuma longa L. cv. Roma from the plains of Northern India. Flavour and Fragrance Journal. 17(2): 99-102.
Raut, J.S. and Karuppayil, S.M. 2014. A status review on the medicinal properties of essential oils. Industrial Crops and Products. 62: 250-264.
Ray, A., Jena, S., Kar, B., Sahoo, A., Panda, P.C., Nayak, S., Mahapatra, N. et al. 2018. Volatile metabolite profiling of ten Hedychium species by gas chromatography mass spectrometry coupled to chemometrics. Industrial Crops and Products. 126: 135-142.
Saeio, K., Chaiyana, W., and Okonogi, S. 2011. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discoveries & Therapeutics. 5(3): 144-149.
Sharma, A., Kumar, V., Mittal, C., Rana, V., Dabral, K., and Parveen, G. 2023. Role of essential oil used pharmaceutical cosmetic product. Journal for Research in Applied Sciences and Biotechnology. 2(3): 147-157.
Singh, M., McKenzie, K., and Ma, X. 2017. Effect of dimethyl sulfoxide on in vitro proliferation of skin fibroblast cells. Journal of Biotechnology Research. 8: 78.
Somwongin, S., Sirilun, S., Chantawannakul, P., Anuchapreeda, S., Yawootti, A, and Chaiyana, W. 2023. Ultrasound-assisted green extraction methods: An approach for cosmeceutical compounds isolation from Macadamia integrifolia pericarp. Ultrasonics Sonochemistry. 92: 106266.
Steiling, W., Bracher, M., Courtellemont, P., and De Silva, O. 1999. The HET–CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicology In Vitro. 13(2): 375-384.
Thring, T.S., Hili, P., and Naughton, D.P. 2009. Anti-collagenase, anti-elastase and antioxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine. 9(1): 111.
Zhao, J., Xu, Z., Gao, P., and Liu, X. 2023. Chemical composition, in vitro antioxidant activities, and inhibitory effects of the acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. essential oil. Biomolecules. 13(7): 1089.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pattiya Tammasorn1, Watchara Kanjanakawinkul2, and Wantida Chaiyana1,*
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy, Phlu Ta Luang, Sattahip, Chon Buri 20180, Thailand.
Corresponding author: Wantida Chaiyana, E-mail: wantida.chaiyana@cmu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 3, 2023;
Revised: February 4, 2024;
Accepted: February 13, 2024;
Published online: February 20, 2024

