
Growth, Hematological Evaluation and Heavy Metal Bioaccumulation in Nile Tilapia (Oreochromis niloticus) from A Municipal Waste Landfill Reservoir
Kannawee Swangneat, Lamyai Neeratanaphan, Kraiwuth Kallawicha, and Bundit Tengjaroensakul*Published Date : February 12, 2024
DOI : https://doi.org/10.12982/NLSC.2024.018
Journal Issues : Number 2, April-June 2024
Abstract The aim of this study was to evaluate the growth performance, mortality rate, and blood profile of Cd-, Pb-and Cr-contaminated Nile tilapia from the Khon Kaen municipal waste landfill reservoir and compare the parameters with those of fish from unpolluted sites. Human bioaccumulation factor (BAF) determination was also performed. Thirty samples of Nile tilapia were taken from the Khon Kaen municipal waste landfill reservoir (CN) and from unpolluted (TN) ponds. Water and soil were collected from these sites, and tilapia muscle, gill, and liver were obtained for heavy metal evaluation. The Cd concentrations in the muscle, gill and liver were 0.003 ± 0.001, 0.006 ± 0.008, and 0.004 ± 0.003 mg/kg; the Pb concentrations were 0.078 ± 0.173, 0.880 ± 1.360 and 1.110 ± 1.862 mg/kg; and the Cr concentrations were 0.067 ± 0.135, 0.572 ± 1.098 and 0.636 ± 1.272 mg/kg, respectively. The total fish length, body weight and survival rate were significantly lower in the TN group (P <0.05), and alanine aminotransferase and creatinine were significantly (P <0.05) higher in the TN group. The hematocrit count of the TN group was also lower than that of the CN group but without a significant difference. The BAF ranged from 0.1-4.3, especially in the gills and liver, which are major heavy metal accumulation organs. Heavy metal contamination in landfill leachate negatively affects fish health and its production.
Keywords: Aquaculture, Nile tilapia, Heavy metal, Pathology, Toxicity
Funding: The authors are grateful for the research funding provided by the Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand.
Citation: Swangneat, K., Neeratanaphan, N., Kallawicha, K., and Tengjaroensakul, B. 2024. Growth, hematological evaluation and heavy metal bioaccumulation in Nile tilapia (Oreochromis niloticus) from a municipal waste landfill reservoir. Natural and Life Sciences Communications. 23(2): e2024018.
INTRODUCTION
Many natural water resources, such as seawater and freshwater, have become polluted by heavy metals (Abiona et al., 2019; Shahjahan et al., 2022). Heavy metals such as cadmium (Cd), lead (Pb) and chromium (Cr) are the heavy metals primarily found in municipal landfills (Madu et al., 2011; Carolin et al., 2017; Thitiyan et al., 2021) that can limit fish growth, survival rates and hematological parameters. For example, heavy metals can elevate alanine aminotransferase (ALT) and serum creatinine (SCr) levels (Phoonaploy et al., 2019; Javed and Usmani, 2019; Tabrez et al., 2021) and lower hematocrit counts (HTCs) (Velma et al., 2011; Parekh et al., 2015; Dos Santos et al., 2019).
Among the heavy metals, Cd, Pb and Cr are ranked 2nd, 7th and 17th for causing serious harm to human health (Agency for Toxic Substances and Disease Registry (ATSDR, 2007). This is because they contaminate soil, water or air before they enter animal and human food chains. Animals and humans are exposed to these heavy metals through the consumption of contaminated food since most heavy metals are absorbed easily through the gastrointestinal tract. Heavy metals are absorbed more than other metals, they have high toxicity, and they are used in many industries, including those producing electronics and agriculture. Over 40% to nearly 100% of waste sites are contaminated with the three heavy metals, these metals remain in environmental systems (except cadmium, less than 10% of which absorbs) (ATSDR, 2007; WHO/FAO, 2015; Jing et al., 2019), and they can be toxic to several body systems. The accumulation of heavy metals in organisms from soil or water that is inhabited or consumed by animals can be measured in an index called bioaccumulation or bioconcentration, which is evaluated by calculating the concentration of heavy metals detected in the tissues of sampled animals per unit of heavy metals in the sampled soil or water inhabited by those living organisms (Oost et al., 2003). This is helpful in monitoring the residues of heavy metals in the tissues of animals living in soil and water environments without directly gathering samples of the animal tissue. The advantage of this method is that it reduces the use of experimental animals and the bioaccumulation index can be used to determine toxicity to animals (Oost et al., 2003; Dhanakumar et al., 2015).
The Khon Kaen province has approximately 800,000 tons of residual waste and is listed 8th in the country and 1st in the northeast region of Thailand in terms of waste. In addition, 1,224 tons of waste is added per day to be disposed. Among this daily waste, there are 4.12 tons of infectious wastes and another 300 kilograms of hazardous wastes (Thai Pollution Control Department, 2022). The waste landfill area of the Khon Kaen Municipality is large and has 8 sewage wells containing fish; these are used as tilapia ponds by some villagers. The fish from these sewage ponds are caught to be consumed or sold to people; tilapia is a major fish species for consumption in Thailand. Recently, Poonaploy et al. (2019), Ruchuwararak et al. (2020) and Neeratanaphan et al. (2020) showed via mathematical models that the major heavy metal contaminants in the sediments, surface water and underground water around the Khon Kaen landfill leachate over an area of 2 km2 were Pb (0.50-5.00 mg/kg), Cr (2.00-5.00 mg/kg), and Cd (0-0.25 mg/kg). The heavy metals were localized with a 2 m in depth from the soil surface and within 500 m from the landfill site. Although the heavy metal concentrations did not exceed the Thai Pollution Control Department standards, there is still a possibility that the heavy metals will be dispersed from the topsoil to deeper layers over the next 20 years, which may affect the environment and human health.
Studies in the literature have reported heavy metal contamination in flora and some aquatic species, e.g., rice (Oryza sativa), snakehead fish (Channa striata), flying barb fish (Esomus metallicus) and frogs (Sylvirana nigrovittata) (Poonaploy, 2016; Neeratanaphan et al., 2017; Neeratanaphan et al., 2020; Ruchuwararak et al., 2020; Ruksachat et al., 2023), but the heavy metal effects on Nile tilapia (Oreochromis niloticus) are still unclear. Therefore, obtaining scientific data to assess the risk of fish consumption from the sewage ponds is important and necessary, particularly data on the adverse effects to human health caused by the consumption of the heavy metal contaminants present in tilapia tissues.
MATERIAL AND METHODS
Experimental sites
Khon Kean City, located in the northeastern of Thailand, which highest produced household waste in northeastern region of Thailand with additional 300 kg of electronic waste daily (Thai Pollution Control Department, 2022). The main landfill waste dumping site is municipal landfill at Kham Bon village, Muang District of Khon Kean Province, Thailand, at GPS location 16059’46.83” N, 102080’49.78” E, 10 km north of Khon Kean City (Figure 1A). There were 3 public reservoirs closed to landfill site where leachate directly contaminated (Poonaploy et al., 2019; Neeratanaphan et al., 2020). The north pond was assigned as experimental site of treatment group (TN). The site for unpolluted control group (CN) was Taphra fish farm, Thaphra, Muang District of Khon Kean Province, Thailand (Figure 1B). This site is located 10 km. south of Khon Kaen City, at GPS location 16334’41.71” N, 10279’69.88” E.
Fish maintenance
A total of 360 Nile tilapia (O. niloticus) were equally divided into control Nile tilapia group (CN) and treatment Nile tilapia group (TN) groups. First 180 Nile tilapia were equally divided and cultured in 3 2.5x8x1 m nylon cages in the Khon Kaen municipal landfill reservoir, Kambon village, Khon Kaen province, for 90 days. Another 180 Nile tilapia were also equally divided into 3 2.5x8x1 m nylon cages in fish farm in Thaphra village, Khon Kaen province in the same duration. The cultured period was from August to the end of October due to Thailand’s rainy season. Commercial feed pellets were given ad libitum twice per day with small size carnivorous fish feeding regime (≥ 32% crude protein, ≥ 4% fat, 33% carbohydrate, ≤ 6% fibers, ≤ 12% moist and others). Sampling sizes were determined using the G-Power statistical analysis program by calculating the effect size from the mean value of heavy metals reported from on-site sampling of contaminated heavy metals in the landfill leachate around Kambon village, Khon Kaen, Thailand, relative to unpolluted sites (Neeratanaphan et al., 2020); the heavy metal contamination amounts from the mentioned report were 1.41 and 0.72 mg/kg, respectively. Hence, the total number of Nile tilapia sampled for heavy metal analysis was 10.
Sampling
Water sampling
Ten water samples for heavy metal and water quality evaluation were randomly taken with a water sampler from 10 locations around the reservoir on the first day of the study for heavy metal evaluation. And first, 30th, 60th and the last (90th) day of the study for water quality evaluation between 09:00 and 11:00 a.m. at a depth of 30 cm below the water surface. An amount of 2 mL of 10% sulfuric acid was added per 200 mL water sample, and the samples were filtered with filter paper and stored at 4°C according to Davies et al. (2005).
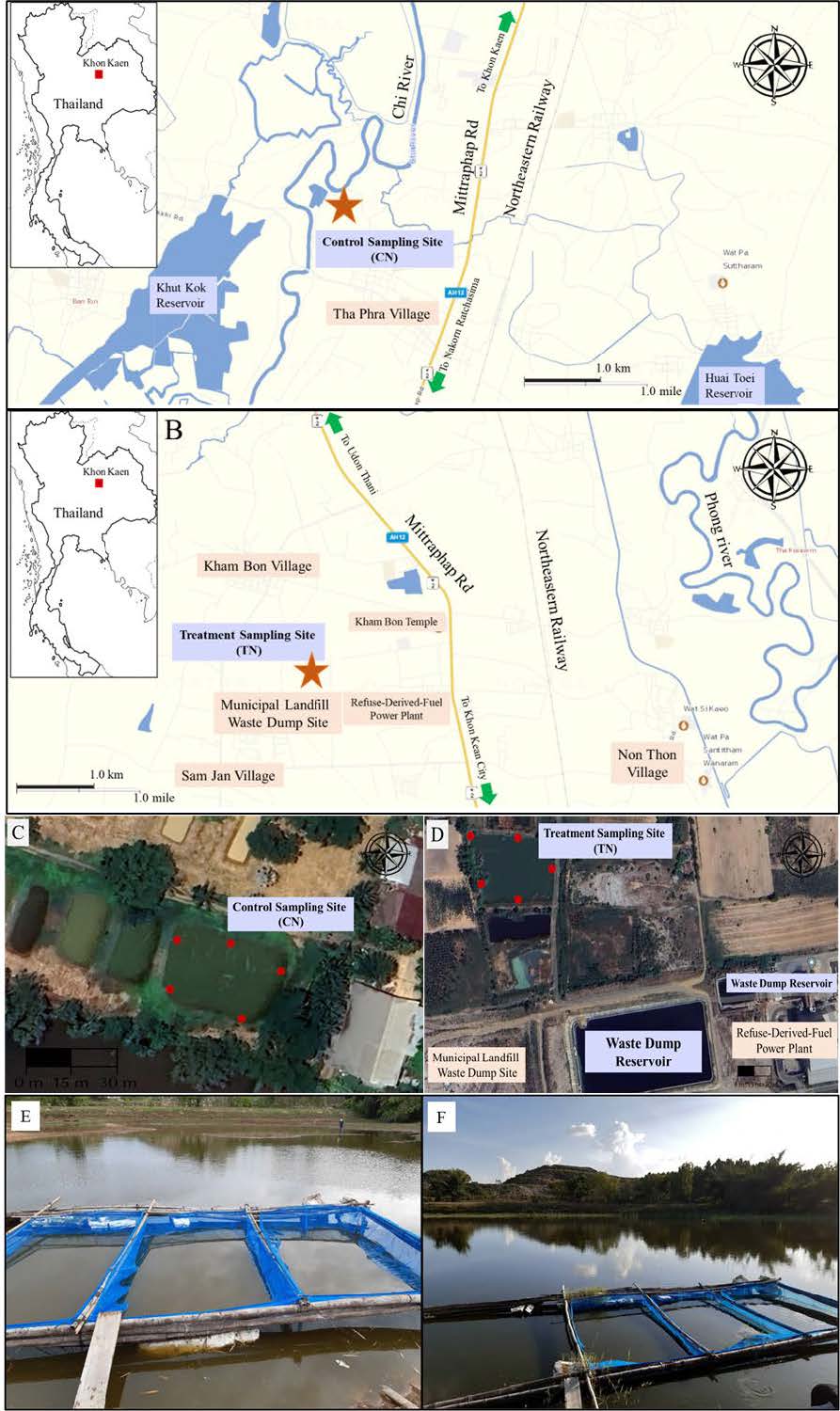
Figure 1. (A) Control sampling site for unpolluted Nile tilapia control group (CN) near Thaphra village, Khon Kaen. (B) Municipal landfill site, Kham Bon village, Muang District, Khon Kaen, where treatment group (TN) were conducted. (C) and (D) Red dots represent sampling area for water and sediments evaluations. (E) and (F) were triplicate fish culture cages in each CN and TN groups, respectively.
Sediment sampling
Ten sediment samples were taken on the first day of the study for heavy metal evaluation. Up to 20 cm in depth were randomly collected from the banks around the reservoir; approximately 200 g was taken from the sites using an Ekman grab sampler. The samples were hot dried at 103°C-105°C and passed through a 2x2 mm wire mesh as described in Pyle et al. (2005). The samples were preserved under dry conditions for further heavy metal analysis.
Fish sampling
Survival rate
All Nile tilapia in each cage were counted in day 0, day 30th, day 60th and end of the experiment (day 90th) for the purpose of monthly survival examination.
Growth performance
The wet weight and body length of 10 Nile tilapia in each group were triplicately measured monthly. External pathological examination was also performed.
Blood and tissue sampling
5 Nile tilapia were triplicately collected from each CN and TN. Blood and internal organs were collected at the beginning (day 0), day 30th and end of the experiment. According to Pollard et al., 2022, those fishes were performed venipuncture via the caudal vasculature for hematological analysis. Then, the individuals were euthanized by an overdose of clove oil (Sigma®, USA) for collection of the liver, gill and muscle. The organs were placed in 10% buffered formalin and preserved at room temperature for 1 week for further heavy metal analysis (Roberts R.J., 2012).
Water quality parameters
According to Roberts et al. (2012), water quality parameters including temperature, pH and DO were measured in TN and CN sites using mobile digital meters at 09.00 a.m., whereas ammonia was measured using titration methods (Table 1).
Table 1. Analytical methods used for measurement of water quality parameters.
|
Water quality parameters |
Analytical methods |
|
Temperature |
Eutech thermometer |
|
pH |
pH meter, Model EcoScan, pH 5 |
|
Dissolved oxygen (DO) |
DO meter model 966, Mettler, Toledo |
|
Ammonia |
Titration method |
Hematological analysis
Blood samples were measured for hematocrit by centrifugation at 2,300 rpm for 5 min. Then, the samples were placed on a hematocrit reader, smeared in a thin film, and stained with Wright-Giemsa for leukocytic differential count and hematological abnormality observation. Blood chemical parameters, ALT and SCr, were determined by Reflovet® Plus (Roche, Basel, Switzerland) (Pollard et al., 2022).
Analysis of heavy metals in water, sediment and fish samples
A total of 2.5 g of each sample was predigested with 3 mL of concentrated nitric acid overnight at 40°C. After cooling, 2 mL of 30% hydrogen peroxide was added. The container was covered, placed in a high-pressure stainless-steel bomb and incubated in an oven at 160°C for 4 h. After the samples cooled, the solutions were diluted with Milli-Q water, and 50 g was transferred into PET bottles. The heavy metal concentrations in each sample were determined using an inductively coupled plasma-optical emission spectrometer (Perkin Elmer PinAAcleTM 900Z) (Ju et al., 2017). The ICP‒OES calibrations and measurements for Pb, Cd and Cr were taken at 220.353, 226.502 and 267.716 nm, respectively. The accuracy of the heavy metal results were evaluated with a certified reference material (CRM) via the 3111C method (APHA 2012). Two aliquots of the CRM with a known amount of heavy metal was spiked into the samples. One spike was analyzed according to the 3111C method, and the other was analyzed with the 3111B method (APHA, 2012).
Bioaccumulation calculation
The bioaccumulation factor (BAF) was analyzed by the equation of Crookes and Brooke (2011) as follows:
BAF = (Ct ÷ Cw) x 100
Where Ct = Concentration of chemical in the tissues, mg/kg
Cw = Concentration of chemical in water; mg/L
Data analysis
Descriptive statistics were used to describe the mean and standard deviation of heavy metal concentrations in the water, sediments and fish. The Mann‒Whitney U test was used to compare the mean heavy metal concentration differences and BAF between the different types of samples. Kaplan-Meiler survival analysis was used for survival analysis by XLSTAT® 2023 program. Water quality, growth, hematological analysis were analyzed using one-way ANOVA. Statistical significance was set to P <0.05. Except for survival analysis, all statistical analysis were analyzed using SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY, USA).
Ethical consideration
This study was approved by the Animal Ethics Committee of Khon Kaen University, with the number No. KKU 107/63.
RESULTS
Water quality
Water quality parameters (temperature, pH, DO, ammonia) in landfill leachate reservoir (TN) and control (CN) sites are reported in Table 2. The temperature range was 29.04-31.06°C, the pH range was 7.4-8.84, DO values were 7.576-8.546 mg/L and ammonia was 0.01-0.02 mg/L in both control and treatment sites.
Table 2. Water quality parameters (temperature, pH, DO, ammonia) in landfill leachate reservoir (TN) and control (CN) sites.
|
|
Control (CN) |
Treatment (TN) |
Standard* |
||||||
|
Day0 |
Day30 |
Day60 |
Day90 |
Day0 |
Day30 |
Day60 |
Day90 |
|
|
|
Mean temperature |
30.32 ± 0.46 |
29.66 ± 0.72 |
29.56 |
29.04 ± 0.99 |
31.06 ± 0.59 |
30.34 ± 0.56 |
29.4 |
29.14 |
23-32 |
|
(°C) |
8.84 ± 0.11* |
7.96 ± 0.40 |
7.62 ± 0.19 |
7.52 ± 0.23 |
8.22 ± 0.26 |
7.52 ± 0.29 |
7.56 ± 0.21 |
7.4 ± 0.27 |
6.5-8.5 |
|
Mean pH |
7.576 ± 0.28 |
8.034 ± 0.15 |
8.542 |
8.366 ± 0.21 |
8.496 ± 0.27 |
8.186 ± 0.15 |
8.4 |
8.546 |
≥4 |
|
Mean DO mg/L |
0.01 |
0.014 ± 0.03 |
0.02 |
0.01 |
0.02 |
0.012 ± 0.01 |
0.02 |
0.01 |
≤0.02 |
Remark (*): Good aquaculture practice for tilapia farm (National Bureau of Agricultural Commodity and Food Standards, 2013)
Cd, Pb and Cr concentrations in the water and sediment of the municipal landfill reservoir
The concentrations of Cd, Pb, and Cr in the sediments of the Khon Kaen municipal landfill reservoir ranged from 16.082-92.096 mg/kg, as described in Table 3, and none of the values exceeded the contamination standard of the Ministry of Industry of Thailand, as shown in Table 4. The concentrations of heavy metals in water ranged from 0.006-0.041 mg/mL, and all samples (100%) exceeded the contamination standards for all heavy metals (Cd, Pb and Cr). The mean concentrations of Cd, Pb and Cr in the sediments were 20.474 ± 3.616, 86.663 ± 4.321 and 49.767 ± 6.308 mg/kg, respectively. The mean concentrations of Cd, Pb and Cr in the water were 0.009 ± 0.003, 0.032 ± 0.004 and 0.017 ± 0.004 mg/L, respectively. The concentrations of all metals in the water exceeded the Thai quality standard (MOI). This also indicates that among the metals, Pb concentration was highest in both the sediment and water in the Khon Kaen municipal landfill reservoir.
Table 3. Concentration of Cd, Pb and Cr in the water and sediments of unpolluted site (CN) and municipal landfill reservoir (TN).
|
Group |
Samples (n=10) |
water (mg/L) |
sediment (mg/kg) |
||
|
Mean ± SD |
Min-Max |
Mean ± SD |
Min-Max |
||
|
CN
|
Cd Pb Cr |
0* 0* 0.022 ± 0.001* |
- - 0.012-0.025 |
0* 0** 0.136 ± 0.004* |
- - 0.092-0.244 |
|
|
Cd |
0.009 ± 0.003* |
0.006-0.015 |
20.474 ± 3.616* |
16.082-25.936 |
|
TN |
Pb |
0.032 ± 0.004* |
0.028-0.041 |
86.663 ± 4.321* |
78.984-92.096 |
|
|
Cr |
0.017 ± 0.004* |
0.011-0.021 |
49.767 ± 6.308* |
40.985-60.016 |
Note: * significant difference in the same heavy metal (P<0.05)
Table 4. Environmental regulatory limit for heavy metal contamination in sediment and water (Ministry of Industry of Thailand, MOI, 2016).
|
Heavy metals |
Sediment standard (mg/kg) |
Water standard (mg/L) |
Heavy metal concentration in TN group |
Percent of samples that exceed the limit |
|
Cd |
37 mg/kg |
≤ 0.01 mg/L (MOI) |
16.082-25.936 mg/kg (Sediment) 5.586-15.256 mg/L (Water) |
Below limit (Sediment) 100 (Water) |
|
Pb |
400 mg/kg (MOI) |
≤ 0.05 mg/L (MOI) |
78.984-92.096 mg/kg (Sediment)27.560-40.838 mg/l (Water) |
Below limit (Sediment) 100(Water) |
|
Cr |
300 mg/kg |
≤ 0.05 mg/L (MOI) |
40.985-60.016 mg/kg (Sediment) 10.520-21.096 mg/l (Water) |
Below limit (Sediment) 100 (Water) |
Cd, Pb and Cr concentrations in Nile tilapia
Three parts of Nile tilapia, i.e., muscle, gill and liver, were sampled for heavy metal concentration analysis. The mean concentrations (mean ± SD, range) of Cd in the muscle, gill and liver (n=30) were 0.040 ± 0.102 (0.003-0.528), 0.345 ± 0.834 (0.003-4.106) and 0.391 ± 1.078 (0.003-5.726) mg/kg, respectively. The values were 0.078 ± 0.173 (0.010-0.826), 0.880 ± 1.360 (0.002-4.456), and 1.110 ± 1.862 (0.010-6.766) mg/kg for Pb, respectively, and 0.067 ± 0.135 (0.010-0.562), 0.572 ± 1.098 (0.010-4.802), and 0.636 ± 1.272 (0.010-5.022) mg/kg for Cr, respectively (Table 3). Therefore, there were showed significant differences in all heavy metal concentrations in the muscle, gill, and liver tissue (P <0.05). It was also found that these heavy metals accumulated mainly in the liver and gills but less in the muscles.
According to our results (Table 5), 13.33% of the total samples exceeded the standard limit for Cd contamination in food by the Ministry of Public Health of Thailand (MOPH), which set a limit of 0.05 mg/kg (MOPH, 2020).
Table 5. The mean (minimum-maximum) concentrations of Cd, Pb and Cr in the muscle, gill and liver of Nile tilapia cultured in a landfill reservoir compared with the Ministry of Public Health (MOPH) standard for heavy metals in fish (mg/kg).
|
Heavy metal
|
Muscle |
Gill |
Liver |
Samples that exceed the limits (%) |
|||
|
Mean ± SD (mg/kg) |
Median (Min-Max) |
Mean ± SD (mg/kg) |
Median (Min-Max) |
Mean ± SD (mg/kg) |
Median (Min-Max) |
||
|
Cd |
0.040 ± 0.102 |
0.004 (0.003-0.528) |
0.354 ± 0.834* |
0.029 (0.003-4.106) |
0.391 ± 1.078 |
0.014 (0.003-5.726) |
13.33
|
|
Standard |
≤ 0.05 |
||||||
|
Pb |
0.078 ± 0.173a |
0.010 (0.010-0.826) |
0.880 ± 1.360*a |
0.137 (0.002-4.456) |
1.110 ± 1.862a |
0.010 (0.010-6.766) |
0
|
|
Standard |
≤ 1 mg/kg |
||||||
|
Cr |
0.067 ± 0.135 |
0.010 |
0.572 ± 1.098* |
0.097 (0.010-4.802) |
0.636 ± 1.272 |
0.047 (0.010-5.022) |
0
|
Note: * significant difference in the same heavy metal (P <0.05), a significant difference in different heavy metals in the same tissue (P <0.05)
Growth
Length and weight
The total length and weight of TN Nile tilapia were significantly lower than those of CN Nile tilapia (P <0.05). The CN and TN lengths from the beginning to the end of the experiment were 9.68 cm. to 23.03 cm. and 9.50 cm. to 19.02 cm, respectively. The weights of CN and TN from the beginning to the end of the experiment were 26.28 g to 264.61 g and 27.798 cm to 154.46 cm, respectively (Figure 2).
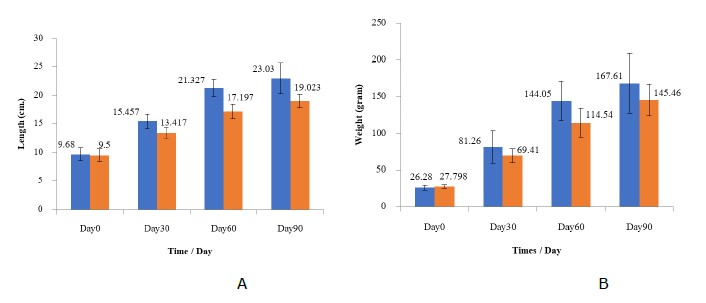
Figure 2. Mean growth performance; A: length (cm) and B: weight of Nile tilapia in the CN (blue) and TN (orange) groups at the beginning of the study (Day 0), Day 30, Day 60 and end of the study (Day 90)
Survival rate
The survival rates and Kaplan-Meier survival analysis of CN Nile tilapia from Day 0 to Day 90 were 100% to 93.89%, whereas those of TN were 100% to 79.44%, with significant differences (P <0.05) from Day 30 until the last day after culture for both parameters (Figure 3). Kaplan-Meier survival analysis described significant different between CN and TN and the mean survival time of each group were 88.5 and 86.861 months, respectively.
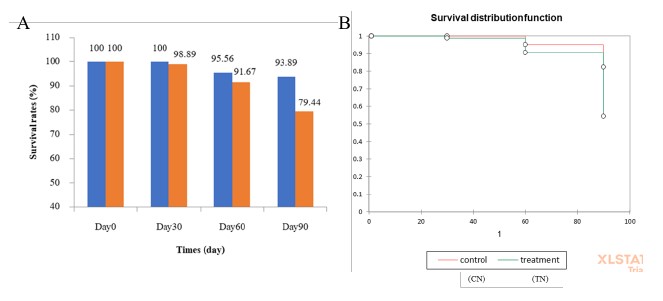
Figure 3. (A) Survival rates (%) of Nile tilapia in the CN (blue) and TN (orange) groups at the beginning of the study (Day 0), Day 30, Day 60 and at the end of the study (Day 90). (B) Kaplan–Meier analysis of CN (──) and TN (──) groups.
Table 6. COX survival analysis in CN and TN groups.
|
Group |
Mean survival time (days) |
95% CI |
P-value for |
|
CN |
88.5 |
86.713-90.287 |
0.015 |
|
TN |
86.861 |
84.371-89.351 |
Hematological analysis
Hematocrit (HTC)
The mean HTC (%) of Nile tilapia in the CN and TN groups at the beginning of the study (Day 0), Day 30 and at the end of the study (Day 90) (Figure 4.) were described as follows: CN showed values of 33.75, 34.42 and 38.67%; and TN showed values of 34.92, 29.83 and 37.37%. The TN group had a lower HTC than the control group, but the difference was not significant (P <0.05).
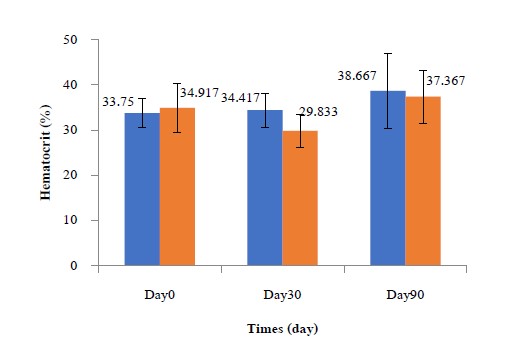
Figure 4. Mean hematocrit count (HTC) (%) of Nile tilapia in the CN and TN groups at the beginning of the study (Day 0), Day 30, Day 60 and at the end of the study (Day 90).
Serum creatinine (SCr)
The mean SCr concentrations in both the CN and TN groups are shown in Table 7 and were found to have ranges of 0.068 and 0.127. The leachate-treated group (TN) showed a higher creatinine concentration without a significant difference (P < 0.05).
Alanine aminotransferase (ALT)
The mean ALT concentrations in both the CN and TN groups are shown in Table 7 and were found to have ranges of CN = 16.583 g/dL and TN = 73.750 g/dL. The mean creatinine concentrations in the current study were significantly different (P <0.05) and were higher in the TN group.
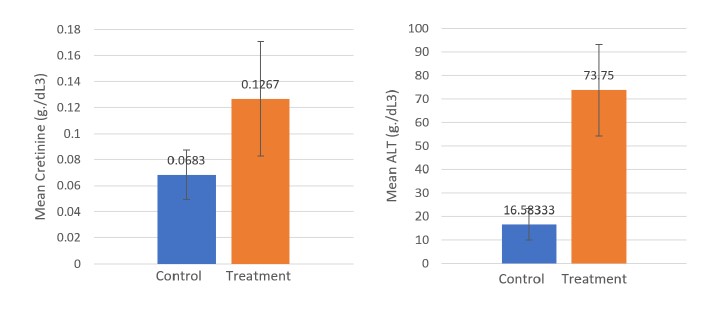
Figure 5. Graphs show Mean creatinine concentration (SCr) and mean ALT concentration (g/dl3) of O. niloticus of CN and TN groups at the end of the study (day 90)
Table 7. Mean SCr concentration and mean ALT concentration (g/dL) of Nile tilapia in the CN and TN groups at the end of the study (Day 90).
|
Group |
Mean SCr (g/dL) |
SD |
Mean ALT (g/dL) |
SD |
|
CN |
0.0683 |
± 0.019 |
16.5833 |
± 6.653 |
|
TN |
0.1267 |
± 0.044 |
73.75 |
± 19.358 |
|
P value |
0.098 |
|
0.000 |
|
Bioaccumulation of Cd, Pb and Cr
Bioaccumulation Cd, Pb and Cr in Nile tilapia compared with Cd, Pb and Cr concentrations in the sediment
The mean bioaccumulation of Cd, Pb and Cr compared with Cd, Pb and Cr concentrations in sediment was 0.1-1.9 (min. 0.9 - max. 27.9) (Table 8). The bioaccumulation of Cd was greater than or equal to that of Pb and Cr. However, the bioaccumulation of Cd was significantly higher than that of Cr (P <0.01). Moreover, heavy metal bioaccumulation was higher in the gill and liver tissue than in the muscle (P <0.05).
Bioaccumulation of Cd, Pb and Cr in Nile tilapia compared with Cd, Pb and Cr concentrations in the water
The mean bioaccumulation of Cd, Pb and Cr compared with Cd, Pb and Cr concentrations in water was 0.2-4.3 (min. 0.2- max. 64) (Table 8).
Table 8. Bioaccumulation of Cd, Pb and Cr in Nile tilapia compared with Cd, Pb and Cr concentrations in the sediment and water.
|
Heavy Metals/ Organs |
Comparing source
|
Cd |
Pb |
Cr |
||||
|
Mean±SD |
Min-Max |
Mean±SD |
Min-Max |
Mean± SD |
Min-Max |
|||
|
Muscle |
|
0.22 ± 0.11a |
0.01-2.58 |
0.14 ± 0.30 |
0.02-1.18 |
0.18 ± 0.17 |
0.01 - 0.91 |
|
|
Gill |
Sediment |
1.69 ± 1.41a |
0.01-20.10 |
1.31 ± 1.08 |
2.00-9.60 |
1.20 ± 1.57 |
0.01 - 5.14 |
|
|
Liver |
|
1.89 ± 1.23*a |
0.00-27.90 |
1.39 ± 1.56* |
2.41-10.09 |
1.28 ± 0.42* |
0.01 - 7.84 |
|
|
Muscle |
|
0.41 ± 0.79 |
3.31-30.06 |
0.43 ± 0.53 |
0.02-5.90 |
0.42 ± 0.5 |
0.03 - 2.61 |
|
|
Gill |
Water |
3.34 ± 2.45 |
0.06-28.30 |
3.40 ± 1.93 |
0.01-50.00 |
2.78 ± 1.24 |
0.006 - 13.8 |
|
|
Liver |
|
3.82 ± 2.54 |
0.06-29.66 |
4.29 ± 1.22 |
0.03-64.00 |
3.41 ± 2.64 |
0.03 - 20.91 |
|
Note: * significant difference in the same heavy metal (P <0.05), a significant difference in different heavy metals in the same tissues (P <0.05)
DISCUSSION
Water quality
Water quality parameters (temperature, pH, DO, ammonia) in landfill leachate reservoir (TN) and control (CN) sites are reported in Table 2. The temperature range was 29.04–31.06 °C, which are not exceed the maximum recommendation for Nile tilapia aquaculture. FAO (2020) reported that the lower and upper lethal temperatures for Nile tilapia are 11-12 °C and 42 °C, respectively, while the preferred temperature ranges from 31 to 36 °C whereas recommended range for in Thailand is 23-32 °C, which reported by Whangchai et al. (2018).
DO values were 7.576-8.546 mg/L in both control and treatment sites, which still over the minimum requirement of Nile tilapia aquaculture. Popma and Lovshin (1996) and Ebeling et al. (2006) reported that DO is important factor for production for intensive aquaculture systems. Therefore, DO is one of essential needs for Nile tilapia aquaculture, and should be monitored daily or twice per day. Oxygen solubility is depended on water temperature which higher soluble in cooler water (Swann, 1997). El-Sayed (2006) indicated that Nile tilapia are able to tolerance lower DO levels than other cultured species and also withstand DO levels lower than 0.5 mg O2/L.
The pH range was 7.4-8.84 in both control and treatment sites, which still in acceptable range, pH is also fundamental factor for aquaculture. Nile tilapia can be cultured in pH ranged from 4-11 (Mustapha and Atolagbe, 2018). The best growth in tilapia is seen at pH levels which are neutral or slightly alkaline which are 7-8 (Popma and Lovshin 1996, El-Sherif and El-Feky, 2009. Acidic aquaculture water effect growth performance and productivities of Nile tilapia (Popma and Lovshin, 1996). Some heavy metal e.g., Cr toxicity depends on the pH of the water in which the fish resides. Rainbow trout demonstrating variable susceptibility to some heavy metals at pH values of 7.8 and 6.5 (Putte et al., 1981). Although, further investigations by the same authors revealed histologic changes at both pH values, the predominant changes occurred in the gills at pH 6.5 (Putte et al., 1981). In another study in young rainbow trout, Cr toxicity was 50-200 times higher at pH 6.4 to 7.4 than at pH 7.8 to 8.0 (Hogendoorn-roozemond et al., 1977).
The ammonia concentration in both CN and TN groups were between 0.01-0.02 mg/L, which were not exceed maximum limitation for aquaculture. Several types of nitrogenous waste, ammonia (NH3) is most dangerous for aquaculture, which are one of the most important factors for aquaculture and can be harmful to most tropical organisms (Crab et al., 2007, Effendi et al., 2015, Wang and Leung, 2015) which can cause several pathological lesions on fish’s organs, risen FCR and can be lethal (LD50) when NH3-N reached to 7.1 mg/L (El-Sherif and El-Feky, 2008). Nitrogen species are final products of protein metabolism which majorly produced from cultured species, uneaten feed etc. Ionized ammonium (NH4+ or NH4+-N or IA-N) and unionized ammonia (NH3 or NH3-N or UIA-N) levels are in equilibrium, being reliant on the pH and temperature of the water (Timmons et al. 2002) which is more toxic to aquatic organisms when decreasing of pH and rising of temperature. These two forms together make up total ammonia nitrogen (TAN). Although, the results of this study indicated that the water quality parameters did not exceed standard values, and DO and TAN values are acceptable for aquatic life. The pH of both sites did not exceed the standard defined by the Thailand Pollution Control Department (2022).
Heavy metals
The current heavy metal contamination results are consistent with the results of Prabpai et al. (2007) and Chuangcham et al. (2008), who reported that Pb was the most abundant heavy metal in the water in the Khon Kaen municipal landfill reservoir but was least abundant in the sediments. This may be because batteries, paint and electronics are major wastes at this site. However, current data on waste disposal at the study site is limited. The concentration of each heavy metal depends on the type of waste disposed, which was not classified in our study. Kamollerd et al., 2019 indicated that Cd, Pb and Cr concentrations in both water and sediments exceeded the MOI standard, and Neeratanaphan et al. (2020) reported that all heavy metals in the water exceeded the MOI standard, but only the Cd concentration in the sediments exceeded the MOI standard. However, the regulatory limit of heavy metal residue indicated by the MOI (2016) is only for residential and agricultural zones and does not include landfill waste reservoirs; thus, heavy metal contamination monitoring should be performed to avoid bioaccumulation in humans. Heavy metal distribution and bioaccumulation from the environment to the food chain can be predicted by mathematical equations or by monitoring and evaluation (Doyi et al., 2018). Previous studies examined the concentrations of Cd, Pb, and Cr in Nile tilapia. Variation in heavy metal concentrations were observed in each study. The differences may have resulted from various factors, e.g., pollution, fish size, fish age, type of heavy metal compound, and evaluation method (El-Sadaawy et al., 2013; Ling et al., 2013; Ju et al., 2020; Somparn et al., 2020).
The current study shows significant differences in all heavy metal concentrations in the muscle, gill, and liver tissue (P <0.05). It was also found that these heavy metals accumulated mainly in the liver and gills but less in the muscles, which in accordance with Neeratanaphan et al. (2020), who indicated that the concentrations of these metals in fish muscles exceeded Thailand’s food quality standards. This may occur because heavy metals are absorbed mainly via the gills and gastrointestinal tract to the liver, and small amounts are absorbed through the skin (Velma et al., 2009). Furthermore, 13.33% of the total samples exceeded the standard limit for Cd contamination in food by the Ministry of Public Health of Thailand (MOPH), which set a limit of 0.05 mg/kg (MOPH, 2020). This is in accordance with Intamat et al. (2017) and Thitiyan et al. (2021), who indicated that Cd, Pb and Cr exceeded the MOPH standard. In contrast, Sriuttha et al. (2017) reported that only Pb exceeded the international limits.
In fish, heavy metals are absorbed via the gills and gastrointestinal tract, and small amounts are absorbed through the skin, which is a phenomenon that is still not well studied (Velma et al., 2009). Heavy metal absorbability depends on the pH, temperature, alkalinity, hardness, concentration of sulfate in the water and the oxidation stage of heavy metals (Soudani, 2010; Aslam and Yousafzai, 2017). Gills represent metal ion exchange sites from water due to the large gill surface area; metals can rapidly diffuse into the gills by penetrating the gill membrane and cytoplasm (Qadir et al., 2011; Dhaneesh et al., 2012). In the liver, metallothioneins (MTT), glutathione (GSH), alpha-tocopherol and selenium (Se) and antioxidant enzyme such as cytochromes (CYP450) and glutathione S-transferase (GST) play first mechanism against heavy metals. Thus, the bioaccumulation of metals in the liver may be linked to the metabolic functions of the liver (Bawuro et al., 2018). The current study revealed that heavy metals are mainly localized in the gill and liver, and minor amounts were present in the muscle. This is in accordance with Annabi et al. (2013), who reported that the highest Cd concentrations were present in the gills rather than other organs. Rajeshkumar and Li (2018) noted that the highest Pb concentration was detected in fish liver. This may be because the gills are the frontline sites for heavy metal uptake from the water into the fish circulatory system, and the liver is the main site of heavy metal metabolism and accumulation in fish. Recently, Neeratanaphan (2017), Poonaploy et al. (2019) and Neeratanaphan et al. (2020) reported that heavy metal bioaccumulation in each organ/tissue was as follows: gill > liver > muscle, which may affect human health due to the internal organ consumption patterns of fish in northeastern Thailand.
Heavy metal contaminants present in water and sediments can be absorbed by organisms. The accumulation of heavy metals in organisms is called bioaccumulation, which can be calculated based on heavy metal concentrations in organisms relative to those in the sediments or water inhabited by the organisms via mathematical equations (Oost et al., 2003). The evaluation of bioaccumulation can be used for the prediction of heavy metal contamination in plant or animal tissue without direct, invasive animal tissue collection, which can reduce the utilization of experimental animals (Oost et al., 2003; Dhanakumar et al., 2015).
Growth
The total length and weight of TN Nile tilapia were significantly lower than those of CN (P <0.05), which are in accordance with previous reports showing that heavy metal overexposure has negative effects on the development and growth of aquatic species such as phytoplankton, zooplankton, and fish (Atici et al., 2010; Bere et al., 2012). Exposure to Cd altered the fish average wet weight and body length and increased FCR (Fazio et al., 2021; Paul et al., 2021). Weight gain, length gain and feed conversion ratio (FCR) declined significantly in many fish exposed to the highest Pb concentration (Javed et al., 2020; Zulfahmi et al., 2021), and the mean body weight of a treated Labeo rohita group gradually decreased significantly from week 1 to week 5 after exposure to 1.4 mg/L lead acetate compared to a control group (P <0.001) due to the disruption of growth hormone production, which ultimately affected growth performance and productivity (Sajjad et al. 2018). Previous reports also support this finding from the current study, e.g., tilapia showed a toxic effect from exposure to more than 4.57 mg Cr6+/L, causing subchronic lesions and retarding the sustainable growth rate (SGR) (Mohamed et al., 2020). Farag et al. (2006) reported that Chinook salmon exposed to increasing Cr concentrations for 5 to 134 days showed changes in both growth and survival rates, and physiological modifications occurred at a concentration of 24 μg/L. In contrast, a report from the National Academy of Science (1974) showed that mammals, e.g., rats, take up more than 5 mg/L Cr. Bioaccumulation was observed, but it caused no changes in growth rate or food intake.
The survival rates of CN comparing with TN by Kaplan-Meier survival analysis were significant higher (P <0.05). TN group was also significantly higher in mean survival time. The survival rate of the TN group decreased gradually but significantly from Day 0 until the end of the culture because the Cr-contaminated landfill leachate altered their survivability via various subchronic effects; for example, a reduction in cell viability and a stimulation of free radical production have been observed in the hepatocytes of goldfish after exposure to 250 μM Cr(VI) (Krumschnabel, 2004). Moreover, a study by Farag et al. (2006) reported that increasing the concentration of Cr from 0.024 to 0.120 mg and 0.054-0.266 mg/L for 105-134 days of exposure significantly affected both the survival and growth rate of Chinook salmon. Physiological alterations have also been observed after exposure to over 120 μg/L Cr. Genetic changes caused by heavy metals has been reported in several studies (Tengjaroenkul et al., 2017; Phunaploy et al., 2019; Neeratanaphan et al., 2020; Suchana et al., 2021), including chromosomal abrasion, alterations in DNA repair processes, DNA damage, mutagenesis, deletion, point mutations, lipid deposition and alterations in lipid peroxidation. Moreover, alterations in the immune system induce pathological changes in various organs, such as the gills and liver. Kidney dysfunction and cardiovascular disorders have also been found in fish exposed to heavy metals (Islam et al., 2020; Sarkar et al., 2021), which could decrease fish survivability (Mohammad et al., 2015; Suchana et al., 2021).
Hematocrit (HTC)
HTC in both CN and TN were not significant different (P <0.05). These findings are in accordance with Abdel-Tawwab et al. (2017) reporting that 8 weeks, 0.5 mg/L Cd-exposed Nile tilapia showed no effect on hematological parameters compared with those unaffected fishes. Moreover, Abdel-Tawwab et al. (2017) and Carvalho et al. (2019) explained that higher temperature (over 32°C) could induce Cd toxicity on hematocrit, meanwhile water temperature in this study was average 29.82°C. Exposure to 5.78 mg/L CdCl for 96 h could cause alteration of HTC in sturgeon (Huso huso) fishes but had no effect to those under 5.78 mg/L CdCl (Valiallahi et al., 2019).
Serum creatinine (SCr)
Urine is the main excretory pathway for heavy metals, which may directly cause kidney damage. Serum Creatinine (SCr) is typical indicators of kidney function (Abdel-Tawwab et al., 2010). In this study, fishes in TN showed higher SCr compared to those in CN without significant difference (P <0.05). These results may be because of too low Cd concentration (0.009 ± 0.003 mg/dL) compared with 1.116 -2.560 mg/L of Cd in previous studies (Almeida et al., 2001; Abdel-Tawwab et al., 2017; Abdelzaher et al., 2022), which revealed the elevation of SCr in Nile tilapia. The action of heavy metal on glomeruli filtration rate and/or Cd may cause pathological changes to the kidney resulting in dysfunction such as rhabdomyolysis, muscular dystrophy, myocardial infarction and acute kidney injury (Oitani et al., 2018).
Alanine aminotransferase (ALT)
Cd and other heavy metal are absorbed via gill and gastrointestinal tract. One of the main target organs is liver (Brungs et al., 1977; Luczynska et al., 2018). Heavy metals play a role in catalyzing the formation of oxidizing agents that cause oxidative tissue damage in macromolecules such as proteins. This is in agreement with previous studies by Soudani et al. (2010) and El-Demerdash et al. (2006), who demonstrated the release of oxidizing agents from the cytoplasm, resulting in damage to fish liver tissue. Liver enzymes such as AST and ALT are wildly used as hepatic damage indicator but ALT is markedly elevated in hepatocyte compared with AST, Thus, ALT is dominantly used for liver injury indication (Mohammad et al., 2015; Suchana et al., 2021). In the current study, The ALT was higher in the TN group comparing with those in CN with significant difference. This may be due to hepatocyte toxicity of Cd. The current results are also supported by Al-Asgah et al. (2015) reported that activity of ALT showed a significant increase (P <0.05) with increasing CdCl2 exposure time and concentrations in Nile tilapia.
Bioaccumulation factor
The bioaccumulation factor in this study corresponded with that in other studies, which highest in liver, gill and muscle, respectively (Soegianto et al., 2012; Rajeshkumar and Li, 2018; Bawuro et al., 2018; Somparn et al., 2020). A number of factors were related to the differences in bioaccumulation results in each region, such as season, sample collection method and the severity of environmental problems. Hence, heavy metal concentrations in the water or sediment are the main drivers of heavy metal bioaccumulation in aquatic species (Soegianto et al., 2012; Rajeshkumar and Li, 2018; Candra et al., 2019).
The bioaccumulation of Cd, Pb and Cr in fish and other aquatic species depends on a number of factors, such as soil and water chemical and biological conditions, severity of heavy metal contamination and the type of aquatic species (Griboff et al., 2018; Strungaru et al., 2018; Ferreira et al., 2019). Furthermore, the differences in the pharmacokinetics of each heavy metal, such as bioavailability, distribution, metabolism, and excretion, also lead to variations in their bioaccumulation.
The bioaccumulation of Cd was greater than or equal to that of As and Pb. However, the bioaccumulation of Cd was significantly higher than that of Cr (P <0.01). Moreover, heavy metal bioaccumulation was higher in the gill and liver tissue than in the muscle (P <0.05). This is in accordance with previous studies; e.g., Thitiyan et al. (2021) reported high BAF in the gill and kidney of silver barb but lower a BAF in the muscle.
In the current study, it was also found that the bioaccumulation of Cd was greater than or equal to that of Cr and greater than that of Pb (Table 4), which corresponded with previous heavy metal bioaccumulation reports. Somparn et al. (2020) stated that the BAFs in Nile tilapia followed the order of Cd > Cr > Pb. The greater bioaccumulation value of Cd may be due to its high absorbability in both the gills and gastrointestinal tract (Niyogi et al., 2008; McGeer et al., 2012). The high binding capacity of Cd with low molecular weight thiols such as metallothionein (MT) and glutathione (GSH), cysteine, and calbindin (Zalups and Ahmed, 2003) as well as albumin, transferrin, ferritin and γ-globulin (Kwong et al., 2011; Sayed et al., 2011; De Smet et al., 2011; Rudneva et al., 2012) also enhances the absorption and distribution of Cd in fish tissue and interferes with Cd detoxification and excretion. Thus, this high tissue accumulation leads to a larger Cd bioaccumulation factor relative to Pb and Cr (Strungaru et al., 2018).
Cr is excreted via the kidney glomerulus but largely reabsorbed in the proximal tubule (Wedeen and Qian, 2001). This result may explain the high amount of Cr detected in fish tissue, which led to a high Cr bioaccumulation factor.
In contrast, the bioaccumulation factor of Pb was the lowest, which may be due to its high accumulation in both the water and sediment. Furthermore, the Pb concentrations were low relative to those of the other heavy metals, especially in the muscle, because the Pb complex is transported via metallothionein and phytochelatin, cysteine-rich proteins, which are water-soluble and are excreted via several mechanisms (Kayaalt et al., 2011; Koh and Lee, 2020) in the kidneys (Radulescu, 2020).
CONCLUSION
In this study, it found that the average heavy metal concentration in soil and water samples exceeded the standards. The heavy metal content in tilapia samples was 13% above the acceptable residual standard, which limited fish growth and exacerbated health conditions. Significantly higher amounts of both ALT and SCr concentrations (P <0.05) were found in polluted fish and showed the effect of heavy metal-contaminated leachate on the fish liver and kidney. Decreases in hematocrit counts in polluted fish showed alterations in the hematological profile caused by heavy metal toxicity. The BAF ranged from 0.1-4.3.
ACKNOWLEDGEMENTS
The authors would like to thank the Research Project on Ecotoxicology, Natural Resources and Environment, Khon Kaen University, Khon Kaen, Thailand.
AUTHOR CONTRIBUTIONS
All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
Abdel-Tawwab, M, and Wafeek, M. 2017. Fluctuations in water temperature affected waterborne cadmium toxicity: Hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus (L.). Aquaculture. 477: 106-111.
Abdel-Tawwab, M, and Wafeek, M. 2010. Response of Nile tilapia, Oreochromis niloticus (L.) to environmental cadmium toxicity during organic selenium supplementation. World aquaculture society. 41(1): 106-114.
Abdelzaher, M.F., Azab, A.M., Authman M.M.N. and Shaban W.M. Effects of Lead and Cadmium Accumulation on Survival and Growth of the Nile Tilapia (Oreochromis niloticus). Egyptian Journal of Aquatic Biology & Fisheries. 26(6): 155-171.
Abiona, O.O., Anifowose, A.J., Awojide, S.H., Adebisi, O.C., Adesina, B.T. and Ipinmoroti, M.O. 2019. Histopathological biomarking changes in the internal organs of tilapia (Oreochromis niloticus) and catfish (Clarias gariepinus) exposed to heavy metals contamination from Dandaru pond, Ibadan. Nigeria. Journal of Taibah University for Science. 13: 903-911.
Al-Asgah, N.A., Abdel-Warith, A.W., Younis, S.M., and Allam, H.Y. 2015. Hematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi Journal of Biological Science. 22(5): 543-50.
Almeida, J. A., Novelli, E.L.B., Dal Pai Silva, M. and Alves, R.J. 2001. Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environmental Pollution. 114: 169-175.
Annabi, A., Said, K. and Messaoudi, I., 2013. Cadmium: Bioaccumulation, Histopathology and Detoxifying Mechanisms in Fish. American Journal of Research Communication. 4(1):60-79.
APHA. 2012. Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation. 22nd edition. Washington, DC, USA. 541.
Aslam, S. and Yousafzai, A. 2017. Chromium toxicity in fish: A review article. Entomology and Zoology Studies. 5(3): 1483-1488.
Atici, T., Obali, O., Altindag, A., Ahiska, S. and Aydin, D. 2010. The accumulation of heavy metals (Cd, Pb, Hg, Cr) and their state in phytoplanktonic algae and zooplanktonic organisms in Beysehir Lake and Mogan Lake, Turkey. African Journal of Biotechnology. 9(4): 475-487.
ATSDR. 2007. Toxicology profiles. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services.
Bawuro, A.A., Voegborlo, R.B. and Adimado, A.A. 2018. Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa State, Nigeria. Environmental and Public Health. 2018: 1-7.
Bere, T., Chia, M.A. and Tundisi, J.G. 2012. Effects of Cr III and Pb on the bioaccumulation and toxicity of Cd in tropical periphyton communities: implications of pulsed metal exposures. Environmental Pollution. 163: 184-191.
Brungs, W.A., McCormick, J.H., Neiheisel, T.W., Spehar and C.E., and Stokes G.N. 1977. Effects of pollution on freshwater fishes. Journal of Water Pollution Control Federation, Washington DC 49: 1425-1493.
Candra, Y., Syaifullah, M., Irawan, B., Putranto, T., Hidayati, D. and Soegianto, A. 2019. Concentrations of metals in mantis shrimp Harpiosquilla harpax (de Haan, 1844) collected from the eastern region of Java Sea Indonesia, and potential risks to human health. Regional Studies in Marine Science. 26: 1-5.
Carolin, F., Ponnusamy, S.K., Saravanan, A. and Naushad, M. 2017. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Environmental Chemical Engineering. 5(3): 2782-2799.
Carvalho, C. and Fernandes, M.N. 2019. Effects of copper toxicity at different pH and temperatures on the in vitro enzyme activity in blood and liver of fish, Prochilodus lineatus. Molecular Biology Reports. 46(5): 4933-4942.
Chuangcham, U., Charusiri, P., Home, W., Lertsirivorakul, R., and Boonsener, M. 2008. Adsorption of heavy metals from landfill leachate in soil: A case study of Kham Bon Landfill, Khon Kaen province, NE Thailand. International Symposia on Geoscience Resources and Environments of Asian Terranes. 501-505.
Crab, R., Avnimelech, Y., Defoirdt, T., Bossier, P. and Verstraete, W., Nitrogen removal techniques in aquaculture for a sustainable production, Aquaculture. 270(1-4): 1-14.
Crookes, M. and Brooke, D.N. 2011. Estimation of fish bioconcentration factor (BCF) from depuration data. SCHO0811BUCE-E-E. Environment Agency, Bristol, UK.
Davies, O.A., Allison, M.E., and Uyi, H.S. 2006. Bioaccumulation of heavy metals in water, sediment and periwinkle (Tympanotonusfuscatusvar radula) from the Elechi Creek, Niger Delta. African Journal of Biotechnology. 5(10): 968-973.
De Smet, H., De Wachter, B., Lobinski, R., and Blust, R. 2001. Dynamics of (Cd, Zn)-metallothioneins in gills, liver and kidney of common carp Cyprinus carpio during cadmium exposure. Aquatic Toxicology. 52(3-4): 269-281.
Dhanakumar, S., Solaraj, G., and Mohanraj, R. 2015. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicology and Environmental Safety. 113: 145-151.
Dhaneesh, V., Gopi, M., Ganeshamurthy, R., Kumar, T.T., and Balasubramanian, T. 2012. Bio-accumulation of metals on reef associated organisms of Lakshadweep Archipelago. Food Chemistry. 131: 985-991.
Dos Santos, C.R., Cavalcante, A.L.M., Hauser-Davis, R.A., Lopes, R.M., and Da Costa Mattos, R.D.C.O. 2019. Effects of sub-lethal and chronic lead concentrations on blood and liver ALA-D activity and hematological parameters in Nile tilapia. Ecotoxicology Environmental Safety. 129: 250-256.
Doyi, I., Essumang, D., Gbeddy, G., Dampare, S., and Saka, D. 2018. Spatial distribution, accumulation and human health risk assessment of heavy metals in soil and groundwater of the Tano Basin, Ghana. Ecotoxicology and Environmental Safety. 165: 540-546.
Ebeling, J.M., Timmons, M.B. and Bisogni, J.J. 2006. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture. 257: 346-358.
Effendi, H., Delis, P.C., Krisanti, M. and Hariyadi, S. (2015). The performance of Nile tilapia (Oreochromis niloticus) and vetiver grass (Vetiveria zizanioides) concurrently cultivated in aquaponic system. Advances in Environmental Biology, 9(24): 382-388.
El-Demerdash, F.M., Yousaf, M.I., and Elaswad, F.A. 2006. Biochemical study on the protective role of folic acid in rabbits treated with chromium (VI). Journal of Environmental Science and Health Part B. 41(5): 731-746.
El-Sadaawy, M.M., El-Said, G.F., and Sallam, N.A. 2013. Bioavailability of heavy metals in fresh water Tilapia nilotica (Oreachromis niloticus Linnaeus, 1758): Potential risk to fishermen and consumers. Environmental Science and Health. 48: 402-409.
El-Sayed, A.F.M. 2006. Tilapia culture in salt water: environmental requirements, nutritional implications and economic potentials. Avances en Nutrición Acuicola, 8: 95-106.
El-Sherif, M.S. and El-Feky A.M.I., 2009. Performance of Nile Tilapia (Oreochromis niloticus) Fingerlings. I. Effect of pH. International Journal of Agriculture and Biology, 11(3): 297-300.
FAO, 2020. Tilapia production and trade with a focus on India WAPI factsheet to facilitate evidence-based policymaking and sector management in aquaculture. http://www.fao.org/3/ca9224en/ca9224en.pdf.
Farag, A.M., May, T., Marty, G.D., Easton, M., Harper, D.D., Little, E.E., and Cleveland, L. 2006. The effect of chronic chromium exposure on the health of Chinook salmon (Oncorhynchus tshawytscha). Aquatic Toxicology. 76(3-4): 246-57.
Fazio, F., Habib, S.S., Naz, S., Hashmi, M.A.H., Saoca, C., and Ullah, M. 2021. Cadmium sub-lethal concentration effect on growth, hematological and biochemical parameters of Mystus seenghala (Sykes, 1839). Biology Trace Element Research. 200: 2432-2438.
Ferreira, N.S., Oliveira, L.H.B., Agrelli, V., de Oliveira, A.F., Nogueira, A.R.A., Oliveira and A., and Gonzalex, M.H. 2019. Bioaccumulation and acute toxicity of As(III) and As(V) in Nile tilapia (Oreochromis niloticus). Chemosphere. 217: 349-354.
Griboff, J., Horacek, M., Wunderlin, D.A., and Monferran M.V. 2018. Bioaccumulation and trophic transfer of metals, as and Se through a freshwater food web affected by antrophic pollution in Córdoba, Argentina. Ecotoxicology and Environmental Safety. 148: 275-284.
Hogendoorn-roozemond A.S., Tenholder J.J.H.M., Stirk J.J.T.W.A., 1977. The influence of pH on the toxicity of hexavalent chromium to rainbow trout (Salmo gairdneri). Aquatic pollutants-transformation and biological effects. Proc the 2nd International Symposium on Aquatic Pollution: 477478.
Intamat, S., Buasriyot, P., Sriuttha, M., Tengjaroenkul, B., and Neeratanaphan, L. 2017. Bioaccumulation of arsenic in aquatic plants and animals near a municipal landfill. International Journal of Environmental Studies. 74(2): 303-314.
Islam, S.M.M., Rohani, M.F., Zabed, S.A., Islam, M.T., Jannat, R., Akter, R., and Shahjahan, M. 2020. Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicology Reports. 7: 664-670.
Javed, M. and Usmani, N. 2019. An overview of the adverse effects of heavy metal contamination on fish health. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 89(2): 389-403.
Jing, W., Lang, L., Lin, Z., Liu, N., and Wang, L. 2019. Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere. 219: 321-327.
Ju, Y.R., Chen, C.W., Chen, C.F., Chuang, X.Y., and Dong, C.D. 2017. Assessment of heavy metals in aquaculture fishes collected from southwest coast of Taiwan and human consumption risk. International Biodeterioration and Biodegradation. 124: 314-325.
Ju, Y.R., Chen, C.F., Chuang, X.Y., Lim, Y.C., Chen, C.W. and Dong, C.D. 2020. Biometry-dependent metal bioaccumulation in aquaculture shellfishes in southwest Taiwan and consumption risk, Chemosphere. 253: 1-9.
Kayaalt, Z., Aliyev, V., and Söylemezoğlu, T. 2011. The potential effect of metallothionein 2A -5 A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels. Toxicology and Applied Pharmacology. 256(1): 1-7.
Khamollerd, C., Tengjaroenkul, B., and Neeratanaphan, L. 2019. Abnormal chromosome assessment of snakehead fish (Channa striata) affected by heavy metals from a reservoir near an industrial factory. International Journal of Environmental Studies. 76(4):1-15.
Koh, J.Y. and Lee, S.J. 2020. Metallothionein-3 as a multifunctional player in the control of cellular processes. Molecular Brain and Diseases. 13: 1-12.
Krumschnabel, G. and Nawaz, M. 2004. Acute toxicity of hexavalent chromium in isolated teleost hepatocytes. Aquatic Toxicology, 70(2): 159-167.
Kwong, R., Andrés, J., and Niyogi, S. 2011. Effects of dietary cadmium exposure on tissue-specific cadmium accumulation, iron status and expression of iron-handling and stress-inducible genes in rainbow trout: Influence of elevated dietary iron. Aquatic Toxicology. 102(1): 1-9.
Ling, M.P., Hsu, H.T., Shie, R.H., Wu, C.C. and Hong, Y.S. 2013. Health risk of consuming heavy metals in farmed Tilapia in Central Taiwan. Environmental Contamination and Toxicology. 83: 558-564.
Luczynska, J., Paszczyk, B., and Luczynski, M.J. (2018). Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer's health. Ecotoxicology and Environmental Safety. 153: 60-67.
Madu, P.C., Akpaiyo, G.D., and Ikoku I. 2011. Biosorption of Cr3+, Pb2+ and Cd2+ ions from aqueous solution using modified and unmodified millet chaff. Chemical Pharmaceutical Research. 3: 467-477.
McGeer, J., Niyogi, S., and Smith, S.D. 2012. Cadmium. In: Wood, C.M., Farell, A.P. and Brauner, C.J. Fish physiology. Homeostasis and toxicology of non-essential metals. 31(B): 126.
Ministry of Industry. 2016. Ministerial Regulation to Control Contamination of Soil and Ground Water in Factory Area.
Ministry of Industry (MOI). 2016. Soil and water chemical contamination standard. Royal Gazette, Announcement and General Publication. 133 (275 Ngo): 4-5.
Ministry of Public Health (MOPH). 2020. Food chemical contamination standard. Royal Gazette, Announcement and General Publication. 414 (118 Ngo): 17-18.
Mohamed, A.A., Houseiny, W., EL-Murr, A.E., Ebraheim, L., Ahmed, A., and El-Hakim, Y.M., 2020. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet, Ecotoxicology and Environmental Safety. 188: 1-10.
Mohammad, M.N., Authman Zaki, M.S., Khallaf, E.A., and Abbas, H.H. 2015. Use of fish as bio-indicator of the effects of heavy metals pollution. Aquatic Resource Development. 328(6): 1-14.
Mustapha, M.K. and Atolagbe, S.D. 2018. Tolerance level of different life stages of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) to low pH and acidified waters. Journal of Basic and Applied Zoology. 79(1): 1-6.
National academy of science. 1974. Chromium: Medical and Biological Effects of Environmental Pollutants. EIFAC Technical Paper. 43, 3-17.
National Bureau of Agricultural Commodity and Food Standards. 2013. Good aquaculture practice for tilapia farm. the Royal Gazette, Announcement and General Publication.130, (76Ngo).
Neeratanaphan, L., Khamlerd, C., Chowrong, S., Intamat, S., Sriuttha, M., and Tengjaroenkul, B. 2017. Assessment of flying barb fish (Esomus metallicus) from a gold mine area with heavy metal contamination. International Journal of Environmental Studies. 74(4): 613-624.
Neeratanaphan, L., Kamollerd, C., Suwannathada, P., Suwannathada, P., and Tengjaroenkul, B. 2020. Genotoxicity and oxidative stress in experimental hybrid catfish exposed to heavy metals in a municipal landfill reservoir. International Journal of Environmental Research and Public Health. 17(6): 1980.
Niyogi, S., Kent, R., Wood and C.M. 2008. Effects of water chemistry variables on gill binding and acute toxicity of cadmium in rainbow trout (Oncorhynchus mykiss): A biotic ligand model (BLM) approach. Comparative Biochemistry and Physiology. 148(C): 305-314.
Oitani, Y., Ishiyama, A., Kosuga, M., Iwasawa, K., Ogata, A., Tanaka, F., Takeshita, E., Shimizu-Motohashi, Y., Komaki, H., Nishino, I., Okuyama, T., and Sasaki, M. 2018. Interpretation of acid α-glucosidase activity in creatine kinase elevation: A case of Becker muscular dystrophy. Brain Development. 40: 837-840.
Oost, R., Beyer J., and Vermeulen, N.P.E. 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology. 13: 57-149.
Parekh, H.M. and Tank, S.K. 2015. Studies of hematological parameters of Oreochromis niloticus exposed to cadmium chloride (CdCl2, 2H2O). International journal of Environment. 4: 116-127.
Paul, J.S. and Small, B.C. 2021. Chronic exposure to environmental cadmium affects growth and survival, cellular stress, and glucose metabolism in juvenile channel catfish (Ictalurus punctatus). Aquatic Toxicology. 230: 1-11.
Phoonaploy, U., Tengjaroenkul, B., and Neeratanaphan, L. 2019. Effects of electronic waste on cytogenetic and physiological changes in snakehead fish (Channa striata). Environmental Monitoring and Assessment. 191(6): 26-38.
Pollard, S., Anderson, J.C., Bah, F., Mateus, M., Sidhu, M., and Simmons, D.B.D. 2022. Non-lethal blood sampling of fish in the lab and field with methods for dried blood plasma spot omic analyses. Frontiers in Genetics. 13: 795348.
Popma, T.J., and Lovshin, L.L. 1996. Worldwide Prospects for Commercial Production of Tilapia; International Center for Aquaculture and Aquatic Environments. Auburn, AL, USA.
Prabpai, S., Charerntanyarak, L., Siri, B., and Moore, M.R. 2007. Agronomic properties and heavy metals content in soil reclaimed from municipal solid waste landfill development of a knowledge-based system for foundry waste recycling. Solid Waste Technology and Management. 33(2): 92-95.
Putte, I.V.D., Brinkhorst, M.A. and Koeman, J.H. 1981. Effect of pH on the acute toxicity of hexavalent chromium to Rainbow trout (Salmo gairdneri). Aquatic Toxicology 1: 129-142.
Pyle, G.G., Rajotte, J.W., and Couture, P. 2005. Effects of industrial metals on wild fish populations along a metal contamination gradient. Ecotoxicology and Environmental Safety. 61(3): 287-312.
Qadir, A., and Malik R.N. 2011. Heavy metals in eight edible fish species from two polluted tributaries (Aik and Palkhu) of the River Chenab, Pakistan. Biological Trace Element Research. 143: 1524-1540.
Radulescu, C., Barboiu, G., Popescu, I., Dulama, I., Bucurica, I., Teodorescu, S., Stirbescu, R., and Tanase, N. 2020. Potential health risk assessment associated with heavy metal accumulation in native Urtica dioica. Romanian Reports in Physics. 72: 1-20.
Rajeshkumar, S. and Li, X. 2018. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicology Reports. 5: 288-295.
Roberts, R.J. 2012. Fish Pathology. 3: 439-481.
Ruksachat, N., Tengjaroensakul, B., Neeratanaphan, L., Tanomtong, A., Srikacha N., Phommavong, T. and Soulivongsa L. 2023. Heavy metals and metalloid effects on cytogenetics in frogs (Sylvirana nigrovittata) around the Sepon gold-copper mine, Lao PDR. Natural and Life Sciences Communications. 22(1): 1-14.
Ruchuwararak, P., Intamat, S., and Neeratanaphan, L. 2020. Genetic difference erentiation and bioaccumulation factor after heavy metal exposure in edible aquatic plants near a municipal landfill. Environment Asia. 13(3): 36-49.
Rudneva, I., Skuratovskaya, E., Dorohova, I., and Kovyrshina, T. 2012. Use of fish blood biomarkers for evaluating of marine environment health. World Journal of Science and Technology. 2(7): 19-25.
Sajjad S., Malik H., Saeed L. and Chaudhary A. 2018. Changes in growth hormone and cortisol profile due to lead induced toxicity in Labeo rohita. Turkish Journal of Fisheries and Aquatic Sciences, 18: 921-926.
Sarkar, M.M., Rohani, M.F., Hossain, M.A.R., and Shahjahan, M. 2021. Evaluation of heavy metal contamination in some selected commercial fish feeds used in Bangladesh. Biology Trace Element Research. 200: 844-854.
Sayed, A., El-Din, H., Mekkawy, I.A.A., and Mahmoud, U. 2011. Effects of 4-nonylphenol on metabolic enzymes, some ions and biochemical blood parameters of the African catfish Clarias gariepinus (Burchell, 1822). African Journal of Biochemistry. 5: 287-297.
Shahjahan, M.D., Taslima, K., Rahman, M.S., Al-Emran, M.D., Alam, S.I., and Faggio, C. 2022. Effects of heavy metals on fish physiology - A review. Chemosphere. 300: 1-4.
Soegianto, A. and Irawan, B. 2012. Bioaccumulation of heavy metals in aquatic animals collected from coastal. Asian Journal of Water, Environment and Pollution. 6(2): 95-100.
Somparn, A., Pamonpol, K., and Tokhun, N. 2020. Health risk assessment and bioaccumulation of heavy metals in surface water and Nile tilapia (Oreochromis niloticus) in the Huai Luang River Basin, Thailand. Public Health and Development. 18: 10-23.
Soudani, N., Sefi, M., Amara, I.B., Boudawara, T., and Zeghal, N. 2010. Protective effects of selenium (Se) on chromium (VI) induced nephrotoxicity in adult rats. Ecotoxicology and Environmental Safety. 73(4): 671-678.
Sriuttha, M., Tengjaroenkul, B., Intamat, S., Phoonaploy, U., Thanomsangad, P., and Neeratanaphan, L. 2017. Cadmium, chromium and lead accumulation in aquatic plantsand animals from a municipal landfill. Human and Ecological Risk Assessment. 23(2): 350-363.
Strungaru, S.A., Nicoara, M., Teodosiu, C., Baltag, E., Ciobanu, C., and Plavan, G. 2018. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere. 207: 192-202.
Suchana, S.A., Ahmed, M.S., Islam, S.M.M., Rahman, M.L., Rohani, M.F., Ferdusi, T., Ahmmad, A.K.S., Fatema, M.K., Badruzzaman, M., and Shahjahan, M. 2021. Chromium exposure causes structural aberrations of erythrocytes, gills, liver, kidney, and genetic damage in striped catfish Pangasianodon hypophthalmus. Biology Trace Elements. Research. 199: 3869-3885.
Swann, L. 1997. A fish farmer’s guide to understanding water quality. Aquaculture Extension Fact Sheet. 503: 1-8.
Tabrez, S., Zughaibi, T.A., and Javed, M. 2021. Bioaccumulation of heavy metals and their toxicity assessment in Mystus species. Saudi Journal of Biological Science. 28(2):1459-1464.
Tengjaroenkul, B., Intamat, S., Boonmee, S., and Neeratanaphan, L. 2017. Chromosomal aberration assessment of Silver Rasbora fish (Rasbora tornieri) living near gold mine area with heavy metal contamination. Human and Ecological Risk Assessment. 23(3): 1-10.
Thai Pollution control department. 2022. https://www.pcd.go.th/wp-content/uploads/2023/04/pcdnew-2023-04-11_03-13-24_292638.pdf
Thitiyan, T., Pongdontri, P., Tengjaroenkul, B., and Neeratanaphan, L. 2021. Bioaccumulation and oxidative stress in Barbonymus gonionotus affected by heavy metals and metalloid in municipal landfill reservoir. International Journal of Environmental Studies. 79(1):1-16.
Timmons, M.B., Ebeling J.M., Wheaton F.W., Summerfelt, S.T. and Vinci, B.J. 2002. Recirculating Aquaculture Systems, 2nd Edition. Ithaca, New York: Cayuga Aquaculture Ventures. 2: 245-401.
Valiallahi, J. and Pourabbasali, M. 2019. The effects of cadmium chloride on the hematological and biochemical parameters in giant Sturgeon fish (Huso huso). Environment Education and Sustainable Development. 12: 83-91.
Velma, V., Vutukuru, S.S. and Tchounwou, P.B. 2009. Ecotoxicology of hexavalent chromium in freshwater fish: a critical review. Reviews on Environmental Health. 24(2): 129-145.
Wang, Z. and Leung, K.M.Y., 2015. Effects of unionized ammonia on tropical freshwater organisms: implications on temperate-to-tropic extrapolation and water quality guidelines. Environmental Pollution. 205, 240-249.
Wedeen, R. and Qian, L.F. 1991. Chromium-induced kidney disease. Environmental Health Perspective. 92: 71-74.
Whangchai, N., Chitmanat, C., Ramaraj, R. and Itayama, T. 2018. Effects of water flow rate and water 1quality on tilapia culture in the Mae Ping River, Thailand. Chiang Mai Journal of Science. 45(3): 1318-1322.
WHO/FAO. 2015. General standard for contaminants and toxins in food and feed. (Codex Alimentarius Commission: 193-1995).
Zalups, R.K. and Ahmad, S. 2003. Molecular handling of cadmium in transporting epithelia. Toxicology and Applied Pharmacology. 186(3): 163-88.
Zulfahmi, I., Rahmi, A., and Muliari, M. 2021. Exposure to lead nitrate alters growth and haematological parameters of milkfish (Chanos chanos). Environmental Contamination Toxicology. 07: 860-867.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kannawee Swangneat1, Lamyai Neeratanaphan2, Kraiwuth Kallawicha3, and Bundit Tengjaroensakul1, *
1 Department of Veterinary Medicine, Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
2 Department of Environmental Science, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
3 College of Public Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand.
Corresponding author: Bundit Tengjaroensakul, E-mail: btengjar@kku.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: August 28, 2023;
Revised: January 28, 2024;
Accepted: February 2, 2024;
Published online: February 12, 2023

