
Phytochemical Contents and Anti-Colon Cancer Activities of Macadamia integrifolia Maiden & Betche Leaves and Husks Extracts
Piyarat Srisawang, Suphunwadee Sawong, Pennapha Suknoppakit, Thitima Sapapak, Adisa Jittrasirichok, Chayanee Saennaronk, Dumrongsak Pekthong*, and Supawadee Parhira*Published Date : February 6, 2024
DOI : https://doi.org/10.12982/NLSC.2024.017
Journal Issues : Number 2, April-June 2024
Abstract The macadamia (Macadamia integrifolia Maiden & Betche, Proteaceae Family) leaves and husks were the by-products from macadamia nut production which have few reports on the phytochemicals and pharmacological activities. This study aimed to investigate the possibility of using these by-products in drug discovery by examining their phytochemicals contents, as well as their anti-colon cancer activities. Dry macadamia leaves and husks were extracted and fractionated to obtain the crude extracts (EtOH), dichloromethane (DCM), ethyl acetate (EtOAc) and water fractions. Colorimetric methods were used to determine the total amounts of phytochemicals. The anti-colon cancer activities of the extracts against human colorectal carcinoma (HCT116) cell line were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results showed that each gram of the samples contained total phenolics between 2.9-12.9 mg equivalent to gallic acid, total flavonoids 30.1-89.7 mg equivalent to rutin, and total triterpenoids 228.4-640.9 mg equivalent to ursolic acid. The DCM and EtOAc fractions of the husks exhibited the highest anti-colon cancer activity, with half maximal inhibitory concentrations (IC50) of 39.1 ± 4.3 and 56.9 ± 15.0 µg/mL, respectively. The EtOAc and water fractions of the leaves had IC50 values of 130.9 ± 17.4 and 127.8 ± 7.9 µg/mL, respectively. The IC50 values of other samples were greater than 200 µg/mL, while the IC50 of sorafenib, a positive control, was 5.95 ± 1.26 µg/mL. In conclusion, the leaves and husks of macadamia contained high amounts of several phytochemicals and exhibited anti-colon cancer activity against HCT116. These findings suggested that the extracts from these wastes may have potential in the discovery of anticancer agent research.
Keywords: Macadamia integrifolia, Leaf, Husk, Colon cancer, Phytochemical
Funding: This work was supported by Naresuan University (NU) and National Scheme, Research and Innovation Fund (NSRF), Grant NO. FRB650022/0179, Project NO. R2565B024. The authors are grateful for the research funding for the undergraduate thesis provided by the Faculty of Pharmaceutical Sciences and the Faculty of Medical Science, Naresuan University, Phitsanulok, Thailand.
Citation: Srisawang, P., Sawong, S., Suknoppakit, P., Sapapak, T., Jittrasirichok, A., Saennaronk, C., Pekthong, D., and Parhira, S. 2024. The phytochemical contents and anti-colon cancer activities of Macadamia integrifolia Maiden & Betche leaves and husks extracts. Natural and Life Sciences Communications. 23(2): e2024017.
INTRODUCTION
Macadamia belongs to the family of Proteaceae. Macadamia integrifolia Maiden & Betche is the accepted scientific name (World flora online, 2022) of the smooth-shelled macadamia nut (Bittenbender and Hirae, 1990; Yang, Liu and Halim, 2009; Abubaker et al., 2017). The appearance of each part of macadamia were illustrated in Figure 1. The macadamia tree (Figure 1A) can grow up to 20 m (Susilowati, Kusuma and Kholibrina, 2019). Its leaves (Figure 1B) are green or dark green in color and elongated with the smooth edges, while the flowers (Figure 1C) are small and spherical, long, bushy and bouquets. The ripe fruit (Figure 1D) is a green in color, single, bushy, bunch, elongated, spherical or round shape with an average of diameter around 2.20 cm. The mature fruits (Figure 1E-H) are brown or green with a thick husk covered a hard and woody shells which contained nuts or kernels inside (Susilowati, Kusuma and Kholibrina, 2019). Macadamia trees were cultivated in some regions in Thailand, such as Chiangmai, Loei, and Phetchabun Provinces (Horticultural Research Institute, 2024). The estimated amounts (in 2019) of imported macadamia with husks and without husks in Thailand were around 1404 and 296 tons, respectively (Horticultural Research Institute, 2021). The macadamia nut production could produce a lot of by-product, i.e., husks and shell up to 70% of the nut’s weight (Adriana, et al., 2015), which might more than 1,000 tons per years.
Macadamia nut is an edible part which can be eaten as raw, fried, roasted and salted nuts. Consumption of macadamia nuts has been reported to reduce total and low density lipoprotein (LDL)-cholesterol in hypercholesterolemic patients resulted in a lowered risk of cardiovascular disease (Griel et al.,2008; Yang, Liu and Halim, 2009). Its nuts showed potential as a good source of an anti-microbial protein, MiAMP1 which inhibited various plant pathogens with no effect on the viability of plant and animal cell lines (McManus, 1999). Moreover, the fermented supernatant of macadamia nut exhibited chemoprotective effects against LT97 colon adenoma cells (Schlörmann et al., 2017). The extract from pericarp of macadamia was reported as a promising material for use as anti-oxidant, anti-aging and whitening active ingredients (Somwonggin et al., 2022). Its nuts and shells contained high contents of phenolic compounds, such as, 2, 6-dihydroxyben-zoic acid and 3, 5 dimethoxy-4-hydroxy-cinnamic acid (Quinn and Tang, 1996). The successful extraction of plant-derived bioactive fractions containing high amounts of phytochemicals have been reported in various researches (Likittrakulwong, et al., 2023; Tengco, et al., 2023; Sirisa-ard, et al., 2023; Maneechai, et al., 2023; Chittasupho, et al., 2023). However, there were few reports on the phytochemical and bioactivity of the leaves and husks of M. integrifolia which were waste from macadamia nut production, especially anticancer activity, for further utilization as a source of bioactive compounds. Therefore, the aims of this study were to determine the total contents of phenolic compounds, flavonoids and triterpenoids in the M. integrifolia leaves and husks extracts along with screening for their cytotoxicity against human colon cancer cell line (HCT116).
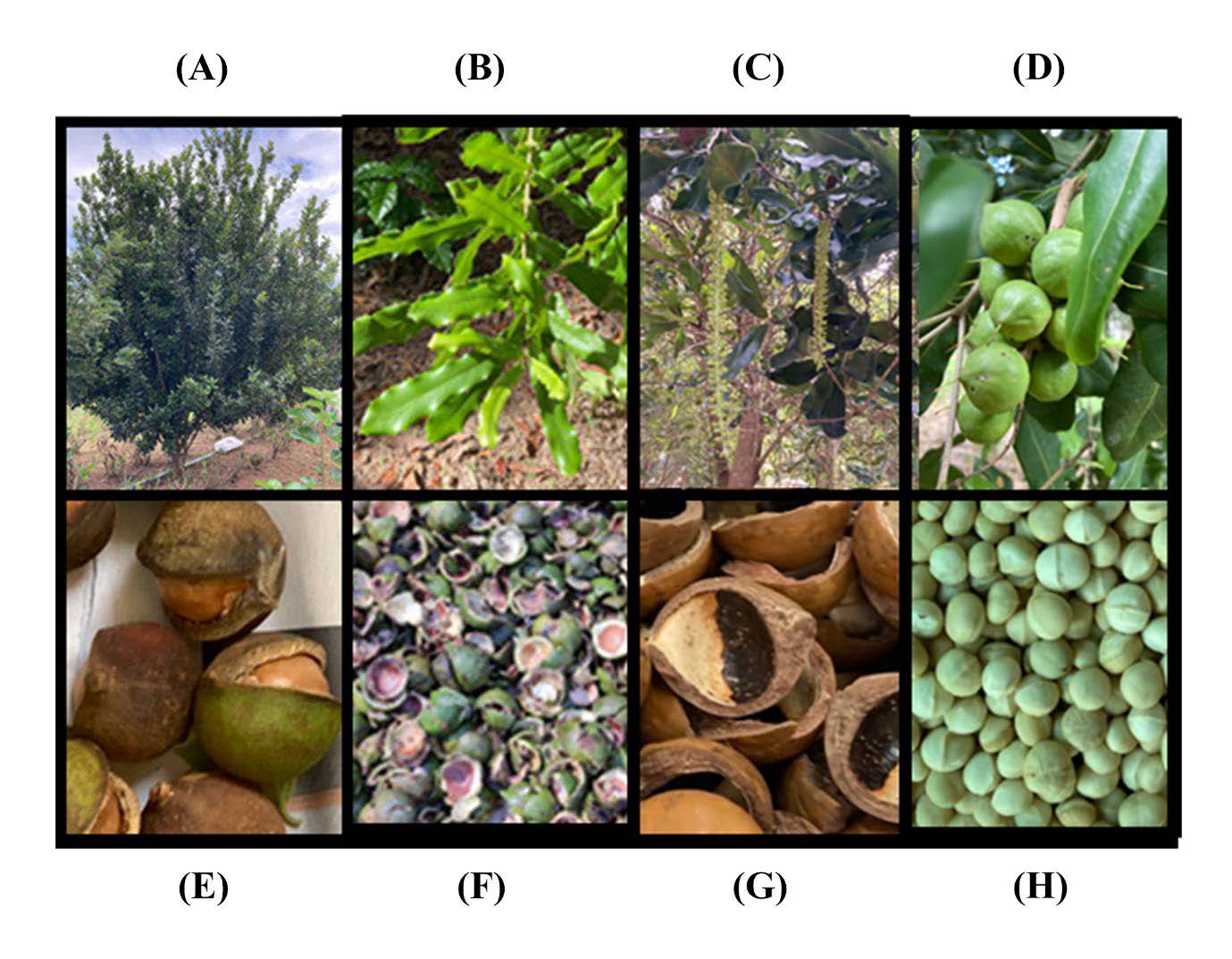
Figure 1. The appearance of each part of M. integrifolia: (A) tree, (B) leaves, (C) flowers, (D) fresh fruits, (E) mature fruits, (F) husks or pericarp, (G) shells, and (H) kernel or nut.
MATERIAL AND METHODS
Materials
Gallic acid was purchased from Sigma-Aldrich, China. Rutin and dimethyl sulfoxide (DMSO) were bought from Sigma-Aldrich, USA. Ursolic acid was purchased from Tokyo Chemical, Japan. Sorafenib was purchased from Med Chem Express, USA. Folin-Ciocalteu’s phenol reagent and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution were purchased from Merck, Germany. Aluminum Chloride hydrated was obtained from Ajax Finechem, Australia. Deionized water, absolute ethanol, methanol, dichloromethane, ethyl acetate, chloroform, glacial acetic acid and sulfuric acid were purchased from LabScan Co. Ltd., Thailand. 95% Ethanol was obtained from the Liquor Distillery organization, Thailand. Human colon cancer cell line, HCT116 (CCL-247), was bought from ATCC, USA. Dulbecco's modified Eagle medium (DMEM) was bought from Corning, USA. The fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco, USA. The 96-well plates were bought from SPL, Korea.
Plant identification
The leaves and husks of M. integrifolia were collected from Phobphra District, Tak Province, Thailand, in December 2019. The plant was identified and authenticated from a taxomonist, Dr. Pranee Nang-ngam. The sample specimen (voucher number 005184) was kept at PNU Herbarium, Department of Biology, Faculty of Science, Naresuan University, Phitsanulok, Thailand, for future reference.
Sample preparation
The macadamia leaves and husks were dried in hot air oven (50 ± 5 °C) for 3 days, then ground by blender to obtain fine dry powders. The dried leaves and husks powders were kept in tight plastic bags then put in a tight plastic box until extraction. The powders of M. integrifolia leaves (160 g) or husks (315 g) were marinated and extracted with 95% ethanol (ratio of dry plant:95% ethanol was 1 g:10 mL) by using ultrasonic assistance (40 KHz, Model 80AL, Shenzhen Jie Tai Ultrasonic Cleaning Equipment Co., Ltd, China) at ambient temperature (27 ± 5 ºC) for 1 h. The mixtures were filtered by a filter paper (Whatman No. 1) to obtain the filtrate solutions of the leaves, the husks and their residues. The residues were extracted with the same protocol for 2 more times. The filtrate solutions from three extractions were combined and evaporated by rotary evaporator (Buchi, Switzerland) at 45°C to furnish the ethanolic crude extracts (EtOH) of M. integrifolia leaves and husks, respectively. Their crude extracts (5.0 g) were subsequently subjected to liquid-liquid chromatography by dispersing in 200 mL of water and fractionated with dichloromethane (400 mL × 3 times) followed by ethyl acetate (400 mL × 3 times). The dichloromethane and ethyl acetate layers were evaporated (45 ºC) to obtain dichloromethane fractions (DCM) and ethyl acetate fractions (EtOAc) of the leaves or husks of macadamia. The rest of water layers were evaporated (60ºC) for 1 h to remove solvent residual then frozen at -20 °C and dried by freeze dryer to gain water fractions of the leaves and husks. The samples were stored in amber glass bottles at 4 ± 2 ºC until used. The percent yields of each fraction were calculated by comparing with 100 g of their ethanolic crude extracts or dry plants.
Phytochemicals determination
Total phenolic content
The measurement of total phenolic contents of the extracts from the M. integrifolia leaves and husks were modified from Sembiring EN et al. (Sembiring, Elya and Sauriasari, 2018). The 25 µL of the EtOH, DCM and water fractions (5 mg/mL) or ethyl acetate fraction (2.5 mg/mL) in methanol were mixed with 100 μL of 1:4 diluted Folin–Ciocalteu reagent in water in a flat-bottom 96-well plate. The reaction mixture was added 75 μL of saturated sodium hydrogen carbonate solution (6 %w/v) then incubated at room temperature (27 ± 5 °C) for 2 h. Their absorbances of tested samples were measured at 765 nm by using the microplate reader (BioTek, USA) then calculated from calibration curve of gallic acid (0–80 µg/mL). Total phenolic contents (mg gallic acid equivalents (GAE) per g of the extract, n=3) were reported as average values ± standard deviation (SD).
Total flavonoid content
An Aluminium chloride assay described by Sembiring EN et al. (Sembiring, Elya and Sauriasari, 2018) with some modifications was used to measure total flavonoid contents of the M. intefrifolia leaves and husks extracts. The stock solutions (50 µL) of EtOH, DCM and water (5 mg/mL) and EtOAc (2.5 mg/mL) in methanol were transferred to a 96 well plate then added 10 µL Aluminium chloride solution in methanol (10%), 150 μL of 96% ethanol and 10 μL of 1 M Sodium acetate. The reaction mixtures were mixed well, incubated for 40 min in the dark place at room temperature (27 ± 2 °C) then measure their absorbance (415 nm) by a microplate reader. Total flavonoid contents of all fractions were calculated from the standard curve of rutin, (5-50 µg/mL) then expressed as an average value ± SD (n=3) in term of mg rutin equivalents (RTE) per g of the extract.
Total triterpenoid content
The colorimetric assay using a vanillin-acetic acid reagent and sulfuric acid described by Chang et al. (Chang, Lin and Lai, 2013) was used to measure the total triterpenoid content of the extracts. Briefly, the tested extracts were dissolved in glacial acetic acid (1 mg/mL 200 µL) followed by adding one mL of 5% vanillin-acetic acid solution and then 1.8 mL of sulfuric acid. The reaction mixture was heated in a water bath (70°C) for 30 min and then cooled to ambient temperature (27 ± 2 °C) before adding 2 mL of glacial acetic acid. The absorbances (548 nm) of the reaction mixture were determined then compared to the calibration curve of ursolic acid (2 − 40 µg/mL). Total triterpenoid contents (mg ursolic acid (UAE) equivalents/ g extract) were performed in triplicate and displayed as the average values and SD.
MTT cell viability assay
Cell culture
Human colorectal carcinoma HCT116 were grown in complete media containing Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. The cells were maintained in an incubator that was 37 °C, 5% CO2, and humidified. When the confluence of the cells reached 80–90%, subcultures were conducted.
Cell viability measurement
On 96-well plates, HCT116 cells were seeded at a density of 15,000 cells/well. Before treating cells, each extract component was diluted with 1% DMSO. Sorafenib was employed as a positive control. After being harvested, the cells were incubated with a 2-mg/mL MTT solution for three hours at 37 °C. MTT (yellow) was converted into formazan crystals (purple) by the enzyme mitochondrial reductase of surviving cells. After dissolving these crystals in DMSO, the optical density (OD) was determined at 595 nm utilizing a microplate reader (SpectraMax iD3, Molecular Devices, USA).
Statistical analysis
The average total phytochemical contents ± standard deviations (SD) of the tested samples from three independent experiments are illustrated. One-way analysis of variance (ANOVA) then Student's t-test with Tukey's post hoc were performed by Microsoft Excel to compare the data that showed statistically significant differences between each fraction at P <0.05. The average IC50 values ± SD of three separate cell culture experiments in triplicate are presented. One-way ANOVA or Student's t-test with Tukey's post hoc test were used to compare the data that showed statistically significant differences between the experimental groups and the vehicle group at P <0.05. All of the cell culture data was examined using GraphPad Prism Software version 9. The correlation coefficients between phytochemical contents and their cytotoxicity against HCT116 were investigated by using Microsoft Excel then interpreted the absolute magnitude of the observed correlation coefficient as recommended by Schober P. et al (Schober, Boer and Schwarte, 2018).
RESULTS
Physical appearance and percent yield of the extracts from M. integrifolia leaves and husks
The ultrasonic-assisted extraction of the M. integrifolia dry leaves (160 g) and husks (315 g) by 95% ethanol, as illustrated in Table 1, yielded the dark green viscous for both parts with the percent yields of the leaves crude extract (12.7 g, 7.91% yield of dry leaves) higher than that of the husks crude extract (7.2 g, 2.27% yield of dry husks). The crude ethanolic extracts of the leaves and husks (5.0 g) were subsequently subjected to liquid-liquid chromatography to yield 3 fractions of the leaves, i.e., DCM (dark green viscous liquid, 1.6 g, 32.00% yield of EtOH and 2.53% yield of dry leaves), EtOAc (dark green viscous liquid, 0.4 g, 7.90% yield of EtOH and 0.63% yield of dry leaves) and water (brown powder, 1.96 g, 39.20% yield of EtOH and 3.10% yield of dry leaves), and 3 fractions of the husks, i.e., DCM (dark green viscous liquid, 0.81 g, 16.20% yield of EtOH and 0.36% yield of dry husks), EtOAc (brown viscous liquid, 0.23 g, 4.60% yield of EtOH and 0.10% yield of dry husks) and water (dark brown viscous liquid, 3.14 g, 62.80% yield of EtOH and 1.86% yield of dry husks).
Table 1. The yields and phytochemical contents of the extracts from the leaves and husks of M. integrifolia.
|
Part of plant |
Fraction |
Physical appearance |
Percent yield (%) |
Phytochemical contents
|
|||
|
of crude extract |
of dry plant |
Phenolics (mg GAE/g extract) |
Flavonoids (mg RTE/g extract) |
Triterpenoids (mg UAE/g extract) |
|||
|
Leaves |
EtOH |
Dark green viscous liquid |
- |
7.91 |
11.5 ± 1.67a |
55.6 ± 7.78a |
447.9 ± 21.04 a |
|
|
DCM |
Dark green viscous liquid |
32.0 |
2.53 |
11.8 ± 1.35a |
59.1 ± 7.71a |
640.9 ± 37.14 b |
|
|
EtOAc |
Dark brown viscous liquid |
7.90 |
0.63 |
3.8 ± 0.40 |
62.4± 5.82 a |
297.3 ± 88.33 c |
|
|
Water |
Brown powder |
39.20 |
3.10 |
12.2 ± 0.20a |
30.1 ± 4.71b |
286.8 ± 61.75 c |
|
Husks |
EtOH |
Dark green viscous liquid |
- |
2.27 |
5.9 ± 0.69b |
41.4 ± 4.06 b |
247.0 ± 9.57 c |
|
|
DCM |
Dark green viscous liquid |
16.20 |
0.36 |
5.5 ± 1.31b |
89.7 ± 16.08 |
633.6 ± 22.29 b |
|
|
EtOAc |
Brown viscous liquid |
4.60 |
0.10 |
12.9 ± 1.11a |
31.1 ± 4.15 b |
373.7 ± 44.85 a |
|
|
Water |
Dark brown viscous liquid |
62.80 |
1.86 |
2.9 ± 0.11 |
31.5 ± 5.51b |
228.4 ± 33.64 c |
Note: The values for “a”, “b”, and “c” (P ≤ 0.05), with equal letters, represented no statistically significant differences.
Phytochemical contents of the extracts from M. integrifolia leaves and husks
Total phenolic contents (mg GAE/ g extract) of the extracts from the macadamia leaves and husks showed in Figure 2A with the detail in Table 1. It revealed that the EtOH (11.5 ± 1.67), DCM (11.8 ± 1.35), and water (12.2 ± 0.2) fractions of the leaves and EtOAc (12.9 ± 1.11) fraction of the husks contained similar amounts of phenolic compounds. Their phenolic contents were statistically significantly higher than those of the EtOAc fraction (3.8 ± 0.40) of the leaves, the EtOH (5.9 ± 0.69), DCM (5.5 ± 1.31) and water (2.9 ± 0.11) fractions of the husks.
Total flavonoid contents in the macadamia extracts were expressed as mg RTE/ g extract in Figure 2B and provided more information about the average values and standard deviations in Table 1. The results indicated that the flavonoid contents of the DCM (89.7 ± 16.08) fractions from the husks were the highest one and statistically significantly higher than those of all tested samples. Triterpenoid contents of the EtOH (55.6 ± 7.78), DCM (59.1 ± 7.71) and EtOAc (62.4± 5.82) extracts from the leaves were similar to each other and significantly higher than those of water (30.1 ± 4.71) fraction from the leaves and the EtOH (41.4 ± 4.06), EtOAc (31.1 ± 4.15) and water (31.5 ± 5.51) fractions from the husks.
Figure 2C and Table 1 illustrated total triterpenoid contents in the unit of mg UAE/ g extract of the extracts from macadamia leaves and husks. The results revealed that the highest contents of triterpenoids were in the DCM fractions of the leaves and the husks with the values of 640.9 ± 37.14 and 633.6 ± 22.29, respectively. They were statistically significantly higher than those of the EtOH (447.9 ± 21.04) fraction from the leaves and EtOAc (373.7 ± 44.85) fraction from the husks and other tested samples. Similar values of total triterpenoid content were found in the EtOAc (297.3 ± 88.33) and water (286.8 ± 61.75) fractions from the leaves, as well as in the EtOH (247.0 ± 9.57) and water (228.4 ± 33.64) fractions from the husks.
Cytotoxic effects against HCT116 cell line of the extracts from M. integrifolia leaves and husks
After 24 hours, the cytotoxic activities of the EtOH, DCM, EtOAc and water fractions of the leaves and husks of M. integrifolia against human colon cell line, HCT116, were determined using the MTT assay. As demonstrated in Figure 3, HCT116 cells responded favorably with dose-response manner to all tested extracts of the macadamia leaves (Figure 3A), the husks (Figure 3B) and sorafenib (Figure 3C) which used as a positive control. The EtOAc and water fractions of leaves extract exhibited the 50% inhibitory concentration (IC50) values of 130.85 ± 17.35 µg/mL and 127.77 ± 7.93 µg/mL, respectively, but the EtOH and DCM fractions exhibited IC50 values against HCT116 cells larger than 200 µg/mL. Compared to the vehicle, the DCM and EtOAc fractions of the husks exerted potent cytotoxic effects on HCT116 cells with IC50 values of 56.89±15.03 µg/mL and 39.08 ± 4.27 µg/mL, respectively, whereas the EtOH and water fractions exhibited IC50 values greater than 200 µg/mL (Figure 3B). Table 2 displayed all IC50 values for the extracts from the leaves and husks and sorafenib (an IC50 value of 5.95 ± 1.26 µg/mL or 13.04 ± 2.98 µM).
It was found that the IC50 values of the DCM and EtOAc fractions of the husks were statistically significantly lower than those of the EtOAc and water fractions of the leaves, indicating their higher potency.
The correlation between their phytochemicals and their cytotoxicity against HCT116 cell line were shown in Table 3. The absolute magnitude of the observed correlation coefficients between total phenolic, total flavonoid and total triterpenoid contents, as well as their IC50 values were 0.15, 0.20 and 0.21, respectively, indicating a weak correlation.
Table 2. Cytotoxicity (IC50 values, µg/mL) against HCT116 of the extracts from M. integrifolia leaves and husks and sorafenib.
|
Macadamia extracts |
IC50 (µg/mL) |
|
|
HCT116 |
||
|
Leaves |
EtOH |
> 200a |
|
DCM |
> 200a |
|
|
EtOAc |
130.85 ± 17.35b |
|
|
Water |
127.77 ±7.93b |
|
|
Husks |
EtOH |
> 200a |
|
DCM |
56.89 ± 15.03c |
|
|
EtOAc |
39.08 ± 4.27c |
|
|
Water |
>200a |
|
|
Sorafenib |
5.95 ± 1.26 (13.04 ± 2.98 µM) |
|
Note: EtOH, DCM, EtOAc and water represented the ethanolic crude extract, dichloromethane fraction, ethyl acetate fraction and water fraction of the macadamia of each part as indicated, respectively. The values for “a”, “b”, and “c” (P ≤ 0.05), with equal letters, represent no statistically significant differences.
Table 3. The absolute magnitude of the observed correlation coefficients between phytochemical contents in the extracts from M. integrifolia leaves and husks and their cytotoxicity (IC50 values) against HCT116.
|
Phytochemicals-Cytotoxicity (HCT116) |
Absolute magnitude of the observed correlation coefficient |
Interpretation |
|
Total phenolic content- HCT116 |
0.15 |
weak correlation |
|
Total flavonoid contents-HCT116 |
0.20 |
weak correlation |
|
Total triterpenoid content-HCT116 |
0.21 |
weak correlation |
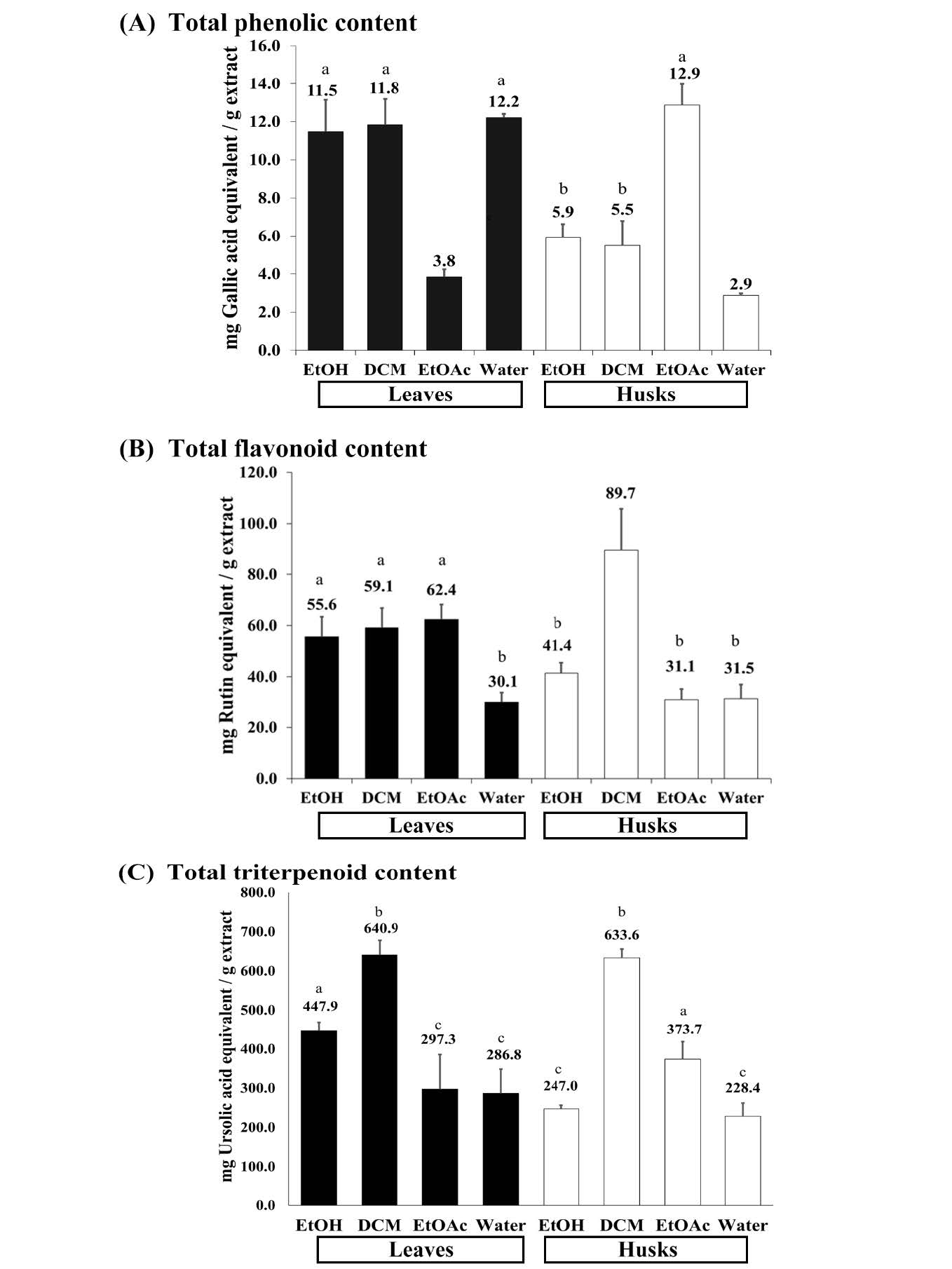
Figure 2. The Phytochemical contents of the extracts from M. integrifolia leaves and husks. (A) total phenolic content (B) total flavonoid content and (C) total triterpenoid content.
Note: EtOH, DCM, EtOAc and water represented the ethanolic crude extract, dichloromethane fraction, ethyl acetate fraction and water fraction of each part from macadamia as indicated, respectively. The values for “a”, “b”, and “c” (P ≤ 0.05), with equal letters, represented no statistically significant differences.
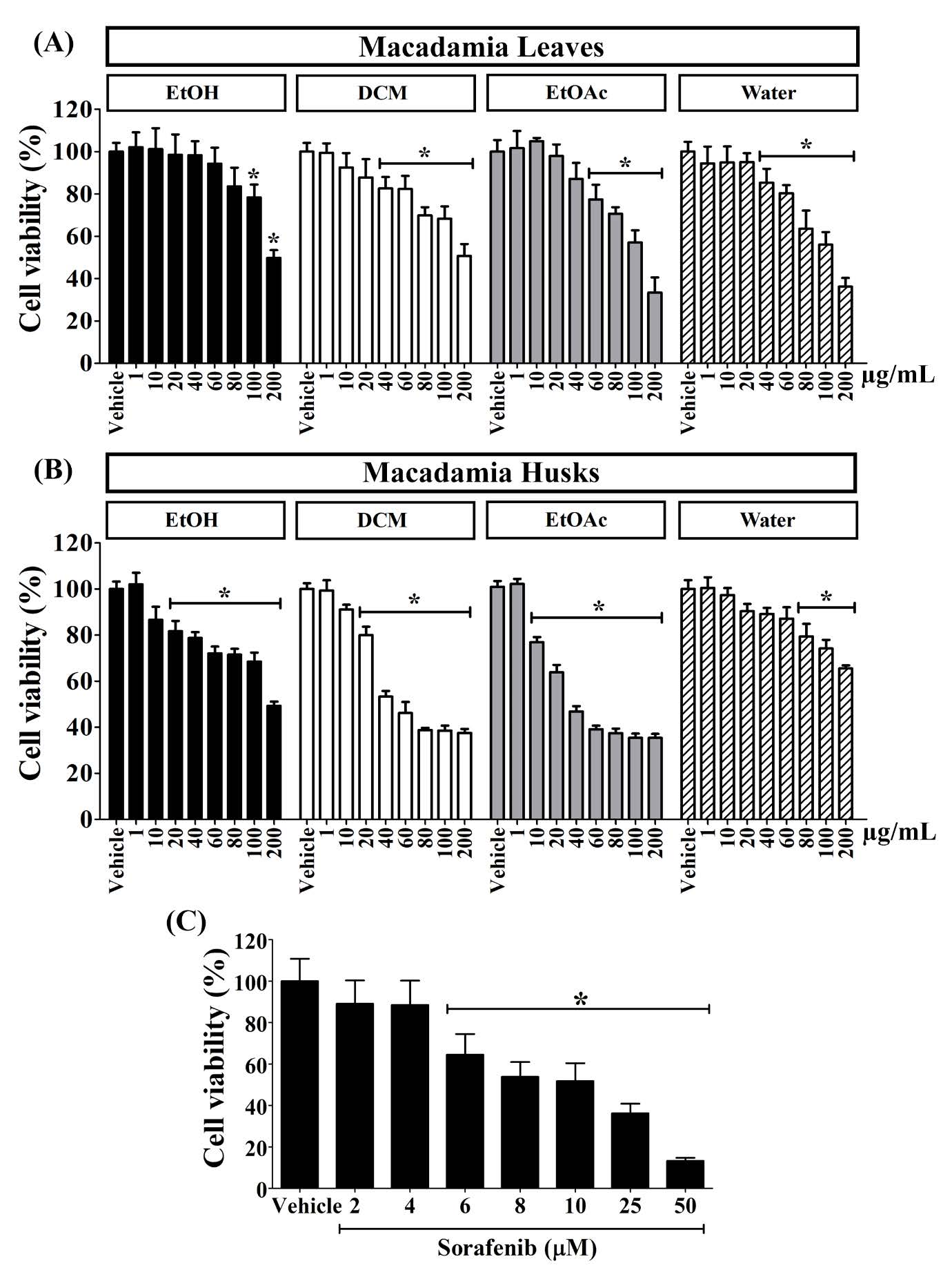
Figure 3. The cell viability of HCT116 after treated with the extracts (1-200 µg/mL) from M. integrifolia (A) leaves and (B) husks and (C) sorafenib (2-50 µM). The “*” symbol indicated a statistically significant difference from vehicle control (P < 0.05).
DISCUSSION
The results from this research revealed that the crude extracts and consequent fractions with different polarity of the used organic solvents, DCM, EtOAc and water, obtaining from liquid-liquid partition of the crude extracts of the macadamia leaves and husks were found to contain high amounts of phenolic compounds, flavonoids and triterpenoids with different magnitudes. The highest content of phenolic compounds was found in the EtOAc fraction of the husks similar to those of EtOH, DCM and water fractions of the leaves at around 11.5-12.9 mg GAE/ g extract. The other fractions consisted of phenolic compounds in the ranged of 2.9 – 5.9 mg GAE/ g extract which were statistically significantly lower than those of the highest one around 2.1-4.4 times. Some of the reported phenolic compounds found in macadamia nut oil might be possible to find in the leaves and husks were 2,6-dihydroxybenzoic acid; 2'-hydroxy-4'-methoxyacetophenone; 3',5'-dimethoxy-4'-hydroxyacetophenone and 3,5-dimethoxy-4-hydroxycinnamic acid and other unusual phenolic compounds (Quinn & Tang, 1996). The high content of phenolic compounds in the leaves and husks of macadamia extracts might promote their anti-colon cancer activities similar to the previous report on high efficacy against HCT116 cell line of the phenolic-rich-fraction from Calotropis gigantea of our research team (Winitchaikul et al., 2021).
The DCM fraction of the husks was shown to hold a significant different and highest value of total flavonoid content among tested fractions at 89.7 mg RTE/g extract which was 1.4-2.9 times of others, followed by the EtOAc, DCM and EtOH of the leaves around 55.6-62.4 mg RTE/ g extract and EtOH, EtOAc and water fractions of the husks around 31.1-41.4 mg RTE/g extract. The very high contents of triterpenoid in the DCM fractions of the leaves and husks around 633.6-640.9 mg UAE/g extract which were 1.4-2.8 times more than other fractions were observed along with high amounts in other fractions ranged in 228.4-447.9 mg UAE/g extract.
The high levels of flavonoids and triterpenoids in the extracts from the leaves and husks of M. integrifolia led our team to hypothesize that these fractions might exhibit anti-colon cancer activity. A previous publication claimed that flavonoids and terpenoids from the leaves of C. gigantea, specifically the 70% ethanol crude extract and its DCM and EtOAc fractions, showed cytotoxicity in a human colon cancer cell line, WiDr. (Mutiah, Sukardiman and Widyawaruyanti, 2017). Our current results support the hypothesis that we have proposed for EtOAc fractions obtained from both the leaves and husks of macadamia. We observed high potencies, with IC50 values below 200 µg/mL, for the EtOAc (39.08 ± 4.27 µg/mL) and DCM (56.89 ± 15.03 µg/mL) fractions of macadamia husks, along with the water (127.77 ± 7.93 µg/mL) and EtOAc (130.85 ± 17.35 µg/mL) fractions of macadamia leaves against HCT116 cell line. Their cytotoxicities were significantly greater than those of other tested fractions, which exhibited inhibitory activity against HCT116 in a dose-dependent manner but the IC50 values exceeded 200 µg/mL. However, after studying the correlation between the total phenolic, flavonoid, and triterpenoid contents of the extracts from the leaves or husks of macadamia and their cytotoxicity against HCT116, the correlation values ranged from 0.15 to 0.21, indicating some correlation but quite weak (Schober, Boer and Schwarte, 2018).
These findings explored the new era of using the by-products from macadamia nut production and agriculture to the new drug discovery, especially anti-colon cancer agent. However, various important experiments such as investigation the underlying mechanism of action, purification of potential bioactive markers or lead compounds, quality control of the extracts and etc., may require to support the utilization of the leaves and husk of M. integrifolia in the future.
CONCLUSION
In conclusion, the extracts from the leaves and husks of M. integrifolia contained high amounts of phenolic, flavonoid and triterpenoid compounds. The extracts from leaves and husks contained total phenolic compounds in the range of 3.8 – 12.2 and 2.9-12.9 mg GAE/ g extract, total flavonoids between 30.1 -62.4 and 31.5 -89.7 mg RTE/ g extract and total triterpenoids around 286.8 – 640.9 and 228.4-633.6 mg UAE/ g extract, respectively. All of the extracts from macadamia leaves and husks (1-200 µg/ mL) exhibited dose-dependent manner to inhibit cell viability of HCT116 cell line. The cytotoxicity (IC50 values) against the colon cancer cell line of the DCM (56.89± 15.03 µg/mL) and EtOAc (39.08 ± 4.27 µg/mL) fractions of the macadamia husks exhibited higher potencies than those of the EtOAc (130.85±17.35 µg/mL) and water (127.77±7.93 µg/mL) fractions of the macadamia leaves, while the IC50 of other fractions were higher than 200 µg/mL. The findings from this research shed light to the utilization of the by-products from macadamia nut production with high content of some phytochemicals and promising cytotoxicity against colon cancer to the development and discovery of new anti-colon cancer compounds in the future.
ACKNOWLEDGEMENTS
The authors are grateful to Ms. Ampai Siriraknavee (Romklao Garden, Phobphra District, Tak Province, Thailand) and KhaoKho District Agicultural Extension Office (Khaokho District, Phetchabun Province, Thailand) for providing macadamia leaves and husks in our preliminary study.
AUTHOR CONTRIBUTIONS
Piyarat Srisawang designed, conducted, performed the statistical analysis and data visualization all of cell culture experiments and wrote the manuscript. Suphunwadee Sawong, Pennapha Supnoppakit and Thitima Sapapak conducted, and performed the statistical analysis all of cell culture experiments and wrote the manuscript. Adisa Jittrasirichok and Chayanee Saennaronk conducted and performed all of extraction and determination of phytochemicals experiments and wrote the manuscript. Dumrongsak Pekthong and Supawadee Parhira designed, conducted, performed the statistical analysis all of extraction and determination of phytochemicals experiments and data visualization of extraction, determination of phytochemicals and cell culture experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abubaker, M., Hawary, S., Mahrous, E., and El-Kader, E. 2017. Study of nutritional contents Macadamia integrifolia Maiden and Betche leaves, kernel and pericarp cultivated in Egypt. International Journal of Pharmacognosy and Phytochemical Research. 9(12):1442-1445.
Adriana Dailey, A. Dailey, Quan V. Vuong, Q. V. Vuong, and Fatih Yildiz, F. Yildiz. 2015. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food and Agriculture. 1: 1115646.
Bittenbender, H.C. and Hirae, H.H. 1990. Common problems of macadamia nut in Hawaii. Reseach Extension Series. 112.
Chang, C.L., Lin, C.S., and Lai, G.H. 2012. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evidence-Based Complementary and Alternative Medicine. 2012: 984295.
Chittasupho, C., Samee, W., Tadtong, S., Jittachai, W., Managit, C., and Athikomkulchai, S. 2023. Cytotoxicity, apoptosis induction, oxidative stress, and cell cycle arrest of Clerodendrum chinense flower extract nanoparticles in HeLa cells. Natural and Life Sciences Communications. 22(4): e2023057.
Griel, A.E., Cao, Y., Bagshaw, D.D., Cifelli, A.M., Holub, B., and Kris-Etherton, P.M. 2008 A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. The Journal of Nutrition. 138(4):761-767.
Horticultural Research Institute. Department of Agriculture. Ministry of Agriculture and cooperatives of Thailand. 2023. Macadamia (in Thai). Accessed online on January 3rd, 2024, from https://www.doa.go.th/hort/?page_id=52678.
Horticultural Research Institute. Department of Agriculture. Ministry of Agriculture and cooperatives of Thailand. 2021. Macadamia production situation (in Thai). Accessed online on January 3rd, 2024, reported date on January 12th, 2021, from https://www.doa.go.th/hort/wp-content/uploads/2021/01/%E0%B8%AA%E0%B8%96%E0%B8%B2%E0%B8%99%E0%B8%81%E0%B8%B2%E0%B8%A3%E0%B8%93%E0%B9%8C%E0%B8%81%E0%B8%B2%E0%B8%A3%E0%B8%9C%E0%B8%A5%E0%B8%B4%E0%B8%95%E0%B8%A1%E0%B8%B0%E0%B8%84%E0%B8%B2%E0%B9%80%E0%B8%94%E0%B9%80%E0%B8%A1%E0%B8%B5%E0%B8%A2_%E0%B8%A1%E0%B8%81%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%A164.pdf
Information on WFO. 2022. Macadamia integrifolia in World Flora Online. Version 2022. [12]. 2022. Published on the Internet; http://www.worldfloraonline.org. Accessed on:[5 Mar 2023].
Likittrakulwong, W., Chanburee, S., Kitpot, T., Ninjiaranai, P., and Pongpamorn, P. 2023. Phytochemical properties, in vitro antimicrobial, and bioactive compounds of banana peel extractions using GC-MS. Natural and Life Sciences Communications. 22(2): e2023021.
Maneechai, P., Leelapornpisid, P., and Poomanee, W. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Natural and Life Sciences Communications. 22(2): e2023030.
McManus, A.M., Nielsen, K.J., Marcus, J.P., Harrison, S.J., Green, J.L., Manners, J.M., et al. 1999. MiAMP1, a novel protein from Macadamia integrifolia adopts a Greek key β-barrel fold unique amongst plant antimicrobial proteins. Journal of Molecular Biology. 293(3): 629-638.
Mutiah, R., Sukardiman, S., and Widyawaruyanti, A. 2017. Cytotoxic effect of crude extract and fraction from Calotropis gigantea leaves on human colon cancer WiDr cell lines. International Journal of Pharmacy and Pharmaceutical Sciences. 9(1): 83–86.
Quinn, L.A. and Tang H.H. 1996. Antioxidant properties of phenolic compounds in Macadamia nuts. Journal of the American Oil Chemist’ Society. 73(11):1585-1588.
Yang. J., Liu, R., and Halim, L. 2009. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Science and Technology. 42:1-8.
Schlörmann, W., Dinc, T., Lorkowski, S., and Glei, M. 2017. Influence of roasting on the chemopreventive potential of Macadamis nuts. Journal of Food Science and Technology. 2(1): 177–185.
Schober, P., Boer, C., and Schwarte, L.A. 2018. Correlation Coefficients: Appropriate Use and Interpretation. Anesthesia and Analgesia, 126(5): 1763–1768.
Sembiring, E.N., Elya, B., and Sauriasari, R. 2018. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacognosy Journal. 10(1): 123-127.
Sirisa-ard, P., Pholsonklam, K., Satchachai, A., Tragoolpua, Y., and Kaewkod, T. 2023. Antioxidant, antibacterial activities and cytotoxicity of garlic leaf extract from garlic waste. Natural and Life Sciences Communications. 22(4): e2023059.
Somwonggin, S., Sirilun, S., Chantawannakul, P., Anuchapreeda, S., & Chaiyana, W. 2022. Potential antioxidant, anti-aging enzymes, and anti-tyrosinase properties of Macadamia (Macadamia integrifolia) pericarp waste products. Asia-Pacific Journal of Science and Technology. 27(02): Mar-Apr (TSB online 2021).
Susilowati, A., Kusuma, Y.S., and Kholibrina, C.R. 2019. Seed morphology and germination of Macadamia (Macadamia integrifolia) from north Sumatra. IOP Conference Series: Earth and Environmental Science. 260 (1) 012164.
Tengco, J.M.J., Atienza, L.M., Sunico, D.J.A., Cayetano, A.C., Barrion, A.S.A., Estacio, M.A.C., and Castillo-Israel, K.A.T. 2023. Nutritional and phytochemical content of freeze-dried fruits of two Philippine bignay (Antidesma bunius (L.) Spreng) cultivars. Natural and Life Sciences Communications. 22(2): e2023028.
Winitchaikul, T., Sawong, S., Surangkul, D., Srikummool, M., Somran, J., Pekthong, D., et al. 2021. Calotropis gigantea stem bark extract induced apoptosis related to ROS and ATP production in colon cancer cells. PLoS One, 16(8): e0254392.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Piyarat Srisawang1, 2, 3, Suphunwadee Sawong1, Pennapha Suknoppakit1, Thitima Sapapak1, Adisa Jittrasirichok4, Chayanee Saennaronk4, Dumrongsak Pekthong2, 5, 6, *, and Supawadee Parhira2, 4, 6,*
1 Department of Physiology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand.
2 Center of Excellence for Innovation in Chemistry, Naresuan University, Phitsanulok 65000, Thailand.
3 Center of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand.
4 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
5 Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
6 Center of Excellence for Environmental Health and Toxicology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok, 65000, Thailand.
Corresponding author: Dumrongsak Pekthong, E-mail: dumrongsakp@nu.ac.th,
Supawadee Parhira, E-mail: supawadeep@nu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: June 8, 2023;
Revised: June 26, 2023;
Accepted: February 2, 2024;
Online First: February 6, 2024

