
Potential use of Polyphenol-Enriched Extract from Moringa oleifera Leaves as an Active Ingredient in Sunscreen
Thi Phuong Anh Tran*, Thi Thao Vy Tran, Thi Lan Pham, and Thi Khanh Vinh PhanPublished Date : February 5, 2024
DOI : https://doi.org/10.12982/NLSC.2024.016
Journal Issues : Number 2, April-June 2024
Abstract Moringa oleifera is commonly referred to as the “tree of life” due to its high nutrient content and valuable biological activities. Widely found in tropical zones, this plant is usually used in local cuisine, cosmetic products and nutraceuticals. In this paper, we explored the potential of Moringa oleifera leaf alcoholic extract as a bioactive ingredient in sunscreen. Characterizations of extract were firstly investigated, including total polyphenol content, antioxidant activity. Formulations of extract containing creams were then created in order to examine its ability to protect against UVB radiation in the presence of other ingredients. Various evaluations of cream were carried out, including stability testing, UV absorption capacity, in vitro SPF values and microbial testing. The results revealed that Moringa oleifera extract showed a promising active ingredient in sunscreen, with a moderate antioxidant activity. Cream containing 2% of extract incorporated with 2% of oxybenzone demonstrated good in vitro SPF values.
Keywords: Moringa oleifera, polyphenol, sunscreen, UV radiation, oxybenzone.
Citation: Tran, T.P.A., Tran, T.T.V., Pham, T.L., and Phan, T.K.V. 2024. Potential use of polyphenol-enriched extract from Moringa oleifera leaves as an active ingredient in sunscreen. Natural and Life Sciences Communications. 23(2): e2024016.
INTRODUCTION
Sunscreens refer to topical products that absorb or deflect a portion of the sun ultraviolet (UV) radiation, thus protecting people against sunburn and, reduce the risk of skin cancer (Stern et al., 1986; Gasparro et al., 1998). There are currently several photo-protective agents bearing many diverse structures used in sunscreen, such as oxybenzone, avobenzone, homosalate, octinoxate… (organic agents) or titan dioxide, zinc oxide… (inorganic agents) (Ngoc et al., 2019). Different agents have different effectiveness against UVA and UVB areas. Hence, producers make formulations by combining different agents in order to get a broad-spectrum protection sunscreen (Moloney et al., 2002). Nevertheless, numerous studies have demonstrated that certain chemical UV-filters, which are prone to photolysis and degradation, can pose potentially harm the skin by generating photodegradants. These chemicals also have the ability to be absorbed into the bloodstream and can result systemic toxicological effects (Benson et al., 2000; Nash et al., 2006; Kockler et al.; 2016). Regarding the environment impact, studies have detected the presence of chemical UV filter in surface water globally, including in the Arctic. This is believed to be linked to limitations in wastewater treatment and long-distance transport (Adler et al., 2020). Furthermore, sunscreens released into coastal waters by swimmers, can stress benthic communities in some reef areas (Danovaro et al., 2008; Downs et al., 2016). Therefore, developing new and effective anti-UV agents that are safe and environmentally friendly represents an attractive trend in the cosmetic field (Korać and Khambholja, 2011; Jimtaisong, 2013; H. C. Polonini et al. 2014; Radice et al., 2016; Yakaew et al., 2020; Pawarisa et al., 2023).
Plant uses sunlight to do photosynthesis, while controlling extreme fluctuations of temperature thanks to the presence of secondary metabolites. Polyphenol is a group of these compounds which have been extensively studied on their antioxidant, antimicrobial, anti-proliferative, anti-inflammatory properties (Jimtaisong, 2013; Saewan and Jimtaisong, 2015; Hu et al. 2017). They all have in common the presence of one or several benzenic cycles bearing one or several hydroxyl groups which make them able to absorb radiation with wavelengths in the range of 200 to 400 nm (UV radiation) (Jimtaisong, 2013). Therefore, utilization of natural polyphenol-containing extracts as anti-UV agents might be a future choice, to gradually replace or reduce the amount of synthesized material needed in sunscreens (Jimtaisong, 2013; Cefali et al., 2016). There are various examples among this class of compounds which have already been used in cosmetic formulations, but there is ongoing research to identify new compounds with even greater photoprotective activity. Green tea contains a group of polyphenols called catechins, is a popular ingredient in many cosmetic products, including sunscreens, to help protect skin from UV-induced damage (Li et al., 2009; Bhattacharya and Sherje, 2020). Resveratrol – a polyphenol found in grapes, berries and peanuts, has been shown to have anti-UV activities, including reducing UV-induced inflammation and DNA damage in skin cells (Reagan-Shaw et al., 2008; Polonini et al., 2013; Freitas et al., 2015; Bhattacharya and Sherje, 2020). Similarity, coffeeberry, pomegranates, berry or grape seed extract also represent potential candidates for use in sunscreen formulations due to their strong antioxidant as well as anti-inflammatory properties (Edgington et al., 2000; Weerakkody et al., 2010; Martincigh and Ollengo, 2016; khammitham et al., 2023).
Moringa oleifera Lam. (M. oleifera), a specie of the family Moringaceae, is a fast-growing, deciduous tree that widely grows in the wild or is cultivated in subtropical or tropical areas, such as South Asia, Africa, Central America...(Moyo et al., 2011). It is mainly cultivated for its seed pods and leaves, used as vegetables and for traditional herbal medicine. Extracts from leaves are particularly rich in polyphenol and other bioactive compounds, such as quercetin, kaempferol, myricetin …which have been shown to have a range of health benefits and used as active ingredients in food, nutraceutical fields (Stohs and Hartman, 2015). Furthermore, the polyphenol antioxidant may work as radical scavengers being thus able to prevent UV induced oxygen free radical generation and lipid peroxidation (Baldisserotto et al., 2018). It is such a fort point to apply extracts in personal care products with multifunctions.
Hence, the present work is to explore anti-UV potential of polyphenol-enriched extract from M. oleifera leaves for designing a broad-spectrum sunscreen. The characterization of the extract, including phenolic content, antioxidant activity and absorption spectrum in the UV ranger was an important first step in understanding its potential as an anti-UV agent. By incorporating the extract into the cream formulations, we evaluated its anti-UV effectiveness on an applicable matrix in different concentrations or in combination with oxybenzone – the FDA UV filter by measuring SPF values, using an in vitro method. Formulations with highest photoprotective activity were finally evaluated for microbial contamination during storage.
MATERIALS AND METHODS
Materials
2,2-diphenyl-1-picrylhydrazyl (DPPH) and Folin Ciocalteu solution, ascorbic acid, gallic acid (GA) were purchased from Asia Laboratory Instrument Company Limited; absolute ethanol was come from Hoa Nam, Viet Nam; reagents: oxybenzone (OB); cetostearyl alcohol, stearic acid, petroleum jelly, potassium hydroxide, propylparaben, methylparaben, petroleum jelly (Vaseline), glycerin, iron (III) chloride were from Organic International Stock Company, Viet Nam.
M. oleifera leaves were collected in My Hai, Ninh Thuan Province, in March 2021. Leaves were naturally dried and avoided direct sunlight. Samples were then crushed into fine powder and stored at 4℃ in dark glass bottles. The M. oleifera leaves powder has a dark green color, a bitter taste and a plant aroma.
Extraction Method
M. oleifera extract from leaves was carried out by following procedure of Vongsak with some modifications (Vongsak et al., 2013). 2 g of leaves powder was mixed with 40 ml of ethanol 96% (a material/solvent ratio 1:20 w/v). Mixture was then sonicated at room temperature for 60 mins in ultrasonic tank Branson CPX 1800-E-USA (paused 10 mins for each 20 mins of sonication). Residue was filtered and added solvent for two more supplemented extractions. Solution of 10% FeCl3 was used to detect polyphenol traces remaining in the residue part. Supernatants were collected, concentrated under vacuum to obtain dried extract using rotary evaporator (IKA RV 10, Germany).
Total Phenolic Content Determination
The Folin-Ciocalteu method was used to determine the total phenolic content (TPC) of the extract with slight modifications (Vongsak et al., 2013). 0.5 ml of the sample (2.4 mg/ml ethanol) was mixed with 2.5 ml of Folin-Ciocalteu reagent solution (diluted at a ratio of 1:10 v/v with distilled water); then added 0.8 ml of 7.5% of sodium carbonate solution to adjust the mixture at pH 10. The mixture was incubated in the dark at room temperature for 30 minutes. The final solution was made up to 25 ml by distilled water and the absorbance at 750 nm was recorded by UV-vis Spectrophotometer Libra S50 (USA). TPC was calculated using a calibration curve performed with GA in the concentration range from 12 to 44 µg/ml. TPC was expressed as a µg of GA equivalent per mg of dried extract (µg GAE/ mg dried extract).
In vitro Antioxidant Activity
DPPH scavenging activity was used to evaluate the antioxidant capacity of samples, following the method of Pothitirat with small modifications (Pothitirat et al., 2009). From the extract at different concentrations, 3.0 ml of DPPH (0.2 mM in pure ethanol) was added and then incubated in the dark for 30 minutes. The absorbance of the samples was measured at 519 nm using the UV-vis Spectrophotometer. The capacity of inhibition was calculated with the following equation (1) and expressed as percentage of inhibition (I%) comparing to the blank (ethanol):
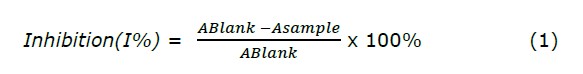
where ABlank is the absorbance of the control reaction (containing all reagents except the test extract), and Asample is the absorbance of the test extract.
The determination of IC50 values were in µg/ml. As a positive control, ascorbic acid was analyzed alongside the test sample.
UV Absorbance Spectrum Profile
The ethanolic extract was diluted to a concentration of 500 µg/ml and its UV spectrum was scanned between wavelengths of 200 and 400 nm using a spectrophotometer. OB – an UV-filter approved by FDA was also screened for its UV spectrum at concentration of 30 µg in 1 ml of absolute ethanol.
Development of sunscreen Formulation
Preparation of six oil in water (o/w) emulsions were followed the protocol of Baldisserotto with some modifications (Figure 1) (Baldisserotto et al., 2018). The emulsifier (cetostearyl alcohol) and other oil soluble components, main active ingredient (OB) were dissolved in the oil phase and heated to 70°C. The base and glycerin were dissolved in the aqueous phase and heated to 70°C. After heating, the aqueous phase was added in portions to the oil phase with continuous stirring; homogenized the mixture for 30 minutes at 70°C. Before adding the extract, it is essential to allow the mixture temperature to decrease to 45°C. The mixture was then stirred in 10 minutes, cooled down to room temperature and stored at 4°C.
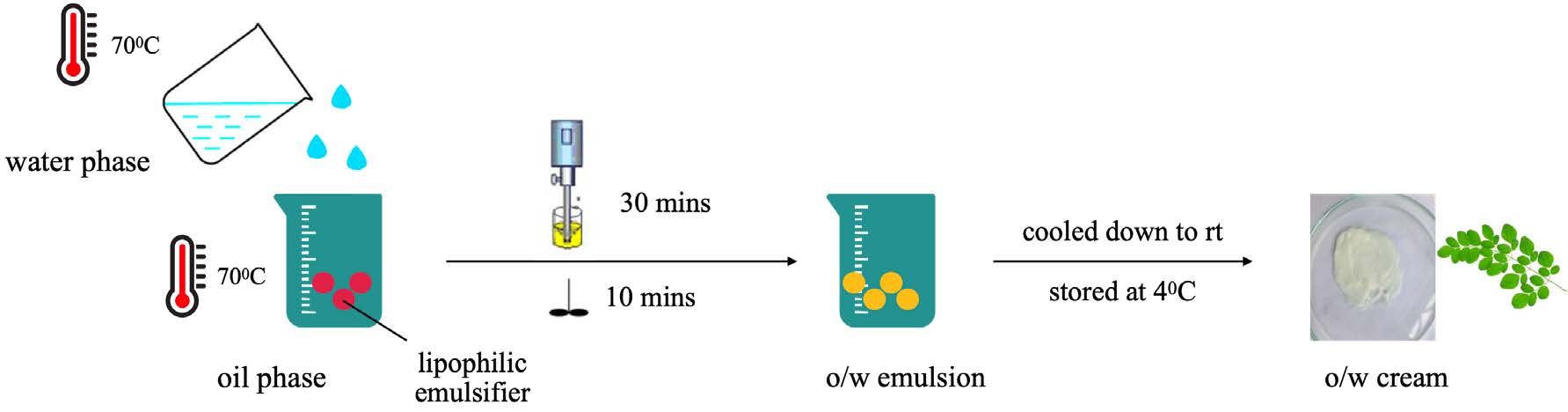
Figure 1. The preparation of o/w formulations.
The extract and the OB at two different concentrations (2% and 4% w/w) were separately integrated or combined with each other into creams (R2-R6). R1 formula was also prepared by not including an active ingredient. The detailed compositions of the formulations were presented in Table 1. Each formula has a total mass of 100 g.
Table 1. Formulations of six o/w sunscreens containing M. oleifera extract and oxybenzone.
|
Ingredient |
Sample (%w/w) |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
|
|
Oil phase |
||||||
|
Cetostearyl alcohol |
5 |
5 |
5 |
5 |
5 |
5 |
|
Stearic acid |
4 |
4 |
4 |
4 |
4 |
4 |
|
Vaseline |
1 |
1 |
1 |
1 |
1 |
1 |
|
Extract |
- |
- |
- |
2 |
4 |
2 |
|
Oxybenzone (OB) |
- |
2 |
4 |
- |
- |
2 |
|
Probylparaben |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Methylparaben |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Aqueous phase |
||||||
|
Potassium hydroxide |
1 |
1 |
1 |
1 |
1 |
1 |
|
Glycerin |
5 |
5 |
5 |
5 |
5 |
5 |
|
Distilled water (ml) |
83,96 |
81,96 |
79,96 |
81,96 |
79,96 |
79,96 |
|
Total (g) |
100 |
100 |
100 |
100 |
100 |
100 |
Evaluation of Prepared Formulations
Organoleptic evaluation
The organoleptic properties of creams, including odor, color and appearance were assessed visually using the method described by Mishra (Mishra et al., 2014). The creams were evaluated both at time of preparation and after being stored for 7, 14, 21 and 28 days.
pH evaluation
The pH values of the creams were monitored during 28 days of storage following the procedure of Huy (Huy et al., 2020). 1 g of each cream was added to the beaker containing 99 ml of distilled water. The mixture was stirred until a uniform consistency was achieved, and then the pH values were measured using a pH meter (Cyberscan pH 1,500, Singapore).
Centrifugation stability
In order to assess the physical stability of creams under mechanical stress, centrifugation tests were conducted using the method of Moshin for 28 days storage (Mohsin et al., 2016). 5 g for each of the six samples were individually weighed and placed into 25 ml centrifuge tubes. The tubes were the centrifuged at 5000 rpm for 30 minutes at 30°C in a centrifugation machine (Hettich EBA 21, Germany). The samples were examined for any evidence of phase separation.
Thermal stability
To evaluate the thermal stability of creams, 5 grams of cream samples were placed in a thermostatic tank at three different temperatures (8°C, 25°C and 40°C) with a relative humidity of 60-70% for a period of 28 days. The samples were examined at regular time intervals to observe if any phase separation or liquefaction occurred as well as changes in color or odor which can indicate a loss of stability in the emulsion (Smaoui et al., 2017).
In vitro UVB photoprotection evaluation
Six creams were evaluated for their UVB photoprotection efficacy by calculating in vitro Sun Protection Factor (SPF) values based on the method of Dutra (Dutra et al., 2004). The samples were prepared at three different concentrations: 500, 1,000 and 2,000 µg/ml in absolute ethanol, subjected to 5 minutes of sonication, and then filtered through cotton. Absorbance data of sample solutions were recorded in the wavelength range from 290 – 320 nm (UVB rays), at 5 nm of intervals using the UV-Visible Spectrophotometer. Ethanol was used as a blank. Determinations were made in triplicate at each point. SPF values of each cream were calculated by the application of Mansur equation (2) (Mansur et al., 1986):
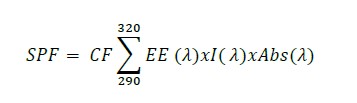
Where: EE: Erythema effect spectrum; I: solar intensity spectrum; Abs: absorbance of formulation; CF: correction factor (= 10). The values EE & I are constant and determined based on the recommendation of Sayre (Sayre et al., 1979).
Microbial testing
Microbial analysis was carried out by following the procedure of FDA guideline, described in the Chapter 23 - Methods for cosmetics of Bacteriological Analytical Manual; ISO 18416:2016 and ISO 22717:2015. 1 g of samples was mixed with 1 ml of Tween 80 and subsequently diluted in a ratio 1:10 in the modified Letheen-Broth (MLB). The mixture was then incubated at 32.5°C ± 2.5°C for 24 hours. Take out a loopful of cultured broth and streak it on the Petri dishes containing SS agar (for P.aeruginosa) and SDA agar (for C.albicans). The presence or absence of P.aeruginosa and C.albicans were determined after incubation for 24 hours at 37°C and for 5 days at room temperature, respectively.
Statistical Analysis
The experiment was carried out no less than three times, with the results reported as the average ± standard deviation. Data and graphs were treated by using Microsoft Excel Software.
RESULTS
The dried extract was obtained after the extraction of M. oleifera leaves powder in environmentally friendly solvent ethanol under ultrasonic technique assistance. Solution of 10% w/w FeCl3 was used as an indicator for the trace of polyphenol remaining in the sample. The dried extract obtained with about 30% of yield was stored in a dark bottle at 4°C for further analysis.
Characterization of the extract
The characterization of M. oleifera extract was firstly performed. Results of TPC, DPPH activity shown in table 2.
Table 2. TPC and DPPH IC50 of the extract. Each value was obtained from three analyses (mean ± SD).
|
Sample |
TPC, µg GAE/mg |
DPPH (IC50), µg/ml |
|
Extract |
10.70 ± 0.15 |
371.00 ± 8.60 |
|
Ascorbic acid |
- |
2.63 ± 0.04 |
Table 2 shows the concentration of polyphenols is about 10.70 µg GAE/mg in the dry extract. The DPPH IC50 value of the extract represents 371.0 ± 8.6 µg/ml. When compared to the positive control, ascorbic acid, which had an IC50 value of 2.63 ± 0.04 µg/ml, the free radical scavenging ability of the studied M. oleifera leaves was weaker by approximately 141 times.
To continue exploring UV absorption capacity of the extract, UV-spectrum profile was recorded and shown in Figure 2.
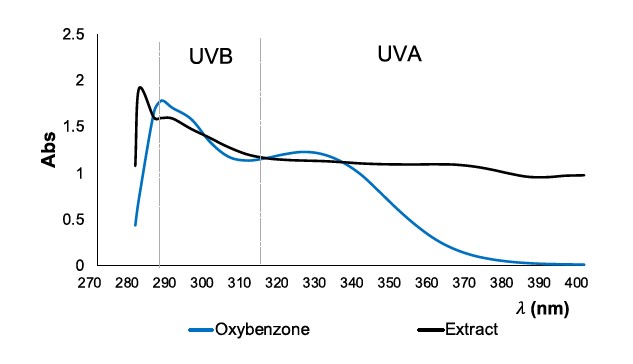
Figure 2. UV Spectrum profile of: oxybenzone (30 µg/ml, blue curve) and extract (500 µg/ml, black curve).
In Figure 2, the UV absorbance spectrum profile of the reference OB at 30 µg/ml showed maximum absorbance at around 285 nm which is at the border of the UVC and UVB area. Its next strong absorbances are around 290 nm and 300 nm, which are in a range of UVB and UVA, respectively. The extract spectrum exhibits an absorbance profile that is similar to OB, with minor variations. At around 280 nm, which corresponds to the UVC range, the extract demonstrates maximum absorbance. A secondary strong absorbance is seen at approximately 290 nm. The extract shows substantial absorbance in both the UVB and UVC ranges at a concentration of 500 µg/ml, which is 17 times more than that of OB.
In the next step, the extract will be incorporated into creams with the aim to discover its compatibility with other components of creams as well as its efficacy for sun protection.
Evaluation of prepared formulations
Organoleptic evaluation
The obtained formulations were submitted to a set of organoleptic (color, odor and appearance) analysis (Table 3).
Table 3. Organoleptic evaluations of freshly prepared creams.
|
Parameters |
Formulation |
|||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
|
|
Color |
White |
Yellow white |
Yellow white |
Green |
Moss green |
Moss green |
|
Flavor |
Mild leaf scent |
Mild leaf scent |
Mild leaf scent |
Mild leaf scent |
Mild leaf scent |
Mild leaf scent |
|
Appearance |
Good |
Good |
Good |
Good |
Good |
Good |
|
pH |
5.8 |
6.2 |
5.9 |
6.3 |
6.1 |
6.4 |
The findings revealed that the freshly prepared formulations had color ranging from white to moss green, as shown in Figure 3. The color of the creams varied depending on the active ingredients and their concentrations, with two creams appearing yellow -white (R2, R3) and others appearing green (R4, R5, R6). All of the creams had a dried leaves aroma. When a small amount of cream was applied to the skin, it can provide a feeling of softness and smoothness.

Figure 3. Cream samples: R1 (base); R2 (2% w/w OB); R3 (4% w/w OB); R4 (2% w/w Extract); R5 (4% w/w Extract); R6 (2% w/w OB and 2% w/w Extract).
Stability of creams
Formulations were placed in different storage conditions (8, 25 and 40°C) in thermostatic tanks for 28 days. The samples were periodically evaluated at day 7, 14, 21, and 28 for changes in color, pH, liquefaction and phase separation. Data for these parameters measured at day 7 were presented in table 4.
Table 4. Stability evaluations of creams after 28-day storage at 8°C, 25°C and 40°C.
|
Parameters |
Formulation |
||||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
||
|
Color |
8°C |
- |
- |
- |
- |
- |
- |
|
25°C |
- |
- |
- |
- |
- |
- |
|
|
40°C |
- |
- |
- |
+ |
+ |
+ |
|
|
pH |
8°C |
- |
- |
- |
- |
- |
- |
|
25°C |
- |
- |
- |
- |
- |
- |
|
|
40°C |
- |
- |
- |
- |
- |
- |
|
|
Liquefaction |
8°C |
- |
- |
- |
- |
- |
- |
|
25°C |
- |
- |
- |
- |
- |
- |
|
|
40°C |
- |
- |
- |
- |
- |
- |
|
|
Centrifugation test |
8°C |
- |
- |
- |
- |
- |
- |
|
25°C |
- |
- |
- |
- |
- |
- |
|
|
40°C |
- |
- |
- |
- |
- |
- |
|
Note: -: no change; +: slight change
Color changes
After 28 days of storage at different temperatures, the color of most of the creams remained unchanged. However, some slight changes in color were observed in formulas R4, R5, and R6, which contained M. oleifera extracts at 40°C. The green color of these creams has faded, as shown in Figure 4.
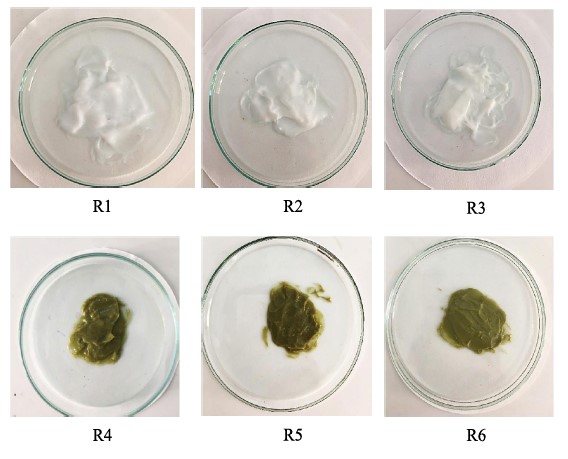
Figure 4. The color of six formulations after 28- days storage.
pH changes
Monitoring the pH values represents very important work to determine the stability of the emulsion. In fact, a change in pH indicates the occurrence of chemical reactions that can affect the quality of a product (Smaoui et al., 2017). Additionally, the pH level of human skin is typically around 5.5, which is slightly acidic. Therefore, emulsions that are formulated with a pH that is similar to that of the skin, can help to maintain the skin’s natural acidity and support its barrier function (Lambers, et al., 2006). In this study, pH values of the freshly prepared emulsion were given in Table 3, which ranged from 5.8 to 6.4. These values remained consistent with the pH of the skin, which is generally around 5.5. During the storage investigation, the pH values of emulsions varied slightly, but these variations were not significantly different.
Liquefaction
The viscosity of an emulsion is an important factor in determining its flow properties, and can vary depending on temperature and storage time. High temperatures can cause a decrease in viscosity, which can lead to an increase in liquefaction (Boyd, et al., 1972). However, in the study, no liquefaction was observed in any of the storage conditions for the emulsion, even at a moderately high temperature 40°C for 28 days storage. This result provides strong evidence for the stability of the emulsion under investigation.
Centrifugation test
Emulsions are thermodynamically unstable systems and can readily separate into distinct oil and water layers. This instability is attributed to the difference in densities between the oil and aqueous phases and the unfavorable contact between oil and water molecules (Maphosa et al., 2018). In this study, no evidence of phase separation was observed in any of the samples after centrifugation, regardless of the storage condition for up to 28 days. These results suggest that all of the emulsions were physically stable. The use of an appropriate emulsifier, i.e cetostearyl alcohol, and proper homogenization speed during emulsion formation can aid in the creation and maintenance of a stable emulsion.
Sun protective activity
UVB absorbance spectrum of creams at 500 µg/ml in absolute ethanol are shown in Figure 5.
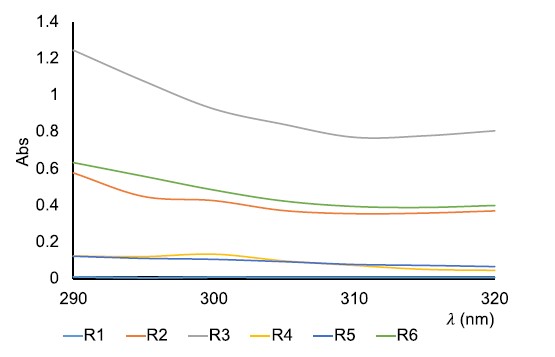
Figure 5. UVB absorption spectrum of creams at 500 µg/ml.
Out of the six creams that were evaluated for their ability to absorb UVB radiation capacity, the formula R3 demonstrated the most effective UVB absorption (indicated by the gray line). This cream contained 4% of OB. The R6 formula (indicated by the green line), which contained 2% mixture of OB and extract, also showed good UVB absorption activity. Cream, which only contained 2% of OB (indicated by the orange line), was absorbed less effectively than R6. Creams R4 and R5, which contained 2 and 4% extract, respectively, demonstrated similar absorption spectra. Cream R1, which was the matrix, did not absorb UVB radiation. All of the absorption spectra exhibited a similar trend, with no peak and a decrease in absorption with increasing wavelength. Based on this spectrum, the combination between OB and extract in cream produced the best results compared to the other formulations.
In this study, the SPF values of six creams were evaluated at concentrations of 500, 1,000, and 2,000 µg/ml using the Mansur equation. Results were compared in Figure 6 to assess the anti-UVB activity of the samples.
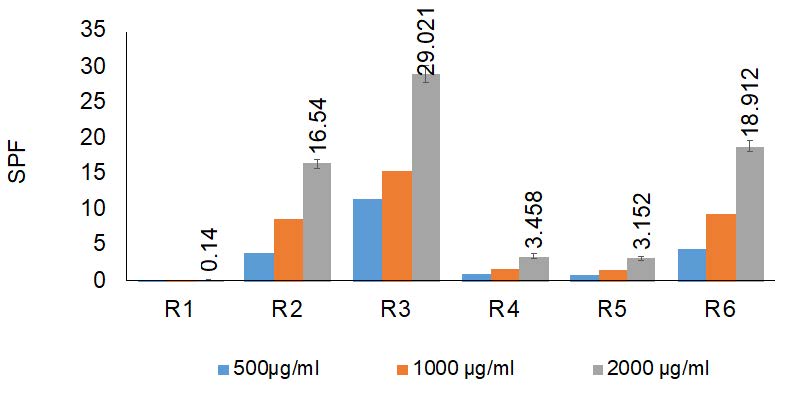
Figure 6. The in vitro SPF values of samples at concentration 500, 1,000, and 2,000 µg/ml.
Based on Figure 6, the in vitro SPF values of the creams increased with increasing concentrations of the creams. To facilitate comparison, the SPF values of the cream at 2,000 µg/ml are discussed. These values ranged from 0.14 to 29.021 of the six creams. Specifically, R3 had the highest SPF value (29.021 ± 1.16), which is consistent with its UVB absorption ability. R4 and R5 exhibited similar effects against UVB radiation, with SPF values of 3.458 and 3.152, respectively. R6 had a relatively high SPF value of 18.912 ± 0.742.
Microbial evaluation
Two formulations with the highest SPF values, R3 and R6, were chosen for the microbiological analysis to detect the presence of Candida albicans and Pseudomonas aeruginosa, which are recognized as the major potential pathogens in cosmetics (Lundov and Zachariae, 2008). Moreover, Pseudomonas aeruginosa is known to be highly resistant to antibacterial agents and many broad-spectrum antibiotics. Figure 7 displays the outcomes of the C. albicans and P. aeruginosa tests at dilution 1:10.
The figure shows that, following an incubation period of 1-3 days at 37°C, there was no growth of C. albicans and P. aeruginosa observed in either of the samples at a dilution factor of 10-3 or at dilution factors of 10-4 and 10-5 (data not shown). This suggests that both cream samples have the ability to resist these strains due to the presence of 0.04% total of propylparaben and methylparaben acting as the primary preservatives.
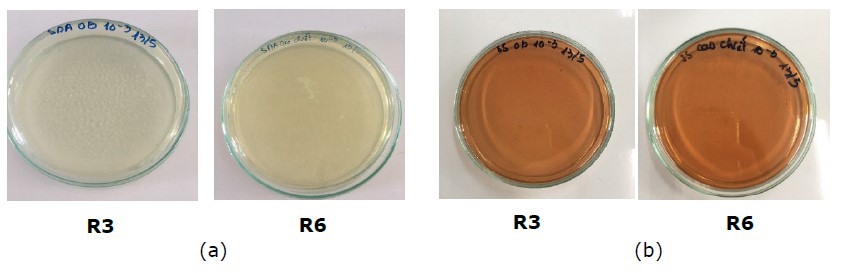
Figure 7. Microbial testing for C. albicans (a) and P. aeruginosa (b) in the two cream samples R3 and R6.
DISCUSSION
With the trend of using plant extracts as an active ingredient, alternative to synthetic chemicals in sunscreen, discovering the one that has not only photoprotection activity but also other activities such as antioxidant activity, antimicrobial activity for skin could be a promising approach. Natural polyphenol might represent a suitable candidate for this approach. Bearing poly-aromatic rings in its structure, polyphenol absorbs UV irradiation of sunlight and prevents its absorption through skin. Moreover, it is well-known that this kind of compound comes from secondary metabolism of plants which possess many biological activities.
In this study, M. oleifera was chosen as a plant of interest, given its popularity in Vietnam. The extract was obtained from M. oleifera leaves using ethanol as an environment-friendly solvent through ultrasound- assisted extraction. The TPC of the extract was found to be equivalent to the study of Trâm et al. (2016) on the same plant in Can Tho (Viet Nam), (Trâm and My, 2016). They got about 9.68 µg GA/mg of TPC according to the Soxhlet extraction method. However, the TPC obtained from M. oleifera leaves collected in Senegal, as reported by Baldisserotto et al. (2018), was higher than TPC obtained in our study. In their study, the TPC was found to be approximately 47.7 ± 1.58 µg GAE/mg (Baldisserotto et al., 2018). Similarity, the study conducted by Prasajak in Thailand, the TPC was reported to be 41, 49 µg GAE/mg (Prasajak et al., 2021). The differences between the results may be due to different raw materials sources and extraction methods employed, leading to different polyphenol content in the samples.
Antioxidant activity, which plays an important role in photoprotection due to its usefulness against UV-exposure-mediated oxidative stress, was also evaluated. The anti-DPPH efficacy of the M. oleifera extract was compared to similar studies. Trâm et al. (2016) reported the maximum anti-DPPH efficacy with an IC50 of 537 µg/ml in Can Tho (Viet Nam), which was lower than the value obtained in this study (Trâm and My, 2016). Vongsak’s study on Thai M. oleifera leaves revealed a greater antioxidant activity than other studies, with a value of 62, 94 µg/ml (Vongsak et al., 2013). The DPPH value suggests a moderate degree of activity when compared to other plant extracts recognized for their fascinating antioxidant profile.
Next, the anti-UV capacity of the extract was assessed and compared to OB – a commercial UV filter. The obtained UV-absorbance spectrum of the extract showed similarities to the study conducted by Abdul Rahman Abid et al., which demonstrated that the OB provides a broad -spectrum sun protection in the UV range from 280 nm to 390 nm (Abid et al., 2017).
OB, a synthesized organic compound, is widely utilized in sunscreen due to its excellent absorption of UV rays from sunlight and its high solubility in most organic solvents. Growing evidence shows that OB may be harmful to human health, affecting hormone production and causing abnormal physiological processes such as children growth, metabolism, atopic dermatitis, coral ecosystems (Mirsky et al., 2018). Hawaii has banned tourists from using sunscreen containing OB while swimming (Levine, 2020). Based on the obtained spectrum, the extract has the potential to serve as an active ingredient, either alone or in conjunction with synthetic chemicals, such as OB, in sunscreen or other photoprotective products.
The extract was then incorporated into cream formulations and their physical properties and stability were evaluated. The results indicated that the emulsions remained chemically stable throughout the storage period, with minimal pH changes. However, color changes were observed, likely due to the oxidation of chlorophylls present in the extract at higher temperatures. The emulsions showed good compatibility with other ingredients, but the green color may pose a challenge for aesthetic appeal.
The sun protective activity of the formulations was assessed through in vitro SPF testing, which can be a valuable screening tool during product development. Products with higher in vitro SPF values are more efficient at preventing sunburn (Dutra et al., 2014; Lourith et al., 2017). The R6 formula, which contained a 2% mixture of OB and extract, demonstrated promising results in terms of UVB absorption capacity and a high SPF value of 18.912. The study of Dutra et al. (2014) examined the SPF values of commercially available creams using a similar method to that used in our study. These creams contained a combination of at least two popular photoprotective agents and the determined SPF values ranged 10 to 20 in a solution with a concentration 10 times lower than that used in our study (Dutra et al., 2004).
The accurate determination of the SPF for photoprotective products is typically achieved through the ISO 24444:2019 in vivo method. However, in our ongoing study, we have not yet reached that stage. In the interim, in vitro SPF values can serve as a predictive measure for assessing sunscreen efficacy during the production process. One such method, based on Mansur’s equation, offers several advantages, including rapidity, cost-effectiveness, and user-friendliness, making it a valuable tool for estimating and screening SPF values during sunscreen development (Acsova et al., 2021).
Microbial testing was conducted to ensure the safety of the formulations, and the utilization of 0.02% propylparaben and 0.02% methylparaben was found to be within the regulated limits considered safe for consumer by the SCCS (Scientific Committee on Consumer Safety). Furthermore, the ethanol extract of M. oleifera leaves, which contains compounds, such as zeatin, quercetin, kaempferom, tannins exhibited activity against P. aeruginosa (Bukar et al., 2010).
These results suggest that the combination of OB and extract in the cream gives positive outcomes, including: (1) effective sun protection; (2) the ability to gradually reduce the OB content in the cream; and (3) compatibility of extracts with other ingredients in the cream. While the SPF values of this combination may not be as high as that of R3 (which contained only OB), it is an improvement over R5 (which contained only extract). However, further research and testing are necessary to fully evaluate the efficacy, safety, and stability of the formulation.
CONCLUSION
The research has successfully studied five sunscreen creams which contain (1) active ingredient OB and (2) polyphenol enriched extract from M. oleifera leaves. The evaluation of UV absorption activity, stability and SPF values of creams were carried out. The results showed high potential application of M. oleifera dried extract used as active ingredient in creams: (1) its UV absorbance spectrum was similar to OB with concentration 17 times lower; (2) compatibility to other ingredients in creams; (3) R6 (mixture of 2% OB and 2% Extract) has a positive SPF value (18.92). Owning moderate DPPH scavenging radical activity, M. oleifera extract has been shown to have a synergistic effect in protecting the skin against UVB radiation. This is achieved through both the absorption of UVB radiation and the supply of antioxidant agents, which work together to prevent UVB oxidative damages to the skin.
The use of natural sunscreen active ingredients is becoming increasingly popular in the cosmetic industry, in order to promote public health and protect the environment. The polyphenol-enriched extract from M. oleifera leaves shows promise as a potential candidate for this purpose.
ACKNOWLEDGEMENTS
We acknowledge the support of time and facilities from Nha Trang University for this study.
AUTHOR CONTRIBUTIONS
Thi Phuong Anh Tran: Study conception and design, data collection, analysis and interpretation of results, and manuscript preparation; Thi Thao Vy Tran: Performed experiments of cream formulations and physical evaluation; data collection, analysis and interpretation of results; Thi Lan Pham: Performed experiments of microbial testing & collection data; Thi Khanh Vinh Phan: analysis and interpretation of results, review and editing manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abid, A.R., Marciniak, B., Pędziński, T., and Shahid, M. 2017. Photo-stability and photo-sensitizing characterization of selected sunscreens’ ingredients. Journal of Photochemistry and Photobiology A: Chemistry. 332: 241–250.
Acsova, A., Hojerova, J., Janotkova, L., Bendova, H., Jedličková, L., Hamranova, V. et al., 2021. The real UVB photoprotective efficacy of vegetable oils: In vitro and in vivo studies. Photochemical and Photobiological Sciences. 20: 139-151.
Adler, B. L. and DeLeo, V. A. 2020. Sunscreen safety: A review of recent studies on humans and the environment. Current Dermatology Reports. 9: 1-9.
Baldisserotto, A., Buso, P., Radice, M., Dissette, V., Lampronti, I., Gambari, R. et al. 2018. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules. 23(3): 664.
Bhattacharya, S. and Sherje, A.P. 2020. Development of resveratrol and green tea sunscreen formulation for combined photoprotective and antioxidant properties. Journal of Drug Delivery Science and Technology. 60: 102000.
Boyd, J., Parkinson, C., and Sherman, P. 1972. Factors affecting emulsion stability, and the HLB concept. Journal of Colloid and Interface Science. 41(2): 359–370.
Benson, H.A. 2000. Assessment and clinical implications of absorption of sunscreens across skin. American Journal of Clinical Dermatology. 1: 217-224.
Bukar, A., Uba, A., and Oyeyi, T. 2010. Antimicrobial profile of Moringa oleifera Lam. extracts against some food – borne microorganisms. Bayero Journal of Pure and Applied Sciences. 3(1): 43-48.
Cefali, L.C., Ataide, J.A., Moriel, P., Foglio, M.A., and Mazzola, P.G. 2016. Plant-based active photoprotectants for sunscreens. International Journal of Cosmetic Science. 38(4): 346–353.
Danovaro, R., Bongiorni, L., Corinaldesi, C., Giovannelli, D., Damiani, E., Astolfi, P. et al. 2008. Sunscreens cause coral bleaching by promoting viral infections. Environmental Health Perspectives. 116(4): 441–447.
Downs, C.A., Kramarsky-Winter, E., Segal, R., Fauth, J., Knutson, S., Bronstein, O. et al. 2016. Toxicopathological effects of the sunscreen UV filter, oxybenzone (Benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Archives of Environmental Contamination and Toxicology. 70(2): 265–288.
Dutra, E.A., Oliveira, D.A.G. da C.e, Kedor-Hackmann, E.R.M., and Santoro, M.I.R.M. 2004. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas. 40: 381–385.
Edgington, S., Segura, H., De la Rosa, W., and Williams, T. 2000. Photoprotection of Beauveria bassiana: Testing simple formulations for control of the coffee berry borer. International Journal of Pest Management. 46(3): 169–176.
Food and Drug Administration (FDA). Bacteriological analytical manual chapter 23: Methods for Cosmetics. 2021.
Freitas, J.V., Praça, F.S.G., Bentley, M.V.L.B., and Gaspar, L.R. 2015. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. International Journal of Pharmaceutics. 484(1): 131–137.
Gasparro, F.P., Mitchnick, M., and Nash, J.F. 1998. A Review of Sunscreen Safety and Efficacy. Photochemistry and Photobiology. 68(3): 243–256.
Hu, S., Zhang, X., Chen, F., and Wang, M. 2017. Dietary polyphenols as photoprotective agents against UV radiation. Journal of Functional Foods. 30: 108–118.
Huy Đ.Q., Thịnh N.Q., Minh N.N., and Hoài L.T. 2020. Study on formulation and evaluation of burn removal cream from Aloe vera l., Centella asiatica Urb., Coptis chinensis Franch. TNU Journal of Science and Technology. 225(08): 161–167.
ISO 18416:2015. Cosmetics - Microbiology - Detection of Candida albicans (ISO 18416:2015, Corrected version 2016-12-15). International Organization for Standardization (ISO): Geneva, Switzerland. 2015.
ISO 22717:2015. Cosmetics - Microbiology - Detection of Pseudomonas aeruginosa. International Organization for Standardization (ISO): Geneva, Switzerland. 2015.
ISO 24444:2019 (SPF), Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor 2019. Available online: https://www.iso.org/standard/72250.html (accessed on 16 September 2022).
Jimtaisong, N.S. and A. 2013. Photoprotection of natural flavonoids. Journal of Applied Pharmaceutical Science. 3(9): 129–141.
Khammitham, T., Leelapornpisid, P., Chansakaow, S., Leelapornpisid, W., and Poomanee, W. 2023. Cleistocalyx nervosum var. paniala extract: Anti-acne, anti-oxidant, anti-inflammatory activities and safety for cosmeceutical applications. Natural and Life Sciences Communications. 22(4): e2023064.
Kockler, J., Oelgemöller, M., Robertson, S., and Glass, B.D. 2012. Photostability of sunscreens. Journal of Photochemistry and Photobiology C: Photochemistry Reviews. 13(1): 91-110.
Korać, R.R. and Khambholja, K.M. 2011. Potential of herbs in skin protection from ultraviolet radiation. Pharmacognosy Reviews, 5(10): 164–173.
Lambers, H., Piessens, S., Bloem, A., Pronk, H., and Finkel, P. 2006. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. International Journal of Cosmetic Science. 28(5): 359–370.
Levine, A. 2020. Sunscreen use and awareness of chemical toxicity among beach goers in Hawaii prior to a ban on the sale of sunscreens containing ingredients found to be toxic to coral reef ecosystems. Marine Policy. 117: 103875.
Li, Y.-H., Wu, Y., Wei, H.-C., Xu, Y.-Y., Jia, L.-L., Chen, J. et al. 2009. Protective effects of green tea extracts on photoaging and photommunosuppression. Skin Research and Technology. 15(3): 338–345.
Lourith, N., Kanlayavattanakul, M., and Chingunpitak, J. 2017. Development of sunscreen products containing passion fruit seed extract. Brazilian Journal of Pharmaceutical Sciences. 53: e16116.
Lundov, M.D. and Zachariae, C. 2008. Recalls of microbiologically contaminated cosmetics in EU from 2005 to May 2008. International Journal of Cosmetic Science. 30(6): 471–474.
Mansur, J. de S., Breder, M.N.R., Mansur, M.C. d’Ascençäo, and Azulay, R.D. 1986. Determinaçäo do fator de proteçäo solar por espectrofotometria. Anais Brasileiros de Dermatologia. 61(3): 121–124.
Maphosa, Y., Jideani, V.A., Maphosa, Y., and Jideani, V.A. 2018. Factors affecting the stability of emulsions stabilised by biopolymers. In Science and Technology Behind Nanoemulsions. IntechOpen.
Martincigh, B.S. and Ollengo, M.A. 2016. The photostabilizing effect of grape seed extract on three common sunscreen absorbers. Photochemistry and Photobiology. 92(6): 870–884.
Mirsky, R.S., Prado, G., Svoboda, R.M., and Rigel, D.S. 2018. Oxybenzone and sunscreens: A critical review of the evidence and a plan for discussion with patients. SKIN The Journal of Cutaneous Medicine. 2(5): 264-268.
Mishra, A.P., Saklani, S., Milella, L., and Tiwari, P. 2014. Formulation and evaluation of herbal antioxidant face cream of Nardostachys jatamansi collected from Indian Himalayan region. Asian Pacific Journal of Tropical Biomedicine. 4: S679–S682.
Mohsin, S., Akhtar, N., Mahmood, T., Khan, H., and Mustafa, R. 2016. Formulation and stability of topical water in oil emulsion containing corn silk extract. Tropical Journal of Pharmaceutical Research. 15(6): 1115–1121.
Moloney, F.J., Collins, S., and Murphy, G.M. 2002. Sunscreens. American Journal of Clinical Dermatology. 3(3): 185–191.
Moyo, B., Masika, P.J., Hugo, A., and Muchenje, V. 2011. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnolog. 10(60): 12925–12933.
Nash, J.F. 2006. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatologic Clinics. 24(1): 35-51.
Ngoc, L.T.N., Tran, V.V., Moon, J.-Y., Chae, M., Park, D., and Lee, Y.-C. 2019. Recent trends of sunscreen cosmetic: An update review. Cosmetics. 6(4): 64.
Pawarisa M., Pimporn L., and Worrapan P. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Natural and Life Sciences Communications, 22(2).e2023030.
Polonini, H.C., Brandão, M.a.F., and Raposo, N.R.B. 2014. A natural broad-spectrum sunscreen formulated from the dried extract of Brazilian Lippia sericea as a single UV filter. RSC Advances. 4(107): 62566–62575.
Polonini, Hudson Caetano, Lima, L.L., Gonçalves, K.M., do Carmo, A.M.R., da Silva, A.D., and Raposo, N.R.B. 2013. Photoprotective activity of resveratrol analogues. Bioorganic and Medicinal Chemistry. 21(4): 964–968.
Pothitirat, W., Chomnawang, M. T., Supabphol, R., and Gritsanapan, W. 2009. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 80(7): 442–447.
Prasajak, P., Renumarn, P., Sriwichai, W., and Detchewa, P. 2021. Antioxidant and antimicrobial properties of Moringa oleifera leaves and pods extracts in pork meatballs during cold storage. Chiang Mai University Journal of Natural Sciences. 20(2): e2021033.
Radice, M., Manfredini, S., Ziosi, P., Dissette, V., Buso, P., Fallacara, A., and Vertuani, S. 2016. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia. 114: 144–162.
Reagan-Shaw, S., Mukhtar, H., and Ahmad, N. 2008. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells†. Photochemistry and Photobiology. 84(2): 415–421.
Saewan, N. and Jimtaisong, A. 2015. Natural products as photoprotection. Journal of Cosmetic Dermatology. 14(1): 47–63.
Sayre, R.M., Agin, P.P., LeVee, G.J., and Marlowe, E. 1979. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochemistry and Photobiology. 29(3): 559–566.
SCCS (Scientific Committee on Consumer Safety), Opinion on parabens, 14 December 2010, revision of 22 March 2011.
Smaoui, S., Ben Hlima, H., Ben Chobba, I., and Kadri, A. 2017. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arabian Journal of Chemistry. 10: S1216–S1222.
Stern, R.S., Weinstein, M.C., and Baker, S.G. 1986. Risk reduction for nonmelanoma skin cancer with childhood sunscreen use. Archives of Dermatology. 122(5): 537–545.
Stohs, S.J. and Hartman, M.J. 2015. Review of the safety and efficacy of Moringa oleifera. Phytotherapy Research. 29(6): 796–804.
Trâm P.T.B., and My N.T.D. 2016. Khảo sát hoạt tính các hợp chất kháng oxy hóa trong lá và thân cây chùm ngây (Moringa oleifera). Tạp chí Khoa học Đại học cần Thơ. (CĐ Nông nghiệp 2016): 179–184.
Vongsak, B., Sithisarn, P., Mangmool, S., Thongpraditchote, S., Wongkrajang, Y., and Gritsanapan, W. 2013. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Industrial Crops and Products. 44: 566–571.
Weerakkody, P., Jobling, J., Infante, M. M. V., and Rogers, G. 2010. The effect of maturity, sunburn and the application of sunscreens on the internal and external qualities of pomegranate fruit grown in Australia. Scientia Horticulturae. 124(1): 57–61.
Yakaew, S., Phimnuan, P., Tiensomjitr, K., Nakyai, W., Nuengchamnong, N., and Ross, G. 2020. Hom–Kularb–Dang rice bran extract for the prevention of UVB-damage against human skin fibroblast. Chiang Mai University Journal Natural Sciences. 19(1): 34-50.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Thi Phuong Anh Tran1, *, Thi Thao Vy Tran1, Thi Lan Pham2, and Thi Khanh Vinh Phan3
1 Deparment of Chemical Engineering, Nha Trang University, Viet Nam.
2 Institut of Biotechnology and Environment, Nha Trang University, Viet Nam.
3 Department of Food Technology, Nha Trang University, Viet Nam.
Corresponding author: Thi Phuong Anh Tran, E-mail: anhttp@ntu.edu.vn
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: November 23, 2023;
Revised: January 12, 2024;
Accepted: January 29, 2024;
Online First: February 5, 2024

