
Significantly Elevated Urinary OH-PAHs and Oxidative Damage Concentrations Attributable to PM2.5 Exposure: A Panel Study of Preaging Females in Chiang Mai, Thailand
Pitakchon Ponsawansong, Tippawan Prapamontol*, Jaras Singkaew, Guoxing Li, and Xiaochuan PanPublished Date : January 19, 2024
DOI : https://doi.org/10.12982/NLSC.2024.015
Journal Issues : Number 1, January-March 2024
Abstract Exposure to ambient particulate matter with an aerodynamic diameter ≤ 2.5 µm (PM2.5) has been associated with oxidative damage. This study investigated the PM2.5-bound polycyclic aromatic hydrocarbon (PAH) exposure levels during elevated PM2.5 levels and the oxidative damage among a preaging females in Chiang Mai, Thailand. The 64 female participants, aged between 50 and 65 years old, were followed for 5 weeks consecutively during the non-haze rainy season as the baseline and haze episodes. The morning void urine sample was collected weekly and analyzed for 8 species of hydroxylated-polycyclic aromatic hydrocarbons (OH-PAHs) and malondialdehyde (MDA). The results showed that the high increase in PM2.5 levels was positively associated with OH-PAH levels. In addition, for every 1 µg/gCre increase in individual urinary OH-PAH concentrations, the MDA concentration increased from 0.073 to 2.071 µg/gCre. Therefore, the present study suggested that detected urinary OH-PAHs were attributable to PM2.5-bound PAH exposure, particularly from inhalation. These study results provide useful information for policy makers and public health agencies to mitigate the chain effects of PM2.5 exposure from haze pollution.
Keywords: Air pollution, Hydroxylated-PAHs, Oxidative stress, Urinary biomarkers
Citation: Ponsawansong P., Prapamontol, T., Singkaew, J., Li, G., and Pan, X. 2024. Significantly elevated urinary OH-PAHs and oxidative damage concentrations attributable to PM2.5 exposure: A panel study of preaging females in Chiang Mai, Thailand. Natural and Life Sciences Communications. 23(1): e2024015.
INTRODUCTION
The elevated airborne particulate matter particularly with an aerodynamic diameter ≤ 2.5 µm (PM2.5) level has occurred annually in upper northern Thailand from January to April for over a decade due to intensive biomass burning, i.e., agricultural waste and forest fires. The increase in PM2.5 levels during this time exceeded the 24-h Thai National Ambient Air Quality Standards (NAAQSs) at 37.5 µg/m3 approximately 2 to 6 folds. This period is commonly known as the smoke haze episode or biomass burning season (Khamkaew et al. 2016b; Thepnuan and Chantara 2020a; Kawichai et al. 2020b).
Exposure to ambient PM2.5 was reported to have adverse health effects on cardiovascular and respiratory diseases (Son et al. 2012; Kim et al. 2013; Mueller et al. 2020; Paoin et al. 2021a), particularly in vulnerable populations, including infants, children, and the elderly (Chen et al. 2018; Pothirat et al. 2019; Mueller et al. 2020). These effects are mainly from the cellular response through the mechanism of oxidative stress and inflammation (Miller et al. 2012; Gamon and Wille 2016; Abdal Dayem et al. 2017). In addition, recent studies have shown that chemicals bound to PM2.5, rather than PM size alone, play a crucial role in health effects due to their variation from different sources of emissions (Son et al. 2012; Wu et al. 2012a; Wu et al. 2012b).
Polycyclic aromatic hydrocarbons (PAHs) are the product of incomplete combustion, are frequently found on PM2.5, and are well reported for their association with PM2.5 levels (Khamkaew et al. 2016a; Pongpiachan et al. 2017; Thepnuan and Chantara 2020a; Kawichai et al. 2020b). The increase in PM2.5 levels in northern Thailand during haze episodes has been found to correlate with the concentration of polycyclic aromatic hydrocarbons (PAHs) bound to its surface. The proportion of PAHs bound to PM2.5 ranges from approximately 2.3% to 15.7% (w/w), increasing by two to three-fold compared to the non-haze rainy season(ChooChuay et al. 2020; Kawichai et al. 2020a; Thepnuan and Chantara 2020b). This is concerning due to the carcinogenic nature of PAHs, and inhalation of PAH-bound PM2.5 may be a significant population-based exposure in the affected city. (Chen et al. 2018; Mueller et al. 2020; Paoin et al. 2021b; Pothirat et al. 2019).
The PAHs bound on respirable PM, i.e., PM with an aerodynamic diameter ≤ 10 µm (PM10), can enter the human body and metabolize to epoxides and hydroxylated PAHs (OH-PAHs) by activating cytochrome P450 complexes before excretion through urine (Guo et al. 2013; Kweon et al. 2015). This process generates superoxide (O2-) and hyperoxide (H2O2), which are highly reactive compounds and free radicals that can imbalance antioxidant levels, resulting in oxidative stress (Fu et al. 2012; Li et al. 2016; Abdal Dayem et al. 2017; Martinez and Kannan 2018). These ROS and free radicals are highly reactive compounds and can bind to other biomolecules, such as proteins, lipids, and DNA, consequently causing adducts, cell damage, and cell apoptosis (Gamon and Wille 2016; Abdal Dayem et al. 2017). Furthermore, exposure to environmental pollutants such as air pollution, UV radiation, and radiation are also well-known environmental factors associated with ROS generation (Fu et al. 2012; He et al. 2020; Pelletier et al. 2017).
In general, OH-PAHs are predominantly excreted via urine, exhibiting a relatively lower presence in other bodily fluids such as breast milk (Martin-Tornero et al. 2020). The comparatively short half-lives of PAHs range from approximately 6 to 35 hours (Jongeneelen et al. 1990; Huang et al. 2007). After exposure, OH-PAH levels tend to rise rapidly, increasing by 9 to 141 times, and subsequently decline in accordance with first-order kinetics (Li et al. 2012). Typically, these levels revert to baseline within 24 to 48 hours post-exposure. The individual half-life of OH-PAHs may vary due to differences in their molecular weight. For instance, OH-Nap might exhibit a shorter half-life compared to higher-molecular-weight, influencing their accumulation in the body. A pharmacokinetic study revealed that 100%, 60%, 11%, and 6.8% of Nap, Flu, Phe, and Pyr, respectively, were excreted in human urine (Li et al. 2012; Yang et al. 2021; Zhu et al. 2021)
The evaluation of PAH exposure levels often relies on the utilization of urinary 1-hydroxypyrene (1-OHP) as a prevalent biomarker in diverse occupational environments, such as fire fighters (Andersen et al. 2018), coke oven workers (Thi et al. 2014), iron and steel mill foundries (Lee et al. 2007), and smokers (Lee and Byeon 2010). However, it is crucial to acknowledge that 1-hydroxypyrene (1-OHP) represents a metabolite that is exclusively linked to pyrene and may not encompass the complete spectrum of other polycyclic aromatic hydrocarbon (PAH) compounds. Furthermore, exposure to mixtures of PAHs can potentially alter metabolic pathways or elicit synergistic or antagonistic effects (Abdel-Shafy and Mansour 2016; Sochacka-Tatara et al. 2018). Consequently, relying solely on urinary 1-OHP as a surrogate biomarker might not provide a comprehensive evaluation of human exposure, especially in the environmental exposure to aerosols generated from biomass burning.
Malondialdehyde (MDA) is a product of oxidative stress. Generally, MDA is a side product of the decomposition of arachidonic acid (AA) and relatively large polyunsaturated fatty acids (PUFAs) to produce thromboxane A2 through an enzymatic process (Ayala et al. 2014). This low rate of lipid peroxidation can stimulate the cells to maintain and survive through antioxidant defense systems or signaling pathway activation. Nevertheless, excess free radicals, AA, and PUFAs can undergo cleavage to produce MDA through non-enzymatic oxygen radical-dependent reactions. A relatively high rate of lipid peroxidation extends to oxidative damage over the repair capacity, resulting in the induction of cell injury and cell death. Oxidative damage has been shown to be involved in cellular physiological and pathological dysfunctions (Lin et al. 2015).
The upper northern Thailand is currently grappling with an annual air pollution crisis that poses significant concerns for public health. This pollution issue holds implication for the well-being of the elderly population, who have been identified as a vulnerable subgroup in relation to air pollution’s adverse effects. With Thailand transitioning into an aged society, it becomes crucial to prioritize the care and safeguarding of this group’s health. This transition is evidenced by the index of aging (IoA) in which measured 68.77, indicating that Thailand falls within the range of an aged society (IoA between 50 and 119.9). Further details on this transition can be found in the supplementary information provided, Table S1 (source: https://www.dop.go.th/th/know/side/1/1/47). Therefore, this study proposed to investigate the increase in oxidative product, urinary MDA, levels, focusing on ambient PM2.5 exposure and the increase in urinary OH-PAH levels among a pre-aged to aged female subgroup in a suburban area of Chiang Mai, Thailand, during haze episodes compared to the non-haze rainy season.
MATERIALS AND METHODS
Study population
This study was designed as a prospective panel study. A total of 64 females aged between 50 and 65 years old in Saraphi District, Chiang Mai Province, were recruited and followed up for five consecutive weeks during the non-haze rainy season (16 July - 25 August 2018) and haze episode, when daily PM2.5 exceeded Thailand NAAQSs at 37.5 µg/m3 (25 February - 6 April 2019). The morning void urine samples were collected each week, weight and height were measured for BMI, and the participants were interviewed using a structured questionnaire to obtain socioeconomic status and health status. All participants did not smoke and had no alcohol beverage consumption at least two weeks before and during activities (more details of the inclusion and exclusion criteria are shown in the supplementary materials).
The participants in this study gave written informed consent for participation. The research protocol and laboratory of samples analyses were approved and under the regulations by the Office of Research Ethics, Research Institute for Health Sciences, Chiang Mai University (Project no. 21/60, approved on 7 March 2018).
Air pollution data
The average 24-h PM2.5 (µg/m3) level was obtained from the nearest air quality monitoring station of the PCD in Sri Phum District (18°47'28.4"N, 98°59'17.6"E, 366 m MSL), which was approximately 13 km away from the study site (shown in Figure 1).
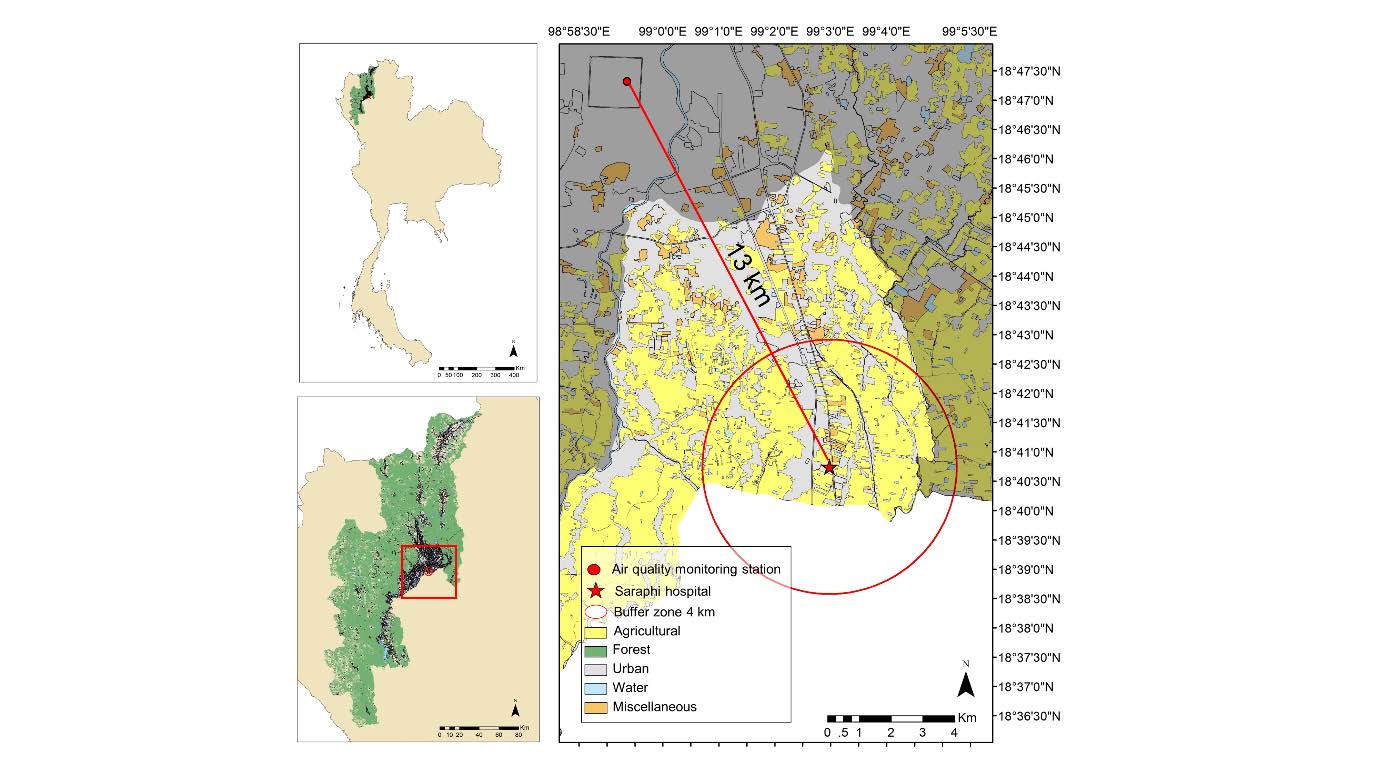
Figure 1. The study site at Saraphi Hospital (suburban area) and the air quality monitoring station (urban area) of the Pollution Control Department (PCD).
Biomarker analysis
Urinary OH-PAHs
Eight species of urinary OH-PAHs, including 1-hydroxynaphthalene (1-OHNap), 2-hydroxynaphthalene (2-OHNap), 2-hydroxyfluorene (2-OHFlu), 2-hydroxyphenanthrene (2-OHPhe), 3-hydroxyphenanthrene (3-OHPhe), 1+9-hydroxyphenanthrene (1+9-OHPhe), 4-hydroxyphenanthrene (4-OHPhe), and 1-hydroxypyrene (1-OHP), were analyzed following the protocol of Chetiyanukornkul et al., 2006 with slight modification. Briefly, 10 mL of urine sample was acidified to pH 5 by 1 M HCl. Then, the solution was mixed with 20 mL of 0.1 M acetate buffer (pH 5) and 25 µL of β-glucuronidase before incubation at 37°C for 2 hours. Then, the sample was loaded into a solid-phase extraction C-18 cartridge (Bond elute-C18 500 mg 3 mL, Agilent, Santa Clara, California, USA.). The loaded sample was eluted using 10 mL of methanol and blown with steam N2 at 45°C to dryness. The extracted sample was finally reconstituted with 200 µL of methanol before analysis (Chetiyanukornkul et al. 2006).
The sample was analyzed using high-pressure liquid chromatography (Agilent Technologies 1,260 Infinity II, Santa Clara, California, USA.) with an RP-Amide C16 column (Discovery: 25 cm × 4.6 mm, 5 µm, Supelco, Bellefonte, Pennsylvania, USA.) with a fluorescence detector (Agilent Technologies 1,260 Infinity II, Santa Clara, California, USA.). The mobile phase was a gradient between 10 mM phosphate buffer pH 7 (A) and acetonitrile (B) with a flow rate of 1 mL/min. The ratio of A: B was 55:45 from the beginning to 20 minutes. % B was then increased to 60 in 37 minutes. The total running time was 45 minutes. The excitation and emission wavelengths are shown in Table S2.
All samples were controlled by adding 1-hydroxypyrene-d9 (1-OHP-d9) as the internal standard. Quality control was performed by testing the precision (3 batches; 15 samples/batch) of known concentrations to obtain %RSD < 15% and %recovery = 80-120%. The spiked-pool urine with exact concentration was used as an internal quality control between batches to keep the %CV in the range of mean ± 2S.D. The concentration of OH-PAHs was subtracted by a blank sample before conversion to micrograms per gram of creatinine (µg/gCre).
Urinary malondialdehyde (MDA)
Urinary MDA, an oxidative marker, was analyzed using HPLC-UV detector following Yoon et al., 2012's protocol with some modification. Briefly, 50 µL of the sample was mixed with 300 µL of 0.5 M phosphoric acid and 150 µL of 23 mM thiobarbituric acid. The mixture was incubated for 1 hour at 95°C before adding 500 µL of methanol. After centrifugation at 3,000 rpm for 5 minutes. The mixture was filtered through a 0.25 µm PTFE filter before analysis (Yoon et al. 2012).
The sample was analyzed using HPLC-UV on a 3 mm × 150 mm × 5 µm C18-reversed-phase column (Discovery-C18, Supelco, Bellefonte, Pennsylvania, USA.) with a 412 nm wavelength UV detector (Agilent Technologies Hewlett Packard series 1100, Santa Clara, California, USA.). The mobile phase comprised potassium phosphate (0.05 M; pH 6.8) and methanol (60:40, v/v). The flow rate was 0.8 mL/min at 35°C.
Quality control was performed by testing the precision (3 batches; 15 samples/batch) of known concentrations to obtain %RSD < 15% and %recovery = 80-120%. The spiked-pool urine with exact concentration was used as an internal quality control between batches to keep the %CV in the range of mean ± 2S.D. shown in Table S2.
Urinary creatinine
Urinary creatinine determination method was modified from Zuo et al., 2008. Briefly, the urine sample was diluted 100 times with deionized water before adjusting the pH to 2.35 with phosphoric acid. After centrifugation at 5,000 × g for 15 minutes, the supernatant was filtered through a 0.45 µm PTFE filter, and the pH was adjusted to 6.85 with 0.01 M sodium hydroxide before analysis (Zuo et al. 2008).
The sample was analyzed using HPLC-UV on a 3 mm × 150 mm × 5 µm C18-reversed-phase column (Discovery-C18, Supelco, Bellefonte, Pennsylvania, USA.) with a 205 nm wavelength UV detector (Agilent Technologies Hewlett Packard series 1100, Santa Clara, California, USA.). The mobile phase was a gradient of sodium phosphate (0.05 M; pH 4.75) and acetonitrile.
Quality control was performed by testing the precision (3 batches; 15 samples/batch) of known concentrations to obtain %RSD < 15% and %recovery = 80-120%. The spiked-pool urine with exact concentration was used as an internal quality control between batches to keep the %CV in the range of mean ± 2S.D. shown in Table S2. In addition, quality assurance of urinary creatinine was performed by using standard creatinine, bought from German External Quality Assessment Scheme (G-EQAUS). Following this protocol, the concentration of urinary creatinine was 0.55 g/l (tolerance range: 0.52 - 0.64) and 2.43 g/l (tolerance range: 2.41 - 2.83). The protocol was certified by G-EQUAS, issued on 28 January 2020 (the intercomparison programmed 64/2019 for occupational/ environmental medical toxicological analysis).
Statistical analysis
Estimated changes in individual biomarker concentrations
The concentrations of urinary OH-PAHs and urinary MDA were adjusted with the concentration of urinary creatinine and are presented in µg/gCre, other unit conversion is shown in Table S3. Student’s T-test was performed to compare all biomarker parameters to the different levels between the non-haze rainy season and haze episodes.
The association of PM2.5 concentration with all biomarkers was analyzed by Pearson’s correlation, and the estimated concentration changes in the urinary biomarkers due to PM2.5 levels were analyzed to present the increment of each urinary biomarkers by a mixed-effect model.
Next, urinary OH-PAHs were categorized as exposure biomarkers, and urinary MDA was a responsive biomarker. The estimated concentration changes of urinary MDA due to the increase of individual urinary OH-PAH concentration, adjusted by age, BMI, NCDs, and season i.e., non-haze and haze seasons, were performed to demonstrate the relationship between internal dose and response. The results were expressed for increasing every 1 µg/gCre of individual urinary OH-PAH concentration increased the urinary MDA concentration. Statistical significance was indicated by a P value lower than 0.05 (P < 0.05).
RESULTS
Demographic data
A total of 64 participants were recruited for this study. The average age was 58 years, with a range of 51 to 65 years old. In the non-haze rainy season as the baseline (2018), the number of participants was 60, shown in Table 1. Some of them left the study in the subsequent follow-up. Thus, new participants were recruited in the haze episode (2019) with the same inclusion criteria to maintain the sample size. The number of participants who participated in both study periods was 48, shown in Figure S1.
Table 1. Demographic data of participants in the two study periods.
|
Characteristics |
Non-haze rainy season (July - August 2018) N = 60 |
Haze episode (March - April 2019) N = 52 |
|
Average age ± SD |
58.4 ± 4.0 (51-65) |
58.9 ± 4.3 (51-66) |
|
< 60 (n) |
34 (57%) |
25 (48%) |
|
≥ 60 (n) |
26 (43%) |
27 (52%) |
|
Body Mass Index (BMI; kg/m2) |
24.7 ± 4.1 |
23.9 ± 3.9 |
|
Hypertension (systolic/diastolic |
14 (23%) |
13 (25%) |
|
Diabetes Mellitus (8-hr fasting plasma glucose level |
1 (2%) |
1 (2%) |
|
Hyperlipidemia (12-hr fasting cholesterol level |
4 (7%) |
6 (11%) |
The participants had lived or worked in the study area (Saraphi District, Chiang Mai Province) for at least 3 years. Approximately 20% of them were diagnosed with noncommunicable diseases (NCDs), including hypertension, diabetes, and hyperlipidemia. Most NCDs were hypertension (23 and 25% of total NCDs in the groups in non-haze rainy season and haze episode follow-up, respectively). The NCD participants were all in a stable condition. All the recruited participants had only one NCD. Although this study recruited only nonsmoking participants, 59% of them reported having second-hand smoke exposure, and the majority reported that exposure being from family members. The protective behaviors of some participants were observed during the haze episode, such as opening windows and not using an air purifier, as shown in Table S4.
Air pollution level
The haze episode was classified due to the high elevation of PM2.5 levels above the 24-h Thai National Ambient Air Quality Standards (NAAQSs) at 37.5 µg/m3. In the haze episode, the mean ± SD of the PM2.5 levels (58.0 ± 48.9 µg/m3) was significantly (P = 0.000) higher than that in the non-haze rainy season (37.1 ± 14.7 µg/m3). The PM2.5 levels decreased below Thailand NAAQSs between June and December because of the precipitation shown in Figure 2.
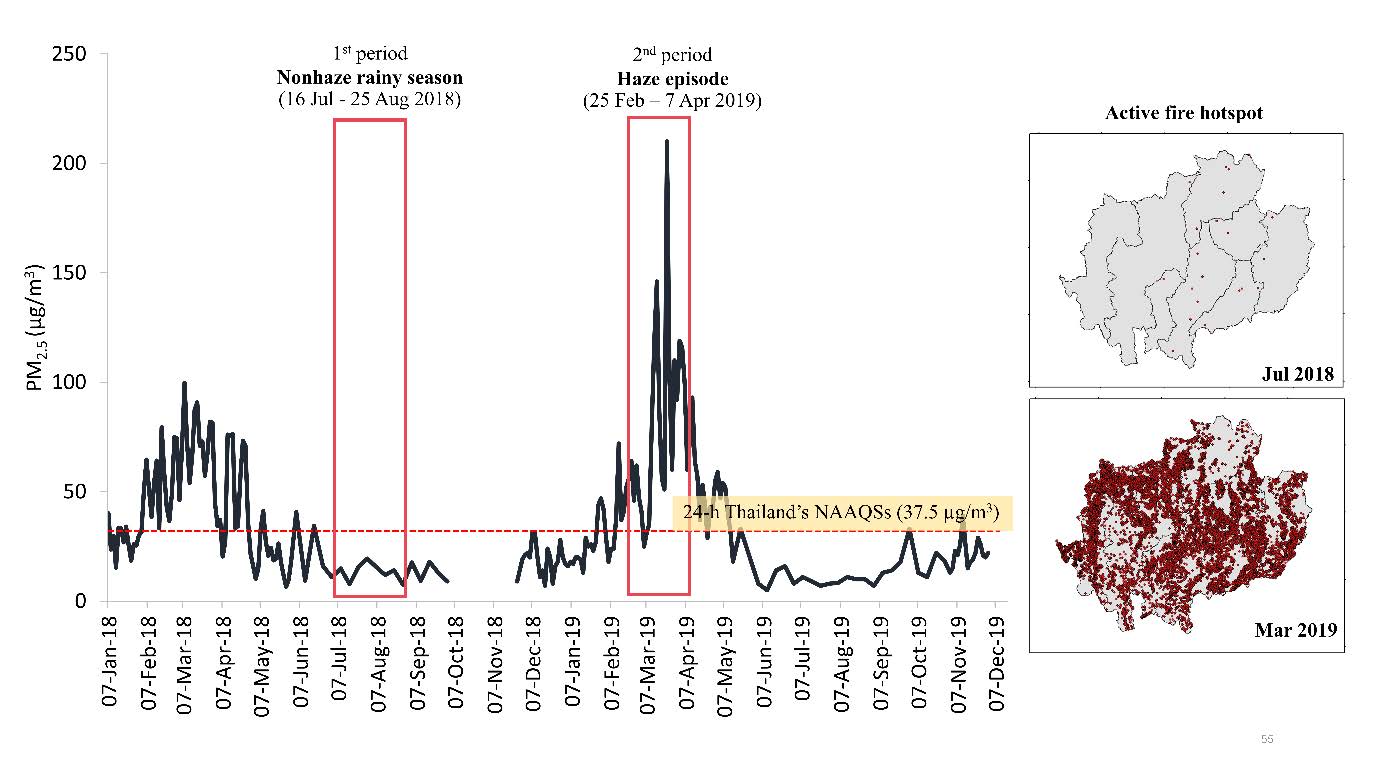
Figure 2. The 24-hr concentration of PM2.5 (µg/m3) from the air quality monitoring station of the Pollution Control Department (PCD) in Chiang Mai City in 2018-2019 and the active fire hotspot counts in the upper northern region of Thailand during the non-haze rainy season (July 2018) and haze episode (March 2019).
The measurement of gaseous pollutant levels, including NO, NO2, and NOx, showed no difference in both seasons, whereas the concentrations of O3 and SO2 were significantly higher in the haze episode, as shown in Table 2. However, this increases in O3 and SO2 levels did not exceed 8-h Thai NAAQSs (O3 ≤ 70 ppb, 120 µg/m3; SO2 ≤ 200 ppb, 786 µg/m3) (Thailand royal gazette volume 135 special issue 283; 25 September 2018).
Table 2. The concentration of air pollutants (µg/m3) in the non-haze rainy season and haze episode and meteorological conditions.
|
Pollutant (µg/m3) |
Non-haze rainy season (2018) |
Haze episode (2019) |
P value |
||
|
Mean±SD |
Min-Max |
Mean±SD |
Min-Max |
||
|
PM2.5 |
37.10 ± 14.70 |
13.80–70.80 |
58.00 ± 48.90 |
7.60–310.80 |
0.000 |
|
NO |
2.91 ± 1.33 |
0.00–6.78 |
2.14 ± 1.84 |
0.00–9.66 |
0.385 |
|
NO2 |
15.70 ± 5.35 |
3.60–27.40 |
14.50 ± 10.50 |
0.00–39.30 |
0.337 |
|
NOx |
20.10 ± 6.47 |
4.58–32.60 |
17.80 ± 11.80 |
0.25–41.80 |
0.109 |
|
O3 |
54.10 ± 14.3 |
17.20–81.40 |
69.40 ± 23.10 |
19.00–115.90 |
0.000 |
|
SO2 |
1.81 ± 1.99 |
0.00–5.58 |
2.81 ± 1.97 |
0.00–8.43 |
0.003 |
|
Meteorological conditions |
|||||
|
Temperature (°C) |
24.40 ± 2.89 |
16.90–31.70 |
26.20 ± 2.69 |
16.80–31.50 |
0.000 |
|
RH (%) |
71.70 ± 8.96 |
48.20–97.00 |
61.60 ± 13.00 |
35.50–93.80 |
0.000 |
|
Wind speed (km/hr) |
18.80 ± 5.43 |
6.00–31.00 |
19.80 ± 6.42 |
7.00–43.00 |
0.279 |
|
Precipitation (mm) |
4.00 ± 11.20 |
0.00–57.70 |
0.45 ± 2.02 |
0.00–15.40 |
0.004 |
Sources: Air quality monitoring station of Pollution Control Department (PCD) and meteorological
Concentration of urinary OH-PAHs
The mean concentration of urinary OH-PAHs, except urinary 1- and 2-OHNap, in the haze episode was significantly higher than that in the non-haze rainy season. Although the highest concentration was urinary 1-OHNap (19.5 ± 17.7 µg/gCre in the non-haze rainy season and 16.8 ± 16.1 µg/gCre in the haze episode), there was no difference between these two seasons, as shown in Table 3. The weekly descriptive data of urinary biomarkers is shown in Table S5 and S6.
Table 3. The mean concentration (µg/gCre) of individual urinary biomarkers in both the non-haze rainy season and haze episodes.
|
Individual Urinary OH-PAHs |
Non-haze rainy season |
Haze episode |
P value |
|||
|
Mean ± SD |
Min – Max |
Mean ± SD |
Min – Max |
|
||
|
1-OHNap |
19.50 ± 17.70 |
2.68–88.00 |
16.80 ± 16.10 |
2.34–87.20 |
0.241 |
|
|
2-OHNap |
4.73 ± 3.27 |
0.67–21.00 |
4.52 ± 3.02 |
0.66–16.90 |
0.497 |
|
|
2-OHFlu |
0.25 ± 0.20 |
0.01–1.04 |
0.39 ± 0.32 |
0.01–1.62 |
0.000 |
|
|
2-OHPhe |
0.25 ± 0.18 |
0.03–0.94 |
0.30 ± 0.20 |
0.05–1.28 |
0.005 |
|
|
3-OHPhe |
0.21 ± 0.14 |
0.03–0.69 |
0.26 ± 0.19 |
0.05–1.05 |
0.001 |
|
|
4-OHPhe |
0.11 ± 0.08 |
0.02–0.53 |
0.16 ± 0.13 |
0.02–0.85 |
0.001 |
|
|
1+9-OHPhe |
0.23 ± 0.15 |
0.03–0.76 |
0.26 ± 0.18 |
0.04–0.83 |
0.037 |
|
|
1-OHP |
0.20 ± 0.18 |
0.02–1.10 |
0.23 ± 0.24 |
0.01–1.36 |
0.497 |
|
|
MDA |
1.87 ± 0.79 |
0.76–5.28 |
2.05 ± 0.89 |
0.60–5.57 |
0.016 |
|
Association between concentration of PM2.5, urinary OH-PAHs and urinary MDA
Exposure to ambient PM2.5 concentrations was positively correlated (P < 0.05) with individual urinary OH-PAH concentrations, i.e., 2-OHFlu (r = 0.242), 2-OHPhe (r = 0.129), 3-OHPhe (r = 0.170), 4-OHPhe (r = 0.162) and 1-OHP (r = 0.148), but PM2.5 concentrations was not statistically correlated with urinary MDA concentration. However, urinary MDA concentration was positively correlated (P < 0.05) with individual urinary OH-PAH concentrations, including 2-OHNap (r = 0.316), 2-OHFlu (r = 0.292), 2-OHPhe (r = 0.278), 3-OHPhe (r = 0.377), 4-OHPhe (r = 0.300), 1+9-OHPhe (r = 0.380) and 1-OHP (r = 0.285), as shown in Table 4.
Table 4. Pearson’s correlation between concentration of PM2.5 and individual urinary biomarkers.
|
|
PM2.5 |
1-OHNap |
2-OHNap |
2-OHFlu |
2-OHPhe |
3-OHPhe |
4-OHPhe |
1+9-OHPhe |
1- OHP |
|
PM2.5 |
1 |
|
|
|
|
|
|
|
|
|
1-OHNap |
-0.083 |
1 |
|
|
|
|
|
|
|
|
2-OHNap |
-0.054 |
0.331** |
1 |
|
|
|
|
|
|
|
2-OHFlu |
0.242* |
0.203** |
0.476** |
1 |
|
|
|
|
|
|
2-OHPhe |
0.129** |
0.105 |
0.421** |
0.568** |
1 |
|
|
|
|
|
3-OHPhe |
0.170** |
0.138* |
0.510** |
0.666** |
0.757** |
1 |
|
|
|
|
4-OHPhe |
0.162** |
0.168* |
0.479** |
0.430** |
0.546** |
0.441** |
1 |
|
|
|
1+9-OHPhe |
0.083 |
0.150* |
0.570** |
0.646** |
0.427** |
0.668** |
0.591** |
1 |
|
|
1-OHP |
0.148** |
0.099 |
0.524** |
0.489** |
0.339** |
0.509** |
0.393** |
0.542** |
1 |
|
MDA |
0.067 |
0.095 |
0.316** |
0.292** |
0.278** |
0.377** |
0.300** |
0.380** |
0.285** |
*Significance at P – value < 0.05, **Significance at P – value < 0.01
The estimated concentration changes of individual urinary biomarkers from ambient PM2.5 exposure were determined by a mixed-effect model. After adjusting the model with covariables (age, BMI, NCDs and season), the increase in PM2.5 concentration by 10 µg/m3 increased the concentrations of urinary 2-OHFlu by 0.014 (95% CI: 0.008 to 0.014), urinary 2-OHPhe by 0.005 (95% CI: 0.001 to 0.009), urinary 3-OHPhe by 0.006 (95% CI: 0.003 to 0.010), urinary 4-OHPhe by 0.004 (95% CI: 0.001 to 0.006), and urinary 1-OHP by 0.007 (95%CI: 0.002 to 0.011) µg/gCre, as shown in Table 5.
Table 5. The estimated concentration changes in individual urinary biomarkers (µg/gCre) from 10 µg/m3 increases in PM2.5 concentration.
|
Urinary |
Estimated concentration changes (µg/gCre) |
95% CI |
P value |
|
1-OHNap |
-0.028 |
-0.092 to 0.035 |
0.379 |
|
2-OHNap |
-0.139 |
-0.637 to 0.360 |
0.585 |
|
2-OHFlu |
0.014 |
0.008 to 0.014 |
0.000 |
|
2-OHPhe |
0.005 |
0.001 to 0.009 |
0.007 |
|
3-OHPhe |
0.006 |
0.003 to 0.010 |
0.000 |
|
4-OHPhe |
0.004 |
0.001 to 0.006 |
0.004 |
|
1+9-OHPhe |
0.003 |
-0.001 to 0.006 |
0.065 |
|
1-OHP |
0.007 |
0.002 to 0.011 |
0.003 |
|
MDA |
0.014 |
-0.002 to 0.030 |
0.089 |
Urinary OH-PAHs, the metabolites of PAHs, were categorized as the internal dose, and urinary MDA was a responsive biomarker of oxidative stress. The 1 µg/gCre concentration increases of individual urinary OH-PAHs significantly increased urinary MDA concentration, including 2-OHNap (0.073, 95% CI: 0.048 to 0.098 µg/gCre MDA), 2-OHFlu (0.979, 95% CI: 0.700 to 1.257 µg/gCre MDA), 2-OHPhe (1.369, 95% CI: 0.998 to 1.741 µg/gCre MDA), 3-OHPhe (1.971, 95% CI: 1.549 to 2.393 µg/gCre MDA), 1+9-OHPhe (1.849, 95% CI: 1.410 to 2.288 µg/gCre MDA), 4-OHPhe (2.071, 95% CI: 1.288 to 2.865 µg/gCre MDA) and 1-OHP (1.008, 95% CI: 0.640 to 1.376 µg/gCre MDA), as shown in Figure 3.
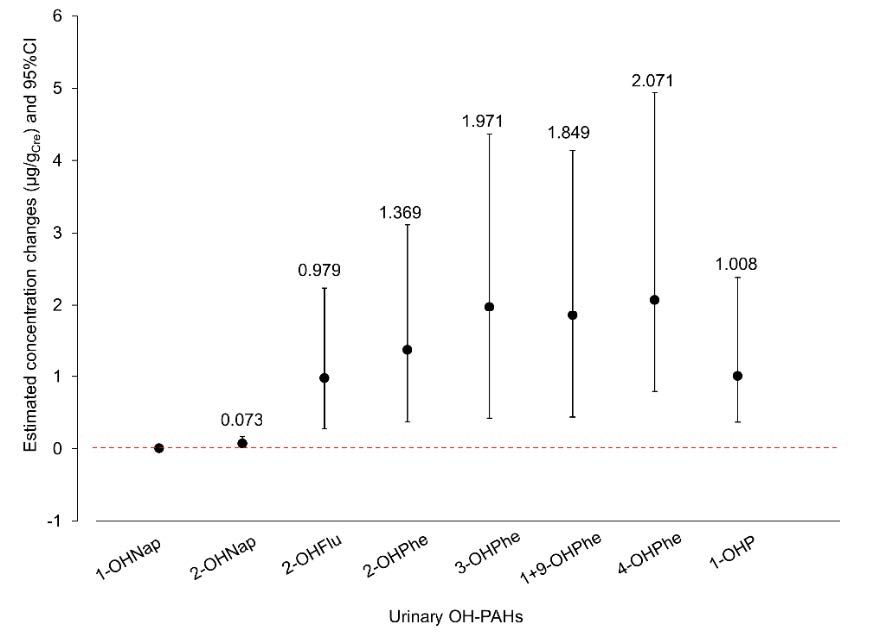
Figure 3. The estimated concentration changes of urinary MDA concentration due to the increase of individual urinary OH-PAH concentrations by 1 µg/gCre.
DISCUSSION
The intensive burning associated with biomass combustion releases a significant amount of PM2.5 and gaseous pollutants. These emissions tend to accumulate in Chiang Mai between January and April, primarily due to the temperature inversion and the unique topography of the valley (Ruttanawongchai et al. 2018; Solanki et al. 2019). The PM2.5 levels exhibit a noticeable decrease from approximately June to November, primarily attributable to the presence of precipitation. It is important to note that the Thai NAAQSs and WHO guideline specify a threshold of 24-h PM2.5 at 37.5 and 15 µg/m3, respectively. However, during the haze episode, the PM2.5 levels exceeded the Thai NAAQSs by approximately 3-fold and surpassed the WHO guideline by approximately 4-fold. In addition, the annual mean PM2.5 level (52.4 ± 41.0 µg/m3) exceeded Thai NAAQSs (15 µg/m3) and WHO guideline (5 µg/m3) by approximately 5 and 10-fold, respectively. Given the significant deviations from the desired target levels and the critical importance of safeguarding public health and well-being, it is imperative to urgently implement strategic plans aimed at mitigating air pollution problems.
The investigation of urinary OH-PAH profiles showed that the levels of 2-OHFlu, 2-OHPhe, 3-OHPhe, 4-OHPhe, and 1+9-OHPhe were significantly higher in the haze episode than in the non-haze rainy season. The mean concentration of urinary OHNap, the most abundant of urinary OH-PAHs, was 22.1 ± 18.3 µg/gCre in the haze episode, accounting for 93% of the total urinary OH-PAHs. Urinary OHNap was reportedly attributed to vehicular emissions, indoor household activities, and naphthalene-containing mothballs (Soghoian et al. 2012; Bortey-Sam et al. 2017). The high concentration of urinary OHNap could be due to both inhalation and ingestion. Remarkably, urinary 2-OHNap was proposed as a biomarker of airborne PAH inhalation (Bortey-Sam et al. 2017). The increase in urinary OHNap concentration was positively associated with exhaled carbon monoxide level (eCO), a mediator of an inflammatory response among the ex-smoker group (Zhou et al. 2018), induced chromosome aberration and translocation (Orjuela et al. 2012).
The concentrations of urinary 2-OHFlu and 5 isomers of urinary OHPhe (2-, 3-, 4-, 1+9-OHPhe) in the haze episode were significantly higher than those in the non-haze rainy season. Fluorene and phenanthrene are low-molecular-weight PAHs with 4 aromatic rings (Humans 2012). These PAHs are primarily generated at low combustion temperatures. Therefore, their possible source might be biomass burning (Bortey-Sam et al. 2017). Multiple metabolites of urinary OHPhe were reportedly markers of PAH exposure in both occupation and non-occupation groups and were associated with levoglucosan bound to PM2.5 (Seidel et al. 2008; Adetona et al. 2017).
The concentration of urinary 1-OHP was slightly higher in the haze episode (0.16 ± 0.13 µg/gCre) than in the non-haze rainy season (0.11 ± 0.08 µg/gCre), but the difference was not significant. Although urinary 1-OHP is a common biomarker of occupational PAH exposure in fire fighters (Andersen et al. 2018), coke oven workers (Thi et al. 2014), iron and steel mill foundries (Lee et al. 2007), and smokers (Lee and Byeon 2010), the present study did not find a higher concentration of urinary 1-OHP in haze episodes,. This is attributed to the insufficiency of relying solely on 1-OHP as a measure of the internal dose for highly volatile polycyclic aromatic hydrocarbons (PAHs) in the context of environmental exposure (Hansen et al. 2008; Campo et al. 2010).
The individual urinary OH-PAH levels compared with worldwide studies, where the air pollution background was different, from New York (Zhu et al. 2021), Poland (Sochacka-Tatara et al. 2018), Ghana (Bortey-Sam et al. 2017), China, Japan, Korea, Vietnam, Malaysia, and Kuwait (Guo et al. 2013) showed a comparable level of each urinary OH-PAHs, but the level of urinary 1-OHNap in the present study was much higher than in other reports. The exposure level in a polluted area and a city with rapid growth as well as occupational exposure tended to exhibit increased urinary OH-PAH levels more than in the present study (Guo et al. 2013; Zhou et al. 2018).
This study has revealed an increase in individual urinary OH-PAH levels from ambient PM2.5 exposure in haze episodes, as shown in Table 6, suggesting that exposure to ambient PM2.5 cannot be neglected for PAH exposure. Similar to other studies, the urinary OH-PAH levels were positively associated with ambient PM2.5 levels (Adetona et al. 2017; Salami et al. 2021). However, exposure to PAHs might be confounded by other routes since PAHs can be in other environments, i.e., air, soil, water, grilled food, and tobacco smoking, that might enter the human body by personal lifestyle and unintentional consumption (Krzyszczak and Czech 2021).
The effect of the increase in individual urinary OH-PAHs on oxidative damage was investigated using an oxidative marker, urinary MDA. The estimated concentration changes of urinary MDA by every 1 µg/gCre increase of individual urinary OH-PAHs are shown in Figure 2. The highest increase was from urinary 4-OHPhe. This result was similar in firewood exposure in the firefighter group, in which urinary 4-OHPhe might be the most sensitive biomarker to vegetative and biomass burning (Adetona et al. 2017).
The other urinary biomarkers were also positively associated with increased urinary MDA levels, yet urinary 1-OHNap. This might be because the relative high rate of naphthalene excretion in urine was 100% compared to OHFlu, OHPhe, and OHP, whose excretion rates were 60, 11, and 6.8%, respectively (Li et al. 2012). However, this study cannot investigate high molecular weight PAHs (> 4 rings) since they might accumulate in the human body rather than excrete through urine since most of them are carcinogenic PAHs and might be excreted through feces (Guo et al. 2013).
It should be noted that the increase in urinary MDA level was found to be higher in females than males (Yoon et al. 2012; Li et al. 2019), but there was no difference between ages. This might be due to the difference in hormones that upregulate cytochrome P450 enzyme activity in different sexes (Uppstad et al. 2011; Guo et al. 2014). Coincidently, the exclusion of males in this study helped diminish the gender confounder in the data analysis, provided there were approximately equal numbers of genders. However, the presence of NCDs and the medication used could not be excluded from the study plan due to the high prevalence of NCDs among the preaging (50-59 years) and aging (> 60 years) groups in Thailand (VatcharaVongVan and Puttawanchai 2017). Therefore, the participants who had only 1 NCD with stable conditions were enrolled in the study.
The socioeconomic status of the studied population is shown in Table S4. Among this study group, the second-hand smoking exposure among 59% of participants and the usage of charcoal (20%) and wood (17%) for cooking might be other essential exposure sources of PAHs. In addition, personal behavior and family financial status might be other factors that discourage people from having clean indoor air because 44% of them had an air conditioner that was mostly installed only in the bedroom, 17% of them had an air purifier, and 63% of them opened windows all the time, even in haze episodes.
Fifty-five percent of participants had a monthly household income of approximately 5,001 to 20,000 THB (approximately 155 to 620 USD; average exchange rate in 2018: 1 THB = 0.031 USD). Concerning the national statistical office (NSO), Thailand reported that the monthly household expense in Chiang Mai was 15,468 THB with a poverty line (expense) of 2,475THB/person/month (http://statbbi.nso.go.th/staticreport/page/sector/th/08.aspx). Therefore, approximately one-third (36% of them) had an income higher than the average household expense (income > 20,000 THB), while approximately two-thirds of them with lower income might be difficult to afford the additional personal protection equipment or achieve clean indoor air during the haze episode. With this status, the participants who represented the suburban population of Chiang Mai might not be able to avoid air pollution exposure. Consequently, the OH-PAH concentration was found to be higher in the haze episode than in the non-haze period.
CONCLUSION
This study investigated the increased urinary OH-PAH concentrations and their oxidative damage among a preaged female subgroup in suburban Chiang Mai during haze episode. The results showed that the concentration of all urinary OH-PAHs and urinary MDA, except for urinary 1-OHNap, urinary 2-OHNap, and urinary 1-OHP, increased significantly in the haze episode. The mixed-effect model illustrated that exposure to ambient PM2.5 during haze episodes increased urinary OH-PAH levels. The present study suggested that inhalation exposure of PM2.5 was a major route of PAH exposure. The increased individual urinary OH-PAH levels consequently increased the urinary MDA levels, suggesting that oxidative damage was a common mechanism underlying health inflammation. Our results suggested that a strategic plan to reduce sources of biomass burning should be mandatory to mitigate the chain effects, particularly human health, from air pollution exposure.
ACKNOWLEDGMENTS
We acknowledge the Thailand Science Research and Innovation (TSRI, formerly the Thailand Research Fund, TRF to Dr. Tippawan Prapamontol) [grant number: RDG 6030019]; PhD-Royal Golden Jubilee Scholarship [grant number: PHD/0150/2559 to Pitakchon Ponsawansong] and the National Natural Science Foundation of China [grant number: 41761144056 to Prof. Dr. Yalin Zhang]. We are grateful to all Village Health Volunteer participants, medical staff, and health-promoting hospital staff in Saraphi District of Chiang Mai Province for their kindly cooperation and support.
AUTHOR CONTRIBUTIONS
P.P. (Ph.D. student) designed and conducted all the experiments and prepared the manuscript writing. T.P. designed the study and edited and reviewed the manuscript. J.S. supported data acquisition and advised on participant recruitment. G.L. and X.P. advised on statistical analysis.
CONFLICT OF INTERESTS
The authors declare no competing interests.
REFERENCES
Abdal Dayem, A., Hossain, M.K., Lee, S.B., Kim, K., Saha, S.K., Yang, G-M., Choi, H.Y., and Cho, S-G. 2017. The role of reactive oxygen species (ros) in the biological activities of metallic nanoparticles. International Journal of Molecular Sciences. 18(1): 120.
Abdel-Shafy, H.I. and Mansour, M.S. 2016. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum. 25(1): 107-123.
Adetona, O., Simpson, C.D., Li, Z., Sjodin, A., Calafat, A.M., and Naeher, L.P. 2017. Hydroxylated polycyclic aromatic hydrocarbons as biomarkers of exposure to wood smoke in wildland firefighters. Journal of Exposure Science & Environmental Epidemiology. 27(1): 78-83.
Andersen, M.H.G., Saber, A.T., Clausen, P.A. Pedersen, J.E., Løhr, M., Kermanizadeh, A., Loft, S., Ebbehøj, N., Hansen, Å.M., and Pedersen, P.B. 2018. Association between polycyclic aromatic hydrocarbon exposure and peripheral blood mononuclear cell dna damage in human volunteers during fire extinction exercises. Mutagenesis. 33(1): 105-115.
Ayala, A., Muñoz, M.F., and Argüelles, S. 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. (2014):360438.
Bortey-Sam, N., Ikenaka, Y., Akoto, O., Nakayama, S.M., Asante, K.A., Baidoo, E., Obirikorang, C., Saengtienchai, A., Isoda, N., and Nimako, C. 2017. Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (PAHs) in Kumasi, Ghana. Environmental Pollution. 228: 311-320.
Campo, L., Rossella, F., Pavanello, S., Mielzynska, D., Siwinska, E., Kapka, L., Bertazzi, P.A, and Fustinoni. S. 2010. Urinary profiles to assess polycyclic aromatic hydrocarbons exposure in coke-oven workers. Toxicology Letters. 192(1): 72-78.
Chen, C., Li, C., Li, Y., Liu, J., Meng, C., Han, J., Zhang, Y., and Xu, D. 2018. Short-term effects of ambient air pollution exposure on lung function: A longitudinal study among healthy primary school children in China. Science of the Total Environment. 645: 1014-1020.
Chetiyanukornkul, T., Toriba, A., Kameda, T., Tang, N., and Hayakawa, K. 2006. Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Analytical and Bioanalytical Chemistry. 386(3): 712-718.
ChooChuay, C., Pongpiachan, S., Tipmanee, D., Deelaman, W., Iadtem, N., Suttinun, O., Wang, Q., Xing, L., Li, G., and Han, Y. 2020. Effects of agricultural waste burning on pm2.5-bound polycyclic aromatic hydrocarbons, carbonaceous compositions, and water-soluble ionic species in the ambient air of Chiang-Mai, Thailand. Polycyclic Aromatic Compounds. 42: 749-770.
Fu, P.P., Xia, Q., Sun, X., and Yu, H. 2012. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)—light-induced reactive oxygen species, lipid peroxidation, and DNA damage. Journal of Environmental Science and Health, Part C. 30(1): 1-41.
Gamon, L.F. and Wille, U. 2016. Oxidative damage of biomolecules by the environmental pollutants no2• and no3•. Accounts of Chemical Research. 49(10): 2136-2145.
Guo, H., Huang, K., Zhang, X., Zhang, W., Guan, L., Kuang, D., Deng, Q., Deng, H., Zhang, X., and He, M. 2014. Women are more susceptible than men to oxidative stress and chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environmental and Molecular Mutagenesis. 55(6): 472-481.
Guo, Y., Senthilkumar, K., Alomirah, H., Moon, H-B., Minh, T.B., Mohd, M.A., Nakata, H., and Kannan, K. 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environmental Science & Technology. 47(6): 2932-2938.
Hansen, Å.M., Mathiesen, L., Pedersen, M., and Knudsen, L.E. 2008. Urinary 1-hydroxypyrene (1-hp) in environmental and occupational studies—a review. International Journal of Hygiene and Environmental Health. 211(5-6): 471-503.
He, L., Cui, X., Xia, Q., Li, F., Mo, J., Gong, J., Zhang, Y., and Zhang, J.J. 2020. Effects of personal air pollutant exposure on oxidative stress: Potential confounding by natural variation in melatonin levels. International Journal of Hygiene and Environmental Health. 223(1):116-123.
Huang, W., Smith, T.J., Ngo, L., Wang, T., Chen, H., Wu, F., Herrick, R.F., Christiani, D.C., and Ding, H. 2007. Characterizing and biological monitoring of polycyclic aromatic hydrocarbons in exposures to diesel exhaust. Environmental science & Technology. 41(8):2711-2716.
Humans IWGotEoCRt. 2012. Chemical agents and related occupations. IARC monographs on the evaluation of carcinogenic risks to humans. 100(PT F): 9.
Jongeneelen, F., van Leeuwen, F.E., Oosterink, S., Anzion, R., Van der Loop, F., Bos, R., and Van Veen, H. 1990. Ambient and biological monitoring of cokeoven workers: Determinants of the internal dose of polycyclic aromatic hydrocarbons. British Journal of Industrial Medicine. 47(7): 454.
Kawichai, S., Prapamontol, T., Chantara, S., Kanyanee, T., Wiriya, W., and Zhang, Y.L. 2020a. Seasonal variation and sources estimation of pm2.5bound PAHs from the ambient air of Chiang Mai city: An all-year-round study in 2017. Chiang Mai Journal of Science. 47(5): 958-972.
Khamkaew, C., Chantara, S., Janta, R., Pani, S.K., Prapamontol, T., Kawichai, S., Wiriya, W., and Lin, N-H. 2016a. Investigation of biomass burning chemical components over northern southeast Asia during 7-seas/baseline 2014 campaign. Aerosol and Air Quality Research. 16(11): 2655-2670.
Khamkaew, C., Chantara, S., and Wiriya, W. 2016b. Atmospheric PM2.5 and its elemental composition from near source and receptor sites during open burning season in Chiang Mai, Thailand. International Journal of Environmental Science and Development. 7(6):436.
Kim, K-H., Jahan, S.A., Kabir, E., and Brown, R.J. 2013. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment International. 60: 71-80.
Krzyszczak, A. and Czech, B. 2021. Occurrence and toxicity of polycyclic aromatic hydrocarbons derivatives in environmental matrices. Science of the Total Environment. 788: 147738.
Kweon, O., Kim, S-J., Blom, J., Kim, S-K., Kim, B-S., Baek, D-H., Park, S.I., Sutherland, J.B., and Cerniglia, C.E. 2015. Comparative functional pan-genome analyses to build connections between genomic dynamics and phenotypic evolution in polycyclic aromatic hydrocarbon metabolism in the genus mycobacterium. BMC Evolutionary Biology. 15(1):21.
Lee, K. and Byeon, S. 2010. The biological monitoring of urinary 1-hydroxypyrene by PAH exposure among smokers. International Journal of Environmental Research. 4(3): 439-442.
Lee, M-S., Eum, K-D., Zoh, K-D., Kim, T-S., Pak, Y-S., and Paek, D. 2007. 1-hydroxypyrene as a biomarker of PAH exposure among subjects living in two separate regions from a steel mill. International Archives of Occupational and Environmental Health. 80(8): 671-678.
Li, J., Zhou, C., Xu, H., Brook, R.D., Liu, S., Yi, T., Wang, Y., Feng, B., Zhao, M., and Wang, X. 2019. Ambient air pollution is associated with hdl (high-density lipoprotein) dysfunction in healthy adults. Arteriosclerosis, Thrombosis, and Vascular Biology. 39(3): 513-522.
Li, W., Wilker, E.H., Dorans, K.S., Rice, M.B., Schwartz, J., Coull, B.A., Koutrakis, P., Gold, D.R., Keaney, Jr. J.F., and Lin, H. 2016. Short‐term exposure to air pollution and biomarkers of oxidative stress: The Framingham heart study. Journal of the American Heart Association. 5(5): e002742.
Li, Z., Romanoff, L., Bartell, S., Pittman, E.N., Trinidad, D.A., McClean, M., Webster, T.F., and Sjödin, A. 2012. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chemical Research in Toxicology. 25(7): 1452-1461.
Lin, W., Zhu, T., Xue, T., Peng, W., Brunekreef, B., Gehring, U., Huang, W., Hu, M., Zhang, Y., and Tang, X. 2015. Association between changes in exposure to air pollution and biomarkers of oxidative stress in children before and during the Beijing Olympics. American Journal of Epidemiology. 181(8): 575-583.
Martin-Tornero, E., Luque-Uría, A., Durán-Merás, I., and Espinosa-Mansilla, A. 2020. A novel analytical methodology for the determination of hydroxy polycyclic aromatic hydrocarbons in breast and cow milk samples. Journal of Chromatography B. 1136:121912.
Martinez, M.P. and Kannan, K. 2018. Simultaneous analysis of seven biomarkers of oxidative damage to lipids, proteins, and DNA in urine. Environmental Science & Technology. 52(11): 6647-6655.
Miller, M.R., Shaw, C.A., and Langrish, J.P. 2012. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiology. 8(4): 577-602.
Mueller, W., Loh, M., Vardoulakis, S., Johnston, H.J., Steinle, S., Precha, N., Kliengchuay, W., Tantrakarnapa, K., and Cherrie, J.W. 2020. Ambient particulate matter and biomass burning: An ecological time series study of respiratory and cardiovascular hospital visits in northern Thailand. Environmental Health. 19(1):1-12.
Orjuela, M.A., Liu, X., Miller, R.L., Warburton, D., Tang, D., Jobanputra, V., Hoepner, L., Suen, I.H., Diaz-Carreño, S., and Li, Z. 2012. Urinary naphthol metabolites and chromosomal aberrations in 5-year-old children. Cancer Epidemiology and Prevention Biomarkers. 21(7): 1191-1202.
Paoin, K., Ueda, K., Ingviya, T., Buya, S., Phosri, A., Seposo, X.T., Seubsman, S-a., Kelly, M., Sleigh, A., and Honda, A. 2021a. Long-term air pollution exposure and self-reported morbidity: A longitudinal analysis from the Thai cohort study (tcs). Environmental Research. 192:110330.
Paoin, K., Ueda, K., Ingviya, T., Buya, S., Phosri, A., Seposo, X.T., Seubsman, S-a., Kelly, M., Sleigh, A., and Honda, A. 2021b. Long-term air pollution exposure and self-reported morbidity: A longitudinal analysis from the Thai cohort study (tcs). Environmental Research. 192: 110330.
Pelletier, G., Rigden, M., Kauri, L.M., Shutt, R., Mahmud, M., Cakmak, S., Kumarathasan, P., Thomson, E.M., Vincent, R., and Broad, G. 2017. Associations between urinary biomarkers of oxidative stress and air pollutants observed in a randomized crossover exposure to steel mill emissions. International Journal of Hygiene and Environmental Health. 220(2): 387-394.
Pongpiachan, S., Hattayanone, M., and Cao, J. 2017. Effect of agricultural waste burning season on PM2.5-bound polycyclic aromatic hydrocarbon (PAH) levels in northern Thailand. Atmospheric Pollution Research. 8(6): 1069-1080.
Pothirat, C., Chaiwong, W., Liwsrisakun, C., Bumroongkit, C., Deesomchok, A., Theerakittikul, T., Limsukon, A., Tajarernmuang, P., and Phetsuk, N. 2019. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. Journal of Thoracic Disease. 11(7): 3070.
Ruttanawongchai, S., Raktham, C. and Khumsaeng, T. 2018. The influence of meteorology on ambient PM2.5 and PM10 concentration in Chiang Mai. Journal of Physics: Conference Series. 1144: 1-11.
Salami, F., Hajizadeh, Y., Yadegarfar, G., Ebrahimpour, K., Pourzamani, H., and Poursafa, P. 2021. Urinary levels of PAH metabolites in pregnant women and their correlation with sociodemographic factors and pm2.5 exposure in an urban and a suburban area. Air Quality, Atmosphere & Health. 14(5): 653-665.
Seidel, A., Spickenheuer, A., Straif, K., Rihs, H-P., Marczynski, B., Scherenberg, M., Dettbarn, G., Angerer, J., Wilhelm, M., and Brüning, T. 2008. New biomarkers of occupational exposure to polycyclic aromatic hydrocarbons. Journal of Toxicology and Environmental Health, Part A. 71(11-12): 734-745.
Sochacka-Tatara, E., Majewska, R., Perera, F.P., Camann, D., Spengler, J., Wheelock, K., Sowa, A., Jacek, R., Mróz, E., and Pac, A. 2018. Urinary polycyclic aromatic hydrocarbon metabolites among 3-year-old children from Krakow, Poland. Environmental Research. 164: 212-220.
Soghoian, S., Nyadedzor, C., Ed Nignpense, B., Clarke, E., and Hoffman, R. 2012. Health risks of using mothballs in greater Accra, Ghana. Tropical Medicine & International Health. 17(1): 135-138.
Solanki, R., Macatangay, R., Sakulsupich, V., Sonkaew, T., and Mahapatra, P.S. 2019. Mixing layer height retrievals from miniMPL measurements in the Chiang Mai valley: Implications for particulate matter pollution. Frontiers in Earth Science. 7: 308
Son, J-Y., Lee, J-T., Kim, K-H., Jung, K., and Bell, M.L. 2012. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environmental Health Perspectives. 120(6): 872-878.
Thepnuan, D. and Chantara, S. 2020a. Characterization of PM2.5–bound polycyclic aromatic hydrocarbons in Chiang Mai, Thailand during biomass open burning period of 2016. Applied Environmental Research. 42(3): 11-24.
Thepnuan, D. and Chantara, S. 2020b. Characterization of pm2. 5–bound polycyclic aromatic hydrocarbons in Chiang Mai, Thailand during biomass open burning period of 2016. Applied Environmental Research. 42(3): 11-24.
Thi, T-U.N., Kawanami, S., Kawai, K., Kasai, H., Li, Y-S., Inoue, J., Le, T.N., and Horie, S. 2014. Urinary 1-hydroxypyrene and 8-hydroxydeoxyguanosine levels among coke-oven workers for 2 consecutive days. Journal of Occupational Health. 13-0222-OA.
Uppstad, H., Osnes, G.H., Cole, K.J., Phillips, D.H., Haugen, A., and Mollerup, S. 2011. Sex differences in susceptibility to pahs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer. 71(3):264-270.
VatcharaVongVan, P. and Puttawanchai, V. 2017. Polypharmacy, medication adherence and medication management at home in elderly patients with multiple non-communicable diseases in Thai primary care. Family Medicine & Primary Care Review. (4): 412-416.
Wu, S., Deng, F., Huang, J., Wang, H., Shima, M., Wang, X., Qin, Y., Zheng, C., Wei, H., and Hao, Y. 2012a. Blood pressure changes and chemical constituents of particulate air pollution: Results from the healthy volunteer natural relocation (hvnr) study. Environmental Health Perspectives. 121(1): 66-72.
Wu, S., Deng, F., Huang, J., Wang, H., Shima, M., Wang, X., Qin, Y., Zheng, C., Wei, H., and Hao, Y. 2012b. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: A prospective panel study. Particle and Fibre Toxicology. 9(1): 1-13.
Yang, Z., Guo, C., Li, Q., Zhong, Y., Ma, S., Zhou, J., Li, X., Huang, R., and Yu, Y. 2021. Human health risks estimations from polycyclic aromatic hydrocarbons in serum and their hydroxylated metabolites in paired urine samples. Environmental Pollution. 290: 117975.
Yoon, H-S., Lee, K-M., Lee, K-H., Kim, S., Choi, K., and Kang, D. 2012. Polycyclic aromatic hydrocarbon (1-ohpg and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. International Journal of Hygiene and Environmental Health. 215(4): 458-464.
Zhou, Y., Liu, Y., Sun, H., Ma, J., Xiao, L., Cao, L., Li, W., Wang, B., Yuan, J., and Chen, W. 2018. Associations of urinary polycyclic aromatic hydrocarbon metabolites with fractional exhaled nitric oxide and exhaled carbon monoxide: A cross-sectional study. Science of the Total Environment. 618: 542-550.
Zhu, H., Martinez-Moral, M-P., and Kannan, K. 2021. Variability in urinary biomarkers of human exposure to polycyclic aromatic hydrocarbons and its association with oxidative stress. Environment International. 156: 106720.
Zuo, Y., Wang, C., Zhou, J., Sachdeva, A., and Ruelos, V.C. 2008. Simultaneous determination of creatinine and uric acid in human urine by high-performance liquid chromatography. Analytical Sciences. 24(12): 1589-1592.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pitakchon Ponsawansong1, 2, Tippawan Prapamontol1, *, Jaras Singkaew3, Guoxing Li4, and Xiaochuan Pan4
1 Research Institute for Health Sciences, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Program in Environmental Science, Environmental Science Research Center, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Saraphi Hospital, Chiang Mai, 50140, Thailand.
4 Department of Occupational and Environmental Health Sciences, School of Public Health, Peking University, Beijing 100191.
Corresponding author: Tippawan Prapamontol, E-mail: tippawan.prapamontol@cmu.ac.th
Total Article Views
Editor: Waraporn Boonchieng,
Chiang Mai University, Thailand
Article history:
Received: November 1, 2023;
Revised: January 13, 2024;
Accepted: January 16, 2024;
Online First: January 19, 2024

