
Evaluation of Antioxidant Activities, Total Phenolic and Total Flavonoid Contents of Aqueous Extracts of Leaf, Stem, and Root of Aerva lanata. Savita Chewchinda, Sumet Kongkiatpaiboon, and Pongtip Sithisarn*
Published Date : 2019-08-23
DOI : https://doi.org/10.12982/CMUJNS.2019.0024
Journal Issues :
Number 3 , July-September 2019
ABSTRACT
Aerva lanata (Amaranthaceae) is a tropical weed commonly found in fields and wasteland. Several biological activities of this plant have been re- ported, such as antihyperglycemic, antimicrobial, and anticancer activities. Different antioxidant assays including DPPH radical scavenging, ferric re- ducing antioxidant power (FRAP), and ABTS radical scavenging assays were assessed to compare antioxidant potentials of plant extracts. The total phe- nolic and flavonoid contents were determined. HPLC analysis was used to quantify the amount of ferulic acid. From the results, the leaf extract showed the strongest radical scavenging activity as measured by DPPH and ABTS assays with IC50 values of 136 μg/ml and 58 mg TEAC/g extract, respectively. Similarly, the highest reducing power of the leaf extract was observed at 70 mmol FeSO4 /100 g extract. HPLC quantification of ferulic acid yielded values of 1.58, 1.53, and 1.33 µg/100 g extract for the leaf, stem and root extracts, respectively. Thus, A. lanata leaf extract may be suitable for further develop- ment and application as pharmaceutical and nutraceutical products due to its potent in vitro antioxidant activities and high phenolic contents.
Keywords: ABTS, Aerva lanata, Antioxidant activity, DPPH, FRAP, Total phenolic
INTRODUCTION
An excess of oxidative stress can cause many inflammatory diseases such as arthritis, diabetes, cancer, Alzheimer’s disease and many others. An imbalance between free radical production and antioxidant defenses in the human body can lead to oxidative stress condition (McCord, 2000). Antioxi- dants are involved in the chain-breaking mechanism by donating an electron to the free radicals present in the system and/or removal of reactive oxygen and nitrogen species initiators by quenching the chain-initiating catalyst (Lobo et al., 2010). Many phytochemicals such as polyphenolics and flavonoids from natural sources are well known antioxidants (Kantappa et al., 2016).
Aerva lanata (L.) Juss. ex Schultes (Amaranthaceae) is a tropical weed which commonly grows in fields and wasteland. It is native to South Asia, South East Asia, Africa, and Australia (Rajesh et al., 2011a). Traditionally, many parts of A. lanata have been used in several applications. The leaves have been used for the treatment of malaria and urinary troubles (Kakrani and Saluja, 1994). The roots has also been used for headaches, scabies, coughs, jaundice, cholera, dysentery and as a diuretic (Joshi, 2000). In Indian and Thai traditional medi- cines, a decoction of the whole plant of A. lanata has been used for the treatment of diabetes mellitus (Vertichelvan et al., 2000; Khunchalee et al., 2002). The reported biological activities of the whole plant are antihyperglycemic, antidia- betic, antimicrobial, hepatoprotective, immunomodulatory and anticancer activ- ities (Chowdhurya et al., 2002; Vetrichelvan and Jegadeesan, 2002; Deshmukh et al., 2008; Rajesh et al., 2011b; Krishna et al., 2012; Siveen and Kutan, 2012). As for its phytochemical constituents, A. lanata is comprised of flavonoids, alkaloids, triterpenes, steroids, polysaccharides, tannins and saponins. The aerial part contains β-sitosterol, α-amyrin, betulin, kaempferol-3-galactoside and kaempferol-3-rhamnogalactoside (Payal et al., 2015). The roots are reported to contain phytoecdysteroids, feruloylamines, flavonoid glycosides, β-sitosterol, palmitic acid and canthin-6-one derivative alkaloids (Goyal et al., 2011). The leaves contain phenolic compounds, alkaloids and steroids (Akanji et al., 2018). Ferulic acid, a phenolic derivatives hydroxycinnamic acid, is regarded as a major active compound present in A. lanata (Rajasekaran and Gebrekidan, 2018). The chemical structure of ferulic acid is shown in Figure 1. Nowadays, functional and nutraceutical foods are gaining popularity worldwide owing to their health-promoting properties. Plants are a great dis- coverable resource for valuable compounds because of their diversity of active constituents. Therefore, the aim of the present study is to determine the antioxidant acitivities, total phenolic and total flavonoid contents of leaf, stem and root extracts of A. lanata. The results could support the traditional use of A. lanata and provide essential data for the development of nutraceu- tical product in order to use them as an alternative antioxidant treatment.
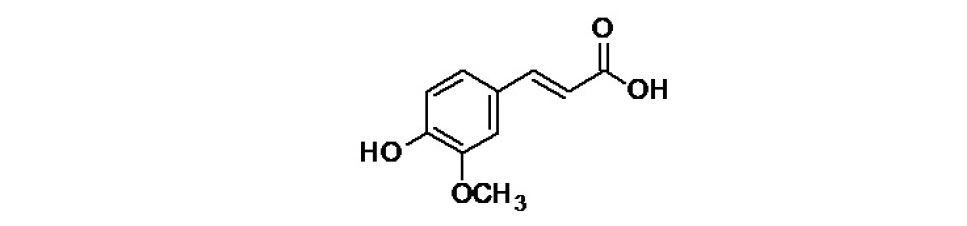
Figure 1. Chemical structure of ferulic acid.
MATERIAL AND METHODS
Chemicals and reagents
Folin-Ciocalteu reagent, DPPH (2, 2’-diphenyl-1- picrylhydrazyl) and ABTS (2, 2’-Azino-bis (3-ethylbenzothiazoline-6-sulfoni acid) Diammonium salt were purchased from Sigma-Aldrich, U.S.A. Glacial acetic acid was purchased from Labscan, Thailand. Ferulic acid reference standard was purchased from Sigma-Aldrich, U.S.A. All reagent and solvents used were of analytical grade.
Plant material
Fresh whole plants of A. lanata were collected from Saraburi province in August, 2016. The plants were authenticated by Prof. Dr. Wongsatit Chuakul, Department of Pharmaceutical Botany, Faculty of Pharmacy, Mahidol University. The voucher specimens (specimen #0816-001-3) were deposited in the Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Thailand.
Sample preparation
The whole plants were washed with tap water and dried in a hot air oven at 50°C for 24 h. The plants were separated into 3 parts including leaf, stem and root. Each dried part was pulverized into a powder and kept at -20°C for further studies.
Extraction method
For decoction method, 20 g of powdered leaf and stem were boiled with 500 ml and 200 ml of distilled water at 100°C for 15 min in a water bath, respectively. The powdered root (10 g) was also boiled with 150 ml of distilled water at 100°C for 15 min in a water bath. The extraction was performed in triplicate. The pooled extract was filtered through Whatman no. 4 paper with a Buchner funnel. After filtration, the extract was dried in a water bath until a constant weight was achieved. The extract was kept at 4°C until further analysis.
Antioxidant activity assay
DPPH free radical scavenging assay. The DPPH free radical scav- enging assay was carried out according to the method described by Sithisarn et al. (2015). DPPH radical was freshly prepared in methanol at a final con- centration of 152 μM. Each plant extract was diluted in methanol at varying concentrations. In a 96-well plate, each extract (100 μl) was added to each well, followed by 100 μl of methanolic DPPH solution. Ascorbic acid was used as a positive control. The mixtures were allowed to stand at room tem- perature in the dark for 30 min. The absorbance was recorded at 517 nm using a microplate reader (Tecan, Switzerland). Each sample was performed in triplicate. The percentage of scavenging activity is calculated as follows:
% inhibition= [(Ac-As)/Ac ] × 100
where Ac is the absorbance of the control solution at 517 nm and As is the absorbance of the sample solution at 517 nm.
Ferric reducing antioxidant power (FRAP) assay. FRAP assay is adapted from the method of Lim and Quah (2007). Each sample (500 µl) was mixed with 500 µl of 0.2 M potassium phosphate buffer (pH 6.6) and 500 µl of a 1% w/v potassium ferricyanide solution. The mixture was incubated at 50°C for 20 min. Then, 2 ml of trichloroacetic acid was added to the mix- ture. The supernatant of the mixture (500 µl) was mixed with 500 µl of de- ionized water and 100 µl of 0.1% w/v ferric chloride solution. The procedure was carried out in triplicate and allowed to stand for 30 min before measur- ing the absorbance at 700 nm using a microplate reader (Tecan, Switzerland). Ferrous sulfate was used for the standard curve. The FRAP value was ex- pressed as mmol FeSO4 equivalent per gram of extract (mmol FeSO4/g extract).
ABTS radical scavenging assay. The procedure for ABTS assay follow the method of Thaipong et al. (2006). The working solution was pre- pared by mixing 7 mM of ABTS solution and 2.45 mM of potassium persul- fate solution in equal quantities. The mixture was allowed to react for 12-16 h in the dark at room temperature. The solution was then diluted by mix- ing 1 ml ABTS solution with 24 ml of methanol to obtain an absorbance of 1.100±0.020 units at 734 nm using a microplate reader (Tecan, Switzerland). In 96-well plates, each sample (10 µl) was mixed with of ABTS▪+ radical cation solution (200 µl). The absorbance was taken at 734 nm after 6 min in a mi- croplate reader. All determinations were carried out in triplicate. Results were expressed in mg Trolox equivalents antioxidant capacity (TEAC)/g extract.
Total phenolic content
The total phenolic content in A. lanata extracts were determined by using the Folin-Ciocalteu assay (Stanković, 2011). Each sample (20 µl) was mixed with 50 µl of Folin-Ciocalteu’s solution (diluted 1:10 with deionized water) in a 96-well plate for 3 min. Then 80 µl of 7.5% w/v sodium carbon- ate solution was added and incubated for 2 h in the dark at room temperature. The absorbance was recorded by using a microplate reader (Tecan, Switzerland) at λmax 765 nm. The assay was performed in triplicate. Gallic acid was used to establish the standard curve. Total phenolic content in the extracts was ex- pressed as gallic acid equivalent per gram of extract (mg of GAE/g of extract).
Total flavonoid content
According to the method of Stanković (2011), each sample (500 µl of 1 mg/ml) was mixed with 100 µl of 2% w/v aluminium chloride in methanol solu- tion. The mixture was incubated for 10 min at room temperature. The absorbance was analyzed using a microplate reader (Tecan, Switzerland) at λmax 415 nm. The procedure was analyzed in triplicate. The standard solution using quercetin was diluted serially to the range of 6.25 to 100 μg/ml and treated in the same proce- dure as sample to create a standard curve. The flavonoid content in each extract was expressed as quercetin equivalent per gram of extract (mg QE/g of extract).
Thin layer chromatographic (TLC) fingerprint
The TLC fingerprint of A. lanata was obtained according to the method of Srivastava et al. (2012). A TLC chromatogram was carried out on an aluminum sheet of silica gel60 F254 (E. Merck, Germany). Toluene:ethylacetate:formal- dehyde(6:3:1, v/v/v) was employed as the solvent system. The developed plate was detected under UV 254 and 366 nm and was sprayed with a natural product spraying reagent (NP-PEG).
HPLC analysis
HPLC apparatus and chromatographic conditions. HPLC analysis was analyzed according to the method of Voncina et al. (2009). HPLC was performed on a Shimadzu Technologies modular model Class VP system con- sisting of a SCL-10A system, a UV-vis SPD-10A detector, LC-10 AD and an auto injector SIL-10A (Shimadzu, Japan). The separation was done on a BDS Hypersil C18 column (150×4.6 mm, i.d. 5 µm) (Thermo Fisher Scientific Inc., USA) with a BDS Hypersil C18 guard column (10×4 mm, i.d. 5 µm) (Thermo Hypersil-Keystone, USA). The elution was analyzed by isocratic solvent system using 2% aqueous acetic acid solution and methanol (82:18, v/v). The total running time was 30 min and a flow rate was 1.0 ml/min. The UV detector was set at the wavelength of 320 nm while the injection volume was 20 µl.
Stock and working solution of ferulic acid. The ferulic acid standard solutions were accurately weighed to a concentration of 1 mg/ml. The ferulic acid working standard solutions were obtained by diluted the stock solution with methanol to concentrations of 1.875-30 µg/ml.
Sample preparation. Each crude extract was accurately weighed and dissolved in methanol. Then adjusted to 10 mL in a volumetric flask. Each sample solution was filtered through a 0.45 µm nylon syringe filters before injection. All samples were measured in triplicate.
Statistical analysis
Results are expressed as the mean values±standard deviation (n=3). Data were analyzed by one-way analysis of variance (ANOVA) and Scheffe’s test using SPSS software. Differences at the 95% level were considered to be significant.
RESULTS
Antioxidant activities, total phenolic and total flavonoid contents of aqueous extracts from the leaves, stems and roots of A. lanata
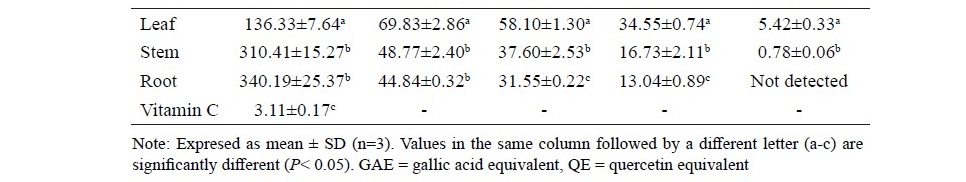
In the present study, a decoction method was chosen due to its tradi- tional usage. Decoction is a well-established technique for use with natural product extraction and offer advantages like rapidity, efficiency and cost ef- fectiveness (Li et al., 2007). The percentages of extraction yield were 42.45, 25.90 and 20.98 % w/w for the leaf, stem and root extracts, respectively. Antioxidant activities, total phenolic and total flavonoid contents of aqueous extracts from the leaves, stems and roots of A. lanata are shown in Table 1. Among all extracts, the leaf extract significantly exhibited the high- est antioxidant activities (P<0.05). The DPPH and ABTS radical scaveng- ing activities of the leaf extract were reported with IC50 values of 136 µg/ ml and 58 mg TEAC/g extract. For the determination of the reducing power, the leaf extract also significantly displayed the highest antioxidant poten- tial (P<0.05). The FRAP value of the leaf, stem and root were 69.83, 48.77 and 44.84 mmol FeSO4/100 g extract, respectively. Similarly, the leaf extract contained the significantly highest amounts of phenolic and flavonoid com- pounds (P<0.05). The amounts of the total phenolic and flavonoid compounds of the leaf extract were 34.55 mg GAE/g extract and 5.42 mg QE/g extract, respectively. There were high correlations between the antioxidant activities and the total phenolic/flavonoid contents in all A. lanata extracts (r2>0.99).
Table 1. DPPH radical scavenging activity, ferric reducing antioxidant power (FRAP), ABTS assay, total phenolic and total flavonoid content of the aqueous extracts of leaf, stem and root of A. lanata.
Thin layer chromatographic (TLC) fingerprint
The TLC fingerprints of the extracts from different parts of A. lanata are shown in Figure 2. It was confirmed that the leaf extract mainly constituted of polyphenolic and flavonoid compounds. Both of which were detected after the TLC plate was sprayed with an NP-PEG spraying reagent and appeared as bright blue or yellow chromatographic bands. TLC analysis suggested that the leaf extract contained higher amounts of phenolics and flavonoids than extracts from the roots and stem. Ferulic acid was present in the TLC chromatograms of all extracts, which was indicated as a bright blue fluorescence band after the TLC plate was sprayed with an NP-PEG spraying reagent, which corresponded with the chromatographic band of standard ferulic acid at an Rf value of 0.58.
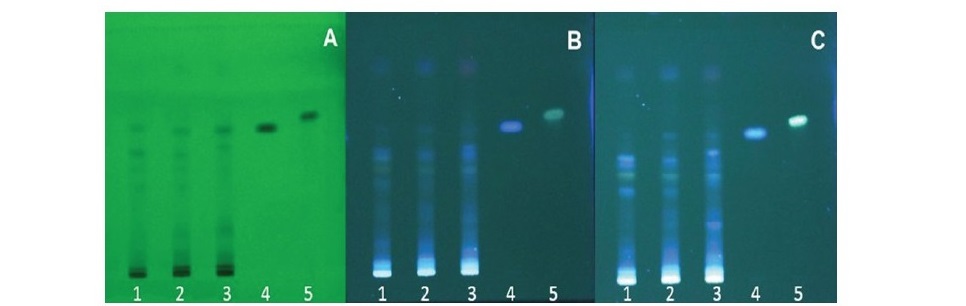
Figure 2. TLC fingerprints of A. lanata extracts (Silica gel F254, toluene:ethyl acetate: formaldehyde (6:3:1 v/v/v); track 1: root extract, track 2: stem extract, track 3: leaf extract, track 4: standard ferulic acid, track 5: standard kaempferol. (A) detected under UV 254 nm, (B) detected under UV 366 nm and (C) detected under UV 366 nm after being sprayed with an NP-PEG spraying reagent.
HPLC analysis
For HPLC analysis, ferulic acid was identified by its retention time and by spiking with the standard under the same conditions. It showed a prominent peak at a retention time of 19.22 min, which corresponded to ferulic acid reference standard. The HPLC chromatograms of different parts of A. lanata extracts are shown in Figure 3. The calibration curve of ferulic acid standard generated the regression equation, y = 49652x - 9243, r2= 0.999. The content of ferulic acid were 1.58, 1.53, and 1.33 µg/100 g extract for the leaf, stem and root extracts, respectively.
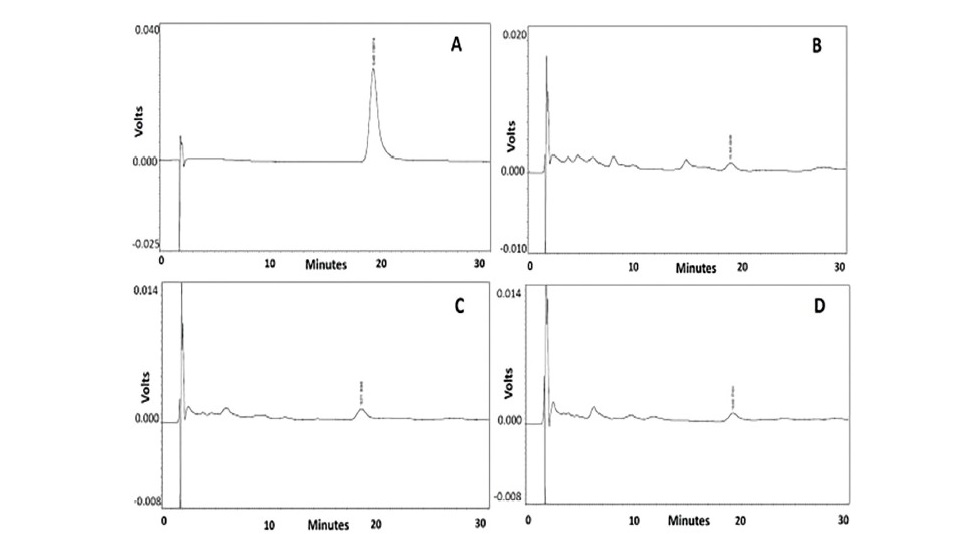
Figure 3. HPLC chromatogram from different parts of A. lanata extracts (A) Ferulic acid reference standard, (B) A.lanata leaf extract,
(C) A.lanata stem extract and (D) A.lanata root extract.
DISCUSSION
The results of antioxidant activities obtained in the present study were in accordance with the previous report which indicated that the flavonoid contents in A. lanata extracts were in the following order: leaves > root > stem determined using the HPTLC method (Mariswamy et al., 2012). However, scavenging activ- ity of ascorbic acid was significantly higher than all of A. lanata extracts. From a previous LC-MS study, it was found that the major compounds of aqueous A. lanata leaf extract were ferulic acid, kaempferol and β-carboline (Poonkuzhali et al., 2014). Many studies reported the strong antioxidant activities of polypheno- lic and flavonoid compounds from medicinal plants (Kantappa et al., 2016). It was reported that the free radical scavenging and antioxidant activities of each pheno- lic compound depends on the number and configuration of H-donating hydroxyl groups in their structures. Phenolic compounds possess antioxidant activities via several pathways such as free radical scavenging, chelation of redox active metal ions, modulation of gene expression and interaction with the cell signaling path- ways (Soobrattee et al., 2005). There were some studies indicating the antioxidant effects of ferulic acid (Graf, 1992; Srinivasan et al., 2007). Moreover, there was also a report that indicated the antioxidant properties of β–carboline alkaloid. It showed antioxidant activity over many pathways including the inhibition of lipid peroxidation, attenuating the oxidative damage of hyaluronic acid, cartilage and collagen. The β–carboline exerts a protective effect on oxidative neuronal dam- age through a scavenging action on reactive oxygen species (Moura et al., 2007).
The results of the present study support the traditional use of A. lanata. It may provide essential information for the utilization of this plant as a source of low-cost natural antioxidants or nutraceutical products.
CONCLUSION
The present study reported the evaluation of in vitro antioxidant activ- ities of the leaf, stem and root extracts of A. lanata. From above results, the leaf extract exhibited the significantly (P< 0.05) strongest free radical scav- enging activity in all tested assays. The TLC fingerprints of extracts from different parts of A. lanata suggested the presence of phenolic and flavonoid compounds. HPLC analysis showed considerable quantities of ferulic acid in the extracts. Therefore, A. lanata leaf extract can serve as a natural antioxi- dant source, which could be further applied in nutraceutical and pharmaceu- tical production. Ferulic acid could be regarded as the marker compound for the quality control of both the raw material and the extract from A. lanata. Further phytochemical studies, determination of related biological activi- ties and a toxicity test on A. lanata extracts should be conducted in the future.
ACKNOWLEDGEMENTS
The author thanks Miss Pattarin Patarakijavanich for her participation in this research.
REFERENCES
Akanji, A.A., Olukolu, S.O., and Kazeem, M.I. 2018. Leaf extracts of Aerva lanata inhibit the activities of type 2 diabetes-related enzymes and possess antioxidant properties. Oxidative Medicine and Cellular Longevity. Article ID 3439048. https://doi.org/10.1155/2018/3439048
Chowdhurya, D., Sayeed, A., Islam, A., Sham Alam Bhuiyan, M., and Astaq Mohal Khan, G.R.M. 2002. Antimicrobial activity and cytotoxicity of Aerva lanata. Fitoterapia.73: 92-94. https://doi.org/10.1016/S0367- 326X(01)00369-0
Deshmukh,T.A.,Yadav, B.V., Badole, S.L., Bodhankar, S.L., and Dhaneshwar, S.R. 2008. Antihyperglycaemic activity of alcoholic extract of Aerva lanata (L.) A. L. Juss. ex J. A. Schultes leaves in alloxan induced diabetic mice. Journal of Applied Biomedicine. 6: 81-87.
Goyal, M., Pareek, A., Nagori, B.P., and Sasmal, D. 2011. Aerva lanata: A review on phytochemistry and pharmacological aspects. Pharmacognosy Reviews. 5(10): 195-198. https://dx.doi.org/10.4103%2F0973-7847.91120
Graf, E. 1992. Antioxidant potential of ferulic acid. Free Radical Biology and Medicine. 13(4): 435-448.
Joshi, S.G. 2000. Medicinal plants. New Delhi: Oxford & IBH Publishing. Kakrani, H.K.N., and Saluja, A.K. 1994. Traditional treatment through herbs in Kutch district, Gujarat state, India. Part II. Analgesic, anti-inflammatory, antirheumatic, antiarthritic plants. Fitoterapia. 65: 427–430.
Kantappa, H., Mallinath, B., and Jonghwi, L. 2016. Structural implications of polyphenolic antioxidants. Journal of Industrial and Engineering Chemistry. 35: 1-7. https://doi.org/10.1016/j.jiec.2016.01.003
Khunchalee, J., Kongkathip, B., Kongkatip, N., Kemsri, W., and Ittipanichpong, C. 2002. Hypoglycemic effect of Aerva lanata. Proceedings of the 40th Kasetsart University Annual Conference Science, Natural Resources and Environmental Economics; 2002 Feb 4-7; Bangkok: Thai National AGRIS Center. 425 p.
ThaiKrishna, C.A., Challa, S.R., and Reddy, M.A. 2012. Hepatoprotective effect of biherbal ethanolic extract against paracetamol-induced hepatic damage in albino rats. Journal of Ayurveda & Integrative Medicine. 3(4): 198-203. https://dx.doi.org/10.4103%2F0975-9476.104436
Li, H.B., Jiang, Y., Wong, C.C., Cheng, K.W., and Chen, F. 2007. Evaluation of two methods for the extraction of antioxidants from medicinal plants. Analytical and Bioanalytical Chemistry. 388(2): 483-488.
Lim, Y.Y, and Quah, E.P.L. 2007. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chemistry. 103(3): 734-740. http://doi.org/ 10/1016/j.foodchem.2006.09.025
Lobo, V., Patil, A., Phatak, A., and Chandra, A. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4(8): 118-126. https://dx.doi.org/10.4103%2F0973-7847.70902
Mariswamy, Y., Gnaraj, W.E., and Antonisamy, J.M. 2012. Chromatographic fingerprint analysis on flavonoids constituents of the medicinally important plant Aerva lanata L. by HPTLC technique. Asian Pacific Journal of Tropical Biomedicine. S8-S12. https://doi.org/10.1016/ S2221-1691(11)60112-3
McCord, J.M. 2000. The evolution of free radicals and oxidative stress. American Journal of Medicine. 108(8): 652-659. https://doi.org/10. 1016/S0002-9343(00)00412-5
Moura, D.J., Richter, M.F., Boeira, J.M., Henriques, J.A.P., and Saffi, J. 2007. Antioxidant properties of β-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis. 22(4): 293–302. https://doi.org/10.1093/mutage/gem016
Payal, C., Gurlaganjeet, K., Davinder, K., Gagan, S., Amit, C., and Dhawan, R.K. 2015. A review on phytochemistry and biological activities of Aerva. Medicinal & Aromatic Plants 4: 2. http://dx.doi.org/10.4172/2167- 0412.1000187
Poonkuzhali, K., Rajeswari, V., Saravanakumar, T., Viswanathamurthi, P., Park, S.M., and Govarthananc, M. 2014. Reduction of hexavalent chromium using Aerva lanata L.: Elucidation of reduction mechanism and identification of active principles. Journal of Hazardous Materials. 272: 89–95. https://doi.org/10.1016/j.jhazmat.2014.03.001
Rajasekaran, R., and Gebrekidan, Y. 2018. A review on antibacterial phytochemical constitutions present in Aerva lanata and their mode of action against bacterial biofilm. International Journal of Pharmaceutical & Biological Archives. 9(1): 16-30.
Rajesh, R., Chitra, K., and Paarakh, P.M. 2011a. Aerva lanata (Linn.) Juss. ex Schult. An overview. Indian Journal of Natural Products and Resources. 2: 5-9.
Rajesh, R., Chitra, K., Paarakh, P.M., and Chidambaranathan, N. 2011b. Anticancer activity of aerial parts of Aerva lanata Linn Juss ex Schult against Dalton’s Ascitic Lymphoma. European Journal of Integrative Medicine. 3: e245-250. https://doi.org/10.1016/j.eujim.2011.05.001
Sithisarn, P., Rojsanga, P., Sithisarn, P., and Kongkiatpaiboon, S. 2015. Antioxidant activity and antibacterial effects on clinical isolated Streptococcus suis and Staphylococcus intermedius of extracts from several parts of Cladogynos orientalis and their phytochemical screenings. Evidence-Based Complementary and Alternative Medicine. http://dx.doi.org/10.1155/2015/908242
Siveen, K.S., and Kuttan, G. 2012. Effect of Aerva lanata on cell-mediated immune response and cytotoxic T-lymphocyte generation in normal and tumor-bearing mice. Journal of Immunotoxicology. 9: 25–33. https:// doi.org/10.3109/1547691X.2011.609191
Soobrattee, M.A., Neergheen, V.S., Luximon-Ramma, A., Aruoma, O.I., and Bahorun, T. 2005. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 579(1-2): 200-221. https://doi.org/ 10.1016/j.mrfmmm.2005.03.023
Srinivasan, M., Sudheer, A.R., and Menon, V.P. 2007. Ferulic Acid: Thera- peutic potential through its antioxidant property. Journal of Clinical Biochemistry and Nutrition. 40(2): 92–100. https://dx.doi.org/10.3164% 2Fjcbn.40.92
Srivastava, S., Singh, A.P., and Rawat, A.K.S. 2012. A HPTLC method for the identification of ferulic acid from Lycopodium clavatum. Asian Pacific Journal of Tropical Biomedicine. S12-S14. https://doi.org/ 10.1016/S2221-1691(12)60121-X
Stanković, M.S. 2011. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac Journal of Science. 33: 63-72.
Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., and Hawkins Byrne, D. 2006. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 19(6–7): 669-675.https://doi.org/10.1016/j.jfca.2006.01.003
Vertichelvan, T., Jegadeesan, M., Senthil, P.S., Murali, N.P., and Sasikumar, K. 2000. Diuretic and anti-inflammatory activities of Aerva lanata in rats. Indian Journal of Pharmaceutical Sciences. 62: 300–302.
Vetrichelvan, T., and Jegadeesan, M. 2002. Anti-diabetic activity of alcoholic extract of Aerva lanata (L.) Juss. ex Schultes in rats. Journal of Ethnopharmacology. 80: 103–107. https://doi.org/10.1016/S0378-8741(01)00412-3
Voncina, D.B., Razborsek, M.I., and Simonic, M. 2009. High performance liquid chromatographic determination of selected phenolic acids in wine. Nova Biotechnologica. 9(2): 113-118.
Savita Chewchinda1, Sumet Kongkiatpaiboon2, and Pongtip Sithisarn3*
1Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
2Drug Discovery and Development Center, Thammasat University Pathum Thani 12121, Thailand
3Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University. Bangkok 10400, Thailand
*Corresponding author: E-mail: pongtip.sit@mahidol.ac.th
Total Article Views
Article history:
Received: August 10, 2018;
Revised: January 17, 2019;
Accepted: January 29, 2019

