
Design of Functional Yogurts with Microencapsulated Biologically Active Compounds
Yulian Tumbarski *, Nadezhda Petkova, Ivan Ivanov, Mina Todorova, Petya Ivanova, Lazar Nikolov, Neli Grozeva, and Krastena NikolovaPublished Date : January 17, 2024
DOI : https://doi.org/10.12982/NLSC.2024.011
Journal Issues : Number 1, January-March 2024
Abstract The development of functional foods providing health benefits above the basic nutritional needs is of great interest to the food industry. This research aimed to: design functional yogurts with agave inulin and extracts from propolis, rose petals and Spirulina platensis; observe the physicochemical and microbiological changes during storage at 4°C for 21 days; evaluate the sensory characteristics of the new functional products. Three kinds of calcium alginate microcapsules (agave inulin + ethanolic propolis extract; agave inulin + ethanolic propolis extract + rose petals extract; agave inulin + ethanolic propolis extract + Spirulina extract) were prepared and applied in yogurts before the coagulation. During the storage, pH in all experimental groups gradually decreased (reaching values between 3.94 and 4.02 on the 21st day), which corresponded to the increasing titratable acidity (reaching values from 94.17°T to 108.78°T on the 21st day). The application of microcapsules did not affect the coagulation process and number of lactic acid bacteria in yogurts until the end of storage period. To the best of our knowledge, this is the first study to investigate the application of agave inulin, propolis, rose petals and S. platensis extracts in microencapsulated form in traditional Bulgarian yogurt in order to obtain a dairy product with enhanced functional properties.
Keywords: Functional foods, Yogurt, Microencapsulation, Propolis, Rose petals, Spirulina platensis
Citation: Tumbarski, Y., Petkova, N., Ivanov, I., Todorova, M., Ivanova, P., Nikolov, L., Grozeva, N., and Nikolova, K. 2024. Design of functional yogurts with microencapsulated biologically active compounds. Natural and Life Sciences Communications. 23(1): e2024011.
INTRODUCTION
Milk and dairy products are essential components of the daily human diet, and can be considered functional foods, which provide the necessary amounts of the basic nutrients and energy, and may reduce the risk of some diseases. In parallel with their health benefits, dairy products represent an excellent matrix for incorporation of viable beneficial microorganisms (probiotics) and substances supporting different metabolic functions – prebiotics and bioactive compounds, which is of paramount importance in designing novel functional products or improving the functional properties of existing ones (Özer and Kirmaci, 2010; Ali et al., 2021). On the other hand, many biologically active compounds are insoluble or unstable under various environmental conditions; therefore, their encapsulation represents an innovative approach to overcome these limitations and to increase their efficiency (Gruskiene et al., 2021). In addition to the incorporation of bioactive substances, the encapsulation in alginate, gelatin and chitosan beads is an efficient method applied to protect probiotic strains from the adverse conditions in the gastrointestinal tract (Khimmakthong et al. 2020).
The traditional Bulgarian yogurt, also known as kiselo mlyako, is a coagulated milk product, characterized by smooth texture, thick consistency and pleasant sour taste. Yogurt is one of the most famous and important dairy products in Bulgaria, produced by bacterial fermentation of cow’s, buffalo’s, sheep’s and goat’s milk using a symbiotic culture of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus, which contribute to its specific flavour characteristics (Tropcheva et al., 2014). It has been known on the Balkan Peninsula since antiquity, when the Thracians obtained it from self-fermented sheep’s milk. Nowadays, it takes an important role in the diet of the Bulgarians due to its excellent nutritional characteristics and beneficial health effects. Besides the high nutritional value, yogurt possesses many therapeutic effects on lactose intolerance and gastrointestinal disorders (McKinley, 2005). Likewise, Bulgarian yogurt is a dairy product recommended for improving the metabolism and body weight maintenance (Jacques and Wang, 2014), for treatment and prevention of Parkinson’s disease and cancer (Velikova et al., 2018). Other studies have revealed that yogurt consumption exerts positive health effects by reducing the cholesterol levels and blood pressure, as well as decreases the risk of cardiovascular diseases and type 2 diabetes (Visioli and Strata, 2014).
Prebiotics are non-digestible compounds (oligosaccharides and polysaccharides) that exert health benefits by stimulating the gastrointestinal microbiota. Inulin is a reserve plant polysaccharide, which is known as a source of dietary fibers and serves as a substrate for the growth of beneficial gut bacteria. The low sweetness of inulin and its properties similar to sucrose allow it to be used as a sugar substitute in some food products (Bouaziz et al., 2014). Incorporated into yogurt, prebiotics stimulate the lactic acid bacteria, leading to higher production of volatile compounds, which positively affects the organoleptic properties of the product (Costa et al., 2019). Agave inulin is a highly soluble prebiotic with the potential to improve the balance of the gastrointestinal flora by stimulating the growth of bifidobacteria, thus exerting its immunomodulatory effects, controlling body weight and improving calcium absorption by the organism (Melo et al., 2018).
Propolis is a complex biological mixture produced by European honey bees (Apis mellifera L.), characterized by a rich chemical composition and possessing antibacterial, antiviral, antiparasitic, antioxidant, immunomodulatory, antidiabetic, anti-inflammatory, anticarcinogenic, hepatoprotective, astringent, antihypertensive, anti-ulcerogenic, anti-allergic and anaesthetic effects (Cauich-Kumul and Campos, 2019). As a safe natural product and a valuable source of many bioactive compounds, propolis has been successfully applied as a remedy in medicine (Pasupuleti et al., 2017), a functional food ingredient (Irigoiti et al., 2021), a promising food biopreservative (Tosi et al., 2007) and a nutritional value enhancer (Morsy
et al., 2021).
The rose petals have been reported to possess a number of pharmacological activities, which can be attributed to the high concentrations of polyphenolic compounds, vitamins B1, B2, B3, C and K, carotenoids, minerals, citric acid, malic acid, pectin and tannins. Rose petals are known to possess antibacterial, antiviral, antioxidant, antidiabetic, antitussive, analgesic, anticonvulsant, hypnotic, anti-depressant, anti-inflammatory, cardioprotective and digestive effects (Nayebi et al., 2017). The rich phytochemical composition and the pleasant aroma of rose petals have been used in food production to improve the organoleptic properties and nutritional value of various products (Gateva et al., 2022).
Spirulina platensis are multicellular, filamentous, prokaryotic, blue-green microalgae, growing in water with high concentrations of salt and alkaline, which usually inhabit the lakes in Central America, Central Africa and Asia. Due to the high amounts of proteins and phycocyanins, essential fatty acids, sterols, vitamins, macro- and microelements, nowadays Spirulina microalgae are widely known as a “super food” and a functional food ingredient. Spirulina platensis exerts many health benefits such as cardioprotective, immunomodulatory and anti-inflammatory effects, but also reduces the risk of hyperglycaemia, hypercholesterolemia, hypertension and obesity (AlFadhly et al., 2022). Some publications highlighted the positive effects of Spirulina consumption on the brain functions by preventing or delaying various neurodegenerative conditions, such as Parkinson’s disease, Alzheimer’s disease and multiple sclerosis (Trotta et al., 2022).
Despite the large number of publications about the health benefits of propolis, rose petals and Spirulina platensis, the information regarding their application as functional components in encapsulated form in yogurt is still very limited. Therefore, this research aimed to: a) develop a functional yogurt based on traditional Bulgarian yogurt, combining the prebiotic effects of agave inulin with the biological activities of propolis, organic rose petals and Spirulina extracts in microencapsulated form; b) evaluate the sensory characteristics of the newly obtained functional product; c) observe the physicochemical and microbiological changes in the product during storage at 4°C for 3 weeks.
MATERIAL AND METHODS
Materials
Test microorganisms
Twenty microorganisms including eight Gram-positive bacteria (Bacillus subtilis ATCC 6633, Bacillus amyloliquefaciens 4BCL-YT, Staphylococcus aureus ATCC 25923, Listeria monocytogenes NBIMCC 8632, Listeria innocua ATCC 33090, Enterococcus faecalis ATCC 19433, Enterococcus faecium ATCC 19434, Micrococcus luteus 2YC-YT), five Gram-negative bacteria (Salmonella enterica serovar Enteritidis ATCC 13076, Klebsiella sp. – clinical isolate, Escherichia coli ATCC 25922, Proteus vulgaris ATCC 6380, Pseudomonas aeruginosa ATCC 9027), two yeasts (Candida albicans NBIMCC 74, Saccharomyces cerevisiae ATCC 9763) and five fungi Aspergillus niger ATCC 1015, Aspergillus flavus, Penicillium sp., Rhizopus sp. – plant isolates, Fusarium moniliforme ATCC 38932) from the collection of the Department of Microbiology at the University of Food Technologies, Plovdiv, Bulgaria, were selected for the antimicrobial activity test.
B. subtilis, B. amyloliquefaciens and M. luteus were cultured on Luria-Bertani agar with glucose (LBG agar) at 30°C for 24 h, while S. aureus, L. monocytogenes, L. innocua, E. faecalis, E. faecium, S. enterica serovar Enteritidis, Klebsiella sp., E. coli, P. vulgaris and P. aeruginosa were cultured on LBG agar at 37°C for 24 h. C. albicans was cultured on Malt extract agar (MEA) at 37°C, while S. cerevisiae was cultured on MEA at 30°C for 24 h. The fungi A. niger, A. flavus, Penicillium sp., Rhizopus sp. and F. moniliforme were grown on MEA at 30°C for 7 days or until sporulation.
Culture media
Luria-Bertani agar with glucose (LBG agar)
LBG agar (Laboratorios Conda S.A., Spain) was used for cultivation of bacteria. A quantity of 50 g of LBG agar base was dissolved in 1 L of deionized water (pH 7.5 ± 0.2).
Malt extract agar (MEA)
MEA (Scharlab S.L., Spain) was used for cultivation of yeasts and fungi. A quantity of 48 g of MEA base was dissolved in 1 L of deionized water (pH 5.6 ± 0.2).
Chloramphenicol glucose agar (CGA)
CGA (Scharlab S.L., Spain) is a selective medium for enumeration of yeasts and fungi. A quantity of 40 g of CGA base was dissolved in 1 L of deionized water (pH 6.6 ± 0.2).
De Man, Rogosa and Sharpe agar (MRS)
MRS agar is a selective medium for cultivation and enumeration of lactobacilli. A quantity of 55.2 g of MRS-broth (Merck, Germany) was dissolved in 1 L of deionized water (pH 6.4 ± 0.2), and then 15 g of agar (Scharlab S.L., Spain) was added.
M 17 agar with glycerophosphate
M 17 agar (Himedia, India) is a selective medium for cultivation and enumeration of lactic streptococci. A quantity of 52.25 g of M17 agar base was dissolved in 1 L of deionized water (pH 7.1 ± 0.2). All the culture media were prepared according to the manufacturers’ instructions and autoclaved at 121°C for 20 min before use.
Starter culture
Symbiotic starter culture LBB BY (LB Bulgaricum, Sofia, Bulgaria), containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus (1×109 cfu/g) was used.
Milk
Fresh, homogenized and pasteurized (at 82-85°C/10-15 min) cow’s milk was used in the experimental procedure. The milk was delivered by the United Milk Company Ltd., Plovdiv, Bulgaria. The milk (10 mL sample) was analysed using an automatic milk analyzer MilkoScope Expert (Etcon Analytical, Nigeria) at room temperature (23°C).
The obtained physicochemical parameters of the milk were as follows:
pH 6.6, fat 4.88%, solids-non-fat (SNF) 8.47%, density 28.98 kg/m3, protein 3.11%, lactose 4.65%, solids 0.69%, added water 0.00%, freezing point -0.547°C, titratable acidity 16.5°T (determined according to the titration method).
Propolis extract
Fresh propolis obtained in 2022 from a beekeeper located in the village of Burya, Gabrovo district, Bulgaria (43°02’N 25°19’E) was used. To obtain 2% propolis extract, a mass of 0.4 g of the sample was finely chopped, placed in a plastic tube and macerated with 20 mL of 70% ethanol (Sigma-Aldrich, Merck, Germany). Next, the sample was vigorously shaken on a vortex V-1 (Biosan, Latvia) for 15–20 s and left at room temperature (23°C) for 72 h in darkness. During the extraction, the sample was periodically vortexed. The obtained ethanolic extract was filtered through filter paper and stored at 4 °C for further analyses (Tumbarski et al., 2021).
To conduct the antimicrobial activity assay, the ethanol was vacuum evaporated and the propolis extract was re-dissolved in the same concentration in methanol (Sigma-Aldrich, Merck, Germany), which does not have antimicrobial activity against the used test microorganisms.
Agave inulin
Inulin powder from organic Mexican agave (Agave tequilana Weber) was purchased from the Zoya online market (Sofia, Bulgaria). Agave inulin was characterized with the average degree of polymerization (DP) 18, Mn 2993 Da, Mw 2916 Da and 91 % purity.
Rose petals
The organic rose petals Rosa damascena × Rosa gallica were picked up in the morning (between 6:00 and 8:00 a.m.) within a day at the beginning of June 2022 from the village of Skobelevo (42°40’N 25°10’E), located in the Rose Valley near Kazanlak, Stara Zagora district, Bulgaria (Todorova et al, 2022). The samples of rose petals were dried and finely grown into a powder. Then, a cold water extraction was performed and the obtained aqueous extract was analysed for total phenols, total flavonoids and antioxidant activity as previously described by Petkova et al. (2020b).
Spirulina platensis
Dry Spirulina platensis was purchased from the Zoya online market (Sofia, Bulgaria). Phycocyanin from the microalgae was extracted by the method of Minchev et al. (2021) by water extraction in an ultrasonic bath (IsoLab, Germany) operating at frequency 40 kHz, temperature 40°C for 1 h. The obtained phycocyanin was with purity of 1.6%.
Methods
Total phenolic content
The total phenolic content (TPC) was determined using a Folin-Ciocalteu reagent. The reaction mixture contained 1 mL of Folin-Ciоcalteu reagent (Sigma-Aldrich, Merck, Germany), 0.8 mL of 7.5% sodium carbonate (Sigma-Aldrich, Merck) and 0.2 mL of the tested propolis/rose petals extract. After incubation at room temperature for 20 min (in darkness), the absorbance was measured by a spectrophotometer Camspec M107 (Spectronic-Camspec Ltd., UK) at 765 nm against a blank (distilled water). The results were presented as mg equivalent of gallic acid (GAE)/g of dry weight sample (Ivanov et al., 2014).
Total flavonoid content
The total flavonoid content (TFC) was evaluated according to the method described by Ivanov et al. (2014). An aliquot of 1 mL of the propolis/rose petals extract was added to 0.1 mL of 10% Al(NO3)3, 0.1 mL of 1 M CH3COOK (Sigma-Aldrich, Merck, Germany), and 3.8 mL of distilled water. After incubation at room temperature for 40 min, the absorbance was measured at 415 nm. Quercetin (QE) was used as a standard and the results are expressed as mg quercetin equivalents (QE)/g of dry weight sample.
Antioxidant activity
DPPH radical scavenging assay
The reaction mixture containing 2.85 mL of DPPH reagent (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich, Merck, Germany) and 0.15 mL of the tested propolis/rose petals/Spirulina extract was kept at 37°C for 15 min. The absorbance was measured at 517 nm against a blank (methanol). The antioxidant activity was expressed as mM Trolox equivalents (TE)/g of dry weight sample (Ivanov et al., 2014).
Ferric-reducing antioxidant power (FRAP) assay
The FRAP reagent was freshly prepared with 300 mM acetate buffer with pH 3.6, 10 mM 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM hydrochloric acid and 20 mM Iron (III) chloride hexahydrate (Sigma-Aldrich, Merck, Germany) in distilled water in a ratio of 10:1:1. The reaction mixture (3 mL of FRAP reagent and 0.1 mL of the propolis/rose petals/Spirulina extract) was incubated at 37°C for 10 min in darkness. The absorbance was measured at 593 nm against a blank (distilled water). The antioxidant activity was expressed as mM TE/g of dry weight sample (Ivanov et al., 2014).
Antimicrobial activity assay
The antimicrobial activity of propolis, rose petals and Spirulina extracts was determined by the agar well diffusion method (Tumbarski et al., 2017). The inocula were prepared by homogenization of a small amount of bacterial/yeast biomass in 5 mL of sterile 0.5% NaCl. The fungal inocula were prepared by the addition of 5 mL of sterile 0.5% NaCl directly into the cultivation tubes. After stirring by vortex V-1 plus (Biosan, Riga, Latvia), the fungal inocula were filtered and replaced in new tubes before use. The number of viable cells and fungal spores was determined using a bacterial counting chamber Thoma (Poly-Optik, GmbH, Germany) and then suspensions containing approximately 1×108 cfu/mL for bacterial/yeast cells and 1×105 cfu/mL for fungal spores were prepared. Subsequently, the inocula were distributed in preliminarily melted and tempered at 45-48°C LBG/MEA media and transferred in quantity of 18 mL in sterile Petri plates (d = 90 mm) (Gosselin, France). After allowing the inoculated agar media to harden at room temperature, six wells (d = 6 mm) per Petri plate were bored and duplicates of 60 μL of the propolis/rose petals/Spirulina extracts were pipetted into the agar wells. The plates were incubated at identical conditions.
The antimicrobial activity was determined by measuring the diameter of the inhibition zones (IZ) around the wells on the 24th and 48th hour of incubation. Test microorganisms with IZ of 18 mm or more were considered sensitive; moderately sensitive were those in which the IZ were from 12 to 18 mm; resistant were those in which the IZ were up to 12 mm or completely missing.
Minimum inhibitory concentration (MIC)
MIC was determined by the dilution method (Tumbarski et al., 2017). The tested propolis extract was serially diluted two-fold in methanol ranging from 100 to 0.05 mg/mL. The microorganisms from the starter culture L. delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus were preliminarily pour-plated into MRS and M 17 agar media in Petri plates, in which after the agar hardening six wells per plate were bored. Samples of each dilution were pipetted in quantity of 60 μL into the agar wells. The plates were incubated at 37°C for 24-48 h. The MIC values were determined as the lowest concentrations of the propolis extract inhibiting completely the growth of each test microorganism around the agar well.
Preparation of calcium alginate microcapsules
The calcium alginate microcapsules were prepared by the method of Ivanova et al. (2018) with slight modification. For this purpose, 1% solution of sodium alginate – sodium salt of alginic acid with M-block 61%, G-block 39%, and M/G ratio 1.55 (Sigma-Aldrich, Germany) was prepared and heated at 60°C for 3 min. Next, 10% of agave inulin was added and the mixture was stirred by a laboratory homogenizer IKA T18 (IKA – Werke GmbH & Co. KG, Germany) for 2–3 min. The obtained mixture was cooled at room temperature and then 0.03% of ethanolic propolis extract was added. The mixture was divided into three equal portions. To the second portion 1% of rose petals extract was added, while to the third one – 1% of Spirulina extract. The obtained suspensions were left for 1 h in order to remove the air bubbles. The calcium alginate microcapsules (d = 2–2.5 mm) were prepared as the suspensions were introduced dropwise from single-use syringes with size 20 G needles into 100 mL of a cold aqueous calcium chloride solution (2%) and stirred at 400 rpm. Thus, three types of calcium alginate microcapsules were obtained: 10% agave inulin + 0.03 % ethanolic propolis extract; 10% agave inulin + 0.03% ethanolic propolis extract + 1% rose petals extract; 10% agave inulin + 0.03% ethanolic propolis extract + 1% Spirulina extract (Figure 1 A, B, C). The microcapsules were harvested by filtration, washed twice with distilled water and used for incorporation in yogurt.

Figure 1. Overall appearance of the calcium alginate microcapsules (A – 10% agave inulin + 0.03% ethanolic propolis extract; B – 10% agave inulin + 0.03% ethanolic propolis extract + 1% rose petals extract; C – 10% agave inulin + 0.03% ethanolic propolis extract + 1% Spirulina extract).
Acidity resistance of calcium alginate microcapsules
In order to test the acidity resistance in simulated gastric conditions, 1 g of calcium alginate microcapsules was placed into 20 mL of artificial gastric juice with pH 1.5, containing 2.5 g pepsin and 4 mL concentrated HCl (Sigma-Aldrich, Merck, Germany) dissolved in 0.5 L of deionized water, incubated at 37°C for 6 h and periodically stirred.
Experimental procedure
The yogurt was prepared in laboratory conditions according to the Bulgarian State Standard BSS 12:2010. The technological scheme is presented in Figure 2.

Figure 2. Technological scheme for the preparation of functional yogurt with microencapsulated biologically active compounds.
The homogenized and pasteurized milk was heated at 45–46°C and transferred in quantity of 200 mL into sterile ceramic jars (220 mL volume). Next, 10 g of calcium alginate microcapsules and 1.5% of fresh symbiotic starter culture (1.0×109 cfu/mL) were added to the jars and mixed. The yogurts were separated into four experimental groups (Table 1). The yogurts were incubated at 42-44°C for 2.5–3.5 h. After coagulation, all samples were cooled at room temperature and stored at 4°C (Figure 3). The physicochemical and microbiological changes in yogurts were monitored once a week, for a period of 3 weeks (21 days).
Table 1. Description of the experimental groups.
|
# |
Yogurt sample |
Bioactive components of the microcapsules |
|
1 |
KM1 |
10% agave inulin + 0.03% ethanolic propolis extract |
|
2 |
KM2 |
10% agave inulin + 0.03% ethanolic propolis extract + 1% rose petals extract |
|
3 |
KM3 |
10% agave inulin + 0.03% ethanolic propolis extract + 1% Spirulina extract |
|
4 |
K (control) |
Without microcapsules |

Figure 3. Overall appearance of the yogurts (up); cutting surface and structure of the coagulum (down). From left to right: 1 - KM1, 2 - KM2, 3 - KM3 and K - control.
Determination of pH
The pH values were determined using a pH-meter WTW pH 7110 (InoLab, Germany) equipped with a glass electrode (Tumbarski et al., 2021).
Determination of titratable acidity
The determination of titratable acidity was implemented according to the Bulgarian State Standard BSS 1111:1980. The titratable acidity was determined by titration of each sample with 0.1 N NaOH using phenolphthalein as an indicator until the appearance of a pale pink colour persisting over 1 min. The results were expressed as Torner degrees (°T).
Enumeration of characteristic microorganisms in yogurt
The enumeration of lactobacilli and lactic streptococci in yogurt was implemented according to the Bulgarian State Standard BSS ISO 7889:2005 using the spread-plating method on MRS and M 17 agar media, respectively, and incubation at 37 °C for 48 h. The results were expressed as colony-forming units/g (cfu/g).
Enumeration of yeasts and/or fungi in yogurt
The enumeration of yeasts and/or fungi in yogurt was implemented according to the Bulgarian State Standard BSS ISO 6611:2006 using the pour-plating method on a CGA medium and incubation at 25°C for 48 h. The results were expressed as cfu/g.
Organoleptic analysis
To conduct the organoleptic analysis, 20 unbiased testers over 18 years old were invited. The analysis was performed by testing a small amount of the four yogurt samples placed in plastic cups. The yogurt samples KM1, KM2, KM3 and the control were evaluated on the following organoleptic parameters: surface, colour, coagulum appearance, cutting surface, consistency, flavour and aroma. For this purpose, the 9-points Hedonic scale was used: 9 = liked extremely, 8 = liked very much, 7 = liked moderately, 6 = liked slightly, 5 = neither liked nor disliked, 4 = disliked slightly, 3 = disliked moderately, 2 = disliked very much, 1 = disliked extremely. The results from the analysis were recorded on individual questionnaire cards and then an organoleptic profile of each type of yogurt was made using MS Excel 2010 software (Tumbarski et al., 2021).
Statistical analysis
Results from triplicates were presented as mean values ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed using Statgraphics Centurion statistical program version XVI, 2009 (Stat Point Technologies, Inc., Warrenton, VA, USA). Mean differences were established by Fisher’s least significant difference test for paired comparison with a significance level of P ≤ 0.05.
RESULTS
Total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity of propolis, rose petals and Spirulina extracts
As seen from the results presented in Table 2, the ethanolic propolis extract, organic rose petals and Spirulina extracts demonstrated great variations in TPC, TFC and antioxidant activity (determined by two different methods – DPPH and FRAP). The propolis extract showed the highest TPC and TFC values that corresponded to the highest antioxidant activity.
The TPC, TFC and antioxidant activity of the organic rose petals extract are given in Table 2. The Spirulina phycocyanin extract showed limited antioxidant activity determined by the two methods (Table 2).
Table 2. Total phenolic content, total flavonoid content and antioxidant activity of ethanolic propolis, rose petals and Spirulina extracts
|
Extract |
Total phenols (mg GAE/g) |
Total flavonoids |
Antioxidant activity |
|
|
DPPH |
FRAP |
|||
|
Propolis (20 mg/mL) |
233.00 ± 0.11* |
71.30 ± 0.10* |
1407.10 ± 2.37* |
950.60 ± 1.02* |
|
Rose petals |
54.65 ± 0.56 |
5.43 ± 0.25 |
316.46 ± 11.81 |
436.97 ± 17.67 |
|
Spirulina (0.6 mg/mL) |
n.d. |
n.d. |
1.33 ± 0.42 |
3.57 ± 0.21 |
Note: *- according to our previous study (Tumbarski et al., 2023); n.d. – not determined
Antimicrobial activity of propolis, rose petals, and Spirulina extracts
As seen from the results presented in Table 3, the propolis extract (20 mg/mL) demonstrated higher antimicrobial activity against Gram-positive bacteria than Gram-negative ones. The inhibitory effect was most pronounced on M. luteus 2YC-YT, B. subtilis ATCC 6633, L. monocytogenes NBIMCC 8632 and L. innocua ATCC 33090. The tested propolis extract also exhibited significant inhibitory effect on S. aureus ATCC 25923, B. amyloliquefaciens 4BCL-YT as well as on the starter culture microorganisms (L.delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus). The antimicrobial activity against E. faecalis ATCC 19433 and E. faecuim ATCC 19434 was moderate. The propolis extract exhibited high antimicrobial activity against P. aeruginosa ATCC 9027 and E. coli ATCC 25922, moderate antimicrobial activity against S. enterica serovar Enteritidis ATCC 13076, and low activity against Klebsiella sp. The propolis extract did not inhibit P. vulgaris ATCC 6380, but demonstrated high antifungal activity against the strain A. flavus. The inhibitory effect on the yeasts S. cerevisiae ATCC 9763 and C. albicans NBIMCC 74 as well as on the other fungal species (A. niger ATCC 1015, Penicillium sp., Rhizopus sp., and F. moniliforme ATCC 38932) was moderate. Methanol used as a solvent after the evaporation of ethanol from the extract did not show antimicrobial effect on the test microorganisms.
The organic rose petals extract (0.03 mg/mL) demonstrated limited antimicrobial activity – moderate inhibitory effect on L. monocytogenes NBIMCC 8632 and P. vulgaris ATCC 6380, and weak inhibitory effect on B. subtilis ATCC 6633 and S. aureus ATCC 25923 was observed. The Spirulina phycocyanin extract in concentration of 0.6 mg/mL showed antimicrobial activity only against the fungus Rhizopus sp. (Table 3).
Table 3. Antimicrobial activity of ethanolic propolis, rose petals and Spirulina extracts.
|
Test microorganism |
Inhibition zones, mm* |
||
|
Propolisextract (20 mg/mL) |
Rose petalsextract (0.03 mg/mL) |
Spirulina extract (0.6 mg/mL) |
|
|
B. subtilis ATCC 6633 |
21.0 ± 0.0 |
8.0 ± 0.0 |
- |
|
B. amyloliquefaciens 4BCL-YT |
18.0 ± 0.0 |
- |
- |
|
S. aureus ATCC 25923 |
19.0 ± 0.0 |
8.0 ± 0.0 |
- |
|
L. monocytogenes NBIMCC 8632 |
21.0 ± 0.7 |
14.0 ± 0.7 |
- |
|
L. innocua ATCC 33090 |
21.0 ± 0.0 |
- |
- |
|
E. faecalis ATCC 19433 |
15.0 ± 0.7 |
- |
- |
|
E. faecium ATCC 19434 |
15.0 ± 0.0 |
- |
- |
|
M. luteus 2YC-YT |
22.0 ± 0.7 |
- |
- |
|
L. delbrueckii subsp. bulgaricus ** |
20.0 ± 0.7 |
- |
- |
|
S. salivarius subsp. thermophilus ** |
22.0 ± 1.4 |
- |
- |
|
S. enterica serovar Enteritidis ATCC 13076 |
15.0 ± 0.0 |
- |
- |
|
Klebsiella sp. (clinical isolate) |
9.0 ± 0.0 |
- |
- |
|
E. coli ATCC 25922 |
20.0 ± 0.0 |
- |
- |
|
P. vulgaris ATCC 6380 |
- |
13.0 ± 0.7 |
- |
|
P. aeruginosa ATCC 9027 |
22.0 ± 2.1 |
- |
- |
|
C. albicans NBIMCC 74 |
15.0 ± 0.7 |
- |
- |
|
S. cerevisiae ATCC 9763 |
14.0 ± 0.0 |
- |
- |
|
A. niger ATCC 1015 |
13.0 ± 0.0 |
- |
- |
|
A. flavus (plant isolate) |
18.0 ± 2.1 |
- |
- |
|
Penicillium sp. (plant isolate) |
14.0 ± 1.4 |
- |
- |
|
Rhizopus sp. (plant isolate) |
14.0 ± 1.4 |
- |
15.0 ± 1.4 |
|
F. moniliforme ATCC 38932 |
14.0 ± 1.4 |
- |
- |
* - dwell = 6 mm; ** - starter culture microorganisms; “-” - no inhibition
Minimum inhibitory concentration (MIC)
As seen in Table 3, the propolis extract (20 mg/mL) inhibited the growth of L. delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus from the starter culture. In order to overcome this limitation, the MIC value was determined. The MIC value of propolis extract for L. delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus was 0.39 mg/mL, and this concentration was taken into account in the dosage of propolis extract in the calcium alginate microcapsules. To avoid the inhibitory effect on the starter culture microorganisms and coagulum formation, respectively, the ethanolic propolis extract was applied in the composition of microcapsules under its MIC value (0.3 mg/mL of alginate mixture or 0.03%).
Acidity resistance of calcium alginate microcapsules
The results from the acidity resistance test demonstrated that the three types of alginate microcapsules retained their integrity in artificial gastric juice more than 6 hours. This ensures their stability under the adverse gastric conditions and unimpeded passage to the intestines, where they dissolve in the alkaline environment.
Physicochemical changes in yogurts
During the 3-week period of storage under refrigeration conditions (4°C), the pH values as well as the titratable acidity of the four yogurt samples – KM1, KM2, KM3 and the control – were monitored. As seen from the results summarized in Table 4, the pH values in all yogurt samples gradually decreased with prolongation of the storage period, which was in correspondence with the increasing titratable acidity. The yogurt sample KM3 (with 10% agave inulin + 0.03% ethanolic propolis extract + 1% Spirulina extract microcapsules) maintained higher pH and lower titratable acidity values by the end of monitoring period, compared to the other three samples. By the end of the monitoring period (21st day), the titratable acidity of the yogurts reached values of 108.78°T, 101.30, 94.17°T and 103.73°T for KM1, KM2, KM3 and the control, respectively, which were in compliance with the allowable limit for Bulgarian yogurt (up to 150°T).
Table 4. Physicochemical changes in yogurt samples during the storage at 4°C for 21 days.
|
Day |
Functional yogurt samples |
||||||||
|
KM1 |
KM2 |
KM3 |
Control |
||||||
|
pH |
TA, °T |
pH |
TA, °T |
pH |
TA, °T |
pH |
TA, °T |
||
|
1 |
4.22 ± 0.01a |
94.75 ± 3.61a |
4.23 ± 0.00a |
94.35 ± 2.04a |
4.25 ± 0.01a |
87.31 ± 1.78b |
4.17 ± 0.03b |
95.80 ± 1.79a |
|
|
7 |
4.05 ± 0.01bc |
102.38 ± 4.46a |
4.09 ± 0.02b |
97.42 ± 0.51ab |
4.22 ± 0.01a |
90.21 ± 0.52b |
4.06 ± 0.01c |
101.12 ± 3.95a |
|
|
14 |
4.00 ± 0.01b |
104.90 ± 0.38a |
4.02 ± 0.02b |
100.12 ± 0.51b |
4.15 ± 0.01a |
92.55 ± 1.28c |
4.00 ± 0.01b |
103.37 ± 0.51a |
|
|
21 |
4.02 ± 0.00a |
108.78 ± 4.34a |
3.97 ± 0.00b |
101.30 ± 2.93ab |
4.02 ± 0.00a |
94.17 ± 4.34b |
3.94 ± 0.03b |
103.73 ± 3.05ab |
|
Note: TA – titratable acidity a-c: Means in a row (for pH/TA values) without a common letter differ significantly (P < 0.05)
Microbiological changes in yogurts
During the 3-week period of storage under refrigeration conditions (4°C), two microbiological parameters – characteristic microorganisms (lactic acid bacteria L. delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus) counts, and yeasts and fungal counts in the four yogurt samples (KM1, KM2, KM3 and control) – were monitored. The results given in Table 5 show that on the 1st, 7th and 14th day of the observation period, L. delbrueckii subsp. bulgaricus and S. salivarius subsp. thermophilus in all experimental groups retained high number of viable cells, indicating that the addition of three types of calcium alginate microcapsules did not affect the number of characteristic microorganisms from the starter culture and their normal ratio in the product. These values remained relatively constant until the end of the second week.
During the third week of the storage period (21st day), a decrease of L. delbrueckii subsp. bulgaricus counts with 2 log units in all yogurt samples was detected, while S. salivarius subsp. thermophilus maintained high number of viable cells. These results can be explained by the faster death of L. delbrueckii subsp. bulgaricus cells in the fermented milk products at the end of their storage. Yeasts and fungi as unwanted microflora in all samples until the end of the storage period were not detected.
Table 5. Microbial counts in yogurt samples during the storage at 4°C for 21 days.
|
Day |
Microorganism |
Functional yogurt samples |
|||
|
KM1 |
KM2 |
KM3 |
Control |
||
|
1 |
Lb, cfu/g* |
3.6 × 108 |
1.6 × 108 |
4.5 × 108 |
1.9 × 108 |
|
St, cfu/g** |
6.3 × 109 |
3.1 × 109 |
3.6 × 109 |
2.8 × 109 |
|
|
Y/F, cfu/g*** |
˂ 10 |
˂ 10 |
˂ 10 |
˂ 10 |
|
|
7 |
Lb, cfu/g |
1.5 × 108 |
2.5 × 108 |
2.2 × 108 |
5.5 × 108 |
|
St, cfu/g |
1.3 × 109 |
1.6 × 109 |
1.8 × 109 |
6.1 × 109 |
|
|
Y/F, cfu/g |
˂ 10 |
˂ 10 |
˂ 10 |
˂ 10 |
|
|
14 |
Lb, cfu/g |
1.0 × 108 |
2.8 × 108 |
1.0 × 108 |
8.0 × 108 |
|
St, cfu/g |
4.4 × 109 |
1.0 × 109 |
2.2 × 109 |
3.6 × 109 |
|
|
Y/F, cfu/g |
˂ 10 |
˂ 10 |
˂ 10 |
˂ 10 |
|
|
21 |
Lb, cfu/g |
3.0 × 107 |
1.5 × 107 |
6.5 × 107 |
5.2 × 107 |
|
St, cfu/g |
1.1 × 109 |
1.0 × 109 |
1.2 × 109 |
1.1 × 109 |
|
|
Y/F, cfu/g |
˂ 10 |
˂ 10 |
˂ 10 |
˂ 10 |
|
Note: *-Lb – L. delbrueckii subsp. bulgaricus; **-St - S. salivarius subsp. thermophilus; ***-Y/F – yeasts and fungi
Organoleptic analysis
The results from organoleptic analysis of yogurts demonstrated that the sample KM1 received the highest average score from the tasters in comparison with KM2, KM3 and the untreated sample (the control). Compared to the control, the application of 10% agave inulin + 0.03% propolis extract microcapsules (KM1), 10% agave inulin + 0.03% propolis extract + 1% rose petals extract microcapsules (KM2) and 10% agave inulin + 0.03% propolis extract + 1% Spirulina extract microcapsules (KM3) led to better acceptance of five from six organoleptic parameters – surface, colour, coagulum appearance, consistency, and flavour and aroma. The control sample kept the highest score or acceptance rate only for the cutting surface parameter. Based on the results from organoleptic analysis, the four experimental groups can be arranged as follows: KM1 (average score 8.74) > KM2 (average score 8.59) > KM3 (average score 8.37) > the control (average score 8.36) from maximum 9 points (Figure 4).
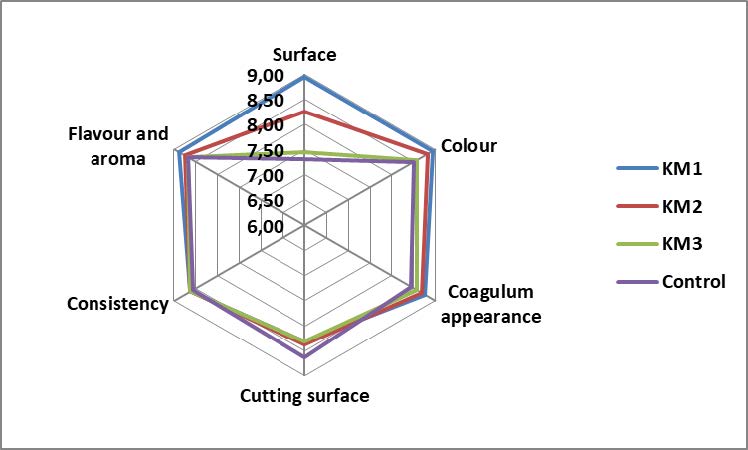
Figure 4. Organoleptic characteristics of the functional yogurts.
DISCUSSION
The TPC and TFC values of propolis extract were similar to the previously reported data for propolis of Bulgarian origin – 220 mg GAE/g and 157 mg QE/g, respectively (Kumazawa et al., 2004) as well as for five Bulgarian commercial propolis extracts – TPC from 193.74 mg GAE/g to 250.02 mg GAE/g, and TFC between 130.60 QE/g and 176.56 QE/g, respectively (Topuzova et al., 2021). The antioxidant potential of the studied propolis extract was similar to those of the commercial propolis extracts obtained by DPPH and FRAP methods by the same authors. In contrast, Socha et al. (2015) analysed the phenolic and flavonoid contents, as well as the antioxidant activity of nine propolis samples from Poland and found that the TPC of propolis samples ranged from 150.05 to 197.14 mg/g GAE, while TFC varied from 35.64 to 62.04 mg QE/g, whose values were lower compared to those obtained for our propolis extract. The propolis samples from Poland also exhibited lower antiradical activity measured by the DPPH method (1.92–2.69 mM TE/g) and lower reducing power determined by the FRAP method (6.23–9.19 mM Fe(II)/g) in comparison with propolis sample used in the study.
Similar TPC and TFC values were obtained for an aqueous leaf extract from Rosa gallica (Abdelbaky et al., 2021). Results close to our measurements were reported by Petkova et al. (2020b) who examined aqueous extracts from R. damascena petals grown by conventional and organic agriculture systems in the Rose Valley, Kazanlak, Bulgaria. However, other authors (Petkova et al., 2020a) reported higher phenolic and flavonoid contents of extracts of R. damascena petals obtained by infusion (TPC 70.29 mg GAE/g and TFC 9.36 mg QE/g) and by decoction (TPC 135.82 mg GAE/g and TFC 15.87 mg QE/g). The same samples demonstrated higher antioxidant activity than our extract, whose values were 472.55 mM TE/g (DPPH) and 904.40 mM TE/g (FRAP) for the extract obtained by infusion, and 1,031.70 mM TE/g (DPPH) and 1,804.88 mM TE/g (FRAP) for the extract obtained by decoction. Consequently, the temperature regime and extraction method affect the levels of TPC, TFC and antioxidant activity.
For comparison, Wu et al. (2016) examined the antioxidant activity of the food-grade phycocyanin isolated from S. platensis by the DPPH and ABTS assays, and found that its radical-scavenging activity determined by both methods increased in a concentration-dependent manner. The Trolox equivalent antioxidant capacity (TEAC) of phycocyanin was 0.75 mmol TE/g. The IC50 for the DPPH method was 1.86 mg/mL, while the same parameter for the ABTS method had a value of 0.56 mg/mL.
The results from the antimicrobial activity test of the propolis extract were in agreement with some previous studies. Popova et al. (2011) determined high antimicrobial activity against S. aureus and moderate activity against C. albicans of 17 propolis samples originating from Malta and one sample from Bulgaria. Comparable to our results were also those obtained by Velikova et al. (2000), who examined the antimicrobial properties of propolis from Bulgaria, Greece, Turkey and Algeria. The authors determined high antimicrobial activity against S. aureus and moderate activity against C. albicans and E. coli. However, Afrouzan et al. (2018) noted low antimicrobial activity of propolis originating from Iran against E. coli ATTC 25922, S. aureus ATCC 6538 and C. albicans ATCC 10231.
The antimicrobial activity of R. damascena was described in some previous studies. Abu-Shanab et al. (2006) examined four plant extracts used in Palestinian folkloric medicine and found that the rose petals water (WE) and ethanolic (EE) extracts in concentration of 100 mg/mL possessed high inhibitory effect on methicillin-resistant S. aureus strains with diameter of inhibition zones of 18 mm (WE) and 34 mm (EE). In another research, Chroho et al. (2022) tested a hydroethanolic extract obtained from R. damascena petals against some foodborne pathogens and stated that MIC values ranged between 20.83 mg/mL for E. coli, S. aureus and L. monocytogenes, and 41.66 mg/mL for Salmonella enterica serovar Typhimirium.
Despite the growing interest in biologically active substances in the production of innovative dairy products, the information about their application in encapsulated form in functional yogurt is still very limited. Most of the existing publications consider the direct addition of propolis, rose petals and Spirulina platensis in yogurts in the form of extracts, improving their stability and organoleptic properties, or as natural preservatives, enhancing their storage life. Santos et al. (2020) evaluated the application of red Brazilian propolis as a natural additive in yogurt. The results showed that the addition of propolis extract in concentrations of 0.05% and 0.046% improved the rheological and sensorial characteristics of yogurt, increased the consumers’ acceptance and enhanced the shelf life of the product during storage at 4°C for 28 days. Qiu et al. (2021) developed yogurt fortified with edible rose petals extract (Rosa rugosa cv. Plena) and found that the addition of rose water extract in concentrations of 0.1 %, 0.3 % and 0.5 % increased the antioxidant activity of yogurt, exerted positive effect on its physicochemical, rheological, functional and sensory properties, and enhanced its storage life. Barkallah et al. (2017) determined the effects of four concentrations (0.25%, 0.5%, 0.75% and 1%) of S. platensis on the physicochemical and organoleptic properties of yogurt during refrigerated storage for 28 days. The authors reported that S. platensis fortification improved the physicochemical, textural and sensory features of yogurt during the fermentation process and storage, and at the same time improved the nutraceutical value of the product by increasing its antioxidant potential. To overcome the unpleasant flavor of S. platensis by its direct application in the food matrix, Nourmohammadi et al. (2020) used the microencapsulation method with alginate and whey protein to incorporate Spirulina extract in functional yogurt. The results demonstrated that during the storage period for 15 days, Spirulina application affected positively some physicochemical properties (increased pH and viscosity, lowered syneresis) and sensorial characteristics of yogurt in comparison with the untreated sample. Sharifi et al. (2023) incorporated carrot pomace extract, Lactobacillus plantarum, and microcapsules based on Alyssum homolocarpum seed gum and sodium alginate in order to create a functional synbiotic yogurt. The evaluation of functional yogurts indicated that the pH values in all experimental groups gradually decreased, reaching values from 4.07 to 4.38 at the end of the storage period. On the 28th day of the storage, the TPC values varied between 16.13 and 48.30 µg GAE/mL, while DPPH radical scavenging activity ranged between 7.4 and 14.64%. The authors also stated that the bead production by A. homolocarpum seed gum and sodium alginate as well as the use of carrot pomace extract significantly enhanced the survival of probiotic bacteria in yogurt compared to the free cells on the 28th day of the storage period. Santos et al. (2022) designed functional yogurts with the addition of blackberry pomace microcapsules obtained by three different methods (spray-drying, freeze-drying and ionic gelation). Yogurts with addition of spray-dried microcapsules presented higher cyanidin-3-glucoside bioavailability levels than those with addition of microcapsules obtained by freeze drying and ionic gelation methods. Moreover, all functional yogurts showed greater post-gastrointestinal digestion bioavailability than the pristine microcapsules. The encapsulation method of biologically active substances was evaluated as an effective approach for preservation of natural antioxidants in dairy desserts. The authors stated that the microencapsulation of blackberry, chokeberry and Cornelian cherry juices significantly retained higher amounts of polyphenols and antioxidants in the dairy desserts during the storage period at 4°C for 20 days (Ivanova et al., 2018).
CONCLUSION
To the best of our knowledge, this is the first study to investigate the application of microencapsulated agave inulin, propolis, organic rose petals and Spirulina platensis extracts in traditional Bulgarian yogurt in order to develop a functional dairy product. The application of calcium alginate microcapsules with agave inulin, ethanolic propolis, organic rose petals and Spirulina platensis extracts did not affect the physicochemical properties and microbiological characteristics of the functional yogurts, which showed that the new products were stable until the end of the storage period for 21 days. The results from the organoleptic evaluation demonstrated that the newly obtained functional yogurts with microencapsulated biologically active compounds received high acceptance scores from the consumers. The application of bioactive substances in encapsulated form can be considered a promising approach in the manufacture of innovative dairy products with extra beneficial properties, such as functional yogurt.
ACKNOWLEDGEMENTS
The authors are thankful to the support of the Bulgarian Ministry of Education and Science under the National Research Program “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17.08.2018, and to the MU-Varna with the Project code: # 21001 “Development of a green method for the production of phycocyanin from Spirulina with potential applicability in pharmacy and food technology”.
AUTHOR CONTRIBUTIONS
Conceptualization - Y. T. and N. P.; Samples provision - Y. T. (propolis), N. P., N. G. and K. N. (agave inulin, organic rose petals and Spirulina platensis); Methodology - Y. T., N. P., I. I. and M. T.; Formal analysis - Y. T., N. P., I. I., M. T. and L. N.; Investigation - Y. T., N. P., I. I., M. T. and L. N.,; Software, data curation and statistical analysis - P. I.; Writing (manuscript preparation) - Y. T. and M. T.; Projects administration - N. G. and K. N.
All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdelbaky, A.S., Mohamed, A.M.H.A., and Alharthi S.S. 2021. Antioxidant and antimicrobial evaluation and chemical investigation of Rosa gallica var. aegyptiaca leaf extracts. Molecules. 26: 6498.
Abu-Shanab, B., Adwan, G.M., Jarrar, N., Abu-Hijleh A., and Adwan K. 2006. Antibacterial activity of four plant extracts used in Palestine in folkloric medicine against methicillin-resistant Staphylococcus aureus. Turkish Journal of Biology. 30(4): 195-198.
Afrouzan, H., Tahghigh, A., Zakeri, S., and Es-haghi, A. 2018. Chemical composition and antimicrobial activities of Iranian propolis. Iranian Biomedical Journal. 22(1): 50-65.
AlFadhly, N.K.Z., Alhelfi, N., Altemimi, A.B., Verma, D.K., Cacciola, F., and Narayanankutty, A. 2022. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A review. Molecules. 27(17): 5584.
Ali, M.A., Kamal, M.M., Rahman, M.H., Siddiqui, M.N., Haque, M.A., Saha, K.K., and Rahman, M.A. 2021. Functional dairy products as a source of bioactive peptides and probiotics: Current trends and future prospectives. Journal of Food Science and Technology. 59: 1263–1279.
Barkallah, M., Dammak, M., Louati, I., Hentati, F., Hadrich, B., Mechichi T., Ayadi, M.A., Fendri, I., Attia, H., Abdelkafi, S. 2017. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage, LWT - Food Science and Technology. 84: 323–330.
Bouaziz, M.A., Rassaoui, R., Besbes, S. 2014. Chemical composition, functional properties, and effect of inulin from Tunisian agave americana L. leaves on textural qualities of pectin gel. Journal of Chemistry. 758697: 1–11.
Bulgarian State Standard BSS 12:2010. “Bulgarian Yogurt”. The Bulgarian Institute of Standardization, Sofia, Bulgaria (in Bulgarian).
Bulgarian State Standard BSS 1111:1980. “Milk and milk products - Determination of acidity”. The Bulgarian Institute of Standardization, Sofia, Bulgaria (in Bulgarian).
Bulgarian State Standard BSS ISO 7889:2005. “Yogurt - Enumeration of characteristic microorganisms - Colony-count technique at 37 degrees C”. The Bulgarian Institute of Standardization, Sofia, Bulgaria (in Bulgarian).
Bulgarian State Standard BSS ISO 6611:2006. “Milk and milk products – Enumeration of colony-forming units of yeasts and/or moulds - Colony-count technique at 25 degrees C”. The Bulgarian Institute of Standardization, Sofia, Bulgaria (in Bulgarian).
Cauich-Kumul, R., and Campos, M.R.S. 2019. Bee propolis: Properties, chemical composition, applications, and potential health effects. p. 227-243. In M.R.S. Campos (ed) Bioactive compounds: Health benefits and potential applications. Elsevier.
Chroho, M., Bouymajane, A., Oulad, E.M.Y., Cacciola, F., Mondello, L., Aazza M., Zair, T., and Bouissane, L. 2022. Phenolic composition, antioxidant and antibacterial activities of extract from flowers of Rosa damascena from Morocco, Separations, 9(9): 247.
Costa M.F, Pimentel T.C., Guimaraes J.T., Balthazar C.F., Rocha R.S., Cavalcanti R.N., Esmerino, E.A., Freitas, M.Q., Raices, R. S. L., Silva, M.C., and Cruz, A.G. 2019. Impact of prebiotics on the rheological characteristics and volatile compounds of Greek yogurt, LWT – Food Science and Technology. 105: 371-376.
Gateva, S., Jovtchev, G., Angelova, T., Dobreva, A., and Mileva, M. 2022. The anti-genotoxic activity of wastewaters produced after water-steam distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. essential oils. Life. 12(3): 455.
Gruskiene, R., Bockuviene, A., and Sereikaite J. 2021. Microencapsulation of bioactive ingredients for their delivery into fermented milk products: A review. Molecules. 26: 4601.
Irigoiti, Y., Navarro, A., Yamul, D., Libonatti, C., Tabera, A., and Basualdo, M. 2021. The use of propolis as a functional food ingredient: A review. Trends in Food Science & Technology. 115: 297–306.
Ivanov, I., Vrancheva, R., Marchev, A., Petkova, N., Aneva, I., Denev P., Georgiev, V., and Pavlov, A. 2014. Antioxidant activities and phenolic compounds in Bulgarian fumaria species. International Journal of Current Microbiology and Applied Sciences. 3(2): 296-306.
Ivanova, M., Petkova, N., Balabanova, T., Ognyanov, M., and Vlaseva, R. 2018. Food design of dairy desserts with encapsulated cornelian cherry, chokeberry and blackberry juices. Analele Universitatii Dunarea de Jos din Galati. 42: 137-146.
Jacques, P.F., Wang, H. 2014. Yogurt and weight management. The American Journal of Clinical Nutrition. 99(5): 1229S–1234S.
Khimmakthong, U., Khumpouk, P., Saichanaphan, N., Intarasin, Y., and Tirawanichakul K. 2020. The efficiency of microencapsulation with alginate, gelatin, and chitosan on the survival of Bacillus subtilis. Chiang Mai University Journal of Natural Sciences. 19(4): 684.
Kumazawa, S., Hamasaka, T., and Nakayama, T. 2004. Antioxidant activity of propolis of various geographic origins. Food Chemistry. 84(3): 329–339.
McKinley, M.C. 2005. The nutrition and health benefits of yoghurt. International Journal of Dairy Technology. 58(1): 1-12.
Melo, D.R., da Silva, P.H.T., Rigoto, R.P., Sottoriva, H.M., Cintra, F.F., Trento, J.P., de Castro, A.L., and Alves, G. 2018. Quark cheese produced with kefir and Agave inulin. Arquivos de Ciências Veterinárias e Zoologia. 21(3): 87-92.
Minchev I., Petkova N., and Milkova-Tomova I. 2021. Ultrasound-assisted extraction of chlorophylls and phycocyanin from Spirulina platensis. Biointerface Research in Applied Chemistry. 11(2): 9296-9304.
Morsy, A.S., Soltan, Y.A., El-Zaiat, H.M., Alencar, S.M., and Abdalla, A.L. 2021. Bee propolis extract as a phytogenic feed additive to enhance diet digestibility, rumen microbial biosynthesis, mitigating methane formation and health status of late pregnant ewes. Animal Feed Science and Technology. 273: 114834.
Nayebi, N., Khalili, N., Kamalinejad, M., and Emtiazy, M. 2017. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complementary Therapies in Medicine. 34: 129–140.
Nourmohammadi, N., Soleimanian-Zad, S., and Shekarchizadeh H. 2020. Effect of Spirulina (Arthrospira platensis) microencapsulated in alginate and whey protein concentrate addition on physicochemical and organoleptic properties of functional stirred yogurt. Journal of the Science of Food and Agriculture, 10576.
Özer, B.H, and Kirmaci, H.A. 2010. Functional milks and dairy beverages. International Journal of Dairy Technology. 63(1): 1–15.
Pasupuleti, V.R, Sammugam, L., Ramesh, N., and Gan S.H. 2017. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxidative Medicine and Cellular Longevity. 2017:1259510.
Petkova, D., Mihaylova, D., Denev, P., and Krastanov, A. 2020a. Antioxidant activity of some edible flowers water extracts from Bulgaria. Bulletin of University of Agricultural Sciences and Veterinary Medicine. 77(1): 54-61.
Petkova, N., Todorova, M., Grozeva N., and Gerdzhikova, M. 2020b. Phenolic content and antioxidant activity of water extracts from Rosa damascena petals grown in Kazanlak valley, Bulgaria. Scientific Papers, Series B, Horticulture. LXIV (2): 345–352.
Popova, M., Trusheva, B., Antonova, D., Cutajar, S., Mifsud, D., Farrugia C., Tsvetkova, I., Najdenski, H., and Bankova, V. 2011. The specific chemical profile of Mediterranean propolis from Malta. Food Chemistry. 126(3): 1431–1435.
Qiu, L., Zhang, M., Mujumdar, A.S., Chang, L. 2021. Effect of edible rose (Rosa rugosa cv. Plena) flower extract addition on the physicochemical, rheological, functional and sensory properties of set-type yogurt. Food Bioscience. 43: 101249.
Santos, M.S., Estevinho, L.M., de Carvalho, C.A.L., da Silva Conceição, A.L., de Castro Almeida, R.C. 2020. Rheological and sensorial evaluation of yogurt incorporated with red propolis. Journal of Food Science and Technology. 57: 1080–1089.
Santos, S.S., Paraíso, C.M., Romanini, E.B., Correa, V.G., Peralta, R.M., da Costa, S.C., de Oliveira Santos Junior, O., Visentainer, J.V., Reis, M.H.M., and Madrona, G.S. 2022. Bioavailability of blackberry pomace microcapsules by using different techniques: An approach for yogurt application. Innovative Food Science & Emerging Technologies. 81: 103111.
Sharifi, Z., Javan, A.J., Hesarinejad, M.A., Parsaeimehr, M. (2023), Application of carrot waste extract and Lactobacillus plantarum in Alyssum homalocarpum seed gum-alginate microcapsules to create a functional synbiotic yogurt. Chemical and Biological Technologies in Agriculture. 10: 3.
Socha, R., Gałkowska, D., Bugaj, M., and Juszczak L. 2015. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Natural Product Research. 29(5): 416–422.
Todorova, M., Dobreva, A., Petkova, N., Grozeva, N., Gerdzhikova, M., and Veleva, P. 2022. Organic vs conventional farming of oil-bearing rose: Effect on essential oil and antioxidant activity. BioRisk. 17: 271-285.
Topuzova, M., Todorova, M., Tumbarski, Y., Petkova, N., and Ivanov, I. 2021. Antioxidant properties and antimicrobial activity of commercial propolis extracts from Bulgaria. Academic Journal Industrial Technologies. 8(1): 117-124.
Tosi, E.A., Ré, E., Ortega, M.E., and Cazzoli, A.F. 2007. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chemistry. 104(3): 1025-1029.
Tropcheva, R., Georgieva, R., Paskov, V., Karsheva, M., and Danova, S. 2014. Sensory properties of Bulgarian yogurts, supplemented with lactobacilli as probiotic adjuncts. Journal of Texture Studies. 45(3): 187-194.
Trotta, T., Porro, C., Cianciulli, A., and Panaro, M.A. 2022. Beneficial effects of Spirulina consumption on brain health. Nutrients. 14: 676.
Tumbarski, Y., Lincheva, V., Petkova, N., Nikolova, R., Vrancheva, R., and Ivanov I. 2017. Antimicrobial activity of extract from aerial parts of potentilla (Potentilla reptans L.). Academic Journal Industrial Technologies. 4(1): 37-43.
Tumbarski, Y.D., Todorova, M.M., Topuzova, M.G., Georgieva, P.I., Ganeva, Z.A., Mihov, R.B., and Yanakieva, V. 2021. Antifungal activity of carboxymethyl cellulose edible films enriched with propolis extracts and their role in improvement of the storage life of kashkaval cheese. Current Research in Nutrition and Food Science Journal. 9(2): 487-499.
Tumbarski, Y. D., Petrova, N., Ivanov, I., Petkova, N., Ivanova, P., Yanakieva, V., Vasilev, K., and Mihalev, K. 2023. Assessment of the bioactivity, preservation potential and sensory acceptance of a propolis extract applied in a functional fruit-herbal beverage. Food Science and Applied Biotechnology. 6(2):320-330.
Velikova, M., Bankova, V., Sorkun, K., Houcine, S., Tsvetkova, I., and Kujumgiev A. 2000. Propolis from the Mediterranean region: Chemical composition and antimicrobial activity. Zeitschrift für Naturforschung C. 55c: 790-793.
Velikova, P., Petrov, K., Lozanov, V., Tsvetanova, F., Stoyanov, A., Wu, Z., Liu, Z., and Petrova, P. 2018. Microbial diversity and health-promoting properties of the traditional Bulgarian yogurt. Biotechnology & Biotechnological Equipment. 32(5): 1205-1217.
Visioli F., and Strata A. 2014. Milk, dairy products, and their functional effects in humans: a narrative review of recent evidence. Advances in Nutrition. 5(2): 131–143.
Wu, H-L., Wang, G-H., Xiang, W-Z., Li, T.,and He, H. 2016. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. International Journal of Food Properties. 19(10): 2349-2362.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Yulian Tumbarski1, *, Nadezhda Petkova2, Ivan Ivanov2, Mina Todorova3, Petya Ivanova4, Lazar Nikolov1, Neli Grozeva5, and Krastena Nikolova6
1 Department of Microbiology, University of Food Technologies, Plovdiv, Bulgaria.
2 Department of Organic Chemistry and Inorganic Chemistry, University of Food Technologies, Plovdiv, Bulgaria.
3 Department of Organic Chemistry, “Paisii Hilendarski” University of Plovdiv, Plovdiv, Bulgaria.
4 Department of Biochemistry and Molecular Biology, University of Food Technologies, Plovdiv, Bulgaria.
5 Department of Biological Sciences, Trakia University, Stara Zagora, Bulgaria.
6 Department of Physics and Biophysics, Medical University – Varna, Varna, Bulgaria.
Corresponding author: Yulian Tumbarski, E-mail: tumbarski@abv.bg
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 26, 2023;
Revised: December 25, 2023;
Accepted: January 2, 2024;
Online First: January 17, 2024

