
Development and Stability Study of Oseltamivir Reconstitutable Dry Suspension from Capsules
Tasana Pitaksuteepong*, Kitiya Kesornmalee and Natthareephon PhanaphaiPublished Date : January 16, 2024
DOI : https://doi.org/10.12982/NLSC.2024.010
Journal Issues : Number 1, January-March 2024
Abstract Oseltamivir phosphate (OSEL) is recommended for the prevention and treatment of influenza virus infection. In a hospital pharmacy, OSEL liquid extemporaneous preparation is generally compounded on a case-by-case basis from the capsules because it can be stored for a certain period. Therefore, the objective of the current research was to develop an oseltamivir reconstitutable dry suspension (ORDS), and to evaluate the stability of the ORDS. The dry-suspending vehicles were first developed and subjected to stability tests. The stable vehicle was selected to mix with the content of OSEL capsules and dry powder was termed ORDS. The stability of ORDS was then tested in both dried and reconstituted states. It was found that the dry vehicle using xanthan gum as a suspending agent (at 0.25% w/v of the reconstituted suspension) was the most stable formulation at 25°C and 45°C under 75% relative humidity (RH) for 28 days. Upon mixing with OSEL capsule contents, ORDS in the dried state showed no change in either physical or chemical properties after storing at room temperature for 7 days. For the reconstituted state, the ORDS showed no change in odor and pH after storing at 2°C to 8°C in a refrigerator, and 25°C and 45°C under 75%RH for 14 days. However, its color was slightly darker after storing at 45°C. The percentage remaining OSEL at the refrigerator temperatures was 98.13% ± 1.63%, at 25°C was 100.30% ± 0.71% and at 45°C was 97.54% ± 1.38%. In conclusion, ORDS may be a convenient alternative to extemporaneous preparations.
Keywords: Oseltamivir capsule, Reconstitutable dry suspension, Extemporaneous, Physico-chemical stability, Influenza
Funding: The authors are grateful for the research funding provided by the Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok, Thailand.
Citation: Pitaksuteepong, T., Kesornmalee, K., and Phanaphai, N. 2024. Development and stability study of oseltamivir reconstitutable dry suspension from capsules. Natural and Life Sciences Communications. 23(1): e2024010.
INTRODUCTION
Influenza is a worldwide disease caused by the influenza virus. In 2022, it was reported that the risk group for influenza in Thailand was mostly found in the age group 0-4 years, followed by the age group 5-14 years and the age group 15-24 years (Division of Epidemiology, 2022).
Oseltamivir (OSEL), a neuraminidase inhibitor, has been approved for the prevention and treatment of influenza in children aged ≥ 14 days. It is recommended to be given within 2 days of illness onset. It is marketed in the form of capsules under the trade names: Tamiflu® (Roche) and GPO-A-Flu™ (Government Pharmaceutical Organization, GPO). Tamiflu® is also marketed as a powder for oral suspension in a strength of 6 mg/ml. However, in Thailand, GPO-A-Flu™ capsules (strength 30, 45 and 75 mg) are the most feasible and accessible product in government healthcare settings. However, they are not suitable for children and patients who cannot swallow capsules. For the treatment of these groups of patients, pharmacists prepare the extemporaneous suspension for each patient in the strength of 10 mg/ml using the formulation developed by the Queen Sirikit National Institute of Child Health (QSNICH), Bangkok, Thailand (Medical Treatment Working Group, 2011). To prepare the extemporaneous suspension, four OSEL capsules (75 mg) are opened and the contents are emptied into a mortar. The contents are then triturated to a fine powder and levigated with a small amount of vehicle to form a smooth paste. The vehicle is gradually added with constant mixing and the suspension is transferred to a calibrated container (30 ml). The mortar is rinsed with a sufficient vehicle and transferred to the container. Finally, the volume is adjusted to the calibration mark using the vehicle, and the suspension is mixed well. The vehicle developed by QSNICH consists of 80% w/v sucrose and 0.1% w/v sodium benzoate in water.
From the literature review, oseltamivir is the most stable in a solid state (Oliyai et al., 1998). It undergoes hydrolysis in aqueous solution under acid and alkaline conditions. The maximal stability is found to be at pH 4.0 (Oliyai et al., 1998). In addition, reducing sugars such as glucose, fructose, and galactose react with the amino group of OSEL which causes degradation via Maillard reactions. Therefore, it is no surprise that the QSNICH vehicle was subsequently changed at a later time. The pH of the new vehicle by QSNICH is controlled using citric acid. The ingredients of the new vehicle are 0.1 g citric acid, 2 ml distilled water, 0.0006 ml sala cider and syrup q.s. to 100 ml (Xayavong et al., 2018). The syrup for this vehicle consists of glycerin 0.1 g, sodium benzoate 0.1 g, sucrose 50 g and distilled water q.s. to 100 ml. The stability of this formulation was studied and the results were published in 2018 (Xayavong et al., 2018). It was found that the percentage remaining of OSEL in the extemporaneous suspension after storing at room temperature for 12 weeks was 98.17%, at refrigerated temperature (7°C ± 3°C) was 98.24% and at accelerated temperature (45°C ± 5°C) the percentage remaining was 62.91% (Xayavong et al., 2018). Due to the time-consuming process of this method, oseltamivir extemporaneous suspension is now often prepared by simply mixing the capsule contents with syrup or flavoured syrup such as Hale’s Blue Boy. However, homogeneity and stability are major concerns. Winiarski et al. (2007) investigated stability of extemporaneous oral liquid formulations which were prepared by mixing the contents of Tamiflu™ 75 mg capsule with Cherry Syrup (Humco) and Ora-Sweet SF (Paddock Laboratories). The suspensions were filled in amber glass and amber polyethyleneterephthalate (PET) bottles. The bottles were stored at 5°C ± 2°C or 25°C ± 2°C at 60% ± 5% relative humidity (RH), or at 30°C ± 2°C at 65% ± 5%. The results showed that OSEL in Cherry Syrup was stable under 5°C for up to 35 days but under 25°C for only 5 days. It was unstable under 30°C. For OSEL in Ora-Sweet SF, it was stable under 5°C or 25°C for up to 35 days and under 30°C for shorter period (i.e. 13 days) (Winiarski et al., 2007). This formulation is therefore should be freshly prepared on the day that the doctor prescribes the medication and is suggested to be used immediately.
Considering the limitations of these various methods, the objective of the current study was to develop oseltamivir reconstitutable dry suspension (ORDS) from capsules. The processes used to prepare ORDS in this study are simple and take into account the equipment normally available in hospitals.
MATERIAL AND METHODS
Materials
The chemicals used were oseltamivir phosphate capsule 75 mg (Reg. No. 1A 3/50 (NG), Lot No. K625238, Exp 20/06/24, the Government pharmaceutical organization, Bangkok, Thailand), citric acid anhydrous (Sigma-Aldrich Co., Ltd., Vienna, Austria), microcrystalline cellulose (Avicel PH 101, Mingtai Chemical Co., Ltd., Taoyuan, Taiwan), silicon dioxide (Jinsha Precipitated Silica Manufacturing Co.,Ltd., Fujian, China), sodium benzoate (Sigma-Aldrich Co., Ltd., Buchs, Switzerland), sodium carboxymethylcellulose (Qingdao SINOCMC Chemical Co.,Ltd., Shandong, China), sucrose (Mitr Phol Co., Ltd, Bangkok, Thailand) and xanthan gum (Henan Tianfu Chemical Co.,Ltd., Henan, China).
Development of dry-suspending vehicles
The compositions of dry-suspending vehicles were selected based on safe use criteria in pediatrics and children. They should be effective at pH of about 4.0. The ingredients of the dry-suspending vehicles developed include suspending agents, sweetening agents, an anticaking agent, a preservative and a pH adjusting agent. These ingredients were weighed and mixed by geometric dilution.
Stability test of dry-suspending vehicles
Tests of the stability of the vehicles in dry powder form were performed to ensure that the vehicles could be prepared in advance and were in a ready-to-mix form with the content of OSEL capsules. The vehicles developed were stored at 25°C and 45°C, with relative humidity (RH) of 75% for 28 days.
Evaluation of dry-suspending vehicles
Before reconstitution
During storage stability testing, the dry-suspending vehicles were sampled and evaluated for physical properties on days 0, 14 and 28. The properties evaluated were color, odor, consolidation and flowability.
Color and odor: Color and odor were inspected by the same observer.
Consolidation: The consolidation of the dry-suspending vehicle was assessed by gradually turning the bottle upside-down and the cluster of powders was observed. If no agglomerations were observed, or the agglomerations that were observed were easily broken when turning the bottle, the results were recorded as a minus (-) sign. If, however, persistent agglomerations were observed even if the bottle was turned upside-down, the results were recorded as plus (+) sign.
Flowability: The flowability of the dry-suspending vehicle was evaluated based on the Hausner ratio and Carr’s compressibility index. A 20-25 g sample was weighed and gently filled in a dry graduated cylinder of 50 ml (readable to 1 ml). The bulk volume was recorded after manually tapping the cylinder three times on a flat surface. The drop height was approximately 2.5 cm. The tapped volume was recorded after every 50 taps till it showed no further reduction in volume. The bulk and tapped densities were calculated from the ratio of the mass of the powders to their volume before (i.e. bulk volume) and after tapping (i.e. tapped volume). The experiments were performed in triplicate.
The Hausner ratio and Carr’s compressibility index were calculated using the following equations:
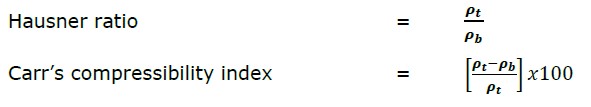
where ρ t is the tapped density and ρ
t is the tapped density and ρ b is the bulk density.
b is the bulk density.
The ratings of flowability for the Hausner ratio and Carr’s compressibility index are shown in Table 1.
Table 1. Rating of flowability for Hausner ratio and Carr’s compressibility index (USP-NF 2012; Carr, 1965).
|
Hausner ratio |
Flow character |
Carr’s index (%) |
|
1.00-1.11 |
Excellent |
≤10 |
|
1.12-1.18 |
Good |
11-15 |
|
1.19-1.25 |
Fair |
16-20 |
|
1.26-1.34 |
Passable |
21-25 |
|
1.35-1.45 |
Poor |
26-31 |
|
1.46-1.59 |
Very poor |
32-38 |
|
>1.60 |
Very, Very poor |
>38 |
After reconstitution
The vehicles in dry powder form sampling on days 0, 14 and 28 were reconstituted with water and evaluated for the physicochemical properties including wettability, color, odor, sedimentation ratio, re-dispersibility and pH.
Wettability: During the reconstitution process, the dry powder is first wetted, submerged, dispersed and (some) solubilized (Fournaise et al., 2020). Wetting is the first step in the reconstitution to ensure complete reconstitution.
In practice, the recommended technique to reconstitute a dry powder mixture is performed in a bottle using a 3-step method to ensure complete mixing. First, the bottle is tapped a few times to loosen the powder. Then, approximately half the volume of the water is added and the bottle is shaken. Finally, the remaining half of the water is added and the suspension is shaken well. This wetting process was performed by adding approximately 30 ml of distilled water into a bottle containing approximately 19 g of the dry-suspending vehicle, the bottle was tapped on a palm. The number of taps necessary to thoroughly wet and uniformly distributed the powder was recorded using the criteria shown in Table 2.
Table 2. Criteria for wettability testing.
|
Wettability |
Criteria |
|
Good (+++) |
Number of tapping ≤ 20 times |
|
Fair (++) |
Number of tapping 21-40 times |
|
Poor (+) |
Number of tapping > 40 times |
Color, odor, Sedimentation ratio: From the wettability test, further water was added to the powder in the bottle to adjust the suspension volume to the 50-ml mark on the bottle, which was then closed with a cap and shaken until a uniform dispersion was obtained. Color and odor were inspected by the same observer. The suspension was left undisturbed. The sedimentation volume (F) was determined at t = 0, 30 min, 1 hr and 24 hr. The experiments were performed in triplicate.
F = Hu/Ho
where Ho is the initial height of the suspension and Hu is the height of the sediment at each time.
Re-dispersibility: The dry-suspending vehicles were reconstituted with distilled water as described above. The suspension (50 ml) was left undisturbed for 24 hr. The re-dispersibility was determined by counting the number of 180° inversions required to disperse the solid to form a uniform suspension (Nyandoro et al., 2019).
pH: The pH of reconstituted vehicles was measured using a pH meter (SevenMulti, Mettler Toledo Co., Ltd., Schwerzenbach, Switzerland).
Compounding of ORDS from Capsules
The dry-suspending vehicle with good physicochemical properties in both dry and liquid forms was selected. The OSEL capsules were opened and their contents were poured into the vehicle and mixed well. The obtained product was named ORDS. The strength of oseltamivir in ORDS was 10 mg/ml.
Evaluation of ORDS
ORDS was evaluated in both dry and liquid forms. For dry form, the physicochemical properties of ORDS, including color, odor, wettability, sedimentation ratio, re-dispersibility and pH were evaluated before and after storing at 25°C, 75% RH for 7 days, using the methods described above.
For liquid form, the ORDS was immediately reconstituted with distilled water and stored in a refrigerator (2°C-8°C), and at 25°C and 45°C, 75% RH for 14 days. The physicochemical properties of the reconstituted ORDS including color, odor and pH were evaluated on days 0, 1, 3, 5, 7, 10 and 14, using the methods described above. The percentage remaining of oseltamivir was also determined using a UV spectrophotometer.
Validation of analytical method for determination of the percentage remaining of oseltamivir
The method validation was performed based on ICH guidelines (Q2(R1)) (2005).
Calibration curve
The OSEL capsules’ contents were weighed at the quantity equivalent to oseltamivir 10 mg and dissolved in distilled water to obtain a stock solution at a concentration of 100 µg/ml. The stock solution was filtered through a cellulose acetate membrane, pore size 0.45 µm (Sartorius Co., Ltd., Goettingen, Germany). The filtrate was further diluted to seven concentrations in the range of 10 – 40 µg/ml. The OSEL solutions were measured for absorbance at wavelength 220 nm using a UV-Vis spectrophotometer (Model UV 1800, Shimadzu Corp., Kyoto, Japan). The calibration curve was plotted between absorbance and concentration. The linear regression equation and correlation coefficient (r) value were calculated using the least-square method. The linearity was considered based on the r value and residual plot. The r value ≥ 0.99 indicates a high correlation coefficient. The residual plot, predicting the differences between the observed and the predicted values, was also performed to confirm the linearity.
Limit of detection (LOD)
The limit of detection (LOD) is the lowest concentration of oseltamivir in a sample that can be reliably detected. It was calculated by the following equation (ICH, 2005):
LOD = 3.3(Sy/S)
where Sy is the standard deviation of the y-intercept and S is the slope of the regression line.
Limit of quantitation (LOQ)
The limit of quantitation (LOQ) is the lowest concentration of oseltamivir in a sample that can be accurately quantified. It was calculated by the following equation (ICH, 2005):
LOQ = 10(Sy/S)
Where Sy is the standard deviation of the y-intercept and S is the slope of the regression line.
Precision
Precision testing was performed to ensure that the analytical method generates reproducible results. The precision of the analytical method was determined at three different levels of target concentrations. For intra-day precision, a total of 3 replicates of each concentration were analysed on the same day whereas the inter-day precision was carried out for three consecutive days. The results were expressed as relative standard deviation (%RSD).
Accuracy
Accuracy testing was performed to ensure that the analytical method could accurately quantify OSEL in a sample using spiking method. A known quantity of OSEL capsules’ contents was added to reconstitutable dry suspension vehicle (blank). The mixture was dissolved in distilled water and filtered. The solutions were diluted to obtain concentrations of 20, 25 and 30 µg/ml. The results were expressed as percentage recovery (% recovery) of the added drug.
Determination of the percentage remaining of oseltamivir
The percentage remaining of oseltamivir in reconstituted ORDS was quantified on days 0, 1, 3, 5, 7, 10 and 14, using the method described by Thatte et al. (2011), with modifications. The reconstituted ORDS was diluted with distilled water and the solution was filtered. The filtrate was analyzed for oseltamivir using the UV-Vis spectrophotometer.
RESULTS
The dry-suspending vehicles were developed based on the study of Pumpuang and Boonwiset (2017). The suspending agents that were used in the study included xanthan gum, sodium carboxymethylcellulose (SCMC) and microcrystalline cellulose (MCC). These were chosen because they were stable at pH 4 (Rown et al., 2009). Sucrose and sorbitol were used as sweeteners. Sodium benzoate was used as a preservative, silicon dioxide was used as an anti-caking agent, and citric acid was used as a pH adjuster. The ingredients of all formulations developed are shown in Table 3.
Table 3. Ingredients of dry-suspending vehicles for the total volume after reconstitution of 100 ml of suspension.
|
Ingredients |
Amount (g) |
||
|
F1 |
F2 |
F3 |
|
|
Xanthan gum |
0.25 |
0.50 |
- |
|
Sodium carboxymethylcellulose (SCMC) |
- |
- |
0.24 |
|
Microcrystalline cellulose (MCC) |
- |
- |
0.96 |
|
Sucrose |
30 |
30 |
30 |
|
Sorbitol |
7 |
7 |
7 |
|
Sodium benzoate |
0.1 |
0.1 |
0.1 |
|
Silicon dioxide |
1 |
1 |
1 |
|
Citric acid |
0.064 |
0.064 |
0.064 |
Table 4. Consolidation and flowability of dry-suspending vehicles during storage stability testing on days 0, 14 and 28 at 25°C and 45°C, 75% relative humidity.
|
Day |
Test condition |
Formulation |
Consolidation* |
Flowability |
|
|
Hausner’s ratio |
Carr’s index |
||||
|
0 |
- |
F1 |
- |
1.09±0.03 |
8.0±2.4 |
|
F2 |
- |
1.08±0.02 |
7.4±0.5 |
||
|
F3 |
- |
1.12±0.02 |
10.2±1.4 |
||
|
14 |
25°C, 75%RH |
F1 |
- |
1.05±0.02 |
5.4±1.2 |
|
F2 |
- |
1.05±0.02 |
5.3±1.6 |
||
|
F3 |
- |
1.06±0.01 |
5.6±0.8 |
||
|
45°C, 75%RH |
F1 |
- |
1.05±0.01 |
5.0±0.8 |
|
|
F2 |
- |
1.07±0.02 |
5.4±0.6 |
||
|
F3 |
- |
1.06±0.01 |
6.0±0.6 |
||
|
28 |
25°C, 75%RH |
F1 |
- |
1.08±0.00 |
7.4±0.2 |
|
F2 |
- |
1.08±0.01 |
7.9±0.3 |
||
|
F3 |
- |
1.12±0.01 |
10.5±1.1 |
||
|
45°C, 75%RH |
F1 |
- |
1.06±0.01 |
5.4±0.4 |
|
|
F2 |
- |
1.07±0.01 |
6.7±0.9 |
||
|
F3 |
+ |
1.10±0.01 |
8.7±0.6 |
||
|
Note: *Consolidation: Symbol (-) means no agglomeration or cluster of powder; Symbol (+) means agglomerations or clusters of powder are observed. |
|||||
All formulations of the powder were white and odorless and did not change after stability testing. The consolidation was observed only in F3 when stored at 45°C, 75% RH for 28 days (Table 4). The differences among all formulations are types and amounts of suspending agents. F1 and F2 contain xanthan gum at 0.25 and 0.50 g for the total volume after reconstitution of 100 ml of suspension. While F3 contains sodium carboxymethylcellulose (0.24 g) and microcrystalline cellulose (0.96 g). These compounds possess hygroscopic property to some extent. The higher the amount of the suspending agents, the more powders will absorb moisture. In addition, it has been reported that cellulose was naturally hygroscopic and the extent of moisture absorbed depended on temperature and relative humidity (Garg et al., 2021). However, its flowability still showed to be excellent based on rating showed in Table 1.
The wettability of powder F1 was good while the wettability of all formulations decreased with storage time and temperature (Table 5).
Table 5. Wettability of the dry-suspending vehicles during storage stability testing on days 0, 14 and 28 at 25°C and 45°C, 75% relative humidity.
|
Day |
Test condition |
Formulation |
Wettability |
|||
|
0 |
- |
F1 |
Good (+++) |
|||
|
F2 |
Fair (++) |
|||||
|
F3 |
Fair (++) |
|||||
|
14 |
25°C, 75%RH |
F1 |
Fair (++) |
|||
|
F2 |
Fair (++) |
|||||
|
F3 |
Poor (+) |
|||||
|
45°C, 75%RH |
F1 |
Fair (++) |
||||
|
F2 |
Fair (++) |
|||||
|
F3 |
Fair (++) |
|||||
|
28 |
25°C, 75%RH |
F1 |
Poor (+) |
|||
|
F2 |
Poor (+) |
|||||
|
F3 |
Poor (+) |
|||||
|
45°C, 75%RH |
F1 |
Fair (++) |
||||
|
F2 |
Poor (+) |
|||||
|
F3 |
Fair (++) |
|||||
F1 and F2, stored at 25°C, 75% RH for 14 and 28 days, yielded reconstituted suspensions with an off-white color which changed to pale yellow after storing at 45°C, 75% RH for 14 days (Figure 1). On day 28, the color of the reconstituted suspensions of F1 and F2 became darker (Table 6). The color of F3 in the reconstituted state did not change with storage time and temperature.
The sedimentation ratio of all formulations decreased with time. The sedimentation rate of F2 was the slowest, followed by F1 and F3 (Table 6). However, F1 and F2 were more readily redispersed (Table 7). The pH of F1 before and after stability testing was well controlled at approximately pH 4 (Table 7).
Table 6. Color and sedimentation ratio of the dry-suspending vehicles in the reconstituted state during storage stability testing on days 0, 14 and 28 at 25°C and 45°C, 75% relative humidity.
|
Day |
Test condition |
Formulation |
Color |
Sedimentation ratio |
|||
|
0 min |
30 min |
1 hr |
24 hr |
||||
|
0 |
- |
F1 |
Off-white |
1.00 ±0.00 |
0.90 ±0.02 |
0.82 ±0.05 |
0.45 ±0.03 |
|
F2 |
Off-white |
1.00 ±0.00 |
1.00 ±0.00 |
1.00 ±0.00 |
0.83 ±0.05 |
||
|
F3 |
Off-white |
1.00 ±0.00 |
1.00 ±0.00 |
0.78 ±0.19 |
0.38 ±0.06 |
||
|
14 |
25°C, 75%RH |
F1 |
Off-white |
1.00 ±0.00 |
0.90 ±0.02 |
0.82 ±0.03 |
0.43 ±0.03 |
|
F2 |
Off-white |
1.00 ±0.00 |
1.00 ±0.00 |
1.00 ±0.00 |
0.88 ±0.02 |
||
|
F3 |
Off-white |
1.00 ±0.00 |
1.00 ±0.00 |
0.90 ±0.09 |
0.41 ±0.01 |
||
|
45°C, 75%RH |
F1 |
Pale yellow |
1.00 ±0.00 |
0.84 ±0.12 |
0.74 ±0.04 |
0.40 ±0.03 |
|
|
F2 |
Pale yellow |
1.00 ±0.00 |
1.00 ±0.00 |
0.99 ±0.01 |
0.84 ±0.06 |
||
|
F3 |
Off-white |
1.00 ±0.00 |
0.69 ±0.09 |
0.53 ±0.06 |
0.38 ±0.02 |
||
|
28 |
25°C, 75%RH |
F1 |
Off-white |
1.00 ±0.00 |
0.86 ±0.03 |
0.82 ±0.01 |
0.40 ±0.02 |
|
F2 |
Off-white |
1.00 ±0.00 |
1.00 ±0.00 |
0.97 ±0.03 |
0.84 ±0.03 |
||
|
F3 |
Off-white |
1.00 ±0.00 |
0.82 ±0.01 |
0.71 ±0.02 |
0.39 ±0.02 |
||
|
45°C, 75%RH |
F1 |
slightly darker yellow compared to day 14 |
1.00 ±0.00 |
0.79 ±0.03 |
0.68 ±0.02 |
0.38 ±0.03 |
|
|
F2 |
slightly darker yellow compared to day 14 |
1.00 ±0.00 |
1.00 ±0.00 |
0.99 ±0.02 |
0.71 ±0.02 |
||
|
F3 |
Off-white |
1.00 ±0.00 |
0.36 ±0.01 |
0.33 ±0.05 |
0.26 ±0.05 |
||
Table 7. Re-dispersibility and pH of the dry-suspending vehicles in the reconstituted state during storage stability testing on days 0, 14 and 28 at 25°C and 45°C, 75% relative humidity.
|
Day |
Test condition |
Formulation |
Re-dispersibility |
pH |
|
0 |
- |
F1 |
2.0±0.6 |
4.04±0.09 |
|
F2 |
2.0±0.6 |
4.08±0.04 |
||
|
F3 |
26.0±5.5 |
4.29±0.07 |
||
|
14 |
25°C, 75%RH |
F1 |
3.0±0.6 |
4.01±0.02 |
|
F2 |
3.0±1.2 |
4.11±0.03 |
||
|
F3 |
39.0±8.1 |
4.42±0.12 |
||
|
45°C, 75%RH |
F1 |
3.0±1.0 |
4.05±0.02 |
|
|
F2 |
3.0±1.0 |
4.13±0.02 |
||
|
F3 |
22.0±3.2 |
4.32±0.07 |
||
|
28 |
25°C, 75%RH |
F1 |
4.0±0.6 |
4.00 ±0.03 |
|
F2 |
4.0± 0.6 |
4.08±0.03 |
||
|
F3 |
36.0±15.3 |
4.29 ±0.11 |
||
|
45°C, 75%RH |
F1 |
4.0±1.2 |
4.07±0.04 |
|
|
F2 |
4.0±1.2 |
4.15±0.03 |
||
|
F3 |
11.0±1.5 |
4.40±0.08 |

Figure 1. Appearance of the dry-suspending vehicles in the reconstituted state during storage stability testing on days 0, 14 and 28 at 25°C and 45°C, 75% relative humidity.
Among the three formulations, F1 showed the best physicochemical properties. Therefore, it was selected to be used as the dry-suspending vehicle in further studies. The vehicle was mixed with the content of OSEL capsules to obtain ORDS with the strength of OSEL 10 mg/ml. Under storage at 25°C, 75% RH for 7 days, changes in color and odor of the ORDS powder were not observed and wettability did not change (Table 8). The sediment formed at the bottom of the bottle could be easily redispersed by shaking. The pH of the reconstituted ORDS remained the same (Table 8).
Table 8. Physicochemical properties of oseltamivir reconstitutable dry suspension (ORDS) before and after storing at 25°C, 75% RH for 7 days.
|
Day |
Wettability |
Sedimentation ratio |
Re-dispersibility |
pH |
|||
|
0 min |
30 min |
1 h |
24 h |
||||
|
0 |
+++ |
1.00 ± 0.00 |
0.78 ± 0.04 |
0.65 ± 0.04 |
0.42 ± 0.02 |
5.0 ± 0.0 |
4.01 ± 0.01 |
|
7 |
+++ |
1.00 ± 0.00 |
0.88 ± 0.04 |
0.78 ± 0.07 |
0.41 ± 0.02 |
6.0 ± 1.5 |
3.96 ± 0.02 |
For reconstituted form, after storage at refrigerator temperature and at 25°C for 14 days, the color of the reconstituted ORDS was not changed. However, at 45°C the color had changed from white to yellow after storing for 7 days. At all testing conditions, the reconstituted ORDS remained odorless and its pH did not change (Figure 2).
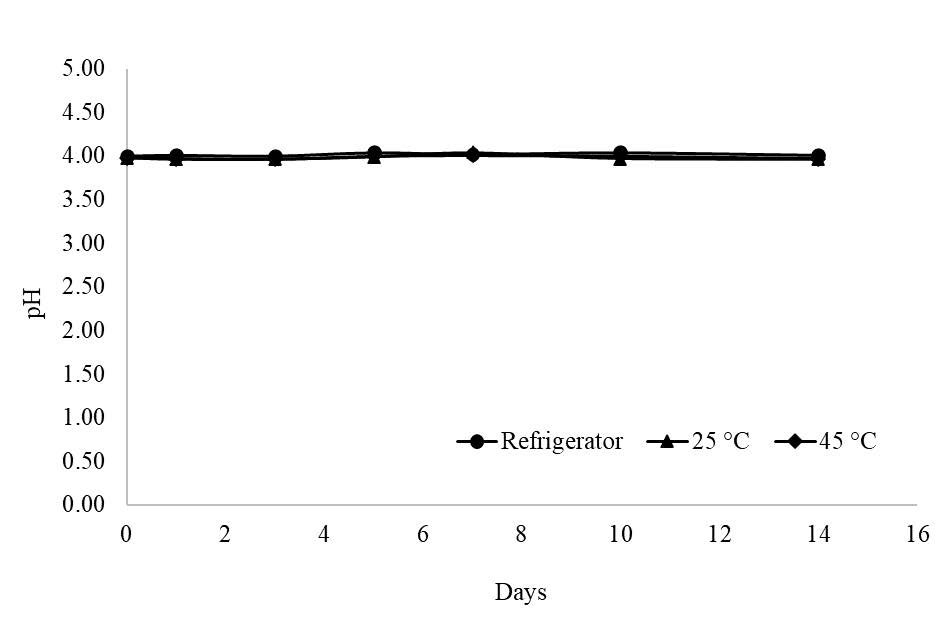
Figure 2. pH of oseltamivir reconstitutable dry suspension (ORDS) in reconstituted state after storing at refrigerator temperature, 25°C and 45°C, 75% RH for 14 days.
Determination of the percentage remaining of oseltamivir
Linearity of the analytical method was performed to investigate its ability within a specified range to obtain test results which were directly proportional to the concentration of the oseltamivir in ORDS. The linear regression equation was found to be y = 0.0253x + 0.0209. The correlation coefficient (r) was 0.9988 indicating linear relationship between concentration (x) and absorbance (y). LOD and LOQ for the analysis method were 1.71 and 5.18 µg/ml. Intra-day and inter-day precision are shown in Table 9. The %RSD was within the acceptance criteria according to AOAC (2002), indicating that the analytical method generates reproducible results.
Table 9. Intra-day and inter-day precision for the analysis method of oseltamivir using UV spectrophotometer (n=3).
|
Concentration (µg/ml) |
Intra-day Precision |
Inter-day Precision |
||||
|
Absorbance measured |
% RSDr |
Acceptance criteria for RSDr* |
Absorbance measured |
% RSDR |
Acceptance criteria for RSDR* |
|
|
20 |
0.5142 ± 0.0060 |
1.17 |
5.07 |
0.5227 ± 0.0093 |
1.79 |
10.14 |
|
25 |
0.6415 ± 0.0103 |
1.61 |
4.90 |
0.6567 ± 0.0225 |
3.42 |
9.80 |
|
30 |
0.7842 ± 0.0079 |
1.01 |
4.77 |
0.7826 ± 0.0130 |
1.66 |
9.54 |
Note: *Acceptance criteria for repeatability (RSDr) and reproducibility (RSDR) were calculated from Horwitz equations:
%RSDr = C-0.15 and %RSDR = 2C-0.15, where C is mass fraction (AOAC, 2002).
The percentage recovery of OSEL determined at concentrations of 20 µg/ml was 99.36%, 25 µg/ml was 99.24% and 30 µg/ml was 101.61%. The %recovery was within the acceptable range of 85%-115%, indicating that the analytical method could accurately quantify OSEL in ORDS. In addition, these results indicated the specificity/selectivity of the analytical method.
For liquid form, ORDS was reconstituted with water and stored at refrigerator temperature, and at 25°C and 45°C, 75% RH. The percentage remaining of OSEL was within 90%-110% (Figure 3), suggesting that it remained stable in the reconstituted ORDS in three different conditions.
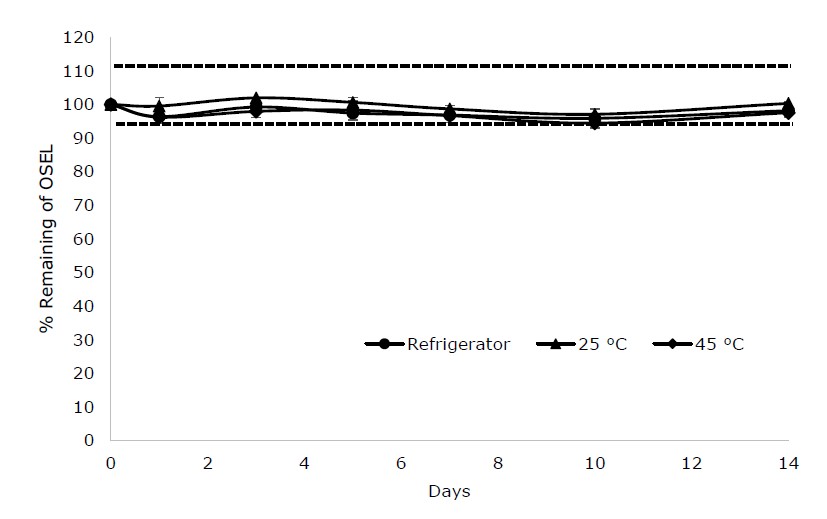
Figure 3. Percentage remaining of oseltamivir in reconstituted ORDS after storing at refrigerator temperature, 25°C and 45°C, 75% RH for 14 days.
DISCUSSION
The objective of this study was to develop oseltamivir reconstitutable dry suspension (ORDS) from capsules. The dry suspension was aimed because oseltamivir in the solid state is the most stable (Oliyai et al., 1998). First, dry-suspending vehicles were developed using suspending agents, sweetening agents, an anticaking agent, a preservative and a pH-adjusting agent. The optimum pH to stabilize OSEL was 4.0.
The suspending agents, including xanthan gum and a mixture of SCMC and MCC (at 1:4 ratio) are studied because they can be easily dispersed and hydrated in water (Pumpuang and Boonwiset, 2017). In addition, they possess pseudoplastic with thixotropic property which gives high viscosity under static conditions and becomes less viscous when shaken.
The advantage of xanthan gum is that its viscosity remains constant over a wide range of pH (pH 1-13), temperature and ionic strength (Singhvi et al., 2019). The combination of SCMC and MCC gives a good suspending property because the network structures formed by MCC in water are stabilized by SCMC through hydrogen bonding and ionic interactions (Zhao et al., 2011).
The vehicles were subjected to stability testing under 25°C and 45°C, 75% RH for 28 days. The design of this stability protocol is based on the following reasons: according to U.S. Pharmacopeia (USP-NF, 2017), 25°C is the correct storage condition for drugs stored in a pharmacy department. In addition, storage at 45°C was tested to ensure that the vehicle developed was stable when stored at room temperature in patients’ homes, especially during the summer. The relative humidity of 75% ± 5% refers to the humidity usual in Thailand’s climatic zone IVb, according to the ASEAN Guideline on Stability Study of Drug Products (2010). The duration of 28 days is set to ensure that the vehicle can be prepared in advance. As well, the short-term expiry date of 28 days for oral products is recommended for extemporaneously compounded medicines in hospitals to minimise the risk of deterioration (Falconer and Steadman, 2017).
For ORDS, its stability in dry form was again tested at 25°C and 75% RH for 7 days to mimic storage conditions in the pharmacy department, according to U.S. Pharmacopeia (USP-NF, 2017). For reconstituted form, ORDS was reconstituted with water and stored in a refrigerator (2°C-8°C), at 25°C and 45°C, 75% RH for 14 days. The storage condition for reconstituted ORDS covers 2°C-8°C because refrigeration is the recommended storage condition for oral liquid preparation. In addition, the dose for pediatric patients is 3 mg/kg, twice daily while the dose for children or adults weighing more than 40 kg is 75 mg, twice daily. The duration of treatment is 5 days for use in the treatment and 7 days for chemoprophylaxis. Thus, the required number of bottles (OSEL 10 mg/ml, 60 ml/bottle) dispensed will be 1-2 bottles. It is also generally recommended to reconstitute ORDS one bottle at a time. Therefore, the timeframe for the stability test of ORDS in dried form and reconstituted form is sufficient to verify the stability of the developed product.
These results suggested that the dry-suspending vehicle using xanthan gum showed better wettability and re-dispersibility compared to that using the combination of SCMC and MCC. However, the wettability of the dry-suspending vehicle slightly decreased with increasing xanthan gum content while re-dispersibility showed no difference. Therefore, the dry vehicle (F1) consisting of xanthan gum, sucrose, sorbitol, sodium benzoate, silicon dioxide and citric acid at 0.25% w/v, 30% w/v, 7% w/v, 0.1% w/v, 1.0% w/v and 0.064% w/v of the reconstituted suspension was selected for further studies. The stability tests showed that the dry-suspending vehicle could be prepared and filled in bottles in advance and should be stored at 25°C (i.e. room temperature in the hospital pharmacy department). At 45°C, it turns pale-yellow and darker when kept beyond 14 days.
The dry-suspending vehicle was weighed into bottles and the contents of OSEL capsules were simply added to yield the strength of OSEL of 10 mg/ml. The bottles were shaken well to obtain ORDS. It was observed that ORDS in a dry state was physically and chemically stable. After reconstitution, ORDS could be kept at either refrigerator (2°C-8°C), 25°C, or 45°C. However, after long storage at 45°C, the suspension turned yellow in color, but the OSEL content was not affected.
CONCLUSION
Oseltamivir reconstitutable dry suspension with the strength of OSEL 10 mg/ml was successfully developed. The dry-suspending vehicle, consisting of xanthan gum, sucrose, sorbitol, sodium benzoate, silicon dioxide and citric acid at 0.25% w/v, 30% w/v, 7% w/v, 0.1% w/v, 1.0% w/v and 0.064% w/v of the reconstituted suspension, can be prepared in advance and ready to mix with capsule contents when required. The ORDS in a dry state should be kept at room temperature. The ORDS in the reconstituted state could be stored in either refrigerator (2-8°C), 25°C, or 45°C. However, if the storage period is longer than 7 days, reconstituted ORDS should be kept at lower temperatures. ORDS developed in this study may be a convenient alternative to the extemporaneous preparation in government health care settings.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Pharmaceutical Sciences, Naresuan University for providing financial support and facilities. Thanks also to Mr Roy Morien of the Naresuan University Graduate School for his editing of the grammar, syntax and general English expression in this manuscript.
AUTHOR CONTRIBUTIONS
Kitiya Kesornmalee and Natthareephon Phanaphai designed and conducted the experiments and performed data analysis. Tasana Pitaksuteepong supervised the project, wrote the manuscript and performed data analysis. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no conflicts of interest.
REFERENCES
AOAC. 2002. Guidelines for single- laboratory validation of chemical methods for dietary supplements and botanicals.
ASEAN Guideline on Stability Study of Drug Product 2010. Revision 25th ACCSQ-PPWG, ASEAN, Thailand. https://asean.org/wp-content/uploads/2018/01/25PPWG-ANNEX-7-iv-Final-ASEAN-Guideline-on-Stability-Study-Drug-Product-R2.pdf. Accessed March 2023.
Carr, R.L. 1965. Evaluating flow properties of solids. Chemical Engineering. 72: 163-168.
Division of Epidemiology, Department of Disease Control, Ministry of Public Health. National Disease Surveillance (Report 506). Thailand Influenza Situation Report 2022. Retrieved on 12 February 2023, from https://ddc.moph.go.th/uploads/ckeditor2//files/DOE_flu_46.2565.pdf
Falconer, J.R. and Steadman, K.J. 2017. Extemporaneously compounded medicines. Australian Prescriber. 40(1): 5-8.
Fournaise, T., Burgain, J., Perroud, C., Scher, J., Gaiani, C., and Petit, J. 2020. Impact of formulation on reconstitution and flowability of spray-dried milk powders. Powder Technology. 372(15): 107-116.
Garg, M., Apostolopoulou-Kalkavoura, V., Linares, M., Kaldéus, T., Malmström, E., Bergström, L., and Zozoulenko, I.V. 2021. Moisture uptake in nanocellulose: The effects of relative humidity, temperature and degree of crystallinity. Cellulose. 28(14): 1-15.
International Conference on Harmonization (ICH). 2005. Validation of analytical procedures: Text and methodology Q2(R1). Geneva: IFPMA.
Medical Treatment Working Group, Department of Medical Services, Ministry of Public Health. 2011. Clinical Practice Guideline for Influenza. Bangkok: Ministry of Public Health (Thai).
Nyandoro, V.O., Ogaji, J.I., and Audu-Peter, J.D. 2019. Effect of particle size of okra gum as a suspending agent on some physicochemical properties of reconstituted dry paracetamol suspension. World Journal of Pharmaceutical Research. 8(11): 129-141.
Oliyai, R., Yuan, L.C., Dahl, T.C., Swaminathan, S., Wang, K.Y., and Lee, W.A. 1998. Biexponential decomposition of a neuraminidase inhibitor prodrug (GS-4104) in aqueous solution. Pharmaceutical Research. 15(8): 1300-1304.
Pumpuang, P. and Boonwiset, U. 2017. Suspending vehicle: dry powder and ready-to-use. Undergraduate Thesis. Naresuan University, Phitsanulok. (Thai)
Rown, R.C., Sheskey, P.J., and Quinn, M.E. 2009. Handbook of pharmaceutical excipients. 6th edition. Washington D.C.: Pharmaceutical Press and American Pharmacists Association.
Singhvi, G., Hans, N., Shiva, N., and Dubey, S.K. 2019. Xanthan gum in drug delivery applications. In Hasnain, M.S., and Nayak, A.K. [eds] Natural Polysaccharides in Drug Delivery and Biomedical Applications. Academic Press. P.121-144.
Thatte, A.A., Kadam, R.J., Pramila, T., Bhoi, U.A., and Deshpande, K.B. 2011. Development, validation and application of UV spectrophotometric method for the determination of oseltamivir phosphate in bulk and pharmaceutical dosage form. International Journal of ChemTech Research. 3(2): 569-573.
United States Pharmacopeia and National Formulary. 2012. <1174> Powder Flow. Rockville, MD: United States Pharmacopeial Convention.
United States Pharmacopeia and National Formulary. 2017. <659> Packaging and Storage Requirements. Rockville, MD: United States Pharmacopeial Convention.
Winiarski, A.P., Infeld, M.H., Tscherne, R., Bachynsky, M., Rucki, R., and Nagano-Mate, K. 2007. Preparation and stability of extemporaneous oral liquid formulations of oseltamivir using commercially available capsules. Journal of the American Pharmacists Association. 47:747-755.
Xayavong, T., Vimolsarawong, N., and Rittirod, T. 2018. Stability study of oseltamivir extemporaneous suspension. Asia-Pacific Journal of Science and Technology. 1(24): 1-4.
Zhao, G.H., Kapur, N., Carlin, B., Selinger, E., and Guthrie, J.T. 2011. Characterisation of the interactive properties of microcrystalline cellulose-carboxymethyl cellulose hydrogels. International Journal of Pharmaceutics. 415 (1-2): 95-101.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tasana Pitaksuteepong1,*, Kitiya Kesornmalee2 and Natthareephon Phanaphai3
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences and Center of Excellence for Innovation in Chemistry, Naresuan University, Phitsanulok 65000, Thailand
2 Mae Ramat Hospital, Mae Ramat District, Tak 63140, Thailand
3 Samut Prakan Hospital, Samut Prakan District, Samut Prakan 10270, Thailand.
Corresponding author: Tasana Pitaksuteepong, E-mail: tasanap@nu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 17, 2023;
Revised: December 29, 2023;
Accepted: January 2, 2024;
Online First: January 16, 2024

