
Development of Mucoadhesive Buccal Films Containing Triamcinolone Acetonide by using Co-solvent Systems
Benchawan Chamsai, Chutima Sinsuebpol, Praneet Opanasopit and Wipada Samprasit*Published Date : January 15, 2023
DOI : https://doi.org/10.12982/NLSC.2024.012
Journal Issues : Number 1, January-March 2024
Abstract This work aimed to develop mucoadhesive buccal films containing triamcinolone acetonide (TA), which shows local anti-inflammatory activity. A thin film layer was formulated from polyvinyl alcohol (PVA) by the solvent casting method. The solubility of TA and the ability of the co-solvents to produce films were evaluated. The optimal co-solvents were chosen to prepare the film. Pharmaceutical properties of the film, including uniformity of weight, thickness, mechanical property, wetting time, mucoadhesion, TA content, and release, were evaluated. Furthermore, the cytotoxicity and scratch assay were assessed for the in vitro cell line. Among the co-solvents, the system consisted of ethanol, distilled water, and an individual plasticizer (glycerin and polyethylene glycol 400 (PEG 400)), which showed promising co-solvents for TA solubility and film formation. The films were clear and colorless, with a smooth surface and good mechanical properties. PEG 400-contained film formulation had higher mechanical properties and faster wetting time than glycerin. Both films had similar mucoadhesive properties, although the TA content differed slightly, and a rapid release of TA from the films was observed. However, PEG 400-contained film offered a slightly faster TA release than glycerin. The films were non-toxic to gingival fibroblast cells. However, they decreased the cell growth and migration compared to the control. According to this research, co-solvents increased TA solubility and produced good characteristic buccal mucoadhesive films containing TA.
Keywords: Triamcinolone acetonide, Co-solvents, Film, Mucoadhesive property
Funding: The authors are grateful for the research funding provided by the National Research Council of Thailand (NRCT): N42A650551.
Citation: Chamsai, B., Sinsuebpol, C., Opanasopit, P. and Samprasit, W. 2024. Development of mucoadhesive buccal films containing triamcinolone acetonide by using co-solvent systems. Natural and Life Sciences Communications. 23(1): e2024012.
INTRODUCTION
Oral ulcers are often painful lesions related to numerous conditions developing within the oral cavity. Oral aphthosis is a painful inflammatory process of the oral mucosa that has an important effect on the patient’s life, causing much pain and difficulty with mastication and speech (Gasmi Benahmed et al., 2021). Therefore, treating oral ulcers and oral aphthous aims to control the ulcer's pain and healing. Currently, triamcinolone acetonide (TA) at concentrations ranging from 0.05% to 0.5% is used as an ointment or emollient paste and is applied to the ulcer site. Although their physical properties enable them to make intimate contact with larger mucosal surface area (Chinna Reddy et al., 2011), they are dislodged by speaking, swallowing, drinking, eating, or salivation, as well as tongue movement, resulting in dose inaccuracy (Alhallak et al., 2023) and short duration of action (Sen et al., 2015). Thus, several applications may be required to ensure the TA remains in place.
Films as dosage forms are the polymeric systems from various types of polymers. Mucoadhesive films are retentive dosage forms and release the drug directly into the oral cavity or the oral mucosa (Morales and McConville, 2011), exhibiting longer periods on the mucosal tissue (Chinna Reddy et al., 2011) and improving the local drug concentration by preventing loss (Sen et al., 2015). Films that release the drug into the oral mucosa increase local bioavailability by blocking non-targeted drug absorption in the gastrointestinal tract, thereby significantly reducing drug dose, systemic toxicity, and frequency of applications (Dubashynskaya and Skorik, 2022). Compared to creams and ointments, films also offer advantages in delivering a measured drug dose to the site (Chinna Reddy et al., 2011). The solvent casting method, which involves casting aqueous solutions and/or organic solvents to form films, is the most frequently used method to prepare films. This is mainly due to the ease of the process and the low cost. The first step is to prepare the casting solution, in which the drug and polymer are dissolved. TA has low aqueous solubility, whereas film former polymers generally dissolve in water. Choosing an appropriate solvent to dissolve is, therefore, challenging. Several previous works developed dosage forms for TA delivery using discs (Sen et al., 2015) and mucoadhesive film (Ahn et al., 2002; Alhallak et al., 2023). TA's solubility was increased using beta-cyclodextrin and organic solvents. Thus, the finding of co-solvents for producing mucoadhesive films and improving the solubility of drugs with low aqueous solubility, like TA, was the novelty of this work. This research study aimed to study TA solubility and film formation in various solvents and co-solvents. The potential of co-solvents to produce the mucoadhesive films using the solvent casting technique was assessed. Film characterization in terms of uniformity of weight, thickness, mechanical property, wetting time, mucoadhesion, TA content, and release were investigated. Also, the cytotoxicity and scratch assay were evaluated.
MATERIAL AND METHODS
Materials
The TA was a gift from Millimed Co., Ltd., Thailand. Polyvinyl alcohol (PVA, degree of polymerization ≈1600, degree of hydrolysis ≈97.5–99.5 mol %) was purchased from Fluka, Switzerland. Ethanol, glycerin, and polyethylene glycol 400 (PEG 400) were purchased from S. Tong Chemicals Co., Ltd., Thailand. The fresh porcine buccal mucosa was purchased from a local market (Ang Thong, Thailand). All other chemicals and reagents were of analytical grade.
Solubility test
Briefly, 0.01 g of TA (0.1%w/w) was added to 10 g of various solvents, varying the dielectric constant, including distilled water, 40 and 50 %w/w ethanol in distilled water. The dielectric constant of the solvents was obtained by calculation from the individual component product’s weight composition (Prakongpan and Nagai, 1984), which was followed by Equation 1:
εmixture = (ε1 × weight fraction1) + (ε2 × weight fraction2) + … + (εn × weight fractionn) eq.1
where εmixture, ε1, ε2, and εn are the dielectric constant at 25°C of the mixture of co-solvents, individual solvents 1, 2, and n, respectively. The dielectric constant at 25°C of water, ethanol, glycerin, and PEG 400 is 78.4, 24.3. 42.5 and 12.4 (Babu et al., 2008), respectively.
The mixtures were mixed by vortexing for 1 min and then by shaking at 100 rpm overnight for the solubility at ambient temperature. The TA solubility was observed by visual inspection. The completely soluble TA appeared as a transparent solution. The co-solvents of ethanol, water, and an individual plasticizer (glycerin and PEG 400) were then tested for TA solubility at the dielectric constant, in which the TA was completely soluble. Glycerin and PEG 400 were ranged from 5 to 30 %w/w. The 5 %w/w of PVA was also evaluated for its solubility in the various solvents and co-solvents. Table 1 shows the composition and dielectric constant of the solvents and co-solvents used for the solubility test.
Preparation of films containing TA
Co-solvents that could dissolve TA and PVA were selected to prepare the films by the solvent casting method. A combination of co-solvents was divided into two parts. Deionized water at 80°C was for dispersing PVA, whereas a mixture of ethanol and individual plasticizer (glycerin and PEG 400) was for dissolving TA. PVA and TA solution were then mixed and allowed to form a solution under agitation for 4 h. The homogeneous solutions were poured onto 10-cm plastic plates with equal polymer weight and left to dry overnight at 45°C to allow solvent evaporation. Co-solvents that provided the dried and transparent film were further used to prepare films containing TA.
Two film formulations containing 0.1%w/w of TA were prepared with different co-solvents of glycerin system (G) and PEG 400 system (P) (Table 2). Films containing TA were produced following the above-described method. The dried films were visually inspected for morphology. The films were cut into square dimensions of 1 cm × 1 cm (1 cm2), packed in aluminum foil, and stored in a desiccator (30% of relative humidity) at room temperature (30°C).
Table 1. Solubility study of TA and PVA in different aqueous systems.
|
System |
Co-solvents (%w/w) |
Dielectric constant |
Solubility of TA |
Appearance of PVA solution |
||||
|
Distilled water |
Ethanol |
Glycerin |
PEG 400 |
|||||
|
Ethanol-water |
||||||||
|
0 %w/w ethanol |
100 |
- |
- |
- |
78.5 |
Incompletely soluble |
Transparent and low viscosity |
|
|
40 %w/w ethanol |
60 |
40 |
- |
- |
56.8 |
Incompletely soluble |
Precipitate |
|
|
50 %w/w ethanol |
50 |
50 |
- |
- |
51.4 |
Completely soluble |
Precipitate |
|
|
Glycerin system |
||||||||
|
5 %w/w glycerin |
48.3 |
46.7 |
5 |
- |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
10 %w/w glycerin |
46.6 |
43.4 |
10 |
- |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
15 %w/w glycerin |
45 |
40 |
15 |
- |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
20 %w/w glycerin |
43.3 |
36.7 |
20 |
- |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
30 %w/w glycerin |
39.9 |
30.1 |
30 |
- |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
PEG 400 system |
||||||||
|
5 %w/w PEG 400 |
51.1 |
43.9 |
- |
5 |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
10 %w/w PEG 400 |
52.2 |
37.8 |
- |
10 |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
15 %w/w PEG 400 |
53.3 |
31.7 |
- |
15 |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
20 %w/w PEG 400 |
54.4 |
25.6 |
- |
20 |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
22.5 %w/w PEG 400 |
54.9 |
22.6 |
- |
22.5 |
51.4 |
Completely soluble |
Transparent and low viscosity |
|
|
Note: *Transparent solution = absorbance at 650 nm was less than 0.0100, and low viscosity = 12–35 cPs |
|
|||||||
Table 2. Formulations of films containing TA.
|
Ingredients (% w/w) |
Formulation |
|
|
G film |
P film |
|
|
TA |
0.1% w/w to film weight |
0.1% w/w to film weight |
|
PVA |
5.0 |
5.0 |
|
Glycerin |
5.0 |
- |
|
PEG 400 |
- |
5.0 |
|
Evaporating solvent |
- |
- |
|
Ethanol |
46.7 |
43.9 |
|
Distilled water |
48.3 |
51.1 |
Evaluation of films containing TA
Weight and thickness of films
The films' uniformity in weight and thickness was evaluated. Ten samples from each film formulation were weighed with an analytical weighing balance (Scientific Promotion Co., LTD, Thailand), and the average weight with standard deviation was determined. A micrometer measured the films’ thickness on a single patch. (Mitutoyo No.7301, Japan). The mean values were calculated.
Mechanical properties
The mechanical properties of films in terms of tensile strength and Young's modulus were evaluated by using a texture analyzer (TA.XT plus, Stable Micro Systems, UK) with a 5 kg load cell equipped with a tensile grips holder. The films were cut into a rectangle (20 × 30 mm). At a speed of 2 mm/s, the tensile strength and Young's modulus were recorded. The flexibility of the films was also evaluated by folding endurance. The films were repeatedly folded in the same place until a visible crack was observed. The number of times the film was folded without breaking was recorded as its folding endurance value.
Wetting time
The wetting time of the films was performed under a simulated saliva fluid (SSF) pH 6.8. The SSF was prepared by dissolving 2.38 g Na2HPO4, 0.19 g KH2PO4, and 8 g NaCl in a liter of deionized water, which was adjusted to pH 6.8. The dried films were put into the SSF pH 6.8. The time required for the solution to diffuse to the film was recorded as the wetting time.
Mucoadhesive property
The mucoadhesive property of films was evaluated by the in vitro wash-off method using fresh porcine buccal mucosa according to the previous method (Chanburee and Tiyaboonchai, 2017). Briefly, the mucosa was mounted. Then, films were placed on the mucosa. Afterward, a continuous flow of SSF pH 6.8 was dropped over the mucosa. The time required for the films to fall out from the mucosa was recorded as the mucoadhesive time. The mucoadhesive force of films onto porcine buccal mucosa was also evaluated using a texture analyzer with a 50 N load cell equipped with a mucoadhesive holder. The films were cut into squares (1 × 1 cm) and attached to an aluminum probe (10 mm in diameter) by using double-sided adhesive tape. The porcine buccal mucosa was securely placed onto the holder stage of the mucoadhesive holder. A constant force (0.3 N) was imposed for 15 s when making contact between the film and the buccal mucosa. The mucoadhesive performance was measured by recording the maximum detachment force of films from the tissue, indicating mucoadhesive force.
TA content
Each film was dissolved overnight in 50% ethanol using a shaking incubator at a speed of 100 rpm. Then, the soluble film was filtrated through a syringe filter (0.45 μm) and analyzed using high-performance liquid chromatography (HPLC). A C18 column (5 mm, 4.6 nm × 250 mm) was used. The mobile phase, acetonitrile/ultrapure water (50/50, v/v), with acetic acid added to adjust the pH to 4, and methanol/phosphoric acid/ultrapure water (75/0.5/25, v/v) was used for P and G films, respectively (Ahn et al., 2002). The flow rate was 1 mL/min, and the UV detection wavelength was 240 and 252 nm for P and G films, respectively. The analyses were carried out in triplicate. The TA content was calculated as the percentage of weight (%w/w) and the percentage of the labeled amount (%LA).
TA release
The study was conducted in a shaking incubator at 37 ± 0.5°C at 100 rpm. Initially, A 1 cm × 1 cm (1 cm2) piece of film was placed into a 15 mL medium of 50 % ethanol with SSF pH 6.8. At given time points, samples (1 mL) were withdrawn from the medium and replaced by fresh solution. HPLC directly determined the amount of TA in the medium. The obtained data were carefully analyzed to determine the cumulative amount of TA release from the specimens at each immersion time point.
Cytotoxicity
The cytotoxicity of the films was evaluated for normal human gingival fibroblast (HGF). The cell was purchased from the American Type Culture Collection (ATCC), USA. The cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acid solution, and 0.1% penicillin-streptomycin solution. The films were sterilized by UV radiation for 1 h before submerging in a serum-free medium (SFM) solution for 24 h to ensure complete film dissolution. The cells were seeded in a 96-well plate. When the cultures had reached confluence, the cells were treated with media containing different film concentrations ranging from 0 to 4,000 μg/mL and further incubated for 24 h. After treatment, cytotoxicity was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Cell viability (%) was calculated based on absorbance at 550 nm using a microplate reader. The viability of non-treated control cells was arbitrarily defined as 100%.
In vitro scratch assay
The migration abilities of the HGF cells were also employed to test the wound-healing effect using the scratch assay. The cells were seeded in a 6-well plate. When the monolayer formed, a sterile pipette tip was used to make a linear scratch, which was subsequently washed with phosphate-buffered saline (PBS). After the scratch defect was made, the cells were exposed to the extraction media with the films at 1,000 μg/mL concentration. Non-treated cells served as the control. Images were taken of the cells after the scratch defect was produced, after which the cells were immediately returned to the incubator at 37˚C. Subsequent images of the scratch defects were obtained at 6, 24, 48 h, and 72 h. The photographs for each sample were then quantitatively examined by using image analysis software (JMicro Vision V.1.2.7, Switzerland). The percent migration rate was obtained after measuring the distance of each scratch closure.
Statistical analysis
All experimental measurements were collected at least triplicate. The data were presented as the means ± standard deviation (SD). The Student's T-test analyzed the statistical data; the significance level was P < 0.05.
RESULTS
Solubility in solvents and co-solvents
TA was completely soluble in 40 and 50%w/w of ethanol, whereas it was incompletely soluble in water (Table 1). However, PVA only provided a transparent solution in water with low viscosity. It precipitated immediately when the ethanol was added at 40 and 50 %w/w. Using glycerin and PEG 400 as the additional co-solvents at several weight ratios could completely dissolve TA and provide a transparent PVA solution with low viscosity (Table 1).
Films containing TA
Films containing 0.1 %w/w TA were prepared using the solvent casting method by dissolving TA and PVA in the various ratios of co-solvents. All TA and PVA solutions were transparent with low viscosity. After drying, only the films from the lowest glycerin and PEG 400 content were satisfactory. The dried and transparent films were observed. Otherwise, the transparent but patchy layer film that dries out was found when the glycerin and PEG 400 were increased. Therefore, the optimal co-solvents for film formation of glycerin and PEG 400 systems were ethanol (46.7%w/w)/glycerin (5%w/w)/water (46.3%w/w) and ethanol (43.9%w/w)//PEG 400(5%w/w)/water (51.1%w/w), respectively which was shown as the formulations of films containing TA in Table 2.
Evaluation of films containing TA
The thin, smooth, and transparent films of G and P, 1 cm × 1 cm, were evaluated for weight, thickness, mechanical property, wetting time, mucoadhesive property, and TA content, as shown in Table 3. Based on their solid contents, the films' average weight and thickness ranged from 7.83 to 8.36 mg and 0.061 to 0.065 mm. There were no significant differences in weight and thickness in each film (P > 0.05). Folding endurance, tensile strength, and elongation at break were conducted to determine the film's mechanical properties. Folding endurance provides the flexibility of the films, which is required for easy handling. Excellent folding endurance was demonstrated in both films (>200 times, P > 0.05, Table 3). Tensile strength and elongation at the break of the P film were higher than the G film (P = 0.016 and 0.000, respectively). In addition, P film wetted faster than G film by two times (P = 0.000). However, both films had a similar mucoadhesive property (P > 0.05 for mucoadhesive time and force). Films were attached to the buccal mucosa for a minute. The TA content in the film was expressed as the percentage of weight and the labeled amount. TA content of the two films differed from the theoretical TA content (0.10 %w/w), which was outside the limits of the pharmacopeia range in the TA dental paste and ointment monograph (USP, 2017). Furthermore, the TA content in the P film was significantly lower than in the G film (P = 0.007 and 0.009 for the percentage of weight and the labeled amount, respectively), suggesting that the solution’s viscosity might impact TA settling. Figure 1 shows the in vitro TA release profiles from the P and G films, in which the P film provided faster TA release than the G film, especially in 5 min (P = 0.001). The complete TA release of the G and P films was 60 and 30 min, respectively.
Table 3. Evaluation of films containing TA. * Values are presented as mean ± SD (n = 3). # Statistically significant between G and P film.
|
Evaluation |
Formulation |
|
|
G film |
P film |
|
|
Weight (mg)* |
7.83 ± 2.98 |
8.36 ± 2.66 |
|
Thickness (mm)* |
0.061 ± 0.081 |
0.065 ± 0.015 |
|
Folding endurance (time) |
> 200 |
> 200 |
|
Tensile strenght (N)*,# |
3.92 ± 1.66 |
7.96 ± 1.41 |
|
Young's modulus (Pa)*,# |
7.19 ± 1.03 |
22.60 ± 1.18 |
|
Wetting time (s)*,# |
55.00 ± 3.78 |
28.17 ± 5.34 |
|
Mucoadhesive time (s)* |
65.40 ± 9.29 |
67.40 ± 18.48 |
|
Mucoadhesive force (N)* |
0.32 ± 0.00 |
0.32 ± 0.01 |
|
TA content (%w/w)*,# |
0.14 ± 0.03 |
0.08 ± 0.01 |
|
TA content (% LA)*,# |
144 ± 25 |
81 ± 13 |

Figure 1. TA release profiles from the P and G films. Data are presented as the mean ± SD (n = 3). # Statistically significant between G and P film.
The G and P films were tested for cytotoxicity for 24h. Figure 2 exhibits the cell viability of the various film concentrations. When the cells were incubated with both films at the tested doses (0-4,000 µg/mL), there was no significant reduction in cell viability compared to the non-treated cells. Although there was a continuous decrease in cell viability when the dose of film was increased, particularly in the G film, there was no significant difference between the G and P films at any given dose.
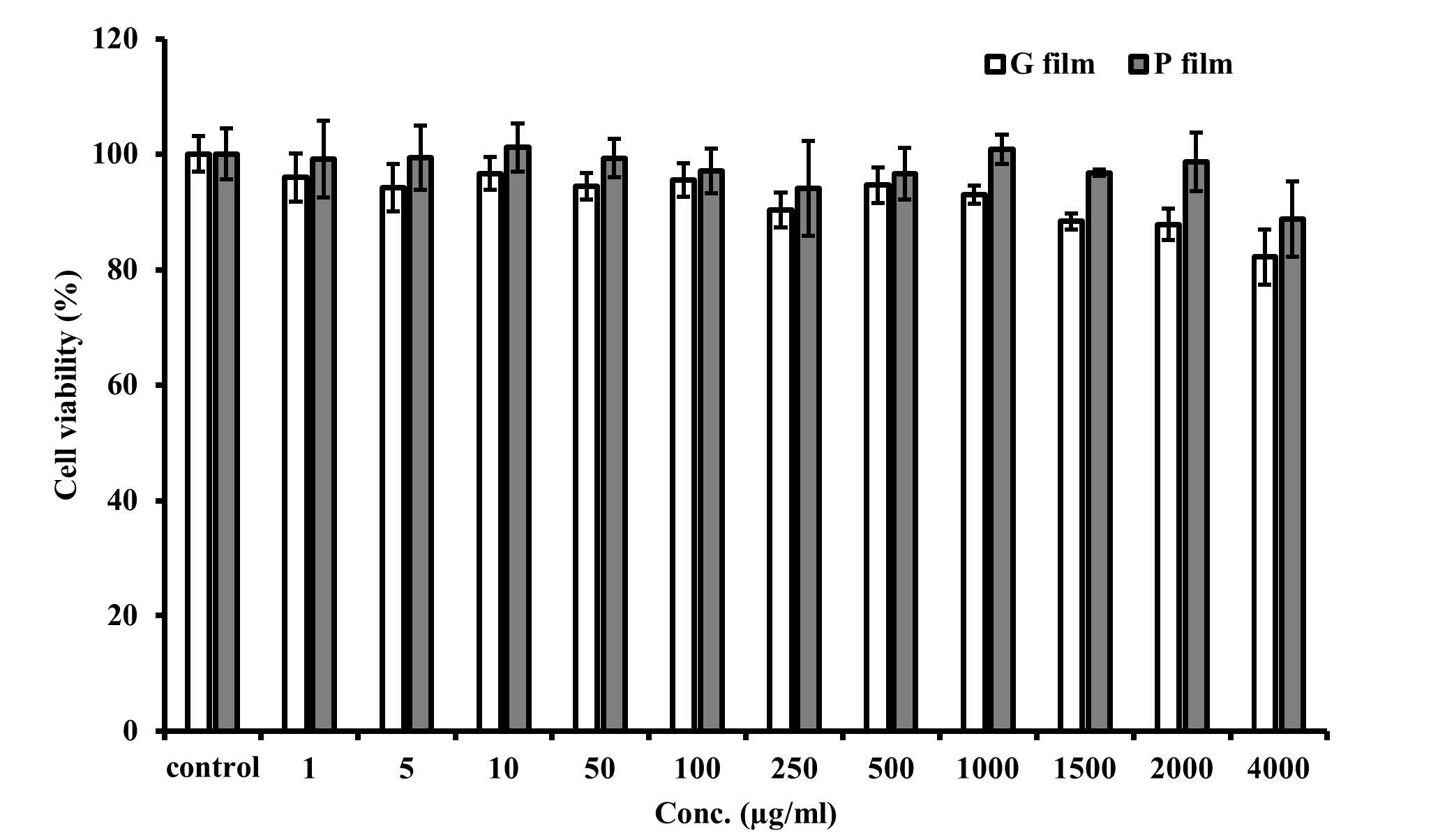
Figure 2. Cytotoxicity of the films to the HGF cells. Data are presented as the mean ± SD (n = 5).
The G and P films were evaluated for the rate of migration of the HGF cells. Graphical data of gap closure rates are shown in Figure 3a. According to the wound healing assay, both films had an inhibitory effect on HGF migration. The migration rate obtained from both films was significantly lower than the control cells after 24, 48, and 72 h of treatment (Figure 3b).
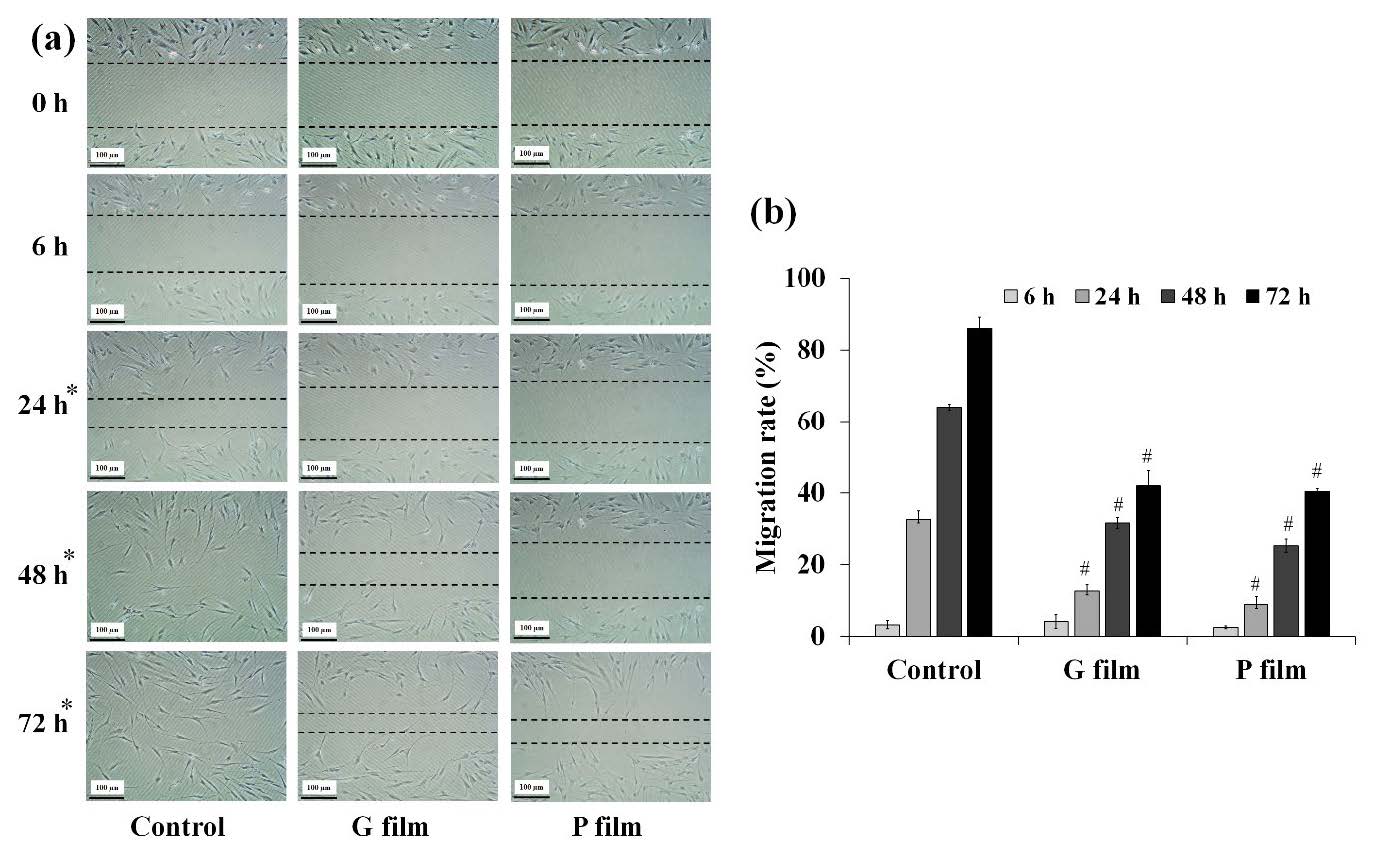
Figure 3. Cell migration in the in vitro scratch assay; (a) Graphical data and (b) time-dependent effects in the gap closure rates after treatment with the control and films. * Time where films had an inhibitory effect on HGF migration and # statistical significance (P < 0.05) with the control cells.
DISCUSSION
TA was usually used as an anti-inflammatory at a concentration of 0.1%w/w. According to low-aqueous-solubility of TA (Block and Patel, 1973) and the nature of PVA that required to dissolve in distilled water at 80°C and then allowing the solution to be stirred for 4 h (Rowe et al., 2009), the solvents and co-solvents need to be found. Glycerin and PEG 400 were added as additional co-solvents to the ethanolic solution to dissolve the TA and produce a transparent PVA solution at the dielectric constant in which the TA was entirely soluble. TA was completely dissolved in any solvents at this dielectric constant, while the PVA was swelling in the aqueous part of co-solvents. There was sufficient water to enable the PVA to swell completely. However, a transparent TA and PVA solution did not ensure that a film would form after drying. Since a high concentration of non-evaporating solvents was left in the films, films were humidified until wet. The lowest concentration of non-evaporating solvents that could dissolve TA and PVA produced a dried, transparent layer in the co-solvent system.
Films made of G and P were found to be similar in weight and thickness. However, it was additionally observed that P film had a slightly higher weight and thickness than G film, which might have been due to the high viscosity of the polymeric solution (Bodiguel and Fretign, 2006). Regarding mechanical properties, the higher folding endurance values of both films indicate the higher mechanical strength of a film (Bala and Sharma, 2018). Tensile strength also refers to the resistance of the films to a tearing force, which is determined by the maximum tension that the films can sustain without tearing (Sudhakar et al., 2006). Young’s modulus indicates the rigidity of the film; a higher value corresponds to a more rigid material (Campo et al., 2016). The glass transition temperature (Tg) and the intermolecular interaction between the polymer chains of films were lowered by incorporating glycerin and PEG 400 as co-solvents and plasticizers after the films had formed (Bala and Sharma, 2018). The hydroxyl group in glycerin and PEG 400 inhibited the intermolecular hydrogen bonding of PVA (Fong et al., 2018). The molecular conformation of glycerin molecules might sterically hinder insertion between the PVA chains, resulting in less effectiveness in disrupting the PVA-PVA interruptions (Wittaya, 2013), tensile strength, and elongation at break of the G film than the P film. However, the previous study reported that the plasticizing efficiencies of glycerin and PEG 400 were similar, although the tensile strength of the film containing PEG 400 was larger than that of the glycerin-containing film (Domján et al., 2009).
The water-soluble polymers of PVA allowed the film to rapidly penetrate SSF pH 6.8, resulting in a rapid wetting of the film (Xiaoqiang et al., 2013). In addition, PEG 400 and glycerin improved the surface hydrophilicity of the film by adsorbing water into the film. After the films were wetted, buccal mucoadhesion occurred. PVA contained the hydroxyl group with favorable features for binding with the mucin by hydrogen bonding (Ikeuchi-Takahashi et al., 2017); therefore, both film’s mucoadhesion time and force were similar. TA content of the two films was defined as the percentage of TA content loaded into the films and the percentage of the labeled amount. Because of the variation and homogeneity of films, there may be a difference in the TA content between the two film formulations. The non-uniform distribution of TA throughout the films might have occurred (Panraksa et al., 2020). TA has very poor solubility in a specific buffer mimicking the oral environment, including SSF pH 6.8; a co-solvent was required to provide the desired sink conditions for TA release. This procedure was in accordance with previous studies that investigated TA release with a co-solvent such as methanol at a concentration of 10% v/v (Abou-ElNour et al., 2019) and ethanol at a concentration of 50% v/v (Hamishehkar et al., 2015). It was clear that a faster TA release was observed from the P film. Many factors, such as wettability, polymer swelling, and mechanical properties of films, might affect the rate and amount of TA release (Borgquist et al., 2006). The rapid wetting time of P film might also result in the rapid TA release. The polymeric network could relax due to the flexibility of the films, allowing for rapid TA diffusion across the matrix, resulting in high and rapid TA diffusion from the films (Tuntiyasawasdikul et al., 2018).
In general, topical dosage forms should be low in cytotoxicity to the contact cells. HGFs were chosen to analyze the cytotoxicity because fibroblasts are essential to wound healing. Exactly, PVA is biocompatible, non-toxic, and non-irritant. Thus, there was no significant reduction in cell viability when the cells were incubated with both films at the investigated concentrations. Moreover, when used as excipients, glycerin and PEG 400 are not usually associated with adverse effects and are generally considered nontoxic and nonirritant materials (Rowe et al., 2009). These results revealed that both films did not affect the cell viability during the 24-hour incubation period, demonstrating excellent biocompatibility. However, TA attenuated the proliferation and migration of cells, including vascular cell proliferation (Otsuka et al., 2023). Our study also indicated that TA delayed the migration of HGF cells. TA-loaded G and P films delayed the growth of HGF cells, with a 50% reduction in migration, compared to control at 72 h. Steroids hinder fibroblast growth, which leads to insufficient granulation tissue healing (Chhabra et al., 2017), which is negative for wound healing. Nonetheless, topical low-dose corticosteroids accelerated wound healing and reduced pain in 79% of clinical treatments (Chhabra et al., 2017).
CONCLUSION
TA-loaded PVA films were successfully prepared by the solvent casting method. TA and PVA were dissolved in co-solvents of 5%w/w glycerin or 5%w/w PEG 400 and aqueous ethanolic solution; the dielectric constant was adjusted to 51.4. In addition to acting as co-solvents, glycerin and PEG 400 additionally functioned as plasticizers once the film had formed. The G and P films were clear and colorless, with a smooth surface and similar mucoadhesive properties. However, P film had higher mechanical properties and faster wetting time than G film, resulting in a faster TA release. Both films were non-toxic toward the HGF with the delay in the wound healing process. In summary, co-solvents created beneficial features of mucoadhesive films and increased TA solubility.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the College of Pharmacy, Rangsit University for the materials and laboratory facilities support. We also thank Ms. Patcharaphan Theeranithi, Ms. Wichayanil Lertthawinphadee, and Ms. Punchanit Wilaiwan as the research assistants on preparation and physical evaluation of films.
AUTHOR CONTRIBUTIONS
Benchawan Chamsai assisted in conducting the experiments, reviewed and edited the manuscript. Chutima Sinsuebpol provided material and reagents, reviewed and edited the manuscript. Praneet Opanasopit acquired the financial support and provided the cell experimental tools. Wipada Samprasit designed all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abou-ElNour, M., Ishak, R.A.H., Tiboni, M., Bonacucina, G., Cespi, M., Casettari, L., Soliman, M.E., and Geneidi, A.S. 2019. Triamcinolone acetonide-loaded PLA/PEG-PDL microparticles for effective intra-articular delivery: Synthesis, optimization, in vitro and in vivo evaluation. Journal of Controlled Release. 309: 125–144.
Ahn, J.S., Choi, H.K., Chun, M.K., Ryu, J.M., Jung, J.H., Kim, Y.U., and Cho, C.S. 2002. Release of triamcinolone acetonide from mucoadhesive polymer composed of chitosan and poly(acrylic acid) in vitro. Biomaterials. 23: 1411–1416.
Alhallak, M., Karpukhina, N., and Patel, M. 2023. Triamcinolone acetonide release modelling from novel bilayer mucoadhesive films: An in vitro study. Dental Materials. 39(6): 595–602.
Babu, P.R., Subrahmanyam, C.V., Thimmasetty, J., Manavalan, R., Valliappan, K., and Kedarnath, S. 2008. Solubility enhancement of Cox-II inhibitors by cosolvency approach. Dhaka University Journal of Pharmaceutical Sciences. 7(2):119-126.
Bala, R. and Sharma, S. 2018. Formulation optimization and evaluation of fast dissolving film of aprepitant by using design of experiment. Bulletin of Faculty of Pharmacy, Cairo University. 56: 159–168.
Block, L.H. and Patel, R.N. 1973. Solubility and dissolution of triamcinolone acetonide. Journal of Pharmaceutical Sciences. 62: 617-621.
Bodiguel, H. and Fretign, C. 2006. Reduced viscosity in thin polymer films. Physical Review Letters. 97: 266105.
Borgquist, P., Korner, A., Piculell, L., Larsson, A. and Axelsson, A. 2006. A model for the drug release from a polymer matrix tablet-effects of swelling and dissolution. Journal of Controlled Release. 113: 216–225.
Campo, C. de., Costa, T. M. H., Rios, A. de O., and Flôres, S. H. 2016. Effect of incorporation of nutraceutical capsule waste of safflower oil in the mechanical characteristics of corn starch films. Food Science and Technology. 36: 33–36.
Chanburee, S. and Tiyaboonchai, W. 2017. Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Development and Industrial Pharmacy. 43: 432–440.
Chhabra, S., Chhabra, N., Kaur, A., and Gupta, N. 2017. Wound healing concepts in clinical practice of OMFS. Journal of Maxillofacial and Oral Surgery, 16(4): 403–423.
Chinna Reddy, P., Chaitanya, K. S., and Madhusudan Rao, Y. 2011. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru journal of Faculty of Pharmacy, Tehran University of Medical Sciences, 19(6), 385–403.
Domján, A., Bajdik, J., and Pintye-Hódi, K. 2009. Understanding of the plasticizing effects of glycerol and PEG 400 on chitosan films using solid-state NMR spectroscopy. Macromolecules. 42(13): 4667-4673.
Dubashynskaya, N.V. and Skorik, Y.A. 2022. Patches as polymeric systems for improved delivery of topical corticosteroids: Advances and future perspectives. International Journal of Molecular Sciences. 23: 12980.
Fong, R.J., Robertson, A., Mallon, P.E., and Thompson, R.L. 2018. The impact of plasticizer and degree of hydrolysis on free volume of poly(vinyl alcohol) films. Polymers. 10: 1036.
Gasmi Benahmed, A., Noor, S., Menzel, A., and Gasmi, A. 2021. Oral aphthous: pathophysiology, clinical aspects and medical treatment. Archives of Razi Institute. 76(5), 1155–1163.
Hamishehkar, H., Nokhodchi, A., Ghanbarzadeh, S., and Kouhsoltani, M. 2015. Triamcinolone acetonide oromucoadhesive paste for treatment of Aphthous stomatitis. Advanced pharmaceutical bulletin. 5(2): 277–282.
Ikeuchi-Takahashi, Y., Ishihara, C., and Onishi, H. 2017. Evaluation of polyvinyl alcohols as mucoadhesive polymers for mucoadhesive buccal tablets prepared by direct compression. Drug Development and Industrial Pharmacy. 43(9): 1489-1500.
Morales, J.O. and McConville, J.T. 2011. Manufacture and characterization of mucoadhesive buccal films. European Journal of Pharmaceutics and Biopharmaceutics. 77: 187–199.
Otsuka, T., Masuda, T., Takahashi, Y., Suzuki, A., Uemura, A., Arakawa, R., Okabe, T., and Naito, A. 2023. Effect of triamcinolone acetonide on retinal inflammation and angiogenesis induced by pericyte depletion in mouse. Journal of Pharmacological Sciences. 151(1): 28-36.
Panraksa, P., Tipduangta, P., Jantanasakulwong, K., and Jantrawut, P. 2020. Formulation of orally disintegrating films as an amorphous solid solution of a poorly water-soluble drug. Membranes. 10(12): 376.
Prakongpan, S. and Nagai, T. 1984. Solubility of acetaminophen in cosolvents. Chemical and Pharmaceutical Bulletin. 32: 340–343.
Rowe, R.D., Sheskey, P.J., and Owen, S.C. 2009. Handbook of pharmaceutical excipients, 6th ed., Pharmaceutical Press, United Kingdom.
Şen, T., Amasya, G., and Tarimci, N. 2015. Triamcinolone acetonide buccal bilayered discs for treatment of erosive oral lichen planus: Design and in vitro characterization. Turkish Journalof Pharmaceutical Sciences. 12(2): 137.
Sudhakar, Y., Kuotsu, K., and Bandyopadhyay, A.K. 2016. Buccalbioadhesive drug delivery-A promising option for orally less efficient drugs. Journal of Controlled Release. 114: 15–40.
Tuntiyasawasdikul, S., Limpongsa, E., Jaipakdee, N., and Sripanidkulchai, B. 2018. Development and evaluation of topical films containing phytoestrogenic diaryheptanoids from Curcuma comosa extract. Drug Development and Industrial Pharmacy. 44: 1385–1394.
USP The United States Pharmacopeia 41/National Formulation 36. Rockville (MD): The United States Pharmacopoeial Convention; 2018.
Wittaya, T. 2013. Influence of Type and concentration of plasticizers on the properties of edible film from mung bean proteins. KMITL Science and Technology Journal. 13(1): 51-58.
Xiaoqiang, L., Kanjwal, M.A., Lin, L., and Chronakis, I.S. 2013. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids and Surfaces B: Biointerfaces. 103: 182–188.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Benchawan Chamsai1, Chutima Sinsuebpol1, Praneet Opanasopit2 and Wipada Samprasit1, *
1 Department of Pharmaceutical Technology, College of Pharmacy, Rangsit University, Pathum Thani, 12000, Thailand.
2 Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, 73000, Thailand.
Corresponding author: Wipada Samprasit, E-mail: wipada.s@rsu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 3, 2023;
Revised: November 21, 2023;
Accepted: January 9, 2024;
Online First: January 15, 2024

